An Overview of the Molten Salt Nanofluids as Thermal Energy Storage Media
Abstract
:1. Introduction
- (i)
- Improved specific heat, latent heat, and thermal conductivity when compared to those of the base fluid alone.
- (ii)
- Stability over time of the nanoparticles in the molten salt.
- (iii)
- Thermal stability and evidence of no significant deterioration under thermal cycling conditions.
- (iv)
- Reduced increase of the viscosity caused by the inclusion of the nanoparticles, which allows for efficient pumping within the thermal management systems.
- (v)
- Chemical compatibility with the container materials and absence of erosion in the fluidic systems.
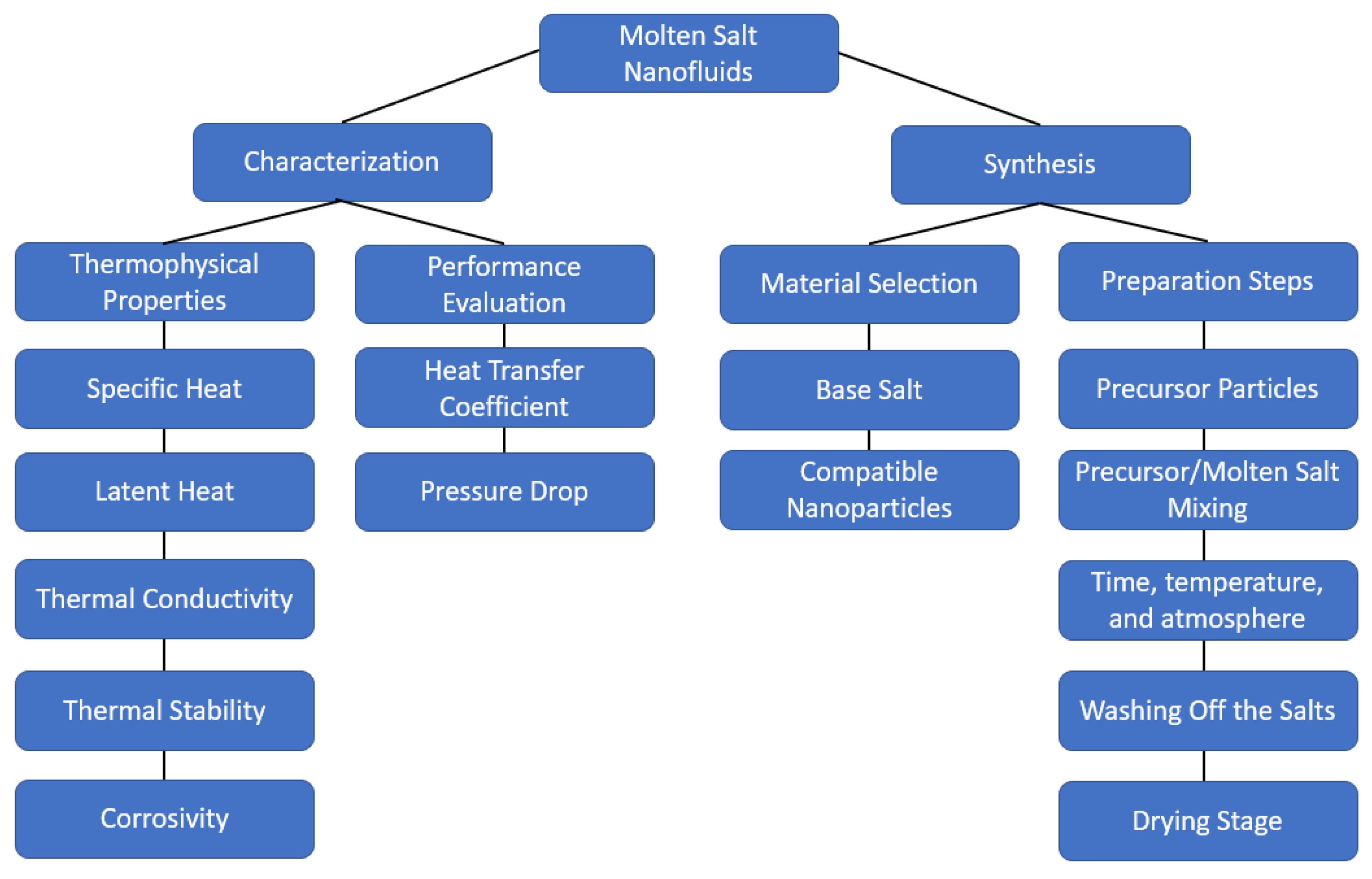
2. Types of Molten Salt Nanofluids
3. Preparation Methods
- (i)
- Weighing and mixing of the salt and nanopowders.
- (ii)
- Water dissolution of the base salt.
- (iii)
- Stabilization of the nanoparticles by ultrasonication procedures.
- (iv)
- Dehydration by evaporation of the water content.
