Abstract
The depletion of fossil fuels has led to a search for new methods of fuel and chemical production from biomass. One of the methods of converting biomass into valuable products is the process of pyrolysis. This process has been extensively researched in recent years due to the rising prices of energy and chemicals. This work contains basic information on the pyrolysis process concerning the individual components present in the biomass and the types of biomass used in the pyrolysis process. Particular attention was paid to sewage sludge, the management of which is a big challenge. The influence of the most important process parameters (temperature, heating rate, residence time of the solid and vapor, reaction atmosphere) on the pyrolysis products (char, oil, and gas) was presented. The paper presents an overview of the reactors used in the pyrolysis process, from slow to fast pyrolysis, together with their efficiency, advantages, and disadvantages. The analysis of the application of other thermochemical processes for producing the energy used in the process of pyrolysis and in the drying of the biomass was carried out. Two industrial-scale installations for the pyrolysis of sewage sludge were presented.
1. General Knowledge of the Pyrolysis Process
The demand for fossil fuels is growing all the time, causing the degradation of the natural environment, which has a negative impact on human health. Due to the depletion of natural resources, not only new energy sources are being sought, but also substrates for the production of chemicals and high-value-added materials. Thermochemical processes are much faster than biochemical ones and require lower space for the reactor [1,2]. Additionally, biochemical processes produce a waste stream that contains a huge amount of water (>90%) and organic matter, which is hardly biodegradable [3,4]. Pyrolysis is the most promising process compared to the other thermochemical processes (gasification, combustion) used for converting biomass to fuel and sources of chemicals [5,6].
This article constitutes the second part of a literature review of pyrolysis reactors and the parameters influencing the pyrolysis process. The first part of the work described the torrefaction process, which takes place at temperatures in the range of 200–300 °C, and the reactors used for its conducting [7,8]. The pyrolysis process differs from torrefaction mainly in the temperature at which it occurs, i.e., above 300 °C. This process is very complex and consists of parallel and series of consecutive reactions (dehydration, depolymerization, isomerization, aromatization, decarboxylation) [9,10] as a result of which char, oil, and gas are formed [11,12]. Generally, the pyrolysis process is divided into three steps: moisture evaporation, primary decomposition, and secondary decomposition (oil cracking and polymerization) (Figure 1) [13]. This process is endothermic and occurs under anaerobic conditions [14], while some decomposition reactions may be exothermic [15]. Moreover, it is worth noting that the pyrolysis process is also important because it is conceptually the first phase of other thermochemical processes (gasification and combustion) [16].
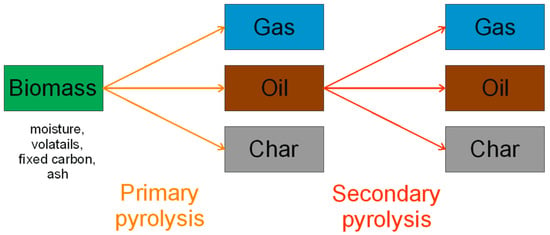
Figure 1.
Decomposition of biomass in the pyrolysis process.
The char produced in the pyrolysis process is also known as carbon-rich solid residue (also named as biochar, charcoal, or coke), the oil is a condensable gas (also known as bio-oil, crude, and pyrolysis liquid), and the gas is non-condensable gas (also known as pyrogas, pyrolytic gas). More detailed properties of pyrolysis products are discussed in Section 4.1, Section 4.2 and Section 4.3. In the pyrolysis process, oil is given the most attention, followed by char. The great interest in oil is caused by the desire to replace fossil fuels. The pyrolysis process is influenced by many parameters, such as temperature, heating rate, residence time, and particle size [12]. A detailed description of the influence of individual parameters on the pyrolysis process is presented in Section 3. The substrate for the pyrolysis process is organic matter, which may contain mineral compounds. The substrate’s composition significantly affects the pyrolysis process and, thus, the products produced. Increasingly, two or more substrates are used in the pyrolysis process, which may cause synergistic effects on the reaction rate and end product quality and quantity [17]. Table 1 presents the basic advantages and disadvantages of the pyrolysis process.

Table 1.
General advantages and disadvantages of pyrolysis process [18,19,20].
This review is the second part of a literature review focused on the reactors used for the torrefaction and pyrolysis processes. So far, the processes of torrefaction and pyrolysis have not been discussed together. Due to their different process parameters (mainly temperature) and the resulting products, there is a significant difference between these processes. In the majority of the literature reviews concerning biomass pyrolysis installations, examples of the use of sewage sludge for the pyrolysis process are often overlooked, while this substrate is de facto biomass.
This review aims to present the influence of process parameters such as temperature, heating rate, and residence time on the pyrolysis process and its products, and also an overview of the reactors used in the pyrolysis process, with a particular emphasis on sewage sludge as a feedstock.
2. Feedstock for the Pyrolysis Process
Among the feedstocks for the pyrolysis process, we can distinguish terrestrial biomass (e.g., wood, energy crops), marine biomass (e.g., algae), and waste biomass (e.g., food waste, sewage sludge) [12]. Biomass is a renewable source of organic matter from which not only energy but also biochemicals and high value-added materials can be produced, and it is very versatile in terms of its morphology and physical characteristics [21].
Biomass consists mainly of cellulose, hemicellulose, lignin, extractives, and ash [22]. Cellulose provides the structure of cell walls and contains glucose units. This polymer decomposes in temperatures in the range of 315–400 °C. Hemicellulose, generally represented by xylan, decomposes at temperatures in the range of 220–315 °C. However, lignin, which strengthens the lignocellulosic structure, is the most stable component of biomass for pyrolytic degradation and occurs in temperatures ranging from 160 to 900 °C [13,23,24]. Cellulose and hemicellulose are responsible for oil production, and lignin contributes to the char production yield [25]. A high lignin content causes an increase in the viscosity and molecular weight and decreases the water content of the oil [26]. During the pyrolysis process, the three main components of biomass can interact with each other [27]. For example, the interaction between hemicellulose and lignin promotes the production of phenols and inhibits the generation of hydrocarbons [28]. Lignin and cellulose interact by decreasing the production of char [29]. Extractives in biomass act as intermediate metabolites, energy reserves, and a defense against microbial and insect attacks and consist of tannins, fatty acids, and resins [30,31]. Biomass also contains inorganic components (ash) such as sodium, potassium, calcium, silicon, phosphorous, and magnesium [9]. The ash content in the biomass should be low, because its high content reduces the efficiency of char production [32,33]. The details of the influence of mineral compounds on pyrolysis will be discussed later in this study.
Another biomass group is marine biomass (algae), which contains proteins and lipids apart from carbohydrates, extractives, and ash [34,35]. Proteins and lipids decompose in the temperatures range of 200–350 °C and 200–600 °C, respectively [36,37]. The use of algae in the pyrolysis process is problematic due to the high water content and the costs associated with its evaporation. Additionally, algae may contain up to 48% ash [38].
2.1. Sewage Sludge
Special attention is directed to sewage sludge in the waste biomass category, which is constantly increasing [39]. In Poland in 2021, 580 thousand tons of dry sewage sludge were created. The amount of sewage sludge being produced increased by 63% in the period of time from 2000 to 2021 [40]. Presently, sewage sludge is disposed of via thermochemical processes (mainly incineration), landfilling, and biological processes (biogas production), or used in agriculture as fertilizer. Using sewage sludge as fertilizer is prohibited without employing an appropriate pre-treatment and meeting many restrictions. Sewage sludge includes several types of sludge (primary, secondary, and digested sludge) produced during wastewater treatment. The types of generated sewage sludge are closely related to the technology used (Figure 2). Detailed information on individual types of sludge can be found in Gao et al. [41].
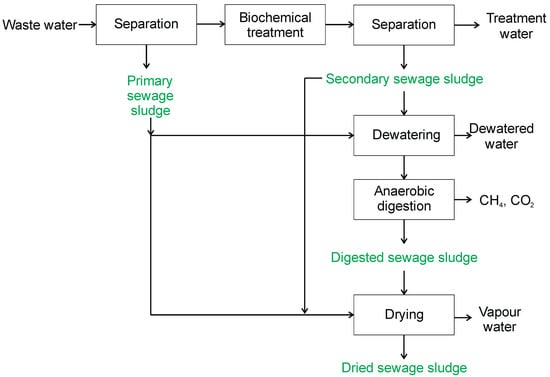
Figure 2.
Various types of sewage sludge.
Sewage sludge is a by-product of the process of the biological and chemical treatment of waste water and is composed of microorganisms (also pathogenic), organic matter (e.g., paper, fecal material, microplastics), and inorganic substances [42]. The organic matter consists of polysaccharides, lipids, proteins, and other organic pollutants such as polycyclic aromatic compounds, pharmaceuticals, adsorbable organohalogens, perfluorinated substances, polychlorinated biphenyls, surfactants, and hormones [41,43]. Depending on its origin, sewage sludge may contain heavy metals that make it difficult to use [44].
Sewage sludge is characterized by a high water content (up to 98%). When using sewage sludge as fertilizer or during landfilling, the water content should be below 60%, and it should be <15% when using it in thermal processes [45]. The waste drying process can be carried out using natural (solar), mechanical, biodrying, or thermal drying methods [46]. The sewage sludge’s higher heating value (HHV) ranges from 7 to 16 MJ/kg [42,47]. Its low calorific value is also associated with a high ash content, ranging from 19 to 50% [39,48].
In recent years, new methods of sewage sludge management have been sought due to the regulations of the European Union, as well as the new methods for recovery of raw materials [49]. The increased interest in recovering raw materials from sewage sludge is influenced by shrinking reserves of fossil fuels, fuel prices, climate change, public awareness, and advancements in renewable energy technologies [50]. The two components of the sludge that can be technically and economically recovered are nutrients (N and P) and energy (C) [41,51]. Nutrients can be recovered particularly from pyrolysis processes using a thermochemical process. Special attention should be paid to the fact that sewage sludge contains more nitrogen than lignocellulosic biomasses [52]. The high nitrogen content in sewage sludge is associated with undecomposed proteins and bacteria and ranges from 3.5 to 9% (on a dry ash free basis) [43,53]. The general advantages and disadvantages of sewage sludge pyrolysis are presented in Table 2.

Table 2.
General advantages and disadvantages of sewage sludge pyrolysis.
The energy recovery from sewage sludge in the pyrolysis process depends strictly on its composition (organic and inorganic matter) and water. The high content of heavy metals in sewage sludge makes the pyrolysis process preferrable the gasification and combustion processes due to its lower temperature, in which less migration of metals from the solid to the gas phase takes place (boiling point) [54]. Both the kinetics of the sludge pyrolysis process and the resulting products on a laboratory scale have been thoroughly tested [55]. The study of pyrolysis process on the laboratory scale enables the proposal of reaction mechanisms for decomposition for given substrates. Based on results from pyrolysis on a small scale, the yield of char, oil, and gas, and their properties (e.g., composition of pyrolysis products; grindability, surface are of char; density, viscosity of oil), can be determined. On an industrial scale, heat and mass transfer, which are dependent on reactor construction, has a huge impact on the pyrolysis products and their properties.
