Slow Pyrolysis of Specialty Coffee Residues towards the Circular Economy in Rural Areas
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pyrolysis Methodology
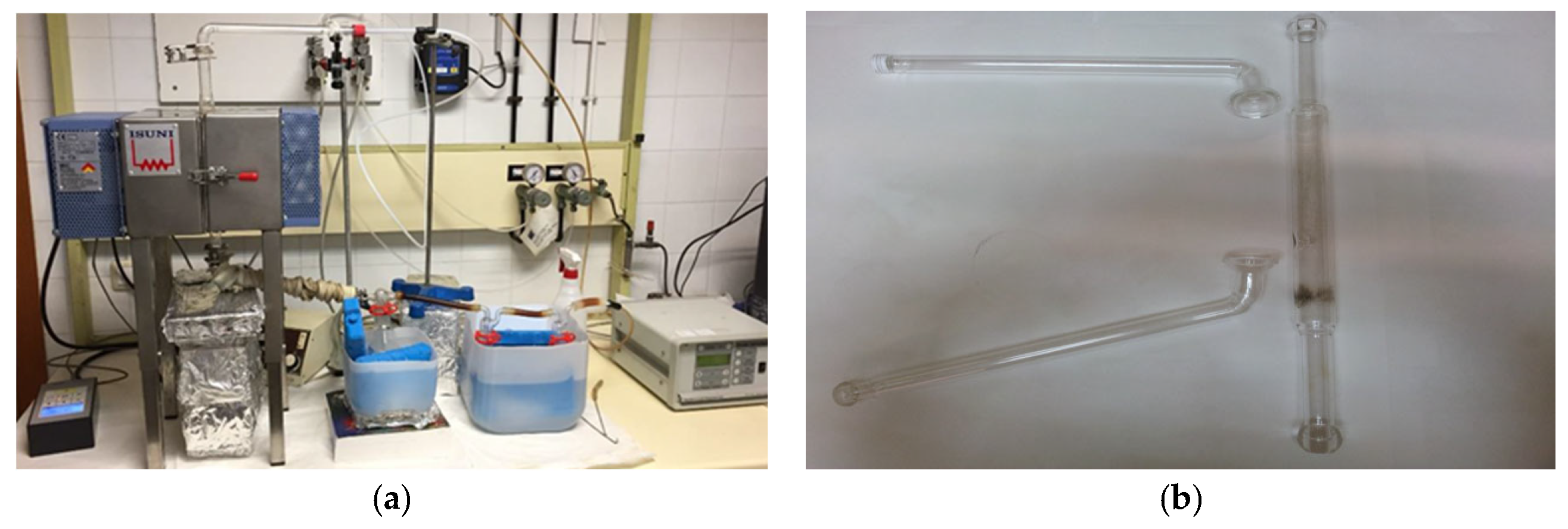
2.3. Characterization of CSS, SC, and Pyrolysis Fractions
2.3.1. Particle Size Distribution
2.3.2. pH and Density of the Pyrolysis Liquid Fractions
2.3.3. Chemical Characterization
2.3.4. Proximate Analysis and Evolved Gas Analysis (EGA)
2.3.5. FTIR Spectra
2.3.6. Ultimate Analysis and Calorific Value
3. Results
3.1. Characterization of Raw Materials
3.1.1. Particle Size Distribution

3.1.2. Chemical Composition
| wt.%, Dry Basis | CSS | SC |
|---|---|---|
| Cellulose | 33.7 | 14.9 |
| Hemicellulose | 4.7 | 41.1 |
| Lignin | 33.1 | 33.3 |
| Extractives | 15.8 | 22.8 |
| Ash | 6.3 | 2.2 |
| Moisture | 9.4 | 10.5 |
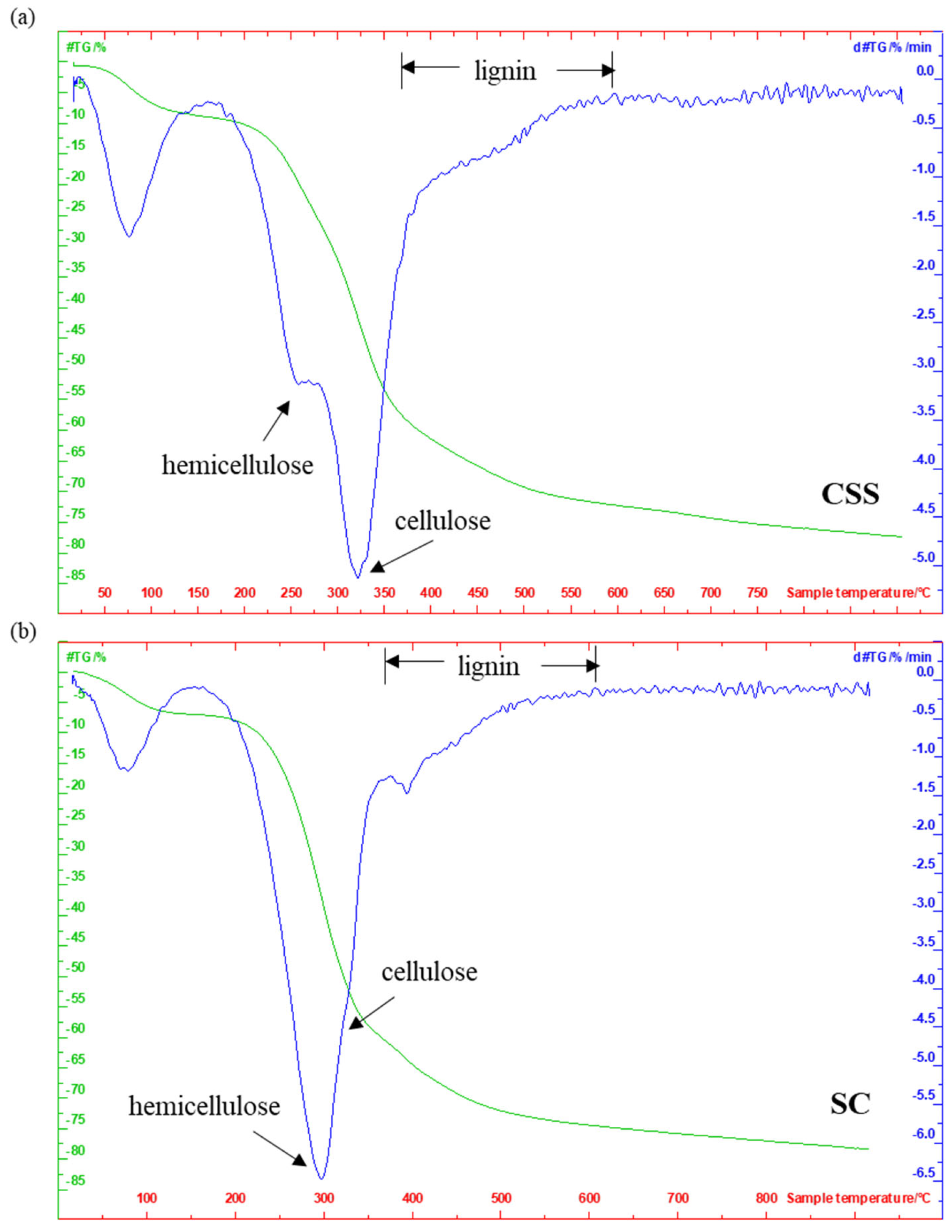
3.1.3. Proximate Analysis and Evolved Gas Analysis (EGA)
| m/z | Assignment | CSS (°C) | SC (°C) |
|---|---|---|---|
| 1 | H | 390 | 350 |
| 2 | H2 | 366 | 314 and 418 |
| 12 | C | 336 and 480 | 322 and 470 |
| 15 | CH3 | 357 and 540 | 331 and 531 |
| 16 | O | 366 | 331 |
| 18 | H2O | 122 and 349 | 131 and 322 |
| 26 | Acetylene, C2H2 | 288, 375, and 505 | 331, 418, and 488 |
| 28 | Ethylene C2H4 | 400 | 366 |
| 30 | Formaldehyde CH2O | 270 and 462 | 322, 400, 436 and 497 |
| 42 | Propylene C3H6 | 357 and 489 | 322 and 418 |
| 44 | CO2 | 288, 350, 453, and 723 | 323 and 453 |
| 45 | -COOH | 340 and 480 | 322 and 453 |
| 46 | Formic acid, ethanol | 350 and 470 | 322 and 462 |
| 58 | Acetone | 357 | 314 and 400 |
| 60 | Acetic acid, propanol | 350 | 322 |
| 68 | Furan | 383 | 340 |
| 78 | Benzene | 410 | 418 |
| 82 | Pyran | 366 | 340 |
| 84 | Cyclopentanone | 366 and 410 | 331 and 418 |
| 92 | Toluene | 392 | 410 |
| 93 | Aniline | 392 | 410 |
| 94 | Phenol | 392 | 357 |
| 96 | Furfural | 357 | 314 |

3.2. Results of Pyrolysis Experiments

3.2.1. Comparison of Grounded and Ungrounded CSS
3.2.2. Comparison of CSS and SC
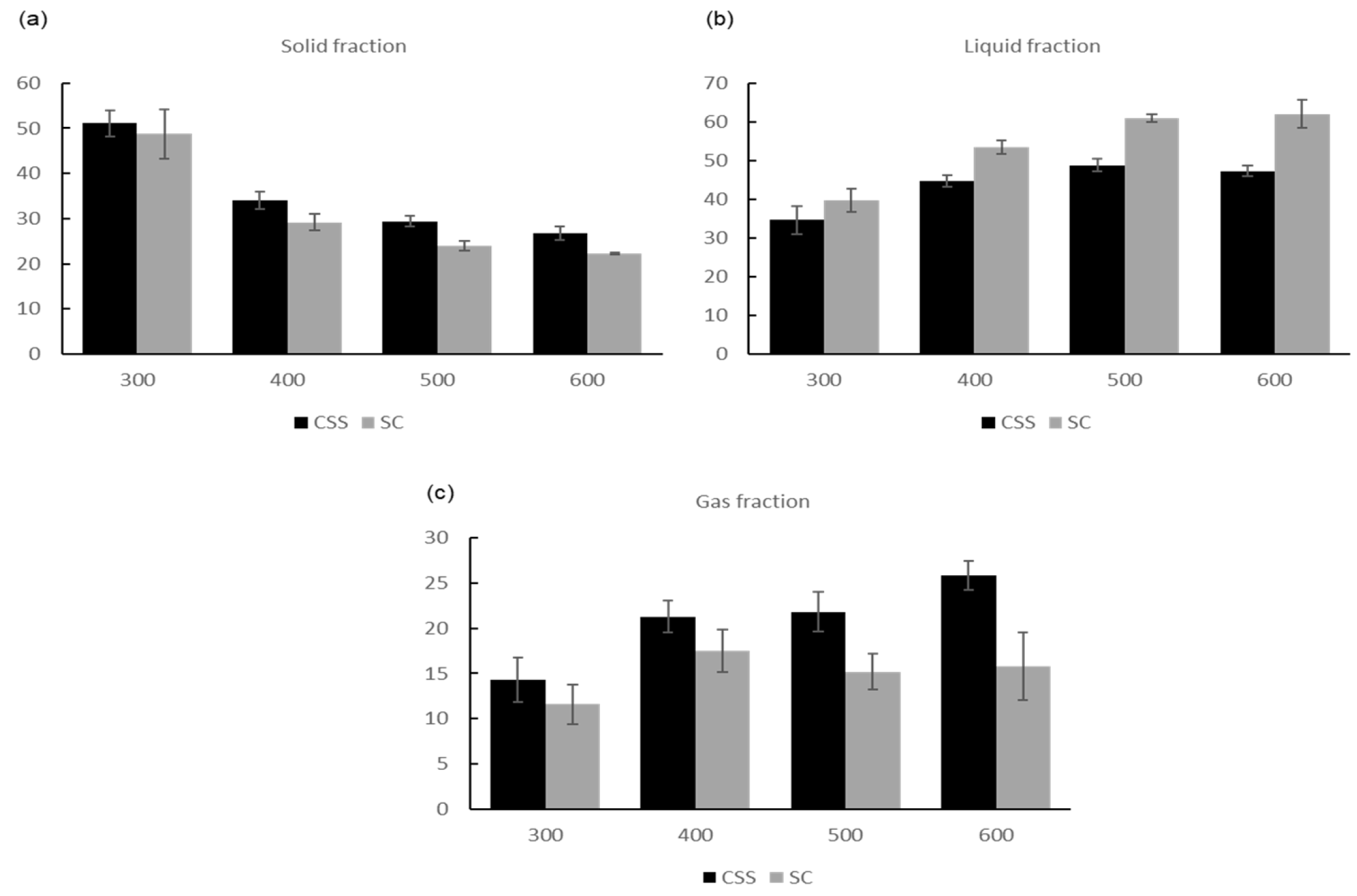
3.3. Results of Characterization of Pyrolysis Fractions
3.3.1. pH and Density of the Liquid Fraction
| Temperature (°C) | CSS | SC | ||
|---|---|---|---|---|
| pH | Density (g/cm3) | pH | Density (g/cm3) | |
| 300 | 3.9 ± 0.1 | 1.0015 ± 0.0003 | 3.5 ± 0.1 | 1.0037 ± 0.0002 |
| 400 | 3.9 ± 0.1 | 1.0027 ± 0.0001 | 3.8 ± 0.1 | 1.0118 ± 0.0003 |
| 500 | 4.2 ± 0.1 | 1.0177 ± 0.0006 | 4.0 ± 0.1 | 1.0360 ± 0.0009 |
| 600 | 4.3 ± 0.1 | 1.0185 ± 0.0007 | 3.7 ± 0.1 | 1.0581 ± 0.0005 |
