Experimental Study on Mineral Dissolution and Carbonation Efficiency Applied to pH-Swing Mineral Carbonation for Improved CO2 Sequestration
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
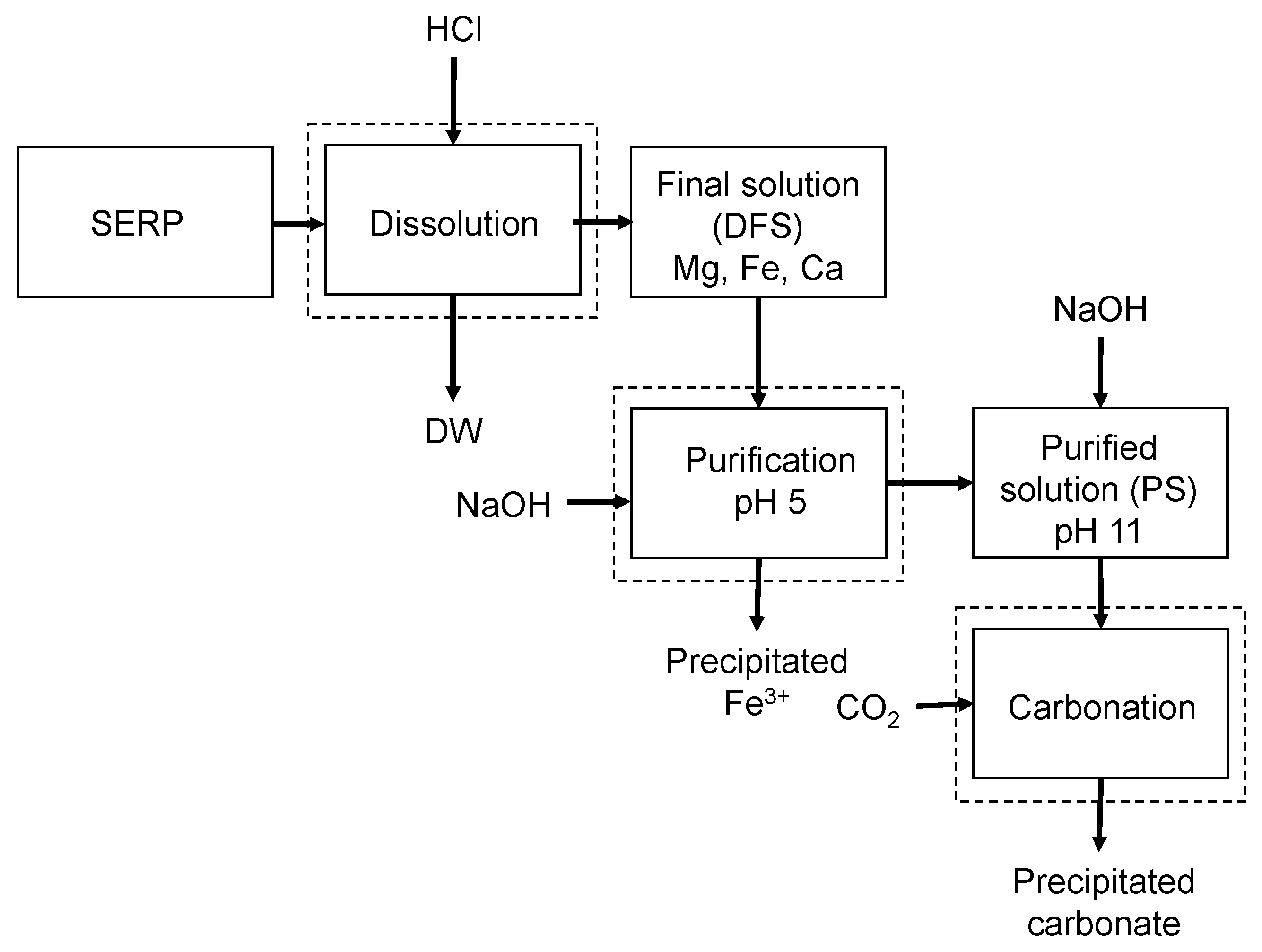
2.2. pH Swing Process
2.2.1. Dissolution of Minerals
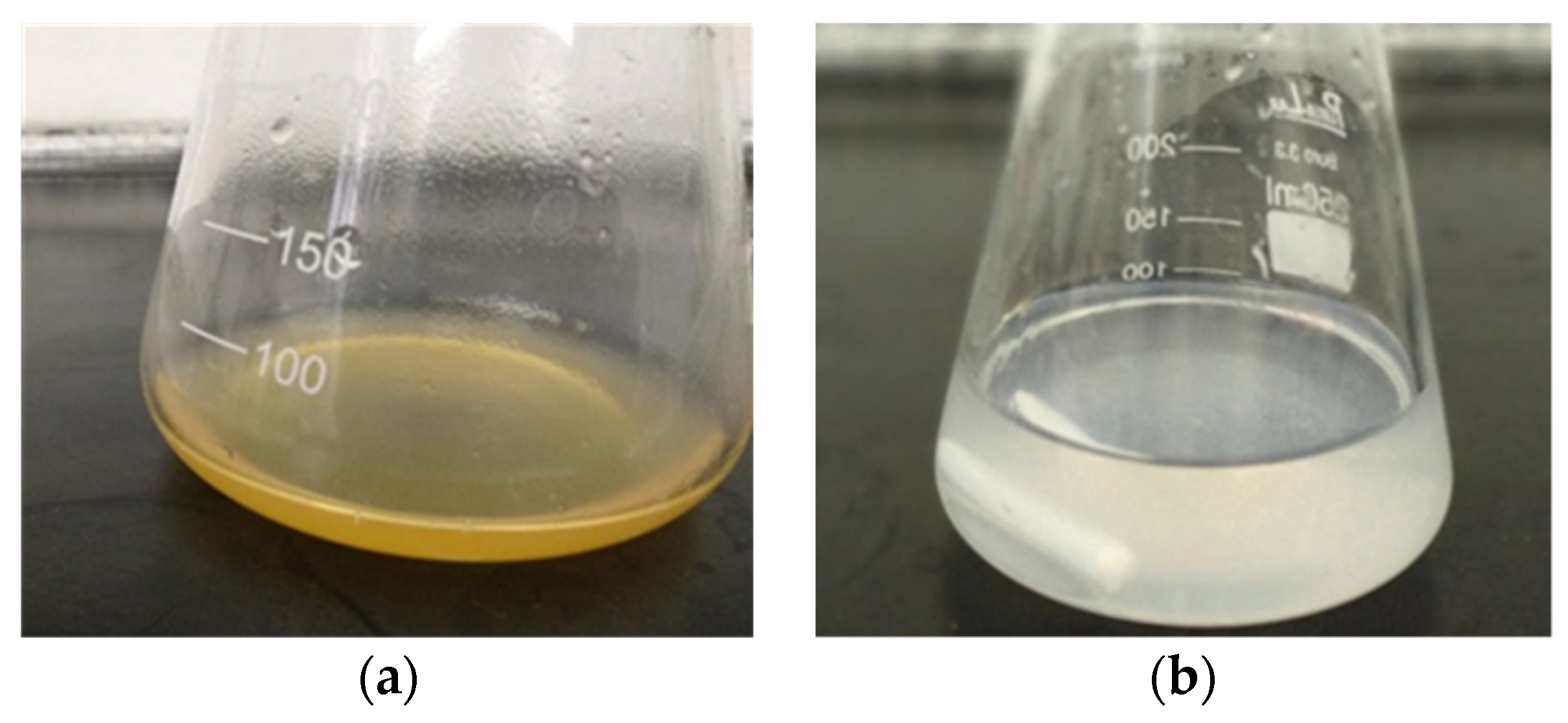
2.2.2. Purification
2.2.3. Mineral Carbonation
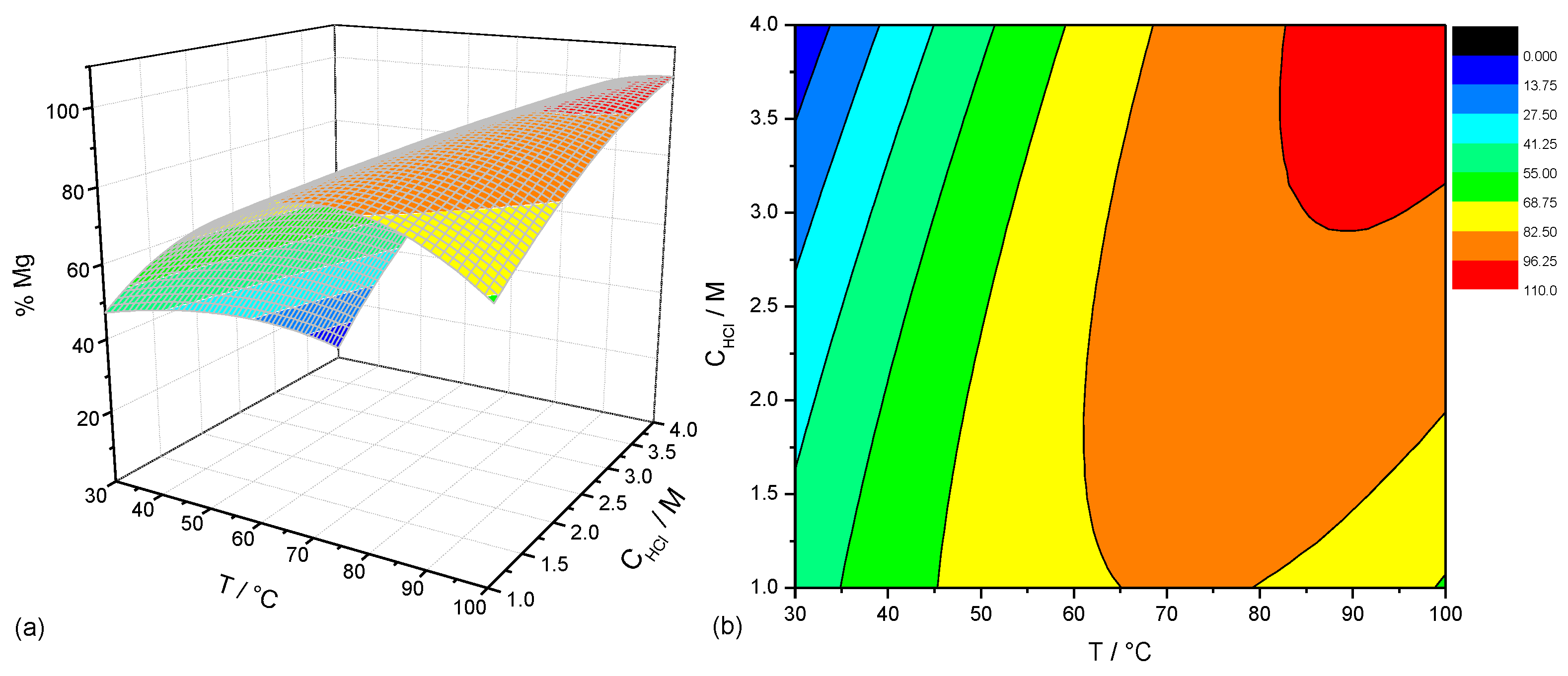
2.3. Experimental Design: Dissolution Stage
3. Results and Discussion
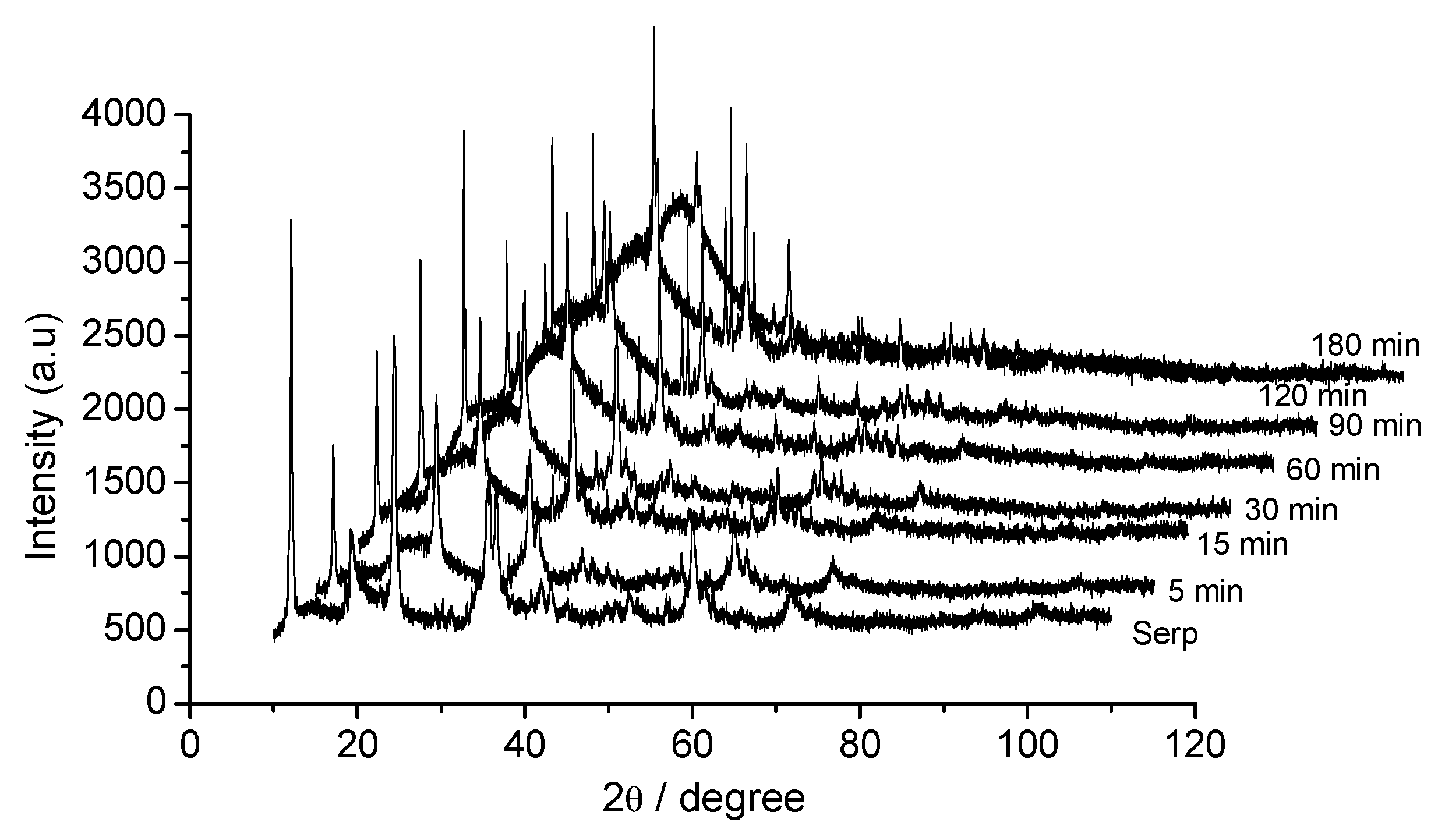
3.1. Dissolution Step
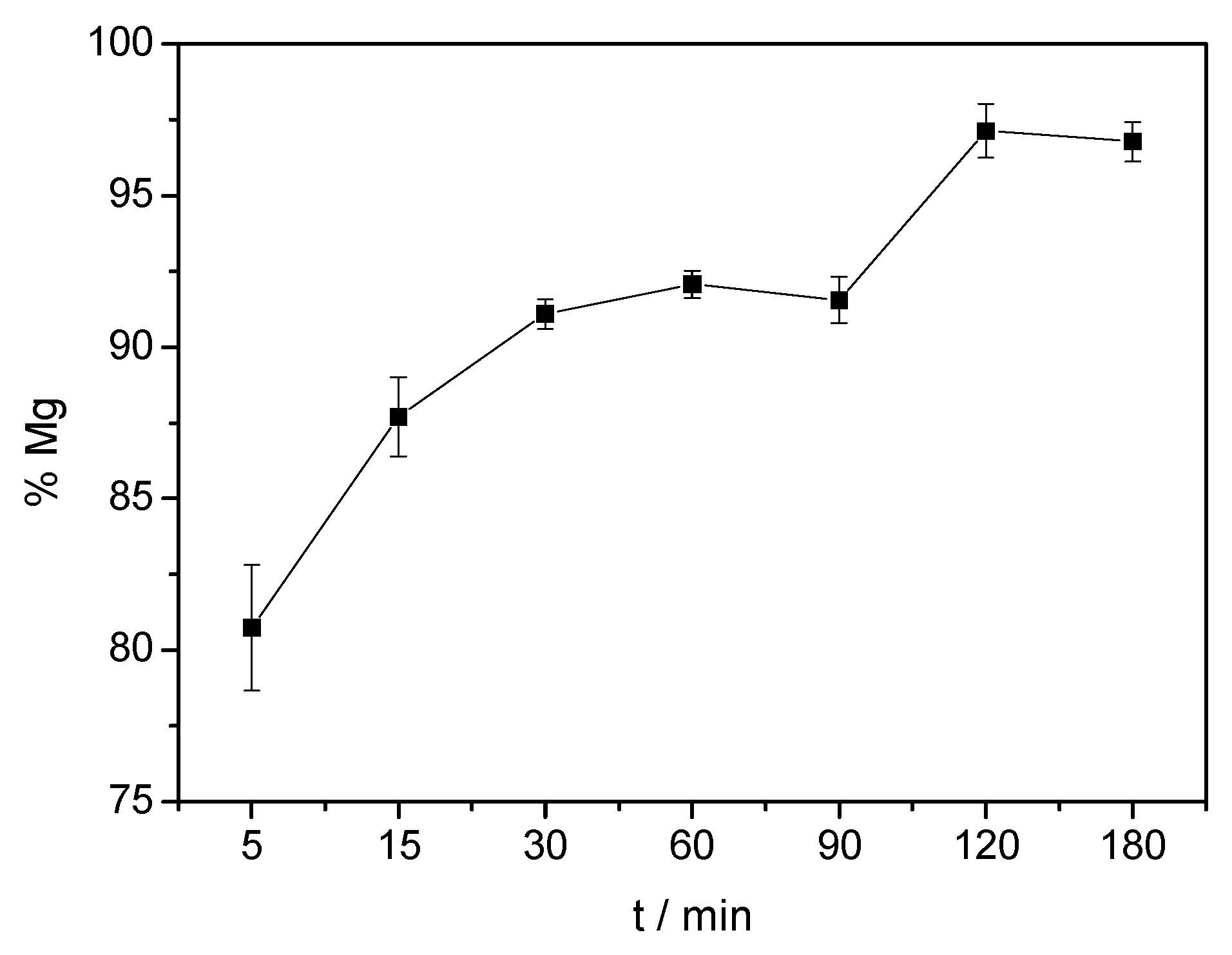
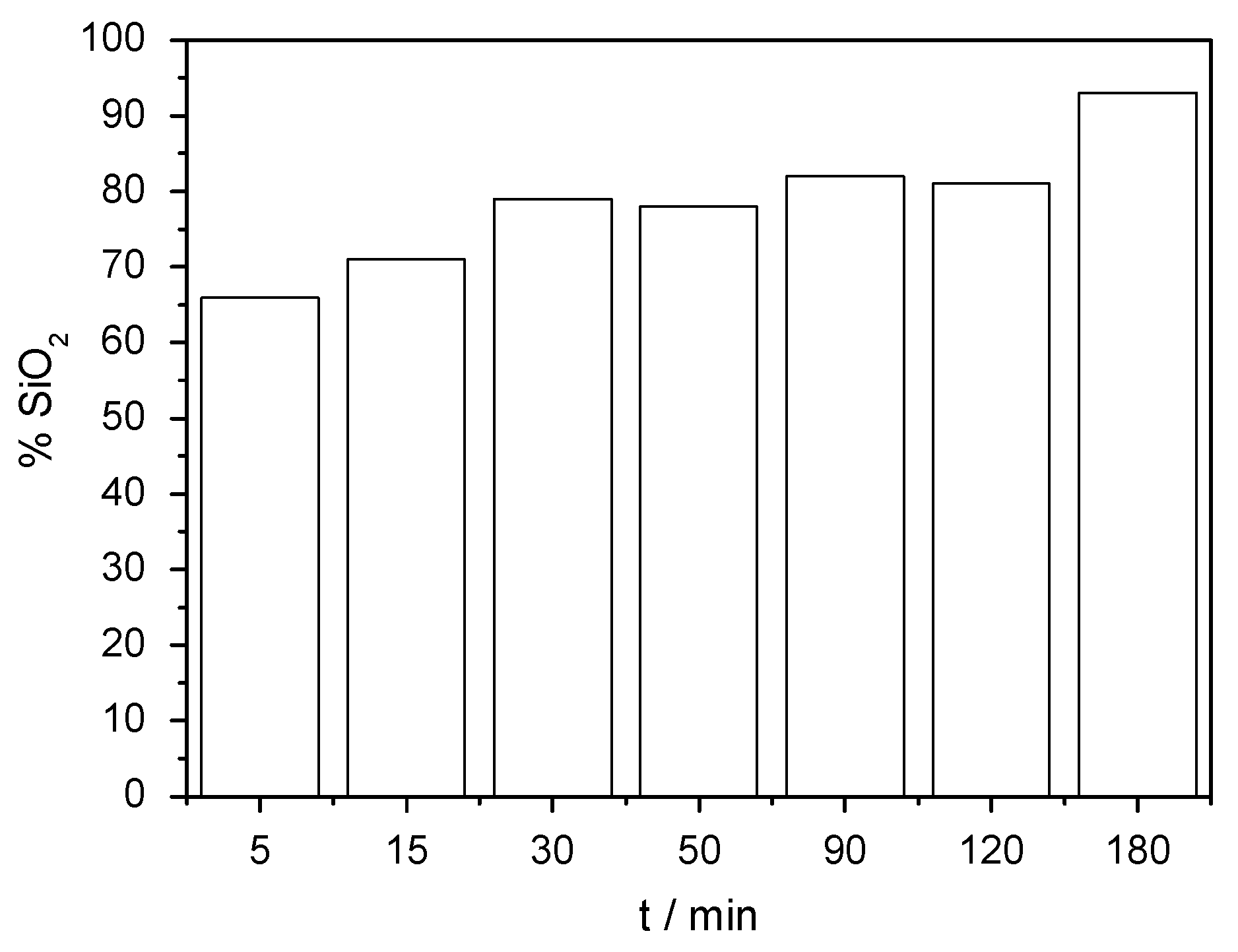
3.2. Effect of Time on Acid Dissolution
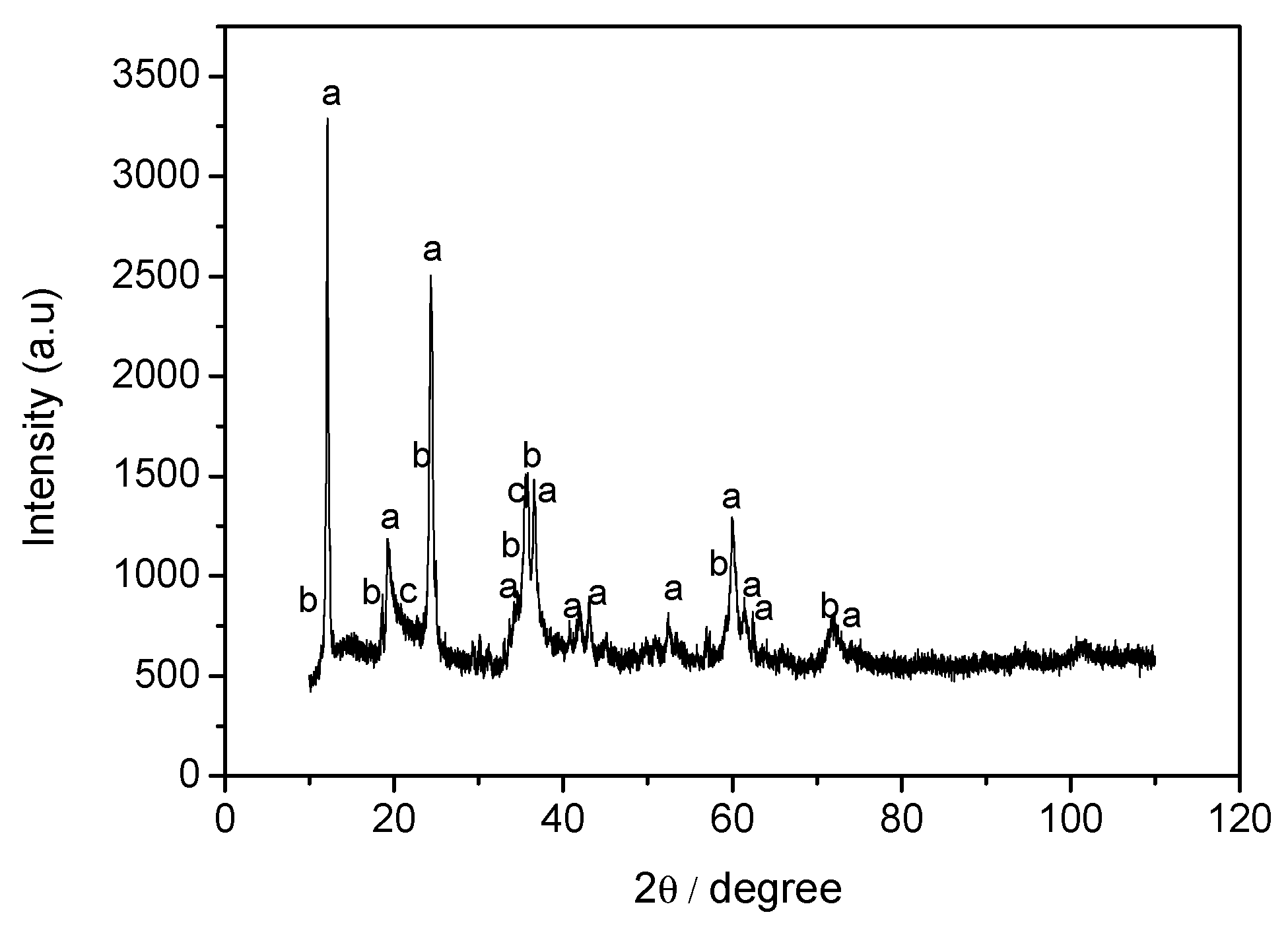
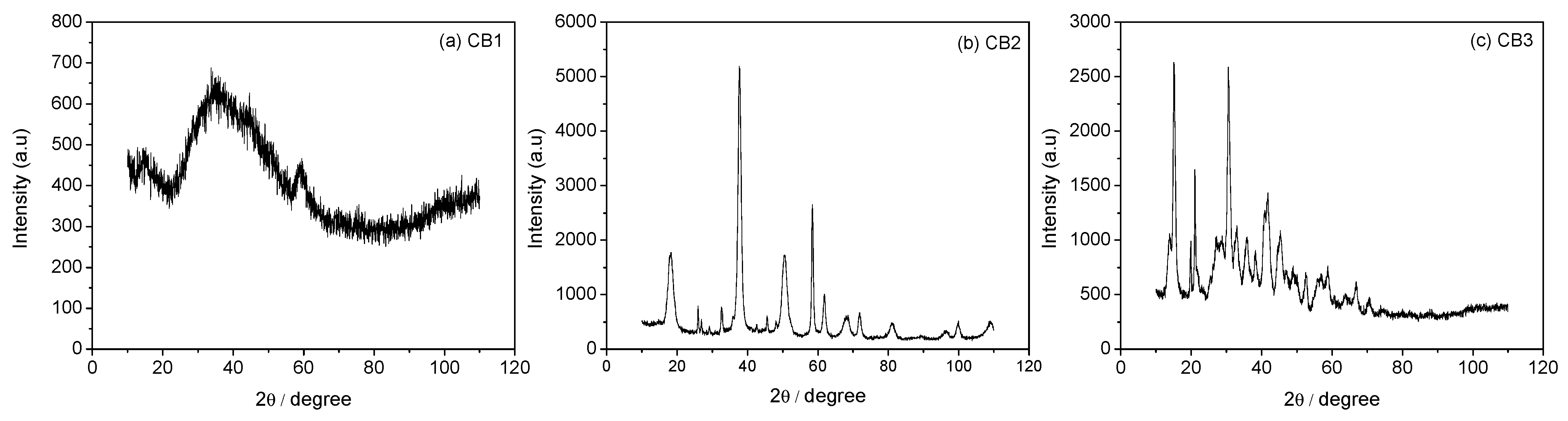
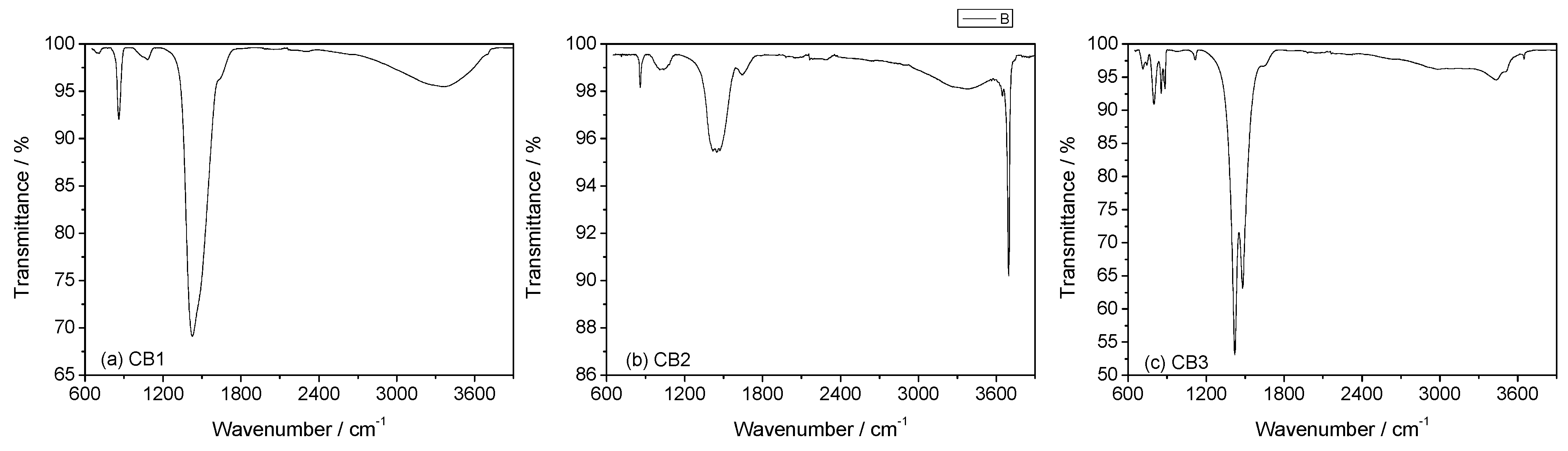
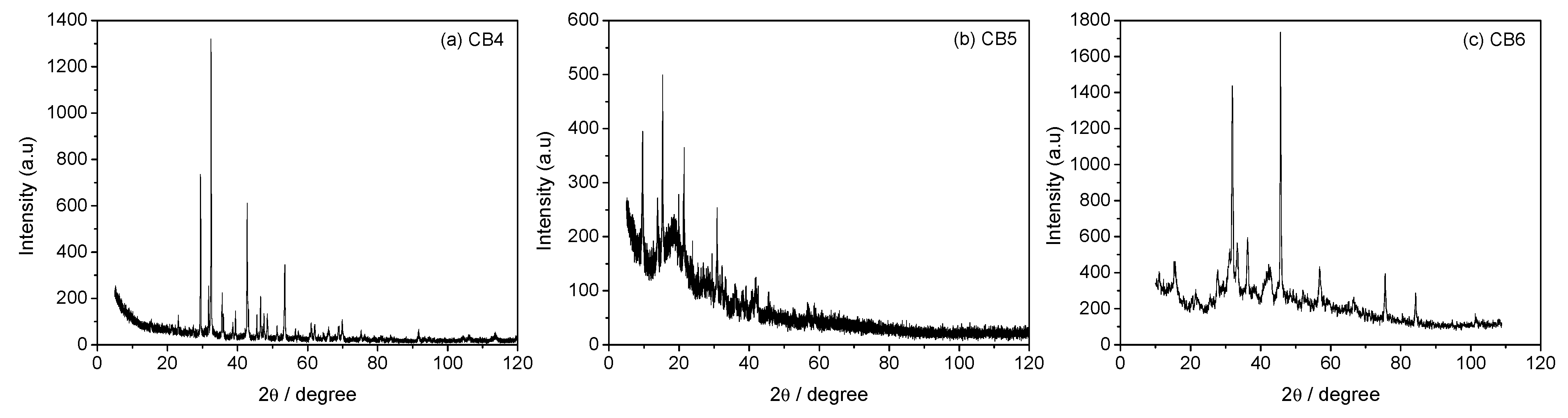
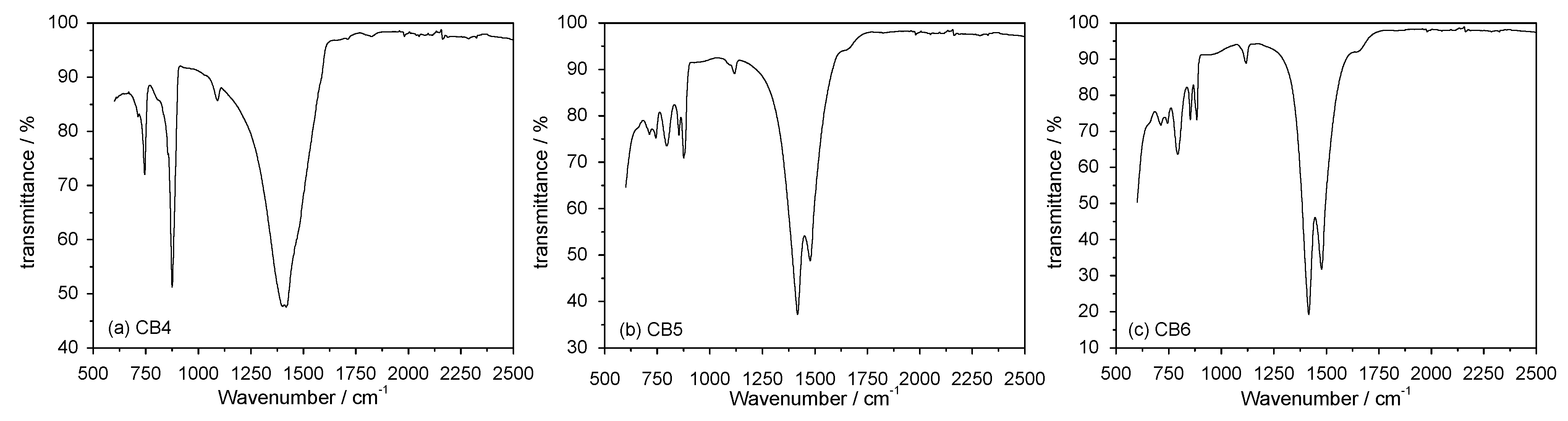
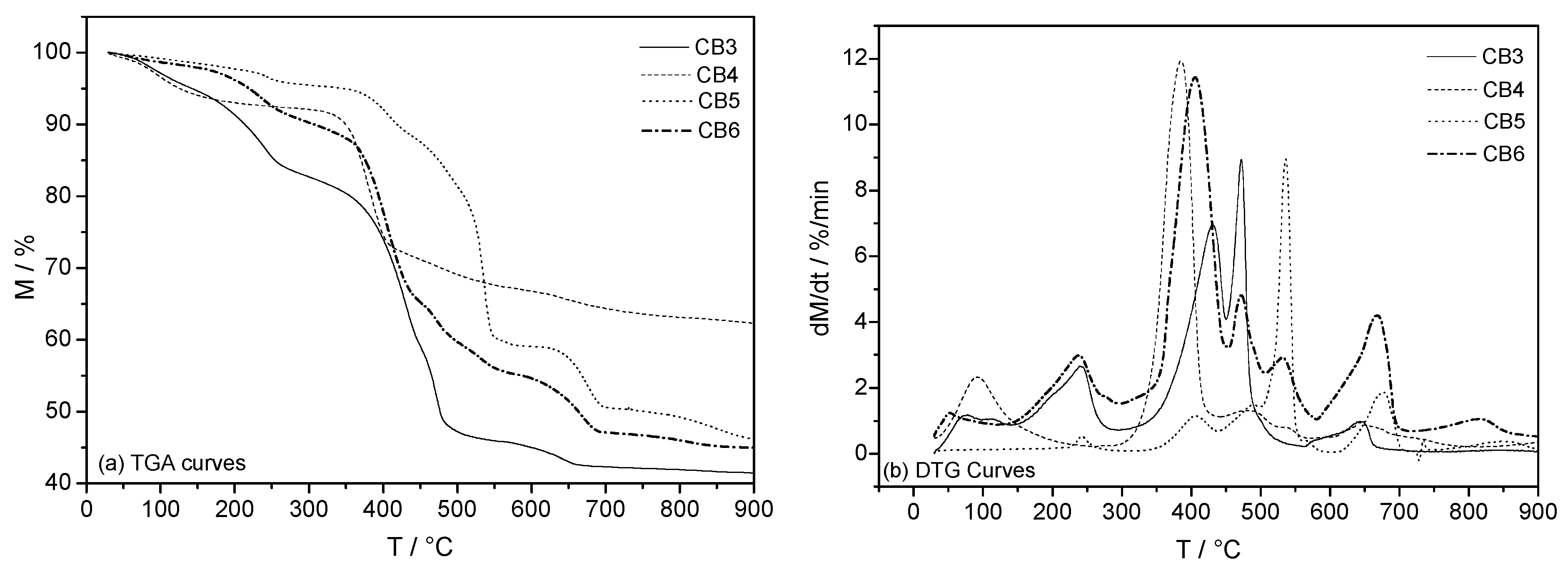
3.3. Mineral Carbonation Step
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mac Dowell, N.; Fennell, P.S.; Shah, N.; Maitland, G.C. The Role of CO2 Capture and Utilization in Mitigating Climate Change. Nat. Clim. Chang. 2017, 7, 243–249. [Google Scholar] [CrossRef]
- Renforth, P. The Negative Emission Potential of Alkaline Materials. Nat. Commun. 2019, 10, 1401. [Google Scholar] [CrossRef] [PubMed]
- Kanniche, M.; Gros-Bonnivard, R.; Jaud, P.; Valle-Marcos, J.; Amann, J.M.; Bouallou, C. Pre-Combustion, Post-Combustion and Oxy-Combustion in Thermal Power Plant for CO2 Capture. Appl. Therm. Eng. 2010, 30, 53–62. [Google Scholar] [CrossRef]
- Kheirinik, M.; Ahmed, S.; Rahmanian, N. Comparative Techno-Economic Analysis of Carbon Capture Processes: Pre-Combustion, Post-Combustion, and Oxy-Fuel Combustion Operations. Sustainability 2021, 13, 13567. [Google Scholar] [CrossRef]
- Galina, N.R.; Arce, G.L.A.F.; Ávila, I. Evolution of Carbon Capture and Storage by Mineral Carbonation: Data Analysis and Relevance of the Theme. Miner. Eng. 2019, 142, 105879. [Google Scholar] [CrossRef]
