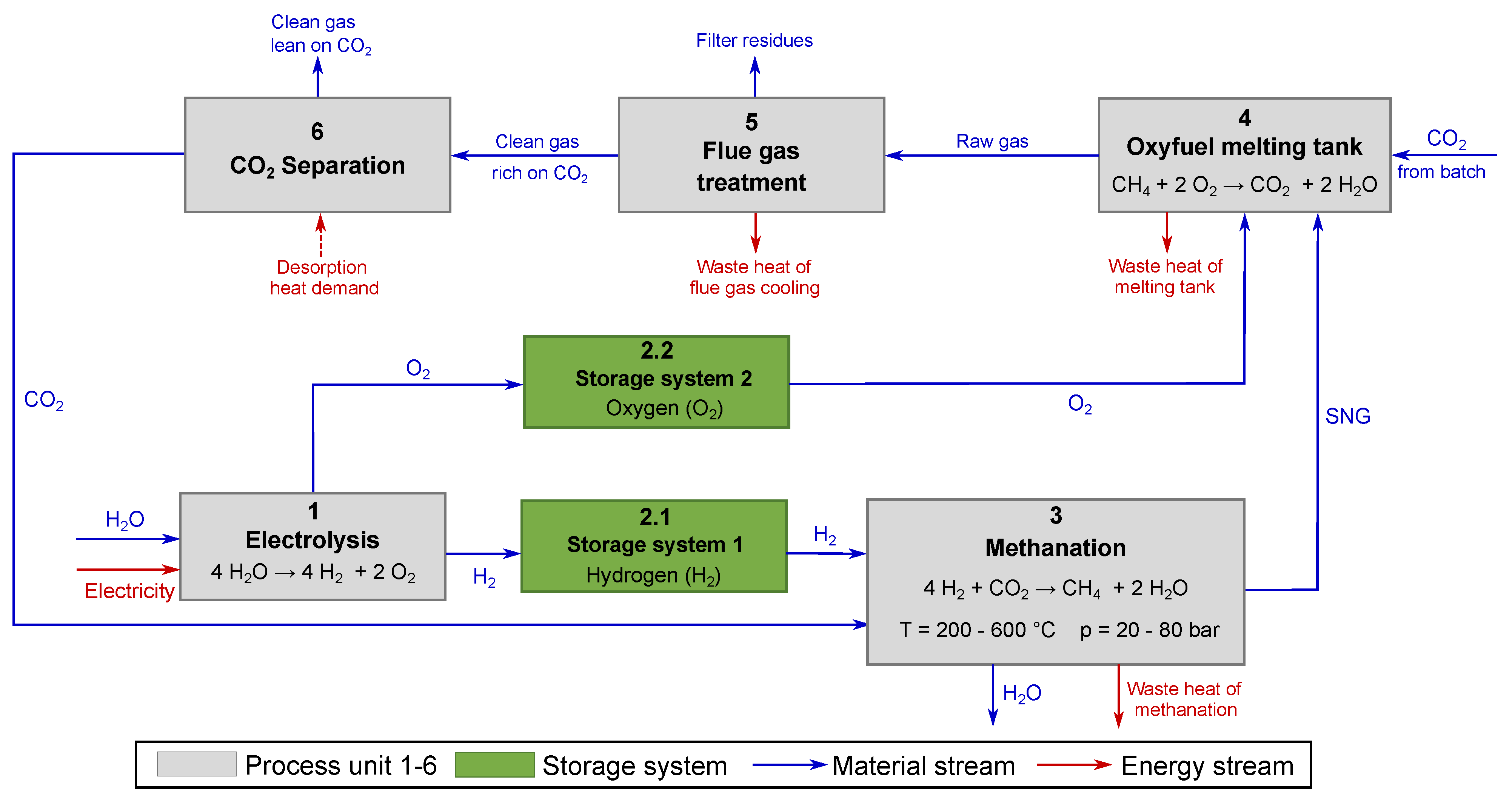
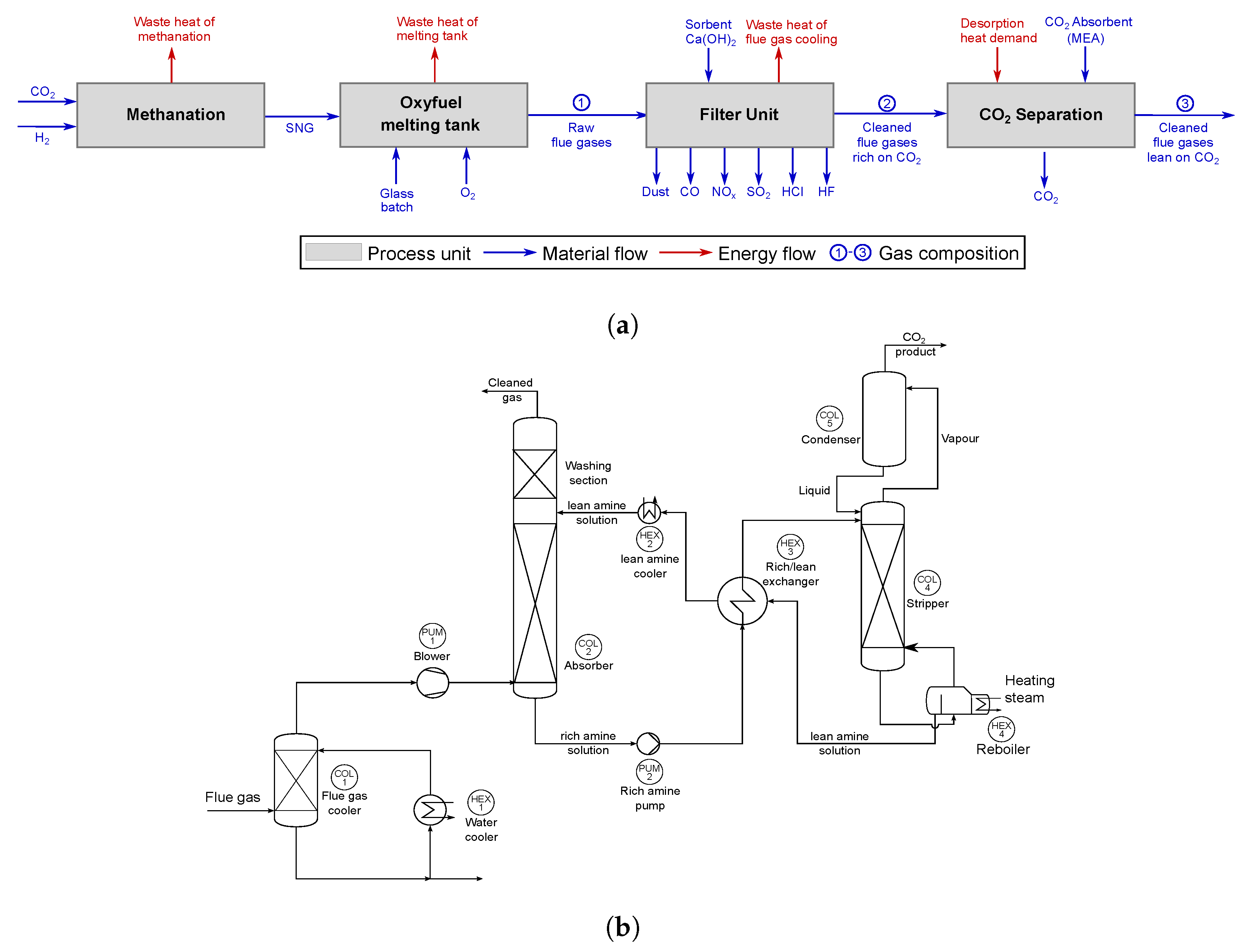
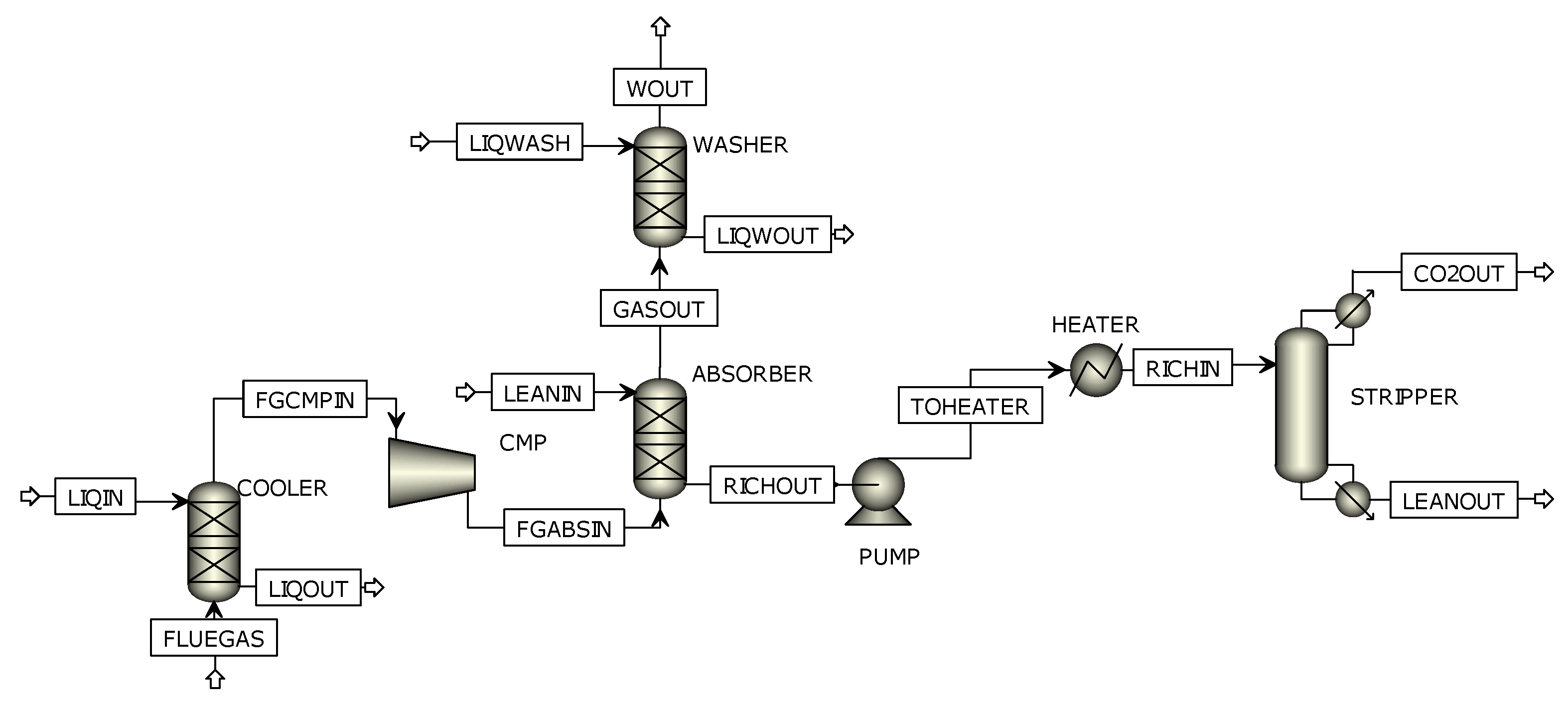
5.2.1. Equipment Cost
Table 7 gives an overview of the equipment cost for both the modeled CO
absorption plants. Details concerning the reference plant are given in [
49]. The plant for this work was designed for the same CO
capture rate of 90% and stripper operating conditions of 2 bar and 130 °C.
The absorber column is the most cost-intensive plant component, both in the original CO
2 separation plant and in the newly designed plant for the glass industry. The costs for the absorber are approximately EUR 340,000. The second most important contribution to equipment costs is related to the stripper column, at approximately EUR 110,000. Accordingly, another 20% of the total costs are contributed by the stripper. To date, 85% of the total equipment costs can be attributed to the most important columns in the process. Other important and cost-relevant items are the cold water pump, blower and DC water cooler with a combined investment cost of approximately EUR 51,000. The previously described parts cover 96% of the total component costs. The remaining plant components such as rich/lean heat exchanger, solvent pumps, reboiler, or the chiller for the exhaust gas cooler cooling supply can be attributed a total cost of EUR 20, 000, based on the given percentage share of [
49]. Since these components account for only 4% of the total cost, this summarized cost estimate is sufficient for these components. This cost allocation pattern is consistent with the data in [
49] and can thus be considered valid.