3.1. Liquid Dispersion
3.2. Mechanical Dispersion
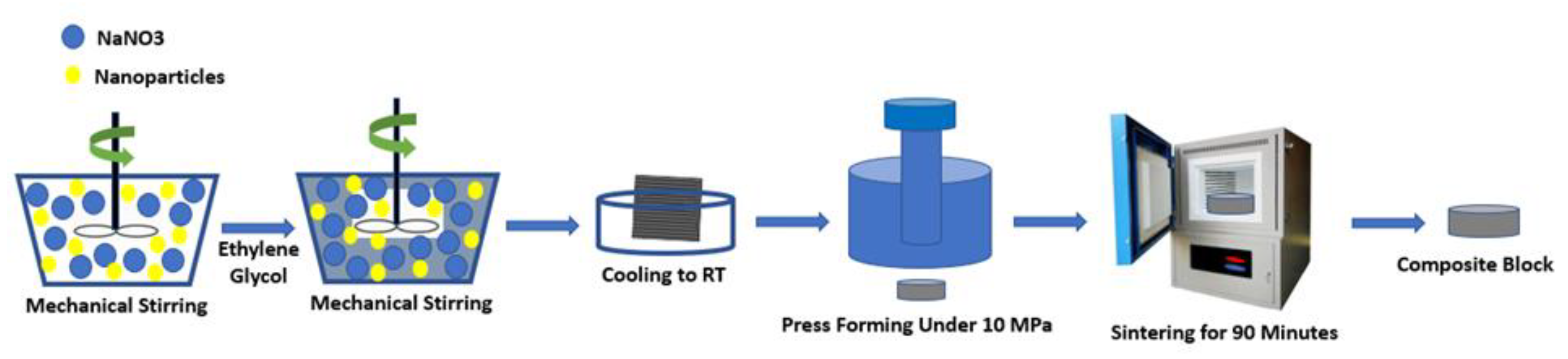
3.3. In Situ Production
4. Thermophysical Properties
4.1. Thermal Conductivity and Diffusivity
4.2. Viscosity
4.3. Latent Heat
4.4. Thermal Stability
4.5. Corrosivity
5. Specific Heat Enhancement Mechanisms
5.1. Salt Composition Ratio
5.2. Size and Shape of the Nanoparticles
5.3. Concentration of the Nanoparticles
5.4. Preparation Methods
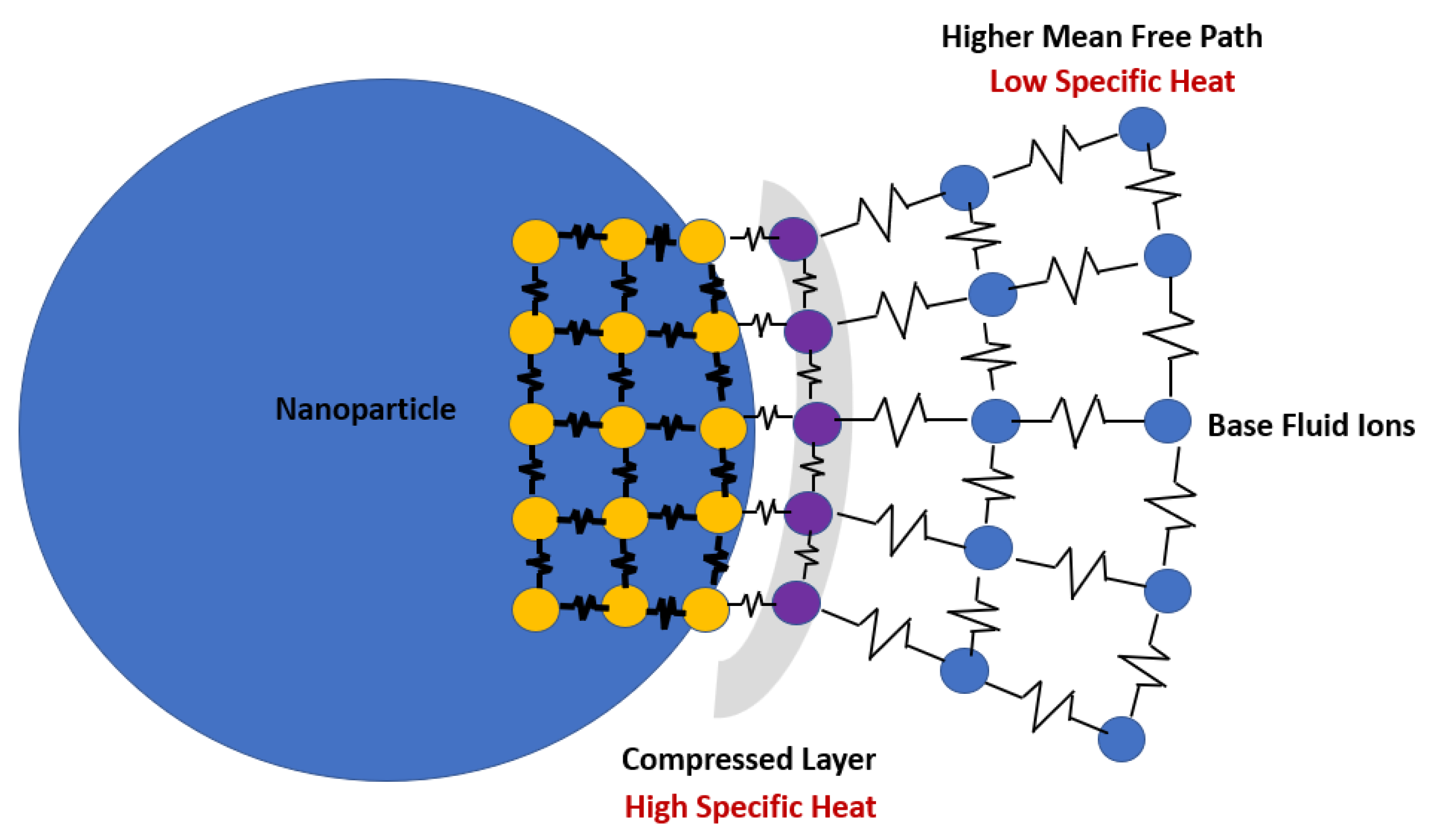
5.5. Interfacial Thermal Resistance
5.6. Compressed Liquid Layer
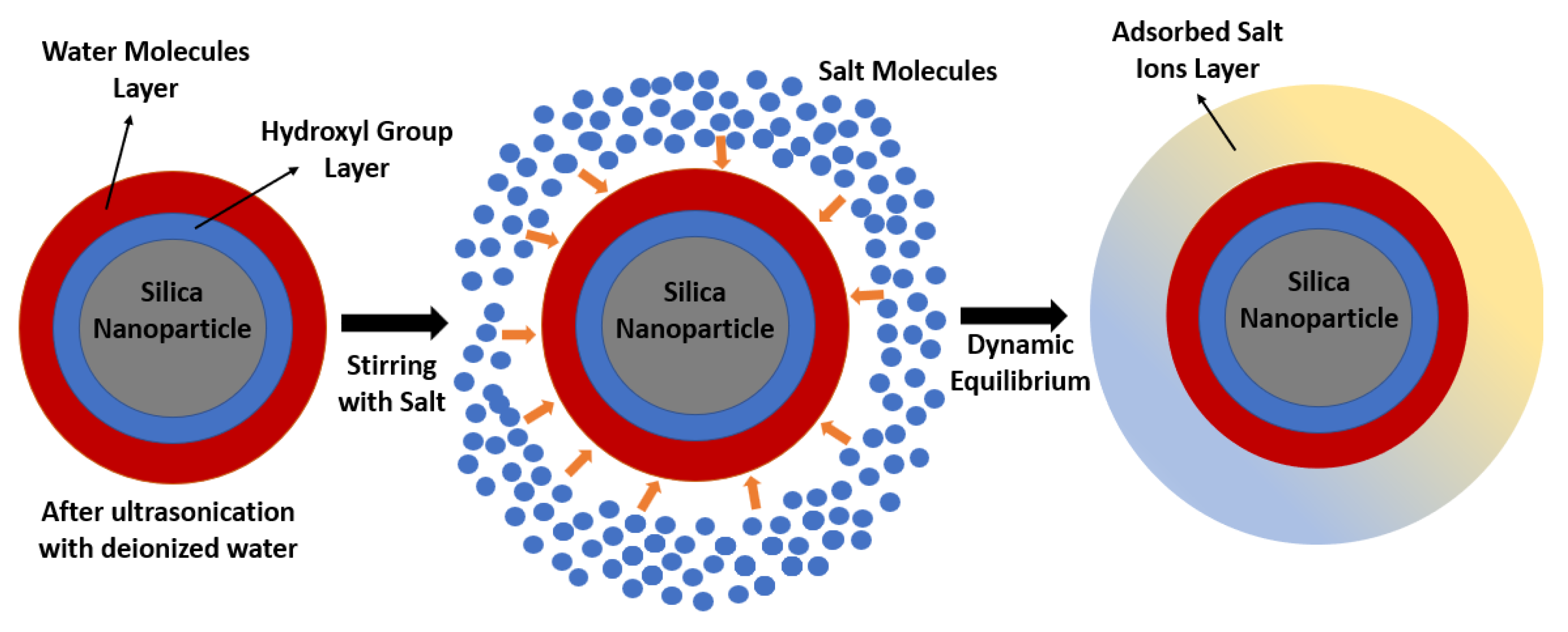
5.7. Ionic Exchange Capacity
5.8. Secondary Nanostructures
5.9. Cloud Nuclei
6. Agglomeration and Sedimentation Over Time
7. Applications in Concentrated Solar Power Plants
7.1. Concentrated Solar Power Plants
7.2. Molten Salt Nanofluids in Concentrated Solar Power Plants
- (i)
- Sensible heat storage that regards the heat that is stored through the addition of kinetic energy to a material, enhancing its temperature without any phase change. The energy is absorbed with the increase of the temperature of the material heat charge—and is released when the material cools down—heat discharge. The sensible heat can be determined by Equation (6):
- (ii)
- Latent heat storage that concerns the thermal energy storage through the phase change without any temperature variation. The operation of the latent heat materials is based on storing the thermal energy during the melting process (heat charge) and on releasing the thermal energy in the solidification process (heat discharge). The quantity of energy that is exchanged can be calculated by Equation (7):
- (iii)
- Thermochemical energy storage that deals with the energy harvested by the breaking-up of the chemical bonds during the reversible chemical reactions and sorption systems. It has the highest storage capacity but possesses some limitations that hinders its large-scale applicability, such as the chemical stability, durability, and the need for separate storage systems.
7.3. Modeling and Practical Case Studies
7.4. Life Cycle Assessment
- (i)
- definition of the involved system.
- (ii)
- collection of data of interest.
- (iii)
- risk characterization and impacts quantification.
- (iv)
- interpretation of the results.
8. Potential Geothermal Applications
9. Limitations and Future Prospects
- The available data on the properties of the inorganic salts and the nanoparticles is still rather scarce. The specific information of the nanoparticles size and morphology and consequent effect on the final characteristics of the molten salt nanofluids should be addressed in-depth.
- The scalability and reproducibility of the preparation methods of the molten salt nanofluids should be further studied. The most used method to produce molten salts nanofluids usually requires the employment of large amounts of water and powered ultrasonication. This strongly hinders the large-scale industrial possible uses and the reproducibility of the preparation method. Hence, some innovative methods are required to tackle these limitations, such as the mixing and the uniform dispersion of the nanoparticles within the liquid salts under high-temperature scenarios.
- The underlying mechanisms for the strong enhancement of the specific heat capacity and thermal conductivity of the molten salt nanofluids should be better understood. For instance, the arrangement/packaging of the ions in the secondary nanostructures in the compressed layer as well as in the percolation network is of relevance. Additionally, the nucleation, growth and assembly of the secondary nanostructures are mechanisms that required further analysis. The correlations between the structure and the properties for these secondary nanostructures should also be numerically modeled and validated in a laboratory environment.
