Influence of Copper Foam on the Thermal Characteristics of Phase Change Materials
Abstract
1. Introduction
2. Experimental System
2.1. Experimental Set-Up
2.2. Experimental Error Analysis
3. Models and Research Methods
3.1. The Copper Foam Model
3.2. The Composite PCM Model
- (1)
- The thermal properties of the paraffin wax and the metal foams do not change with the temperature. They are set as constant values.
- (2)
- The Paraffin density is defined based on the Boussinesq hypothesis, of which the volume expansion during the melting process is ignored.
- (3)
- The liquid paraffin wax is incompressible Newtonian fluid and the flow is laminar flow in the study.
3.3. Rayleigh Number
3.4. Error Analysis
4. Results and Analysis
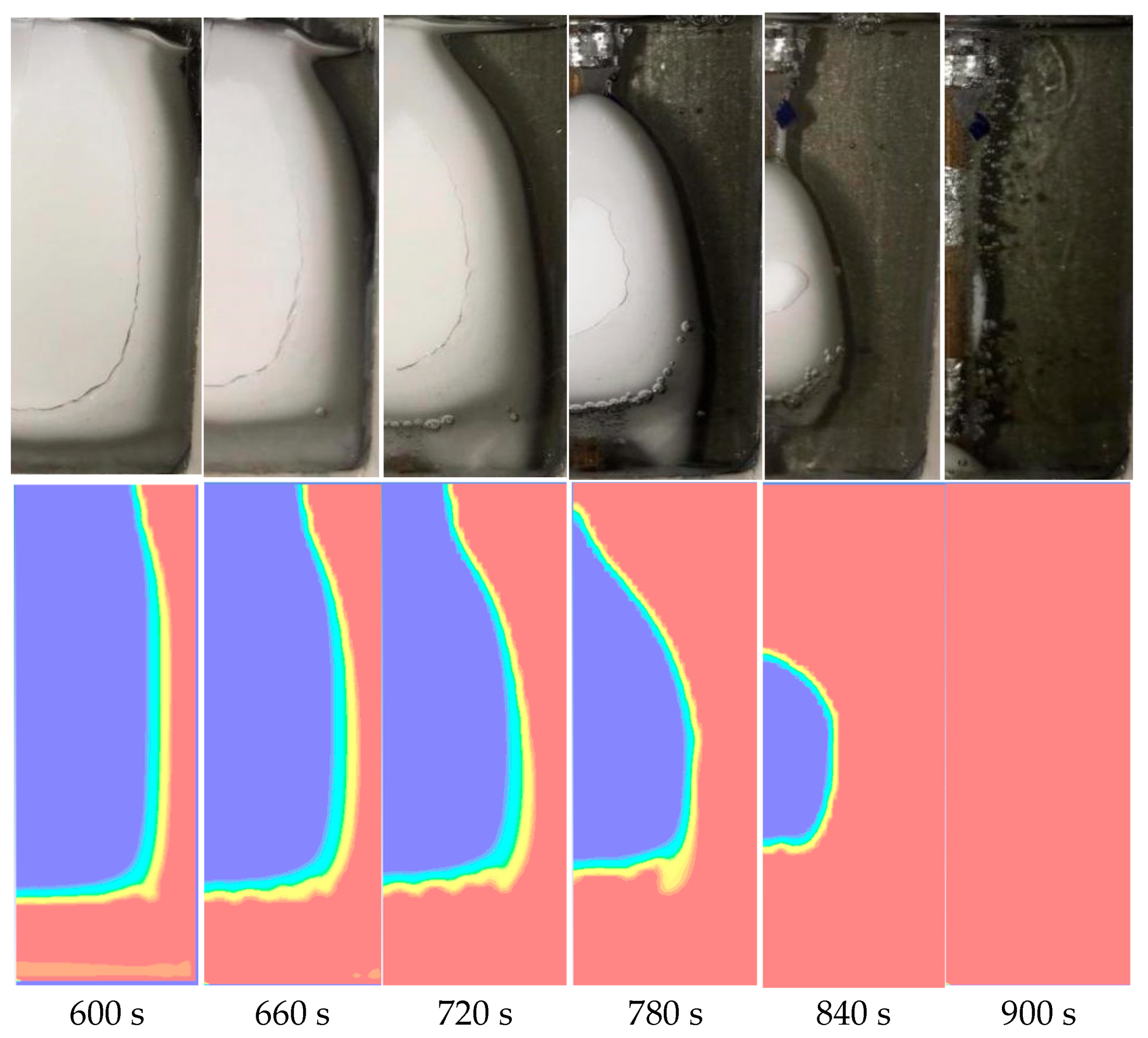
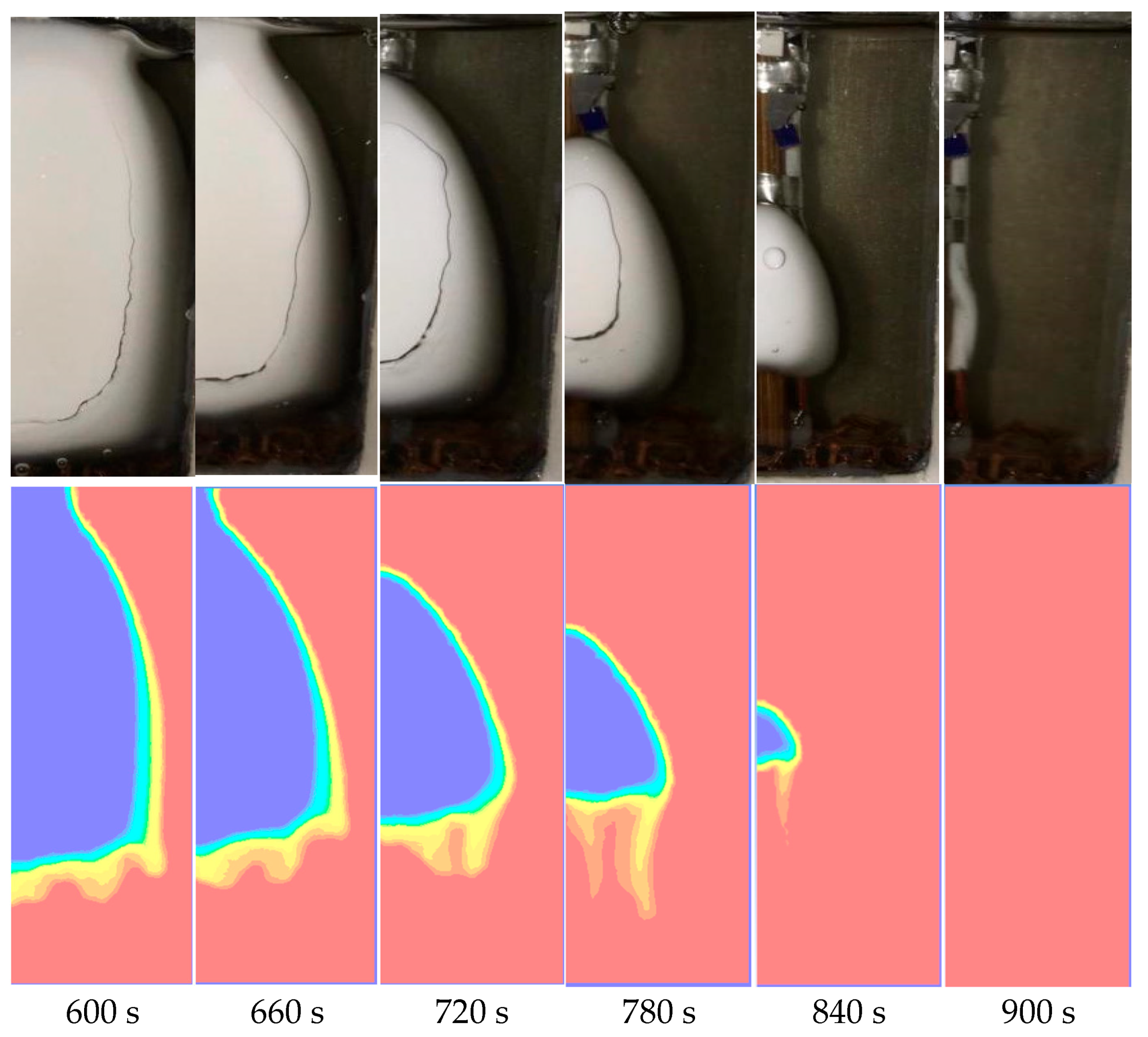
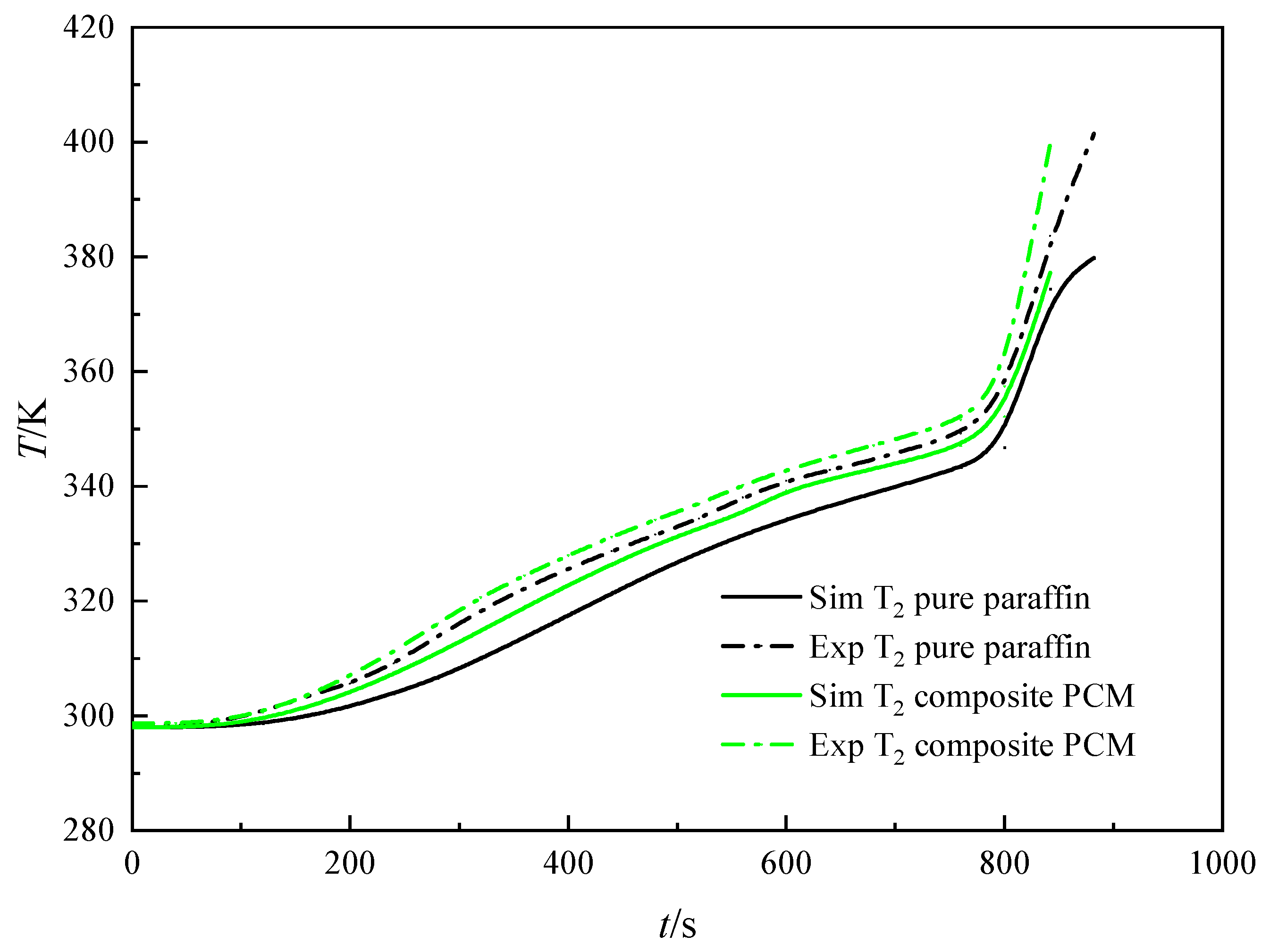
4.1. Temperature Field Analysis
4.2. Numerical Simulation
4.3. Analysis of the Internal Convection
5. Conclusions
- (1)
- A direct numerical simulation of melting process of composite phase change materials was developed using a simplified three-dimensional model of tetradecahedron metal foam. The simulation results agree well with the experimental results. The root mean square errors of the temperature for pure paraffin and composite PCM are 0.0223 and 0.0179, respectively.