3.3.2. FTIR of Liquid and Solid Fractions
3.3.3. Ultimate Analysis and Calorific Value
| % C | % H | % N | % S | % O | HHV (MJ/kg) | LHV (MJ/kg) | |
|---|---|---|---|---|---|---|---|
| Raw CSS | 44.02 | 5.63 | 1.91 | 0.15 | 36.15 | 16.54 | 16.29 |
| CSS-300 °C | 56.9 | 4.92 | 2.79 | 0.12 | 22.06 | 22.41 | 21.93 |
| CSS-400 °C | 57.21 | 3.55 | 2.53 | 0.12 | 19.27 | 21.05 | 20.80 |
| CSS-500 °C | 60.63 | 2.37 | 2.31 | 0.04 | 16.8 | 20.95 | 20.89 |
| CSS-600 °C | 64.68 | 1.63 | 2.5 | 0.04 | 13.78 | 21.81 | 21.73 |
| Raw SC | 47.11 | 7.05 | 1.89 | 0.11 | 34.08 | 19.99 | 19.36 |
| SC-300 °C | 62.63 | 5.72 | 3.32 | 0.05 | 19.66 | 25.92 | 25.18 |
| SC-400 °C | 69.56 | 3.95 | 3.37 | 0.03 | 13.16 | 26.89 | 26.38 |
| SC-500 °C | 72.76 | 2.7 | 3.21 | 0.01 | 9.87 | 26.78 | 26.45 |
| SC-600 °C | 74.4 | 1.97 | 3.32 | 0.01 | 7.9 | 26.64 | 26.42 |
3.3.4. HPLC Results of the Liquid Fraction
| Sample | Caffeine | Formic | Acetic | Levulinic | HMF | Furfural |
|---|---|---|---|---|---|---|
| CSS T300 | 4.76 ± 0.01% | 0.51 ± 0.04% | 4.13 ± 0.04% | 2.01 ± 0.02% | 0.10 ± 0.00% | 0.06 ± 0.00% |
| CSS T400 | 4.98 ± 0.01% | 0.70 ± 0.03% | 3.94 ± 0.03% | 1.83 ± 0.03% | 0.12 ± 0.01% | 0.05 ± 0.00% |
| CSS T500 | 4.25 ± 0.01% | 0.58 ± 0.04% | 3.54 ± 0.00% | 1.62 ± 0.00% | 0.11 ± 0.03% | 0.04 ± 0.00% |
| CSS T600 | 4.01 ± 0.02% | 0.58 ± 0.04% | 3.32 ± 0.01% | 1.54 ± 0.01% | 0.09 ± 0.00% | 0.03 ± 0.01% |
| SC T300 | 2.18 ± 012% | 1.04 ± 0.03% | 1.55 ± 0.04% | 1.99 ± 0.03% | 0.08 ± 0.00% | 0.02 ± 0.00% |
| SC T400 | 2.47 ± 0.03% | 1.53 ± 0.08% | 1.96 ± 0.04% | 2.57 ± 0.00% | 0.14 ± 0.00% | 0.02 ± 0.00% |
| SC T500 | 2.32 ± 0.09% | 1.62 ± 0.25% | 1.94 ± 0.18% | 2.52 ± 0.01% | 0.14 ± 0.00% | 0.02 ± 0.00% |
| SC T600 | 2.23 ± 0.01% | 1.45 ± 0.04% | 1.69 ± 0.12% | 2.21 ± 0.04% | 0.12 ± 0.00% | 0.01 ± 0.00% |
3.3.5. Biorefinery Options of the Liquid Fractions
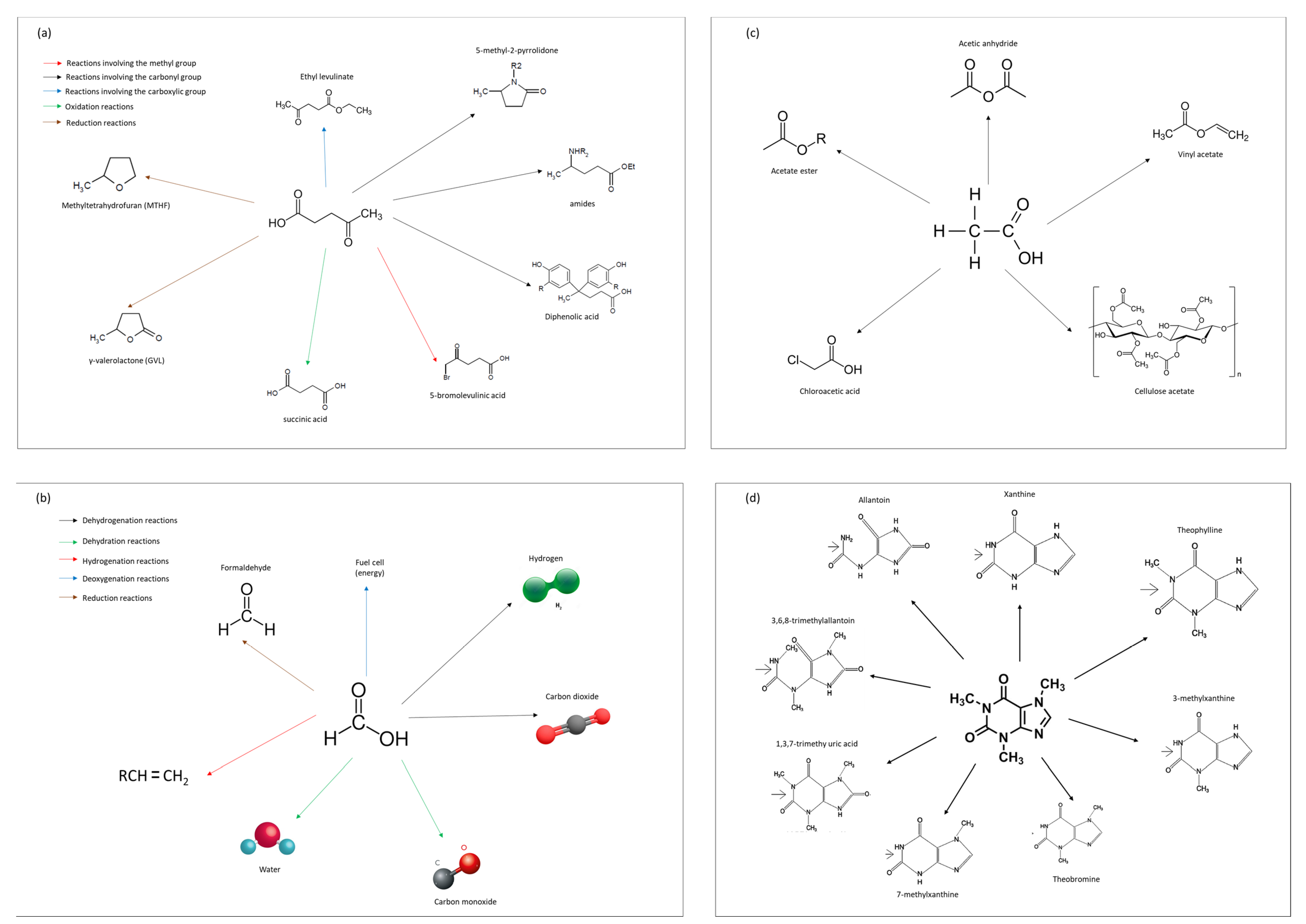
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Garcia, C.V.; Kim, Y.-T. Spent coffee grounds and coffee silverskin as potential materials for packaging: A review. J. Polym. Environ. 2021, 29, 2372–2384. [Google Scholar] [CrossRef]
- Behrouzian, F.; Amini, A.M.; Alghooneh, A.; Razavi, S.M.A. Characterization of dietary fiber from coffee silverskin: An optimization study using response surface methodology. Bioact. Carbohydrates Diet. Fibre 2016, 8, 58–64. [Google Scholar] [CrossRef]