- The long-term stability of the molten salt nanofluids should be assessed deeper. Some studies revealed that the nanoparticles in a molten salt agglomerate and settle only after a few hours. A deeper understanding of the interaction mechanisms between the molten salts and nanoparticles at a molecular level is needed to proceed in the search of the most stable dispersions. The employment of auxiliary dispersing techniques, such as sonication or vigorous stirring to break up the agglomerates, may be the secure pathway to follow. Additionally, the use of surface modification functionalized nanoparticles may appreciably diminish their clustering into the molten salt.
- It is suggested to obtain a correlation between the ion adsorption onto the surface of the nanoparticles and the long-term stability of the molten salt nanofluids. For instance, in the case of nitrate salt nanofluids, the adsorption of the nitrate ions onto the surface of the nanoparticles induces a strong electrostatic repulsive force that reduces the possibility of agglomeration and settling.
- Innovative numerical models and experimental methods should be implemented to determine the behavior between the molten salt nanofluids and an electrically charged heating surface at high temperatures. In this direction, sophisticated equipment operating at temperatures superior to 500 °C will need to be developed to obtain improved knowledge of the involved transport mechanisms.
- The measuring methods of the thermophysical properties of the molten salts nanofluids should be standardized to allow a reliable comparison of results between the different research teams. The methods usually encountered in the literature of the field to measure the specific heat capacity, latent heat capacity, and dynamic viscosity of the molten salts nanofluids made any concluding comparison or tendency of the intrinsic properties of certain suspensions difficult.
- It is recommended to develop methods and equipment suitable to the high-temperature conditions and to the corrosive character of the molten salts. For instance, the current thermal conductivity measuring procedures are not appropriate for molten salts. A similar limitation occurs in measuring the size of the nanoparticles, given that the indirect measurements through scanning electronic microscopy and other techniques, or the implementation of in-house developed protocols and apparatuses have been the followed so far. However, these routes do not present a high level of accuracy and representativeness, hindering the comparison of results.
- The influence of the nanoparticle size in the specific heat of the molten salt nanofluids should be clarified. The published articles do not usually report the initial size and morphology of the nanoparticles, although their size or agglomerated clusters in the final nanofluids is often summarized. The size of the nanoparticles is commonly measured by SEM imaging observation or, alternatively, by DLS in the solid state, and not in the real state of the nanofluids, thus constraining the evaluation of the impact of the size of the nanoparticles on the specific heat of the molten salt nanofluids.
- The viscosity of the molten salt nanofluids should be further investigated, given that the rheological properties are of vital importance for possible large-scale ends. In this direction, some feasible strategies and solutions should be undertaken to prevent an excessive viscosity. The latter can provoke an increment in the pressured drop of the systems and, hence, an extra pumping power will be needed for the systems to operate.
- There should be more future experimental works on the corrosion concern of the molten salts nanofluids, since the corrosive nature of the salts can induce severe erosion of the heat pipes, heat exchangers, retention tanks, and other equipment with the continuous motion of the nanofluids. Furthermore, it is of relevance to achieve a better knowledge about the corrosion potential modification of the salts caused by the inclusion of the nanoparticles.
