Understanding the Influence of Biochar Augmentation in Anaerobic Digestion by Principal Component Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Data
2.2. Biochar Effect on Anaerobic Digestion
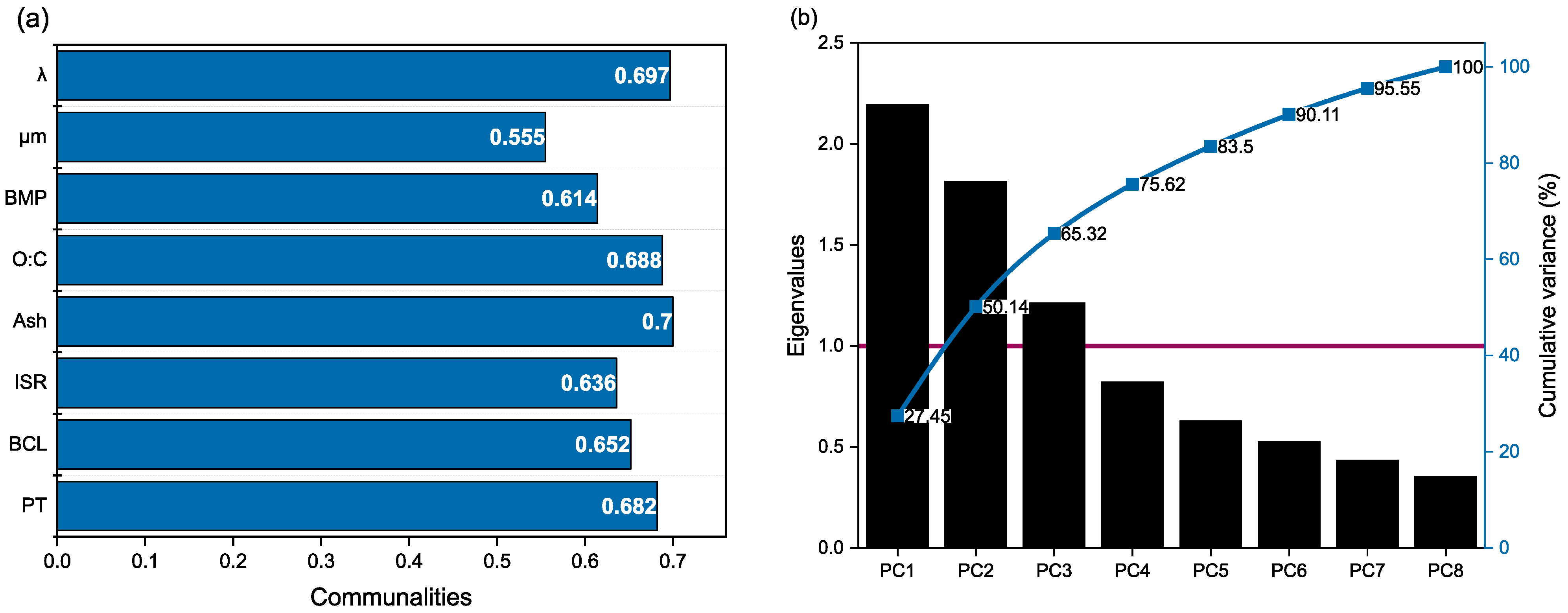
2.3. Principal Component Analysis
Visual Representation of the Principal Component Analysis
2.4. Descriptive Analysis
3. Results and Discussion
3.1. Principal Component Analysis
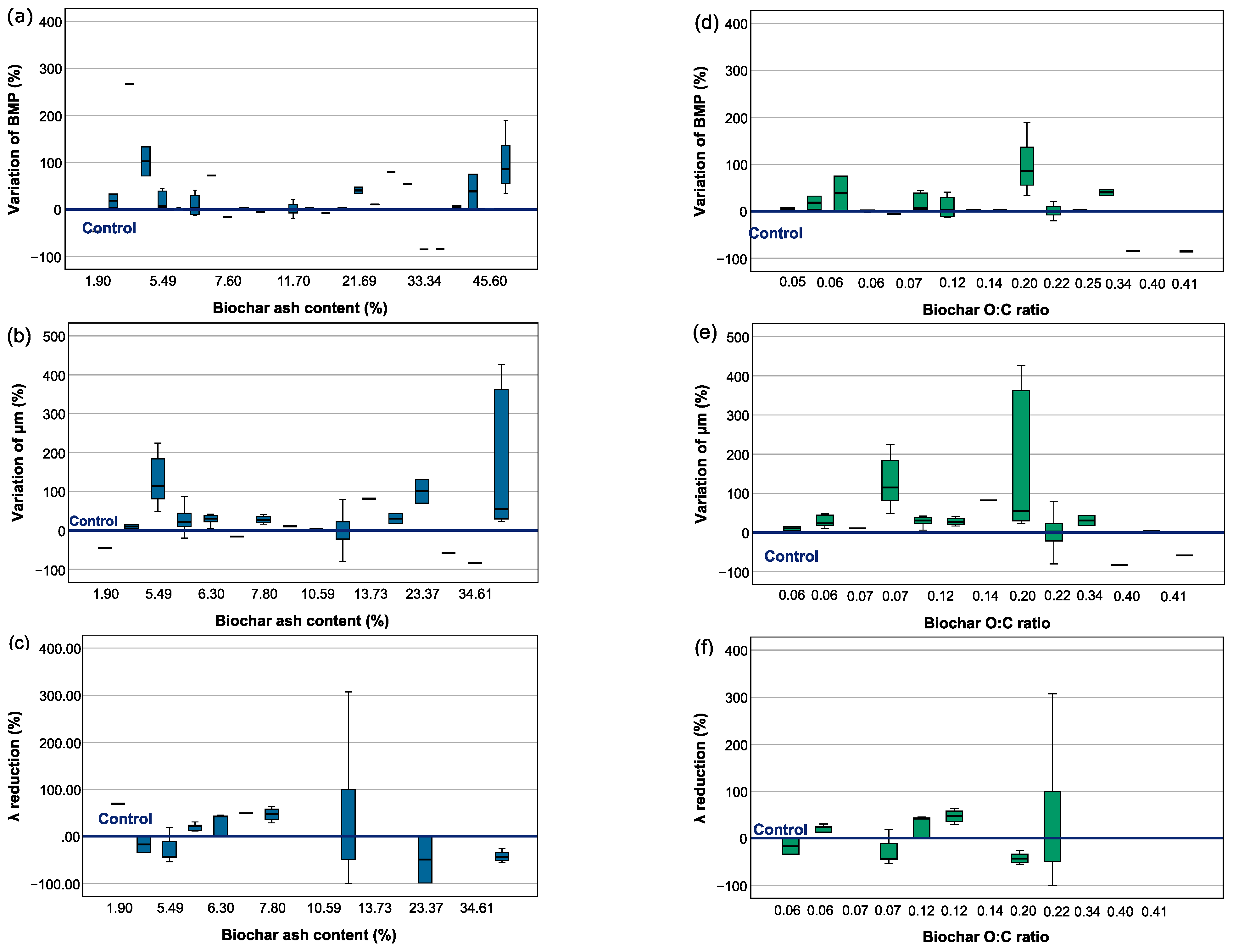
3.2. Biochar Properties and Their Effect on Anaerobic Digestion
3.2.1. Feedstocks Used for Producing the Biochar Added to Anaerobic Digestion
3.2.2. Pyrolysis Temperature
3.2.3. Biochar Composition
3.3. Anaerobic Digestion Conditions
3.3.1. Substrate
3.3.2. Inoculum to Substrate Ratio
3.3.3. Biochar Load
3.3.4. pH and Buffering Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AcoD | Anaerobic co-digestion |
| AD | Anaerobic digestion |
| APL | Aqueous pyrolysis liquid |
| BC | Biochar |
| BCL | Biochar load |
| BMP | Biochemical methane potential |
| BOAP | Bio-oil aqueous phase |
| CEC | Cation exchange capacity |
| COD | Carbon oxygen demand |
| DIET | Direct interspecies electron transfer |
| EC | Electrical conductivity |
| FC | Fixed carbon |
| FDH | Formate dehydrogenases |
| FW | Food waste |
| HR | Heating rate |
| HRT | Hydraulic retention time |
| ISR | Inoculum to substrate ratio |
| KMO | Kaiser-Meyer-Olkin method |
| NR | Not reported |
| OFG | Oxygenated functional groups |
| OFMSW | Organic fraction of the municipal solid waste |
| PC | Principal component |
| PCA | Principal component analysis |
| PS | Particle size |
| PT | Pyrolysis temperature |
| PV | Pore volume |
| SA | Surface area |
| SP | Slow pyrolysis |
| SS | Sewage sludge |
| SW | Softwood |
| TAN | Total ammonia nitrogen |
| TC | Total carbon |
| TS | Total solids |
| VFA | Volatile fatty acids |
| VM | Volatile matter |
| VS | Volatile solids |
| µm | Methane production rate |
| λ | Lag phase |
References
- Pan, J.; Ma, J.; Zhai, L.; Luo, T.; Mei, Z.; Liu, H. Achievements of Biochar Application for Enhanced Anaerobic Digestion: A Review. Bioresour. Technol. 2019, 292, 122058. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Liang, D.; Li, N. Conductive Materials in Anaerobic Digestion: From Mechanism to Application. Bioresour. Technol. 2020, 298, 122403. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, Y.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Enhancing Syntrophic Metabolism in Up-Flow Anaerobic Sludge Blanket Reactors with Conductive Carbon Materials. Bioresour. Technol. 2015, 191, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, F.; Yang, H.; Huang, X.; Liu, H.; Zhang, J.; Guo, S. Graphene Oxide as a Matrix for Enzyme Immobilization. Langmuir 2010, 26, 6083–6085. [Google Scholar] [CrossRef]
- Klüpfel, L.; Keiluweit, M.; Kleber, M.; Sander, M. Redox Properties of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2014, 48, 5601–5611. [Google Scholar] [CrossRef] [PubMed]