The pyrolysis of sewage sludge is an environmentally friendly alternative for both carbon sequestration and nutrient cycle closure. A life cycle assessment (LCA) study was performed by Gievers et al. [56] to investigate whether pyrolysis is more sustainable than mono-incineration. It was observed that biochar is a beneficial type of fertilizer and is useful for carbon sequestration. The LCA calculations suggest that replacing biochar with fossil-based materials and fuel (peat and lignite) has the greatest potential to mitigate the climate change effect of sewage sludge treatment. Larina and Zaichenko [57] have investigated the influence of sewage sludge pyrolysis on the evaporation and solubility of heavy metals. They found that the process reduces the initial solubility of heavy metals by 10–100 times. Several studies have been published on the co-pyrolysis of sewage sludge to increase the product quality, reduce the working volume, and allow for the hazard-free treatment of the sewage sludge. Blending with rice husk and bamboo sawdust [58], recycled low density polyethylene, hard thermoplastics, municipal solid waste, polyester wastes and paper rejects [59], and cotton stalk [60] are a few examples of the approaches in these studies.
2.2. Pre-Treatment of Biomass
In addition to its many advantages, biomass has several disadvantages, such as a high water content, low energy density, poor grindability, hydroscopic nature, high oxygen content, high alkali metal content, and heterogeneity [61]. For these reasons, the biomass before the pyrolysis process should be pretreated using the following methods: physical, thermal, biological, and chemical pre-treatment. In the literature on the subject, you can also find their mutual connection [62,63]. Physical biomass pre-treatment involves milling, grinding to a smaller size, extrusion, and ultrasonic methods. The extrusion of biomass under pressure causes the production of pellets with an increased energy density and a lower water content. Note that the shredding or extrusion of biomass increases the plant’s operating costs [63]. Ultrasound destroys the connection between hemicellulose, cellulose, and lignin structures [64]. Thermal pre-treatment includes the drying of the biomass, torrefaction, and steam extrusion. Drying is an energy-consuming process but is particularly important for biomass with a high moisture content (>70%). A drying process carried out at temperatures from 60 to 200 °C also causes the partial torrefaction of the biomass. Thermal pre-treatment of the biomass causes a higher energy density and an improved grindability, lowers the hygroscopicity, lowers the risk of biodegradability and self-ignition, and makes feeding the biomass to the reactor easier [65]. Torrefied biomass as a feedstock for the pyrolysis process causes the production of higher quality oil (lower acidity and higher HHV) and gas (more H2 and CH4) [30,66,67]. Biological pre-treatment mainly focuses on the hydrolysis of a lignocellulosic biomass by enzymes secreted by microorganisms (bacteria, fungi, etc.). This process is slow but consumes less energy, requires fewer chemicals, and is more environmentally friendly than the others. These processes mainly break down cellulose and hemicellulose. The chemical methods include acid (HNO3 and HF) or water washing, which causes the washing of inorganic compounds from the ash and the partial hydrolysis of the biomass. The washing of the biomass can cause an increased oil yield and a decreased char production yield [68,69]. At the same time, mineral compounds such as alkali (K, Na, etc.) and alkaline earth (Ca, Mg, etc.) can affect the mechanism of the pyrolysis process [70]. They change the decomposition temperature and pyrolysis reaction rate [71]. It should be noted that the build-up of salts on the wall of the reactor and the pipe line can cause corrosion problems [72]. However, mineral compounds can be intentionally added to the pyrolysis process to act as catalysts. Adding Fe2O3 and ZnO increases the efficiency of char production, and Al2O3, CaO, and TiO2 are favorable for promoting oil production [41]. Adding CaO to feedstock causes a decrease in the water and PAHs in the oil content. Al2O3 reduces the oxygen content in oil and can lead to aromatization [41]. Some metals, such as Ni, Co, Fe, and Ca, can reduce the amount of NH3 and HCN in the gas produced in favor of N2 [73]. Noble metals can be used to remove S from oil [39]. Zeolites (HZSM-5) can also be used as catalysts that increase the gas production in the pyrolysis process and improve the quality of the oil by reducing the oxygen and sulfur contents [52,74]. More information about catalysts in the pyrolysis process for sewage sludge was presented in the work of Gao et al. [41].
3. Process Parameters
The pyrolysis process is influenced by the following process parameters: temperature, heating rate, the residence time of the solid and vapor, reaction atmosphere, and particle size. Among these, the most important are temperature and heating rate [75].
3.1. Temperature
The pyrolysis process has three temperature ranges: <200 °C, 200–600 °C, and >600 °C [76,77]. At temperatures below 200 °C, mainly water and light volatile substances evaporate. The proper pyrolysis process takes place in the temperature range of 200–600 °C. It is assumed that, up to a temperature of 380 °C, biodegradable compounds decompose, and, above this temperature, non-biodegradable ones decompose [39]. In the third temperature range (>600 °C), further decomposition of the organic compounds, as well as the char gasification process and the decomposition of minerals such as CaCO3, can be observed [38].
The temperature has a great influence on the products formed during pyrolysis. Char is the dominant product at temperatures below 400 °C (Figure 3). The most significant amount of oil is formed in the temperature range of 400–650°C [78]. Further increases in the temperature increase the gas production yield [79]. However, the greatest amount of gas is produced in the oil decomposition process [80].
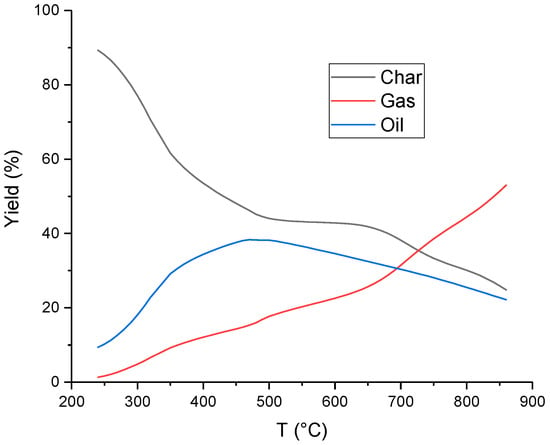
Figure 3.
Effect of temperature on the yield of pyrolysis products (data adapted from ref. [77], slow pyrolysis, feedstock: spent mushroom substrate).
It is assumed that reasonable temperature control is critical in ensuring the quality of the pyrolysis products [81]. The poor quality of pyrolysis products is particularly visible in batch reactors where it is difficult to maintain a low-temperature gradient. This is due to the large size of the feedstock and the lack of mixing. On the one hand, the increase in temperature causes a decrease in the char production yield, which is related to the decomposition of hemicellulose, cellulose, and lignin [82]. On the other hand, it increases the HHV of the char and its surface area [17,83,84]. The low pyrolysis temperatures result in a low yield of oil, which contains a lot of water formed from the oxygen molecules in the biomass [39]. Increasing the pyrolysis temperature increases the gas production efficiency and its HHV due to the increased concentration of CO, H2, CH4 [39]. Unfortunately, increases in the temperature also cause the formation of greater amounts of undesirable products such as H2S, NH3, and HCN [85]. The process temperature must be selected appropriately to optimize the production of the desired products.
3.2. Heating Rate
The second significant process parameter is the heating rate. Depending on the heating rate, there are three types of pyrolysis [81]:
- Slow pyrolysis (carbonization)—temperature: 400 °C, solid residence time: a few minutes to a few hours, heating rate: from 0.1 to 1.0 °C/s;
- Intermediate pyrolysis (conventional)—temperature: 500 °C, solid residence time: up to 10 min, heating rate: 1–100 °C/s;
- Fast pyrolysis—temperature: 400–800 °C, solid residence time: less than 2 s, heating rate: 100–1000 °C/s [5,86,87].
The summary does not include flash pyrolysis since using such high heating rates (>1000 °C/s) is difficult to achieve on an industrial scale.
Rapid heating causes fast biomass fragmentation, resulting in low char production efficiency. It also increases mass and heat transfer, which hinders the secondary reaction and, as a result, increases the oil and gas production [88]. At a slow heating rate, heat transfer controls the pyrolysis process, while, for high heating rates (fast pyrolysis), the pyrolysis process is controlled mainly by the rate of the chemical reactions [81]. The high value of the heating rate causes over 90% of P to remain in the carbonate, which enables its recovery [89]. It was also observed that, at a temperature of <450 °C, the heating rate significantly influences the yield of pyrolysis products, and, at temperatures above 600 °C, the influence of the heating rate is negligible [90].
3.3. Residence Time
The residence time in the pyrolysis process concerns solid particles and vapor. Generally, a shorter residence time for the contact of solid particles with vapor favors oil production. Increased oil production is related to minimizing secondary reactions [63]. Increasing the residence time at high temperatures causes the cracking of oxygenated molecules to produce gas and water [39]. It has been observed that a short residence time and a high heating rate reduce NH3 and HCN [91]. On the other hand, a long residence time and a low heating rate cause a reduction in SOx [92].
3.4. Reaction Atmosphere
The pyrolysis process is carried out under anaerobic conditions. N2, CO2, and water vapor are used as inert gases. Changing the atmosphere from N2 to CO2 in the pyrolysis process results in an increased syngas yield and reduced char and oil production [17,93,94]. Applying CO2 as a pyrolytic agent enhances the thermal cracking and dehydrogenation of hydrocarbons. Char produced in a CO2 atmosphere is characterized by a higher surface area than that produced under a nitrogen atmosphere [94]. Fluidized bed reactors are in high demand when using a carrier gas. Using water vapor or/and CO2 during pyrolysis at temperatures above 700 °C causes partial char gasification [63,95].
3.5. Particle Size
The particle size directly affects the heat transfer in biomass and the mass transfer of oil and gases outside of the particle [81,96]. The smaller the grain diameter, the faster the pyrolysis process due to the even temperature distribution in the particle. It also causes the quick removal of oil from the particles and prevents the formation of additional char due to a secondary reaction [97]. A secondary reaction may occur on the surface of the grain and inside it [98]. The extensive fragmentation of biomass for heat transfer improvement is related to the poor thermal conductivity of the biomass (approx. 0.1 W/(m·K)) [81,99]. On the other hand, pelleted biomass increases the production of char and gas during the pyrolysis process [100].
4. Properties of Pyrolysis Products
Due to the long residence time of the particles, slow pyrolysis is carried out in a batch reactor. Feedstock can contain more moisture, up to 20%, when used in this process. This process is mainly aimed at char production (Figure 4). Biomass with dimensions ranging from a few cm to 1 m is used as a substrate in this process [101]. Intermediate pyrolysis is characterized by high water content in the oil products and their low viscosity. The feedstock for this process should be shredded, chopped, or ground [102]. Fast pyrolysis is focused on the production of oil from biomass. This process is the most technologically advanced, and the process parameters are carefully controlled to achieve high oil production. Biomass for this process should be shredded to the size below 3 mm to enable high heat and mass transfer.
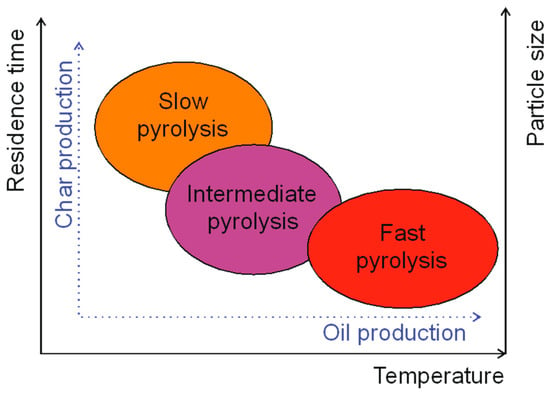
Figure 4.
Influence of pyrolysis parameters onto pyrolysis products.
