Examination of the Structure and Definition of the Mechanism of Formation of Products by Pyrolysis of Tarim Crude Oil
Abstract
1. Introduction
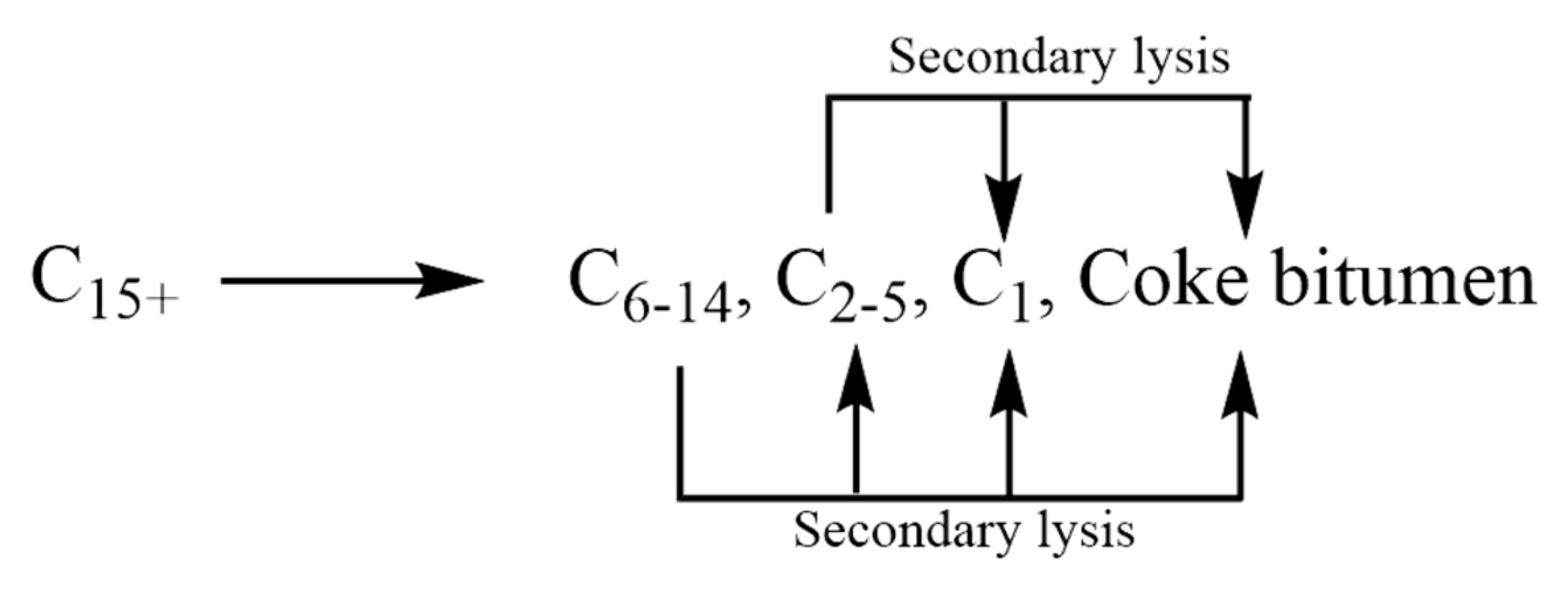
1.1. Pyrolysis Mechanism of Crude Oil
- (1)
- Depolymerization reaction: chain break in recombinant branches is the mainstay, mainly the break of C-O and C-S bonds, mainly generating soluble unsaturated organic matter;
- (2)
- C-C bond breaking reaction: C6+ saturated hydrocarbons crack to generate small-molecule saturated hydrocarbons and C14+ aromatic hydrocarbons;
- (3)
- Demethylation reaction: C14+ aromatic hydrocarbon demethylation to generate gaseous hydrocarbons and coke;
- (4)
- C-C bond breaking reaction: C-C bond breaking in C3-5 produces CH4 and C2.