- Cruz Viggi, C.; Simonetti, S.; Palma, E.; Pagliaccia, P.; Braguglia, C.; Fazi, S.; Baronti, S.; Navarra, M.A.; Pettiti, I.; Koch, C.; et al. Enhancing Methane Production from Food Waste Fermentate Using Biochar: The Added Value of Electrochemical Testing in Pre-Selecting the Most Effective Type of Biochar. Biotechnol. Biofuels 2017, 10, 303. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, W.; Zhang, H.; Wang, Z.; Fan, C.; Zang, L. Recent Achievements in Enhancing Anaerobic Digestion with Carbon- Based Functional Materials. Bioresour. Technol. 2018, 266, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.R.; Adhikari, S.; Nam, H.; Kar Sajib, S. Effect of Bio-Char on Methane Generation from Glucose and Aqueous Phase of Algae Liquefaction Using Mixed Anaerobic Cultures. Biomass Bioenergy 2018, 108, 479–486. [Google Scholar] [CrossRef]
- Quintana-Najera, J.; Blacker, A.J.; Fletcher, L.A.; Ross, A.B. The Effect of Augmentation of Biochar and Hydrochar in Anaerobic Digestion of a Model Substrate. Bioresour. Technol. 2021, 321, 124494. [Google Scholar] [CrossRef]
- Wang, G.; Li, Y.; Sheng, L.; Xing, Y.; Liu, G.; Yao, G.; Ngo, H.H.; Li, Q.; Wang, X.C.; Li, Y.Y.; et al. A Review on Facilitating Bio-Wastes Degradation and Energy Recovery Efficiencies in Anaerobic Digestion Systems with Biochar Amendment. Bioresour. Technol. 2020, 314, 123777. [Google Scholar] [CrossRef]
- Lü, F.; Luo, C.; Shao, L.; He, P. Biochar Alleviates Combined Stress of Ammonium and Acids by Firstly Enriching Methanosaeta and Then Methanosarcina. Water Res. 2016, 90, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, Q.; Gao, X.; Wang, X.C. Synergetic Promotion of Syntrophic Methane Production from Anaerobic Digestion of Complex Organic Wastes by Biochar: Performance and Associated Mechanisms. Bioresour. Technol. 2018, 250, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Li, S.; Cai, J.; He, P.; Lü, F. Ability of Biochar to Facilitate Anaerobic Digestion Is Restricted to Stressed Surroundings. J. Clean. Prod. 2019, 238, 117959. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Gao, X.; Chen, H.; Xu, X.; Zhu, L. Role of Biochar in the Granulation of Anaerobic Sludge and Improvement of Electron Transfer Characteristics. Bioresour. Technol. 2018, 268, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, H.; Li, X.; Cheng, J.J.; Wu, W. Improving Methane Yield from Organic Fraction of Municipal Solid Waste (OFMSW) with Magnetic Rice-Straw Biochar. Bioresour. Technol. 2017, 245, 1058–1066. [Google Scholar] [CrossRef]
- Fernandes, P.; Cabral, J.M.S. Bioreactors. In Multiphase Catalytic Reactors; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 156–170. [Google Scholar]
