Abstract
Recent technological developments have led to a significant increase in energy consumption in daily life. The search for alternative means of energy production has become an important task for applied sciences and modern technology. Hydrogen technology has great potential as a source of clean energy. The production of green hydrogen is a desirable and beneficial way to contribute to the decarbonization of the energy sector. In response to the demand for environmentally friendly and economically feasible approaches, biohydrogen production from waste materials has recently attracted interest. Waste materials from industrial or municipal production can be used as low-cost substrates for biohydrogen production through microbial degradation. Green energy needs could be met through a form of sustainable development that moves hand in hand with the harnessing of the microbial potential of waste biomass. Reuse of waste materials leads to pollution reductions and energy recycling. The aim of this review is to provide informative insights for researchers and engineers to help them better understand microbial biohydrogen production from low-cost waste substrates, such as industrial wastewater and waste activated sludge.
1. Introduction
The increase in energy demand is closely connected to the growth in the population and economic development of the world. The global energy crisis has exacerbated the tensions over the management and sustainability of fossil fuels that are responsible for most of the increases in energy demand [1]. In addition, the limited reserves of fossil fuels, the looming climate changes, the perceived need for energy security and the dangers that the use of petroleum products poses to the environment and human health are the driving factors for the development of green fuels [2]. The growing environmental awareness among the population has raised the question of how to balance energy demand and environmental protection. One important strategy for slowing global warming and reducing harmful environmental impacts is to reduce greenhouse gas emissions by replacing fossil carbon-based energy sources and materials with renewable carbon-free resources [3].
Hydrogen represents one of the most promising energy carriers of the future. It is a potentially sustainable energy source with a high specific energy density. Its combustion produces only water, which makes it environmentally friendly [4,5]. Hydrogen has a notable role to play in soliciting the decarbonization of energy-intensive industrial sectors and enhancing energy security [3,6]. As the development of technologies that contribute to the modernization of society rapidly accelerates, the problems of resources and the environment become more heavily emphasized, which makes the reuse of waste streams an increasingly important activity for sustainable development. Hydrogen can be produced through photochemical and thermochemical processes, electrolysis, and biological processes. Although hydrogen is produced mainly through steam reforming or thermal cracking of natural gas or petroleum fractions, efficient methods to produce hydrogen from renewable sources are being developed [7]. Among these alternatives, aqueous-phase reforming has been proposed as a process that can be carried out under relatively mild conditions and can convert oxygenated molecules into hydrogen. It follows that it can be applied to high-carbon wastewater, increasing the conversion efficiency of plants, reducing the amount of waste to be processed, and generating a valuable product at the same time [8,9]. Many governments, especially in Europe, are turning to low-emission hydrogen to reduce dependence on fossil fuels [6] because conventional methods of hydrogen production are energy-intensive and not beneficial for the environment. Biological processes and renewable sources, such as waste, are being prioritized as they are considered part of green technology.
The production of biohydrogen is advantageous because the reactions are less energy-intensive; specifically, there are mild reaction conditions, such as ambient temperature and pressure [10,11]. Moreover, when organic waste is used as a substrate for biohydrogen production, clean energy production and waste management can be achieved. Some microorganisms can produce hydrogen through their metabolism. Hydrogen-producing microorganisms have the enzymatic potential to utilize different types of substrates. They can be used for the degradation of organic substrates that represent waste streams and require further treatment. The use of microbial metabolism for hydrogen production can contribute to waste management and energy production at the same time, providing both environmental and economic benefits [12,13,14]. In addition, biological processes are the better option for decentralized energy production in smaller plants and in locations where waste substrates are readily available, making it possible to avoid additional energy consumption and transport costs [15]. Research shows that, from a technological point of view, dark fermentation is the most cost-effective biological technology for hydrogen production. In addition, an environmental impact assessment showed that the greenhouse gas emissions of dark and photo-fermentation are low compared to microbial electrolysis cells. Dark fermentation was also found to be the most suitable method when combined with a microbial electrolysis cell [16].
In recent years, emphasis has been placed on exploring waste streams that can be used for biohydrogen production through biological processes. Waste streams consist of organic fractions that can be used as potential substrates for hydrogen production by microorganisms. The choice of substrate depends on several requirements, such as availability and accessibility, the cost of the substrate, the carbohydrate content and biodegradability [15]. Wastes that are suitable substrates for biohydrogen production are municipal solid waste [15,17,18,19], kitchen and food waste [20,21], agro-industrial waste [22], industrial waste and wastewater [10,23,24] and waste activated sludge [10,25]. Due to globally accelerated urbanization and industrial development, the generation of waste streams is increasing; in particular, municipal solid waste and wastewater, which are increasing due to economic growth [26]. As part of a sustainable approach to waste management, the waste-to-energy procedure seems like a promising strategy. The Reduce, Reuse, Recycle, Recover principle, commonly known as the 4Rs, is an approach that can be applied side by side with the solving of problems related to waste management, energy production and environmental protection. This principle can enable an easier transition from a linear to a circular economy and, thus, the realization of beneficial sustainable development [10]. Considering the 4Rs principle, efforts should be made to avoid the production of municipal solid waste and food waste. In any industrial process, the generation of waste streams is unavoidable. Therefore, the focus of waste-to-energy processes should be those waste streams that it is not possible to avoid generating or which cannot be used as animal feed. Hence, wastewater from industry and waste activated sludge, as a by-product of biological wastewater treatment systems, are waste streams that have great potential from the perspective of sustainable development and the circular economy. Resources for industrial production are becoming more expensive by the day, and these waste streams are not only environmentally sound but also represent raw materials that can be used for hydrogen production, thus enabling sustainable industrial growth.
The main aim of this work was to review recent insights concerning biohydrogen production through microbial activity using industrial wastewater and waste activated sludge as substrates. The potential of these waste streams as low-cost biohydrogen substrates is briefly discussed in this review.
2. Biological Processes for Hydrogen Production
The various biological processes used for the production of hydrogen can be classified into photosynthetic processes, fermentative processes and processes with microbial electrolysis cells (MECs). Photosynthetic processes (direct and indirect biophotolysis) are biological processes in which solar energy is used to drive water-splitting photosynthesis and to generate hydrogen from the energy-rich electrons produced in the process by photosynthetic microorganisms (green algae, cyanobacteria) [2]. The MEC process is a biological hydrogen production process in which a hydrogen evolution reaction is catalyzed by electroactive bacteria in the presence of anaerobic conditions [27]. Anaerobic fermentation is one of the most commonly used processes for biohydrogen production from waste substrates [16]. Depending on the types of microorganisms used and whether they require light to maintain their life cycle, fermentation processes can be divided into photo-fermentation, dark fermentation and processes integrating photo- and dark fermentation [28].