In Figure 5, the share of individual products as a function of the heating rate is shown. In slow pyrolysis, it can be observed that the proportion of char, oil, and gas is comparable. In intermediate pyrolysis, an increase in the amount of oil produced of up to about 50% is observed. On the other hand, fast pyrolysis is dominated by oil, with an efficiency of about 75%.
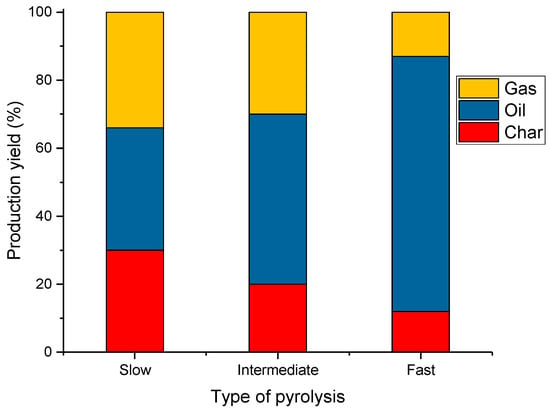
Figure 5.
Typical shares of wood biomass pyrolysis products obtained in different types of pyrolysis (data adapted from ref. [103]).
4.1. Char
Char is the solid residue that remains after the pyrolysis process and is rich in carbon [104,105] and other inorganic compounds, especially when produced from waste biomass [106]. The use of char as a fertilizer may be restricted if the content of heavy metals exceeds the limits. Adding char to the soil causes an increased water-holding capacity but also contributes to carbon sequestration for mitigating atmospheric carbon [107]. The char can also be used as an adsorbent to remove metal ions from wastewater [87]. The adsorption process depends mainly on the surface properties rather than the specific surface area [101]. The activated carbon sorption value can be improved through chemical or physical activation [63]. The content of mineral compounds in the feedstock can lead to chemisorption phenomena [108]. Char can be used as a fuel in the combustion process. The higher heating value (HHV) of char and the char yield range from 14 to 36 MJ/kg and from 30 to 90%, respectively, depending on the process conditions and the composition of the biomass used in the pyrolysis process [87,101]. The resulting char can also be used in the gasification process, resulting in the formation of syngas [109]. Feedstock’s high fixed carbon content increases the char content in the pyrolysis process [21]. The mineral compounds contained in feedstock mainly remain in the char [110]. The high content of minerals in the char (e.g., char from sewage sludge) makes it possible to use it as a fertilizer due to the high concentration of K, P, N, Mg, Ca, and S [89]. Good-quality char is obtained from slow pyrolysis and is characterized by high stability [39]. Char can also be used as an electron conductor in the anaerobic digestion process [111,112].
4.2. Oil
In the pyrolysis process, the oil consists of condensable gases from the decomposition of the feedstocks. They are separated in a condenser, usually located after the cyclone [14]. The oil can be used as fuel in boilers to produce heat or combusted in the engine to produce heat and electricity or syngas [113]. Oil is divided into two fractions: aqueous and organic (tar). After upgrading, the organic fraction can be used as fuel or a chemical [114]. An aqueous fraction consists mainly of water with soluble components such as acetic acids, hydroxyl acetone, or phenol [101]. The resulting tar in the pyrolysis process includes many components, such as acids, alcohols, aromatics, esters, furans, ketone, phenols, and sugars [115]. Before being used as a chemical or fuel, oil requires upgrading to improve its properties [116]. The upgrading process for pyrolytic oil can be realized as a rectification or extraction process. During the upgrading for fuel, it is important to remove organonitrogen compounds from the oil [117]. The oil may also contain solid particles after the pyrolysis process [63]. The oil contains little water when it produced through a fast pyrolysis process, while, when produced by slow and intermediate processes, the oil consists of more water and decanted aqueous phase from tar [5].
The commercial application of the oil is difficult due to the high cost of the separation and refining techniques. Therefore, the oil is currently used in the combustion process to produce heat. However, due to the high content of oxygen in the oil (up to 50%) [118], its higher heating value (HHV) ranges from 13 to 20 MJ/kg [101,119]. The HHV of conventional liquid fuel ranges from 42 to 46 MJ/kg. In addition, mineral compounds in the feedstock can cause water formation from the oxygen contained in the feedstock [39]. The oil produced in the fast pyrolysis process can have an energy density 3–5 times higher than that of the substrate for pyrolysis [120]. The rate of char cooling also influences the yield of the oil production in the pyrolysis process [12]. The best oil yield in the pyrolysis process is achieved from woody biomass [14].
Due to its high content of carboxylic acids and low pH (below 4), it can be potentially corrosive. Pyrolytic oil can be unstable during storage as a result of the ongoing polymerization and esterification reactions [121]. Pyrolytic oils emit odors due to acids, aldehydes, and other volatile compounds [122]. The co-pyrolysis of sewage sludge with wood can reduce the concentration of aldehydes, aliphatic hydrocarbon, amines, ketones, and phenols and increase the concentrations of acids and esters [41].
4.3. Non-Condensable Gases (NCG)
The composition of the gases produced during pyrolysis is strongly dependent on the process parameters and the chemical composition of the biomass. Pyrolytic gas is formed in the primary pyrolysis process from feedstock and in the secondary pyrolysis process from the reforming and cracking of oil [123]. In addition to the basic compounds, such as carbon dioxide (CO2), carbon monoxide (CO), hydrogen (H2), and low hydrocarbons (CxHy), it may contain ammonia (NH3), hydrogen cyanide (HCN), hydrogen sulfide (H2S), nitrogen oxides (NOx), sulfur oxides (SOx), low molecular alcohols, tar, ash, and water vapor [39,63]. Pyrolytic gas can be used for heat, electricity, or chemical production [124].
CO2 and CO are produced mainly in the primary decomposition and reforming of carbonyl and carboxyl groups [125]. CH4 is produced from the decomposition of methoxy and methylene groups and oxygenated compounds. H2 is produced from the secondary decomposition and reforming of aromatic and C-H groups at high temperatures [63,126]. The biomass, which contains more moisture, increases hydrogen production when subjected to the pyrolysis process [127]. In the pyrolysis process, mainly CO2 is produced from hemicellulose because of the contained carboxylic group. CO is primarily produced from cellulose due to the thermal cracking of the carbonyl and carboxylic groups. The chemical structure of lignin is more complex than that of the other compounds and contains aromatic ring and methoxy groups, from which more H2 and CH4 are formed in the pyrolysis process [101]. Feedstock’s high volatile matter content means more oil and gas are produced in the pyrolysis process [21]. NCG can be recycled as a fluidization agent in a fluidized reactor. Due to its calorific value (about 10–20 MJ/m3), NCG can be combusted to provide processing heat [63]. During the pyrolysis of sewage sludge, the amount of NH3 and HCN formed can reach as high as an 80% nitrogen content in the substrate for the pyrolysis process [73]. An increased amount of nitrogen in char can be obtained from the co-pyrolysis of sewage sludge with biomass containing high amounts of hemicellulose, cellulose, and lignin [128]. It was observed that the co-pyrolysis of two substrates can increase the production of H2 [104].
5. Reactors for the Pyrolysis Process
There are many types of reactors for the pyrolysis process. Reactors can be classified according to various criteria, e.g., the rate of heating, the operation mode of the reactor (batch, continuous), the heating system (direct or indirect), the pressure inside the reactor (vacuum, atmospheric, pressurized), and the reactor’s portability (stationary or mobile) [81]. In this work, the classification of reactors for the pyrolysis process was based on the heating rate. The slow pyrolysis process is carried out in a batch reactor (kiln, retort). The intermediate pyrolysis process is carried out in a rotary kiln reactor, vacuum, or screw/auger reactor. However, the fast pyrolysis process requires such reactors as fluidized bed reactors, rotating cones reactors, and ablative reactors [14].
5.1. Slow Pyrolysis
Two types of kiln and retorts reactors can be distinguished in this group. Both reactors are used for char production [81]. The main difference between these reactors is that oil and gas are recovered in retorts, while oil and gas are lost in kilns. In neither reactor does the reactor’s biomass (mainly wood) need to be shredded; wood logs are preferred. Biomass particles that are too small prevent the flow of the generated gas in the pyrolysis process.
Kilns can be built of clay, brick, and metal and are enclosed spaces with an air inlet and an oil and gas outlet [129]. This method has been used for centuries and is still popular. This is a typical batch process and is controlled by measuring the temperature inside the reactor or by observing the color of the vapor produced. Batch processes have a low rate of yield per unit volume of the reactor compared to continuous reactors. The stacked wood logs in the kiln reactor are ignited inside, and the airflow process is limited to prevent complete combustion. The pyrolysis process taking place in kilns is autothermal and may take up to 10 days. The advantages include a low investment cost and no need for special equipment. The main disadvantages are the emission of oil and gases into the environment, a high labor demand, low efficiency, and inferior control of the process [81]. The char production yield is between 10 and 33% [130]. Figure 6 shows kilns for the slow pyrolysis process.
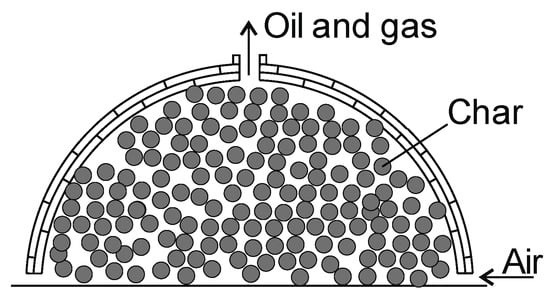
Figure 6.
Kilns made from brick used for the char production.
One example of a retort reactor for wood pyrolysis is the Lambiotte retort. The height of the retort reactor can reach up to 17 m, and the diameter up to 4 m. The pre-dried wood is served into the top of the retort reactors through the valve to keep them always full. Three zones can be distinguished in the retort (drying, pyrolysis, and cooling) (Figure 7) [131]. The pyrolysis process takes place at a temperature of about 550 °C. The heat for the process is provided by the hot gas coming from the combustion of gases from the pyrolysis process. The process is controlled by measuring the temperature inside the reactor. The char leaving the retort does not require additional cooling and can be directed to be stored. The efficiency of the installation reaches 6000 tons/year with a char production yield of 33% [81]. The advantages of the installation include a good product quality and a high yield of char production. However, its disadvantages include sensitivity to the biomass moisture content and corrosivity caused by the acetic acid produced [129].
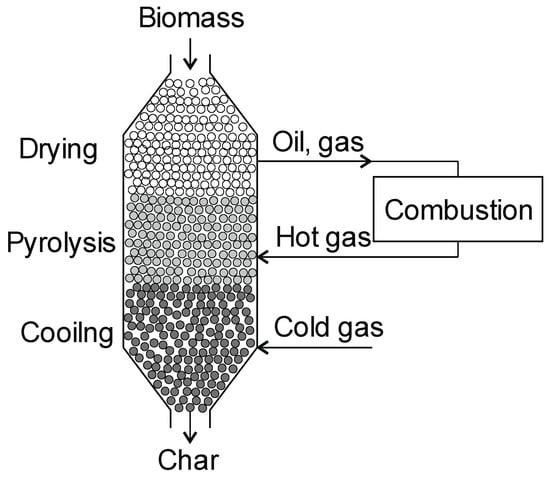
Figure 7.
Lambiotte retort.
Slow pyrolysis is carried out in a fixed bed (tubular) reactor, a simplified diagram of which is shown in Figure 8. These reactors are mainly used in studies of the pyrolysis process on a laboratory scale [17]. The biomass is placed in the reactor, and then the reactor is closed and heated to a set temperature. An inert gas is often passed through the reactor to remove the pyrolysis products (oil and gas). Figure 8. When the process is completed, and the reactor cooled down, the produced char is removed.