- (2)
- The copper foam strengthens the thermal conductivity of the PCM. It takes 870 s for the composite PCM to melt, which is 3.44% less than that of the pure paraffin. Besides, the internal temperature difference is 16 K, which is 48.39% less than that of the pure paraffin.
- (3)
- The copper foam decreases the Rayleigh number of the PCM, which weakens the convection process. The maximum difference of the Rayleigh number is 9.4 × 107.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Roman Symbols | ||
| coefficient | [-] | |
| thermal diffusivity | [m2/s] | |
| specific heat | [J/kg K] | |
| coefficient | [-] | |
| error | [-] | |
| fraction | [-] | |
| gravitational acceleration | [m/s2] | |
| conductivity coefficient | [W/m K] | |
| latent heat | [J/kg] | |
| number | [-] | |
| pressure | [Pa] | |
| Rayleigh number | [-] | |
| coefficient | [-] | |
| time | [s] | |
| temperature | [K] | |
| phase-change temperature | [K] | |
| velocity | [m/s] | |
| equivalent length | [m] | |
| experimental value | [-] | |
| theoretical value | [-] | |
| Greek Symbols | ||
| coefficient of cubic expansion | [1/K] | |
| expansion coefficient | [1/K] | |
| error | [-] | |
| viscosity | [Pa s] | |
| density | [kg/m3] | |
| partial differential | [-] | |
| nabla operater | [-] | |
| Subscripts | ||
| phase change material | ||
| high temperature | ||
| liquid | ||
| phase change temperature | ||
References
- Yang, T.; King, W.P.; Miljkovic, N. Phase Change Material-Based Thermal Energy Storage. Cell Rep. Phys. Sci. 2021, 2, 100540. [Google Scholar] [CrossRef]
- Herbinger, F.; Groulx, D. Experimental Comparative Analysis of Finned-Tube PCM-Heat Exchangers Performance. Appl. Therm. Eng. 2022, 211, 118532. [Google Scholar] [CrossRef]