1.2. Crude Oil Analysis Technology
2. Experimental
2.1. Material
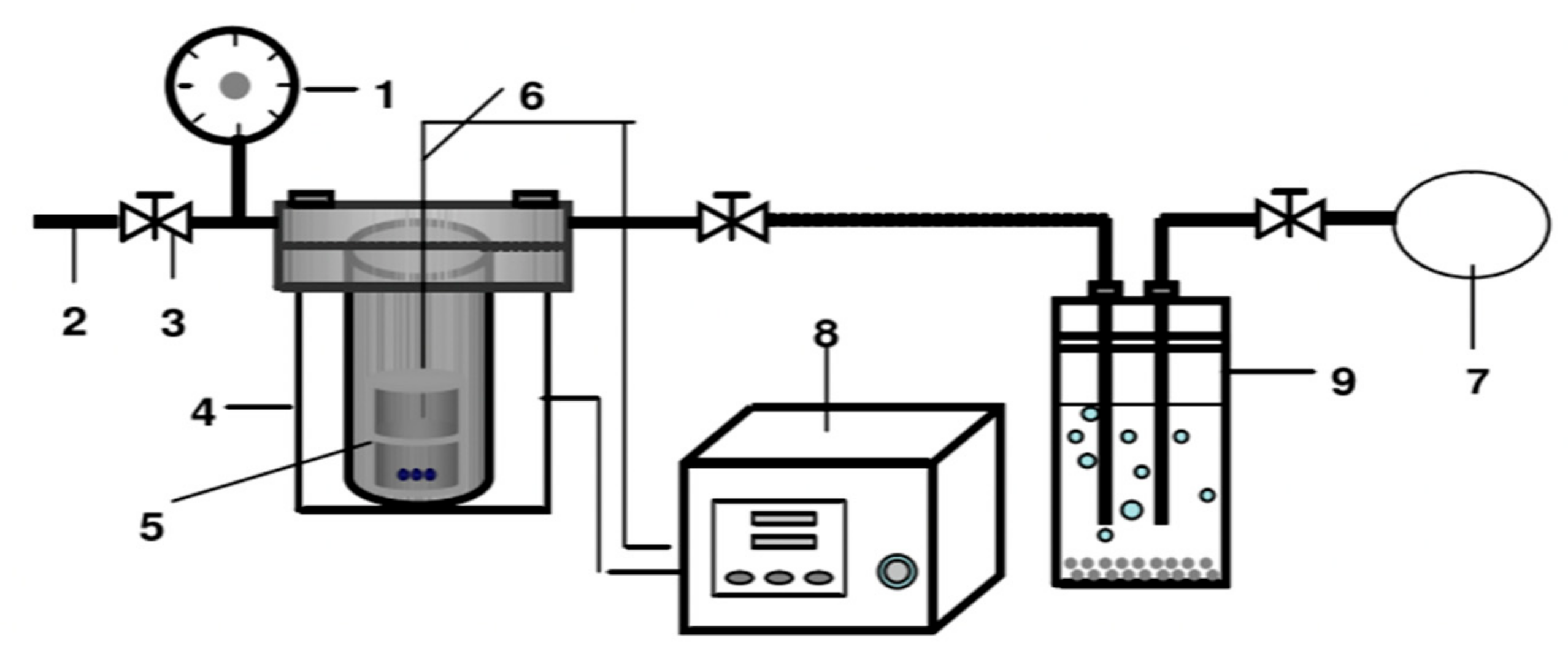
2.2. Pyrolysis Experimental Apparatus and Steps
2.3. Characterization of Feedstock and Gaseous Products
3. Results and Discussion
3.1. FT-IR Analysis of Tarim Crude Oil
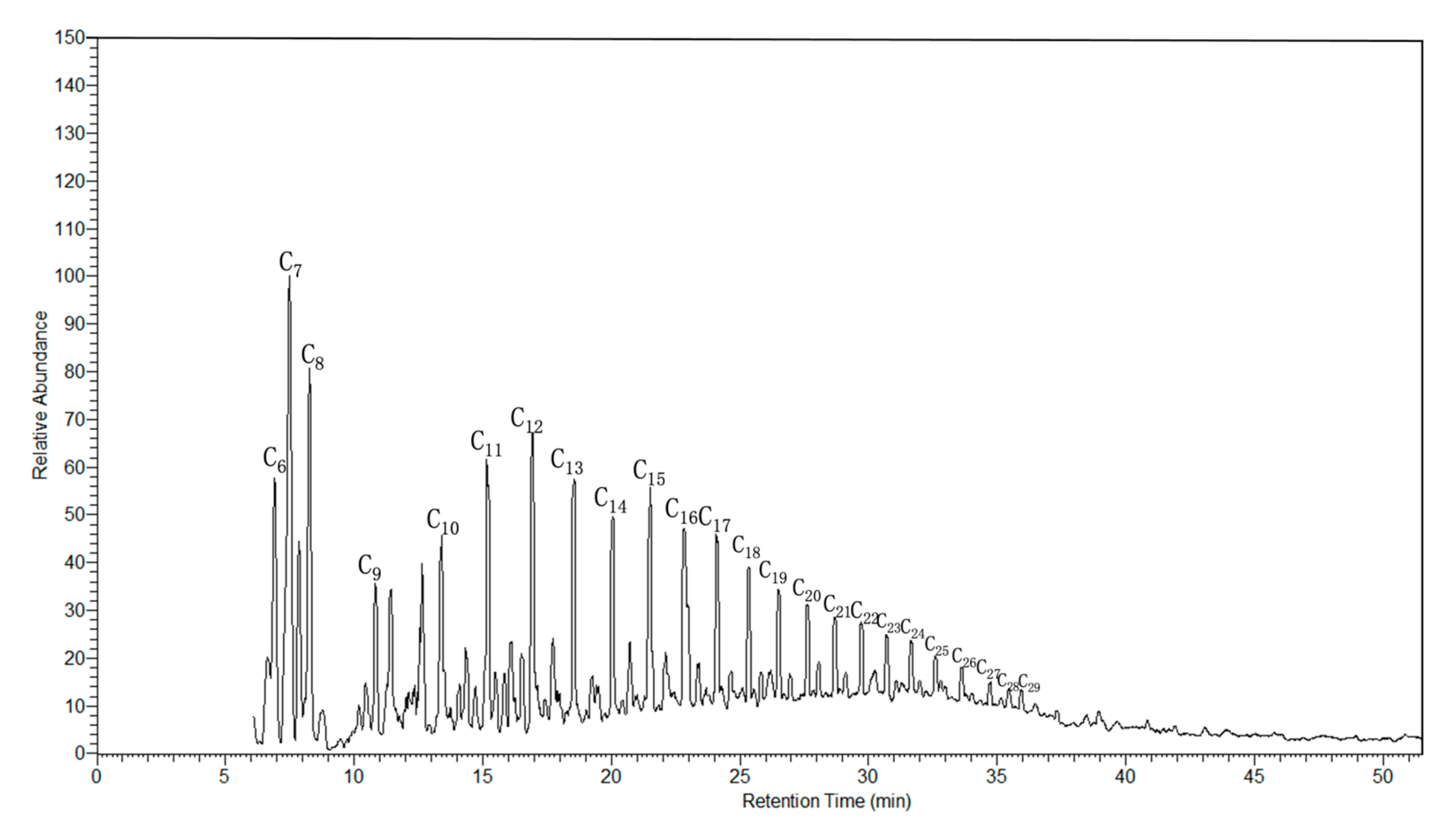
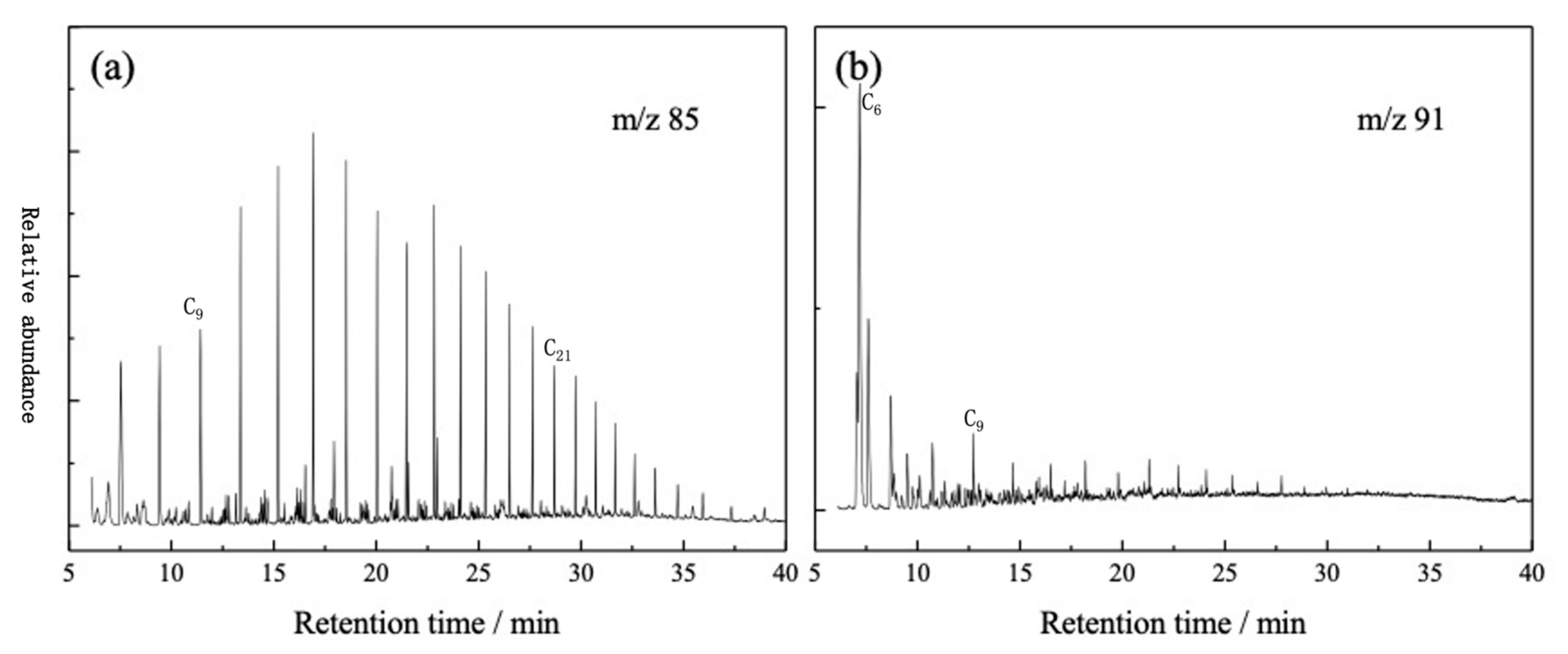
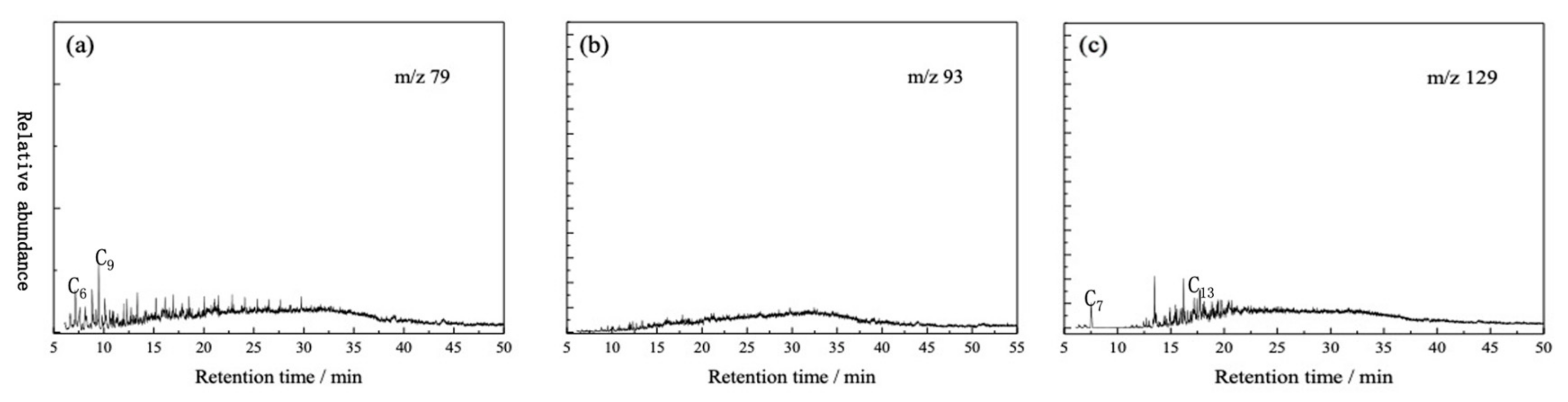
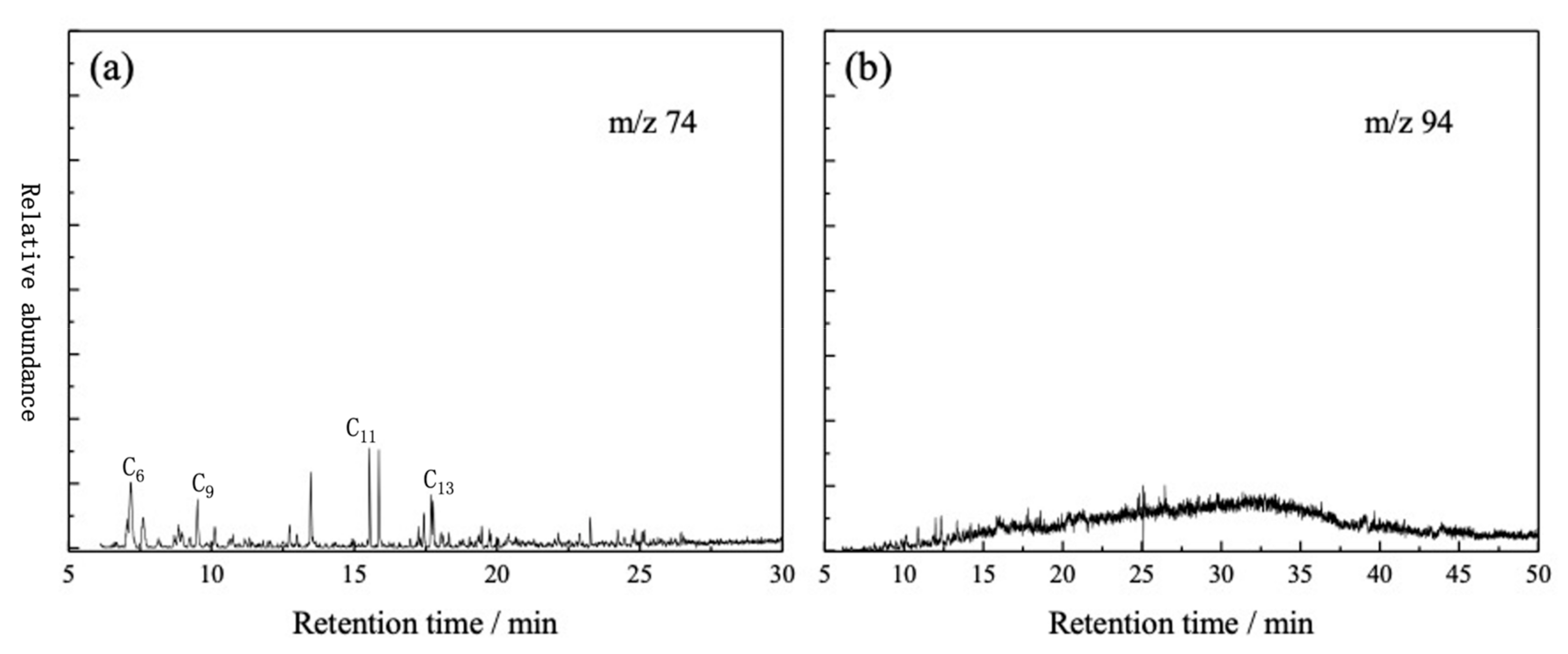
3.2. GC-MS Analysis of Oil Fraction of Tarim Crude
3.3. Composition Analysis of Gaseous Products
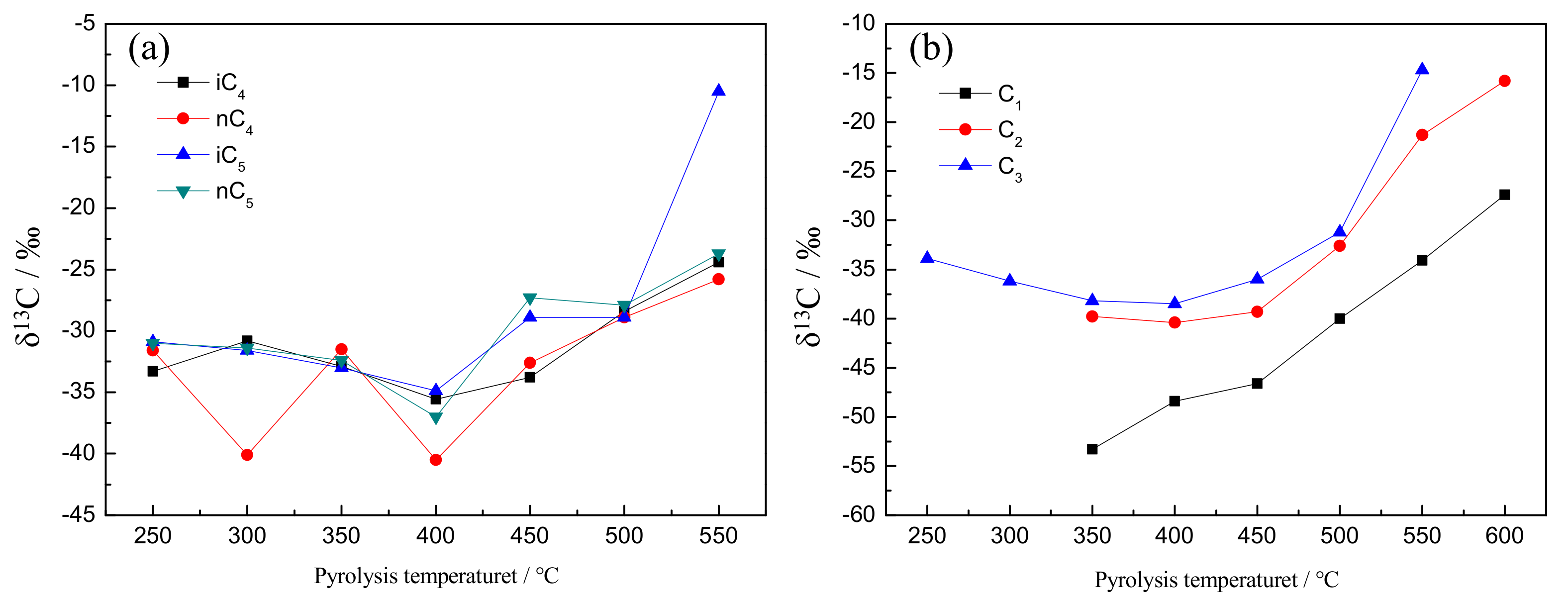
3.4. Carbon Isotope Composition of Gaseous Products
3.5. Formation Mechanism of Gaseous Hydrocarbons
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.; Hu, W.; Sun, Y.; Li, Z.; Zeng, F. Characteristics of gas generation from the pyrolysis of crude oil under the different geological conditions. Pet. Sci. Technol. 2018, 36, 2064–2069. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, Z.; Meng, Q. Genetic types of natural gas and filling patterns in Daniudi gas field, Ordos Basin, China. J. Asian Earth Sci. 2015, 107, 1–11. [Google Scholar] [CrossRef]
- Li, J.; Hou, D.J.; Li, J.H. Characteristics and genetic types of natural gas in the eastern sag of the Liaohe Depression. Nat. Gas Ind. 2011, 31, 43–47. [Google Scholar]
- Liu, Q.; Wu, X.; Wang, X. Carbon and hydrogen isotopes of methane, ethane, and propane: A review of genetic identification of natural gas. Earth Sci. Rev. 2019, 190, 247–272. [Google Scholar] [CrossRef]
- Horsfield, B.; Schenk, H.; Mills, N.; Welte, D. An investigation of the in-reservoir conversion of oil to gas: Compositional and kinetic findings from closed-system programmed-temperature pyrolysis. Org. Geochem. 1992, 19, 191–204. [Google Scholar] [CrossRef]