2.1. Microbiology of Biohydrogen-Producing Systems
The biological processes of biohydrogen production are directly influenced by the process conditions, which must meet the requirements for microbial growth. These processes are catalyzed by microorganisms under optimal environmental conditions. The characteristics of these microorganisms differ greatly depending on the substrate and the process conditions [15]. When substrates with high organic content are used for biohydrogen production, such as industrial wastewater and waste activated sludge, the leading biological method is the process of dark fermentation, which is discussed in this section.
In order to realize the two main advantages of waste management and energy generation—or the principle of “waste-to-biohydrogen”—organic waste is preferred as the substrate for hydrogen production via the fermentation process. Real waste samples typically contain complex compounds, such as polysaccharides, proteins and lipids. As polysaccharides can be more easily converted to hydrogen, a high hydrogen yield can be obtained, while proteins and lipids have lower energy conversion efficiency. However, they are essential for microbial growth [13]. The most favorable condition for biohydrogen production from different types of organic waste is a high concentration of soluble substrate. Mesophilic conditions are also preferred because they require lower temperatures and lower energy consumption compared to thermophilic conditions. An acidic pH, which can be maintained without the addition of chemicals, increases the attractiveness and environmental sustainability of this process. In addition, the short residence time means a smaller reactor volume, which helps to reduce the capital and operating costs of the process [29].
The microorganisms present in biohydrogen-producing systems can be defined as hydrogen producers or non-hydrogen producers (Figure 1). In terms of their hydrogen-producing metabolism, hydrogen producers can be classified as photosynthetic microorganisms or fermentative microorganisms (photo-fermentation—e.g., Rhodobacter, Chromatium; and dark fermentation—e.g., Clostridium, Enterobacter, Citrobacter). Hydrogen-producing systems are heterogeneous and interacting ecosystems. In addition to the dependence of the biohydrogen yield on the hydrogen producers, other microbial groups also contribute to the essential functionality of the ecosystem. Microorganisms that are not able to produce hydrogen are categorized as non-hydrogen producers, with the three subgroups of hydrogen consumers, substrate contenders and beneficial bacteria. The undesirable microbial group of hydrogen consumers can reduce the efficiency of biohydrogen production systems due to their consumption of the produced hydrogen and their forming of methane or acetate. Substrate contenders compete with hydrogen producers for the substrate and in this way negatively influence the biohydrogen yield [13].
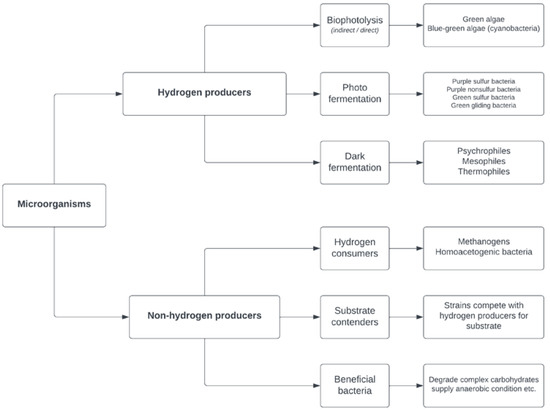
Figure 1.
Microbial groups present in hydrogen-producing systems.
Beneficial bacteria are precursors in hydrogen-producing systems that positively contribute to ecosystem sustainability. They assist in different mechanisms, such as oxygen consumption, pH regulation, substrate hydrolysis, cometabolism and cell granulation [30]. Depleting oxygen traces in hydrogen-producing systems is a key part of sustaining anaerobic conditions, which are mandatory for the growth of strict anaerobic bacteria, such as Clostridium. Facultative anaerobic microorganisms, such as bacteria from the genus Bacillus and Klebsiella sp., have the main roles in oxygen depletion [30,31]. Some microbial species—for instance, Streptococcus sp.—can contribute to the aggregation of microbial biomass in the reactor, resulting in the retention of microbial cells, which prevents biomass washout and increases resistance to undesirable operating conditions [13,30].
A biohydrogen production system consists of complex microbial communities that rely on microbial interactions to create a stable and functioning ecosystem. From an engineering perspective, the production of hydrogen as a desired product can be achieved if the ecosystem is properly managed.
2.2. Role of Microorganisms in Dark Fermentation Process
The dark fermentation process is the most widely studied fermentation process for the utilization of waste materials. In this process, hydrogen-producing microorganisms can use organic fractions of waste materials as a source of carbon and produce biohydrogen without light and oxygen. The production of biohydrogen through dark fermentation has several advantages, such as a high production rate, the ability to efficiently use a variety of organic waste substrates, sustainability [32,33] and no requirement for light energy [34]. The disadvantages of the dark fermentative process include the accumulation of hydrogen in the fermentative system, which can lead to inhibition of bacterial metabolism [35], and the relative sensitivity of the hydrogenate enzyme in dark-fermentative bacteria to oxygen, which can lead to lower hydrogen yields [36]. Microorganisms involved in the dark fermentation process belong to the groups of facultative anaerobic bacteria or obligate anaerobic bacteria.
In dark fermentation, several biochemical processes occur in parallel with those of anaerobic digestion. Different microbial strains act synergistically to contribute to the degradation of organic compounds. The process of anaerobic digestion can be divided into four phases: hydrolysis, acidogenesis, acetogenesis and methanogenesis, with the last phase of methanogenesis being excluded in dark fermentation (Figure 2).
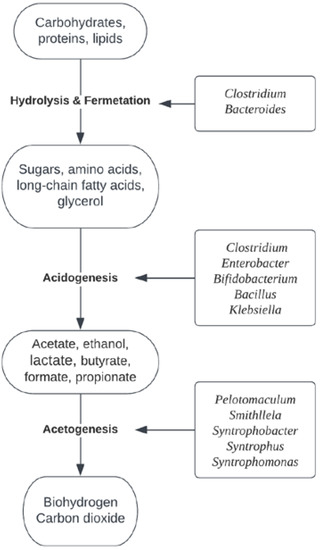
Figure 2.
The biochemical pathway and dominant microbial genera in the dark fermentation process for organic complex compounds.
Complex compounds provide a substrate for microbial degradation in which hydrolysis of carbohydrates, proteins and lipids first occurs. These compounds are hydrolyzed by extracellular hydrolytic enzymes into sugars, amino acids and long-chain fatty acids. The microbial genera Clostridium and Bacteroides are two of the most important microbial strains and contribute to hydrolytic activities with extracellular hydrolytic enzymes (cellulose, lipase, protease) [37]. Members of these groups are less sensitive to changes in environmental conditions and can grow rapidly using hydrolyzed products through fermentation [38].
In the acidogenesis phase, the hydrolyzed products are further metabolized by acidogenic microbial communities into short-chain fatty acids (formate, acetate, propionate, butyrate). The accumulation of short-chain fatty acids leads to a subsequent drop in pH. The microorganisms in the acidogenic phase consist of facultative and obligate anaerobes, such as Clostridium, Enterobacter, Bifidobacterium, Bacillus and Klebsiella [37]. In this phase, carbohydrates are converted into pyruvate via the glycolytic pathway [39].