- Neeraj; Yadav, S. Carbon Storage by Mineral Carbonation and Industrial Applications of CO2. Mater. Sci. Energy Technol. 2020, 3, 494–500. [Google Scholar] [CrossRef]
- Veetil, S.P.; Hitch, M. Recent Developments and Challenges of Aqueous Mineral Carbonation: A Review. Int. J. Environ. Sci. Technol. 2020, 17, 4359–4380. [Google Scholar] [CrossRef]
- Olajire, A.A. A Review of Mineral Carbonation Technology in Sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Mohammadian, E.; Hamidi, H.; Junin, R.; Karaei, M.A. A Review on Carbon Dioxide Mineral Carbonation through PH-Swing Process. Chem. Eng. J. 2015, 279, 615–630. [Google Scholar] [CrossRef]
- Hills, C.D.; Tripathi, N.; Carey, P.J. Mineralization Technology for Carbon Capture, Utilization, and Storage. Front. Energy Res. 2020, 8, 1–14. [Google Scholar] [CrossRef]
- Vieira, K.R.M.; Arce, G.L.A.F.; Luna, C.M.R.; Facio, V.O.; Carvalho, J.A.; Neto, T.G.S.; Ávila, I. Understanding the Acid Dissolution of Serpentinites (Tailings and Waste Rock) for Use in Indirect Mineral Carbonation. South African J. Chem. Eng. 2022, 40, 154–164. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A Review of Mineral Carbonation Technologies to Sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [PubMed]
- Bobicki, E.R.; Liu, Q.; Xu, Z.; Zeng, H. Carbon Capture and Storage Using Alkaline Industrial Wastes. Prog. Energy Combust. Sci. 2012, 38, 302–320. [Google Scholar] [CrossRef]
- Kemache, N.; Pasquier, L.; Cecchi, E.; Mouedhen, I.; Blais, J.; Mercier, G. Aqueous Mineral Carbonation for CO2 Sequestration: From Laboratory to Pilot Scale. Fuel Process. Technol. 2017, 166, 209–216. [Google Scholar] [CrossRef]
- Benhelal, E.; Imran, M.; Rayson, M.S.; Prigge, J.; Molloy, S.; Brent, F.; Cote, A.; Stockenhuber, M.; Kennedy, E.M. Study on Mineral Carbonation of Heat Activated Lizardite at Pilot and Laboratory Scale. J. CO2 Util. 2018, 26, 230–238. [Google Scholar] [CrossRef]
- Sanna, A.; Dri, M.; Maroto-Valer, M. Carbon Dioxide Capture and Storage by PH Swing Aqueous Mineralisation Using a Mixture of Ammonium Salts and Antigorite Source. Fuel 2013, 114, 153–161. [Google Scholar] [CrossRef]