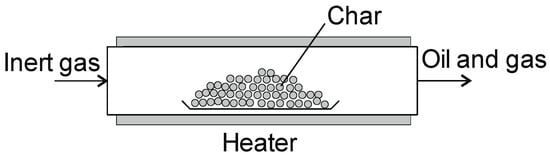
Figure 8.
Fixed bed reactor for batch processes.
5.2. Intermediate Pyrolysis
In this type of pyrolysis, biomass particles from 0.5 cm to several centimeters in diameter are used as a feedstock. This process is carried out continuously and is more technologically advanced. The biomass is rotated and moved to improve the homogeneity of the pyrolysis products. This group includes the following types of reactors: auger or screw reactors, rotary drum reactors, and vacuum reactors.
5.2.1. Auger or Screw Reactor
The reactor is stationary, and the material is transported through a screw conveyor (Figure 9). This provides shorter residence time control and fine feedstock mixing. The gaseous products are not diluted with inert gas as in the case of fluidized bed reactors [81]. The outer wall of the reactor and/or the hollow screw can be heated. Heat for the reactor can also be supplied by hot sand or ceramic balls added to the biomass [12,132]. A disadvantage of the screw feed reactors may be the uneven heating or sticking of the material due to local overheating around the screw. The solution is to use a self-cleaning interlocking screw system or a double screw in such cases. The residence time in such a reactor depends on the length and the speed of rotation of the screw. The screw reactor is relatively cheap, but upscaling limits the ratio of the screw surface to the reactor volume, which decreases for larger reactors. Nevertheless, the high heat transfer coefficients make it very effective even for larger-scale applications [133]. The temperature of the pyrolysis process in this type of reactor is in the range of 400–500 °C [81]. For resident times of vapor in the reactor of 5 to 30 s, the yield for the production of oil is in the range 45–60%, and that for the production of char is 17–30%. The produced oil may contain as much as 55% water [134]. A long resident time for the gases produced during the pyrolysis process causes a lower yield in oil production (25–40%). This type of pyrolizer is compact and favorable for mobile pyrolizer units [135]. The efficiency of these installations can reach 50 tons/day [134]. In the literature, articles can be found that discuss this type of reactor in great detail, e.g., Campuzano et al. [120].
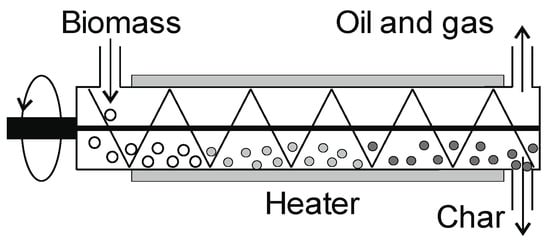
Figure 9.
Diagram of a reactor with a screw.
5.2.2. Rotary Drum Reactor
The reactor rotates along a horizontal axis and is inclined at an angle so that the material flows from the inlet to the end of the reactor due to gravity and to the drum rotation (Figure 10). The heat is supplied by a diaphragm through the outer jacket of the rotating drum and/or by diaphragmless means from the exhaust gas flowing through the reactor. The char production efficiency coefficient is in the range of 20–40%, and that of oil is in the range of 35–60%, while the efficiency can reach as high as 290 tons/day [81]. However, these reactors can also work with low efficiency. The advantage of the reactor is that it does not require a high degree of material fragmentation. However, its disadvantages include a complicated sealing system for the rotating drum.
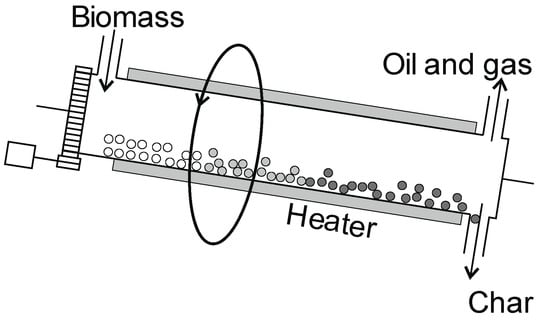
Figure 10.
Rotary drum pyrolysis reactor.
5.2.3. Vacuum Pyrolysis Reactor
This configuration of the reactor (Figure 11) is popular for studying the kinetics of thermochemical changes and for studying the effect of pressure (pyrolysis under reduced pressure). The material is fed from above, flows in co-current with the gas stream, and does not require a high degree of material fragmentation (2–5 cm) [12]. The material is sprinkled by rakes on rotating discs and falls due to gravity to the lower levels while its temperature rises from 200 to 500 °C. The flow of material through the reactor is similar to a plug flow, during which it is possible to control the residence time through the length of the heating zone. The secondary reaction in this reactor is limited because the residence time is reduced by vacuum. The yield of liquid products is usually lower (about 35–50%) than that of fluidized bed reactors [12]. Low pressure in the reactor requires a sealed system for feeding the biomass and discharging the products.
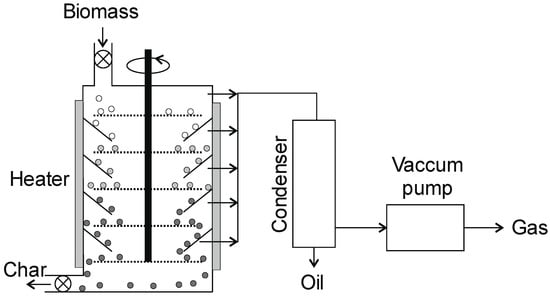
Figure 11.
Entrained flow vacuum reactor (adopted from [136]).
5.3. Fast Pyrolysis
The primary purpose of fast pyrolysis is the production of oil. This process is carried out in reactors such as rotating cone, ablative, bubbling fluidized bed, and circulating fluidized bed reactors. The last reactor type is often the subject of researched aimed at producing the highest possible purity of oil. For most reactors (except the ablative), the biomass should be fragmented to a size below 3 mm [98]. These reactors are used to carry out the pyrolysis process in a continuous manner.
5.3.1. Ablative Pyrolizer
The biomass is mechanically pressed against the heated wall of the reactor (Figure 12). The biomass comes into contact with the hot wall and moves on, and the resulting products are removed. This type of pyrolysis resembles “melting butter in a frying pan” [5]. There is no need for the type of inert gas atmosphere that causes the production of high-quality gas. The temperature of the pyrolysis process in this type of reactor is usually above 500 °C. Another advantage of this technology is that the feed material does not need to be comminuted, but the process is the most efficient when the particle size is about 20 mm. The size reduction of the feedstock causes an increase in operating costs [137]. The efficiency of installations using ablative pyrolysis can reach a value of 2 tons/h [101,138]. The oil yield in this type of reactor reaches 70% [139]. The oil from the ablative pyrolysis process contains lower molecular weights (MWs) due to its cracking and depolymerization on a hot surface [140]. The high velocity of the biomass particles causes reactor erosion and scale-up problems. Another disadvantage of the reactor is the low heat transfer from the spinning disc and raw material feeding system. Its advantages include a compact design and no need for feedstock grinding. For different kinds of biomass, the yield of char, oil, and gas is in the range of 6–32, 40–60, and 12–34%, respectively [81].
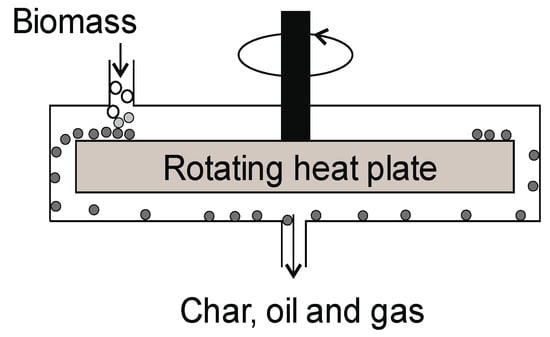
Figure 12.
Ablative pyrolysis reactor.
5.3.2. Rotating Cone Pyrolizer
In this type of reactor, biomass is fed in near the bottom of the rotating cone (Figure 13), and the biomass in the reactor is moved by centrifugal force and must be shredded. In such a reactor, heating is achieved by the heat transfer via the wall or heated sand [12]. Hot pyrolysis vapors leave the reactor at the top, while hot char is removed from the outside stationary cone. No carrier gas is required, which simplifies the installation of additional devices and reduces the operating costs. The oil yield from this pyrolizer is up to 70%. Scaling up this type of reactor is complicated in terms of the configuration of the rotating cone [141]. The capacity of an industrial installation can be as high as 50 tons/day [81].
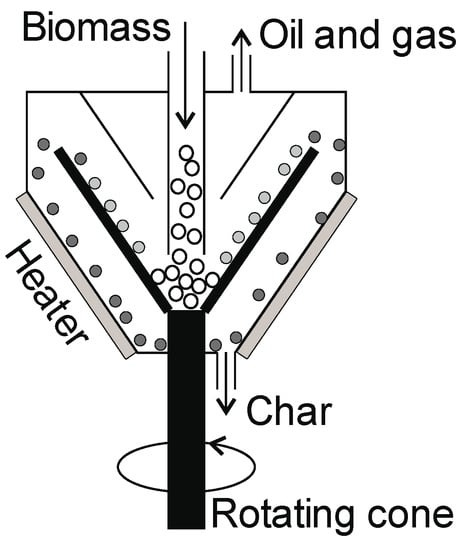
Figure 13.
Rotating cone reactor.
5.3.3. Bubbling Fluidized Bed (BFB)
Fluidized bed reactors are divided into two groups: Bubbling Fluidized Bed (BFB) and Circulating Fluidized Bed (CFB). BFB technology is well known; the reactor is easy to build and operate (on a large scale). The process has very high heat transfer coefficients and is run at temperatures ranging from 450 to 600 °C [81]. In order to obtain high heating rates, small particle sizes are required (2–3 mm) [12]. The gas velocity is in the range of 1.5–2 times the minimum fluidization velocity [101]. This type of reactor operates in the co-current flow of the biomass and fluidizing agent (Figure 14). Sand is often added to the bed to improve the heat and mass transfer [12]. The residence time of the biomass in the BFB is approx. 2–3 s [12]. The efficiency of this type of reactor can be as high as 200 tons/day. The production yields depend on the type of biomass: for char they are 13–45, for oil 35–77, and for gases 3–25% [5,81]. The advantages of this process include accurate temperature control, ease of scaling up, and the fact that it is a well-known process. The main disadvantage is the high demand for inert gas. To extract char from other products, a cyclone is needed [14].
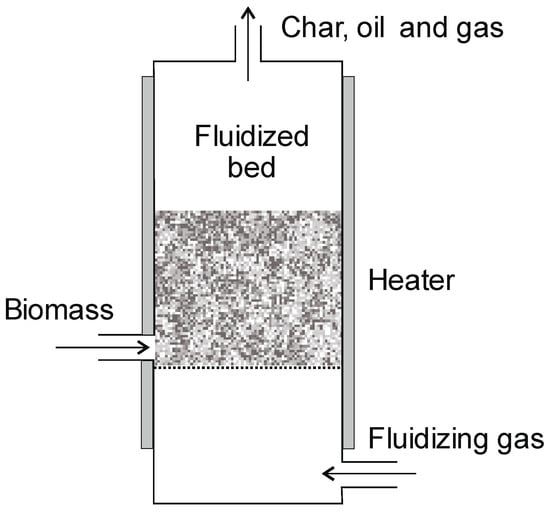
Figure 14.