- Oliveira, G.; Passos, C.; Ferreira, P.; Coimbra, M.; Gonçalves, I. Coffee by-products and their suitability for developing active food packaging materials. Foods 2021, 10, 683. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Iriondo-DeHond, M.; Del Castillo, M. Applications of compounds from coffee processing by-products. Biomolecules 2020, 10, 1219. [Google Scholar] [CrossRef]
- Atabani, A.E.; Mahmoud, E.; Aslam, M.; Naqvi, S.R.; Juchelková, D.; Bhatia, S.K.; Badruddin, I.A.; Khan, T.M.Y.; Hoang, A.T.; Palacky, P. Emerging potential of spent coffee ground valorization for fuel pellet production in a biorefinery. Environ. Dev. Sustain. 2022, 1–39. [Google Scholar] [CrossRef]
- González-Moreno, M.; Gracianteparaluceta, B.G.; Sádaba, S.M.; Urdin, J.Z.; Domínguez, E.R.; Ezcurdia, M.P.; Meneses, A.S. Feasibility of vermicomposting of spent coffee grounds and silverskin from coffee industries: A laboratory study. Agronomy 2020, 10, 1125. [Google Scholar] [CrossRef]
- Costa, A.S.; Alves, R.C.; Vinha, A.F.; Costa, E.; Costa, C.S.; Nunes, M.A.; Almeida, A.A.; Santos-Silva, A.; Oliveira, M.B.P. Nutritional, chemical and antioxidant/pro-oxidant profiles of silverskin, a coffee roasting by-product. Food Chem. 2018, 267, 28–35. [Google Scholar] [CrossRef]
- Polidoro, A.D.S.; Scapin, E.; Lazzari, E.; Silva, A.N.; dos Santos, A.L.; Caramão, E.B.; Jacques, R.A. Valorization of coffee silverskin industrial waste by pyrolysis: From optimization of bio-oil production to chemical characterization by GC × GC/qMS. J. Anal. Appl. Pyrolysis 2018, 129, 43–52. [Google Scholar] [CrossRef]
- Battista, F.; Barampouti, E.M.; Mai, S.; Bolzonella, D.; Malamis, D.; Moustakas, K.; Loizidou, M. Added-value molecules recovery and biofuels production from spent coffee grounds. Renew. Sustain. Energy Rev. 2020, 131, 110007. [Google Scholar] [CrossRef]
- Martinez, C.L.M.; Saari, J.; Melo, Y.; Cardoso, M.; de Almeida, G.M.; Vakkilainen, E. Evaluation of thermochemical routes for the valorization of solid coffee residues to produce biofuels: A Brazilian case. Renew. Sustain. Energy Rev. 2020, 137, 110585. [Google Scholar] [CrossRef]
- Jiménez-Zamora, A.; Pastoriza, S.; Rufián-Henares, J.Á. Revalorization of coffee by-products. Prebiotic, antimicrobial and antioxidant properties. LWT Food Sci. Technol. 2015, 61, 12–18. [Google Scholar] [CrossRef]
- Fernandez-Gomez, B.; Lezama, A.; Amigo-Benavent, M.; Ullate, M.; Herrero, M.; Martín, M.; Mesa, M.D.; del Castillo, M.D. Insights on the health benefits of the bioactive compounds of coffee silverskin extract. J. Funct. Foods 2016, 25, 197–207. [Google Scholar] [CrossRef]