- Arce Ferrufino, G.L.A.; Okamoto, S.; Dos Santos, J.C.; de Carvalho, J.A.; Avila, I.; Romero Luna, C.M.; Gomes Soares Neto, T. CO2 Sequestration by PH-Swing Mineral Carbonation Based on HCl/NH4OH System Using Iron-Rich Lizardite 1T. J. CO2 Util. 2018, 24, 164–173. [Google Scholar] [CrossRef]
- Teir, S.; Kuusik, R.; Fogelholm, C.-J.; Zevenhoven, R. Production of Magnesium Carbonates from Serpentinite for Long-Term Storage of CO2. Int. J. Miner. Process. 2007, 85, 1–15. [Google Scholar] [CrossRef]
- Hemmati, A.; Shayegan, J.; Bu, J.; Yeo, T.Y.; Sharratt, P. Process Optimization for Mineral Carbonation in Aqueous Phase. Int. J. Miner. Process. 2014, 130, 20–27. [Google Scholar] [CrossRef]
- Park, A.-H.H.A.; Fan, L.-S.S. CO2 Mineral Sequestration: Physically Activated Dissolution of Serpentine and PH Swing Process. Chem. Eng. Sci. 2004, 59, 5241–5247. [Google Scholar] [CrossRef]
- Wang, X.; Maroto-valer, M.M. Dissolution of Serpentine Using Recyclable Ammonium Salts for CO2 Mineral Carbonation. Fuel 2011, 90, 1229–1237. [Google Scholar] [CrossRef]
- Teir, S.; Revitzer, H.; Eloneva, S.; Fogelholm, C.-J.J.; Zevenhoven, R. Dissolution of Natural Serpentinite in Mineral and Organic Acids. Int. J. Miner. Process. 2007, 83, 36–46. [Google Scholar] [CrossRef]
- Yuen, Y.T.; Sharratt, P.N.; Jie, B. Carbon Dioxide Mineralization Process Design and Evaluation: Concepts, Case Studies, and Considerations. Environ. Sci. Pollut. Res. 2016, 23, 22309–22330. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Maroto-Valer, M.M. Optimization of Carbon Dioxide Capture and Storage with Mineralisation Using Recyclable Ammonium Salts. Energy 2013, 51, 431–438. [Google Scholar] [CrossRef]
- Dri, M.; Sanna, A.; Maroto-Valer, M.M. Mass and Energy Balance of NH4-Salts PH Swing Mineral Carbonation Process Using Steel Slag. Energy Procedia 2014, 63, 6544–6547. [Google Scholar] [CrossRef]
- Teir, S. Fixation of Carbon Dioxide by Producing Carbonates from Minerals and Steelmaking Slags; Helsinki University of Technology: Espoo, Finland, 2008. [Google Scholar]