- De la Rubia, M.A.; Villamil, J.A.; Rodriguez, J.J.; Mohedano, A.F. Effect of Inoculum Source and Initial Concentration on the Anaerobic Digestion of the Liquid Fraction from Hydrothermal Carbonisation of Sewage Sludge. Renew. Energy 2018, 127, 697–704. [Google Scholar] [CrossRef]
- Linville, J.L.; Shen, Y.; Ignacio-de Leon, P.A.; Schoene, R.P.; Urgun-Demirtas, M. In-Situ Biogas Upgrading during Anaerobic Digestion of Food Waste Amended with Walnut Shell Biochar at Bench Scale. Waste Manag. Res. 2017, 35, 669–679. [Google Scholar] [CrossRef]
- Sunyoto, N.M.S.; Zhu, M.; Zhang, Z.; Zhang, D. Effect of Biochar Addition on Hydrogen and Methane Production in Two-Phase Anaerobic Digestion of Aqueous Carbohydrates Food Waste. Bioresour. Technol. 2016, 219, 29–36. [Google Scholar] [CrossRef]
- Bin Khalid, Z.; Siddique, M.N.I.; Nayeem, A.; Adyel, T.M.; Ismail, S. Bin; Ibrahim, M.Z. Biochar Application as Sustainable Precursors for Enhanced Anaerobic Digestion: A Systematic Review. J. Environ. Chem. Eng. 2021, 9, 105489. [Google Scholar] [CrossRef]
- Chiappero, M.; Norouzi, O.; Hu, M.; Demichelis, F.; Berruti, F.; Di Maria, F.; Mašek, O.; Fiore, S. Review of Biochar Role as Additive in Anaerobic Digestion Processes. Renew. Sustain. Energy Rev. 2020, 131, 110037. [Google Scholar] [CrossRef]
- Codignole Luz, F.; Cordiner, S.; Manni, A.; Mulone, V.; Rocco, V. Biochar Characteristics and Early Applications in Anaerobic Digestion-a Review. J. Environ. Chem. Eng. 2018, 6, 2892–2909. [Google Scholar] [CrossRef]
- Chiappero, M.; Cillerai, F.; Berruti, F.; Mašek, O.; Fiore, S. Addition of Different Biochars as Catalysts during the Mesophilic Anaerobic Digestion of Mixed Wastewater Sludge. Catalysts 2021, 11, 1094. [Google Scholar] [CrossRef]
- Qin, Y.; Yin, X.; Xu, X.; Yan, X.; Bi, F.; Wu, W. Specific Surface Area and Electron Donating Capacity Determine Biochar’s Role in Methane Production during Anaerobic Digestion. Bioresour. Technol. 2020, 303, 122919. [Google Scholar] [CrossRef]
- Luo, C.; Lü, F.; Shao, L.; He, P. Application of Eco-Compatible Biochar in Anaerobic Digestion to Relieve Acid Stress and Promote the Selective Colonization of Functional Microbes. Water Res. 2015, 68, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Sugiarto, Y.; Sunyoto, N.M.S.; Zhu, M.; Jones, I.; Zhang, D. Effect of Biochar Addition on Microbial Community and Methane Production during Anaerobic Digestion of Food Wastes: The Role of Minerals in Biochar. Bioresour. Technol. 2021, 323, 124585. [Google Scholar] [CrossRef]
- Martínez, E.J.; Rosas, J.G.; Sotres, A.; Moran, A.; Cara, J.; Sánchez, M.E.; Gómez, X. Codigestion of Sludge and Citrus Peel Wastes: Evaluating the Effect of Biochar Addition on Microbial Communities. Biochem. Eng. J. 2018, 137, 314–325. [Google Scholar] [CrossRef]
- Cai, J.; He, P.; Wang, Y.; Shao, L.; Lü, F. Effects and Optimization of the Use of Biochar in Anaerobic Digestion of Food Wastes. Waste Manag. Res. 2016, 34, 409–416. [Google Scholar] [CrossRef]