- Mesías, M.; Navarro, M.; Martínez-Saez, N.; Ullate, M.; del Castillo, M.; Morales, F. Antiglycative and carbonyl trapping properties of the water soluble fraction of coffee silverskin. Food Res. Int. 2014, 62, 1120–1126. [Google Scholar] [CrossRef]
- Huang, Y.; Li, B.; Liu, D.; Xie, X.; Zhang, H.; Sun, H.; Hu, X.; Zhang, S. Fundamental advances in biomass autothermal/oxidative pyrolysis: A review. ACS Sustain. Chem. Eng. 2020, 8, 11888–11905. [Google Scholar] [CrossRef]
- Herdem, M.S. Performance investigation of a non-combustion heat carrier biomass gasifier for various reforming methods of pyrolysis products. Int. J. Green Energy 2021, 19, 62–71. [Google Scholar] [CrossRef]
- Fernández-Ferreras, J.; Rueda, C. Sewage sludge pyrolysis conditions assessment to maximize the liquid fraction for its valorization. In Proceedings of the 18th International Symposium on Waste Management and Sustainable Landfilling, Cagliari, Italy, 11–15 October 2021. [Google Scholar]
- Fernández-Ferreras, J.; Quesada-Rumayor, M.E.C.-A. Conventional pyrolysis of sawdust to obtain wood vinegar. In Proceedings of the 2019 International Conference on Green Energy and Environmental Technology GEET-19, Paris, France, 24–26 July 2019. [Google Scholar]
- Fernández-Ferreras, J.; Quesada-Rumayor, M.; Cuesta-Astorga, E. Valorisation of sawdust by conentional pyrolysis. Trends Chem. Eng. 2021, 19, 121–130. [Google Scholar]
- Fernández-Ferreras, J.; Sánchez-Fernández, N.; Llano, T.; Coz, A. Slow pyrolysis of coffee silverskin and spent coffee for its integral valorisation. In Proceedings of the 18th International Symposium on Waste Management and Sustainable Landfilling, Cagliari, Italy, 11–15 October 2021. [Google Scholar]
- Ktori, R.; Kamaterou, P.; Zabaniotou, A. Spent coffee grounds valorization through pyrolysis for energy and materials production in the concept of circular economy. Mater. Today Proc. 2018, 5, 27582–27588. [Google Scholar] [CrossRef]
- Van Soest, P.J.; McQueen, R.W. The chemistry and estimation of fibre. Proc. Nutr. Soc. 1973, 32, 123–130. [Google Scholar] [CrossRef]
- Viel, M.; Collet, F.; Lanos, C. Chemical and multi-physical characterization of agro-resources’ by-product as a possible raw building material. Ind. Crop. Prod. 2018, 120, 214–237. [Google Scholar] [CrossRef]
- ISO 14453:2014; Pulps-Determination of Acetone-Soluble Matter. International Organization for Standardization: Geneva, Switzerland, 2014.
- Pokhrel, P.; Shrestha, S.; Rijal, S.K.; Rai, K.P. A simple HPLC method for the determination of caffeine content in tea and coffee. J. Food Sci. Technol. Nepal 2016, 9, 74–78. [Google Scholar] [CrossRef]
