Feasibility of the Production of Argemone pleiacantha Ultrasound-Assisted Biodiesel for Temperate and Tropical Marginal Areas
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Raw Material
2.1.2. Reagents for Fatty Acid Composition, Transesterification and Analysis of Both Oil and Biodiesel
2.1.3. Equipment to Characterize Oil and Biodiesel Samples
2.1.4. Heater–Stirrer
2.1.5. Ultrasonic Probe
2.1.6. Equipment for Transesterification Energy Consumption Studies
2.1.7. Chromatographic Analysis
2.2. Methods
2.2.1. Location, Seeding and Harvesting
2.2.2. Grain Milling, Moisture Determination and Oil Extraction
2.2.3. Fatty Acid Characterization
2.2.4. Characterization of Oil and Biodiesel Samples
2.2.5. Acid-Catalyzed Esterification Pretreatment
2.2.6. Ultrasonicated Transesterification
2.2.7. Conventional Transesterification
2.2.8. Energy and Power Measurements
2.2.9. Response Surface Methodology Modeling and Desirability Function as Optimization Method
3. Results
3.1. Chicalote Oilseed Yield, Fatty Acid Composition and Oil Characterization
3.2. Previous Acid Esterification
3.3. Basic Transesterification
3.3.1. Ultrasonicated Transesterification
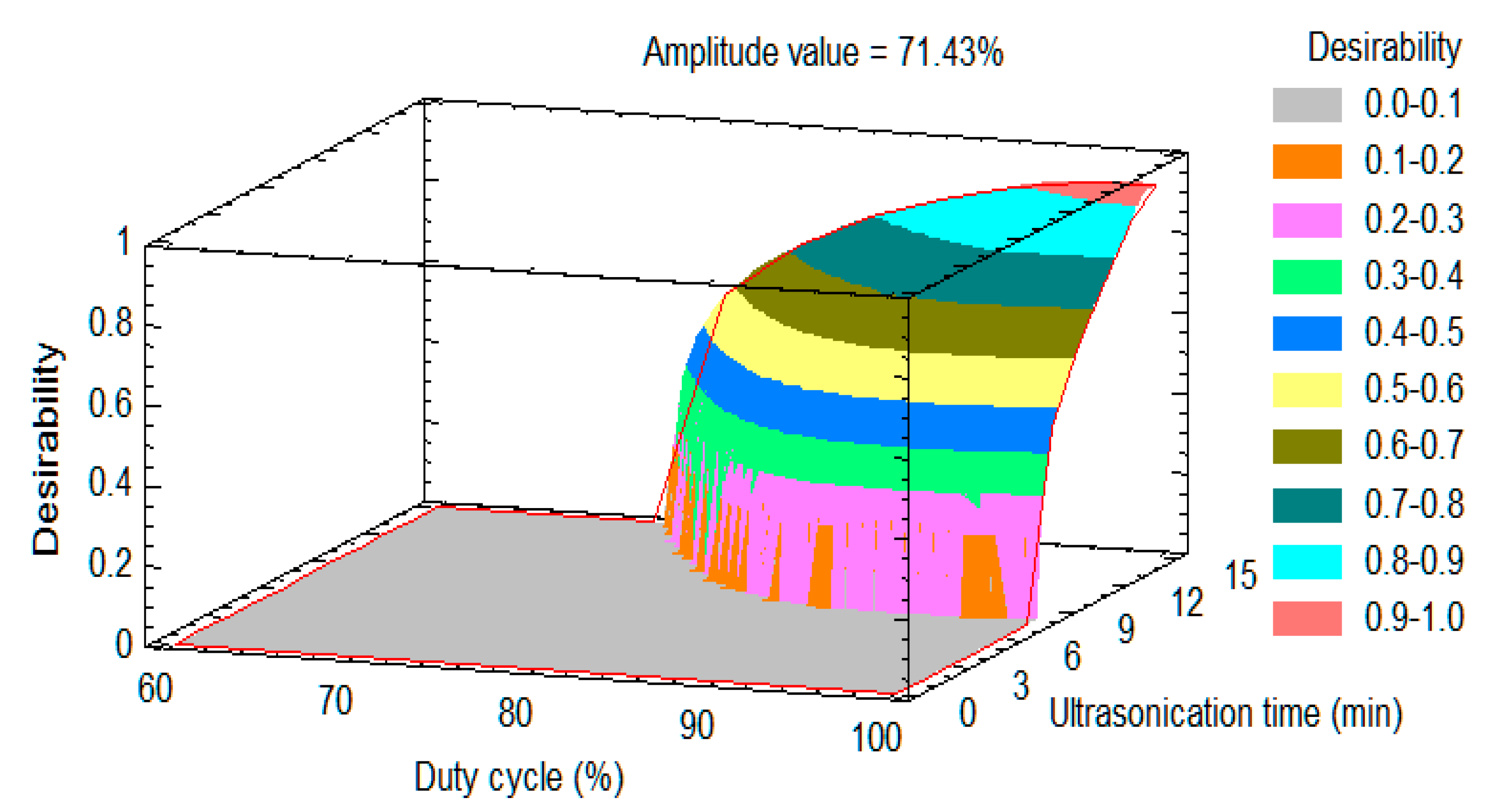
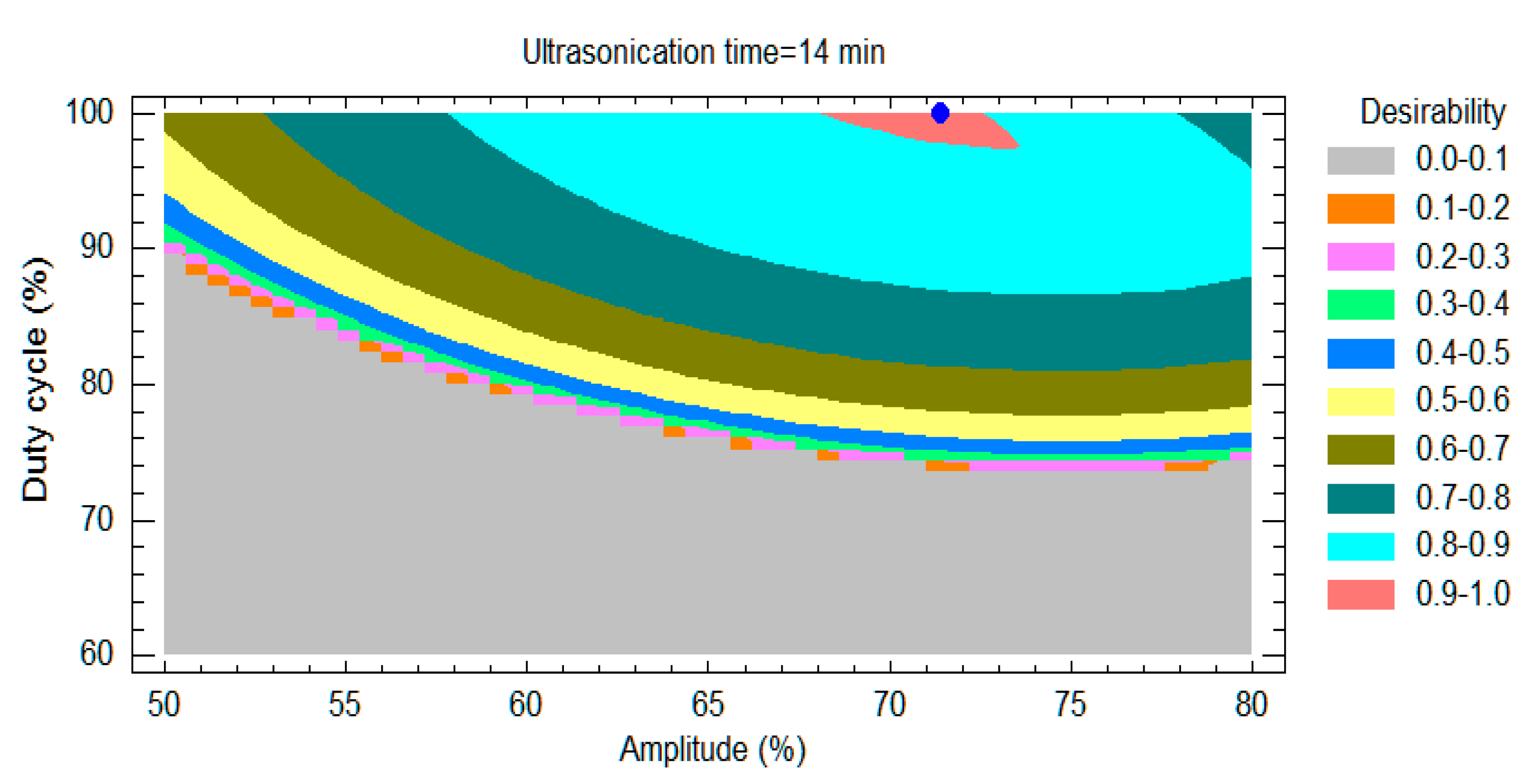
- Response surface modeling
- Desirability approach
- The desirability function converts an estimated response into a scale-free value. Its value varies between 0 and 1 and increases as the corresponding response value becomes more desirable. The overall desirability, D (0 and 1) is defined by combining individual desirability values. The optimal setting is determined by maximizing D. The calculation for this function is expressed in Equation (1).