- Zhao, M.J.; Lu, S.F. Natural gas from secondary cracking of crude oil—An important pattern of gas generation. Geol. Rev. 2000, 46, 645–650. [Google Scholar]
- Zhao, W.; Wang, Z.; Zhang, S.; Wang, H.; Wang, Y. Oil cracking: An important way for highly efficient generation of gas from marine source rock kitchen. Chin. Sci. Bull. 2005, 50, 2628–2635. [Google Scholar] [CrossRef]
- Zhu, D. Application and innovation analysis of oil exploration technology. Chem. Ind. Manag. 2017, 8, 238. [Google Scholar]
- Li, M.; Zhang, M. Development status and progress of crude oil exploitation technology and equipment. China Pet. Chem. Stand. Qual. 2021, 17, 106–110. [Google Scholar]
- Fetisov, V.; Mohammadi, A.H.; Pshenin, V.; Kupavykh, K.; Artyukh, D. Improving the economic efficiency of vapor recovery units at hydrocarbon loading terminals. Oil Gas Sci. Technol. Rev. D’ifp. Energ. Nouv. 2021, 76, 38. [Google Scholar] [CrossRef]
- Ilyushin, Y.V. Development of a Process Control System for the Production of High-Paraffin Oil. Energies 2022, 15, 6462. [Google Scholar] [CrossRef]
- Mahlstedt, N.; Horsfield, B.; Dieckmann, V. Second order reactions as a prelude to gas generation at high maturity. Org. Geochem. 2008, 39, 1125–1129. [Google Scholar] [CrossRef]
- Jackśon, K.J.; Burnham, A.K.; Braun, R.L.; Knauss, K.G. Temperature and pressure dependence of n-hexadecane cracking. Org. Geochem. 1995, 23, 941–953. [Google Scholar] [CrossRef]