- Patil, P.N. Caffeine in various samples and their analysis with HPLC—A review. Int. J. Pharm. Sci. Rev. Res. 2012, 16, 76–83. [Google Scholar]
- Llano, T.; Quijorna, N.; Andrés, A.; Coz, A. Sugar, acid and furfural quantification in a sulphite pulp mill: Feedstock, product and hydrolysate analysis by HPLC/RID. Biotechnol. Rep. 2017, 15, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Malucelli, L.C.; Silvestre, G.F.; Carneiro, J.; Vasconcelos, E.C.; Guiotoku, M.; Maia, C.M.B.F.; Filho, M.A.S.C. Biochar higher heating value estimative using thermogravimetric analysis. J. Therm. Anal. Calorim. 2019, 139, 2215–2220. [Google Scholar] [CrossRef]
- Demirbaş, A.; Demirbaş, A.H. Estimating the calorific values of lignocellulosic fuels. Energy Explor. Exploit. 2004, 22, 135–143. [Google Scholar] [CrossRef]
- Go, A.W.; Conag, A.T.; Cuizon, D.E.S. Recovery of sugars and lipids from spent coffee grounds: A new approach. Waste Biomass Valorization 2016, 7, 1047–1053. [Google Scholar] [CrossRef]
- Kang, S.B.; Oh, H.Y.; Kim, J.J.; Choi, K.S. Characteristics of spent coffee ground as a fuel and combustion test in a small boiler (6.5 kW). Renew. Energy 2017, 113, 1208–1214. [Google Scholar] [CrossRef]
- Del Pozo, C.; Rego, F.; Yang, Y.; Puy, N.; Bartrolí, J.; Fàbregas, E.; Bridgwater, A.V. Converting coffee silverskin to value-added products by a slow pyrolysis-based biorefinery process. Fuel Process. Technol. 2021, 214, 106708. [Google Scholar] [CrossRef]
- Del Pozo, C.; Bartrolí, J.; Alier, S.; Puy, N.; Fàbregas, E. Production of antioxidants and other value-added compounds from coffee silverskin via pyrolysis under a biorefinery approach. Waste Manag. 2020, 109, 19–27. [Google Scholar] [CrossRef]
- Matrapazi, V.; Zabaniotou, A. Experimental and feasibility study of spent coffee grounds upscaling via pyrolysis towards proposing an eco-social innovation circular economy solution. Sci. Total. Environ. 2020, 718, 137316. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioproc. Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, composition, and application of coffee and its industrial residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef]