- Further specific studies should be carried out according to the potential application field that include corrosion resistance evaluation and inherent costs of the salt purification to conclude whether the carbonate salt nanofluids are or not more suitable than the binary nitrate and ternary chloride salts for the same intended applications.
- It is suggested to explore the applicability of the soft computing techniques for modelling the specific heat capacity and other thermophysical properties of the molten salt nanofluids. These techniques should consider the effect of the working temperature and concentration, size, and intrinsic specific heat of the nanoparticles. Additionally, the computing techniques should rely on the modern machine learning prediction models and algorithms such as the optimized feed forward back propagation neural network and its corresponding ANN algorithm.
- More turbulent thermal convection flow modelling with molten salt nanofluids should be carried out. For instance, in the work performed by Harish et al. [140] the melting and turbulent heat transfer of potassium nitrate phase change material dispersed with hybrid nanoparticles of alumina–silica, alumina–titania, and alumina–multi walled carbon nanotubes inside a differentially heated rectangular enclosure was numerically studied. The impact of the high Rayleigh number and concentration of the nanoparticles on the transient melting behavior and melt pool temperature distribution are compared with that of the pure phase change material. Additionally, similar numerical simulations to those reported in [141,142,143] should be further performed considering molten salt nanofluids.
- Efforts should be made to accurately evaluate the economic impact of the use of molten salt nanofluids, given that the eventuality of implementing these fluids depends on its final cost. For instance, the enhancement of the values of the thermal properties and the required quantity of nanoparticles to reach those values should be studied. It should be emphasized that the incorporation of nanoparticles in molten salts can appreciably enhance the specific heat capacity, but the overall system cost could be unacceptable.
- The further performing of Life Cycle Assessment analysis for the molten salt nanofluids is highly recommended. Considering the uncertainties associated with the properties, transportation, manufacturing of materials, disposal, corrosion, and pumping power requirements, the environmental impact of the nanofluids may differ very much. The dynamic viscosity and manufacturing procedures are among the most relevant factors to determine the impact of the incorporation of nanoparticles according to the Life Cycle Assessment analysis.
- It is of relevance to conduct further quantitative studies about the thickness of the compressed liquid layer needed for a more accurate evaluation of the specific heat and latent heat changes for single salt, multi-salt mixtures, and nanoparticles. Hence, additional measurements are required to better understand the underlying mechanisms that cause alterations in the latent heat of the molten salt nanofluids and, furthermore, to the validation of these nanofluids as suitable media for solar thermal applications and latent heat storage systems involving encapsulation.
- Other possible applications of the molten salt nanofluids as thermal energy storage materials should be explored, including the conventional power generation in coal and gas power generation plants, nuclear power industry, generation of power from geothermal resources, industrial process heating, and desalination stations, among others.
- It is also recommended to evaluate other possible applications of the molten salt nanofluids prepared by the one-step method, such as molten salt batteries for electrical energy storage, processing of chemicals, refining of metals, production of ceramic nanoparticles, bio, and medical purposes, among others.
- It is suggested to carry out further experiments in the enhanced oil recovery area of research with molten salt nanofluids, rather than only with aqueous and oil-based nanofluids. The nanofluid flooding with molten salt nanofluids with the addition of silica, carbon, and zirconia nanoparticles should be further tested in pilot projects and oil fields. The enhanced thermophysical properties are promising for shale oil recovery, but bringing some more light on the enhanced oil recovery impacting factors is needed, such as wettability modification, interfacial tension reduction, and recovery magnitude through both laboratory experiments and in-situ practical situations.
- The assessment should be further elaborated for the systems composed of carbonate salts and alumina nanoparticles with various characteristics to elucidate which one is the most suitable in terms of environmental benevolence and sustainability.
10. Conclusions
- Nowadays, the molten salt nanofluids present a great potential for improving the heat transfer capability and energy storage of thermal management and conversion systems, with the main favored industry for the development of this solution being the solar thermal power one.
- Although many considerable advances took their place so far, the knowledge stage is still on its infancy and further coordinated efforts should be made by the scientific community to fully comprehend the features of the molten salt nanofluids. In the medium term, the fully development of these thermal fluids would seriously impact concentrated solar power science and technology.
- In terms of carbonate molten salts nanofluids, the synthesis methods that do not imply the water dissolution of the carbonates are more suitable than the wet methods, given that these ones may lead to the chemical instability of the carbonates and to a heterogeneous mixture in water because of the different solubility. Additionally, this lack of homogeneity may be misleading since it usually conducts to a non-eutectic composition, which may be mistakenly taken as a specific heat capacity enhancement.
- The stability over time of the nitrate and carbonate molten salts nanofluids is usually poor, and the verified thermophysical properties improvement decreased within 3 to 4 h, or even less. However, the mechanical redistribution of the nanoparticles and the consequent recovery of the improved specific heat value is viable. This evidence highlights the need for potential instability solution procedures, such as vigorous mechanical mixing, pumping, and stirring.
- The accurate measurement of the thermophysical characteristics of the molten salt nanofluids requires well-defined methods, statistical analysis, cross-verification, and reproducibility of methods and results.
- A two-fold corrosivity reduction of stainless-steel was found for the (Li,Na,K)2CO3 molten salt with alumina nanoparticles, as compared to the molten salt itself at 600 °C. This corrosion rate decrement is mainly caused by the interaction of the alumina nanoparticles with the stainless-steel and the production of a mixed oxide containing aluminum with a counter-corrosion effect. These findings are consistent with the previous corrosion resistance inferring studies on nitrate salts nanofluids and suggest that some nanoparticles will migrate from the nanofluid to the corrosion layer.
- The published studies on the nanoparticle induce erosion of the thermal storage systems argued that the added nanoparticles did not cause an appreciable deterioration in the equipment, even in these cases where agglomeration occurred.
- The working temperature using the thermal energy storage medium in the solid and liquid phases and the thermal energy storage method using the sensible heat mechanism only or, alternatively, along with the latent heat mechanism, must be taken into account in the selection of a thermal energy storage medium exhibiting maximized thermal energy storage capability.
- When the thermal energy storage capability is the goal, the performing of intense thermostatic and thermal shock stability tests should be seriously considered to infer if the specific heat of the molten salt nanofluids suffer any considerable deterioration after being subjected to those tests.
- A relevant concern about the addition of phase change materials in molten salt nanofluids is the non-congruent melting process that diminishes the phase change reversibility and, consequently, the thermal energy storage capability. Hence, before a nanomaterial can be used as a phase change material, the characterization of its thermal features should be carried out, such as the temperature at which the phase change process occurs, sub-cooling, the rate of nucleation, and the enthalpy trend.
