1. Introduction
Climate change is one of the most urgent issues that require much attention. For some developing countries that heavily depend on agriculture, it can bring about a heavier burden on their population [
1]. Promoting the use of renewable energy sources can reduce fossil fuel use and curtail greenhouse gas emissions [
2], which will lead to curbing climate change. However, their intermittent nature is a challenging issue when it comes to exploiting renewable energy, and building an energy storage system can help overcome the issue [
3,
4]. Storing energy when the demand for energy is low and distributing the stored energy to the population in on-peak hours enables a consistent supply of energy.
Compressed air energy storage (CAES) is one of the few bulk-scale energy storage options that are already commercialized. Due to the economic and environmentally friendly features of CAES, it is perceived as a promising choice for energy storage [
5,
6]. The fundamental mechanism of CAES is to store energy in the form of compressed air. During peak hours, the stored compressed air is expanded to run a turbine to restore the energy [
7,
8]. As a gas is compressed, heat is generated, whereas heat is lost when a gas expands. Depending on how CAES treats the heat generated during compression, it can be classified into three different types: diabatic CAES (D-CAES), adiabatic CAES (A-CAES), and isothermal CAES (I-CAES) [
9,
10]. In D-CAES, compression heat is dissipated before the compressed air is transferred to a storage vessel. In the discharging phase to regenerate the stored energy, an external energy source such as natural gas needs to be brought in to increase the temperature of the gas because gas expansion accompanies a significant temperature drop [
9,
11]. In D-CAES, the heat dissipation leads to the loss of energy. The idea of A-CAES stems from saving the heat energy that is wasted in conventional D-CAES. In A-CAES, a thermal energy storage device stores the compression heat energy generated during the compression step. In the discharging process, the stored heat can be employed to increase the gas temperature instead of using an external energy source. Therefore, it reduces the energy waste and eliminates the need for extra heat supply [
9]. I-CAES is considered to be the most efficient among the three types [
9,
10]. By maximizing heat transfer, the temperature change is minimized during compression/expansion [
12,
13]. Because no energy is compromised by the heat generation, less work input is needed to compress air [
12]. In addition, it does not require an extra device such as a thermal energy storage device of A-CAES, and no extra energy input is needed for the expansion stage. Owing to its promising characteristics, a variety of I-CAES models have been studied. Researchers in [
14] proposed a system equipped with a near-isothermal compression system for wind energy, which serves as one of the major electricity generation sources in the US [
15] and is rapidly growing globally [
16]. According to their quantified evaluation, the suggested model resulted in the size and weight reduction, which leads to cost saving [
14]. Researchers in [
17] focused on the expansion process where the energy discharging takes place. Compared to adiabatic expansion, the introduction of their near-isothermal expander resulted in a 15.7% increase in specific work.
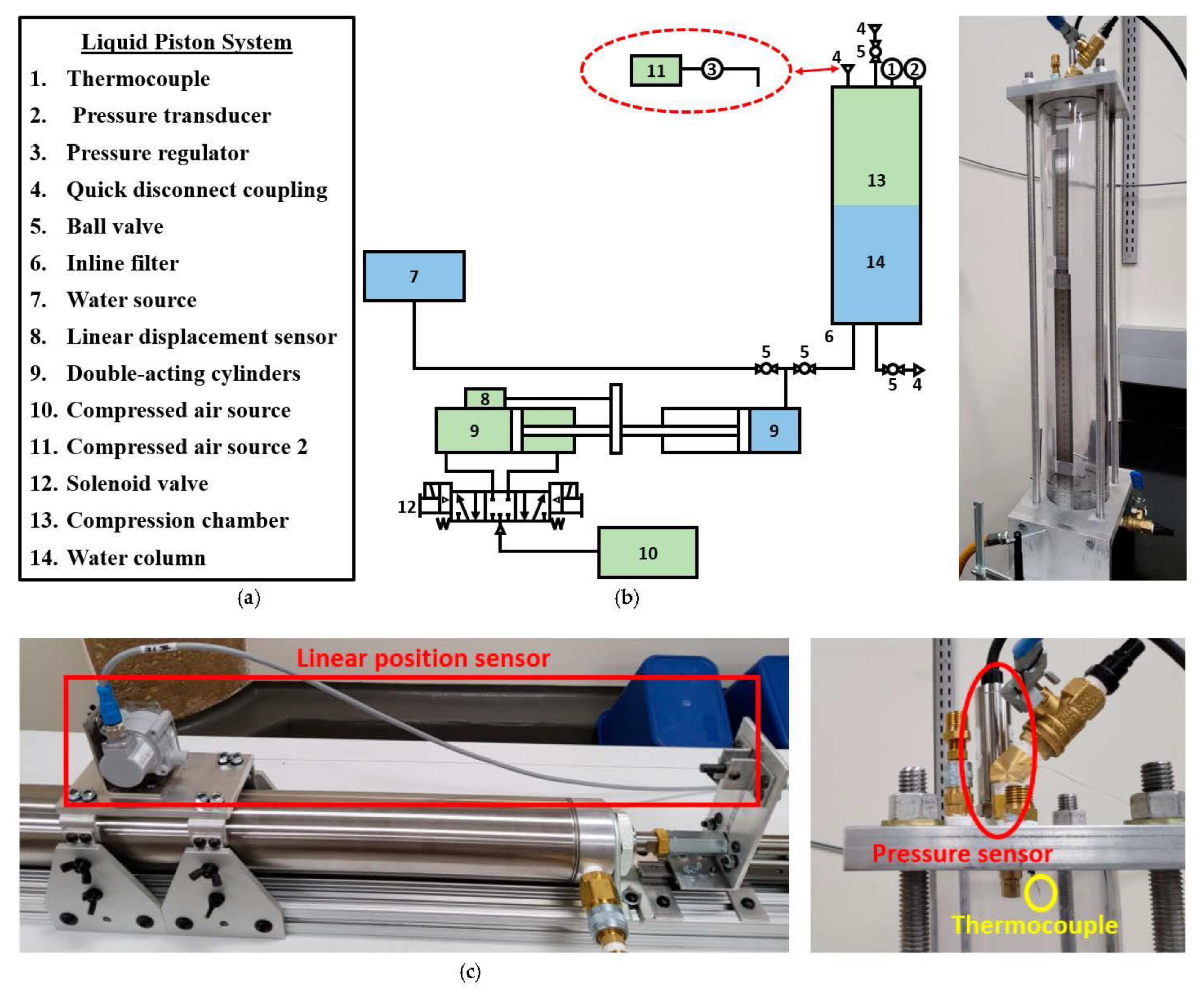
The liquid piston gas compression concept was recently re-introduced by Van de Ven and Li [
12]. The fundamental mechanism of the compressor is that a liquid column functions as the piston of a conventional reciprocating compressor. It has been drawing attention as an innovative compression technique for energy storage applications as a variety of heat transfer improvement techniques can be utilized in the compressor, owing to the conforming nature of a liquid that can take a complicated shape. In other words, it has a great potential to achieve a near-isothermal process. A number of experimental and simulation studies have been performed to investigate the heat transfer improvement techniques in a liquid piston compressor. Placing an insert is a direct way to provide extra surface area for heat transfer. The effects of porous media and interrupted-plate-type inserts were examined in [
18]. For the same level of power density in their test, a 18% efficiency increase was achieved with the inserts. According to [
19], at the much higher target pressure of 210 bar, compression efficiency improvement was observed with the use of porous media. Metal wire mesh inserts were introduced as an insert for the liquid piston in [
20]. An increase of 6–8% in compression efficiency with the metal wire inserts was attained over the baseline liquid piston compression. By testing two different materials, aluminum and copper, the impacts of the thermal property of an insert were also studied, and it turned out that the material does not make a notable efficiency change. Metal plates were also used as an insert in [
21]. By varying the compression speed and the dimensions of inserts, a compression efficiency improvement of up to 8% was observed under their experimental conditions. Liquid-based heat transfer techniques have also been studied. Researchers in [