- Ahmed, S.; Abderrahmane, A.; Saeed, A.M.; Guedri, K.; Mourad, A.; Younis, O.; Botmart, T.; Shah, N. Melting Enhancement of PCM in a Finned Tube Latent Heat Thermal Energy Storage. Sci. Rep. 2022, 12, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lei, K.; Wang, Z.; Wang, H.; Zou, D. Metal-Based Phase Change Material (PCM) Microcapsules/Nanocapsules: Fabrication, Thermophysical Characterization and Application. Chem. Eng. J. 2022, 438, 135559. [Google Scholar] [CrossRef]
- Trivedi, G.V.N.; Parameshwaran, R. Cryogenic Conditioning of Microencapsulated Phase Change Material for Thermal Energy Storage. Sci. Rep. 2020, 10, 423–435. [Google Scholar] [CrossRef]
- Yazici, M.; Saglam, M.; Aydin, O.; Avci, M. Thermal Energy Storage Performance of PCM/Graphite Matrix Composite in a Tube-in-Shell Geometry. Therm. Sci. Eng. Prog. 2021, 23, 100915. [Google Scholar] [CrossRef]
- Li, W.; Dong, Y.; Zhang, X.; Liu, X. Preparation and Performance Analysis of Graphite Additive/Paraffin Composite Phase Change Materials. Processes 2019, 7, 447. [Google Scholar] [CrossRef]
- Falcone, M.; Rehman, D.; Dongellini, M.; Naldi, C.; Pulvirenti, B.; Morini, G.L. Experimental Investigation on Latent Thermal Energy Storages (LTESs) Based on Pure and Copper-Foam-Loaded PCMs. Energies 2022, 15, 4894. [Google Scholar] [CrossRef]
- Noghrehabadi, A.; Behbahan, A.S.; Wong, C.; Behbahani-Nejad, M. Investigation on the Effect of Metal Foam Properties on the PCM Melting Performance Subjected to Various Heat Fluxes. J. Comput. Appl. Mech. 2020, 52, 320–331. [Google Scholar]
- Thakur, A.K.; Ahmed, M.S.; Kang, H.; Prabakaran, R.; Said, Z.; Rahman, S.; Sathyamurthy, R.; Kim, J.; Hwang, J.Y. Critical Review on Internal and External Battery Thermal Management Systems for Fast Charging Applications. Adv. Energy Mater. 2022, 2202944. [Google Scholar] [CrossRef]
- Xu, Y.; He, Y.-L.; Zhu, G.-H.; Lv, S.; Shan, B. Al/Al2O3 Form-Stable Phase Change Material for High Temperature Thermal Energy Storage. Energy Procedia 2017, 2017, 4328–4333. [Google Scholar] [CrossRef]