The metabolic pathway in the dark fermentation process is a limiting parameter that represents a crucial part of the processing steps [22]. Depending on the fermentation pathway and end-products, the production of hydrogen may differ notably in terms of the microbial species (Clostridium, Bacillus, Enterobacter) and the numerous forms of substrates under mesophilic conditions. Two primary pathways via which microorganisms produce hydrogen are acetate and butyrate fermentation. The hydrogen production reactions using acetate and butyrate fermentation are expressed in Equations (1) and (2), for which the theoretical values of 4 mol H2/mol of glucose and 2 mol H2/mol of glucose are obtained for acetate or butyrate as the end-product, respectively [15,22,40].
C6H12O6 + 2H2O → 2CH3COOH + 4H2 + 2CO2
C6H12O6 → CH3CH2CH2COOH + 2H2 + 2CO2
C6H12O6 → CH3COOH + CH3CH2COOH + H2 + CO2
Depending on the dominant formation of volatile fatty acids, there are several other fermentation types for the metabolic pathway. For propionate-type fermentation (Equation (3)) and ethanol-type fermentation, theoretical yields of 1 mol H2/mol of glucose and 2 mol H2/mol of glucose are obtained [41]. Propionate fermentation should be avoided in a hydrogen-producing system due to the low hydrogen yield. Mixed-type fermentation usually occurs at the start-up level in the fermentation process when various fermentation types coexist [13].
The “Thauer limit” is a term that refers to the theoretical hydrogen yield, which is restricted to 4 mol H2/mol of glucose (Equation (1)) [42]. In recent years, studies have been conducted that showed the possibility of increasing hydrogen yield beyond the Thauer limit. Ergal et al. (2020) showed that applying an interdisciplinary approach involving physiology, ecology and biotechnology could, through the designed artificial bacterial consortium, increase the hydrogen yield to 5.6 mol H2/mol of glucose. According to conducted studies [43], the lack of an eco-biotechnological perspective could be the main factor limiting the establishment of advanced hydrogen-producing microbial consortia.
By-products such as butyrate or propionate require bioconversion using syntrophic acetogenesis. In the acetogenesis phase, substrates are converted into acetate, carbon dioxide and hydrogen by fermentative bacteria that have no hydrolytic activities. The most commonly reported syntrophic acetogens in anaerobic digesters belong to the genera Pelotomaculum, Smithllela and Syntrophobacter (propionate degraders) and Syntrophus and Syntrophomonas (degraders of butyrate and other fatty acids) [37]. Acetogenesis is the rate-limiting phase and plays an important role in ensuring stable operating conditions in anaerobic systems.
Obligate anaerobes are the most widely studied bacterial group for biohydrogen production (e.g., Clostridium). However, the combination of obligate anaerobic bacteria and facultative anaerobic bacteria in biohydrogen production may turn out to be a more advantageous approach [44]. In anaerobic mesophilic fermentative ecosystems, the obligate anaerobic species of Clostridium are considered the main hydrogen producers. However, with the development of molecular characterization techniques, other hydrogen-producing communities have been found. These anaerobic bacteria can be categorised as spore-forming obligate anaerobes (Clostridium sp.); non-spore-forming obligate anaerobes (e.g., Ethanoligenens, Acetanaerobacterium, Megasphaera, Acidaminococcus, Prevotella) and fermentative facultative anaerobes (Enterobacter, Citrobacter, Klebsiella) [30].
Most of this microbial diversity in hydrogen-producing systems originates from indigenous microbial cultures in untreated substrates or inocula. Mixed microbial cultures, which can be used as inocula in the dark fermentative process, can come from a great variety of sources, such as soil, sediment, animal manure (cow/poultry), organic waste [13], leachate [45], anaerobic activated sludge [20,32,46,47,48,49], sewage sludge [50,51,52,53], compost [17,54] and wastewater [55,56,57]. Anaerobic activated sludge is a mixed microbial culture that is the primary inoculum for the dark fermentation process due its diversity of hydrogen-producing strains, as well as beneficial microbial strains [13].
Pure cultures can also be applied for biohydrogen production via the dark fermentation process. The most widely studied hydrogen-producing cultures are from the Clostridium genus [58], with leading research on Clostridium beijerinckii and Clostridium butyricum [31,34,59]. Other species, such as Bacillus and Enterobacter, can often be found in coexistence with Clostridium [13]. In addition, other genera, such as Ethanoligenens, Escherichia, Citrobacter and Klebsiella, are also biohydrogen producers according to the literature [37].
Significant efforts have been directed towards isolating and identifying new hydrogen-producing species with specific capabilities, such as species that can be used with different substrates and species that have tolerance to extreme conditions or high efficiency in hydrogen production. Murugan et al. (2018) [60] succeeded in isolating Acinetobacter junii AH4 as a potential bacterial strain that can be used for efficient hydrogen production. This biohydrogen-producing bacterium was isolated from pretreated anaerobic sludge samples from the dairy industry. Murugan et al. (2021) [61] demonstrated biohydrogen production by the abovementioned hydrogen-producing strain from various industrial wastewaters. In the study by Litti et al. (2022) [50], a new hydrogen-producing strain—Thermoanaerobacterium thermosaccharolyticum SP-H2—was isolated with a thermophilic acidogenic reactor inoculated with municipal sewage sludge from the processing of carbohydrate-rich simulated food waste. The newly isolated strain showed promising results in the dark fermentation of carbohydrate-rich wastewater under thermophilic conditions.
To optimize the dark fermentation process, external microbial strains can be inoculated into the hydrogen-producing system. Pretreatment of the inoculum is a common approach used to select specific spore-forming, hydrogen-producing bacteria and inhibit hydrogen-consuming bacteria [62]. Different pretreatment methods can greatly alter the microbial community composition and, thus, microbial activity [63]. Yin et al. (2023) [64] evaluated the effects of the type of inoculum and investigated methods to accelerate the start-up of a hydrogen production system. It was found that, after start-up, the dominant strain for hydrogen production in different types of inoculum sludge was Thermoanaerobacterium thermosaccharolyticum strain TG57. Furthermore, pretreatment of the substrate can lead to an efficient hydrogen production system, as Yang and Wang (2020) [65] found. After chemical pretreatment with sodium citrate followed by ultrasonic pretreatment of waste activated sludge, hydrogen-producing bacteria were enriched, especially those of the genera Clostridium sensu stricto and Paraclostridium, which led to a synergistic increase in hydrogen yield and energy conversion efficiency. An interesting discovery was made by Chen et al. (2021) [66] when they investigated the effect of butyrate on hydrogen production. The results showed that butyric acid inhibited fermentative hydrogen production at pH 5.5–7.0, levels associated with undissociated acids. The addition of butyric acid to the system decreased the substrate utilization rate, as well as the accumulation of volatile fatty acids. The addition of butyric acid affected the microbial ecology of the biohydrogen production system, and the proportion of non-hydrogen-producing bacterial strains, such as Bacillus, Klebsiella, Acinetobacter and Pseudomonas, increased. Recent research [48] has shown that combining waste streams can increase microbial hydrogen production during anaerobic fermentation. An environmentally friendly method using waste such as corncob can enhance solubilization, hydrolysis and acetogenesis in dark fermentation and contribute to the enrichment of hydrolytic microorganisms (e.g., Bacteroides sp. and Leptolinea sp.).