- Fagbohungbe, M.O.; Herbert, B.M.J.; Hurst, L.; Li, H.; Usmani, S.Q.; Semple, K.T. Impact of Biochar on the Anaerobic Digestion of Citrus Peel Waste. Bioresour. Technol. 2016, 216, 142–149. [Google Scholar] [CrossRef]
- Gómez, X.; Meredith, W.; Fernández, C.; Sánchez-García, M.; Díez-Antolínez, R.; Garzón-Santos, J.; Snape, C.E. Evaluating the Effect of Biochar Addition on the Anaerobic Digestion of Swine Manure: Application of Py-GC/MS. Environ. Sci. Pollut. Res. 2018, 25, 25600–25611. [Google Scholar] [CrossRef]
- Jang, H.M.; Choi, Y.-K.K.; Kan, E. Effects of Dairy Manure-Derived Biochar on Psychrophilic, Mesophilic and Thermophilic Anaerobic Digestions of Dairy Manure. Bioresour. Technol. 2018, 250, 927–931. [Google Scholar] [CrossRef]
- Shen, Y.; Linville, J.L.; Ignacio-de Leon, P.A.A.; Schoene, R.P.; Urgun-Demirtas, M. Towards a Sustainable Paradigm of Waste-to-Energy Process: Enhanced Anaerobic Digestion of Sludge with Woody Biochar. J. Clean. Prod. 2016, 135, 1054–1064. [Google Scholar] [CrossRef]
- Shen, Y.; Linville, J.L.; Urgun-Demirtas, M.; Schoene, R.P.; Snyder, S.W. Producing Pipeline-Quality Biomethane via Anaerobic Digestion of Sludge Amended with Corn Stover Biochar with in-Situ CO2 Removal. Appl. Energy 2015, 158, 300–309. [Google Scholar] [CrossRef]
- Torri, C.; Fabbri, D. Biochar Enables Anaerobic Digestion of Aqueous Phase from Intermediate Pyrolysis of Biomass. Bioresour. Technol. 2014, 172, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Najera, J.; Blacker, A.J.; Fletcher, L.A.; Bray, D.G.; Ross, A.B. The Influence of Biochar Augmentation and Digestion Conditions on the Anaerobic Digestion of Water Hyacinth. Energies 2022, 15, 2524. [Google Scholar] [CrossRef]
- Quintana-Najera, J.; Blacker, A.J.; Fletcher, L.A.; Ross, A.B. Influence of Augmentation of Biochar during Anaerobic Co-Digestion of Chlorella Vulgaris and Cellulose. Bioresour. Technol. 2022, 343, 126086. [Google Scholar] [CrossRef]
- Deng, C.; Lin, R.; Kang, X.; Wu, B.; O’Shea, R.; Murphy, J.D. Improving Gaseous Biofuel Yield from Seaweed through a Cascading Circular Bioenergy System Integrating Anaerobic Digestion and Pyrolysis. Renew. Sustain. Energy Rev. 2020, 128, 109895. [Google Scholar] [CrossRef]
- Mumme, J.; Srocke, F.; Heeg, K.; Werner, M. Use of Biochars in Anaerobic Digestion. Bioresour. Technol. 2014, 164, 189–197. [Google Scholar] [CrossRef]
- Swarbrick, B.; Westad, F. An Overview of Chemometrics for the Engineering and Measurement Sciences. In Handbook of Measurement in Science and Engineering; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; Volume 3, pp. 2307–2407. [Google Scholar]
- Plonsky, L.; Loewen, S.; Gonulal, T. Exploratory Factor Analysis and Principal Components Analysis. In Advancing Quantitative Methods in Second Language Research; Routledge: London, UK, 2015; pp. 182–212. [Google Scholar]