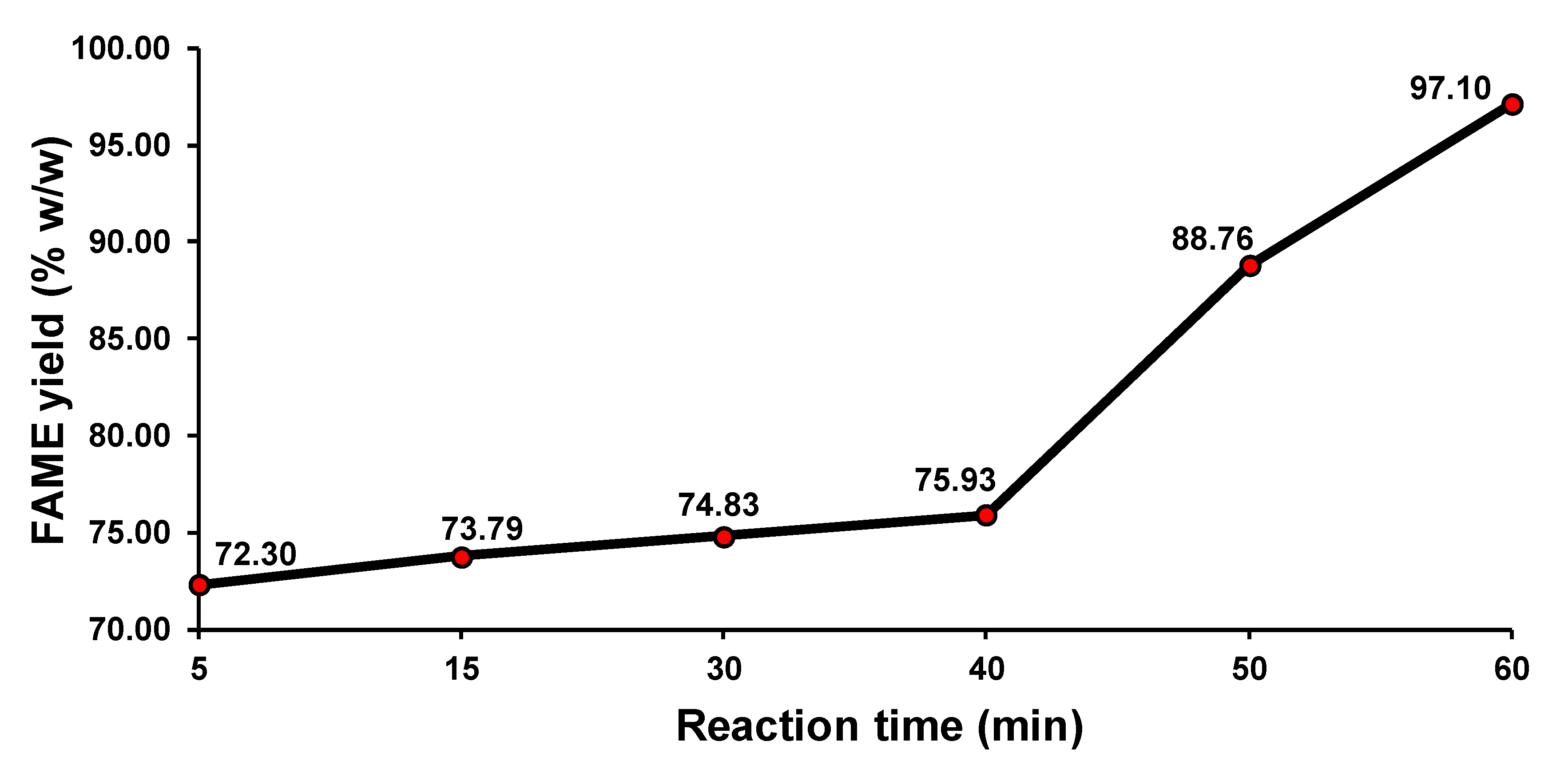
3.3.2. Conventional Transesterification
3.3.3. Energy Consumption Study and Regulated Quality Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costarrosa, L.; Leiva-Candia, D.E.; Cubero-Atienza, A.J.; Ruiz, J.J.; Dorado, M.P. Optimization of the transesterification of waste cooking oil with Mg-Al hydrotalcite using response surface methodology. Energies 2018, 11, 302. [Google Scholar] [CrossRef]
- Sáez-Bastante, J.; Pinzi, S.; Arzamendi, G.; Luque de Castro, M.D.; Priego-Capote, F.; Dorado, M.P. Influence of vegetable oil fatty acid composition on ultrasound-assisted synthesis of biodiesel. Fuel 2014, 125, 183–191. [Google Scholar] [CrossRef]
- Haghighi, S.F.M.; Parvasi, P.; Jokar, S.M.; Basile, A. Investigating the effects of ultrasonic frequency and membrane technology on biodiesel production from chicken waste. Energies 2021, 14, 2133. [Google Scholar] [CrossRef]
- Ho, W.W.S.; Ng, H.K.; Gan, S. Advances in ultrasound-assisted transesterification for biodiesel production. Appl. Therm. Eng. 2016, 100, 553–563. [Google Scholar] [CrossRef]
- Mofijur, M.; Kusumo, F.; Fattah, I.M.R.; Mahmudul, H.M.; Rasul, M.G.; Shamsuddin, A.H.; Mahlia, T.M.I. Resource recovery from waste coffee grounds using ultrasonic-assisted technology for bioenergy production. Energies 2020, 13, 1770. [Google Scholar] [CrossRef]
- Liu, L.; Hong, Y.; Ye, X.; Wei, L.; Liao, J.; Huang, X.; Liu, C. Biodiesel production from microbial granules in sequencing batch reactor. Bioresour. Technol. 2018, 249, 908–915. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sahoo, P.K.; Singhal, S.; Joshi, G. Exploration of upstream and downstream process for microwave assisted sustainable biodiesel production from microalgae Chlorella vulgaris. Bioresour. Technol. 2016, 216, 793–800. [Google Scholar] [CrossRef]
- Chellappan, S.; Aparna, K.; Chingakham, C.; Sajith, V.; Nair, V. Microwave assisted biodiesel production using a novel Brønsted acid catalyst based on nanomagnetic biocomposite. Fuel 2019, 246, 268–276. [Google Scholar] [CrossRef]
- Tagliapietra, S.; Calcio Gaudino, E.; Cravotto, G. 33-The use of power ultrasound for organic synthesis in green chemistry. In Power Ultrasonics; Gallego-Juárez, J.A., Graff, K.F., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 997–1022. [Google Scholar]