3. Waste Materials as Potential Substrates
The principles of the circular economy can contribute to meeting the need for energy, which is the primary driver of economic and industrial development. Industrial processes are becoming more and more expensive due to the reduced amounts and availability of resources. Waste streams are generally considered a type of material available with lower production costs, making them a desirable substrate from a circular economy perspective [67]. The main issue that arises with regard to biohydrogen production is how the efficient conversion of substrates can be achieved while maintaining low costs and circular economy principles. The use of organic waste as a substrate for biohydrogen production is becoming increasingly important for economic reasons, as the production of renewable, clean energy also involves the biological processing of waste [68]. The various kinds of organic wastes differ in their compositions and structures. Carbohydrates can be extracted from pretreated organic waste in order to obtain a source of carbon for hydrogen-production microorganisms [69]. The scientific community continues to exert great effort in optimizing and maintaining sustainable microbial growth to produce biohydrogen from waste streams [70]. As economic growth is accompanied by demands that waste management meet the challenges of environmental protection, the use of industrial effluents and activated sludge as waste streams has been the focus of attention. Due to their quantities, both industrial wastewater and waste activated sludge represent interesting substrates with high organic content that can be used for biohydrogen production. The aim of this review was, therefore, to focus on industrial wastewater and waste activated sludge as potential substrates for biohydrogen production.
3.1. Industrial Wastewater
Dark fermentation is a relatively low-tech, low-cost process that offers a moderate hydrogen production rate while making it possible to remove organic pollutants from wastewater [71]. Industrial effluents consist of various major soluble and bio-available organic compounds, such as short-chain alcohols and volatile fatty acids (acetic, propionic and butyric acid) [72]. The operational conditions of the dark fermentation process are mainly an acidic to neutral pH value (4.5–7.5) and mesophilic temperature (35–37 °C). The concentration of substrate (g COD/L), hydraulic retention time and choosing the optimal inoculum can have wide-ranging effects on the hydrogen production rate [41]. The succession of mixed microbes can lead to different types of fermentation and has a major impact on the fermentation characteristics and production capacity of wastewater fermentation systems [73]. Despite the common use of carbohydrate model substrates for fermentative hydrogen production, recent studies have focused on potential biohydrogen production from various real industrial wastewaters [45,70,74]. These include dairy wastewater [45], confectionery wastewater [74], rice mill wastewater [57,61,75], beverage wastewater [54,76], sugary wastewater [53,61,77], agricultural [47] and agroindustrial wastewater [52,56] and wastewater from the textile [51] and paper industries [78]. The studies indicate great potential for biohydrogen production from carbohydrate-rich wastewater. The biohydrogen yield depends on various parameters; e.g., the type of wastewater and inoculum, as well as the operating conditions and the type of biological process. In order to achieve optimal biohydrogen production, these parameters should be investigated. This is a sustainable approach for simultaneous green energy production and pollution minimization.
A comparison of the biohydrogen yields from different industrial wastewaters using dark fermentation is shown in Table 1.

Table 1.
Comparison of the potential for biohydrogen production of various industrial wastewaters.
Due to its often easily hydrolysable carbohydrates and nutrient content, industrial wastewater requires less pretreatment for biohydrogen production [70]. Recent research has mainly investigated the hydrogen potential of wastewater from the food industry, as it is rich in carbohydrates, such as various sugars; e.g., glucose, starch and fructose [54,57,61]. From a waste management perspective, wastewaters from other non-food industries, such as agriculture or the paper or textile industries [47,51,78], are very interesting and promising substrates for biohydrogen production.
Biohydrogen production is a promising approach for treating industrial wastewater and generating clean energy. Highly polluted industrial wastewater with high organic matter content is a suitable substrate for energy recovery, and microbial cultures can help to increase the efficiency of the processes.
3.2. Waste Activated Sludge
Waste activated sludge (WAS) is produced as an inevitable product of biological wastewater treatment. Due to its immense quantity, potential risk of secondary pollution and significant disposal costs, it has become a critical environmental problem [79]. The production rate is estimated to be about 20–25 kg dry solids per person per year across Europe [80]. Large quantities of WAS significantly increase the cost of biological waste treatment, as disposal costs can account for 40–60% of the total costs of the plant, depending on the size of the plant and the wastewater characteristics [81]. A widely used biological process for the bioconversion of WAS is anaerobic digestion, the main product of which is the biogas methane (CH4). Due to the disadvantages of the process, such as the longer retention time, lower biogas yield and production of the greenhouse gasses methane and carbon dioxide [82,83], the process of methane production can be switched to the process of biohydrogen production. WAS consists of a complex floc structure, with its organic fraction mainly comprising sludge flocs, tightly and loosely bound extracellular polymeric substances and intracellular materials [84]. To increase the efficiency of the process, some modifications can be undertaken.
The literature indicates that waste activated sludge is considered an acceptable substrate for microbial production of hydrogen. Nevertheless, the focus of further research should be investigating the economic aspects of process modifications in the use of WAS or biohydrogen production.
Due to its complex floc structure, waste activated sludge pretreatment is often needed before the dark fermentation process in order to disintegrate the biomass. Due to the nature of the organic fraction of WAS, it is necessary to increase the contact between extracellular organic substances and the enzymes of the microorganisms. This can be achieved through individual or combined physical (dispersion, heat, freezing, ultrasound) [46,65,85,86], chemical (additive, complexing agent, electron shuttle) [46,49,65,84,85,87] and biological (enzyme application) [88] methods of WAS pretreatment. In this way, the sludge structure can be destroyed, which leads to an improvement in the sludge disintegration efficiency, overcoming of the issue with slow WAS hydrolysis and an increase in the dissolution of intracellular organic matter, as well as in the yield of short-chain fatty acids and WAS biodegradability [87].
Since the process of WAS fermentation is generally used for biohydrogen production, some modifications can be introduced to optimize and increase hydrogen production, mainly via WAS pre-treatment and improvement of the inoculum. The potential of waste activated sludge for biohydrogen production is summarized in Table 2, where, in addition to the hydrogen yield, modifications to the substrate and the inoculum are also listed.

Table 2.
Comparison of the potential of waste activated sludge for biohydrogen production.