- Dandikas, V.; Heuwinkel, H.; Lichti, F.; Drewes, J.E.; Koch, K. Correlation between Biogas Yield and Chemical Composition of Grassland Plant Species. Energy Fuels 2015, 29, 7221–7229. [Google Scholar] [CrossRef]
- Amonette, J.E.; Joseph, S. Characteristics of Biochar: Microchemical Properties. In Biochar for Environmental Management: Science and Technology; Routledge: London, UK, 2009; pp. 65–84. [Google Scholar]
- Li, D.C.; Jiang, H. The Thermochemical Conversion of Non-Lignocellulosic Biomass to Form Biochar: A Review on Characterizations and Mechanism Elucidation. Bioresour. Technol. 2017, 246, 57–68. [Google Scholar] [CrossRef]
- Mafu, L.D.; Neomagus, H.W.J.P.; Everson, R.C.; Strydom, C.A.; Carrier, M.; Okolo, G.N.; Bunt, J.R. Chemical and Structural Characterization of Char Development during Lignocellulosic Biomass Pyrolysis. Bioresour. Technol. 2017, 243, 941–948. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of Biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Chintala, R.; Schumacher, T.E.; Kumar, S.; Malo, D.D.; Rice, J.A.; Bleakley, B.; Chilom, G.; Clay, D.E.; Julson, J.L.; Papiernik, S.K.; et al. Molecular Characterization of Biochars and Their Influence on Microbiological Properties of Soil. J. Hazard. Mater. 2014, 279, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; Xu, Z. Biochar: Nutrient Properties and Their Enhancement. In Biochar for Environmental Management: Science and Technology; Routledge: London, UK, 2009; pp. 99–116. [Google Scholar]
- Deng, C.; Lin, R.; Kang, X.; Wu, B.; Wall, D.M.; Murphy, J.D. What Physicochemical Properties of Biochar Facilitate Interspecies Electron Transfer in Anaerobic Digestion: A Case Study of Digestion of Whiskey by-Products. Fuel 2021, 306, 121736. [Google Scholar] [CrossRef]
- Takaya, C.A.; Fletcher, L.A.; Singh, S.; Anyikude, K.U.; Ross, A.B. Phosphate and Ammonium Sorption Capacity of Biochar and Hydrochar from Different Wastes. Chemosphere 2016, 145, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Moset, V.; Al-zohairi, N.; Møller, H.B. The Impact of Inoculum Source, Inoculum to Substrate Ratio and Sample Preservation on Methane Potential from Different Substrates. Biomass Bioenergy 2015, 83, 474–482. [Google Scholar] [CrossRef]
- Meng, X.; Yu, D.; Wei, Y.; Zhang, Y.; Zhang, Q.; Wang, Z.; Liu, J.; Wang, Y. Endogenous Ternary PH Buffer System with Ammonia-Carbonates-VFAs in High Solid Anaerobic Digestion of Swine Manure: An Alternative for Alleviating Ammonia Inhibition? Process Biochem. 2018, 69, 144–152. [Google Scholar] [CrossRef]
- Paritosh, K.; Vivekanand, V. Biochar Enabled Syntrophic Action: Solid State Anaerobic Digestion of Agricultural Stubble for Enhanced Methane Production. Bioresour. Technol. 2019, 289, 121712. [Google Scholar] [CrossRef]
- Achi, C.G.; Hassanein, A.; Lansing, S. Enhanced Biogas Production of Cassava Wastewater Using Zeolite and Biochar Additives and Manure Co-Digestion. Energies 2020, 13, 491. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Thompson, M.L.; Lawrinenko, M. Characterization and Quantification of Biochar Alkalinity. Chemosphere 2017, 167, 367–373. [Google Scholar] [CrossRef]