- Rashid, M.I.; Benhelal, E.; Farhang, F.; Oliver, T.K.; Stockenhuber, M.; Kennedy, E.M. Application of a Concurrent Grinding Technique for Two-Stage Aqueous Mineral Carbonation. J. CO2 Util. 2020, 42, 101347. [Google Scholar] [CrossRef]
- Bu, J.; Bai, P.; Sharratt, P. Carbon Dioxide Capture with Regeneration of Salt. 2012. [Google Scholar]
- Arce, G.L.A.F.; Soares Neto, T.G.; Ávila, I.; Luna, C.M.R.; Carvalho, J.A. Leaching optimization of mining wastes with lizardite and brucite contents for use in indirect mineral carbonation through the pH swing method. J. Clean. Prod. 2017, 141, 1324–1336. [Google Scholar] [CrossRef]
- Stokreef, S.; Sadri, F.; Stokreef, A.; Ghahreman, A. Mineral Carbonation of Ultramafic Tailings: A Review of Reaction Mechanisms and Kinetics, Industry Case Studies, and Modelling. Clean. Eng. Technol. 2022, 8, 100491. [Google Scholar] [CrossRef]
- Sanna, A.; Gaubert, J.; Maroto-valer, M.M. Alternative Regeneration of Chemicals Employed in Mineral Carbonation towards Technology Cost Reduction. Chem. Eng. J. 2016, 306, 1049–1057. [Google Scholar] [CrossRef]
- Hitch, M.; Dipple, G.M. Economic Feasibility and Sensitivity Analysis of Integrating Industrial-Scale Mineral Carbonation into Mining Operations. Miner. Eng. 2012, 39, 268–275. [Google Scholar] [CrossRef]
- Zhang, N.; Chai, Y.E.; Santos, R.M.; Šiller, L. Advances in Process Development of Aqueous CO2 Mineralisation towards Scalability. J. Environ. Chem. Eng. 2020, 8, 104453. [Google Scholar] [CrossRef]
- Bi, R.; Chen, C.; Tang, J.; Jia, X.; Xiang, S. Two-Level Optimization Model for Water Consumption Based on Water Prices in Eco-Industrial Parks. Resour. Conserv. Recycl. 2019, 146, 308–315. [Google Scholar] [CrossRef]
- Sanna, A.; Steel, L.; Maroto-Valer, M.M. Carbon Dioxide Sequestration Using NaHSO4 and NaOH: A Dissolution and Carbonation Optimisation Study. J. Environ. Manag. 2017, 189, 84–97. [Google Scholar] [CrossRef]
- Daval, D.; Hellmann, R.; Martinez, I.; Gangloff, S.; Guyot, F. Lizardite Serpentine Dissolution Kinetics as a Function of PH and Temperature, Including Effects of Elevated PCO2. Chem. Geol. 2013, 351, 245–256. [Google Scholar] [CrossRef]