Recently, more focus has also been placed on modification of WAS by mixing it with other waste substrates to achieve better initial qualities for the WAS as a substrate and to utilize other waste materials. Tunay et al. (2022) [91] studied the effects of the addition of the organic fraction of municipal solid waste to carbon-rich waste activated sludge obtained from a high-rate activated sludge system. They found that waste sludge hydrogen potential was improved with the addition of the organic fraction of municipal solid waste, after which the maximum biohydrogen production of the waste sludge was 18.9 mL H2/g volatile solids (VS). A green method of using corncob ash as a waste material to boost the microbial biohydrogen production from WAS as another waste material was proposed by Wang et al. (2022) [48]. They discovered that the dosage of corncob ash positively impacted the hydrogen production, making it possible to reach a hydrogen yield of up to 46.8 mL/g VS as a result of the enrichment of hydrolytic microorganisms. The new waste-control paradigm proposed by this study could make sludge disposal and wastewater treatment more sustainable.
Industrial waste streams containing biodegradable substrates are an efficient source for biohydrogen production because they minimize the total cost of processing. As it produces no greenhouse gases, hydrogen is an ideal alternative fuel.
4. Conclusions
Waste streams represent an alternative source of raw materials for hydrogen production, as the cost of traditional production of hydrogen from raw materials is high and waste pollution can be decreased. With industrial development on a global scale increasing, the demand for energy is inevitably increasing. Hydrogen is considered a suitable alternative to conventional fossil fuels and a sustainable energy source. It can contribute to a clean, safe and affordable energy future. It is time to harness the potential of hydrogen as a renewable energy source, and it looks promising as the most cost-effective option. By reusing industrial waste streams, as well as waste activated sludge, savings can be achieved in resources that have already become diminished. By utilizing waste streams, the cycle of resource exploitation can be closed, thus applying the principles of the circular economy. The usual by-product of the microbial metabolic pathway from the dark fermentation process is biohydrogen. It can be produced from easily available waste substrates, such as industrial wastewater and waste activated sludge. In order to ensure efficient microbial hydrogen production, modifications to the substrate and inoculum can be introduced. Biohydrogen is an important environmentally friendly industrial feedstock that can reduce dependence on fossil fuels. Biohydrogen production from low-cost industrial waste streams can solve complex industrial challenges through recovery and contribute to sustainability and a circular economy.
Author Contributions
Conceptualization, M.Š.R. and M.V.D.; investigation, M.Š.R. and M.V.D.; data curation, M.Š.R. and M.V.D.; writing—original draft preparation, M.Š.R.; writing—review and editing, M.Š.R. and M.V.D.; visualization, M.Š.R. and M.V.D.; supervision, M.V.D. and A.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nnabuife, S.G.; Ugbeh-Johnson, J.; Okeke, N.E.; Ogbonnaya, C. Present and Projected Developments in Hydrogen Production: A Technological Review. CCST (Carbon Capture Sci. Technol.) 2022, 3, 100042. [Google Scholar] [CrossRef]
- Hallenbeck, P.C.; Zampol Lazaro, C.; Sagir, E. Biology and Physiology of Photobiological Hydrogen Production. In Microalgal Hydrogen Production: Achievements and Perspectives; Seibert, M., Torzillo, G., Eds.; Royal Society of Chemistry: London, UK, 2018; pp. 1–30. [Google Scholar]
- Hermesmann, M.; Müller, T.E. Green, Turquoise, Blue, or Grey? Environmentally friendly Hydrogen Production in Transforming Energy Systems. Prog. Energy Combust. Sci. 2022, 90, 100996. [Google Scholar] [CrossRef]
- Hovorukha, V.; Havryliuk, O.; Gladka, G.; Tashyrev, O.; Kalinichenko, A.; Sporek, M.; Dołhańczuk-Śródka, A. Hydrogen Dark Fermentation for Degradation of Solid and Liquid Food Waste. Energies 2021, 14, 1831. [Google Scholar] [CrossRef]
- Han, W.; Hu, Y.; Li, S.; Li, F.; Tang, J. Biohydrogen production in the suspended and attached microbial growth systems from waste pastry hydrolysate. Bioresour. Technol. 2016, 218, 589–594. [Google Scholar] [CrossRef] [PubMed]
- International Energy Agency. Global Hydrogen Review; International Energy Agency: Paris, France, 2022. [Google Scholar]
- Łukajtis, R.; Hołowac, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen production from biomass using dark fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Pipitone, G.; Zoppi, G.; Pirone, R.; Bensaid, S. A critical review on catalyst design for aqueous phase reforming. Int. J. Hydrog. Energy 2022, 47, 151–180. [Google Scholar] [CrossRef]
- Zoppi, G.; Pipitone, G.; Pirone, R.; Bensaid, S. Aqueous phase reforming process for the valorization of wastewater streams: Application to different industrial scenarios. Catal. Today 2022, 387, 224–236. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S.; Shetti, N.P.; Aminabhavi, T.M. Waste-to-energy nexus for circular economy and environmental protection: Recent trends in hydrogen energy. Sci. Total Environ. 2020, 713, 136633. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lay, C.H. Carbon/nitrogen-ratio effect on fermentative hydrogen production by mixed microflora. Int. J. Hydrog. Energy 2004, 29, 41–45. [Google Scholar] [CrossRef]
- Brar, K.K.; Cortez, A.A.; Pellegrini, V.O.A.; Amulya, K.; Polikarpov, I.; Magdouli, S.; Kumar, M.; Yang, Y.-H.; Bhatia, S.K.; Brar, S.K. An overview on progress, advances, and future outlook for biohydrogen production technology. Int. J. Hydrog. Energy. 2022, 47, 37264–37281. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Progress in microbiology for fermentative hydrogen production from organic wastes. Crit. Rev. Environ. Sci. Technol. 2019, 49, 825–865. [Google Scholar] [CrossRef]