- Carvalho, W.S.; Oliveira, T.J.; Cardoso, C.R.; Ataíde, C.H. Thermogravimetric analysis and analytical pyrolysis of a variety of lignocellulosic sorghum. Chem. Eng. Res. Des. 2015, 95, 337–345. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Choi, Y.S.; Choi, S.K.; Kim, S.J.; Jeong, Y.W.; Soysa, R.; Rahman, T. Fast pyrolysis of coffee ground in a tilted-slide reactor and characteristics of biocrude oil. Environ. Prog. Sustain. Energy 2017, 36, 655–661. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Hu, X.; Liu, Q. A mini review of the specialties of the bio-oils produced from pyrolysis of 20 different biomasses. Renew. Sustain. Energy Rev. 2019, 114, 109313. [Google Scholar] [CrossRef]
- Balmuk, G.; Videgain, M.; Manyà, J.J.; Duman, G.; Yanik, J. Effects of pyrolysis temperature and pressure on agronomic properties of biochar. J. Anal. Appl. Pyrolysis 2023, 169, 105858. [Google Scholar] [CrossRef]
- Lataf, A.; Jozefczak, M.; Vandecasteele, B.; Viaene, J.; Schreurs, S.; Carleer, R.; Yperman, J.; Marchal, W.; Cuypers, A.; Vandamme, D. The effect of pyrolysis temperature and feedstock on biochar agronomic properties. J. Anal. Appl. Pyrolysis 2022, 168, 105728. [Google Scholar] [CrossRef]
- Kazimierski, P.; Kardas, D. Influence of temperature on composition of wood pyrolysis products. Drv. Ind. 2017, 68, 307–313. [Google Scholar] [CrossRef]
- Pereira, L.G.G.; Pires, C.A.M. Bio-oil viscosity of sisal residue: Process and temperature influence. Energy Fuels 2018, 32, 5115–5124. [Google Scholar] [CrossRef]
- Neves, D.; Thunman, H.; Matos, A.; Tarelho, L.; Gómez-Barea, A. Characterization and prediction of biomass pyrolysis products. Prog. Energy Combust. Sci. 2011, 37, 611–630. [Google Scholar] [CrossRef]
- de Wild, P.; Reith, H.; Heeres, E. Biomass pyrolysis for chemicals. Biofuels 2011, 2, 185–208. [Google Scholar] [CrossRef]
- Li, O.L.; Qin, L.; Takeuchi, N.; Kim, K.; Ishizaki, T. Effect of hydrophilic/hydrophobic properties of carbon materials on plasma-sulfonation process and their catalytic activities in cellulose conversion. Catal. Today 2019, 337, 155–161. [Google Scholar] [CrossRef]
- Li, C.; Zhang, L.; Zhang, S.; Gholizadeh, M.; Hu, X. Impacts of temperature on hydrophilicity/functionalities of char and evolution of bio-oil/gas in pyrolysis of pig manure. Fuel 2022, 323, 124330. [Google Scholar] [CrossRef]
- Tóth, A.; Hoffer, A.; Pósfai, M.; Ajtai, T.; Kónya, Z.; Blazsó, M.; Czégény, Z.; Kiss, G.; Bozóki, Z.; Gelencsér, A. Chemical characterization of laboratory-generated tar ball particles. Atmos. Meas. Tech. 2018, 18, 10407–10418. [Google Scholar] [CrossRef]
- Caetano, N.S.; Silva, V.F.M.; Melo, A.C.; Martins, A.A.; Mata, T.M. Spent coffee grounds for biodiesel production and other applications. Clean Technol. Environ. Policy 2014, 16, 1423–1430. [Google Scholar] [CrossRef]