- Burnham, A.K.; Gregg, H.R.; Ward, R.L.; Knauss, K.G.; Copenhaver, S.A.; Reynolds, J.G.; Sanborn, R. Decomposition kinetics and mechanism of n-hexadecane-1, 2-13C2 and dodece-1-ene-1, 2-13C2 doped in petroleum and n-hexadecane. Geochim. Et Cosmochem. Acta 1997, 61, 3725–3737. [Google Scholar] [CrossRef]
- Domine, A.F.; Dessort, D.; Brevart, O. Towards a new method of geochemical kinetic modeling: Implications for the stability of crude oil. Org. Geochem. 1998, 28, 597–612. [Google Scholar] [CrossRef]
- Liao, Y.; Zheng, Y.; Pan, Y.; Sun, Y.; Geng, A. A method to quantify C1–C5 hydrocarbon gases by kerogen primary cracking using pyrolysis gas chromatography. Org. Geochem. 2015, 79, 49–55. [Google Scholar] [CrossRef]
- Uguna, C.N.; Carr, A.D.; Snape, C.E.; Meredith, W. Retardation of oil cracking to gas and pressure induced combination reactions to account for viscous oil in deep petroleum basins: Evidence from oil and n-hexadecane pyrolysis at water pressures up to 900 bar. Org. Geochem. 2016, 97, 61–73. [Google Scholar] [CrossRef]
- Behar, F.S.; Kressmann, S.; Rudkiewicz, J.L.; Vandenbroucke, M. Experimental simulation in a confined system and kinetic modeling of kerogen and oil cracked. Org. Geochem. 1992, 19, 173–189. [Google Scholar] [CrossRef]
- Hill, R.J.; Tang, Y.; Kaplan, I.R. Insights into oil cracking based on laboratory experiments. Org. Geochem. 2003, 34, 1651–1672. [Google Scholar] [CrossRef]
- Brenden, J.; Riley, C.L.; Stephen, F.; Val, S. Pyrolysis-GC-MS analysis of crude and heavy fuel oil asphaltenes for application in oil fingerprinting. Environ. Forensics 2018, 19, 14–26. [Google Scholar]