- The ternary molten salts, including the Hitec and Hitec XL, were found to be very suitable for operating in the concentrated solar power facilities because these salts had low melting points, which decreased the thermal dispersions. The nanoparticles that gave the highest increments in the thermophysical properties of the molten salt nanofluids were found to be titania, silica, alumina, and magnesia. Nevertheless, the multi-walled carbon nanotubes and the single-walled carbon nanotubes were also widely used for increasing the thermal storage density.
- The addition of alumina and silica nanoparticles can decrease the rate of corrosion of the molten salt nanofluids by 50% or more. Additionally, another suitable counter-corrosion measure is the reduction of the chromium content in the molten salts.
- The usage of NaNO3 and KNO3 in a two-tank storage configuration was found to be the most employed thermal energy storage technological approach with enhanced efficiency.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, S.U.S.; Eastman, J.A. Enhancing thermal conductivity of fluids with nanoparticles. In Proceedings of the International Mechanical Engineering Congress & Exposition, San Francisco, CA, USA, 12–17 November 1995. [Google Scholar]
- Eastman, J.A.; Choi, U.S.; Li, S.; Thompson, L.J.; Lee, S. Enhanced Thermal Conductivity through the Development of Nanofluids. MRS Online Proc. Libr. 1996, 457, 3–11. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Siddiqui, S.; Sreedhar, I.; Parameshwaran, R. Molten salts: Potential candidates for thermal energy storage applications. Int. J. Energy Res. 2022, 46, 17755–17785. [Google Scholar] [CrossRef]
- Shin, D.; Banerjee, D. Specific heat of nanofluids synthesized by dispersing alumina nanoparticles in alkali salt eutectic. Int. J. Heat Mass Transf. 2014, 74, 210–214. [Google Scholar] [CrossRef]
- Isaza-Ruiz, M.; Osorio, F.O. Thermal Properties of Hitec Salt-Based Nanofluids Synthesized By New Two-Step Method. Procedia Environ. Sci. Eng. Manag. 2021, 8, 147–155. [Google Scholar]
- Chen, M.; Shen, Y.; Zhu, S.; Li, P. Digital phase diagram and thermophysical properties of KNO3-NaNO3-Ca(NO3)2 ternary system for solar energy storage. Vacuum 2017, 145, 225–233. [Google Scholar] [CrossRef]
- Peng, Q.; Ding, J.; Wei, X.; Jiang, G. Thermodynamic Investigation of the Eutectic Mixture of the LiNO3-NaNO3-KNO3-Ca(NO3)2 system. Int. J. Thermophys. 2017, 38, 142. [Google Scholar] [CrossRef]
- Bauer, T.; Pfleger, N.; Breidenbach, N.; Eck, M.; Laing, D.; Kaesche, S. Material aspects of Solar Salt for sensible heat storage. Appl. Energy 2013, 111, 1114–1119. [Google Scholar] [CrossRef]
- Du, L.; Tian, H.; Wang, W.; Ding, J.; Wei, X.; Song, M. Thermal Stability of the Eutectic Composition in NaCl-CaCl2-MgCl2 Ternary System Used for Thermal Energy Storage Applications. Energy Procedia 2017, 105, 4185–4191. [Google Scholar] [CrossRef]
- Forsberg, C.W.; Peterson, F.; Zhao, H. High-Temperature Liquid-Fluoride-Salt Closed-Brayton-Cycle Solar Power Towers. J. Sol. Energy Eng. 2007, 129, 141–146. [Google Scholar] [CrossRef]
- An, X.-H.; Cheng, J.-H.; Su, T.; Zhang, P. Determination of thermal physical properties of alkali fluoride/carbonate eutectic molten salt. AIP Conf. Proc. 2017, 1850, 070001. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Miliozzi, A.; Crescenzi, T.; Torre, L.; Kenny, J.M. A new phase change material based on potassium nitrate with silica and alumina nanoparticles for thermal energy storage. Nanoscale Res. Lett. 2015, 10, 984. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Cabedo, P.; Mondragon, R.; Hernandez, L.; Martinez-Cuenca, R.; Cabedo, L.; Julia, J.E. Increment of specific heat capacity of solar salt with SiO2 nanoparticles. Nanoscale Res. Lett. 2014, 9, 582. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, D.; Liu, J.; Nian, Y.; Qiu, P. Development of a novel molten-salt filled with nanoparticles for concentration solar plants. In Proceedings of the 2nd IET Renewable Power Generation Conference—RPG 2013, Beijing, China, 9–11 September 2013. [Google Scholar] [CrossRef]
- Shankar, S. Thermal Cycling Effects of the Nanoparticle Distribution and Specific Heat of a Carbonate Eutectic with Alumina Nanoparticles. Master’s Thesis, Texas A&M University, College Station, TX, USA, 2011. [Google Scholar]
- Shin, D.; Banerjee, D. Enhanced thermal properties of SiO2 nanocomposite for solar thermal energy storage applications. Int. J. Heat Mass Transf. 2015, 84, 898–902. [Google Scholar] [CrossRef]
- Lu, M.-C.; Huang, C.-H. Specific heat capacity of molten salt-based alumina nanofluid. Nanoscale Res. Lett. 2013, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Cabedo, P. Mejora de las Propiedades Térmicas de sal Solar Mediante Adición de Nanopartículas; Proyecto del Máster en Eficiencia Energética y Sostenibilidad; Universitat Jaume I: Castelló, Spain, 2014. [Google Scholar]
- Hu, Y.; He, Y.; Zhang, Z.; Wen, D. Enhanced heat capacity of binary nitrate eutectic salt-silica nanofluid for solar energy Storage. Sol. Energy Mater. Sol. Cells 2019, 192, 94–102. [Google Scholar] [CrossRef]
- Akanda, M.A.M.; Shin, D. A synthesis parameter of molten salt nanofluids for solar thermal energy storage applications. J. Energy Storage 2023, 60, 106608. [Google Scholar] [CrossRef]
- Somani, V. Colloidal Stability of Magnetic Nanoparticles in Molten Salts. Master’s Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2010. [Google Scholar]
