Optimization of High-Temperature Electrolysis System for Hydrogen Production Considering High-Temperature Degradation
Abstract
1. Introduction
- A pseudo-dynamic model of SOEC degradation is established and embedded in the HTSE system, which takes into account the high-temperature degradation of typical SOEC materials.
- The effects of the key operating conditions on the hydrogen production efficiency and degradation were studied such as the operating temperature, current density, air-to-fuel feed ratio, etc.
- An optimization was carried out to propose the operation strategies to balance the hydrogen production efficiency and the stack life span.
2. Model Development of SOEC Degradation
- All gas flows are considered as the ideal gases.
- Only the degradation due to the stack materials changes at the high temperature condition is considered, while the mass or heat accumulation inside the cell is not considered.
- The governing equations to describe the structural degradation are only available for specified SOEC materials.
- A planar SOEC stack consists of many single cells, which are regarded as unit cells with the same performance.
2.1. Equilibrium Potential
2.2. Cathode Overpotentials
2.3. Anode Overpotentials
2.4. Electrolyte Overpotentials
2.5. Mass and Heat Balance
3. Simulation
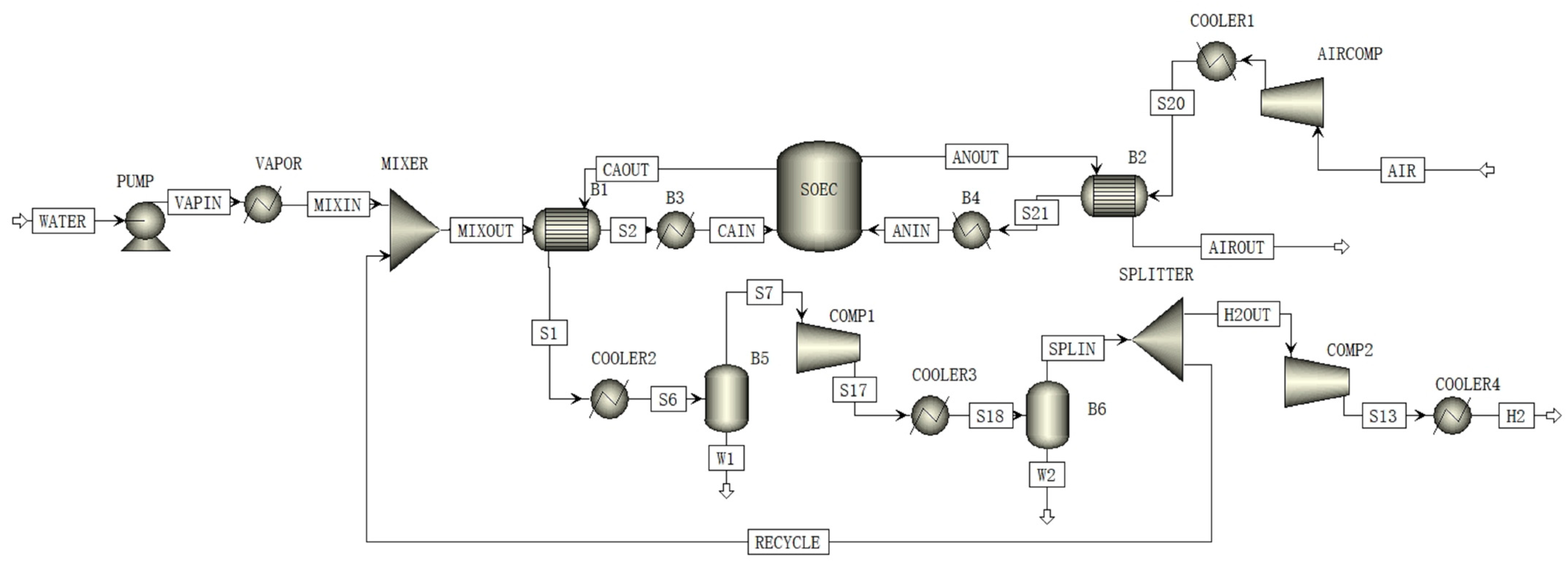
3.1. Process Description and Simulation
3.2. Optimization
- Update the structural parameters of the SOEC stack. The structural parameters (i.e., , and ) at the end of the previous degradation period should be assigned as the structural parameters of the SOEC stack in the current period.
- Perform the optimization. The optimal decision variables for the new degradation period are solved by the MOPSO algorithm.