13] investigated factors that affect droplet heat transfer by varying the mass of injected water and droplet size. To some degree, reducing the size of droplets is beneficial, and enlarging the mass of droplet injection is advantageous for boosting heat transfer. Researchers in [
22] experimentally evaluated the spray injection technique by varying the angle of the nozzle and spray injection pressure. Higher injection pressure leads to higher flowrate and introduces a greater amount of water into the compression chamber. Thus, less temperature increase during compression was observed. However, as higher injection pressure requires higher work input, a higher spray pressure does not always guarantee a better overall efficiency despite the smaller temperature increment. Researchers in [
23] examined the effectiveness of utilizing two proven techniques of metal inserts and spray injection at a time. They performed a more detailed analysis of the impacts of spray injection pressure on droplet heat transfer at different injection pressures. They proved that the spray pressure is critical in determining droplet heat transfer, including the size and velocity of a droplet as well as spray flowrate, all of which have an impact on droplet heat transfer and are dependent on the pressure. Aqueous foam is another liquid-based heat transfer technique, and an experimental investigation was carried out in [
24]. Aqueous foam is generated inside the compression chamber so that extra heat transfer surface area is provided, and it increases the isothermal efficiency to 92% from 86% of the compression without the foam. Compared to the way spray injection is employed in [
22,
23], where the nozzle is continuously in operation throughout the compression, foam generation is carried out once before the compression begins. Therefore, it is expected to save work input compared to the other liquid-based method. A similar gas compression technique that utilizes a liquid and a porous medium was introduced and experimentally assessed in [
25,
26]. They examined the issue of gas dissolution during compression, which needs to be addressed when a liquid is used for compression. Using Henry’s law, the amount of dissolved air was estimated based upon a proportional relationship between the solubility and the pressure. A scaled-up liquid piston has also been tested in [
27]. A chamber with a volume of 24.71
was designed, and exergy-based efficiency evaluation was performed under various experimental conditions. On top of bringing external heat transfer enhancement, a variety of approaches to optimizing the liquid piston itself have also been made. In [
28], chambers designed with diverse design parameters were examined. Because the shape of the chamber affects the flow behavior of the internal air, the change in the chamber shape can result in heat transfer efficiency variations. In [
29], compression speed was varied and different liquid piston chambers were experimentally investigated. The faster compression leads to lowered isothermal performance, and the thermal conductivity of the chamber material has a relatively small impact on heat transfer performance. A chamber that consists of a number of tube arrays was also tested [
30]. Increasing the number of tubes leads to a positive effect on compression efficiency.
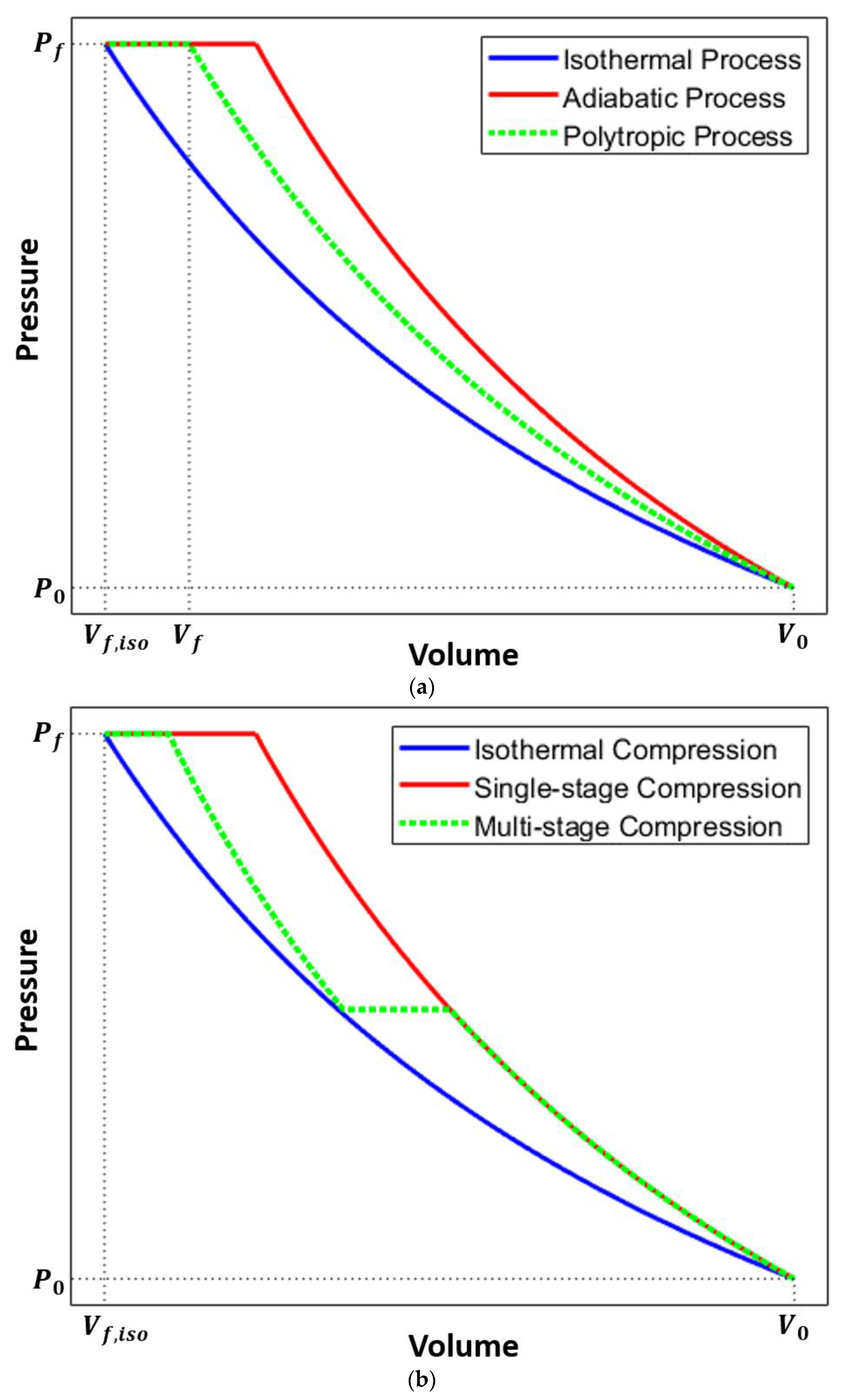
Many experimental studies on liquid piston compression focus on compression starting from atmospheric pressure or compression with a single case of pressure variation. In the current work, liquid piston gas compression at different initial pressure levels is tested, and how the different pressure affects compression efficiency for the same pressure ratio is examined. As multi-stage compression can lead to a better efficiency than single-stage compression [
31], and the CAES plants in the actual operation employ multi-stage compressors [
9,
32,
33], this work can aid in evaluating the real applicability of a liquid piston at an elevated pressure in a multi-stage system. Furthermore, this can pave the way for the future application of pressure-sensitive techniques such as spray injection and aqueous foam by broadening the fundamental understanding of liquid piston efficacy at various pressures. In addition, the dissolution issue of a liquid piston is also investigated by estimating how much air dissolution takes place for each process. It is also identified whether the difference in the dissolution contributes to the compression efficiency. There have been separate studies that examined the impact of the initial pressure [
27,
30] or identified the dissolution issue [
25,
26,
34,
35] in a liquid piston or similar liquid-based compression systems. To the author’s best knowledge, this is the first work that relates the impacts of different pressure levels on liquid piston compression efficiency and air dissolution. Because this work examines relatively moderate pressure ranges, which were not covered in [
27,
30] and where air solubility data can be easily estimated with the data available in [
36], those two issues that are closely associated can be studied simultaneously. Another novelty of the current work is to investigate how the dissolution affects the overall efficiency of the energy storage system. In addition, by taking into consideration the discharging process of CAES, the study allows for a practical evaluation of a liquid piston system for energy storage applications.
