Impact of Adding Bioethanol and Dimethyl Carbonate on Gasoline Properties
Abstract
1. Introduction
- High oxygen content, 53.28% by weight, with an important role in the complete combustion of the fuel, thus reducing greenhouse gas emissions, particularly carbon and hydrocarbon emissions [12];
- A very good miscibility with fossil fuels (diesel and gasoline) [10];
- It can be produced in a cleaner way, and is non-toxic, non-corrosive and environmentally friendly [13].
2. Materials and Methods
2.1. Materials and Blends
2.2. Analysis Methods
2.2.1. Gas Chromatography
2.2.2. Distillation Temperature Measurement Method
2.2.3. Vapor Pressure Measurement Method
2.2.4. Research Octane Number (RON) and Motor Octane Number (MON) Measurement Method
3. Results and Discussion
3.1. Inquiry into Volatility
3.1.1. ASTM Distillation
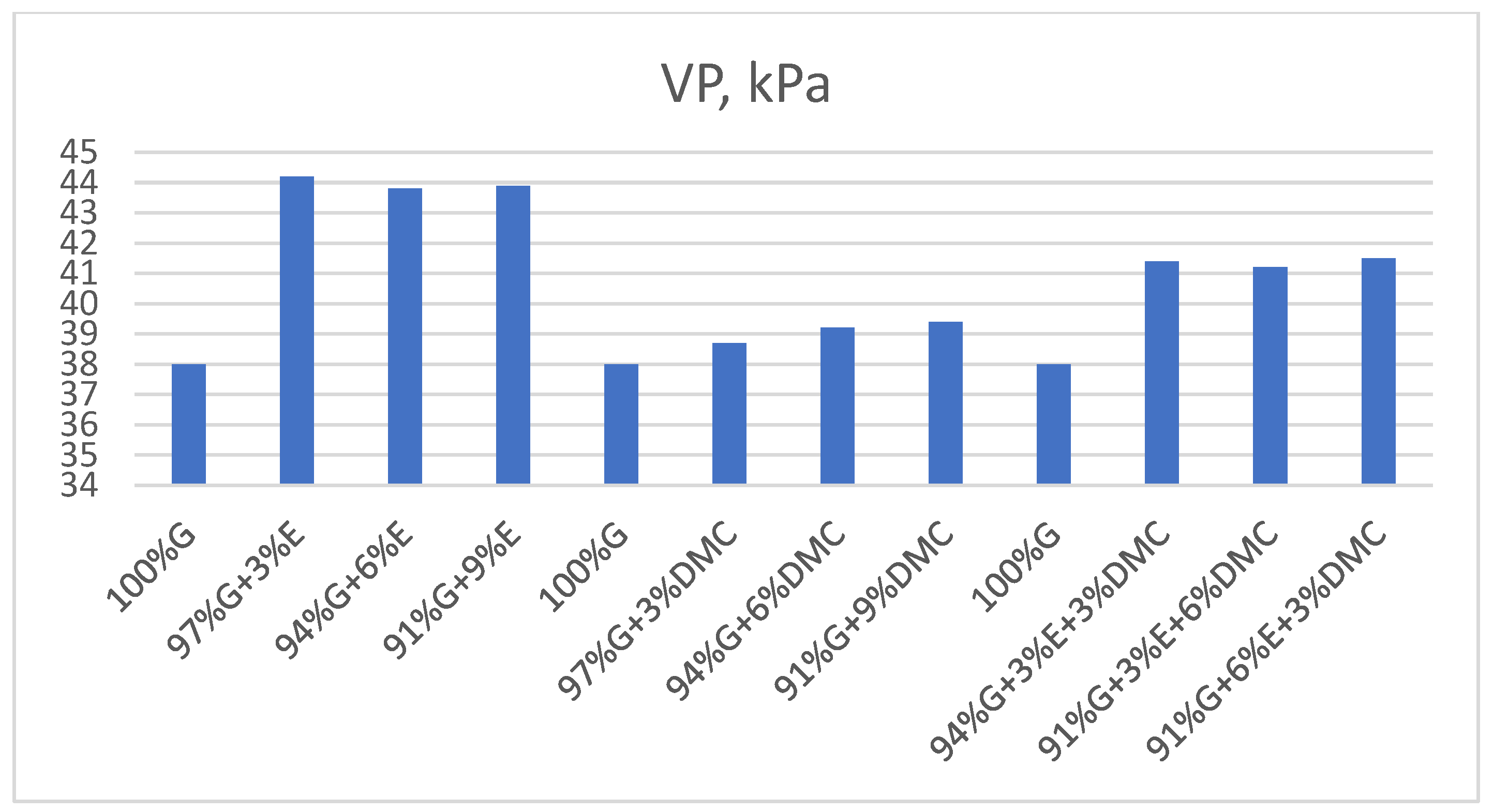
3.1.2. Reid Vapor Pressure
3.1.3. Calculation of Volatility Indexes
Calculation of Vapor Lock Index (VLI)
Calculation of Drivability Index (DI)
Calculation of Temperature for a Vapor–Liquid Ratio of 20 (TV/L =20)
3.2. Inquiry into the Antiknock Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Research Council, Committee on Measuring Lead in Critical Populations. Measuring Lead Exposure in Infants, Children and Other Sensitive Populations; National Academy Press: Washington, DC, USA, 1993; pp. 31–98. [Google Scholar] [CrossRef]
- Kivi, J.; Kytö, M.; Nylund, N.O.; Orre, K. Use of MTBE and ETBE as Gasoline Reformulation Components; Society of Automotive Engineers: San Francisco, CA, USA, 1992. [Google Scholar] [CrossRef]
- Mennear, J.H. Carcinogenicity studies on MTBE: Critical review and interpretation. Risk Anal. 1997, 17, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Clary, J.J. Methyl tert Butyl Ether Systemic Toxicity. Risk Anal. 1997, 17, 661–672. [Google Scholar] [CrossRef] [PubMed]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Ge, J.C.; Wu, G.; Choi, N.J. Comparative study of pilot–main injection timings and diesel/ethanol binary blends on combustion, emission and microstructure of particles emitted from diesel engines. Fuel 2022, 313, 122658. [Google Scholar] [CrossRef]
- Ge, J.C.; Wu, G.; Yoo, B.-O.; Choi, N.J. Effect of injection timing on combustion, emission and particle morphology of an old diesel engine fueled with ternary blends at low idling operations. Energy 2022, 253, 124150. [Google Scholar] [CrossRef]