- Carry out the simulation of the next period. The simulation of the new degradation period is carried out by the new decision variables and structural parameters, and the new structural parameters for the succeeding period are calculated. Repeat steps 1 to 2 until the optimization process is over.
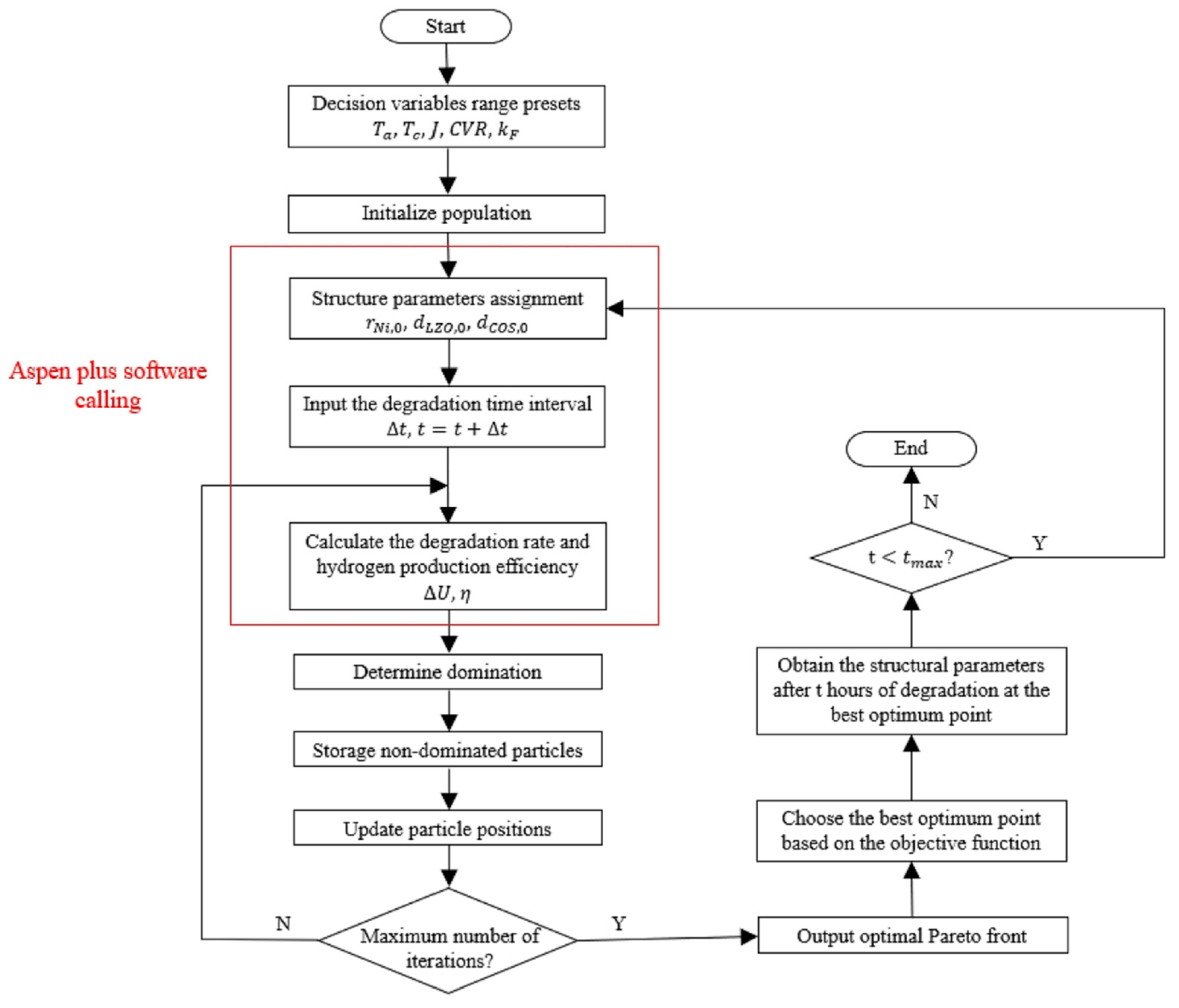
4. Results and Discussion
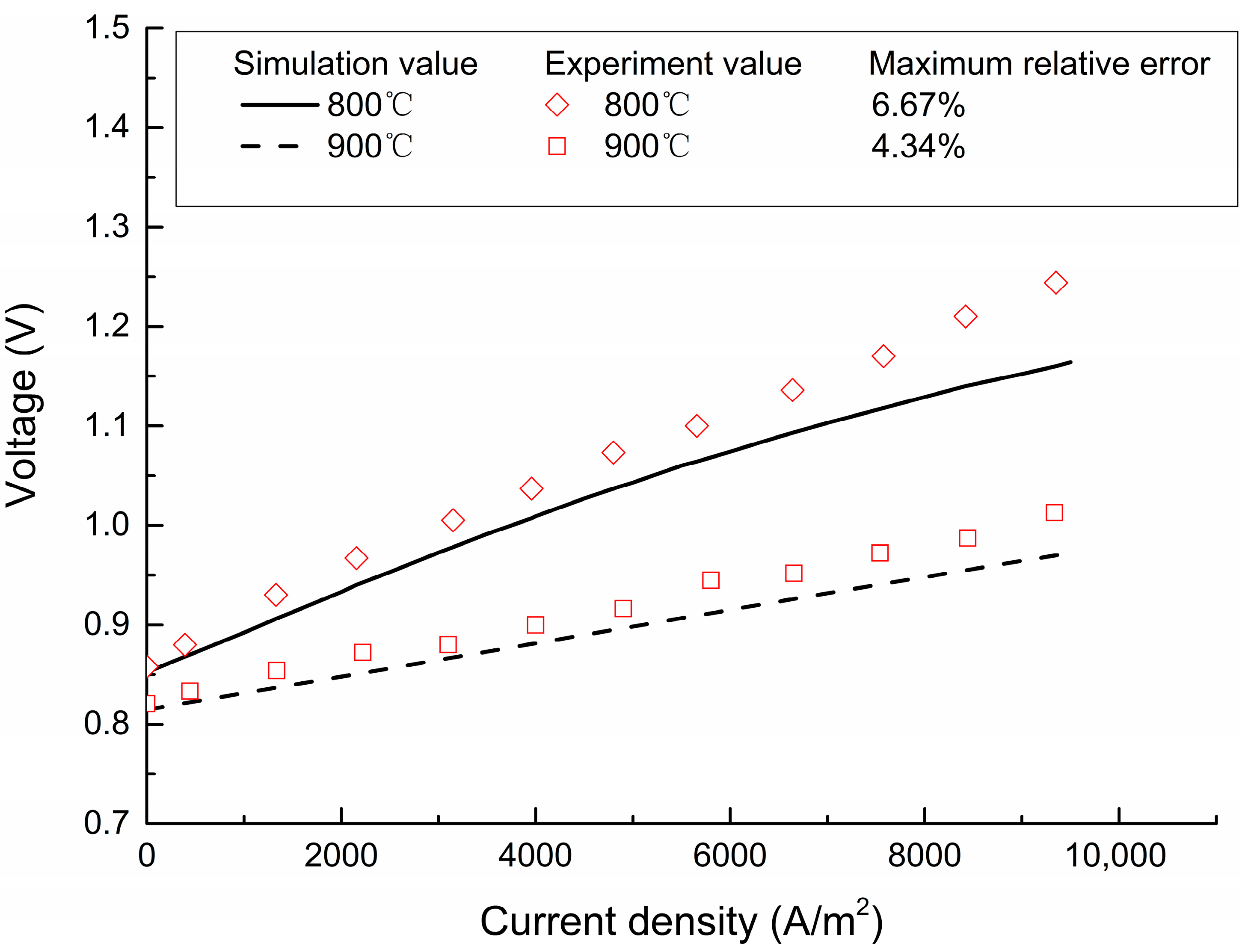
4.1. Model Validation and Analysis
4.2. Sensitivity Analysis