This paper consists of five sections. The current section introduces the background of this research. The following section illustrates the analytical modeling to describe the concept and the methodology with the experimental apparatus description. The next section presents the results of the experiments. Then, analysis of the results is presented in the following section. Lastly, the paper ends with concluding remarks.
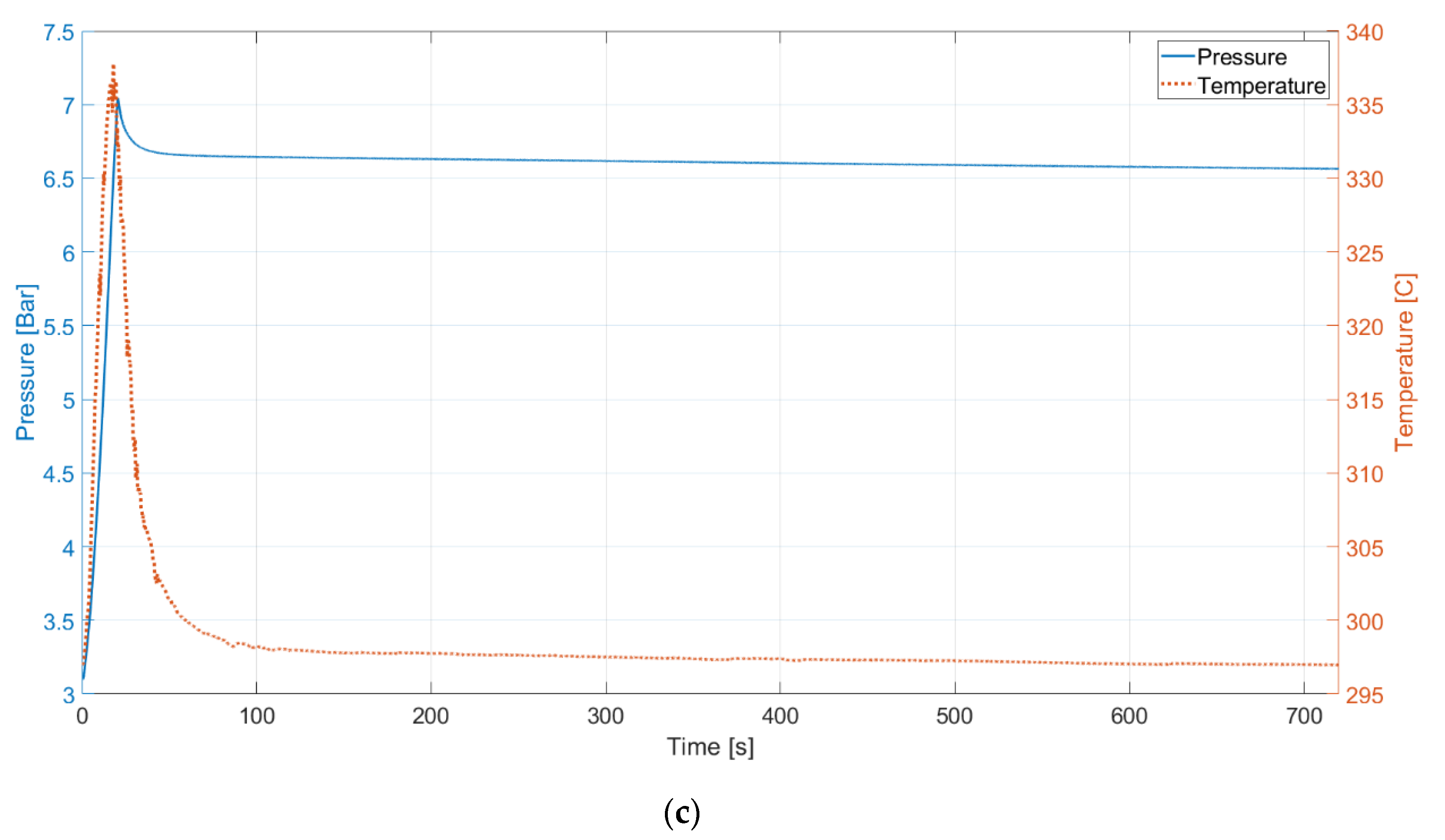
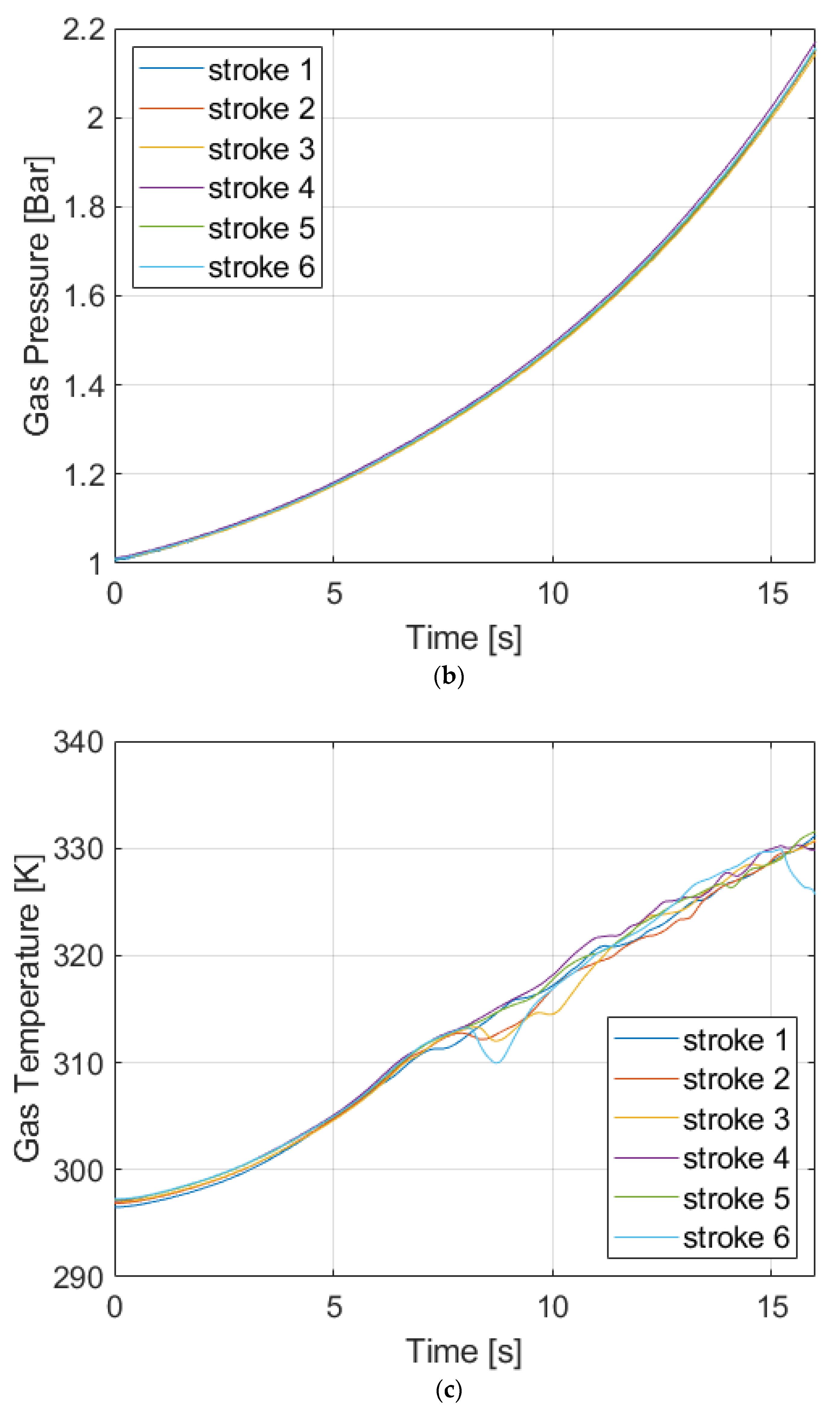