- Asgharzadehahmadi, S.; Raman, A.A.A.; Parthasarathy, R.; Sajjadi, B. Sonochemical reactors: Review on features, advantages and limitations. Renew. Sustain. Energy Rev. 2016, 63, 302–314. [Google Scholar] [CrossRef]
- Gallego-Juárez, J.A.; Rodríguez, G.; Acosta, V.; Riera, E. Power ultrasonic transducers with extensive radiators for industrial processing. Ultrason. Sonochemistry 2010, 17, 953–964. [Google Scholar] [CrossRef]
- Pinzi, S.; Leiva-Candia, D.E.; García, I.L.; Redel-Macías, M.D.; Dorado, M.P. Latest trends in feedstocks for biodiesel production. Biofuels Bioprod. Biorefining-Biofpr. 2014, 8, 126–143. [Google Scholar] [CrossRef]
- Fadhil, A.B.; Al-Tikrity, E.T.B.; Albadree, M.A. Biodiesel production from mixed non-edible oils, castor seed oil and waste fish oil. Fuel 2017, 210, 721–728. [Google Scholar] [CrossRef]
- Jung, J.-M.; Lee, S.-R.; Lee, J.; Lee, T.; Tsang, D.C.W.; Kwon, E.E. Biodiesel synthesis using chicken manure biochar and waste cooking oil. Bioresour. Technol. 2017, 244, 810–815. [Google Scholar] [CrossRef]
- Patiño, Y.; Mantecón, L.G.; Polo, S.; Faba, L.; Díaz, E.; Ordóñez, S. Effect of sludge features and extraction-esterification technology on the synthesis of biodiesel from secondary wastewater treatment sludges. Bioresour. Technol. 2018, 247, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Kamil, M.; Ramadan, K.; Olabi, A.G.; Ghenai, C.; Inayat, A.; Rajab, M.H. Desert palm date seeds as a biodiesel feedstock: Extraction, characterization, and engine testing. Energies 2019, 12, 3147. [Google Scholar] [CrossRef]
- Saez-Bastante, J.; Pinzi, S.; Jimenez-Romero, F.J.; Luque de Castro, M.D.; Priego-Capote, F.; Dorado, M.P. Synthesis of biodiesel from castor oil: Silent versus sonicated methylation and energy studies. Energy Convers. Manag. 2015, 96, 561–567. [Google Scholar] [CrossRef]
- Patel, C.; Chandra, K.; Hwang, J.; Agarwal, R.A.; Gupta, N.; Bae, C.; Agarwal, A.K. Comparative compression ignition engine performance, combustion, and emission characteristics, and trace metals in particulates from waste cooking oil, Jatropha and Karanja oil derived biodiesels. Fuel 2019, 236, 1366–1376. [Google Scholar] [CrossRef]