- Riley, B.J.; Lennard, C.; Fuller, S.; Spikmans, V. An FTIR method for the analysis of crude and heavy fuel oil asphaltenes to assist in oil fingerprinting. Forensic Sci. Int. 2016, 266, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Oudot, J.; Chaillan, F. Pyrolysis of asphaltenes and biomarkers for the fingerprinting of the Amoco-Cadizoil spill after 23 years. Comptes Rendus Chim. 2010, 13, 548–552. [Google Scholar] [CrossRef]
- Wang, G.; Cheng, B.; Wang, T.-G.; Simoneit, B.R.; Shi, S.; Wang, P. Monoterpanes as molecular indicators to diagnose depositional environments for source rocks of crude oils and condensates. Org. Geochem. 2014, 72, 59–68. [Google Scholar] [CrossRef]
- Mair, J.B.; Jack, M.T. Composition of the dinuclear aromatics, C12 to C14, in the light gas oil fraction of petroleum. Anal. Chem. 1964, 36, 351–362. [Google Scholar] [CrossRef]
- Von Mühlen, C.; de Oliveira, E.C.; Zini, C.A.; Caramão, E.B.; Marriott, P.J. Characterization of nitrogen-containing compounds in heavy gas oil petroleum fractions using comprehensive two-dimensional gaschromatography coupled to time-of-flight mass spectrometry. Energy Fuels 2010, 24, 3572–3580. [Google Scholar] [CrossRef]
- Flego, C.; Zannoni, C. N-containing species in crude oil fractions: An identification and quantification method by comprehensive two-dimensional gas chromatography coupled with quadrupole mass spectrometry. Fuel 2011, 90, 2863–2869. [Google Scholar] [CrossRef]
- Li, M.; Wang, T.-G.; Simoneit, B.R.; Shi, S.; Zhang, L.; Yang, F. Qualitative and quantitative analysis of dibenzothiophene, its methylated homologues, and benzonaphthothiophenes in crude oils, coal, and sediment extracts. J. Chromatogr. A 2012, 1233, 126–136. [Google Scholar] [CrossRef]