- Kumar, G.R.; Chowdhary, N. Biotechnological and bioinformatics approaches for augmentation of biohydrogen production: A review. Renew. Sustain. Energy Rev. 2016, 56, 1194–1206. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Sudharsanaa, T.; Bharghavi, A.; Jayamuthunagai, J.; Praveenkumar, R. Biohydrogen and Biogas—An overview on feedstocks and enhancement process. Fuel 2016, 185, 810–828. [Google Scholar] [CrossRef]
- Kanwal, F.; Torriero, A.A.J. Biohydrogen—A Green Fuel for Sustainable Energy Solutions. Energies 2022, 15, 7783. [Google Scholar] [CrossRef]
- Angeriz-Campoy, R.; Fdez-Güelfo, L.A.; Alvarez-Gallego, C.J.; Romero-García, L. Pre-composting of municipal solid wastes as enhancer of bio-hydrogen production through dark fermentation process. Fuel 2023, 333, 12657. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Kargi, F. Bio-hydrogen production from waste materials. Enzyme Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Yeshanew, M.M.; Paillet, F.; Barrau, C.; Frunzo, L.; Lens, P.N.L.; Esposito, G.; Escudie, R.; Trably, E. Co-production of Hydrogen and Methane From the Organic Fraction of Municipal Solid Waste in a Pilot Scale Dark Fermenter and Methanogenic Biofilm Reactor. Front. Environ. Sci. 2018, 6, 41. [Google Scholar] [CrossRef]
- Greses, S.; Tomás-Pejó, E.; González-Fernández, C. Food waste valorization into bioenergy and bioproducts through a cascade combination of bioprocesses using anaerobic open mixed cultures. J. Clean. Prod. 2022, 372, 133680. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Abd_Allah, E.F.; Singh, R.; Hashem, A.; Gupta, V.K. Biohydrogen production using kitchen waste as the potential substrate: A sustainable approach. Chemosphere 2021, 271, 129537. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Aron, N.S.M.; Jeevanantham, S.; Karishma, S.; Yaashikaa, P.R.; Chew, K.W.; Show, P.L. A review on bioconversion processes for hydrogen production from agro-industrial residues. Int. J. Hydrog. Energy 2022, 47, 37302–37320. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.; Yan, L.; Wang, Y.; Li, S.; Yan, X.; Wang, N.; Liang, N.; Li, H. Feasibility of biohydrogen production from industrial wastes using defined microbial co-culture. Biol. Res. 2015, 48, 24. [Google Scholar] [CrossRef] [PubMed]
- Arimi, M.M.; Knodel, J.; Kiprop, A.; Namango, S.S.; Zhang, Y.; Geißen, S.-U. Strategies for improvement of biohydrogen production from organic-rich wastewater: A review. Biomass Bioenergy 2015, 75, 101–118. [Google Scholar] [CrossRef]
- Barghash, H.; AlRashdi, Z.; Okedu, K.E.; Desmond, P. Life-Cycle Assessment Study for Bio-Hydrogen Gas Production from Sewage Treatment Plants Using Solar PVs. Energies 2022, 15, 8056. [Google Scholar] [CrossRef]
- Adeogba, E.; Barty, P.; O’Dwyer, E.; Guo, M. Waste-to-resource transformation: Gradient boosting modeling for organic fraction municipal solid waste projection. ACS Sustain. Chem. Eng. 2019, 7, 10460–10466. [Google Scholar] [CrossRef]
- Jensen, L.S.; Kaul, C.; Juncker, N.B.; Thomsen, M.H.; Chaturvedi, T. Biohydrogen Biohydrogen Production in Microbial Electrolysis Cells Utilizing Organic Residue Feedstock: A Review. Energies 2022, 15, 8396. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Deng, L.; Chen, Z.; Ye, Y.; Bui, X.T.; Hoang, N.B. Advanced strategies for enhancing dark fermentative biohydrogen production from biowaste towards sustainable environment. Bioresour. Technol. 2022, 351, 127045. [Google Scholar] [CrossRef]
- Moussa, R.N.; Moussa, N.; Dionisi, D. Hydrogen Production from Biomass and Organic Waste Using Dark Fermentation: An Analysis of Literature Data on the Effect of Operating Parameters on Process Performance. Processes 2022, 10, 156. [Google Scholar] [CrossRef]
- Cabrol, L.; Marone, A.; Tapia-Venegas, E.; Steyer, J.-P.; Ruiz-Filippi, G.; Trably, E. Microbial ecology of fermentative hydrogen producing bioprocesses: Useful insights for driving the ecosystem function. FEMS Microbiol. Rev. 2017, 41, 158–181. [Google Scholar] [CrossRef]
- Chou, C.H.; Han, C.L.; Chang, J.J.; Lay, J.-J. Co-culture of Clostridium beijerinckii L9, Clostridium butyricum M1 and Bacillus thermoamylovorans B5 for converting yeast waste into hydrogen. Int. J. Hydrog. Energy 2011, 36, 13972–13983. [Google Scholar] [CrossRef]
- Fatima, A.; Basak, B.; Ganguly, A.; Chatterjee, P.K.; Dey, A. Biohydrogen Production through Dark Fermentation of Food Wastes by Anaerobic Digester Sludge Mixed Microbial Consortium. In Recent Developments in Waste Management; Kalamdhad, A., Ed.; Springer: Singapore, 2020; pp. 57–70. [Google Scholar]
- Słupek, E.; Kucharska, K.; Gębicki, J. Alternative methods for dark fermentation course analysis. SN Appl. Sci. 2019, 1, 469. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Thanigaivelan, A.; Das, D.B.; Show, P.L.; Banat, F. Augmented biohydrogen production from rice mill wastewater through nano-metal oxides assisted dark fermentation. Bioresour. Technol. 2021, 319, 124243. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; White, D.; Demirel, Y.; Kelly, R.; Noll, K.; Blum, P. Uncoupling fermentative synthesis of molecular hydrogen from biomass formation in thermotoga maritima. Appl. Environ. Microbiol. 2018, 84, e00998-18. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, A.; Zhou, J.L.; Li, X.; Afsari, M.; Altaee, A. Techno-economic and environmental impact assessment of hydrogen production processes using bio-waste as renewable energy resource. Renew. Sustain. Energy Rev. 2022, 156, 111991. [Google Scholar] [CrossRef]
- Dzulkarnain, E.L.N.; Audu, J.O.; Dagang, W.R.Z.W.; Abdul-Wahab, M.F. Microbiomes of biohydrogen production from dark fermentation of industrial wastes: Current trends, advanced tools and future outlook. Bioresour. Bioprocess 2022, 9, 16. [Google Scholar] [CrossRef]
- Li, W.; Guo, J.; Cheng, H.; Wang, W.; Dong, R. Two-phase anaerobic digestion of municipal solid wastes enhanced by hydrothermal pretreatment: Viability, performance and microbial community evaluation. Appl. Energy 2017, 189, 613–622. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Khoo, K.S.; Show, P.-L.; Carolin, C.F.; Jackulin, C.F.; Jeevanntham, S.; Karishma, S.; Show, K.-Y.; Lee, D.-J.; et al. Biohydrogen from organic wastes as a clean and environment-friendly energy source: Production pathways, feedstock types, and future prospects. Biores. Technol. 2021, 342, 126021. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Das, D.; Kim, S.C.; Cho, B.-K.; Kalia, V.C.; Lee, J.K. Integrating strategies for sustainable conversion of waste biomass into dark-fermentative hydrogen and value-added products. Renew. Sustain. Energy Rev. 2021, 150, 111491. [Google Scholar] [CrossRef]