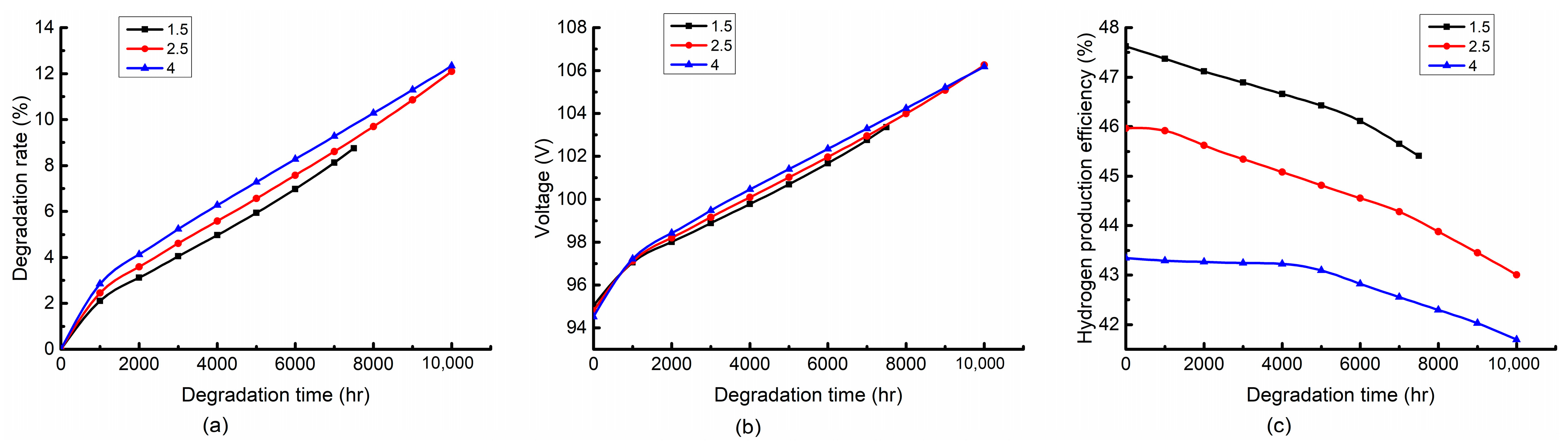
4.2.1. Effect of Inlet Temperature
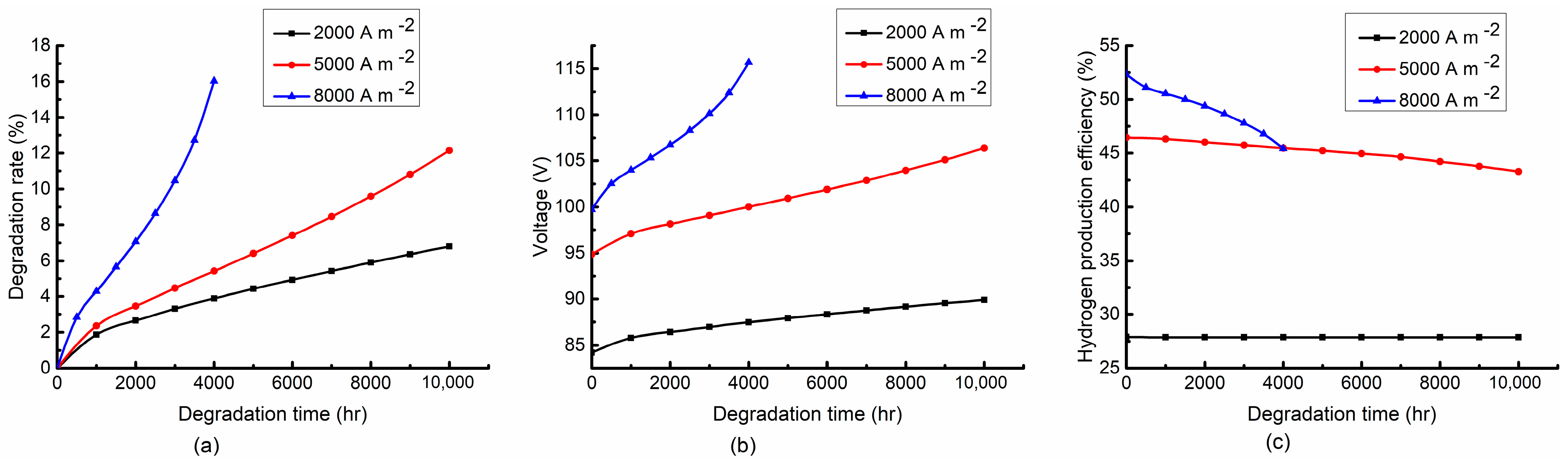
4.2.2. Effect of Current Density
4.2.3. Effect of Air-to-Fuel Feed Ratio
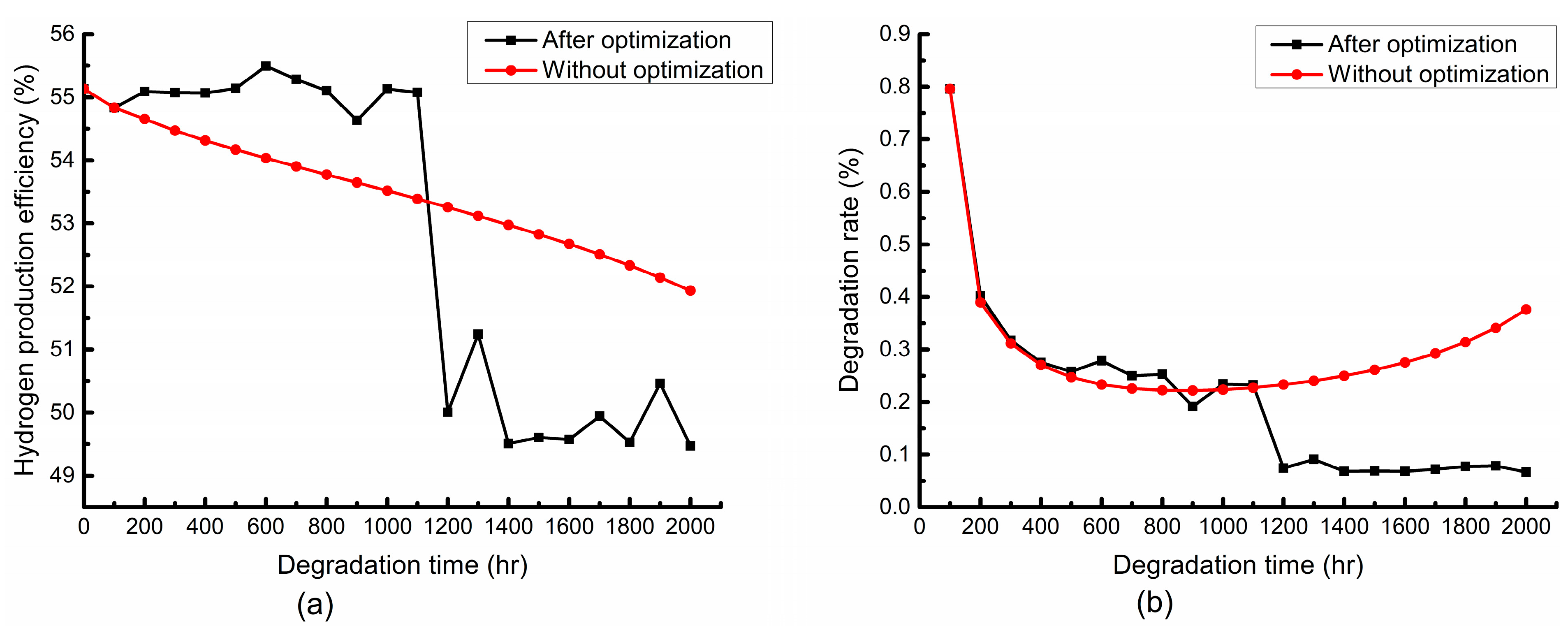
4.3. Multiobjective Optimization
5. Conclusions
- (1)
- The high temperature accelerates the stack degradation. However, it is favorable to the hydrogen production efficiency at the early stage of the degradation process and gradually becomes unfavorable in the late stage. The high current density can improve the hydrogen production efficiency while accelerating the stack degradation. The air-to-fuel feed ratio has a slight effect on the degradation rate and stack voltage, while it has a significant effect on the hydrogen production efficiency. A low air-to-fuel feed ratio is beneficial to the degradation rate and hydrogen production efficiency.
- (2)
- Compared with the nonoptimization, the hydrogen production efficiency after the optimization is significantly larger when taking the hydrogen production efficiency as the objective in the early stage. The degradation rate is significantly lower than that without the optimization when taking the degradation rate into consideration in the late stage. Part of the hydrogen production efficiency has to be sacrificed in order to obtain a lower stack degradation rate.