Bubbling Fluidized Bed (BFB) (adopted from [136]).
5.3.4. Circulating Fluidized Bed (CFB)
In these reactors, the gas flow rate is significantly greater than in the BFB system. Due to the high gas flow velocities, the average residence time of the biomass is about 0.5–1 s [12]. The CFB system is much more complex than the BFB system. In the CFB residence system, the residence time of the char in the reactor is comparable to the gas residence time in this reactor, which results in lower biomass conversion. The char from the CFB pyrolizer can contain approximately 18% unreacted feedstock [2]. As in the BFB reactor, sand is added to the biomass in a proportion of 20–25% of the biomass [31]. In this system, the sand leaves the reactor to be reused. It is separated from the pyrolysis products in a cyclone. Hot sand circulates between the reactor and the inert (sand) heater. The sand that is separated in the cyclone can be burned, which results in a temperature increase (Figure 15). Circulating fluidized bed (CFB) reactors are widely used in the oil industry in very high-capacity plants. CFB are potentially also suitable for high biomass throughputs and are characterized by good temperature control. Their disadvantages include a large amount of inert gas, which dilutes the gas, and difficult oil separation. The capacity of the industrial installations reaches the value of 50 tons/day, and the yield production of oil is 55–70% [81]. Pyrolysis gases can be used as a fluidizing agent after oil or flue gas condensation. The heat for fluidized heating gas can also come from char, oil, or gas combustion. All configurations of the fluidized bed reactors allow greater control of the gas residence time, which is an essential parameter for optimizing the oil production to obtain a high yield.
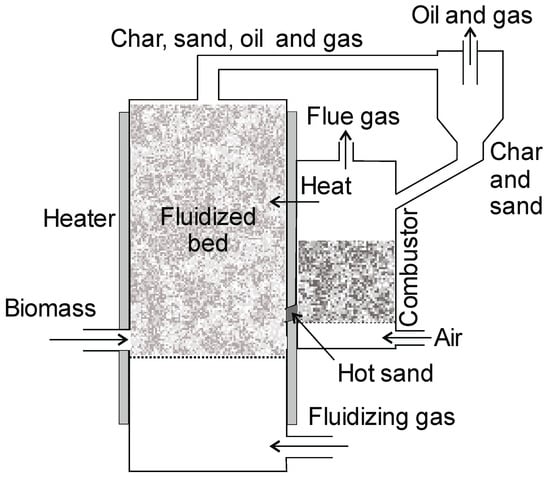
Figure 15.
Circulating Fluidized Bed (CFB) (adopted from [136]).
5.4. Comparison of Pyrolysis Reactors
Table 3 presents a summary of the types of reactors used in the pyrolysis process. The selection of the appropriate type of reactor is related not only to its advantages and disadvantages but to the properties of the substrate and the desired products. The properties of the substrate affect the kinetics of the reaction as well as the heat, mass, and momentum transfer. The microwave pyrolizer was omitted when discussing the reactors because the microwave penetration of the feedstock is limited (to 1–2 cm), which makes scale-up difficult [12]. Among the reactors mentioned, fluidized reactors and rotary kiln reactors are the most frequently used [63]. It is worth noting that the cost of the pyrolysis reactor is 10–25% of the capital cost.

Table 3.
Comparison of different types of reactors for pyrolysis.
6. Combination of Thermochemical Processes
Due to the fact that the pyrolysis process is endothermic (allothermal) and biomass can contain water, a source of energy is required. At the same time, it should be noted that the amount of heat needed to evaporate the water is significantly greater than that required for the pyrolysis process. For the pyrolysis reactor to be autothermal, the additional heat from the feedstock or from pyrolysis products is needed [16]. Theoretically, all products of the pyrolysis process (char, oil, and gas) can be burnt to produce heat (ex situ heat generation). Additional heat in the pyrolysis process may come from the reaction of the partial combustion of the feedstock in the reactor, obtained as a result of introducing a limited amount of oxygen into the reactor (in situ heat generation). Such a pyrolysis process, with partial combustion, is called oxidative pyrolysis [16]. After the pyrolysis process, the char can be used in the gasification process, in which syngas is produced. Syngas can also be burned to produce heat. At the same time, it should be remembered that syngas is a valuable substrate for the production of value-added products via Fischer-Tropsch synthesis [142]. The gasification process takes place at a temperature above 750 °C and may be endo or exothermic depending on the oxidizing agent (oxygen, steam, or carbon dioxide) used [109]. It should also be noted that gasification consists of the following stages: drying, pyrolysis, oxidation, and reduction [143]. The gasification process is much slower than the pyrolysis process. On the other hand, the combustion process is carried out at a temperature above 1000 °C and aims to generate thermal energy, decompose organic matter, and reduce the feedstock volume [143]. It should also be emphasized that pyrolysis is one of the stages of combustion. Figure 16 shows the different possibilities for producing the heat used to dry the biomass and the pyrolysis process.
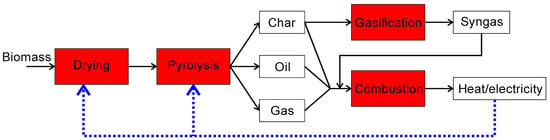
Figure 16.
Heat production in thermochemical processes.
Examples of combining pyrolysis and other thermochemical processes are:
- EDDITh process (pyrolysis and combustion) France, Japan [144];
- PKA technology (pyrolysis and gasification) Germany [145];
- Thermoselect process (pyrolysis and gasification) Germany, Japan, Italy [146];
- WGT technology (pyrolysis and gasification) [147];
- Simens Schwel Brenn technology (pyrolysis and combustion) [148].
7. Review of Sludge Pyrolysis Technology
Among the types of reactors mentioned, the auger and rotary drum reactors are most often used for the sludge pyrolysis process. Other types of sewage sludge pyrolysis reactors are used on the laboratory scale [39,41]. In the next part of the article, examples of two installations using screw and rotary drum reactors are presented. Eisenmann’s installation called “Pyrobustor” (Figure 17) uses rotary drum reactors. The installation’s capacity is about 550 kg/h. It was installed in a sewage treatment plant and works for about 7500 h/year. The dried granulate is directed to a two-stage drum reactor, in which pyrolysis takes place in the first stage, and, in the second stage, the pyrolysis char is burnt. Pyrolysis gases are burned in the external combustion chamber and produced heat is used for drying sewage sludge. Flue gases generated during char combustion are used to heat the pyrolysis reactor, and the air is introduced into the combustion rector. The solid residue is inert ash, which can be safely stored. Detailed information on the installation is available on the manufacturer’s website.
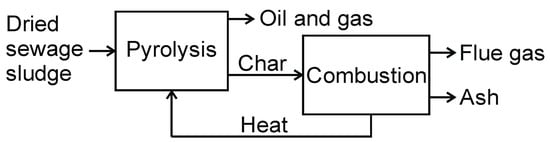
Figure 17.
Two-stage “Pyrobustor” reactor for thermal treatment of sewage sludge.
The screw reactor is used in an installation called “Spirajoule®” by E.T.I.A., France, for the pyrolysis of sewage sludge. Heat to the reactor is supplied by a resistively electrically heated spiral transporting the material in the reactor. The system shown in Figure 18 consists of a material feeder, a gas condenser, a char cooler, and a combustion chamber for non-condensing and/or condensing gases. The residence time of the feedstock in the reactor ranges from 5 to 20 min. The maximum installation capacity is about 16 t/day for a material with a moisture content of 10–20% and particle sizes below 20 mm. Detailed information on the installation is available on the manufacturer’s website, and the results of laboratory- and pilot-scale pyrolysis of sewage sludge are described by Ledakowicz et al. [149].
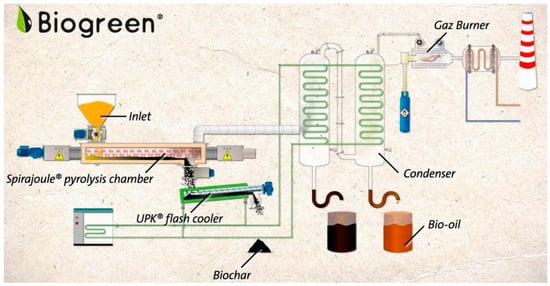
Figure 18.
Scheme of the ETIA “Biogreen” installation for biomass pyrolysis [150].
8. Conclusions
The most important advantages of the pyrolysis process for industry are its simplicity, low emission technology, tolerance for changes, substrate properties, and formation of marketable products. The pyrolysis process is often used in biorefineries or in a circular economy as a pathway for the management of the waste stream. Before selecting the type of pyrolysis reactor, attention should be paid to the availability of biomass for the pyrolysis process. The pyrolysis of two or more substrates may improve the quality of the pyrolysis products. In addition to the composition of the substrate, it is essential to know the kinetics of the pyrolysis of a given substrate and its physicochemical properties that affect the momentum, heat, and mass transfer. Apart from biomass, attention should be paid to the possibility of managing the resulting products (char, oil, and gas). Regardless of the process conditions and reactor type, in addition to the desired product (e.g., oil), by-products (e.g., char and gas) are also formed. Before selecting a reactor, it is important to determine the optimal pyrolysis conditions of a given substrate in order to obtain the desired product with specific properties. The appropriate type of pyrolysis reactor can be selected with information about the substrate, the desired product, and the process conditions. However, each reactor presented in this paper has both advantages and disadvantages. When selecting a reactor, the way of loading and transporting the raw material inside the reactor and the way of receiving the products should be considered. The decision of which reactor to choose is also greatly influenced by the availability of inert gas as well as the method of supplying heat to the reactor. Pyrolysis by-products are often used to the generate heat (e.g., in a combustion process) used by the reactor and to dry the biomass. In the case of biomass with high moisture (above 90%) and ash content (e.g., sewage sludge), the main goal is to dispose of the waste. To achieve this target, heat must be generated from the pyrolysis products which can then be used in the pyrolysis reactor and for drying the substrate.