- Coz, A.; Llano, T.; Cifrián, E.; Viguri, J.; Maican, E.; Sixta, H. Physico-chemical alternatives in lignocellulosic materials in relation to the kind of component for fermenting purposes. Materials 2016, 9, 574. [Google Scholar] [CrossRef]
- Świątek, K.; Gaag, S.; Klier, A.; Kruse, A.; Sauer, J.; Steinbach, D. Acid hydrolysis of lignocellulosic biomass: Sugars and furfurals formation. Catalysts 2020, 10, 437. [Google Scholar] [CrossRef]
- Leong, H.Y.; Chang, C.-K.; Khoo, K.S.; Chew, K.W.; Chia, S.R.; Lim, J.W.; Chang, J.-S.; Show, P.L. Waste biorefinery towards a sustainable circular bioeconomy: A solution to global issues. Biotechnol. Biofuels 2021, 14, 1–15. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. Review on utilization and composition of coffee silverskin. Food Res. Int. 2014, 61, 16–22. [Google Scholar] [CrossRef]
- Barbero López, A. Recovery of antifungal compounds from wood and coffee industry side-streams and residues for wood preservative formulations. Diss. For. 2020. [Google Scholar] [CrossRef]
- Girisuta, B. Levulinic Acid from Lignocellulosic Biomass. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2007. [Google Scholar]
- Signoretto, M.; Taghavi, S.; Ghedini, E.; Menegazzo, F. Catalytic production of levulinic acid (LA) from actual biomass. Molecules 2019, 24, 2760. [Google Scholar] [CrossRef]
- Travália, B.M.; Forte, M. New proposal in a biorefinery context: Recovery of Acetic and formic acids by adsorption on hydrotalcites. J. Chem. Eng. Data 2020, 65, 4503–4511. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Vidra, A.; Németh, Á. Bio-produced acetic acid: A review. Period. Polytech. Chem. Eng. 2017, 62, 245–256. [Google Scholar] [CrossRef]
- Voß, D.; Kahl, M.; Albert, J. Continuous production of formic acid from biomass in a three-phase liquid–liquid–gas reaction process. ACS Sustain. Chem. Eng. 2020, 8, 10444–10453. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Ross, J.R.H. Towards sustainable production of formic acid. ChemSusChem 2018, 11, 821–836. [Google Scholar] [CrossRef]
- Rees, N.; Compton, R.G. Sustainable energy: A review of formic acid electrochemical fuel cells. J. Solid State Electrochem. 2011, 15, 2095–2100. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, W.; Jiang, Z.; Fang, T. A review on liquid-phase heterogeneous dehydrogenation of formic acid: Recent advances and perspectives. Chem. Pap. 2018, 72, 2121–2135. [Google Scholar] [CrossRef]