- Xool-Tamayo, J.F.; Graniel-Sabido, M.; Miron-Lopez, G.; Mena-Rejon, G.J.; Monforte-Gonzalez, M.; Flota, F.V. HPLC-DAD Determination of berberine, chelerythrine and sanguinarine in the Mexican prickly poppy (Argemone mexicana L. papaveraceae), a medicinal plant. Quim. Nova 2017, 40, 1238–1243. [Google Scholar]
- Singh, D.; Singh, S.P. Low cost production of ester from non edible oil of Argemone mexicana. Biomass Bioenergy 2010, 34, 545–549. [Google Scholar] [CrossRef]
- Pramanik, P.; Das, P.; Kim, P.J. Preparation of biofuel from argemone seed oil by an alternative cost-effective technique. Fuel 2012, 91, 81–86. [Google Scholar] [CrossRef]
- Singh, M.; Sandhu, S.S. Performance and emission characteristics of an indirect injection (IDI) multi-cylinder compression ignition (CI) engine using diesel/Argemone mexicana biodiesel blends. RSC Adv. 2015, 5, 91069–91081. [Google Scholar] [CrossRef]
- Parida, M.K.; Rout, A.K. Investigation of performance and emission analysis of Argemone mexicana biodiesel blends as a fuel in a DICI engine at part load conditions. Energy Sources Part A-Recovery Util. Environ. Eff. 2017, 39, 623–629. [Google Scholar] [CrossRef]
- Parida, M.K.; Rout, A.K. Combustion analysis of Argemone mexicana biodiesel blends. Energy Sources Part A-Recovery Util. Environ. Eff. 2017, 39, 698–705. [Google Scholar] [CrossRef]
- Sharma, M.; Khan, A.A.; Dohhen, K.C.; Christopher, J.; Puri, S.K.; Tuli, D.K. A heterogeneous catalyst for transesterification of Argemone mexicana oil. J. Am. Oil Chem. Soc. 2012, 89, 1545–1555. [Google Scholar] [CrossRef]
- Rao, R.Y.; Zubaidha, P.K.; Kondhare, D.D.; Reddy, N.J.; Deshmukh, S.S. Biodiesel production from Argemone mexicana seed oil using crystalline manganese carbonate. Pol. J. Chem. Technol. 2012, 14, 65–70. [Google Scholar] [CrossRef]
- Fatima, A.; Zafar, M.; Ahmad, M.; Sultana, S.; Ali, M.I. Parametric characterization and statistical optimization of Argemone ochroleuca (Mexican Poppy) methyl esters as a renewable source of energy. Energy Sources Part A-Recovery Util. Environ. Eff. 2017, 39, 1963–1969. [Google Scholar] [CrossRef]