Author Contributions
Conceptualization, R.S. and S.S.; data curation, R.S.; writing—original draft preparation, R.S.; writing—review and editing, S.S. and H.U.; visualization, R.S.; supervision, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
The publication was produced as part of the Doctoral Candidate’s participation in a project of the National Agency for Academic Exchange within the framework of the “STER Programme—Internationalisation of Doctoral Schools” as part of the project “Curriculum for advanced doctoral education & training—CADET Academy of Lodz University of Technology”. Grant No: PPI/STE/2020/1/00027/U/00001.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mulchandani, A.; Westerhoff, P. Recovery opportunities for metals and energy from sewage sludges. Bioresour. Technol. 2016, 215, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Wang, Y.; Liu, Y.; Ruan, R.; He, C.; Yu, Z.; Jiang, L.; Zeng, Z.; Tian, X. Integrated process of lignocellulosic biomass torrefaction and pyrolysis for upgrading bio-oil production: A state-of-the-art review. Renew. Sustain. Energy Rev. 2019, 107, 20–36. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; César Torres-Mayanga, P.; Abaide, E.R.; Zabot, G.L.; De Castilhos, F. Hydrothermal carbonization and Liquefaction: Differences, progress, challenges, and opportunities. Bioresour. Technol. 2022, 343, 126084. [Google Scholar] [CrossRef]
- Ünyay, H.; Yılmaz, F.; Başar, İ.A.; Altınay Perendeci, N.; Çoban, I.; Şahinkaya, E. Effects of organic loading rate on methane production from switchgrass in batch and semi-continuous stirred tank reactor system. Biomass Bioenergy 2022, 156, 106306. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Murillo, R.; Aylón, E.; Navarro, M.V.; Callén, M.S.; Aranda, A.; Mastral, A.M. The application of thermal processes to valorise waste tyre. Fuel Process. Technol. 2006, 87, 143–147. [Google Scholar] [CrossRef]
- Piersa, P.; Unyay, H.; Szufa, S.; Lewandowska, W.; Modrzewski, R.; Ślężak, R.; Ledakowicz, S. Review An Extensive Review and Comparison of Modern Biomass Torrefaction Reactors vs. Biomass Pyrolysis—Part 1. Energies 2022, 15, 2227. [Google Scholar] [CrossRef]
- Szufa, S.; Piersa, P.; Junga, R.; Błaszczuk, A.; Modliński, N.; Sobek, S.; Marczak-Grzesik, M.; Adrian, Ł.; Dzikuć, M. Numerical modeling of the co-firing process of an in situ steam-torrefied biomass with coal in a 230 MW industrial-scale boiler. Energy 2023, 263, 125918. [Google Scholar] [CrossRef]
- Collard, F.X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Axelsson, L.; Franzén, M.; Ostwald, M.; Berndes, G.; Lakshmi, G.; Ravindranath, N.H. Perspective: Jatropha cultivation in southern India: Assessing farmers’ experiences. Biofuels Bioprod. Biorefining 2012, 6, 246–256. [Google Scholar] [CrossRef]
- Giudicianni, P.; Gargiulo, V.; Grottola, C.M.; Alfè, M.; Ferreiro, A.I.; Mendes, M.A.A.; Fagnano, M.; Ragucci, R. Inherent Metal Elements in Biomass Pyrolysis: A Review. Energy Fuels 2021, 35, 5407–5478. [Google Scholar] [CrossRef]
- Sharifzadeh, M.; Sadeqzadeh, M.; Guo, M.; Borhani, T.N.; Murthy Konda, N.V.S.N.; Garcia, M.C.; Wang, L.; Hallett, J.; Shah, N. The multi-scale challenges of biomass fast pyrolysis and bio-oil upgrading: Review of the state of art and future research directions. Prog. Energy Combust. Sci. 2019, 71, 1–80. [Google Scholar] [CrossRef]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Roy, P.; Dias, G. Prospects for pyrolysis technologies in the bioenergy sector: A review. Renew. Sustain. Energy Rev. 2017, 77, 59–69. [Google Scholar] [CrossRef]
- Park, W.C.; Atreya, A.; Baum, H.R. Experimental and theoretical investigation of heat and mass transfer processes during wood pyrolysis. Combust. Flame 2010, 157, 481–494. [Google Scholar] [CrossRef]
- Huang, Y.; Li, B.; Liu, D.; Xie, X.; Zhang, H.; Sun, H.; Hu, X.; Zhang, S. Fundamental Advances in Biomass Autothermal/Oxidative Pyrolysis: A Review. ACS Sustain. Chem. Eng. 2020, 8, 11888–11905. [Google Scholar] [CrossRef]
- Lu, J.S.; Chang, Y.; Poon, C.S.; Lee, D.J. Slow pyrolysis of municipal solid waste (MSW): A review. Bioresour. Technol. 2020, 312, 123615. [Google Scholar] [CrossRef]
- Pérez-Elvira, S.I.; Nieto Diez, P.; Fdz-Polanco, F. Sludge minimisation technologies. Rev. Environ. Sci. Biotechnol. 2006, 5, 375–398. [Google Scholar] [CrossRef]
- Schnell, M.; Horst, T.; Quicker, P. Thermal treatment of sewage sludge in Germany: A review. J. Environ. Manag. 2020, 263, 110367. [Google Scholar] [CrossRef]
- Frantzi, D.; Zabaniotou, A. Waste-Based Intermediate Bioenergy Carriers: Syngas Production via Coupling Slow Pyrolysis with Gasification under a Circular Economy Model. Energies 2021, 14, 7366. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Rahman, M.M.; Rasul, M.G.; Mahlia, T.M.I.; Ashrafur, S.M. An overview of recent developments in biomass pyrolysis technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef]
- Hameed, S.; Sharma, A.; Pareek, V.; Wu, H.; Yu, Y. A review on biomass pyrolysis models: Kinetic, network and mechanistic models. Biomass Bioenergy 2019, 123, 104–122. [Google Scholar] [CrossRef]
- Matusiak, M.; Slęzak, R.; Ledakowicz, S. Thermogravimetric kinetics of selected energy crops pyrolysis. Energies 2020, 13, 3977. [Google Scholar] [CrossRef]
- Stefanidis, S.D.; Kalogiannis, K.G.; Iliopoulou, E.F.; Michailof, C.M.; Pilavachi, P.A.; Lappas, A.A. A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J. Anal. Appl. Pyrolysis 2014, 105, 143–150. [Google Scholar] [CrossRef]
- Akhtar, J.; Saidina Amin, N. A review on operating parameters for optimum liquid oil yield in biomass pyrolysis. Renew. Sustain. Energy Rev. 2012, 16, 5101–5109. [Google Scholar] [CrossRef]
- Fahmi, R.; Bridgwater, A.V.; Donnison, I.; Yates, N.; Jones, J.M. The effect of lignin and inorganic species in biomass on pyrolysis oil yields, quality and stability. Fuel 2008, 87, 1230–1240. [Google Scholar] [CrossRef]
- Caballero, J.A.; Conesa, J.A.; Font, R.; Marcilla, A. Pyrolysis kinetics of almond shells and olive stones considering their organic fractions. J. Anal. Appl. Pyrolysis 1997, 42, 159–175. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Wang, K.; Luo, Z. Influence of the interaction of components on the pyrolysis behavior of biomass. J. Anal. Appl. Pyrolysis 2011, 91, 183–189. [Google Scholar] [CrossRef]
- Hosoya, T.; Kawamoto, H.; Saka, S. Cellulose-hemicellulose and cellulose-lignin interactions in wood pyrolysis at gasification temperature. J. Anal. Appl. Pyrolysis 2007, 80, 118–125. [Google Scholar] [CrossRef]
- Zheng, A.; Zhao, Z.; Chang, S.; Huang, Z.; Wang, X.; He, F.; Li, H. Effect of torrefaction on structure and fast pyrolysis behavior of corncobs. Bioresour. Technol. 2013, 128, 370–377. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of wood /biomass for Bio-oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Oasmaa, A.; Sundqvist, T.; Kuoppala, E.; Garcia-Perez, M.; Solantausta, Y.; Lindfors, C.; Paasikallio, V. Controlling the phase stability of biomass fast pyrolysis bio-oils. Energy Fuels 2015, 29, 4373–4381. [Google Scholar] [CrossRef]
- Di Blasi, C.; Galgano, A.; Branca, C. Effects of potassium hydroxide impregnation on wood pyrolysis. Energy Fuels 2009, 23, 1045–1054. [Google Scholar] [CrossRef]
- Yang, C.; Li, R.; Zhang, B.; Qiu, Q.; Wang, B.; Yang, H.; Ding, Y.; Wang, C. Pyrolysis of microalgae: A critical review. Fuel Process. Technol. 2019, 186, 53–72. [Google Scholar] [CrossRef]
- Aravind, S.; Kumar, P.S.; Kumar, N.S.; Siddarth, N. Conversion of green algal biomass into bioenergy by pyrolysis. A review. Environ. Chem. Lett. 2020, 18, 829–849. [Google Scholar] [CrossRef]
- Nyoni, B.; Tsipa, P.; Duma, S.; Shabangu, S.; Hlangothi, S. Modelling of thermal decomposition kinetics of proteins, carbohydrates and lipids using Scenedesmus microalgae thermal data. Asian J. Chem. 2020, 32, 2921–2926. [Google Scholar] [CrossRef]
- Chen, W.H.; Chu, Y.S.; Liu, J.L.; Chang, J.S. Thermal degradation of carbohydrates, proteins and lipids in microalgae analyzed by evolutionary computation. Energy Convers. Manag. 2018, 160, 209–219. [Google Scholar] [CrossRef]
- Slezak, R.; Nawrot, P.; Ledakowicz, S. Pyrolysis of micro- and macroalgae in thermobalance coupled with mass spectrometer. Algal Res. 2022, 66, 102782. [Google Scholar] [CrossRef]
- Djandja, O.S.; Wang, Z.C.; Wang, F.; Xu, Y.P.; Duan, P.G. Pyrolysis of municipal sewage sludge for biofuel production: A review. Ind. Eng. Chem. Res. 2020, 59, 16939–16956. [Google Scholar] [CrossRef]
- Environment 2022. Statistics Poland. Available online: https://stat.gov.pl/obszary-tematyczne/srodowisko-energia/srodowisko/ochrona-srodowiska-2022,1,23.html (accessed on 1 January 2023).