- Teir, S.; Eloneva, S.; Fogelholm, C.-J.; Zevenhoven, R. Carbonation of Minerals and Industrial By-Products for CO2 Sequestration. In Proceedings of the 3rd International Green Energy Conference, Västeras, Sweden, 18–20 June 2007. [Google Scholar]
- Brown, T.L.; LeMay, E.; Bursten, B. Química: A Ciência Central, 9th ed.; Pearson Prentice Hall: São Paulo, Brazil, 2005; ISBN 85-87918-42-7. [Google Scholar]
- Kola, A.K.; Mekala, M.; Goli, V.R. Experimental Design Data for the Biosynthesis of Citric Acid Using Central Composite Design Method. Data Br. 2017, 12, 234–241. [Google Scholar] [CrossRef]
- Venkatesan, L.; Harris, A.; Greyling, M. Optimisation of Air Rate and Froth Depth in Flotation Using a CCRD Factorial Design—PGM Case Study. Miner. Eng. 2014, 66–68, 221–229. [Google Scholar] [CrossRef]
- Mortari, D.A.; Ávila, I.; Crnkovic, P.M. Response Surface Methodology Applied to the Evaluation of the SO2 Sorption Process in Two Brazilian Limestones. Energy Fuels 2013, 27, 2890–2898. [Google Scholar] [CrossRef]
- Bösiger, P.; Richard, I.M.T.; Le Gat, L.; Michen, B.; Schubert, M.; Rossi, R.M.; Fortunato, G. Application of Response Surface Methodology to Tailor the Surface Chemistry of Electrospun Chitosan-Poly(Ethylene Oxide) Fibers. Carbohydr. Polym. 2018, 186, 122–131. [Google Scholar] [CrossRef]
- Sanna, A.; Wang, X.; Lacinska, A.; Styles, M.; Paulson, T.; Maroto-Valer, M.M. Enhancing Mg Extraction from Lizardite-Rich Serpentine for CO2 Mineral Sequestration. Miner. Eng. 2013, 49, 135–144. [Google Scholar] [CrossRef]
- Arce, G.L.A.F.; Neto, T.G.S.; Ávila, I.; Luna, C.M.R.; dos Santos, J.C.; Carvalho, J.A. Influence of Physicochemical Properties of Brazilian Serpentinites on the Leaching Process for Indirect CO2 Mineral Carbonation. Hydrometallurgy 2017, 169, 142–151. [Google Scholar] [CrossRef]
- Hemmati, A.; Shayegan, J.; Sharratt, P.; Yeo, T.Y.; Bu, J. Solid Products Characterization in a Multi-Step Mineralization Process. Chem. Eng. J. 2014, 252, 210–219. [Google Scholar] [CrossRef]