- Agarwal, S.; Chhibber, V.K.; Bhatnagar, A.K.; Srivastav, B.; Kumar, A.; Singhal, S.; Sharma, A.K. Physico-chemical and tribological studies of Argemone biodiesel synthesized using microwave technique. Curr. Sci. 2017, 113, 938–941. [Google Scholar] [CrossRef]
- Sáez-Bastante, J.; Ortega-Román, C.; Pinzi, S.; Lara-Raya, F.R.; Leiva-Candia, D.E.; Dorado, M.P. Ultrasound-assisted biodiesel production from Camelina sativa oil. Bioresour. Technol. 2015, 185, 116–124. [Google Scholar] [CrossRef]
- Sánchez-Avila, N.; Mata-Granados, J.M.; Ruiz-Jiménez, J.; Luque de Castro, M.D. Fast, sensitive and highly discriminant gas chromatography-mass spectrometry method for profiling analysis of fatty acids in serum. J. Chromatogr. A 2009, 1216, 6864–6872. [Google Scholar] [CrossRef]
- Dorado, M.P.; Ballesteros, E.; de Almeida, J.A.; Schellert, C.; Lohrlein, H.P.; Krause, R. An alkali-catalyzed transesterification process for high free fatty acid waste oils. Trans. ASAE 2002, 45, 525–529. [Google Scholar] [CrossRef]
- Saha, R.; Goud, V.V. Ultrasound assisted transesterification of high free fatty acids karanja oil using heterogeneous base catalysts. Biomass Convers. Biorefinery 2015, 5, 195–207. [Google Scholar] [CrossRef]
- Carmona-Cabello, M.; Leiva-Candia, D.; Castro-Cantarero, J.L.; Pinzi, S.; Dorado, M.P. Valorization of food waste from restaurants by transesterification of the lipid fraction. Fuel 2018, 215, 492–498. [Google Scholar] [CrossRef]
- Pinzi, S.; López-Giménez, F.J.; Ruiz, J.J.; Dorado, M.P. Response surface modeling to predict biodiesel yield in a multi-feedstock biodiesel production plant. Bioresour. Technol. 2010, 101, 9587–9593. [Google Scholar] [CrossRef]
- Sáez-Bastante, J.; Pinzi, S.; Reyero, I.; Priego-Capote, F.; de Castro, M.D.L.; Dorado, M.P. Biodiesel synthesis from saturated and unsaturated oils assisted by the combination of ultrasound, agitation and heating. Fuel 2014, 131, 6–16. [Google Scholar] [CrossRef]
- Moser, B.R.; Vaughn, S.F. Evaluation of alkyl esters from Camelina sativa oil as biodiesel and as blend components in ultra low-sulfur diesel fuel. Bioresour. Technol. 2010, 101, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Bastante, J.; Fernández-García, P.; Saavedra, M.; López-Bellido, L.; Dorado, M.P.; Pinzi, S. Evaluation of Sinapis alba as feedstock for biodiesel production in Mediterranean climate. Fuel 2016, 184, 656–664. [Google Scholar] [CrossRef]