- Ge, J.C.; Kim, J.Y.; Yoo, B.-O.; Song, J.H. Effects of Engine Load and Ternary Mixture on Combustion and Emissions from a Diesel Engine Using Later Injection Timing. Sustainability 2023, 15, 1391. [Google Scholar] [CrossRef]
- Ahmed, A.A.; El-Masry, A.M.; Barakat, Y. Azeotrope formation in gasoline–ethanol blends. Part 1–Impact of nonionic on E10 distillation curve. Egypt. J. Pet. 2018, 27, 1167–1175. [Google Scholar] [CrossRef]
- Schifter, I.; Gonzalez, U.; Gonzlez-Macias, C. Effects of ethanol, ethyl-tert-butyl ether and dimethyl-carbonate blends with gasoline on SI engine. Fuel 2016, 183, 253–261. [Google Scholar] [CrossRef]
- Wen, L.B.; Xin, C.Y.; Yang, S.C. The effect of adding dimethyl carbonate (DMC) and ethanol to unleaded gasoline on exhaust emission. Appl. Energy 2010, 87, 115–121. [Google Scholar] [CrossRef]
- Kumar, B.R.; Saravanan, S. Partially premixed low temperature combustion using dimethyl carbonate (DMC) in a DI diesel engine for favorable smoke/NOx emissions. Fuel 2016, 180, 396–406. [Google Scholar] [CrossRef]
- Tundo, P.; Selva, M. The chemistry of dimethyl carbonate. Acc. Chem. Res. 2002, 35, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Rashid, K.T.; Mansour, K.; Abid, M.F.; Ali, S.M.; Abed, K.N. Synthesis of dimethyl carbonate for enhancement of gasoline performance. J. King Saud Univ. Eng. Sci. 2019, 31, 171–177. [Google Scholar] [CrossRef]
- Schifter, I.; Gonzalez, U.; Diaz, L.; Sanchez-Reyna, G.; Mejia-Centeno, I.; Gonzalez-Macias, C. Comparison of performance and emissions for gasoline-oxygenated blends up to 20 percent oxygen and implications for combustion on a spark-ignited engine. Fuel 2017, 208, 673–681. [Google Scholar] [CrossRef]
- Abdalla, A.O.G.; Liu, D. Dimethyl Carbonate as a Promising Oxygenated Fuel for Combustion: A Review. Energies 2018, 11, 1552. [Google Scholar] [CrossRef]
- Wang, C.; Zeraati-Rezaei, S.; Xiang, L.; Xu, H. Ethanol blends in spark ignition engines: RON, octane-added value, cooling effect, compression ratio, and potential engine efficiency gain. Appl. Energy 2017, 191, 603–619. [Google Scholar] [CrossRef]
- Pacheco, M.A.; Marshall, C.L. Review of Dimethyl Carbonate (DMC) Manufacture and Its Characteristics as a Fuel Additive. Energy Fuels 1997, 11, 2–29. [Google Scholar] [CrossRef]
- Huang, S.; Yan, B.; Wang, S.; Ma, X. ChemInform Abstract: Recent Advances in Dialkyl Carbonates Synthesis and Applications. Chem. Soc. Rev. 2015, 44, 3079. [Google Scholar] [CrossRef]