- Wang, G.; Li, Q.; Dzakpasu, M.; Gao, X.; Yuwen, C.; Wang, X.C. Impacts of Different Biochar Types on Hydrogen Production Promotion during Fermentative Co-Digestion of Food Wastes and Dewatered Sewage Sludge. Waste Manag. 2018, 80, 73–80. [Google Scholar] [CrossRef] [PubMed]



| Biochar Feedstock | Pyrolysis Conditions | BC Load (% w/v) | AD Conditions | ISR | BMP | µm | λ (Days) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Oak wood | Commercial SP mono retort reactor 450 °C 650 °C | 0 3 3 | AMPTS, 37 °C, HRT 30 d, cellulose 5 g VS/L | 1 | 265.9 285.5 251.6 | 11.8 28.1 13.1 | 0 1.5 13.1 | [10] |
| Fucus serratus | SP fixed bed, N2 flow, HR 5 °C/min 450 °C, 1 h 600 °C, 1 h | 3 3 | 38.3 41.1 | 4.9 1.9 | 17.3 3.6 | |||
| Water hyacinth | 450 °C, 1 h 600 °C, 1 h | 3 3 | 294.2 266.0 | 27.3 12.3 | 1.2 3.3 | |||
Rice straw Corn stover Bamboo Pine wood Oak wood Apple wood | Hypoxic conditions, PS < 1 mm 500 °C, 2 h | 0 0.5 0.5 0.5 0.5 0.5 0.5 | AMPTS, 35 °C, HRT 25 d, glucose 9 g/L | 0.18 | 142.0 143.6 138.0 145.0 156.4 158.9 163.8 | 6.5 8.2 6.3 9.8 9.7 9.0 9.2 | NR | [25] |
| Fruitwoods | 800–900 °C PS 0.5–1 mm | 0 1 | Serum bottle, 35 °C, HRT 120 d, glucose 6 g/L TAN 0.3 g/L | 0.17 | 13.2 a 12.9 a | 1.3 b 1.5 b | 23.5 16.3 | [12] |
| 800–900 °C PS 0.5–1 mm | 0 1 | Glucose 6 g/L, TAN 3.5 g-N/L | 13.5 a 13.3 a | 0.59 b 0.65 b | 30.5 26.5 | |||
| 800–900 °C PS 0.5–1 mm PS 2–5 mm PS 75–150 µm | 0 1 1 1 | Glucose 6 g/L, TAN 7 g-N/L | 13.6 a 13.8 a 15.2 a 14.0 a | 0.34 b 0.42 b 0.50 b 0.49 b | 63.5 48.4 48.3 59.8 | |||
| Fruitwoods | Kiln reactor 800 °C | 0 1 | Serum bottle, HRT 22 d, glucose 2 g/L | 0.5 | 15.7 a 15.3 a | 2.8 b 2.3 b | 12.7 10.6 | [26] |
| 0 1 | HRT 32 d, glucose 4 g/L | 0.25 | 16.6 a 13.7 a | 1.1 b 2.1 b | 15.8 14.0 | |||
| 0 1 | HRT 38 d, glucose 6 g/L | 0.17 | 14.2 a 13.7 a | 1.3 b 1.5 b | 23.4 16.3 | |||
| 0 1 | HRT 42 d, glucose 8 g/L | 0.125 | 15.1 a 13.3 a | 1.0 b 1.0 b | 25.0 19.6 |
| Feedstock | Pyrolysis Conditions | BC Load (% w/v) | AD Conditions | ISR | BMP | µm | λ (Days) | Ref. |
|---|---|---|---|---|---|---|---|---|
|
Pine sawdust | Indirectly fired kiln, size PS 12–25.9 µm 650 °C, 20 m | 0 1.5 | Serum bottle 100 mL, HRT 40 d, 37 °C, food waste 496 g VS/L | 1487 a 2092 a | 272 b 362 b | 6 6 | [27] | |
| 900 °C, 20 m | 1.5 | 2187 a | 389 b | 6 | ||||
|
Vineyard pruning | Pilot plant semi-continuous electrical reactor, anoxic, no inert gas, 550 °C, 15 min | 0 1 3 | Erlenmeyer flask 250 mL, HRT 54 d, 37 °C, citrus peel waste | 1 | 103 209 298 | 10.9 14.3 14.2 | 16.8 9.8 9.3 | [28] |
| Wallnut shell | Commercial downdraft gasifier 900 °C | 0 0.35 0.70 | Serum bottle 650 mL, HRT 55 d, 37 °C, food waste 4 g VS/L | 1.36 | 484 492 131 | NR | NR | [19] |