- Islam, A.K.M.K.; Dunlop, P.S.M.; Hewitt, N.J.; Lenihan, R.; Brandoni, C. Bio-Hydrogen Production from Wastewater: A Comparative Study of Low Energy Intensive Production Processes. Clean Technol. 2021, 3, 156–182. [Google Scholar] [CrossRef]
- Ergal, I.; Gräf, O.; Hasibar, B.; Steiner, M.; Vukotić, S.; Bochmann, G.; Fuchs, W.; Rittmann, S.K.M.R. Biohydrogen production beyond the Thauer limit by precision design of artificial microbial consortia. Commun. Biol. 2020, 3, 443. [Google Scholar] [CrossRef]
- Ergal, I.; Bochmann, G.; Fuchs, W.; Rittmann, S.K.M.R. Design and engineering of artificial microbial consortia for biohydrogen production. Curr. Opin. Biotechnol. 2022, 73, 74–80. [Google Scholar] [CrossRef]
- Chong, M.L.; Rahim, R.A.; Shirai, Y.; Hassan, M.A. Biohydrogen production by Clostridium butyricum EB6 from palm oil mill effluent. Int. J. Hydrog. Energy 2009, 34, 764–771. [Google Scholar] [CrossRef]
- Wong, Y.M.; Show, P.L.; Wu, T.Y.; Leog, H.Y.; Ibrahim, S.; Juan, J.C. Production of bio-hydrogen from dairy wastewater using pretreated landfill leachate sludge as an inoculum. J. Biosci. Bioeng. 2019, 127, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.N.; Mohan, S.V. Acidogenesis of waste activated sludge—Biohydrogen production with simultaneous short chain carboxylic acids. J. Environ. Chem. Eng. 2018, 6, 2983–2991. [Google Scholar] [CrossRef]
- Cheng, J.; Lin, R.; Xia, A.; Liu, Y.; Zhou, J.; Cen, K. Sequential Generation of Fermentative Hydrogen and Methane from Swine Manure with Physicochemical Characterization. Energy Fuel 2014, 28, 563–570. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, W.; Dai, X.; Ni, B.-J. Corncob ash boosts fermentative hydrogen production from waste activated sludge. Sci. Total Environ. 2022, 807, 151064. [Google Scholar] [CrossRef]
- Zhang, L.; Ban, Q.; Li, J.; Zhang, S. An enhanced excess sludge fermentation process by anthraquinone-2-sulfonate as electron shuttles for the biorefinery of zero-carbon hydrogen. Environ. Res. 2022, 210, 113005. [Google Scholar] [CrossRef]
- Litti, Y.V.; Potekhina, M.A.; Zhuravleva, E.A.; Vishnyakova, A.V.; Gruzdev, D.S.; Kovalev, A.A.; Kovalev, D.A.; Katraeva, I.V.; Parshina, S.N. Dark fermentative hydrogen production from simple sugars and various wastewaters by a newly isolated Thermoanaerobacterium thermosaccharolyticum SP-H2. Int. J. Hydrog. Energy 2022, 47, 24310–24327. [Google Scholar] [CrossRef]
- Li, Y.-C.; Chu, C.-Y.; Wu, S.-Y.; Tsai, C.-Y.; Wang, C.-C.; Hung, C.-H.; Lin, C.-Y. Feasible pretreatment of textile wastewater for dark fermentative hydrogen production. Int. J. Hydrog. Energy 2012, 37, 15511–15517. [Google Scholar] [CrossRef]
- Ghimire, A.; Sposito, F.; Frunzo, L.; Trably, E.; Escudié, R.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Effects of operational parameters on dark fermentative hydrogen production from biodegradable complex waste biomass. Waste Manag. 2016, 50, 55–64. [Google Scholar] [CrossRef]
- Chang, S.; Li, J.; Liu, F. Continuous biohydrogen production from diluted molasses in an anaerobic contact reactor. Front. Environ. Sci. Eng. China 2011, 5, 140–148. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Lin, C.-Y. Biohydrogen Production From Beverage Wastewater Using Selectively Enriched Mixed Culture. Waste Biomass Valorization 2020, 11, 1049–1058. [Google Scholar] [CrossRef]
- Policastro, G.; Carraturo, F.; Compagnone, M.; Guida, M.; Fabbricino, M. Enhancing hydrogen production from winery wastewater through fermentative microbial culture selection. Bioresour. Technol. Rep. 2022, 19, 101196. [Google Scholar] [CrossRef]
- Pachiega, R.; Rodrigues, M.F.; Rodrigues, C.V.; Sakamoto, I.K.; Varesche, M.B.A.; De Oliveira, J.E.; Maintinguer, S.I. Hydrogen bioproduction with anaerobic bacteria consortium from brewery wastewater. Int. J. Hydrog. Energy 2019, 44, 155–163. [Google Scholar] [CrossRef]
- Ramu, S.M.; Dinesh, G.H.; Thulasinathan, B.; Rajan, A.S.T.; Ponnuchamy, K.; Pugazhendhi, A.; Alagarsamy, A. Dark fermentative biohydrogen production from rice mill wastewater. Int. J. Energy Res. 2021, 45, 17233–17243. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Clostridium species for fermentative hydrogen production: An overview. Int. J. Hydrog. Energy 2021, 46, 34599–34625. [Google Scholar] [CrossRef]
- Lertsriwong, S.; Glinwong, C. Newly-isolated hydrogen-producing bacteria and biohydrogen production by Bacillus coagulans MO11 and Clostridium beijerinckii CN on molasses and agricultural wastewater. Int. J. Hydrog. Energy 2020, 45, 26812–26821. [Google Scholar] [CrossRef]
- Murugan, R.S.; Dinesh, G.H.; Swetha, T.R.A.; Boobalan, T.; Jothibasu, M.; Manimaran, P.S.; Selvakumar, G.; Arun, A. Acinetobacter junii AH4-A Potential Strain for Bio-hydrogen Production from Dairy Industry Anaerobic Sludge. J. Pure Appl. Microbiol. 2018, 12, 1761–1769. [Google Scholar] [CrossRef]
- Murugan, R.S.; Dinesh, G.H.; Raja, R.K.; Obeth, E.S.J.; Bora, A.; Samsudeen, N.M.; Pugazhendhi, A.; Arun, A. Dark fermentative biohydrogen production by Acinetobacter junii-AH4 utilizing various industry wastewaters. Int. J. Hydrog. Energy 2021, 46, 11297–11304. [Google Scholar] [CrossRef]
- Parthiba Karthikeyan, O.; Trably, E.; Mehariya, S.; Bernet, N.; Wong, J.W.C.; Carrere, H. Pretreatment of food waste for methane and hydrogen recovery: A review. Bioresour. Technol. 2018, 249, 1025–1039. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Kinetics and microbial community analysis for hydrogen production using raw grass inoculated with different pretreated mixed culture. Bioresour. Technol. 2018, 247, 954–962. [Google Scholar] [CrossRef]
- Yin, T.; Wang, W.; Zhuo, S.; Cao, G.; Ren, H.; Li, J.; Xing, D.; Xie, G.; Liu, B. Thermophilic dark fermentation fast start-up of hydrogen production with substrate concentration regulation and moderate pretreatment inoculum. Fuel 2023, 334, 126748. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Biohydrogen production from waste activated sludge pretreated by combining sodium citrate with ultrasonic: Energy conversion and microbial community. Energy Convers. Manag. 2020, 225, 113436. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, Y.; Wang, J. Influence of butyrate on fermentative hydrogen production and microbial community analysis. Int. J. Hydrog. Energy 2021, 46, 26825–26833. [Google Scholar] [CrossRef]