- Ioppolo-Armanios, M.; Alexander, R.; Kagi, R.I. Identification and analysis of C0–C3 phenols in some Australian crude oils. Org. Geichem. 1992, 18, 603–609. [Google Scholar] [CrossRef]
- Ioppolo-Armanios, M.; Alexander, R.; Kagi, R.I. Identification and origins of isopropylmethylphenols in crude oils. Org. Geochem. 1994, 22, 815–823. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Pan, S. Study on the structure, pyrolysis kinetics, gas release, reaction mechanism, and pathways of Fushun oil shale and kerogen in China. Fuel Process. Technol. 2022, 225, 107058. [Google Scholar]
- Iskenderova, Z.I.; Kurbanov, M.A. Changes in performance characteristics of transformer oil by the action of ionizing radiation. High Energy Chem. 2019, 53, 454–458. [Google Scholar] [CrossRef]
- Kaanagbara, L.; Inyang, H.I.; Wu, J. Aromatic and aliphatic hydrocarbon balance in electric transformer oils. Fuel 2010, 89, 3114–3118. [Google Scholar] [CrossRef]
- Wang, T.; Geng, A.; Li, X. Pyrolysis of one crude oil and its asphaltenes: Evolution of gaseous hydrocarbons and carbon isotope. J. Pet. Sci. Eng. 2010, 71, 8–12. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Zhu, G.; Chi, L.; Han, J. Impacts of Thermochemical Sulfate Reduction, Oil Cracking, and Gas Mixing on the Petroleum Fluid Phase in the Tazhong Area, Tarim Basin, China. Energy Fuels 2019, 33, 968–978. [Google Scholar] [CrossRef]
- Tian, H.; Xiao, X.; Yang, L.G.; Xiao, Z.Y.; Guo, L.G.; Shen, J.G.; Lu, Y.H. Pyrolysis of oil at high temperatures: Gas potentials, chemical and carbon isotopic signatures. Chin. Sci. Bull. 2009, 54, 1217–1224. [Google Scholar] [CrossRef]