- Varatharajan, K.; Pushparani, D.S. Screening of antioxidant additives for biodiesel fuels. Renew. Sustain. Energy Rev. 2018, 82, 2017–2028. [Google Scholar] [CrossRef]
- Karavalakis, G.; Bakeas, E.; Stournas, S. Influence of oxidized biodiesel blends on regulated and unregulated emissions from a diesel passenger car. Environ. Sci. Technol. 2010, 44, 5306–5312. [Google Scholar] [CrossRef] [PubMed]
- Nogales-Delgado, S.; Maria Encinar, J.; Felix Gonzalez, J. Safflower biodiesel: Improvement of its oxidative stability by using BHA and TBHQ. Energies 2019, 12, 1940. [Google Scholar] [CrossRef]
- Pinzi, S.; Mata-Granados, J.M.; Lopez-Gimenez, F.J.; de Castro, M.D.L.; Dorado, M.P. Influence of vegetable oils fatty-acid composition on biodiesel optimization. Bioresour. Technol. 2011, 102, 1059–1065. [Google Scholar] [CrossRef]
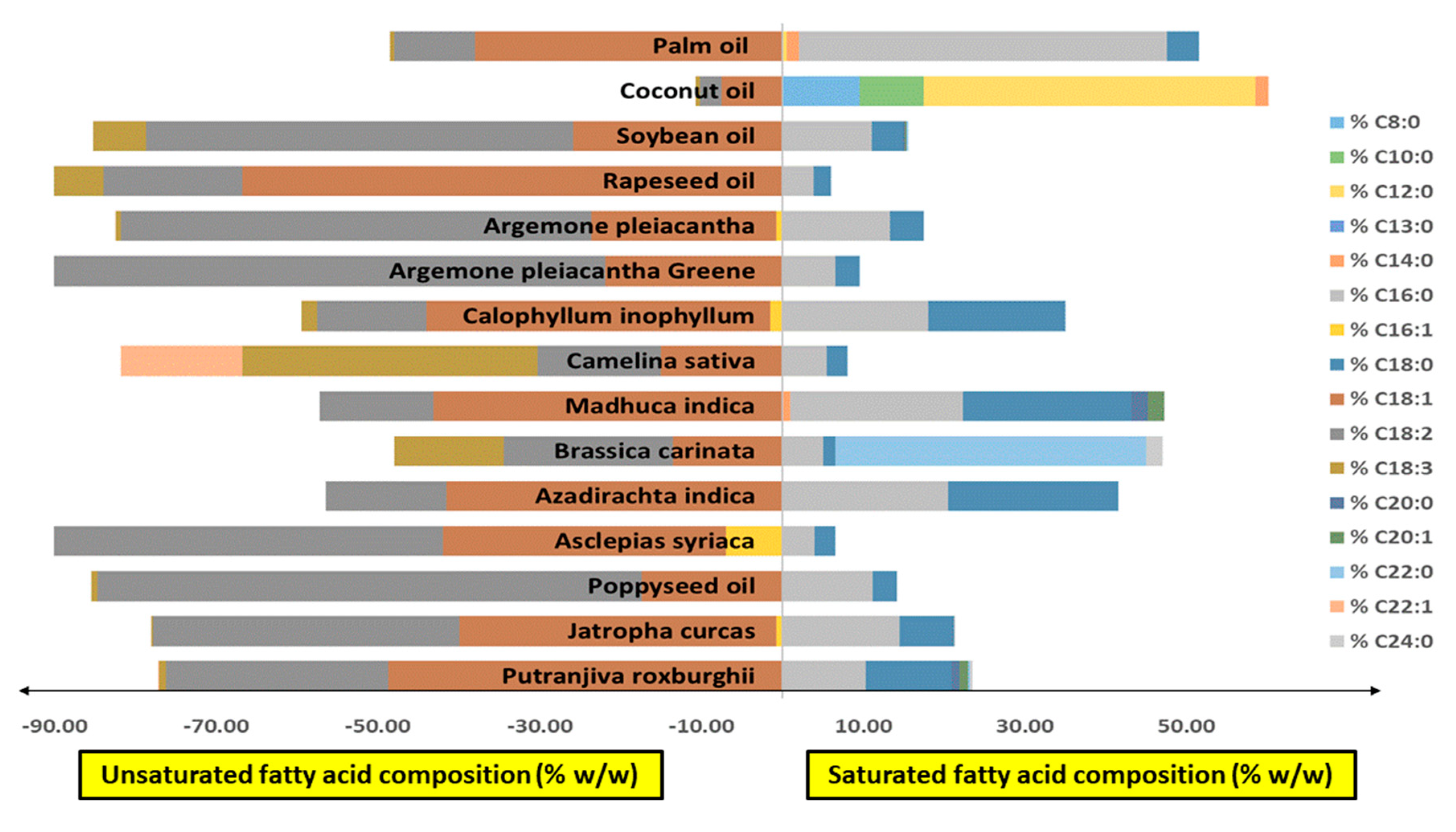
| Oilseed Characterization | ||
|---|---|---|
| Oilseed yield (% w/w) | 39.81 | |
| Moisture (% w/w) | 5.57 | |
| Fatty Acid Composition | ||
| Fatty Acid | Content (% w/w) | |
| Myristic acid (C14:0) | 0.10 | |
| Palmitic acid (C16:0) | 13.22 | |
| Palmitoleic acid (C16:1) | 0.77 | |
| Estearic acid (C18:0) | 4.22 | |
| Oleic acid (C18:1) | 22.79 | |
| Linoleic acid (C18:2) | 58.17 | |
| Linolenic acid (C18:3) | 0.73 | |
| Hydrocarbon Chain Properties | ||
| LC 1 | 17.71 | |
| TUD 2 | 143.55 | |
| PUD 3 | 58.90 | |
| MUD 4 | 23.56 | |
| Physical and Chemical Properties | ||
| Water content (ppm) | 510 | |
| Acid value (mg KOH/g) | Before AT 5 | After AT |
| 14.58 | 0.28 | |
| Density (kg/m3) | 928 | |
| Kinematic viscosity (mm2/s) | 27.15 | |
| Response Variable | Low | High | Goal |
|---|---|---|---|
| MGs (%, w/w) | 0.055 | 0.70 | Minimizing |
| DGs (%, w/w) | 0.12 | 0.20 | Minimizing |
| TGs (%, w/w) | 0.033 | 0.20 | Minimizing |
| FAME (%, w/w) | 96.50 | 98.45 | Maximizing |
| Experimental Parameter | Low | High | Optimum |
|---|---|---|---|
| Amplitude (%) | 50.00 | 80.00 | 71.43 |
| Duty cycle (%) | 60 | 100 | 100 |
| Ultrasonication time (min) | 2 | 14 | 14 |
| Response Variable | Predicted Optimum Value | Experimental Optimum Value | EN 14214 Limit |