- Ricke, S.C.; Dittoe, D.K.; Richardson, K.E. Formic acid as an antimicrobial for poultry production: A review. Front. Veter. Sci. 2020, 7, 563. [Google Scholar] [CrossRef]
- Summers, R.M.; Mohanty, S.K.; Gopishetty, S.; Subramanian, M. Genetic characterization of caffeine degradation by bacteria and its potential applications. Microb. Biotechnol. 2015, 8, 369–378. [Google Scholar] [CrossRef]
- Andrs, M.; Muthna, D.; Rezacova, M.; Seifrtova, M.; Siman, P.; Korabecny, J.; Benek, O.; Dolezal, R.; Soukup, O.; Jun, D.; et al. Novel caffeine derivatives with antiproliferative activity. RSC Adv. 2016, 6, 32534–32539. [Google Scholar] [CrossRef]
- Singh, N.; Shreshtha, A.K.; Thakur, M.; Patra, S. Xanthine scaffold: Scope and potential in drug development. Heliyon 2018, 4, e00829. [Google Scholar] [CrossRef]
- Gummadi, S.N.; Bhavya, B.; Ashok, N. Physiology, biochemistry and possible applications of microbial caffeine degradation. Appl. Microbiol. Biotechnol. 2011, 93, 545–554. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Delgado, G.; Fernández-Arteaga, A.; Fornasier, F.; Mondini, C. Spent coffee grounds by-products and their influence on soil C–N dynamics. J. Environ. Manag. 2021, 302, 114075. [Google Scholar] [CrossRef]
- López-Linares, J.C.; García-Cubero, M.T.; Coca, M.; Lucas, S. A biorefinery approach for the valorization of spent coffee grounds to produce antioxidant compounds and biobutanol. Biomass Bioenergy 2021, 147, 106026. [Google Scholar] [CrossRef]


| wt.%, Dry Basis | CSS | SC |
|---|---|---|
| Moisture | 9.4 | 10.5 |
| VM | 74.4 | 76.7 |
| Ash | 6.7 | 2.4 |
| FC | 18.9 | 20.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Ferreras, J.; Llano, T.; Kochaniec, M.K.; Coz, A. Slow Pyrolysis of Specialty Coffee Residues towards the Circular Economy in Rural Areas. Energies 2023, 16, 2300. https://doi.org/10.3390/en16052300
Fernández-Ferreras J, Llano T, Kochaniec MK, Coz A. Slow Pyrolysis of Specialty Coffee Residues towards the Circular Economy in Rural Areas. Energies. 2023; 16(5):2300. https://doi.org/10.3390/en16052300
Chicago/Turabian StyleFernández-Ferreras, Josefa, Tamara Llano, María K. Kochaniec, and Alberto Coz. 2023. "Slow Pyrolysis of Specialty Coffee Residues towards the Circular Economy in Rural Areas" Energies 16, no. 5: 2300. https://doi.org/10.3390/en16052300
APA StyleFernández-Ferreras, J., Llano, T., Kochaniec, M. K., & Coz, A. (2023). Slow Pyrolysis of Specialty Coffee Residues towards the Circular Economy in Rural Areas. Energies, 16(5), 2300. https://doi.org/10.3390/en16052300