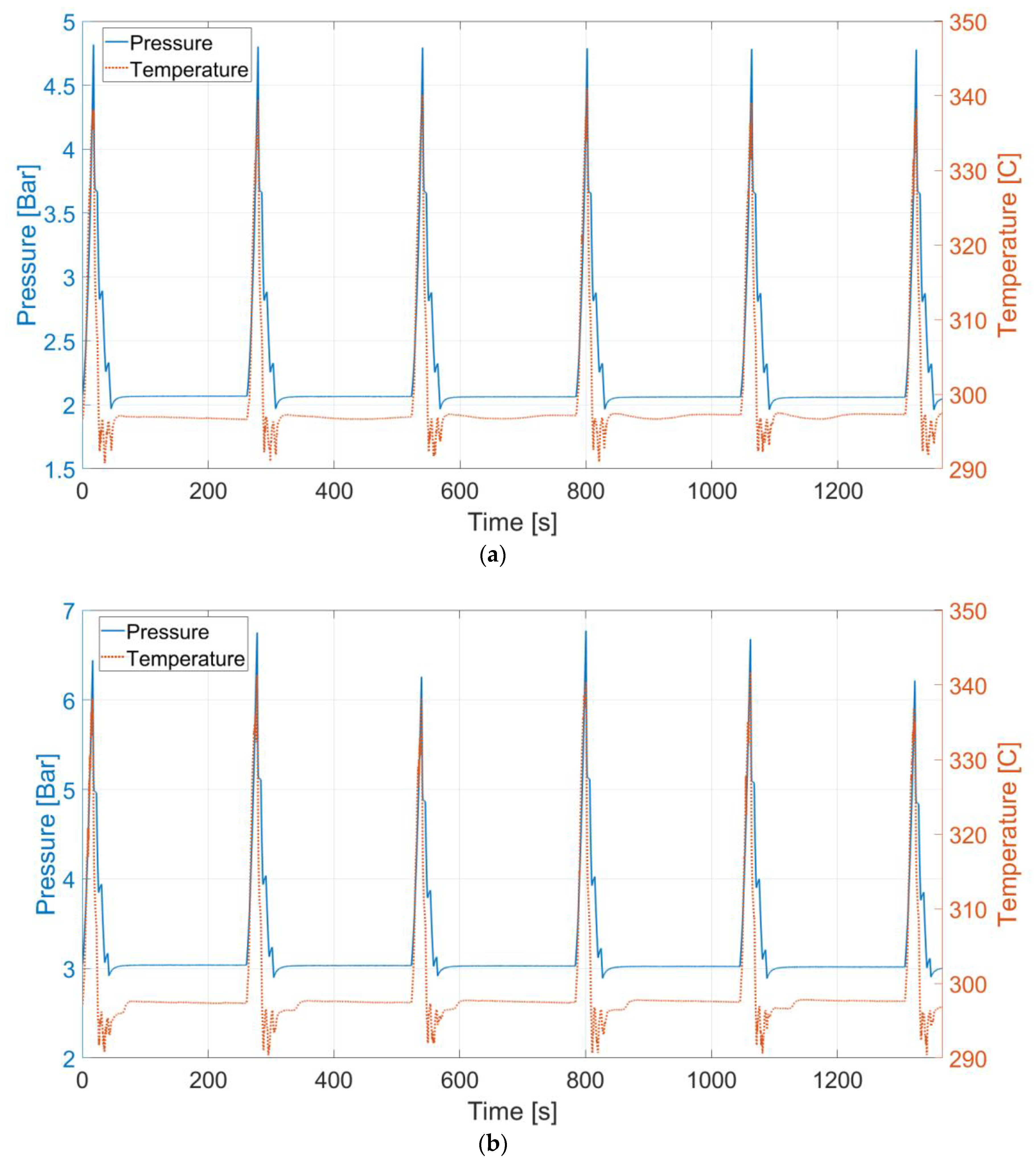
4. Discussion
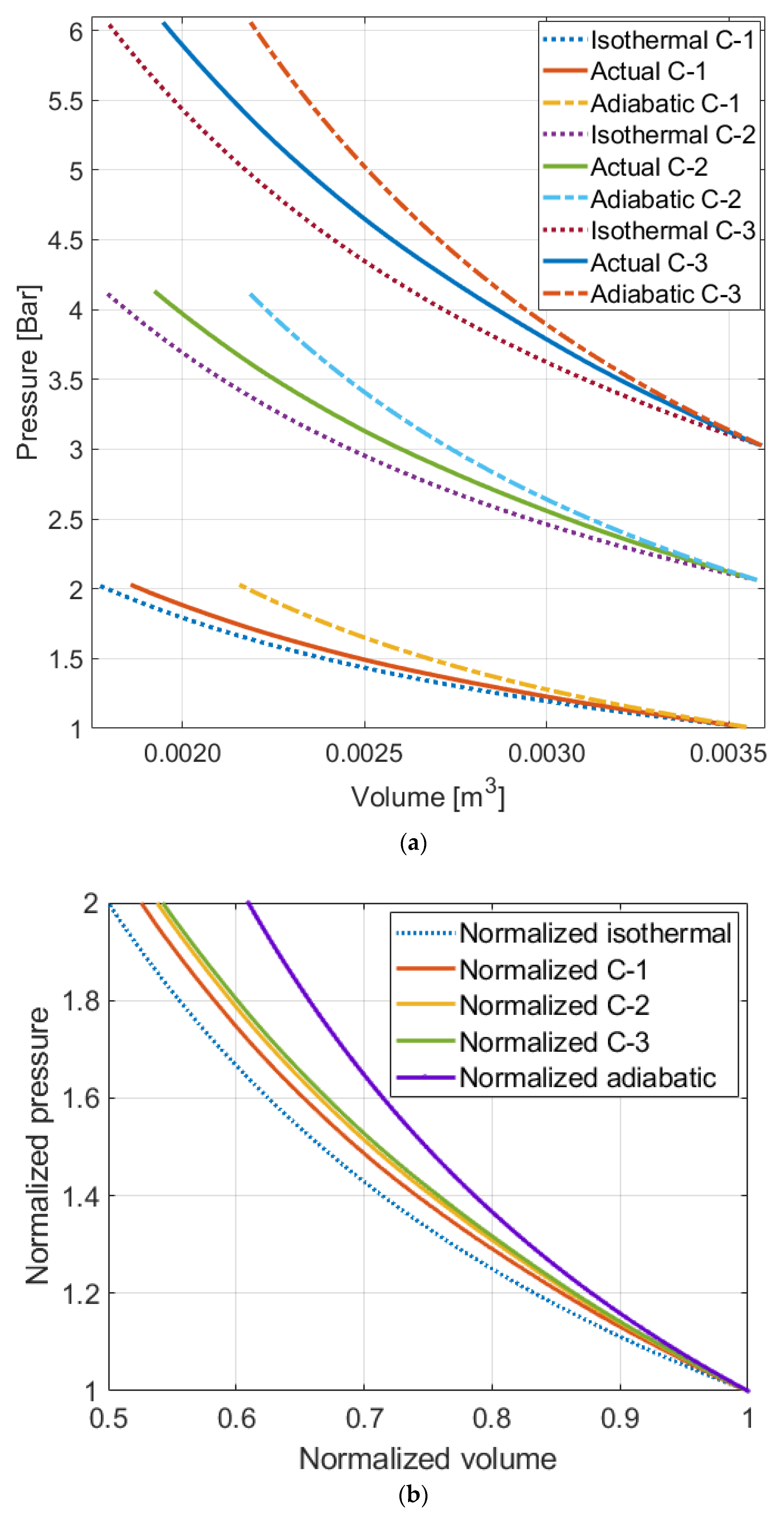
The results show a trend over the tested cases. The higher the pressure level is, the less efficient the process becomes. When pressure is different, one of the phenomena that occurs is gas dissolution. Therefore, to determine whether the dissolution contributes to the variation in the efficiencies at different pressure levels and to properly evaluate the liquid piston performance, the gas dissolution issue needs to be investigated.