| Rice straw | Furnace, N2 flow 500 °C, 2 h | 0 0.5 | AMPTS, HRT 25 d, 35 °C, OFMSW 8.6 g/L | 1 | 174.2 c 92.4 c | 72.5 d 40.1 d | 1.8 1.1 | [16] |
| Fruitwoods | Commercial kiln 800–900 °C PS <1 mm | 0 0.2 0.5 1 | Serum bottle 1100 mL, 210 d, 35 °C, food waste 4 g/L | 2 | 490.0 480.1 493.1 507.5 | 0.05 0.08 0.07 0.15 | 55.4 65.8 51.6 49 | [29] |
| 0 0.2 0.5 1 | Food waste 8 g/L | 1 | 440.0 460.3 530.5 476.6 | 0.03 0.07 0.06 0.07 | 89.9 51 50.5 41.1 | |||
| 0 0.2 0.5 1 | Food waste 10 g/L | 0.8 | 340.0 490.2 478.1 471.9 | 0.03 0.04 0.06 0.05 | 123.9 79 57 68.1 | |||
|
Pine sawdust | Indirectly fired kiln 650 °C, 20 min PS 3.6–25.9 µm | 0 0.83 1.66 2.51 3.33 | Serum bottle 100 mL, HRT 40 d, 35 °C, food waste 13.7 g/L | 1070 a 1137 a 1057 a 956 a 931 a | 113 b 156 b 160 b 145 b 138 b | 10 5.9 5.7 5.5 5.7 | [20] | |
Coconut shell Wood Rice husk | Commercial 450 °C PS 1.7–2.0 mm | 0 0.96 096 0.96 | Serum bottle 500 mL, HRT 30 d, 35 °C, citrus peel waste | 0.3 | 165.9 186.8 171.3 172.1 | 21.8 26.0 18.4 26.6 | 13.4 7.3 6.8 12.8 | [30] |
| Feedstock | Pyrolysis Conditions | BC Load (% w/v) | Substrate | ISR | BMP | µm | λ (Days) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Vineyard pruning | Pilot plant semi-continuous electrical reactor, anoxic, no inert gas, 550 °C, 15 min | 0 1 3 | Erlenmeyer flask 250 mL, HRT 54 d, 37 °C, sludge | 1 | 273 364 425 | 18.7 23.1 33.4 | 7.9 5.2 5.9 | [28] |
| Almond shell residue | Commercial semi-continuous electrically heated, anoxic 550 °C, 15 min | 0 1.2 | Serum bottle 250 mL, HRT 40 d, 35 °C, swine manure 6 g VS/L | 1 | 298.7 395.4 | 21.2 24.5 | 9.2 6.1 | [31] |
| 0 1.2 | Pre-treated swine manure | 1 | 416.7 433.2 | 27.5 28.8 | 5.9 5.8 | |||
| Dairy manure | Muffle furnace HR 10 °C/min 350 °C, 3 h Size 420–600 µm | 0 0.1 1.0 | Serum bottle 280 mL, 35 °C, HRT 35 d, dairy manure | NR | 374.7 394.9 466.5 | 28.2 29.9 37.4 | 2.1 1.9 1.5 | [32] |
| Ashe juniper | Semi-pilot Auger reactor, N2 flow 400 °C, 30 min 600 °C, 30 min | 0 1 1 | Serum bottle 160 mL, 37 °C, HRT 10 d, BOAP 4 g COD/L | 0.24 | 24 296 88 | NR | NR | [9] |
| Canola meal | 700 °C, 2 h 900 °C, 2 h | 1 1 | 43 37 | NR | NR | |||
Pine wood Oak wood | Commercial pilot-scale fluidised bed gasifier, gas recirculation and N2 flow 710 °C, 0.8 sec | 0 3.1 6.3 | 2-step 600 mL digesters: (i) 37 °C/HRT 1.2 d; (ii) 53 °C/HRT 12 d, sludge | NR | 0.31 a 0.31 a 0.31 a | 72.5 b 82.9 b 71.4 b | NR | [33] |
| Oak wood | 2.8 5.6 | NR | 0.33 a 0.32 a | 83.2 b 79.4 b | NR | |||
| Cornstalk | Commercial pilot-scale fluidised bed gasifier, gas recirculation and N2 flow 710 °C, 0.8 sec | 0 0.8 1.1 1.3 1.6 | Serum bottle 600 mL, HRT 25 d, 35 °C, sludge 4.3 g TS/L | 2 | 488.9 494.3 494.9 495.2 494.5 | 125.5 b 160.1 b 144.5 b 143.6 b 131.5 b | NR | [34] |
| Cornstalk pellet | Fixed bed reactor, HR 100 °C/min, N2 flow 400 °C, 10 min | 0 8 | Syringe 100 mL, HRT 225 d, 40°, APL 35 g COD/L | 0.6 | 12 c 20 c | 0.1 d 0.2 d | NR | [35] |