- Pyo, S.H.; Ji, H.P.; Chang, T.S.; Hatti-Kaul, R. Dimethyl carbonate as a green chemical. Curr. Opin. Green Sustain. Chem. 2017, 5, 61–66. [Google Scholar] [CrossRef]
- EFIX 95 Gasoline. Use: Fuel for Spark Ignition Engines. Available online: https://rompetrol-rafinare.kmginternational.com/upload/files/ss_2_1_8_t_efix_95_gasoline_1909.pdf (accessed on 31 December 2022).
- ASTM D5191-15; Standard Test Method for Vapor Pressure of Petroleum Products (Mini Method). ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- Sholes, K.R.; Odaka, M.; Goto, Y.; Ishii, H.; Suzuki, H. Study of the Effect of Boiling Point on Combustion and PM Emissions in a Compression Ignition Engine Using Two-Component n-Paraffin Fuels; SAE Technical Paper 2002-01-0871; SAE International: San Francisco, CA, USA, 2002. [Google Scholar] [CrossRef]
- Smith, B.L.; Bruno, T.J. Improvements in the measurement of distillation curves. 3. Application to gasoline and gasoline + methanol mixtures. Ind. Eng. Chem. Res. 2007, 46, 297–309. [Google Scholar] [CrossRef]
- Smith, B.L.; Ott, L.S.; Bruno, T.J. Composition-explicit distillation curves of diesel fuel with glycol ether and glycol ester oxygenates: Fuel analysis metrology to enable decreased particulate emissions. Environ. Sci. Technol. 2008, 42, 7682–7689. [Google Scholar] [CrossRef]
- Aghahossein, S.S.; Abdollahipoor, B.; Martinson, J.; Windom, B.; Foust, T.D.; Reardon, K.F. Effects of dual-alcohol gasoline blends on physiochemical properties and volatility behavior. Fuel 2019, 252, 542–552. [Google Scholar] [CrossRef]
- Masum, B.M.; Masjuki, H.H.; Kalam, M.A.; Palash, S.M.; Habibullah, M. Effect of alcohol-gasoline blends optimization on fuel properties, performance and emissions of a SI engine. J. Clean. Prod. 2015, 86, 230–237. [Google Scholar] [CrossRef]
- Dalli, D.; Lois, E.; Karonis, D. Vapor Pressure and Octane Numbers of Ternary Gasoline–Ethanol–ETBE Blends. J. Energy Eng. 2014, 140, A4014002. [Google Scholar] [CrossRef]
- Andersen, V.F.; Anderson, J.E.; Wallington, T.J.; Mueller, S.A.; Nielsen, O.J. Vapor pressures of alcohol-gasoline blends. Energy Fuels 2010, 24, 3647–3654. [Google Scholar] [CrossRef]
- Chevron. Motor Gasolines Technical Review. Available online: https://www.chevron.com/-/media/chevron/operations/documents/motor-gas-tech-review.pdf (accessed on 24 January 2023).