The idea that dissolution takes place during compression can be supported by computing polytropic indices of the compression trajectories. Polytropic indices can be calculated by using Equation (7) and pressure and volume data at two different points. The initial point is used for
and
, and the halfway point of the process for
and
.
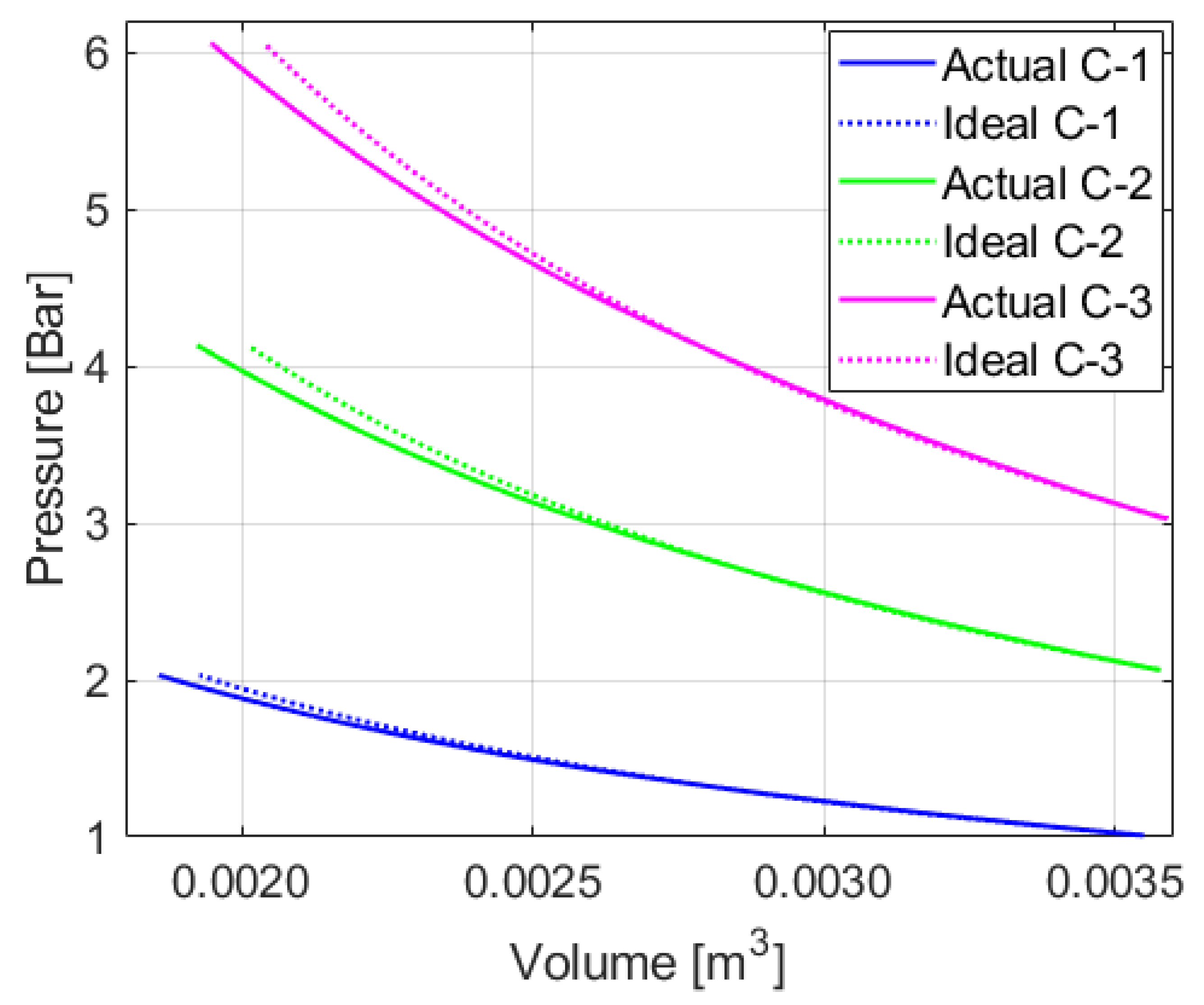
Figure 7 displays the actual profiles plotted with experimental data and ideal polytropic process profiles from the calculated polytropic indices.
There are mismatches between the actual compression profiles and the ideal polytropic process profiles. Although choosing another point for
and
can adjust the shape of the profiles, it does not make them perfectly overlap the actual profiles. A change in air mass during the process can cause this issue. One factor that brings about air mass change may be leakage because the system is not perfectly leakage-free. In order to identify how significant the leakage issue is, extra tests are performed. The system performs a compression stroke and holds the pressurized state for some extended period for the initial pressure of 1, 2, and 3 bars. Once it becomes thermally stable after 2 min from the beginning of the process, there is no more notable pressure drop, due to cooling down of the air. After that, the pressure drop per minute is less than 0.01 bar, which is negligibly small for the elevated pressure level achieved. The pressure drop ratio per minute is, at most, 0.15% among the collected data. The tested pressure is even higher than the maximum pressure of interest in the main experiments. For the lower-pressure cases, the pressure drop is even smaller. Provided that a single compression stroke takes less than 20 s in this work, the mass loss during compression due to leakage is negligible. The measured gas properties and plots of the data to support this assumption are summarized in the figures and tables in
Appendix A.
Another factor that may cause the mismatch is gas dissolution. Because most of gas dissolution takes place within a very short time in such a liquid-based pressurized system [
35], the compression time in this work is long enough for the gas dissolution. Thus, it is reasonable to conclude that the dissolution takes place during the process, and it results in the mismatch between the actual profiles and generated polytropic process profiles. Therefore, although polytropic index is a straightforward indicator that shows how efficient a process is, it should not be used in evaluating the efficiency of compression performed with a liquid piston or a similar system that contains a liquid and a gas.
To determine whether the dissolution is a primary factor that causes the efficiency differences of the processes, the gas solubility changes are estimated. There are two parameters that determine air solubility in water: temperature and pressure. However, Henry’s law given in Equation (11) only includes a pressure variable. Therefore, it is difficult to directly employ Equation (11) to estimate how the air mass changes during the process. Thus, simplified estimations are made in the current work with solubility data available on [
36] and Henry’s law. Two opposing cases are examined based on two different assumptions. First, temperature change is not considered. Owing to the high specific heat of water and the short compression time, the temperature of the water is assumed to be constant throughout compression. Second, the temperature change is counted. With the air pressure and temperature data, the corresponding solubility values at the beginning and the end of the compression can be attained from the solubility table of [
36]. For simplification, the measured temperature data are used in finding the corresponding solubility values. Neither of these cases perfectly simulate the actual circumstances of the system during the compression. However, by examining the two extreme cases, the overall trends can be glanced if there is a consistency.