The total costs for CO
compression after separation, calculated as described in
Section 4.2, were approximately EUR 153,000. Alternatively, using the second approach described in
Section 4.3 would yield investment costs of EUR 69,000, but only for a single-stage compression process. A single-stage compressor, however, would have to achieve a compression ratio of 1:10. Such a high compression ratio is unfavorable for thermodynamic reasons in single-stage design and would lead to high heat generation, high compressor operating power, and thus high energy demand. Thus, the single-stage compressor is extended to a two-stage compression process, yielding a compression ratio of 1:5 in the first compressor stage. Therefore, the costs calculation of method 2 would double, yielding costs of EUR 138,000. Finally, the costs were validated against vendor information, giving estimated costs of EUR 120,000 for a two-stage reciprocating compressor [
52]. For further calculations, the cost estimated by the vendor was used, as this can be considered the most practical approach.
5.2.2. Capital Expenditures
In addition to the equipment cost, additional investments for installation, control engineering, pipes, and electrical installation have to be considered. These cost are calculated according to the methodology shown in
Section 4.3. The cost elements can be divided into direct costs for materials and further equipment, and indirect costs for services such as engineering and construction expenses of the plant. The direct cost elements can be further structured according to inside battery limit (ISBL) cost, and outside battery limit (OSBL) cost. ISBL includes material costs, which are directly associated to the plant, while ISBL are secondary expenses for building, yard improvement, or service facilities.
Ranges for the assumed percentages of ISBL costs are given in [
53].
Table 8 shows the parameters taken into account and their influence on the total CAPEX. Due to the comparably small size of the CO
capture plant, the lower limits of the ranges proposed in [
53] were selected. Thus, the additional costs for construction and installation, instrumentation, and control, as well as piping and electrical equipment are approximately EUR 354,000, or approximately 68% of the component costs. The total material and equipment cost of the CO
separation plant for the glass industry are approximately EUR 875,000.
The OSBL costs for buildings, yard improvements, and service facilities were assumed to be lower than recommended by [
53]. Due to the low column volumes and the resulting compactness of the plant, a container solution for plant housing is attractive. Considering this option, OSBL costs of over EUR 100,000 building infrastructure seems justified, or even on the safe side. The excavation and concrete construction work will be limited with this simplified type of building design. Service facilities such as changing rooms and showers for personnel or workshops can be provided by the existing infrastructure at a glass manufacturer site. The total direct costs thus amount to approximately EUR 980,000.
For indirect cost, ranges suggested by [
53,
58] are used. This results in total indirect costs of approximately EUR 107,000. For the calculation of the total plant investment costs (FCI), the investment costs of the CO
compressor must also be considered. In total, the FCI add to approximately EUR 1.15 million.
In addition to the FCI, costs for labor arising in the company, start-up, and initial material costs must be taken into account. The costs for start-up and initial material costs amount to approximately EUR 92.000. The material cost is mainly for the initial loading of the solvent circuit. This consists in a 30 wt. % solution of monoethanolamine (MEA) in water.
The component costs were determined by cost comparison with a CO
separation plant for coal-fired power plants, which was designed as early as 2007. Since then, due to inflation, as well as global geopolitical events such as the COVID-19 pandemic, non-negligible price increases have occurred in all areas of public life, which also affected chemical engineering. In order to take these developments into account, a price index correction, based on [
56] was applied. This price index correction since 2007 has a significant impact of 21% on total CAPEX. The extent to which prices developed according to current influences such as recent conflicts, should be closely examined. These effects have not yet been taken into account in the current values of [
56] and may require reassessment for future works.
The CAPEX of plants for CO
capture from flue gases of power plants are far above the values calculated in this study. Abu-Zahra and Singh proposed EUR 147 million and EUR 179 million, respectively [
49,
59]. With respect to CAPEX, there is a factor of 90 between both plants, and with respect to the absorber column dimensions, there is even a factor of 300. This is mainly due to the significantly lower flue gas mass flow of the investigated glass melting tank. In the investigated case study, 0.569 kg/s are present at the glass industrial plant, while 616.0 kg/s are present at the reference plant of the coal-fired power plant. To allow a more specific comparison, the required absorber column volume per ton of CO
2 captured is calculated. The glass industry plant needs
m
3 absorber/t CO
captured, while the reference plant needs only
m
3 absorber/t CO
captured. Accordingly, for the specific amount of flue gas occurring in the glass industry, a larger absorber column is necessary. This is most likely due to the high levels of ambient air flowing into the investigated flue gas filter unit. The ambient air increases the exhaust gas volume while reducing the CO
2 partial pressure in the cleaned flue gases rich on CO
2.