- (3)
- Compared with the nonoptimization, the structural degradation after the optimization is more obvious when taking the hydrogen production efficiency as the objective in the early stage, while it decreases to be less than those without the optimization when taking the degradation rate into consideration in the late stage.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, N.; Wang, M.; Shen, Q.; Teng, Y.; Wang, D.; Chen, C.; Zhan, Z. Reduced concentration polarization and enhanced steam throughput conversion with a solid oxide electrolysis cell supported on an electrode with optimized pore structure. Int. J. Hydrogen Energy 2022, 47, 21673–21680. [Google Scholar] [CrossRef]
- Navasa, N.; Yuan, J.; Sundén, B. Computational fluid dynamics approach for performance evaluation of a solid oxide electrolysis cell for hydrogen production. Appl. Energy 2015, 137, 867–876. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A. Recent advances in high temperature electrolysis using solid oxide fuel cells: A review. J. Power Sources 2012, 203, 4–16. [Google Scholar] [CrossRef]
- Hauch, A.; Küngas, R.; Blennow, P.; Hansen, A.B.; Hansen, J.B.; Mathiesen, B.V.; Mogensen, M.B. Recent advances in solid oxide cell technology for electrolysis. Science 2020, 370, 954. [Google Scholar] [CrossRef]
- Hattori, M.; Takeda, Y.; Sakaki, Y.; Nakanishi, A.; Ohara, S.; Mukai, K.; Lee, J.H.; Fukui, T. Effect of aging on conductivity of yttria stabilized zirconia. J Power Sources 2004, 126, 23–27. [Google Scholar] [CrossRef]
- Yan, Y.; Fang, Q.; Blum, L.; Lehnert, W. Performance and degradation of an SOEC stack with different cell components. Electrochim. Acta 2017, 258, 1254–1261. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Q.; Chen, T.; Wu, W.; Xu, C.; Wang, W.G. Comparison of performance and degradation of large-scale solid oxide electrolysis cells in stack with different composite air electrodes. Int. J. Hydrogen Energy 2015, 40, 2460–2472. [Google Scholar] [CrossRef]
- Wolf, S.E.; Vibhu, V.; Tröster, E.; Vinke, I.C.; Eichel, R.-A.; da Haart, L.G.J. Steam electrolysis vs. co-electrolysis: Mechanistic studies of long-term solid oxide electrolysis cells. Energies 2022, 15, 5449. [Google Scholar] [CrossRef]
- Vibhu, V.; Vinke, I.C.; Zaravelis, F.; Neophytides, S.G.; Niakolas, D.K.; Eichel, R.-A.; de Haart, L.G.J. Performance and degradation of electrolyte-supported single cell composed of Mo-Au-Ni/GDC fuel electrode and LSCF oxygen electrode during high temperature steam electrolysis. Energies 2022, 15, 2726. [Google Scholar] [CrossRef]
- Kim-Lohsoontorn, P.; Brett, D.; Laosiripojana, N.; Kim, Y.; Bae, J. Performance of solid oxide electrolysis cells based on composite La0.8Sr0.2MnO3−δ-yttria stabilized zirconia and Ba0.5Sr0.5Co0.8Fe0.2O3−δ oxygen electrodes. Int. J. Hydrogen Energy 2010, 35, 3958–3966. [Google Scholar] [CrossRef]
- Zhang, X.; O’Brien, J.E.; O’Brien, R.C.; Housley, G.K. Durability evaluation of reversible solid oxide cells. J. Power Sources 2013, 242, 566–574. [Google Scholar] [CrossRef]
- Hoerlein, M.P.; Schiller, G.; Tietz, F.; Friedrich, K.A. Systematic Parameter Study on the Influence of Humidification and Current Density on SOEC Degradation. Meet. Abstr. 2015, 68, 3553–3561. [Google Scholar] [CrossRef]
- Jacobsen, T.; Mogensen, M. The Course of Oxygen Partial Pressure and Electric Potentials across an Oxide Electrolyte Cell. ECS Trans. 2008, 13, 259–273. [Google Scholar] [CrossRef]
- Virkar, A.V. Mechanism of oxygen electrode delamination in solid oxide electrolyzer cells. Int. J. Hydrogen Energy 2010, 35, 9527–9543. [Google Scholar] [CrossRef]
- Jensen, S.H.; Hauch, A.; Knibbe, R.; Jacobsen, T.; Mogensen, M. Modeling degradation in SOEC impedance spectra. J. Electrochem. Soc. 2013, 160, F244–F250. [Google Scholar] [CrossRef]