- Gilbert, A.; Yamada, K.; Suda, K.; Ueno, Y.; Yoshida, N. Measurement of position-specific 13C isotopic composition of propane at the nanomole level. Geochim. Cosmochim. Acta 2016, 177, 205–216. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Y.-M.; Liu, Y. Gas evolution and isotopic fractionations during pyrolysis on coals of different ranks. Int. J. Coal Geol. 2018, 188, 136–144. [Google Scholar] [CrossRef]
- Berner, U.; Faber, E.; Stahl, W. Mathematical simulation of the carbon isotopic fractionation between huminitic coals and related methane. Chem. Geol. Isot. Geosci. 1992, 94, 315–319. [Google Scholar] [CrossRef]
- Lorant, F.; Prinzhofer, A.; Behar, F.; Huc, A.-Y. Carbon isotopic and molecular constraints on the formation and the expulsion of thermogenic hydrocarbon gases. Chem. Geol. 1998, 147, 249–264. [Google Scholar] [CrossRef]
- Berner, U.; Faber, E. Empirical carbon isotope/maturity relationships for gases from algal kerogens and terrigenous organic matter based on dry open-system pyrolysis. Org. Geochem. 1996, 24, 947–955. [Google Scholar] [CrossRef]
- Wen, P.; Cui, M.; Li, C.; Hao, H.; Deng, W. Element Distribution and Infrared Spectrum Analysis in Tarim Heavy Oils and Their Sub-Fractions. J. Petrochem. Univ. 2013, 26, 16–21. [Google Scholar]



| Basic Properties of Crude Oil | Crude Oil in Tarim, Xinjiang |
|---|---|
| Saturate, % | 65.54 |
| Aromatics, % | 18.31 |
| Resin, % | 11.33 |
| Asphaltene, % | 4.82 |
| Density (25 °C), g/cm3 | 0.8821 |
| Wax, % | 11.29 |
| Viscosity (25 °C), g/cm3 | 74.40 |
| δ13C, % | −33.00 |
| Wave Number/cm−1 | Absorption Peak Attribution | Functional Groups |
|---|---|---|
| 2975–2800 | Absorption peak of -CH3 | Aliphatic and side chain |