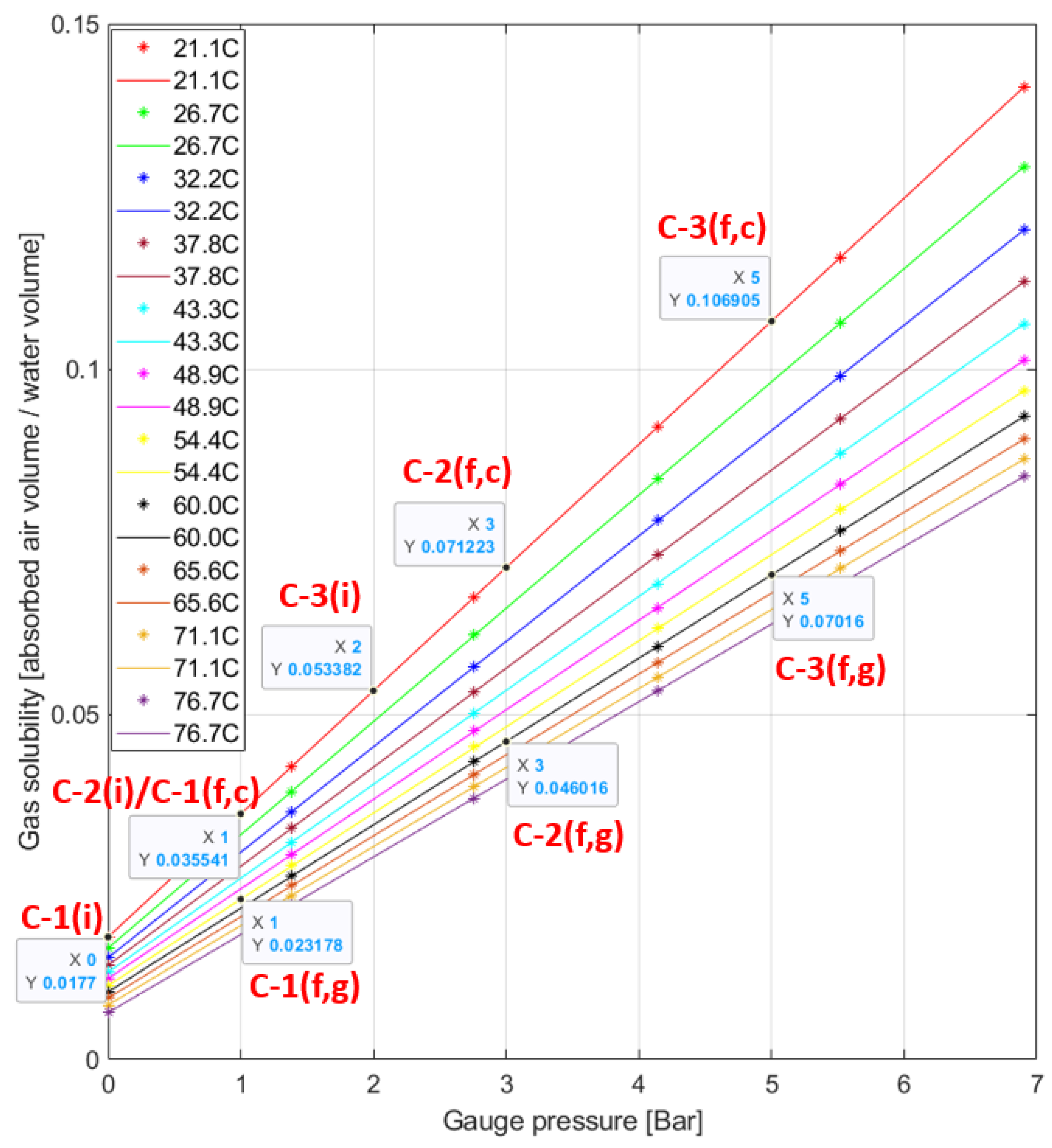
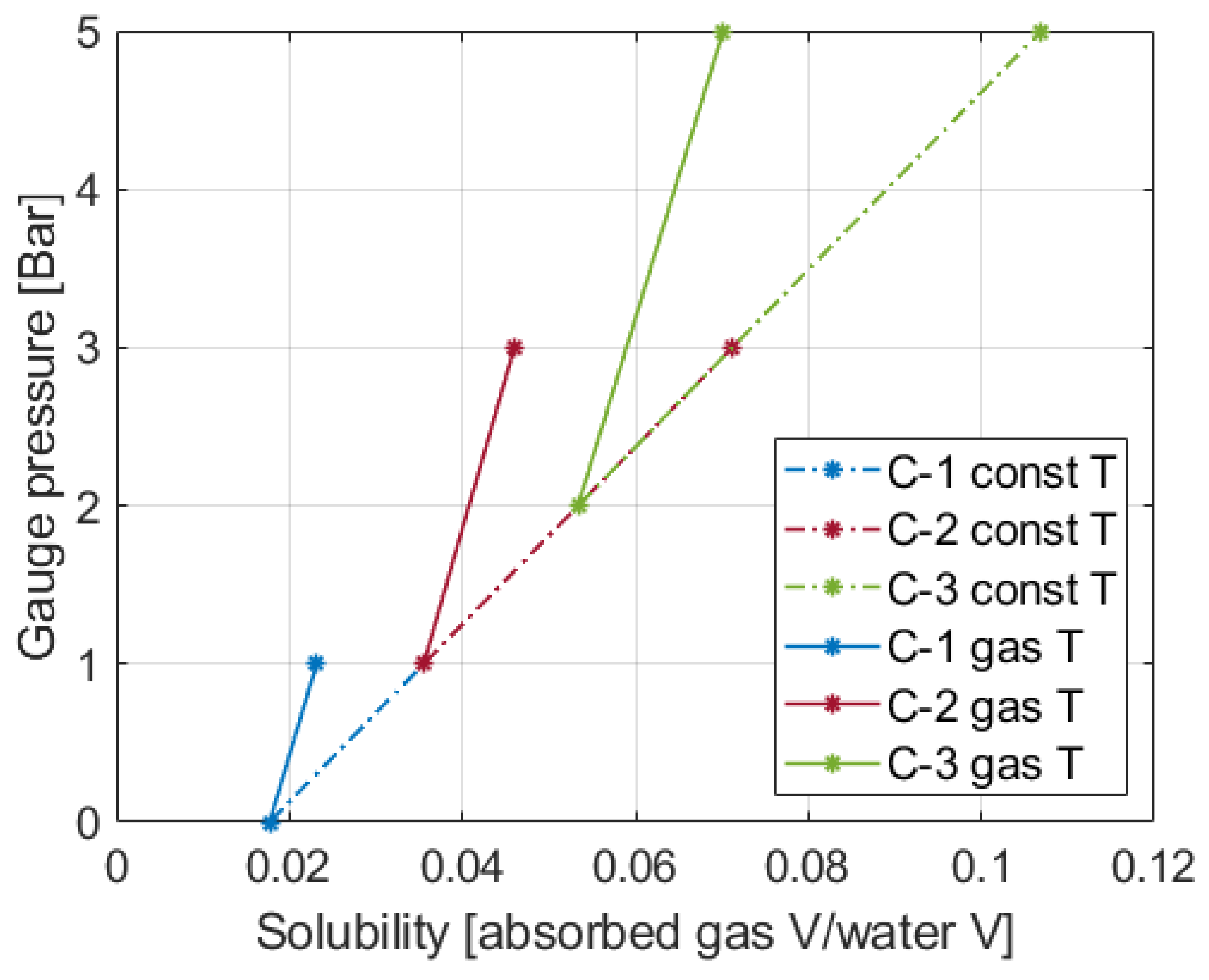
Figure 8 presents the solubilities of air into water at the initial and final points of compression starting from 1, 2, and 3 bars with the pressure ratio of 2. The plot is drawn based upon the data from [
36], and how the data are selected is explained in the
Appendix A with a supplementary figure. As the data are given in the gauge pressure, Compression-1, 2, and 3 start from 0, 1, and 2 gauge bar and end at 1, 3, and 5 gauge bar, respectively. For the final points, there are two points for each process. The points named const T in the legend box are for the constant-temperature assumption, and the points named gas T are for the second assumption that the system temperature changes the same as the gas temperature.