- Tutolo, B.M.; Luhmann, A.J.; Tosca, N.J.; Seyfried, W.E. Serpentinization as a Reactive Transport Process: The Brucite Silicification Reaction. Earth Planet. Sci. Lett. 2018, 484, 385–395. [Google Scholar] [CrossRef]
- Hollingbery, L.A.; Hull, T.R. The Thermal Decomposition of Natural Mixtures of Huntite and Hydromagnesite. Thermochim. Acta 2012, 528, 45–52. [Google Scholar] [CrossRef]
- Winnefeld, F.; Epifania, E.; Montagnaro, F.; Gartner, E.M. Further Studies of the Hydration of MgO-Hydromagnesite Blends. Cem. Concr. Res. 2019, 126, 105912. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Mohammadian, E.; Junin, R.; Hamidi, H.; Manan, M.; Rafizan, A.; Daud, M. Mineral Carbonation of Red Gypsum via PH-Swing Process: Effect of CO2 Pressure on the Efficiency and Products Characteristics. Chem. Eng. J. 2015, 264, 425–436. [Google Scholar] [CrossRef]
- Herring, A.; King, P.L.; Saadatfar, M.; Mahdini, F.; Kemis Yahyah, A.M.; Andò, E. 3D Microstructure Controls on Mineral Carbonation. J. CO2 Util. 2021, 47, 101494. [Google Scholar] [CrossRef]
- Maroto-Valer, M.M.; Fauth, D.J.; Kuchta, M.E.; Zhang, Y.; Andrésen, J.M. Activation of Magnesium Rich Minerals as Carbonation Feedstock Materials for CO2 Sequestration. Fuel Process. Technol. 2005, 86, 1627–1645. [Google Scholar] [CrossRef]
- Krevor, S.C.; Lackner, K.S. Enhancing Process Kinetics for Mineral Carbon Sequestration. Energy Procedia 2009, 1, 4867–4871. [Google Scholar] [CrossRef]
- Pasquier, L.C.; Mercier, G.; Blais, J.F.; Cecchi, E.; Kentish, S. Technical & Economic Evaluation of a Mineral Carbonation Process Using Southern Québec Mining Wastes for CO2 Sequestration of Raw Flue Gas with by-Product Recovery. Int. J. Greenh. Gas Control 2016, 50, 147–157. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, R.; Geerlings, H.; Bi, J. Mg-Silicate Carbonation Based on an HCl- and NH 3-Recyclable Process: Effect of Carbonation Temperature. Chem. Eng. Technol. 2012, 35, 525–531. [Google Scholar] [CrossRef]
| Types of Carbonation | Advantage | Disadvantage | Application |
|---|---|---|---|