- Christensen, E.; Yanowitz, J.; Ratcliff, M.; McCormick, R.L. Renewable oxygenate blending effects on gasoline properties. Energy Fuels 2011, 25, 4723–4733. [Google Scholar] [CrossRef]
- French, R.; Malone, P. Phase Equilibria of Ethanol Fuel Blends. Fluid Phase Equilibria 2005, 228–229, 27–40. [Google Scholar] [CrossRef]
- Mužíková, Z.; Pospíšil, M.; Šebor, G. Volatility and phase stability of petrol blends with ethanol. Fuel 2009, 88, 1351–1356. [Google Scholar] [CrossRef]
- EN 228:2012+A1:2017. Automotive Fuels—Unleaded Petrol—Requirements and Test Methods. Irish Standard: Dublin, Ireland, 2017.
- Manal, A.; Hoda, A.M.; Barakat, Y. Volatility criteria and physicochemical properties of the promising dimethyl carbonate-gasolin blends. Sci. Rep. 2022, 12, 17183. [Google Scholar] [CrossRef]
- Sarathy, S.M.; Oßwald, P.; Hansen, N.; Kohse-Höinghaus, K. Alcohol combustion chemistry. Prog. Energy Combust. Sci. 2014, 44, 40–102. [Google Scholar] [CrossRef]
- Wibowo, C.S.; Adian, F.; Masuku, M.; Nugroho, Y.; Sugiarto, B. The Optimization Performance of Mixed Fuel Gasoline RON 88, 92, 98 with Bioethanol on Spark Ignition Engine. Int. J. Eng. Technol. Res. 2020, 9, 989–992. [Google Scholar] [CrossRef]
- Adian, F.; Sugiarto, B.; Wibowo, C.S.; Zikra, A.; Mulya, T. The effect of 5% ethanol in 88, 92, and 98 RON gasoline on motorcycle engine performance. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019; Volume 2114. [Google Scholar] [CrossRef]

| Component | % v/v | %wt |
|---|---|---|
| n + iP4 | 0.35 | 0.27 |
| n + iP5 | 6.85 | 5.53 |
| n + iP6 | 6.06 | 5.12 |
| n + iP7 | 11.35 | 10.01 |
| n + iP8 | 7.03 | 6.37 |
| n + iP9 | 3.17 | 2.94 |
| n + iP10 | 1.02 | 0.96 |
| n + iP11 | 1.32 | 1.26 |
| Total Paraffins | 37.15 | 32.46 |
| OC4 | 0.27 | 0.22 |
| n+ Cy O C5 | 2.96 | 2.48 |
| n + Cy O C6 | 1.92 | 1.67 |
| n + Cy O C7 | 1.12 | 1.01 |
| n + Cy O C8 | 0.63 | 0.58 |
| n + Cy O C9 | 0.18 | 0.17 |
| n + Cy O 10 | 0.07 | 0.06 |
| Total Olefins | 7.15 | 6.19 |
| N C5 | 0.09 | 0.090 |
| N C6 | 0.96 | 0.965 |
| N C7 | 1.64 | 1.692 |
| N C8 | 1.13 | 1.130 |
| N C9 + C10 | 0.74 | 0.743 |
| Total Naphtens | 4.56 | 4.62 |
| A C6 | 0.73 | 0.82 |
| A C7 | 10.8 | 12.13 |
| A C8 | 16.72 | 18.55 |
| A C9 | 12.63 | 14.04 |
| A C10 + C11 | 10.08 | 11.18 |
| Total Aromatics | 50.96 | 56.72 |
| Oxygenates | 0.01 | 0.04 |
| TOTAL | 99.83 | 100.03 |
| Properties | Gasoline | Ethanol | Dimethyl Carbonate |
|---|---|---|---|
| Molecular weight (g/mol) | 103.07 | 46.07 | 90.08 |
| Oxygen (%) | 0 | 34.73 | 53.28 |