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical conversion of sewage sludge: A critical review. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lo, S.L. Sludge: A waste or renewable source for energy and resources recovery? Renew. Sustain. Energy Rev. 2013, 25, 708–728. [Google Scholar] [CrossRef]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Fijalkowski, K.; Rorat, A.; Grobelak, A.; Kacprzak, M.J. The presence of contaminations in sewage sludge—The current situation. J. Environ. Manag. 2017, 203, 1126–1136. [Google Scholar] [CrossRef]
- Zhang, X.P.; Zhang, C.; Li, X.; Yu, S.H.; Tan, P.; Fang, Q.Y.; Chen, G. A two-step process for sewage sludge treatment: Hydrothermal treatment of sludge and catalytic hydrothermal gasification of its derived liquid. Fuel Process. Technol. 2018, 180, 67–74. [Google Scholar] [CrossRef]
- Collard, M.; Teychené, B.; Lemée, L. Comparison of three different wastewater sludge and their respective drying processes: Solar, thermal and reed beds—Impact on organic matter characteristics. J. Environ. Manag. 2017, 203, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Werle, S.; Wilk, R.K. A review of methods for the thermal utilization of sewage sludge: The Polish perspective. Renew. Energy 2010, 35, 1914–1919. [Google Scholar] [CrossRef]
- Stolarek, P.; Ledakowicz, S.; Slȩzak, R. Influence of Liming on Kinetics of Sewage Sludge Pyrolysis. Ecol. Chem. Eng. S 2019, 26, 175–188. [Google Scholar] [CrossRef]
- Rulkens, W. Sewage sludge as a biomass resource for the production of energy: Overview and assessment of the various options. Energy Fuels 2008, 22, 9–15. [Google Scholar] [CrossRef]
- Cao, Y.; Pawłowski, A. Sewage sludge-to-energy approaches based on anaerobic digestion and pyrolysis: Brief overview and energy efficiency assessment. Renew. Sustain. Energy Rev. 2012, 16, 1657–1665. [Google Scholar] [CrossRef]
- Campbell, H.W. Sludge management—Future issues and trends. Water Sci. Technol. 2000, 41, 1–8. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, Y.; Zhu, X.; Brewer, C.E.; Brown, R.C. Ex-situ catalytic pyrolysis of wastewater sewage sludge—A micro-pyrolysis study. Bioresour. Technol. 2017, 232, 229–234. [Google Scholar] [CrossRef]
- Wei, L.; Wen, L.; Yang, T.; Zhang, N. Nitrogen Transformation during Sewage Sludge Pyrolysis. Energy Fuels 2015, 29, 5088–5094. [Google Scholar] [CrossRef]
- Raheem, A.; He, Q.; Mangi, F.H.; Areeprasert, C.; Ding, L.; Yu, G. Roles of Heavy Metals during Pyrolysis and Gasification of Metal-Contaminated Waste Biomass: A Review. Energy Fuels 2022, 36, 2351–2368. [Google Scholar] [CrossRef]
- Nowicki, L.; Ledakowicz, S. Comprehensive characterization of thermal decomposition of sewage sludge by TG-MS. J. Anal. Appl. Pyrolysis 2014, 110, 220–228. [Google Scholar] [CrossRef]
- Gievers, F.; Loewen, A.; Nelles, M. Life cycle assessment of sewage sludge pyrolysis: Environmental impacts of biochar as carbon sequestrator and nutrient recycler. Detritus 2021, 16, 94–105. [Google Scholar] [CrossRef]
- Larina, O.M.; Zaichenko, V.M. Influence of pyrolysis on evaporation and solubility of heavy metals in sewage sludge. J. Phys. Conf. Ser. 2020, 1556, 012017. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, J.; Wang, M.; Naidu, R.; Liu, Y.; Man, Y.B.; Liang, X.; Wong, M.H.; Christie, P.; Zhang, Y.; et al. Co-pyrolysis of sewage sludge and rice husk/ bamboo sawdust for biochar with high aromaticity and low metal mobility. Environ. Res. 2020, 191, 110034. [Google Scholar] [CrossRef] [PubMed]
- Kubonova, L.; Janakova, I.; Malikova, P.; Drabinova, S.; Dej, M.; Smelik, R.; Skalny, P.; Heviankova, S. Evaluation of waste blends with sewage sludge as a potential material input for pyrolysis. Appl. Sci. 2021, 11, 1610. [Google Scholar] [CrossRef]
- Wang, Z.; Shu, X.; Zhu, H.; Xie, L.; Cheng, S.; Zhang, Y. Characteristics of biochars prepared by co-pyrolysis of sewage sludge and cotton stalk intended for use as soil amendments. Environ. Technol. 2020, 41, 1347–1357. [Google Scholar] [CrossRef]
- Bach, Q.V.; Skreiberg, O. Upgrading biomass fuels via wet torrefaction: A review and comparison with dry torrefaction. Renew. Sustain. Energy Rev. 2016, 54, 665–677. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Fang, S.; Gu, W.; Chen, L.; Yu, Z.; Dai, M.; Lin, Y.; Liao, Y.; Ma, X. Ultrasonic pretreatment effects on the co-pyrolysis of municipal solid waste and paper sludge through orthogonal test. Bioresour. Technol. 2018, 258, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.; Westover, T.L.; Czernik, S.; Jablonski, W. Biomass feedstocks for renewable fuel production: A review of the impacts of feedstock and pretreatment on the yield and product distribution of fast pyrolysis bio-oils and vapors. Green Chem. 2014, 16, 384–406. [Google Scholar] [CrossRef]
- Meng, J.; Park, J.; Tilotta, D.; Park, S. The effect of torrefaction on the chemistry of fast-pyrolysis bio-oil. Bioresour. Technol. 2012, 111, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Lei, H.; Wang, L.; Bu, Q.; Chen, S.; Wu, J.; Julson, J.; Ruan, R. The effects of torrefaction on compositions of bio-oil and syngas from biomass pyrolysis by microwave heating. Bioresour. Technol. 2013, 135, 659–664. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C.; D’Errico, G. Degradation characteristics of straw and washed straw. Thermochim. Acta 2000, 364, 133–142. [Google Scholar] [CrossRef]
- Raveendran, K.; Ganesh, A.; Khilart, K.C. Influence of mineral matter on biomass pyrolysis characteristics. Fuel 1995, 74, 1812–1822. [Google Scholar] [CrossRef]
- Williams, P.T.; Horne, P.A. The role of metal salts in the pyrolysis of biomass. Renew. Energy 1994, 4, 1–13. [Google Scholar] [CrossRef]
- Trendewicz, A.; Evans, R.; Dutta, A.; Sykes, R.; Carpenter, D.; Braun, R. Evaluating the effect of potassium on cellulose pyrolysis reaction kinetics. Biomass Bioenergy 2015, 74, 15–25. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, T.; Che, D. Effect of water washing on fuel properties, pyrolysis and combustion characteristics, and ash fusibility of biomass. Fuel Process. Technol. 2013, 106, 712–720. [Google Scholar] [CrossRef]
- Liu, H.; Yi, L.; Hu, H.; Xu, K.; Zhang, Q.; Lu, G.; Yao, H. Emission control of NOx precursors during sewage sludge pyrolysis using an integrated pretreatment of Fenton peroxidation and CaO conditioning. Fuel 2017, 195, 208–216. [Google Scholar] [CrossRef]
- Kim, Y.; Parker, W. A technical and economic evaluation of the pyrolysis of sewage sludge for the production of bio-oil. Bioresour. Technol. 2008, 99, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Agar, D.A.; Kwapinska, M.; Leahy, J.J. Pyrolysis of wastewater sludge and composted organic fines from municipal solid waste: Laboratory reactor characterisation and product distribution. Environ. Sci. Pollut. Res. 2018, 25, 35874–35882. [Google Scholar] [CrossRef] [PubMed]
- Kan, T.; Strezov, V.; Evans, T. Effect of the Heating Rate on the Thermochemical Behavior and Biofuel Properties of Sewage Sludge Pyrolysis. Energy Fuels 2016, 30, 1564–1570. [Google Scholar] [CrossRef]
- Slezak, R.; Krzystek, L.; Ledakowicz, S. Thermogravimetric analysis coupled with mass spectrometry of spent mushroom substrate and its fractions. J. Anal. Appl. Pyrolysis 2018, 133, 1–8. [Google Scholar] [CrossRef]
- Demirbas, M.F.; Balat, M. Biomass pyrolysis for liquid fuels and chemicals: A review. J. Sci. Ind. Res. 2007, 66, 797–804. [Google Scholar]
- Patwardhan, P.R.; Satrio, J.A.; Brown, R.C.; Shanks, B.H. Influence of inorganic salts on the primary pyrolysis products of cellulose. Bioresour. Technol. 2010, 101, 4646–4655. [Google Scholar] [CrossRef]
- Zhang, B.; Xiong, S.; Xiao, B.; Yu, D.; Jia, X. Mechanism of wet sewage sludge pyrolysis in a tubular furnace. Int. J. Hydrogen Energy 2011, 36, 355–363. [Google Scholar] [CrossRef]
- Garcia-Nunez, J.A.; Pelaez-Samaniego, M.R.; Garcia-Perez, M.E.; Fonts, I.; Abrego, J.; Westerhof, R.J.M.; Garcia-Perez, M. Historical Developments of Pyrolysis Reactors: A Review. Energy Fuels 2017, 31, 5751–5775. [Google Scholar] [CrossRef]
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Demirbas, A. Determination of calorific values of bio-chars and pyro-oils from pyrolysis of beech trunkbarks. J. Anal. Appl. Pyrolysis 2004, 72, 215–219. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of Slow Pyrolysis Biochars: Effects of Feedstocks and Pyrolysis Temperature on Biochar Properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Tian, S.; Zheng, C.; Zhang, Z. Effect of Calcium Hydroxide on the Pyrolysis Behavior of Sewage Sludge: Reaction Characteristics and Kinetics. Energy Fuels 2017, 31, 5079–5087. [Google Scholar] [CrossRef]
- Homagain, K.; Shahi, C.; Luckai, N.; Sharma, M. Biochar-based bioenergy and its environmental impact in Northwestern Ontario Canada: A review. J. For. Res. 2014, 25, 737–748. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Li, J.; Yan, R.; Xiao, B.; Wang, X.; Yang, H. Influence of temperature on the formation of oil from pyrolyzing palm oil wastes in a fixed bed reactor. Energy Fuels 2007, 21, 2398–2407. [Google Scholar] [CrossRef]
- Trinh, T.N.; Jensen, P.A.; Kim, D.J.; Knudsen, N.O.; Sørensen, H.R. Influence of the pyrolysis temperature on sewage sludge product distribution, bio-oil, and char properties. Energy Fuels 2013, 27, 1419–1427. [Google Scholar] [CrossRef]
- Lu, T.; Yuan, H.R.; Zhou, S.G.; Huang, H.Y.; Noriyuki, K.; Chen, Y. On the pyrolysis of sewage sludge: The influence of pyrolysis temperature on biochar, liquid and gas fractions. Adv. Mater. Res. 2012, 518–523, 3412–3420. [Google Scholar] [CrossRef]
- Liu, T.; Guo, Y.; Peng, N.; Lang, Q.; Xia, Y.; Gai, C.; Liu, Z. Nitrogen transformation among char, tar and gas during pyrolysis of sewage sludge and corresponding hydrochar. J. Anal. Appl. Pyrolysis 2017, 126, 298–306. [Google Scholar] [CrossRef]
- Udayanga, W.D.C.; Veksha, A.; Giannis, A.; Lisak, G.; Lim, T.T. Effects of sewage sludge organic and inorganic constituents on the properties of pyrolysis products. Energy Convers. Manag. 2019, 196, 1410–1419. [Google Scholar] [CrossRef]
- Kwon, E.E.; Yi, H.; Castaldi, M.J. Utilizing carbon dioxide as a reaction medium to mitigate production of polycyclic aromatic hydrocarbons from the thermal decomposition of styrene butadiene rubber. Environ. Sci. Technol. 2012, 46, 10752–10757. [Google Scholar] [CrossRef] [PubMed]
- Hornung, A. Intermediate pyrolysis of biomass. In Biomass Combustion Science, Technology and Engineering; Woodhead Publishing: Birmingham, UK, 2013; pp. 172–186. [Google Scholar]