| pH-Swing | Shorter reaction time; greater efficiency | Two or more reactors; regeneration of chemical additives; large water consumption in regeneration stages | It cannot be currently applied due to its large energy consumption in additive regeneration stages |
| Aqueous Mineral Carbonation | A single reactor | Additive regeneration; non-reusable chemical additives; impure carbonates | Difficult applicability due to the level of complexity to regenerate used additives |
| Gas–Solid Mineral Carbonation | A single reactor; simple process | Very slow kinetics | Non-applicable due to very slow reaction rates |
| Oxides | XRF Concentration (%) | Elements | ICP-OES * Concentration (%) |
|---|---|---|---|
| MgO | 37.09 | Mg | 23 |
| Al2O3 | 1.62 | Al | 0.35 |
| SiO2 | 44.23 | Si | 8.4 |
| CaO | 2.79 | Ca | 279.5 |
| Cr2O3 | 0.84 | Cr | n.d |
| Fe2O3 | 12.82 | Fe | 4.49 |
| NiO | 0.58 | Ni | n.d |
| Experiments | Temperature | Pressure | CNaOH | pH |
|---|---|---|---|---|
| CB1 | 70 | 1 atm | 50% | 11 |
| CB2 | 90 | 1 atm | 50% | 11 |
| CB3 | 90 | 1 atm | solid (2.5 g) | 11 |
| CB4 | 70 | 100 bar | solid (0.8 g) | 11 |
| CB5 | 90 | 100 bar | solid (0.8 g) | 11 |
| CB6 | 90 | 150 bar | solid (0.8 g) | 11 |
| Factors | Description | Level | ||||
|---|---|---|---|---|---|---|
| −1 | 0 | +1 | ||||
| x1 | Temperature, T (°C) | 30 | 40 | 65 | 90 | 100 |
| x2 | HCl concentration, CHCl (M) | 1 | 1.44 | 2.5 | 3.5 | 4 |
| Experiments | Coded Factors | Numeric Factors | Response | ||
|---|---|---|---|---|---|
| x1 | x2 | T (°C) | CHCl (M) | % Mg | |
| 1 | −1 | −1 | 40 | 1.4 | 61 |
| 2 | +1 | −1 | 90 | 1.4 | 79 |
| 3 | −1 | +1 | 40 | 3.5 | 34 |
| 4 | +1 | +1 | 90 | 3.5 | 91 |
| 5 | − | 0 | 30 | 2.5 | 29 |
| 6 | 0 | + | 65 | 4 | 84 |
| 7 | 0 | 100 | 2.5 | 96 | |
| 8 | 0 | − | 65 | 1 | 82 |
| 9 | 0 | 0 | 65 | 2.5 | 85 |
| 10 | 0 | 0 | 65 | 2.5 | 85 |
| 11 | 0 | 0 | 65 | 2.5 | 85 |
| 12 | 0 | 0 | 65 | 2.5 | 85 |
| 13 | 0 | 0 | 65 | 2.5 | 85 |
| Variable | Sum of Squares | df | Mean Square | F | p-Value | R2 |
|---|---|---|---|---|---|---|
| x1 | 3549.94 | 1 | 3549.94 | 135.85 | 0 | n.d |
| x2 | 22 | 1 | 22 | 0.84 | 0.389 | n.d |
| x12 | 1118.2 | 1 | 1118.2 | 42.79 | 0 | n.d |