| Density at 15 °C (g/cm3) | 0.7892 | 0.7933 | 1.0754 |
| Viscosity at 40 °C (mm2/s) | 0.64 | 1.17 | 0.56 |
| Flash point (°C) | - | 17 | 17 |
| Vapor pressure (mmHg) la 100 °F | 285.09 | 115 | 143.75 |
| RON | 94.7 | 107 * | 101–116 ** |
| MON | 84.7 | 89 * |
| Second Component | b.p.of Second Component, °C | b.p. of the Azeotropic Mixture, °C | Second Component Concentration, %wt. |
|---|---|---|---|
| n-Pentane | 36.2 | 34.3 | 95 |
| n-Hexane | 68.9 | 58.7 | 79 |
| n-Heptane | 98.5 | 70.9 | 51 |
| Cyclohexane | 80.7 | 64.9 | 69.5 |
| Benzene | 80.2 | 68.2 | 67.6 |
| Toluene | 110.8 | 76.7 | 82 |
| Gasoline and Blends,% v/v | VLI | DI | T(V/L = 20), °C |
|---|---|---|---|
| 100% G | 454.9 | 632.5 | 74.1 |
| 97% G + 3% E | 564.5 | 619.0 | 70.3 |
| 94% G + 6% E | 591.3 | 613.0 | 70.1 |
| 91% G + 9% E | 635.0 | 603.5 | 69.5 |
| 97% G + 3% DMC | 467.2 | 618.0 | 73.1 |
| 94% G + 6% DMC | 477.7 | 613.5 | 72.5 |
| 91% G + 9% DMC | 480.5 | 604.5 | 71.9 |
| 94% G + 3% E + 3% DMC | 540.0 | 612.5 | 70.2 |
| 91% G + 3% E + 6% DMC | 524.0 | 611.5 | 71.3 |
| 91% G + 6% E + 3% DMC | 583.0 | 606.0 | 70.5 |
| Gasoline and Blends, % v/v | RON | MON | AKI | S |
|---|---|---|---|---|
| 100%G | 94.7 | 84.7 | 89.7 | 10.0 |
| 97%G + 3%E | 95.2 | 85.0 | 90.1 | 10.2 |
| 94% G + 6%E | 96.2 | 85.5 | 90.8 | 10.7 |
| 91%G + 9%E | 96.9 | 85.9 | 91.4 | 11.0 |
| 97%G + 3%DMC | 94.9 | 85.0 | 89.9 | 9.9 |
| 94%G + 6%DMC | 95.4 | 85.5 | 90.4 | 9.9 |
| 91%G + 9%DMC | 96.2 | 87.0 | 91.6 | 9.2 |
| 94%G + 3%E + 3%DMC | 96.0 | 85.2 | 90.6 | 10.8 |
| 91%G + 3%E + 6%DMC | 96.4 | 86.3 | 91.3 | 10.1 |
| 91%G + 6%E + 3%DMC | 96.7 | 85.7 | 91.2 | 11.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, S.; Sapunaru, O.V.; Sterpu, A.E.; Chis, T.V.; I.Koncsag, C. Impact of Adding Bioethanol and Dimethyl Carbonate on Gasoline Properties. Energies 2023, 16, 1940. https://doi.org/10.3390/en16041940
Osman S, Sapunaru OV, Sterpu AE, Chis TV, I.Koncsag C. Impact of Adding Bioethanol and Dimethyl Carbonate on Gasoline Properties. Energies. 2023; 16(4):1940. https://doi.org/10.3390/en16041940
Chicago/Turabian StyleOsman, Sibel, Olga Valerica Sapunaru, Ancaelena Eliza Sterpu, Timur Vasile Chis, and Claudia I.Koncsag. 2023. "Impact of Adding Bioethanol and Dimethyl Carbonate on Gasoline Properties" Energies 16, no. 4: 1940. https://doi.org/10.3390/en16041940
APA StyleOsman, S., Sapunaru, O. V., Sterpu, A. E., Chis, T. V., & I.Koncsag, C. (2023). Impact of Adding Bioethanol and Dimethyl Carbonate on Gasoline Properties. Energies, 16(4), 1940. https://doi.org/10.3390/en16041940