- Guo, P.; Zhao, C.; Sheng, N.; Zhu, C.; Rao, Z. Enhancement of Solar Thermal Storage Properties of Phase Change Composites Supported by Modified Copper Foam. Sol. Energy Mater. Sol. Cells 2022, 247, 111950. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, P. Morphologies and Thermal Characterization of Paraffin/Carbon Foam Composite Phase Change Material. Sol. Energy Mater. Sol. Cells 2013, 117, 451–461. [Google Scholar] [CrossRef]
- Filippeschi, S.; Mameli, M.; Di Marco, P. Experimental Analysis of the Melting Process in a PCM/Aluminum Foam Composite Material in Hypergravity Conditions. Interfacial Phenom. Heat Transf. 2018, 4, 451–467. [Google Scholar] [CrossRef]
- Diani, A.; Moro, L.; Rossetto, L. Melting of Paraffin Waxes Embedded in a Porous Matrix Made by Additive Manufacturing. Appl. Sci. 2021, 11, 5396. [Google Scholar] [CrossRef]
- Zhang, Z.; He, X. Three-Dimensional Numerical Study on Solid-Liquid Phase Change within Open-Celled Aluminum Foam with Porosity Gradient. Appl. Therm. Eng. 2017, 113, 298–308. [Google Scholar] [CrossRef]
- Di Giorgio, P.; Iasiello, M.; Viglione, A.; Mameli, M.; Filippeschi, S.; Di Marco, P.; Andreozzi, A.; Bianco, N. Numerical Analysis of a Paraffin/Metal Foam Composite for Thermal Storage. J. Phys. Conf. Ser. 2017, 2016, 12032. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, F.; Gong, X. Experimental and Numerical Study on the Thermal Behavior of Phase Change Material Infiltrated in Low Porosity Metal Foam. J. Energy Storage 2019, 26, 101005. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Z.-G. Numerical Study on the Thermal Behavior of Phase Change Materials (PCMs) Embedded in Porous Metal Matrix. Sol. Energy 2014, 99, 172–184. [Google Scholar] [CrossRef]
- Feng, S.; Shi, M.; Li, Y.; Lu, T.J. Pore-Scale and Volume-Averaged Numerical Simulations of Melting Phase Change Heat Transfer in Finned Metal Foam. Int. J. Heat Mass Transf. 2015, 90, 838–847. [Google Scholar] [CrossRef]
- Yang, X.; Bai, Q.; Guo, Z.; Niu, Z.; Yang, C.; Jin, L.; Lu, T.J.; Yan, J. Comparison of direct numerical simulation with volume-averaged method on composite phase change materials for thermal energy storage. Appl. Energy 2018, 229, 700–714. [Google Scholar] [CrossRef]