| x22 | 44.65 | 1 | 44.65 | 1.71 | 0.232 | n.d |
| x1x2 | 386.32 | 1 | 386.32 | 14.78 | 0.006 | n.d |
| Model | 5081.94 | 5 | 1016.39 | 38.89 | 0 | 96.53% |
| Residue | 182.92 | 7 | 26.13 | n.d | n.d | |
| Total | 5264.86 | 12 | 438.73 | n.d | n.d |
| Experiments | Product | Efficiency |
|---|---|---|
| CB1 | Amorphous u.d. | n.d |
| CB2 | Mg (OH)2 | n.d |
| CB3 | Hydromagnesite | 66% |
| CB4 | Magnesite (MgCO3) | 78% |
| CB5 | Hydromagnesite | 76% |
| CB6 | Hydromagnesite | 90% |
| Process Type | System | t (min) | T (°C) | p (Bar) | Reference | |
|---|---|---|---|---|---|---|
| pH swing | MgCl2-CO2-NaOH-H2O | 30 | 90 | 100 | 76% | This study |
| 150 | 90% | |||||
| pH swing | MgCl2-CO2-NH3-H2O | 60 | 70 | 10 | 43.5% | [51] |
| 20 | 60.9% | |||||
| 30 | 66.7% | |||||
| 60 | 68.6% | |||||
| pH Swing * | MgSO4-NaOH-CO2-H2O | 10 | 20 | 40 | 55% | [52] |
| Aqueous carbonation * | EDTA | 420 | 120 | 20 | 80% | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galina, N.R.; Arce, G.L.A.F.; Maroto-Valer, M.; Ávila, I. Experimental Study on Mineral Dissolution and Carbonation Efficiency Applied to pH-Swing Mineral Carbonation for Improved CO2 Sequestration. Energies 2023, 16, 2449. https://doi.org/10.3390/en16052449
Galina NR, Arce GLAF, Maroto-Valer M, Ávila I. Experimental Study on Mineral Dissolution and Carbonation Efficiency Applied to pH-Swing Mineral Carbonation for Improved CO2 Sequestration. Energies. 2023; 16(5):2449. https://doi.org/10.3390/en16052449
Chicago/Turabian StyleGalina, Natália R., Gretta L. A. F. Arce, Mercedes Maroto-Valer, and Ivonete Ávila. 2023. "Experimental Study on Mineral Dissolution and Carbonation Efficiency Applied to pH-Swing Mineral Carbonation for Improved CO2 Sequestration" Energies 16, no. 5: 2449. https://doi.org/10.3390/en16052449
APA StyleGalina, N. R., Arce, G. L. A. F., Maroto-Valer, M., & Ávila, I. (2023). Experimental Study on Mineral Dissolution and Carbonation Efficiency Applied to pH-Swing Mineral Carbonation for Improved CO2 Sequestration. Energies, 16(5), 2449. https://doi.org/10.3390/en16052449