Nevertheless, the large scaling factor between the reference plant and the designed plant is outside the limit proposed by [
53] for the applicability of the cost comparison method. However, the determined cost frameworks appear plausible by comparing specific CAPEX to other literature sources. Ref. [
35] investigated the CO
capture for carbon-intensive industrial processes and reported a specific CAPEX of 160 EUR/t CO
and year for amine-based post-combustion CO
capture in cement industry processes. The resulting specific CAPEX of this work is 188 EUR/t CO
per year. Considering the price increases since 2012 (approximately 16% according to [
56], as can also be seen in
Figure A1), the deviation of 15% calculated in this work seems justified.
It should be noted that the applied boundary conditions and assumptions of this work may have a significant impact on the implementation costs of a CO capture plant in glass melting processes. However, the figures proposed herein should provide a sufficiently detailed basis for an initial cost estimation that could serve as a valuable basis for further investigations.
5.2.3. Operational Expenditures
The operating expenses (OPEX) for the CO
capture plant can be further divided into fixed charge, direct production costs, plant overhead costs, and general expenses.
Table 9 shows an overview of the calculated OPEX. According to the methodology described in [
53,
58], ranges or fixed percentages for the respective cost categories are given.
The fixed costs of the CO
capture plant include local taxes, fees, and charges, as well as insurance rates. Lower percentages of the range suggested in references [
53,
58] were specified for this purpose, as there are currently no specific tax rates or fees for such plants in Germany. The insurance coverage will primarily include fire insurance, the costs of which are based on the investment volume, fire risk, and the business activity. Based on the investment costs of EUR 1.63 million determined in
Section 5.2.2, the low fire risk of the plant (essentially short circuit and cable fire, hardly flammable materials) and the low fire-prone glass industry, the resulting fixed costs of approximately 18,000 EUR/year (1.1% of CAPEX) appear sufficient.
The direct production cost included the material requirements for plant operation, such as the cooling water, MEA makeup caused by degradation, and maintenance materials. The cooling water demand is based on simulation results. The MEA degradation was calculated based on the data given in
Table 3 and
Table 5. The cost of the MEA makeup is approximately 14,000 EUR/year or 18% of the total OPEX. Other sources have reported an OPEX cost share of up to 32% [
49,
59]. Due to the size of the plant, the reduced cost share for the CO
separation plant in the glass industry seems plausible. However, the high O
content in the flue gas (see
Table 2) may cause increased MEA degradation due to oxidation. For this reason, switching to more stable absorbents such as secondary or tertiary amines appears promising. These exhibit increased thermal and chemical stability against oxidative degeneration, as well as increased energy efficiency (as can be seen in
Section 5.5). Besides these beneficial effects on operating parameters, the reduced energy demand and increased stability of advanced amines may result in a significant cost reduction.
The operating labor cost for the maintenance, operation, and monitoring of the plant, amounting to approximately EUR 39,000 per year, is the largest cost component (approximately 27%) in the annual OPEX. For this work, we assumed a 0.1 job per shift for the operation of the CO
separation plant. In the glass industry, a three-shift operation of 8 h each for 365 days per year is common to maintain labor supply. The assumption that 10% of the working time of a maintenance employee per shift is spent on the maintenance and servicing of the CO
separation plant thus corresponds to a total time expenditure of approximately 2.5 h/day or 912.5 h/year. Other studies such as [
49,
59] assumed a two job per shift staff expenditure for plant operation. However, as the separation plant discussed in this work is much smaller, such a reduction in OL expenses seems justified. In addition, OL costs influence supervision and support labor costs, laboratory charges, and plant overhead costs. Taking all of this influence into account, OL costs contribute to approximately 60% of total OPEX.
Additionally, general expenses for distribution and marketing, as well as ongoing research and development, add up to approximately EUR 11,000 per year. As a result, the annual operating expenses (OPEX) amount to approximately EUR 148,000 in total. This represents approximately 9% of the total CAPEX. [
35] assumed an OPEX range of 7–12% of total CAPEX for a post-combustion MEA separation plant in the cement industry. Therefore, the OPEX calculated in this work can be considered consistent with other literature sources and even indicate potential for further optimization.