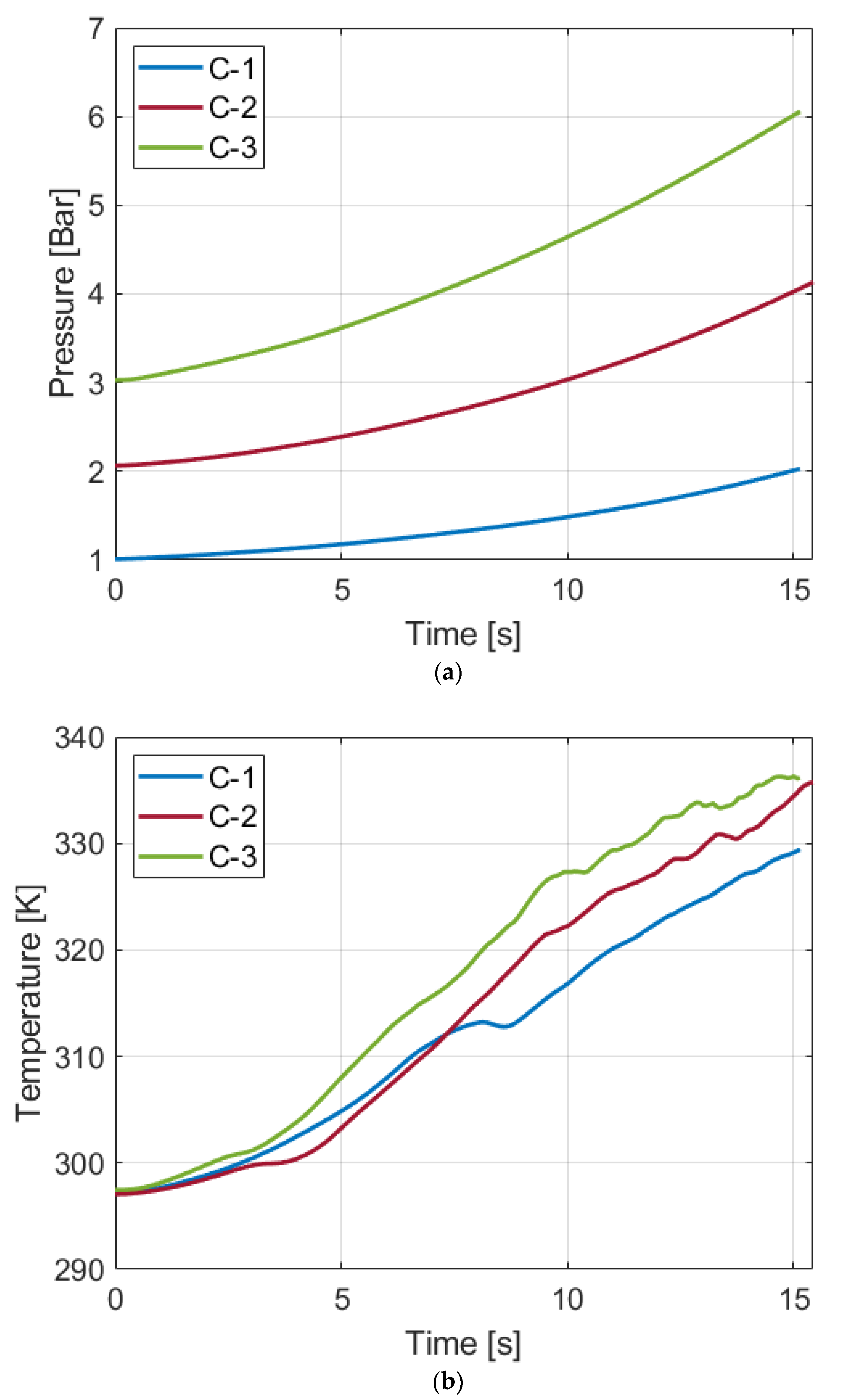
Table 4 summarizes the solubility at the marked points and the amount of increase from the initial points to the final points along with the reduced work input by the dissolution. If the constant temperature is assumed, the solubility increase of Compression-2 is twice as much as Compression-1. For Compression-3, the increase is three times as much as Compression-1. If the temperature is the same as the gas temperature, compared to Compression-1, increases of about 1.91 times for Compression-2 and 3.06 times for Compression-3 are observed, and a lower amount of air is absorbed due to the higher temperature. A similar trend of the proportional increase with the initial pressure is observed under both cases.
Regarding the reduced work input, the higher pressure leads to the greater decrease in work input due to an increased amount of the dissolved gas volume. For both cases, Compression-2 and 3 show roughly 4 times and 9 times the reduced work, compared to Compression-1. Considering that the compression work input of Compression-2 and 3 takes about 2 times and 3 times the work input of Compression-1, the ratios of the reduced work input to the compression work input of Compression-2 and 3 are about 2 and 3 times the ratio of Compression-1, respectively. Despite the greater reduced work of the processes starting from the elevated pressures, Compression-2 and 3 have lower isothermal efficiency than Compression-1. This implies that the gas dissolution is not the primary reason for the different isothermal efficiencies of the present work. Therefore, the results observed in this work of the higher isothermal efficiency of the compression with the lower initial pressure cannot be explained by air dissolution alone. A possible explanation is that air at higher pressure has a greater air mass in a fixed volume. Thus, it may change the motion of air during compression and affect the degree of convection. To accurately identify the full reason for the differences in the efficiency of processes with various initial pressures, microscopic investigation on the dynamics of air inside the chamber should be performed, which is beyond the scope of this work.
The air dissolution complicates the efficiency evaluation with Equation (8). This is because the dissolution compromises the work output. For an energy storage application, the compressed air has to be expanded to restore the energy. When the compressed air is removed from the compressor, the total mass of the compressed air leaving the chamber is less than the original mass before the compression due to the loss caused by dissolution. This will lead to a reduced energy restored in the discharging process. In other words, although the dissolution is beneficial for the isothermal efficiency on paper obtained using Equation (8), the actual efficiency based on how much work can be restored is different from the calculated value. Because each compression process has different dissolved amounts, the compromised work output may vary as well. To evaluate how much efficiency is compromised, the output work needs to be quantified. Because of the difficulty of predicting the exact expansion trajectory without empirical work, the volume of air after the expansion is quantified and compared by estimating the mass changes of the air. The ideal gas equation and the solubility data from [
36] enable the volume quantification. The absorbed gas volume varies with the water volume.