- Chieruzzi, M.; Kenny, J.M.; Miliozzi, A. Studio e Sviluppo di un Mezzo di Accumulo a Calore Latente a Media Temperatura (200–400 °C) Costituito da una Miscela di sali e Nanoparticelle; Report Ricerca di Sistema Elettrico Accordo di Programma Ministero dello Sviluppo Economico—ENE; Università Degli Studi di Perugia, Dipartimento di Ingegneria Civile e Ambientale: Perugia, Italy, 2013. [Google Scholar]
- Lasfargues, M. Nitrate Based High Temperature Nano-Heat-Transfer-Fluids: Formulation & Characterization. Ph.D. Thesis, The University of Leeds, Leeds, UK, 2014. [Google Scholar]
- Song, W.; Lu, Y.; Fan, Z.; Wu, Y. Preparation and Thermophysical Properties of Sodium Nitrate/Nanoparticle/Expanded Graphite Composite Heat Storage Material. Front. Energy Res. 2022, 10, 878747. [Google Scholar] [CrossRef]
- Sang, L.; Ai, W.; Wu, Y.; Ma, C. SiO2-ternary carbonate nanofluids prepared by mechanical mixing at high temperature: Enhanced specific heat capacity and thermal conductivity. Sol. Energy Mater. Sol. Cells 2019, 203, 110193. [Google Scholar] [CrossRef]
- Luo, Y.; Du, X.; Awad, A.; Wen, D. Thermal energy storage enhancement of a binary molten salt via in-situ produced nanoparticles. Int. J. Heat Mass Transf. 2017, 104, 658–664. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, X.; Li, Y.; Yu, G.; Xu, K.; Li, G. Effect of in-situ synthesized nano-MgO on thermal properties of NaNO3-KNO3. Sol. Energy 2018, 160, 208–215. [Google Scholar] [CrossRef]
- Chen, L.-C.; Ho, C.-C. Submerged arc spray synthesis of TiO2 nanoparticles with desired form sphericity using process characterization and optimization. J. Nanosci. Nanotechnol. 2008, 8, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Bang, I.C.; Onoe, J. Characteristic stability of bare Au-water nanofluids fabricated by pulsed laser ablation in liquids. Opt. Lasers Eng. 2009, 47, 532–538. [Google Scholar] [CrossRef]
- Lasfargues, M.; Bell, A.; Ding, Y. In situ production of titanium dioxide nanoparticles in molten salt phase for thermal energy storage and heat-transfer fluid applications. J. Nanopart. Res. 2016, 18, 150. [Google Scholar] [CrossRef] [PubMed]
- Schuller, M.; Little, F.; Malik, D.; Betts, M.; Shao, Q.; Luo, J.; Zhong, W.; Shankar, S.; Padmanaban, A. Molten Salt-Carbon Nanotube Thermal Energy Storage for Concentrating Solar Power Systems Final Report; Texas A & M University: College Station, TX, USA; Texas A & M Engineering Experiment Station: Bryan, TX, USA, 2012. [Google Scholar] [CrossRef]
- Saranprabhu, M.K.; Rajan, K.S. Magnesium oxide nanoparticles dispersed solar salt with improved solid phase thermal conductivity and specific heat for latent heat thermal energy storage. Renew. Energy 2019, 141, 451–459. [Google Scholar] [CrossRef]
- Bellos, E.; Tzivanidis, C.; Tsimpoukis, D. Thermal, hydraulic and exergetic Evaluation of a parabolic trough collector Operating with thermal oil and molten salt based Nanofluids. Energy Convers. Manag. 2018, 156, 388–402. [Google Scholar] [CrossRef]
- Shin, D. Molten Salt Nanomaterials for Thermal Energy Storage and Concentrated Solar Power Applications. Ph.D. Thesis, Texas A & M University, College Station, TX, USA, 2011. [Google Scholar]
- Ueki, Y.; Fujita, N.; Kawai, M.; Shibahara, M. Thermal conductivity of molten salt-based nanofluid. AIP Adv. 2017, 7, 055117. [Google Scholar] [CrossRef]
- Li, Z.; Cui, L.; Li, B.; Du, X. Enhanced heat conduction in molten salt containing nanoparticles: Insights from molecular dynamics. Int. J. Heat Mass Transf. 2020, 153, 119578. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Z.; Wang, W.; Ding, J. Effects of MgO Nanoparticles on Thermo-Physical Properties of LiNO3-NaNO3-KNO3 for Thermal Energy Storage. Energies 2021, 14, 677. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, Y.; Ouyang, L.; Sun, Q.; Cao, X.; Alelyani, S. Enhanced thermal properties of Li2CO3-Na2CO3-K2CO3 nanofluids with nanoalumina for heat transfer in high-temperature CSP systems. J. Therm. Anal. Calorim. 2017, 128, 1783–1792. [Google Scholar] [CrossRef]
- Wei, X.; Yin, Y.; Qin, B.; Wang, W.; Ding, J.; Lu, J. Preparation and enhanced thermal conductivity of molten salt nanofluids with nearly unaltered viscosity. Renew. Energy 2020, 145, 2435–2444. [Google Scholar] [CrossRef]
- Maxwell, J.C. A Treatise on Electricity and Magnetism; Oxford University Press: Oxford, UK, 1881; p. 1. [Google Scholar]
- Qiao, G.; Cao, H.; Jiang, F.; She, X.; Cong, L.; Liu, Q.; Lei, X.; Alexiadis, A.; Ding, Y. Experimental Study of Thermophysical Characteristics of Molten Nitrate Salts Based Nanofluids for Thermal Energy Storage. ES Energy Environ. 2019, 4, 48–58. [Google Scholar] [CrossRef]
- Cui, L.; Yu, Q.; Wei, G.; Du, X. Mechanisms for thermal conduction in molten salt-based nanofluid. Int. J. Heat Mass Transf. 2022, 18, 122648. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Du, X.; Wu, H. Experimental investigation on stability of thermal performances of solar salt based nanocomposite. Renew. Energy 2020, 146, 816–827. [Google Scholar] [CrossRef]
- Yu, Q.; Lu, Y.; Zhang, C.; Wu, Y.; Sunden, B. Research on thermal properties of novel silica nanoparticle/binary nitrate/expanded graphite composite heat storage blocks. Sol. Energy Mater. Sol. Cells 2019, 201, 110055. [Google Scholar] [CrossRef]
- Nithiyanantham, U.; Zaki, A.; Grosu, Y.; González-Fernández, L.; Igartua, J.M.; Faik, A. SiO2@Al2O3 core-shell nanoparticles based molten salts nanofluids for thermal energy storage applications. J. Energy Storage 2019, 26, 101033. [Google Scholar] [CrossRef]
- Nithiyanantham, U.; González-Fernández, L.; Grosu, Y.; Zaki, A.; Igartua, J.M.; Faik, A. Shape effect of Al2O3 nanoparticles on the thermophysical properties and viscosity of molten salt nanofluids for TES application at CSP plants. Appl. Therm. Energy 2020, 169, 114942. [Google Scholar] [CrossRef]