- Kline, S.; McClintock, F.A. The Description of Uncertainties in Single Sample Experiments. Mech. Eng. 1953, 75, 3–9. [Google Scholar]
- Wu, W.; Chang, K.-C. Revisiting Transition to Chaos of Air in a Cubical Cavity Heated from One Vertical Side and Cooled from the Opposite Side. AIAA Thermophys. Conf. 2004, 2004, 2–9. [Google Scholar]



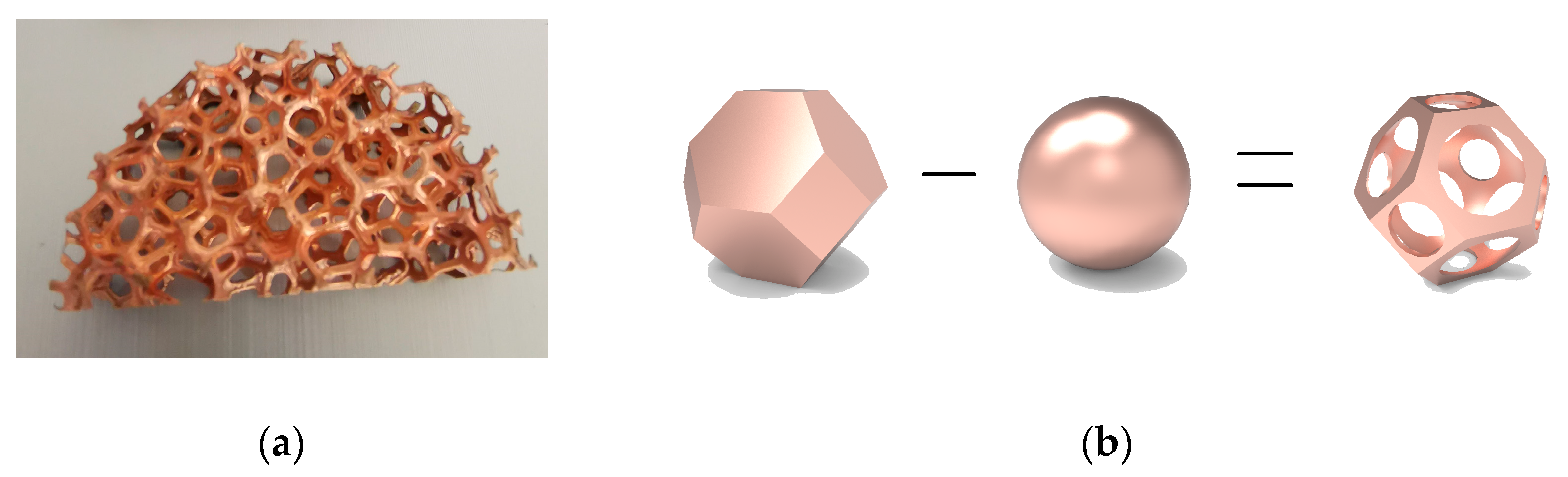





| Name | Density | Solid Specific Heat | Liquid Specific Heat | Thermal Conductivity | Phase Transition Start Point | Phase Change Termination Point | Phase Change Latent Heat | Dynamic Viscosity | Coefficient of Thermal Expansion |
|---|---|---|---|---|---|---|---|---|---|
| kg/m3 | J kg−1·K−1 | J kg−1·K | W m−1·K−1 | K | K | J kg−1 | Pa·s | K−1 | |
| paraffin | 837 | 1750 | 2450 | 0.305 | 348 | 365 | 218,400 | 0.004 | 0.00583 |
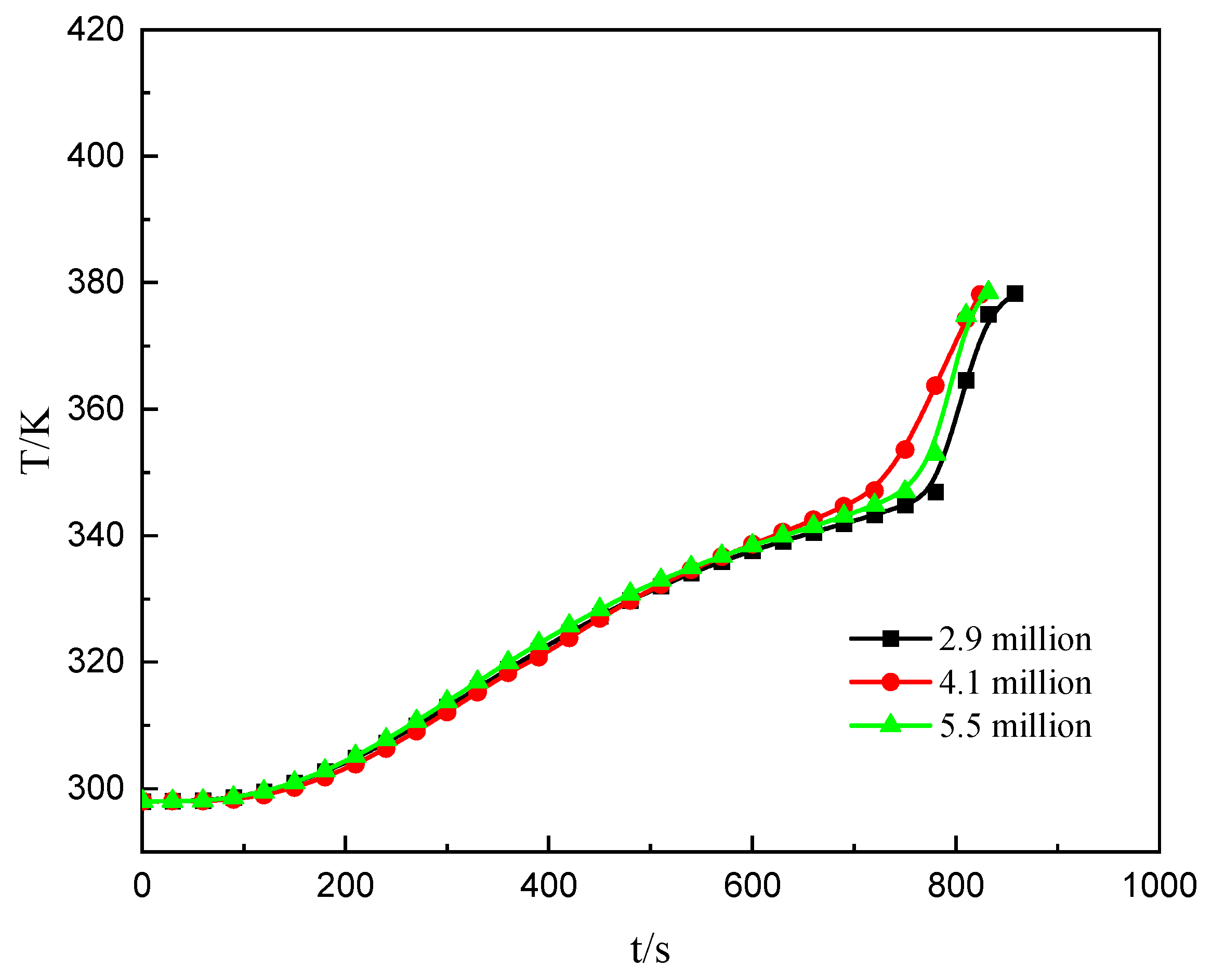
| Girds (Million) | Melting Time (s) | Error Compared to 2.9 Million |
|---|---|---|
| 2.9 | 858 | - |
| 4.1 | 824 | 3.96% |
| 5.5 | 832 | 3.03% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, X.; Sun, X.; Huang, J.; Wang, Z.; Zhang, H. Influence of Copper Foam on the Thermal Characteristics of Phase Change Materials. Energies 2023, 16, 1994. https://doi.org/10.3390/en16041994
You X, Sun X, Huang J, Wang Z, Zhang H. Influence of Copper Foam on the Thermal Characteristics of Phase Change Materials. Energies. 2023; 16(4):1994. https://doi.org/10.3390/en16041994
Chicago/Turabian StyleYou, Xiaokuan, Xiangxin Sun, Jie Huang, Zilong Wang, and Hua Zhang. 2023. "Influence of Copper Foam on the Thermal Characteristics of Phase Change Materials" Energies 16, no. 4: 1994. https://doi.org/10.3390/en16041994
APA StyleYou, X., Sun, X., Huang, J., Wang, Z., & Zhang, H. (2023). Influence of Copper Foam on the Thermal Characteristics of Phase Change Materials. Energies, 16(4), 1994. https://doi.org/10.3390/en16041994