| 1706–1604 | Double bond telescopic vibration peak of C=O and C=C | Unsaturated hydrocarbon |
| 1460–1370 | Methylene variable angle vibration Methyl asymmetric variable angle | Aliphatic compound structure |
| 868 | Benzene toroidal flexural vibration | Monocyclic benzene |
| 783–746 | Amino characteristic peak | Amidogen |
| Material Composition | Content/% | |
|---|---|---|
| Aliphatic compound | N-alkane | 68.02 |
| Branched alkanes | 3.17 | |
| Alpha olefin | 8.32 | |
| Non-alpha olefins | 1.31 | |
| Naphthenic | 2.87 | |
| Aromatic compound | Mononuclear aromatics, PAHs | 14.08 |
| Heteroatom compounds | Oxy-compound | 1.31 |
| Sulfur compounds | 0.64 | |
| Nitrogen compounds | 0.28 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Shao, F.; Wang, J.; Yang, H.; Yue, C. Examination of the Structure and Definition of the Mechanism of Formation of Products by Pyrolysis of Tarim Crude Oil. Energies 2023, 16, 2073. https://doi.org/10.3390/en16042073
Ma Y, Shao F, Wang J, Yang H, Yue C. Examination of the Structure and Definition of the Mechanism of Formation of Products by Pyrolysis of Tarim Crude Oil. Energies. 2023; 16(4):2073. https://doi.org/10.3390/en16042073
Chicago/Turabian StyleMa, Yue, Fan Shao, Jing Wang, Han Yang, and Changtao Yue. 2023. "Examination of the Structure and Definition of the Mechanism of Formation of Products by Pyrolysis of Tarim Crude Oil" Energies 16, no. 4: 2073. https://doi.org/10.3390/en16042073
APA StyleMa, Y., Shao, F., Wang, J., Yang, H., & Yue, C. (2023). Examination of the Structure and Definition of the Mechanism of Formation of Products by Pyrolysis of Tarim Crude Oil. Energies, 16(4), 2073. https://doi.org/10.3390/en16042073