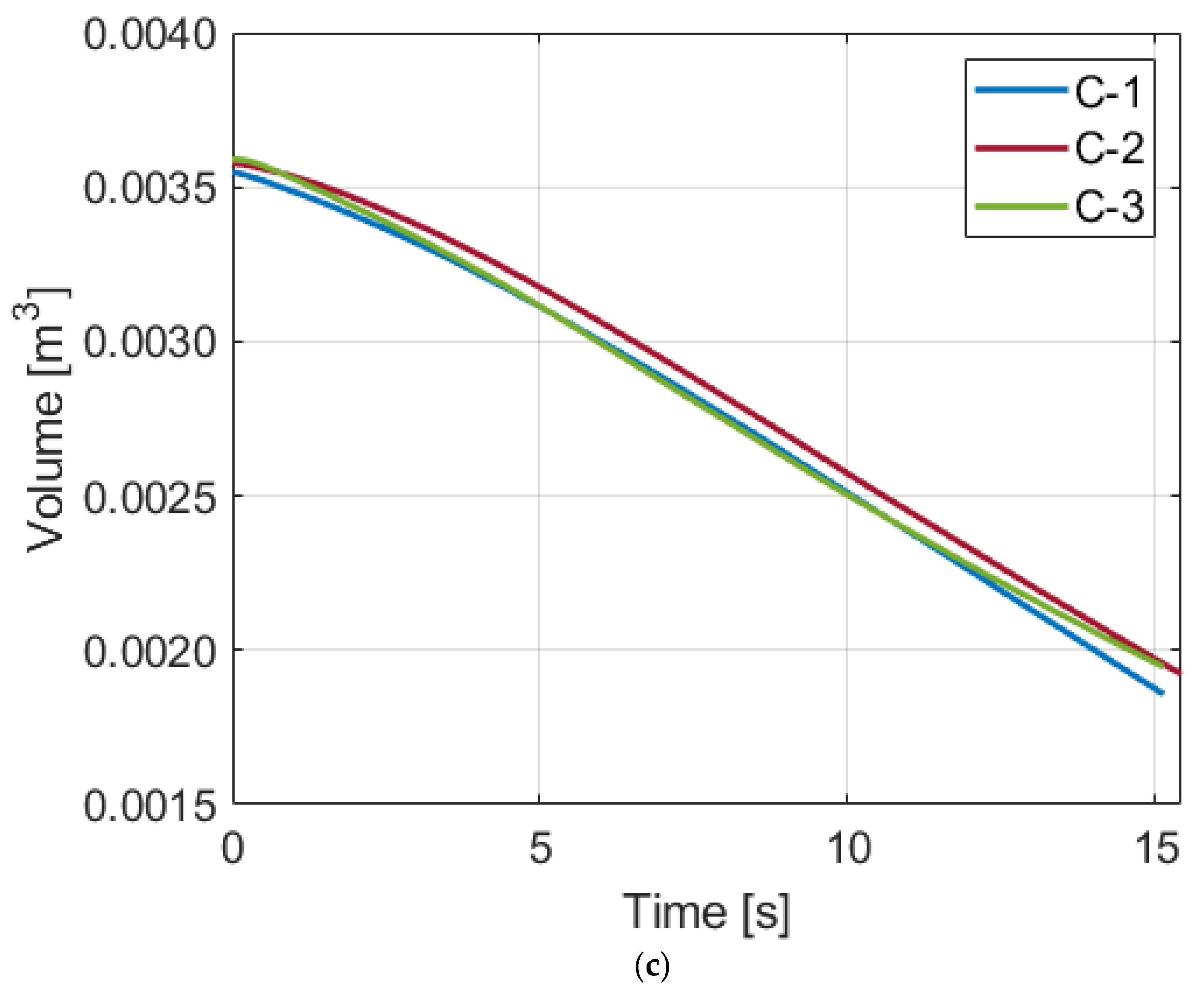
Figure 9 displays the mass of the air that is not dissolved into the water and remains as a gas above the water. This estimation needs water volume in a system and air solubility. For water volume, the volume is assumed to be the same as the initial air volume in the chamber. For solubility changes, the solubilities under the constant-temperature assumption are used. To perform a proportional comparison of the three processes, the mass and pressure values are normalized by dividing by the initial values. At the target pressure, the ratios of the mass to the initial mass for Compression-1, 2, and 3 are 94.57%, 88.96%, and 83.81%, respectively. After expansion up to the initial pressure of the pre-compression status, the restorable volume will also be 94.57%, 88.96%, and 83.81% of the initial volume, respectively. This means that an increased solubility at the higher pressure results in a greater compromise in the work output unless the expansion takes place in the same chamber and the dissolved air is fully restored.
Separately, a significant change in the water volume also has an impact on the soluble amount.
Table 5 presents the variations in the air mass ratios with different water volumes.
When the water volume is smaller, the absolute amount of the dissolved air mass is smaller, leading to a larger air mass ratio. In contrast, when the water volume is larger, the ratios become smaller. Furthermore, the differences in the ratios between the processes become greater, which results in a significantly lower value for Compression-3 when the water volume is 1.5 times the initial air volume. Hence, using the minimum amount of water will help secure the restorable work output. Although those numbers are generated based on a simple simulation model, it implies how the volume of water can affect the system efficiency.
The dissolution is advantageous for the isothermal compression efficiency on paper calculated using Equation (8) because it reduces the volume of the air inside the chamber as the pressure increases. Thus, the process with higher initial pressure benefits more from an increased dissolution. However, despite a greater dissolution, the higher pressure shows the lower efficiency. On top of the lower efficiency at the higher pressure, the greater dissolution will lead to a lower restoration of the output energy. Hence, for the overall system including the energy discharging stage considered, there will be an even larger gap in the actual efficiencies than their calculated values. Although the higher-pressure process has the advantage of having a higher power to perform the charging/discharging process faster, it shows compromised performance with regard to overall efficiency. However, this should not depreciate the efficacy of the liquid piston compressor. The main strength of the liquid piston compressor is that various heat transfer enhancement techniques can be easily integrated regardless of the dissolution.
There are limitations to the current study. As referred earlier, the overall temperature of the air and the dissolved air mass cannot be obtained with the ideal gas equation, because each one requires the value of the other one. As the simplified estimation of the total solubility changes is used for the amount of the dissolved air, errors from the simplification may be inevitable. In addition, there is room for improvement in efficiency analysis. The efficiency, , is calculated by comparing the isothermal work, , and the actual work, . In the current study, the mass change is not considered in calculating , while is calculated from the data collected from the real experiment where dissolution takes place. If the isothermal profile reflects the mass change as well, it could possibly lead to a different conclusion.
Nevertheless, to the author’s best knowledge, this is the first work to experimentally investigate how the initial pressure level affects the isothermal compression efficiency when the pressure ratio is the same and to determine whether the gas dissolution is associated with the different compression efficiencies. Furthermore, by testing compression at different initial pressures, an initial attempt is made to simulate the applicability of the liquid piston compressor to a multi-stage compression system. The examined pressure ranges are quite limited to lower pressures because Henry’s law is not applicable if the pressure goes up high enough [
40]. At higher pressures, the trend may be different from the evaluated range of the current work. Compression at much higher levels to study how the trend changes will be an interesting topic for future research.
5. Conclusions
The impacts of different initial pressures on liquid piston compression are investigated in this paper by performing compressions starting from 1 bar, 2 bars, and 3 bars with the same pressure ratio of 2. At elevated pressures, the processes result in higher temperature increases over the compression that starts from the atmospheric pressure, whereas there is no notable difference in temperature rise between compressions starting at 2 bars and 3 bars. Regarding compression efficiency, the compressions at higher pressure lead to lower isothermal efficiencies. Experimental results have shown that the isothermal efficiencies of Compression-1, 2, and 3 processes are 89.1%, 85.0%, and 83.5%, respectively. To identify the cause of the efficiency differentiation, gas solubility changes of the three compression processes are quantified based on simple estimation models. Because the gas solubility is proportional to the pressure, the higher initial pressure process has more gas absorbed in the water. Despite the increased solubility, which is beneficial for isothermal efficiency calculations on paper, the compression processes at higher pressures have shown lower efficiencies than the compression starting from the atmospheric pressure. Hence, the dissolution is not the primary reason for the lower compression efficiency at higher initial pressure. Because the air behaves differently during compression depending on the pressure and the mass in the chamber, a comprehensive analysis on the dynamics of the air in the chamber may help clarify the cause of the phenomenon.
Although the dissolution is advantageous for the isothermal efficiency calculations on paper, the dissolved mass may further compromise the overall system efficiency than the calculated values. This is because the isothermal efficiency can be calculated in a conventional manner without considering air mass changes caused by the dissolution. For a real application that includes the energy discharging process, dissolved mass results in a reduced volume of air after expansion and it leads to less energy output. Assuming the system contains the same water volume as the initial air volume, the masses of the air after compressions starting at 1, 2, and 3 bars are estimated to be 94.57%, 88.96%, and 83.81% of the initial masses prior to the compression, respectively, which further compromises the system efficiency than the calculated compression efficiencies discounting dissolution. In conclusion, even though the dissolution is not a major factor that results in efficiency decrease at a higher pressure level on paper, it actually further degrades the overall system efficiency.