- Guizani, C.; Escudero Sanz, F.J.; Salvador, S. Effects of CO2 on biomass fast pyrolysis: Reaction rate, gas yields and char reactive properties. Fuel 2014, 116, 310–320. [Google Scholar] [CrossRef]
- Piddubniak, O.; Ledakowicz, S.; Nowicki, L. New approach to a problem of heat transfer with chemical reaction in a cylinder of finite dimensions. Int. J. Heat Mass Transf. 2011, 54, 338–344. [Google Scholar] [CrossRef]
- Sharma, A.; Wang, S.; Pareek, V.; Yang, H.; Zhang, D. Multi-fluid reactive modeling of fluidized bed pyrolysis process. Chem. Eng. Sci. 2015, 123, 311–321. [Google Scholar] [CrossRef]
- Shen, J.; Wang, X.S.; Garcia-Perez, M.; Mourant, D.; Rhodes, M.J.; Li, C.Z. Effects of particle size on the fast pyrolysis of oil mallee woody biomass. Fuel 2009, 88, 1810–1817. [Google Scholar] [CrossRef]
- Shuping, Z.; Yulong, W.; Mingde, Y.; Chun, L.; Junmao, T. Pyrolysis characteristics and kinetics of the marine microalgae Dunaliella tertiolecta using thermogravimetric analyzer. Bioresour. Technol. 2010, 101, 359–365. [Google Scholar] [CrossRef]
- Erlich, C.; Björnbom, E.; Bolado, D.; Giner, M.; Fransson, T.H. Pyrolysis and gasification of pellets from sugar cane bagasse and wood. Fuel 2006, 85, 1535–1540. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Dhyani, V.; Kumar, J.; Bhaskar, T. Thermal decomposition kinetics of sorghum straw via thermogravimetric analysis. Bioresour. Technol. 2017, 245, 1122–1129. [Google Scholar] [CrossRef]
- Panchasara, H.; Ashwath, N. Effects of pyrolysis bio-oils on fuel atomisation—A review. Energies 2021, 14, 794. [Google Scholar] [CrossRef]
- Wang, W.; Sun, K.; Ali, M.; Liu, X.; Huang, Q. Copyrolysis behavior of xylan and polyvinyl chloride plastic. Energy Fuels 2019, 33, 8727–8734. [Google Scholar] [CrossRef]
- Rostocki, A.; Unyay, H.; Ławińska, K.; Obraniak, A. Granulates Based on Bio and Industrial Waste and Biochar in a Sustainable Economy. Energies 2022, 16, 56. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Manyà, J.J. Pyrolysis for biochar purposes: A review to establish current knowledge gaps and research needs. Environ. Sci. Technol. 2012, 46, 7939–7954. [Google Scholar] [CrossRef]
- Mullen, C.A.; Boateng, A.A.; Goldberg, N.M.; Lima, I.M.; Laird, D.A.; Hicks, K.B. Bio-oil and bio-char production from corn cobs and stover by fast pyrolysis. Biomass Bioenergy 2010, 34, 67–74. [Google Scholar] [CrossRef]
- Slezak, R.; Krzystek, L.; Ledakowicz, S. Steam gasification of pyrolysis char from spent mushroom substrate. Biomass Bioenergy 2019, 122, 336–342. [Google Scholar] [CrossRef]
- Dong, Q.; Zhang, S.; Wu, B.; Pi, M.; Xiong, Y.; Zhang, H. Co-pyrolysis of Sewage Sludge and Rice Straw: Thermal Behavior and Char Characteristic Evaluations. Energy Fuels 2020, 34, 607–615. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.C.; Ryu, C.; Jeon, J.K.; Shin, M.C.; Park, Y.K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Lu, J.S.; Chang, J.S.; Lee, D.J. Adding carbon-based materials on anaerobic digestion performance: A mini-review. Bioresour. Technol. 2020, 300, 122696. [Google Scholar] [CrossRef]
- Kalogo, Y.; Monteith, H. Energy and Resource Revovery from Sludge; IWA: London, UK, 2012. [Google Scholar]
- Lehto, J.; Oasmaa, A.; Solantausta, Y.; Kytö, M.; Chiaramonti, D. Review of fuel oil quality and combustion of fast pyrolysis bio-oils from lignocellulosic biomass. Appl. Energy 2014, 116, 178–190. [Google Scholar] [CrossRef]
- Xiao, R.; Yang, W. Influence of temperature on organic structure of biomass pyrolysis products. Renew. Energy 2013, 50, 136–141. [Google Scholar] [CrossRef]
- Yu, G.; Feng, Y.; Chen, D.; Yang, M.; Yu, T.; Dai, X. In Situ Reforming of the Volatile by Char during Sewage Sludge Pyrolysis. Energy Fuels 2016, 30, 10396–10403. [Google Scholar] [CrossRef]
- Huang, X.; Cao, J.P.; Shi, P.; Zhao, X.Y.; Feng, X.B.; Zhao, Y.P.; Fan, X.; Wei, X.Y.; Takarada, T. Influences of pyrolysis conditions in the production and chemical composition of the bio-oils from fast pyrolysis of sewage sludge. J. Anal. Appl. Pyrolysis 2014, 110, 353–362. [Google Scholar] [CrossRef]
- David, K.; Ben, H.; Muzzy, J.; Feik, C.; Iisa, K.; Ragauskas, A. Chemical characterization and water content determination of bio-oils obtained from various biomass species using 31P NMR spectroscopy. Biofuels 2012, 3, 123–128. [Google Scholar] [CrossRef]
- Demirbas, A. The influence of temperature on the yields of compounds existing in bio-oils obtained from biomass samples via pyrolysis. Fuel Process. Technol. 2007, 88, 591–597. [Google Scholar] [CrossRef]
- Campuzano, F.; Brown, R.C.; Martínez, J.D. Auger reactors for pyrolysis of biomass and wastes. Renew. Sustain. Energy Rev. 2019, 102, 372–409. [Google Scholar] [CrossRef]
- Czernik, S.; Johnson, D.K.; Black, S. Stability of wood fast pyrolysis oil. Biomass Bioenergy 1994, 7, 187–192. [Google Scholar] [CrossRef]
- Botella, L.; Sierra, M.; Bimbela, F.; Gea, G.; Sánchez, J.L.; Gonzalo, A. Enhancement of Biodiesel Oxidation Stability Using Additives Obtained from Sewage Sludge Fast-Pyrolysis Liquids. Energy Fuels 2016, 30, 302–310. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Zhang, Y.; Li, A. Pressurized pyrolysis of sewage sludge: Process performance and products characterization. J. Anal. Appl. Pyrolysis 2019, 139, 205–212. [Google Scholar] [CrossRef]
- Gao, N.; Quan, C.; Liu, B.; Li, Z.; Wu, C.; Li, A. Continuous Pyrolysis of Sewage Sludge in a Screw-Feeding Reactor: Products Characterization and Ecological Risk Assessment of Heavy Metals. Energy Fuels 2017, 31, 5063–5072. [Google Scholar] [CrossRef]
- Qu, T.; Guo, W.; Shen, L.; Xiao, J.; Zhao, K. Experimental Study of Biomass Pyrolysis Based on Three Major Components: Hemicellulose, Cellulose, and Lignin. Ind. Eng. Chem. Res. 2011, 50, 10424–10433. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Zheng, Y.; Luo, Z.; Cen, K. Mechanism study of wood lignin pyrolysis by using TG-FTIR analysis. J. Anal. Appl. Pyrolysis 2008, 82, 170–177. [Google Scholar] [CrossRef]
- Hu, G.; Huang, H.; Li, Y. Hydrogen-rich gas production from pyrolysis of biomass in an autogenerated steam atmosphere. Energy Fuels 2009, 23, 1748–1753. [Google Scholar] [CrossRef]
- Ren, Q.; Zhao, C.; Chen, X.; Duan, L.; Li, Y.; Ma, C. NOx and N2O precursors (NH3 and HCN) from biomass pyrolysis: Co-pyrolysis of amino acids and cellulose, hemicellulose and lignin. Proc. Combust. Inst. 2011, 33, 1715–1722. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, J.; Xu, J.; Zhao, S. Biomass carbonization industrial process. In Proceedings of the ICMREE2011—International Conference on Materials for Renewable Energy and Environment, Shanghai, China, 20–22 May 2011; Volume 1, pp. 54–59. [Google Scholar]
- Kammen, D.M.; Lew, D.J. Review of Technologies for the Production and Use of Charcoal; Renewable and Appropriate Energy Laboratory Report. 2005, pp. 1–19. Available online: http://rael.berkeley.edu/old_drupal/sites/default/files/old-site-files/2005/Kammen-Lew-Charcoal-2005.pdf (accessed on 1 January 2023).
- Klavina, K.; Klavins, J.; Veidenbergs, I.; Blumberga, D. Charcoal Production in a Continuous Operation Retort. Experimental Data Processing. Energy Procedia 2016, 95, 208–215. [Google Scholar] [CrossRef]
- Brown, J.N.; Brown, R.C. Process optimization of an auger pyrolyzer with heat carrier using response surface methodology. Bioresour. Technol. 2012, 103, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Ledakowicz, S.; Piddubniak, O. The Non-Stationary Heat Transport inside a Shafted Screw Conveyor Filled with Homogeneous Biomass Heated Electrically. Energies 2022, 15, 6164. [Google Scholar] [CrossRef]
- Meier, D.; Van De Beld, B.; Bridgwater, A.V.; Elliott, D.C.; Oasmaa, A.; Preto, F. State-of-the-art of fast pyrolysis in IEA bioenergy member countries. Renew. Sustain. Energy Rev. 2013, 20, 619–641. [Google Scholar] [CrossRef]
- Anex, R.P.; Aden, A.; Kazi, F.K.; Fortman, J.; Swanson, R.M.; Wright, M.M.; Satrio, J.A.; Brown, R.C.; Daugaard, D.E.; Platon, A.; et al. Techno-economic comparison of biomass-to-transportation fuels via pyrolysis, gasification, and biochemical pathways. Fuel 2010, 89, S29–S35. [Google Scholar] [CrossRef]
- Isahak, W.N.R.W.; Hisham, M.W.M.; Yarmo, M.A.; Yun Hin, T.Y. A review on bio-oil production from biomass by using pyrolysis method. Renew. Sustain. Energy Rev. 2012, 16, 5910–5923. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Peacocke, G.V.C. Fast pyrolysis processes for biomass. Renew. Sustain. Energy Rev. 2000, 4, 1–73. [Google Scholar] [CrossRef]
- Venderbosch, R.H.; Prins, W. Fast pyrolysis technology development. Biofuels Bioprod. Biorefining 2010, 4, 178–208. [Google Scholar] [CrossRef]
- Diebold, J.; Scahill, J. Production of Primary Pyrolysis Oils in a Vortex Reactor. Pyrolysis Oils Biomass 1988, 4, 31–40. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Meier, D.; Radlein, D. An overview of fast pyrolysis of biomass. Org. Geochem. 1999, 30, 1479–1493. [Google Scholar] [CrossRef]
- Wagenaar, B.M.; Venderbosch, R.H.; Carrasco, J.; Strenziok, R.; van der Aa, B.J. Rotating cone bio-oil production and applications. In Progress in Thermochem Biomass Convers; Bridgwater, A.V., Ed.; Blackwell Science: Oxford, UK, 2001; pp. 1268–1280. [Google Scholar]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Oladejo, J.; Shi, K.; Luo, X.; Yang, G.; Wu, T. A review of sludge-to-energy recovery methods. Energies 2019, 12, 60. [Google Scholar] [CrossRef]
- Study of Gasification/Pyrolysis of MSW Residuals. Available online: https://tnrd.civicweb.net/document/4805/ (accessed on 1 January 2023).
- Malkow, T. Novel and innovative pyrolysis and gasification technologies for energy efficient and environmentally sound MSW disposal. Waste Manag. 2004, 24, 53–79. [Google Scholar] [CrossRef]
- Thermoselect—An Advanced Field Proven High Temperature Recycling Process. Available online: http://www.viveracorp.com/index_htm_files/R02_manuscriptThermoselect.pdf (accessed on 1 January 2023).
- Brems, A.; Dewil, R.; Baeyens, J.; Zhang, R. Gasification of Plastic Waste as Waste-to-Energy or Waste-to-Syngas Recovery Route. Solid Waste A Renew. Resour. Methodol. 2015, 5, 241–264. [Google Scholar] [CrossRef]
- Zeng, R.; Wang, S.; Cai, J.; Kuang, C. A Review of Pyrolysis Gasification of MSW. Adv. Eng. Res. 2018, 163, 166–171. [Google Scholar] [CrossRef]
- Ledakowicz, S.; Stolarek, P.; Malinowski, A.; Lepez, O. Thermochemical treatment of sewage sludge by integration of drying and pyrolysis/autogasification. Renew. Sustain. Energy Rev. 2019, 104, 319–327. [Google Scholar] [CrossRef]
- Pyrolysis Process for Biomass and Waste. Available online: https://etia-group.com/operations-for-thermal-processing/pyrolysis/ (accessed on 1 January 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).