| Feedstock | Pyrolysis Conditions | BC Load (% w/v) | Substrate | ISR | BMP | µm | λ (Days) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Oak wood | Commercial SP mono retort reactor 450 °C | 0 0.5 1 | AMPTS, 37 °C, HRT 30 d, water hyacinth 5 g VS/L Samples from different sources | 1 | 208.9 217.7 141.7 | 15.0 24.9 13.0 | 0.0 1.5 0.4 | [36] |
| 0 0.5 1 | 201.3 163.3 196.6 | 20.2 15.8 17.5 | 0.0 0.0 | |||||
| 0 0.5 | 177.1 141.4 | 19.8 32.6 | 0.0 0.2 | |||||
| 0 0.5 | 91.6 53.7 | 6.8 5.0 | 0.0 0.0 | |||||
| Oak wood | Commercial SP mono retort reactor 450 °C | 0 3 | AMPTS, 37 °C, HRT 30 d, C. vulgaris cellulose, 5 g VS/L, C/N 10 | 0.5 | 50.8 232.7 | 23.6 9.5 | 0.4 1.0 | [37] |
| 0 3 | C/N 20 | 0.8 | 91.2 239.1 | 39.5 10.0 | 1.0 0.0 | |||
| 0 3 | C/N 30 | 0.9 | 136.2 241.2 | 22.7 12.4 | 0.5 0.0 | |||
| Waste wood | Commercial continuous rotatory kiln 700 °C, 1 h PS 75–500 µm | 0 0.03 0.06 0.12 0.5 1 | AMPTS, HRT 30 d, 37 °C, L. digitata 5 g VS/L | 2 | 200.1 211.5 212.9 234.0 180.0 179.7 | 22.1 25.8 24.2 24.7 19.5 20.3 | 1.5 0.8 0.8 0.7 1.3 1.4 | [38] |
| Sawdust | Muffle furnace, HR 10 °C/min, anoxic 500 °C, 1.5 h PS 0.25–1.0 mm | 0 0.2 0.6 1.0 1.5 | Serum bottle working volume 90 mL, 35 °C, HRT 55 d, food waste and sludge 2 g VS/L | 0.67 | 111.7 a 114.6 a 116.2 a 112.1 a 109.5 a | 6.7 b 8.7 b 9.4 b 8.2 b 7.8 b | 21.2 15.3 12.1 10.2 7.8 | [13] |
| Vineyard pruning | Pilot plant semi-continuous electrical reactor, anoxic, no inert gas, 550 °C, 15 min | 0 1 3 | Erlenmeyer flask 250 mL, HRT 54 d, 37 °C, citrus peel waste and sludge | 1 | 298 500 704 | 14.4 66.3 75.5 | 7.3 3.6 3.3 | [28] |
| Paper sludge-wheat husk | Commercial screw pyrolyser, no inert gas. Post-outgassed and quenched with water 500 °C, 20 min | 0 2 | Syringe 100 mL, HRT 63 d, 40 °C, (NH4)2CO3 TAN 0.5–5 g/kg | NR | 4.4 c 4.5 c | 0.03 d 0.03 d | NR | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintana-Najera, J.; Blacker, A.J.; Fletcher, L.A.; Ross, A.B. Understanding the Influence of Biochar Augmentation in Anaerobic Digestion by Principal Component Analysis. Energies 2023, 16, 2523. https://doi.org/10.3390/en16062523
Quintana-Najera J, Blacker AJ, Fletcher LA, Ross AB. Understanding the Influence of Biochar Augmentation in Anaerobic Digestion by Principal Component Analysis. Energies. 2023; 16(6):2523. https://doi.org/10.3390/en16062523
Chicago/Turabian StyleQuintana-Najera, Jessica, A. John Blacker, Louise A. Fletcher, and Andrew B. Ross. 2023. "Understanding the Influence of Biochar Augmentation in Anaerobic Digestion by Principal Component Analysis" Energies 16, no. 6: 2523. https://doi.org/10.3390/en16062523
APA StyleQuintana-Najera, J., Blacker, A. J., Fletcher, L. A., & Ross, A. B. (2023). Understanding the Influence of Biochar Augmentation in Anaerobic Digestion by Principal Component Analysis. Energies, 16(6), 2523. https://doi.org/10.3390/en16062523