- Wu, Y.; Li, J.; Wang, M.; Wang, H.; Zhong, Y.; Zhao, Y.; Wei, M.; Li, Y. Solar salt doped by MWCNTs as a promising high thermal conductivity material for CSP. RSC Adv. 2018, 8, 19251. [Google Scholar] [CrossRef]
- Han, D.; Lougou, B.G.; Xu, Y.; Shuai, Y.; Huang, X. Thermal properties characterization of chloride salts/nanoparticles composite phase change material for high-temperature thermal energy storage. Appl. Energy 2020, 264, 114674. [Google Scholar] [CrossRef]
- Tian, H.; Wang, W.; Ding, J.; Wei, X.; Song, M.; Yang, J. Thermal conductivities and characteristics of ternary eutectic chloride/expanded graphite thermal energy storage composites. Appl. Energy 2015, 148, 87–92. [Google Scholar] [CrossRef]
- Tao, Y.B.; Lin, C.H.; He, Y.L. Preparation and thermal properties characterization of carbonate salt/carbon nanomaterial composite phase change material. Energy Convers. Manag. 2015, 97, 103–110. [Google Scholar] [CrossRef]
- Sang, L.; Ai, W.; Wu, Y.; Ma, C. Enhanced specific heat and thermal conductivity of ternary carbonate nanofluids with carbon nanotubes for solar power applications. Int. J. Energy Res. 2020, 44, 334–343. [Google Scholar] [CrossRef]
- Wei, X.; Yin, Y.; Qin, Y.; Ding, J.; Lu, J. Thermal conductivity improvement of liquid Nitrate and Carbonate salts doped with MgO nanoparticles. Energy Procedia 2017, 142, 407–412. [Google Scholar] [CrossRef]
- Lasfargues, M.; Cao, H.; Geng, Q.; Ding, Y. Rheological analysis of binary eutectic mixture of sodium and potassium nitrate and the effect of low concentration CuO nanoparticle addition to its viscosity. Materials 2015, 8, 5194–5204. [Google Scholar] [CrossRef]
- Jo, B.; Banerjee, D. Viscosity measurements of multi-walled carbon nanotubes-based high temperature nanofluids. Mater. Lett. 2014, 122, 212–215. [Google Scholar] [CrossRef]
- Krieger, I.M.; Dougherty, T.J. A Mechanism for Non-Newtonian Flow in Suspensions of Rigid Spheres. J. Rheol. 1959, 3, 137–152. [Google Scholar] [CrossRef]
- Chen, H.; Ding, Y.; He, Y.; Tan, C. Rheological behavior of ethylene glycol based titania nanofluids. Chem. Phys. Lett. 2007, 444, 333–337. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, G.; Ding, Y.; Wen, D. Rheological Characteristics of Molten Salt Seeded with Al2O3 Nanopowder and Graphene for Concentrated Solar Power. Energies 2019, 12, 467. [Google Scholar] [CrossRef]
- Muñoz-Sánchez, B.; Nieto-Maestre, J.; Veca, E.; Liberatore, R.; Sal, S.; Navarro, H.; Ding, Y.; Navarrete, N.; Juliá, J.E.; Fernández, A.G.; et al. Rheology of Solar-Salt based nanofluids for concentrated solar power. Influence of the salt purity, nanoparticle concentration, temperature and rheometer geometry. Sol. Energy Mater. Sol. Cells 2018, 176, 357–373. [Google Scholar] [CrossRef]
- Jiang, Z.; Palacios, A.; Lei, X.; Navarro, M.E.; Qiao, G.; Mura, E.; Xu, G.; Ding, Y. Novel key parameter for eutectic nitrates based nanofluids selection for concentrating solar power (CSP) systems. Appl. Energy 2019, 235, 529–542. [Google Scholar] [CrossRef]
- El Far, B.; Rizvi, S.M.M.; Nayfeh, Y.; Shin, D. Investigation of heat capacity and viscosity enhancements of binary carbonate salt mixture with SiO2 nanoparticles. Int. J. Heat Mass Transf. 2020, 156, 119789. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.-T.; Zhang, L.-D.; Wang, X.; Ma, C.-F. Experimental study on thermophysical properties of molten salt nanofluids prepared by high-temperature melting. Sol. Energy Mater. Sol. Cells 2019, 191, 209–217. [Google Scholar] [CrossRef]
- Grosu, Y.; Anagnostopoulos, A.; Balakin, B.; Krupanek, J.; Navarro, M.E.; González-Fernández, L.; Ding, Y.; Faik, A. Nanofluids based on molten carbonate salts for high-temperature thermal energy storage: Thermophysical properties, stability, compatibility and life cycle analysis. Sol. Energy Mater. Sol. Cells 2021, 220, 110838. [Google Scholar] [CrossRef]
- Lasfargues, M.; Geng, Q.; Cao, H.; Ding, Y. Mechanical dispersion of nanoparticles and its effect on the specific heat capacity of impure binary nitrate salt mixtures. Nanomaterials 2015, 5, 1136–1146. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Cerritelli, G.F.; Miliozzi, A.; Kenny, J.M. Effect of nanoparticles on heat capacity of nanofluids based on molten salts as PCM for thermal energy storage. Nanoscale Res. Lett. 2013, 8, 448. [Google Scholar] [CrossRef]
- Lee, D.; Jo, B. Thermal energy storage characteristics of binary molten salt nanofluids: Specific heat and latent heat. Int. J. Energy Res. 2021, 45, 3231–3241. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Z.; Xu, P.; Hongbing, C.; Wu, Y. Experimental investigation on the thermos-physical properties by dispersing nanoparticles to the nitrates. Energy Procedia 2019, 158, 5551–5556. [Google Scholar] [CrossRef]
- Rizvi, S.M.M.; Shin, D. Specific heat capacity, viscosity, and thermal stability of carbonate-based molten salt nanofluids. J. Energy Storage 2021, 43, 103192. [Google Scholar] [CrossRef]
- Myers, P.D.; Alam, T.E.; Kamal, R.; Goswami, D.Y.; Stefanakos, E. Nitrate salts doped with CuO nanoparticles for thermal energy storage with improved heat transfer. Appl. Energy 2016, 165, 225–233. [Google Scholar] [CrossRef]
- Salim, N.J.; Rahman, M.A. Effect of Nanoparticle Concentration on the Specific Heat Capacity and Thermal Stability of Graphite Nanoparticle-based Molten Salt. In Proceedings of the International Exchange and Innovation Conference on Engineering & Sciences (IEICES), Fukuoka, Japan, 22–23 October 2020; Volume 6, pp. 52–59. [Google Scholar] [CrossRef]