- Kamkeng, A.; Wang, M. Long-term performance prediction of solid oxide electrolysis cell (SOEC) for CO2/H2O co-electrolysis considering structural degradation through modelling and simulation. Chem. Eng. J. 2022, 429, 132158. [Google Scholar] [CrossRef]
- AlZahrani, A.A.; Dincer, I. Modeling and performance optimization of a solid oxide electrolysis system for hydrogen production. Appl. Energy 2018, 225, 471–485. [Google Scholar] [CrossRef]
- Xing, X.; Lin, J.; Song, Y.; Hu, Q.; Zhou, Y.; Mu, S. Optimization of hydrogen yield of a high-temperature electrolysis system with coordinated temperature and feed factors at various loading conditions: A model-based study. Appl. Energy 2018, 232, 368–385. [Google Scholar] [CrossRef]
- Min, G.; Park, Y.J.; Choi, S.; Hong, J. Sensitivity analysis of a solid oxide co-electrolysis cell system with respect to its key operating parameters and optimization with its performance map. Energy Convers. Manag. 2021, 249, 114848. [Google Scholar] [CrossRef]
- Li, G.; Xiao, G.; Guan, C.; Hong, C.; Yuan, B.; Li, T.; Wang, J.Q. Assessment of thermodynamic performance of a 20 kW high-temperature electrolysis system using advanced exergy analysis. Fuel Cells 2021, 21, 550–565. [Google Scholar] [CrossRef]
- Prabhakaran, P.; Giannopoulos, D.; Köppel, W.; Mukherjee, U.; Remesh, G.; Graf, F.; Trimis, D.; Kolb, T.; Founti, M. Cost optimisation and life cycle analysis of SOEC based Power to Gas systems used for seasonal energy storage in decentral systems. J Energy Storage 2019, 26, 100987. [Google Scholar] [CrossRef]
- Cai, Q.; Adjiman, C.S.; Brandon, N.P. Optimal control strategies for hydrogen production when coupling solid oxide electrolysers with intermittent renewable energies. J. Power Sources 2014, 268, 212–224. [Google Scholar] [CrossRef]
- Chen, C.; Xia, Q.; Feng, S.; Liu, Q. A novel solar hydrogen production system integrating high temperature electrolysis with ammonia based thermochemical energy storage. Energy Convers. Manag. 2021, 237, 114143. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, J.; Liu, Q.; Shuai, W.; Sun, A.; Zheng, N.; Han, Y.; Xiao, G.; Xuan, J.; Ni, M.; et al. Multi-objective optimizations of solid oxide co-electrolysis with intermittent renewable power supply via multi-physics simulation and deep learning strategy. Energy Convers. Manag. 2022, 258, 115560. [Google Scholar] [CrossRef]
- Mohammadpour, M.; Houshfar, E.; Ashjaee, M. Sustainability analysis and optimization of innovative geothermal-driven energy storage system for green production of H2, NH3, and pure O2. Int. J. Hydrogen Energy 2022, 47, 26156–26177. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.; Leung, D.Y. Parametric study of solid oxide steam electrolyzer for hydrogen production. Int. J. Hydrogen Energy 2007, 32, 2305–2313. [Google Scholar] [CrossRef]
- Faes, A.; Hessler-Wyser, A.; Presvytes, D.; Vayenas, G.G.; Vanherle, J. Nickel-Zirconia anode degradation and triple phase boundary quantification from microstructural analysis. Fuel Cells 2010, 9, 841–851. [Google Scholar] [CrossRef]
- Larrain, D.; Van Herle, J.; Favrat, D. Simulation of SOFC stack and repeat elements including interconnect degradation and anode reoxidation risk. J. Power Sources 2006, 161, 392–403. [Google Scholar] [CrossRef]
- Schiller, G.; Ansar, A.; Patz, O. High temperature water electrolysis using metal supported solid oxide electrolyser cells (SOEC). J. Appl. Electrochem. 2009, 39, 293–301. [Google Scholar] [CrossRef]
- Cai, Q.; Luna-Ortiz, E.; Adjiman, C.S.; Brandon, N.P. The Effects of Operating Conditions on the Performance of a Solid Oxide Steam Electrolyser: A Model-Based Study. Fuel Cells 2010, 10, 1114–1128. [Google Scholar] [CrossRef]