| MGs (% w/w) | 0.190 | 0.10 | ≤0.70 |
| DGs (% w/w) | 0.055 | 0.12 | ≤0.20 |
| TGs (% w/w) | 0.039 | 0.040 | ≤0.20 |
| FAME (% w/w) | 98.30 | 98.45 | ≥96.50 |
| Desirability optimum value | 0.91 | ||
| Property | Biodiesel Standard EN14214 | Ultrasonicated Reaction | Conventional Reaction |
|---|---|---|---|
| Total glycerol content (% w/w) | EN14105 Max: 0.25 | 0.38 | 0.42 |
| Water content (ppm) | EN ISO 12937 Max: 500 | 390 | 410 |
| Carbon residue remnant (% w/w) | EN ISO 10370 Max: 0.3 | 0.12 | 0.18 |
| Flash point (°C) | EN ISO 2719 Min: 101 | 140 | 140 |
| Higher calorific value (J/g) | ASTM D240 | 38,968 | 38,395 |
| Kinematic viscosity at 40 °C (mm2/s) | EN ISO 3401 Min: 3.5; max: 5 | 3.90 | 4.10 |
| Density at 15 °C (kg/m3) | EN ISO 3675 Min: 860; max: 900 | 870 | 880 |
| Cold filter plugging point (°C) | EN116 | −3 | −3 |
| Oxidation stability at 110 °C (h) | EN14112 Min: 8 h | 1.5 | 1.5 |
| Cetane number * | Min: 51 | 55.30 | 55.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sáez-Bastante, J.; Carmona-Cabello, M.; Villarreal-Ornelas, E.; Trejo-Calzada, R.; Pinzi, S.; Dorado, M.P. Feasibility of the Production of Argemone pleiacantha Ultrasound-Assisted Biodiesel for Temperate and Tropical Marginal Areas. Energies 2023, 16, 2588. https://doi.org/10.3390/en16062588
Sáez-Bastante J, Carmona-Cabello M, Villarreal-Ornelas E, Trejo-Calzada R, Pinzi S, Dorado MP. Feasibility of the Production of Argemone pleiacantha Ultrasound-Assisted Biodiesel for Temperate and Tropical Marginal Areas. Energies. 2023; 16(6):2588. https://doi.org/10.3390/en16062588
Chicago/Turabian StyleSáez-Bastante, Javier, Miguel Carmona-Cabello, Elena Villarreal-Ornelas, Ricardo Trejo-Calzada, Sara Pinzi, and M. Pilar Dorado. 2023. "Feasibility of the Production of Argemone pleiacantha Ultrasound-Assisted Biodiesel for Temperate and Tropical Marginal Areas" Energies 16, no. 6: 2588. https://doi.org/10.3390/en16062588
APA StyleSáez-Bastante, J., Carmona-Cabello, M., Villarreal-Ornelas, E., Trejo-Calzada, R., Pinzi, S., & Dorado, M. P. (2023). Feasibility of the Production of Argemone pleiacantha Ultrasound-Assisted Biodiesel for Temperate and Tropical Marginal Areas. Energies, 16(6), 2588. https://doi.org/10.3390/en16062588