- Ubando, A.T.; Chen, W.-H.; Hurt, D.A.; Conversion, A.; Rajendran, S.; Lin, S.-L. Biohydrogen in a circular bioeconomy: A critical review. Bioresour. Technol. 2022, 366, 128168. [Google Scholar] [CrossRef] [PubMed]
- Boboescu, I.Z.; Ilie, M.; Gherman, V.D.; Mirel, I.; Pap, B.; Negrea, A.; Kondorosi, É.; Bíró, T.; Maróti, G. Revealing the factors influencing a fermentative biohydrogen production process using industrial wastewater as fermentation substrate. Biotechnol. Biofuels 2014, 7, 139. [Google Scholar] [CrossRef]
- Zhang, Q.; He, C.; Li, Y. Outlook of Biohydrogen from Waste: Quo Vadis? In Waste to Renewable Biohydrogen; Zhang, Q., He, C., Ren, J., Goodsite, M.E., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 229–247. [Google Scholar]
- Qyyum, M.A.; Ihsanullah, I.; Ahmad, R.; Ismail, S.; Khan, A.; Nizami, A.-S.; Tawfik, A. Biohydrogen production from real industrial wastewater: Potential bioreactors, challenges in commercialization and future directions. Int. J. Hydrog. Energy 2022, 47, 37154–37170. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar]
- Van Ginkel, S.; Logan, B.E. Inhibition of Biohydrogen Production by Undissociated Acetic and Butyric Acids. Environ. Sci. Technol. 2005, 39, 9351–9356. [Google Scholar] [CrossRef]
- Li, J.; Zheng, G.; He, J.; Chang, S.; Qin, Z. Hydrogen-producing capability of anaerobic activated sludge in three types of fermentations in a continuous stirred tank reactor. Biotechnol. Adv. 2009, 27, 573–577. [Google Scholar] [CrossRef]
- Mikheeva, E.R.; Katraeva, I.V.; Vorozhtsov, D.L.; Kovalev, D.A.; Kovalev, A.A.; Grigoriev, V.S.; Litti, Y.V. Dark fermentative biohydrogen production from confectionery wastewater in continuous-flow reactors. Int. J. Hydrog. Energy 2022, 47, 22348–22358. [Google Scholar] [CrossRef]
- Ramprakash, B.; Muthukumar, K. Comparative study on the performance of various pretreatment and hydrolysis methods for the production of biohydrogen using Enterobacter aerogenes RM 08 from rice mill wastewater. Int. J. Hydrog. Energy 2015, 40, 9106–9112. [Google Scholar] [CrossRef]
- Alvarez, A.J.; Fuentes, K.L.; Arias, C.A.; Chaparro, T.R. Production of hydrogen from beverage wastewater by dark fermentation in an internal circulation reactor: Effect on pH and hydraulic retention time. Energy Convers. Manag. X 2022, 15, 100232. [Google Scholar] [CrossRef]
- Özgür, E.; Mars, A.E.; Peksel, B.; Louwerse, A.; Yücel, M.; Gündüz, U.; Claassen, P.A.M.; Eroğlu, İ. Biohydrogen production from beet molasses by sequential dark and photofermentation. Int. J. Hydrog. Energy 2010, 35, 511–517. [Google Scholar] [CrossRef]
- Vaez, E.; Taherdanak, M.; Zilouei, H. Dark Hydrogen Fermentation from Paper Mill Effluent (PME): The influence of Substrate Concentration and Hydrolysis. Environ. Energy Econ. Res. 2017, 1, 163–170. [Google Scholar]
- Zhang, Z.; Guo, L.; Wang, Y.; Zhao, Y.; She, Z.; Gao, M.; Guo, Y. Application of iron oxide (Fe3O4) nanoparticles during the two-stage anaerobic digestion with waste sludge: Impact on the biogas production and the substrate metabolism. Renew. Energy 2020, 146, 2724–2735. [Google Scholar] [CrossRef]
- EurEau. Waste Water Treatment—Sludge Management, Briefing Note; EurEau: Paris, France, 2021. [Google Scholar]
- Available online: https://www.tpomag.com/online_exclusives/2021/12/rising-cost-of-sludge-handling-and-disposal-necessitates-a-smarter-approach_sc_001jj (accessed on 20 January 2023).
- Liu, H.; Wang, Y.; Wang, L.; Yu, T.; Fu, B.; Liu, H. Stepwise hydrolysis to improve carbon releasing efficiency from sludge. Water Res. 2017, 119, 223–225. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lien, L.S. Sludge: A waste or renewable source for energy and resources recovery. Renew. Sustain. Energy Rev. 2013, 25, 708–728. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Zhang, A.; Wang, L. Enhancing the quantity and quality of shortchain fatty acids production from waste activated sludge using CaO2 as an additive. Water Res. 2015, 83, 84–93. [Google Scholar] [CrossRef]
- Hu, J.; Guo, B.; Li, Z.; Wu, Z.; Tao, W. Freezing pretreatment assists potassium ferrate to promote hydrogen production from anaerobic fermentation of waste activated sludge. Sci. Total Environ. 2021, 781, 146685. [Google Scholar] [CrossRef]
- Preethi, J.R.B.; Kumar, G.; Tyagi, V.K.; Bajhaiya, A.K.; Gugulothu, P.; Gunasekaran, M. Biohydrogen production from waste activated sludge through thermochemical mechanical pretreatment. Bioresour. Technol. 2022, 358, 127301. [Google Scholar] [CrossRef]
- Ding, W.; Fang, Q.; Zhou, W.; Ping, Q.; Xiao, Y.; Wang, Z. Performance and mechanism of sodium citrate pretreatment to promote waste activated sludge disintegration and short-chain fatty acid production during anaerobic fermentation. J. Environ. Chem. Eng. 2023, 11, 109161. [Google Scholar] [CrossRef]
- Arun, C.; Sivashanmugam, P. Enhanced production of biohydrogen from dairy waste activated sludge pre-treated using multi hydrolytic garbage enzyme complex and ultrasound-optimization. Energy Convers. Manag. 2018, 164, 277–287. [Google Scholar] [CrossRef]
- Córdova-Lizama, A.; Carrera-Figueiras, C.; Palacios, A.; Castro-Olivera, P.M.; Ruiz-Espinoza, J. Improving hydrogen production from the anaerobic digestion of waste activated sludge: Effects of cobalt and iron zero valent nanoparticles. Int. J. Hydrog. Energy 2022, 47, 30074–30084. [Google Scholar] [CrossRef]
- Hu, J.; Zuo, Y.; Guo, B.; Shi, H. Enhanced hydrogen production from sludge anaerobic fermentation by combined freezing and calcium hypochlorite pretreatment. Sci. Total Environ. 2023, 858, 160134. [Google Scholar] [CrossRef]
- Tunay, D.; Yildirim, O.; Ozkaya, B.; Demir, A. Effect of organic fraction of municipal solid waste addition to high rate activated sludge system for hydrogen production from carbon rich waste sludge. Int. J. Hydrog. Energy 2022, 62, 26284–26293. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).