- Brisse, A.; Schefold, J.; Zahid, M. High temperature water electrolysis in solid oxide cells. Int. J. Hydrogen Energy 2008, 33, 5375–5382. [Google Scholar] [CrossRef]




| Parameter | Value |
|---|---|
| Cathode inlet gas composition: H2O/H2 (%mol) | 90/10 |
| Anode inlet gas composition: O2/N2 (%mol) | 21/79 |
| Operating temperature (°C) | 750 |
| Operating pressure (bar) | 1.05 |
| Cathode thickness (m) | 3.2 × 10−4 |
| Anode thickness (m) | 1.0 × 10−5 |
| Electrolyte thickness (m) | 1.5 × 10−5 |
| Number of single cells | 75 |
| Cell sizes (m2) | 0.12 × 0.12 |
| YSZ surface area (m2 g−1) | 0.41 [16] |
| Initial Ni radius (m) | 4.5 × 10−6 [16] |
| Initial YSZ radius (m) | 4 × 10−6 [16] |
| Volume Fraction: Ni/YSZ | 40/60 [16] |
| Volume Fraction: LSM/YSZ | 50/50 [16] |
| LSM surface diffusion (cm−2 h−1) | 1.12 × 10−5 [16] |
| Ionic radius (Å) | 1.01 [16] |
| YSZ coordination number | 6 [16] |
| LZO density (g cm−3) | 6.05 [16] |
| COS density (g cm−3) | 5.255 [16] |
| Activation energy for sintering (J/mol) | 3.32 × 105 [16] |
| Decision Variable | Lower Bound | Upper Bound |
|---|---|---|
| (°C) | 700 | 800 |
| (°C) | 700 | 800 |
| (A/m2) | 2000 | 8000 |
| 1 | 4 |
| Degradation Time (h) | Ta (°C) | Tc (°C) | J (A/m2) | kF | η (%) | ΔU (%) | U (V) |
|---|---|---|---|---|---|---|---|
| 100 | 721.68 | 721.68 | 8000.0 | 1.5 | 54.83 | 0.795 | 101.14 |
| 200 | 741.94 | 741.94 | 8000.0 | 1.5 | 55.09 | 0.402 | 100.38 |
| 300 | 746.98 | 746.98 | 8000.0 | 1.5 | 55.07 | 0.317 | 100.41 |
| 400 | 751.81 | 751.81 | 8000.0 | 1.5 | 55.07 | 0.275 | 100.41 |
| 500 | 760.34 | 760.34 | 8000.0 | 1.5 | 55.14 | 0.258 | 100.18 |
| 600 | 783.44 | 783.44 | 8000.0 | 1.5 | 55.49 | 0.279 | 99.15 |
| 700 | 777.47 | 777.47 | 8000.0 | 1.5 | 55.28 | 0.250 | 99.74 |
| 800 | 783.60 | 783.60 | 8000.0 | 1.6 | 55.10 | 0.253 | 99.71 |
| 900 | 752.23 | 752.23 | 8000.0 | 1.5 | 54.63 | 0.191 | 101.59 |
| 1000 | 782.39 | 782.39 | 8000.0 | 1.5 | 55.13 | 0.234 | 100.14 |
| 1100 | 784.07 | 784.07 | 8000.0 | 1.5 | 55.07 | 0.233 | 100.28 |
| 1200 | 700.00 | 700.00 | 6624.8 | 1.5 | 50.00 | 0.075 | 104.11 |
| 1300 | 725.34 | 725.34 | 6800.3 | 1.5 | 51.24 | 0.091 | 102.66 |
| 1400 | 700.00 | 700.00 | 6442.0 | 1.5 | 49.51 | 0.068 | 104.14 |
| 1500 | 700.00 | 700.00 | 6500.4 | 1.5 | 49.61 | 0.069 | 104.25 |
| 1600 | 700.00 | 700.00 | 6500.4 | 1.5 | 49.57 | 0.068 | 104.33 |
| 1700 | 700.00 | 700.00 | 6682.5 | 1.5 | 49.94 | 0.072 | 104.53 |
| 1800 | 746.16 | 746.16 | 5882.5 | 1.5 | 49.53 | 0.078 | 100.62 |
| 1900 | 700.00 | 700.00 | 6965.4 | 1.5 | 50.46 | 0.079 | 104.89 |
| 2000 | 700.00 | 700.00 | 6521.4 | 1.5 | 49.47 | 0.067 | 104.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Li, Z.; Yuan, B.; Xiao, G.; Li, T.; Wang, J.-Q. Optimization of High-Temperature Electrolysis System for Hydrogen Production Considering High-Temperature Degradation. Energies 2023, 16, 2616. https://doi.org/10.3390/en16062616
Yuan J, Li Z, Yuan B, Xiao G, Li T, Wang J-Q. Optimization of High-Temperature Electrolysis System for Hydrogen Production Considering High-Temperature Degradation. Energies. 2023; 16(6):2616. https://doi.org/10.3390/en16062616
Chicago/Turabian StyleYuan, Jiming, Zeming Li, Benfeng Yuan, Guoping Xiao, Tao Li, and Jian-Qiang Wang. 2023. "Optimization of High-Temperature Electrolysis System for Hydrogen Production Considering High-Temperature Degradation" Energies 16, no. 6: 2616. https://doi.org/10.3390/en16062616
APA StyleYuan, J., Li, Z., Yuan, B., Xiao, G., Li, T., & Wang, J.-Q. (2023). Optimization of High-Temperature Electrolysis System for Hydrogen Production Considering High-Temperature Degradation. Energies, 16(6), 2616. https://doi.org/10.3390/en16062616