- Aslfatahhi, N.; Saidur, R.; Sidik, N.A.C.; Sabri, M.F.M.; Zahir, M.H. Experimental Assessment of a Novel Eutectic Binary Molten Salt-based Hexagonal Boron Nitride Nanocomposite as a Promising PCM with Enhanced Specific Heat Capacity. J. Adv. Res. Fluid Mech. Therm. Sci. 2020, 68, 73–85. [Google Scholar] [CrossRef]
- Madathil, P.K.; Balagi, N.; Saha, P.; Bharali, J.; Rao, P.V.C.; Choudary, N.V.; Ramesh, K. Preparation and characterization of molten salt based nanothermic fluid with enhanced thermal properties for solar thermal applications. Appl. Therm. Energy 2016, 109, 901–905. [Google Scholar] [CrossRef]
- Nithiyanantham, U.; Grosu, Y.; González- Fernandéz, L.; Zaki, A.; Igartua, J.M.; Faik, A. Corrosion aspects of molten nitrate salt-based nanofluids for thermal energy storage applications. Sol. Energy 2019, 189, 219–227. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, W.; Ji, C.; Wang, Q. Experimental study on heat storage and corrosion properties of ternary carbonate salt-based ZnO nanofluids for solar thermal energy storage. J. Therm. Anal. Calorim. 2022, 147, 13935–13947. [Google Scholar] [CrossRef]
- Ma, B.; Shin, D.; Banerjee, D. One-step synthesis of molten salt nanofluid for thermal energy storage application—A comprehensive analysis on thermophysical property, corrosion behavior, and economic benefit. J. Energy Storage 2021, 35, 102278. [Google Scholar] [CrossRef]
- Nithiyanantham, U.; Grosu, Y.; Anagnostopoulos, A.; Carbó-Argibay, E.; Bondarchuk, O.; González-Fernández, L.; Zaki, A.; Igartua, J.M.; Navarro, M.E.; Ding, Y.; et al. Nanoparticles as a high-temperature anticorrosion additive to molten nitrate salts for concentrated solar power. Sol. Energy Mater. Sol. Cells 2019, 203, 110171. [Google Scholar] [CrossRef]
- Atkin, R.; Borisenko, N.; Druschler, M.; Endres, F.; Hayes, R.; Huber, B.; Roling, B. Structure and dynamics of the interfacial layer between ionic liquids and electrode materials. J. Mol. Liq. 2014, 192, 44–54. [Google Scholar] [CrossRef]
- Thoms, M.W. Adsorption at the Nanoparticle Interface for Increased Thermal Capacity in Solar Thermal Systems. Masters`s Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2012. [Google Scholar]
- Sang, L.; Ai, W.; Liu, T.; Wu, Y.; Ma, C. Insights into the specific heat capacity enhancement of ternary carbonate nanofluids with SiO2 nanoparticles: The effect of change in the composition ratio. RSC Adv. 2019, 9, 5288–5294. [Google Scholar] [CrossRef]
- Jo, B.; Banerjee, D. Thermal properties measurement of binary carbonate salt mixtures for concentrating solar power plants. J. Renew. Sustain. Energy 2015, 7, 033121. [Google Scholar] [CrossRef]
- Tiznobaik, H.; Shin, D. Enhanced specific heat capacity of high-temperature molten salt-based nanofluids. Int. J. Heat Mass Transf. 2013, 57, 542–548. [Google Scholar] [CrossRef]
- Jung, S.; Banerjee, D. A simple analytical model for specific heat of nanofluid with tube shaped and disc shaped nanoparticles. In Proceedings of the ASME/JSME 8th Thermal Engineering Joint Conference, Honolulu, HI, USA, 13–17 March 2011. [Google Scholar]
- Dudda, B.; Shin, D. Effect of nanoparticle dispersion on specific heat capacity of a binary nitrate salt eutectic for concentrated solar power applications. Int. J. Therm. Sci. 2013, 69, 37–42. [Google Scholar] [CrossRef]
- Riazi, H.; Mesgari, S.; Ahmed, N.A.; Taylor, R.A. The effect of nanoparticle morphology on the specific heat of nanosalts. Int. J. Heat Mass Transf. 2016, 94, 254–261. [Google Scholar] [CrossRef]
- Devaradjane, R.; Shin, D. Nanoparticle dispersions on ternary nitrate salts for heat transfer fluid applications in solar thermal power. J. Heat Transf. 2016, 138, 051901. [Google Scholar] [CrossRef]
- Changla, S. Experimental Study of Quaternary Nitrate/Nitrite Molten Salt as Advanced Heat Transfer Fluid and Energy Storage Material in Concentrated Solar Power Plant; University of Texas at Arlington: Arlington, TX, USA, 2015. [Google Scholar]
- Mondragón, R.; Hernandez, L.; Cabedo, L.; Torro, S.; Julia, J.E. Increment of specific heat of solar salt with SiO2 and Al2O3 nanoparticles. Eurotherm Semin. Adv. Therm. Energy Storage 2014, 99, 1–9. [Google Scholar]
- Ma, B.; Shin, D.; Banerjee, D. Synthesis and Characterization of Molten Salt Nanofluids for Thermal Energy Storage Application in Concentrated Solar Power Plants—Mechanistic Understanding of Specific Heat Capacity Enhancement. Nanomaterials 2020, 10, 2266. [Google Scholar] [CrossRef] [PubMed]
- Miliozzi, A.; Veca, E.; Sal, S.; Grena, R.; Celino, M.; Falconieri, M.; Rondino, F. Individuazione e Caratterizzazione di Miscele di Materiali a Cambiamento di Fase e Nanoparticelle da Impiegare Come Sistemi Alternativi di Accumulo Térmico; Report Rds/2013/079; Agenzia Nazionale per le Nuove Tecnologie, l´Energia e lo Sviluppo Economico Sostenible, Ministero dello Sviluppo Economico: Rome, Italy, 2013. [Google Scholar]
- Shin, D.; Jo, B.; Kwak, H.; Banerjee, D. Investigation of high temperature nanofluids for solar thermal power conversion and storage applications. In Proceedings of the 14th International Heat Transfer Conference, Washington, DC, USA, 8–13 August 2010; pp. 1–9. [Google Scholar]
- Schuller, M.; Shao, Q.; Lalk, T. Experimental investigation of the specific heat of a nitrate alumina nanofluid for solar thermal energy storage systems. Int. J. Therm. Sci. 2015, 91, 142–145. [Google Scholar] [CrossRef]
- Kim, H.J.; Jo, B. Anomalous Increase in Specific Heat of Binary Molten Salt-Based Graphite Nanofluids for Thermal Energy Storage. Appl. Sci. 2018, 8, 1305. [Google Scholar] [CrossRef]
- Ho, M.X.; Pan, C. Optimal concentration of alumina nanoparticles in molten Hitec salt to maximize its specific heat capacity. Int. J. Heat Mass Transf. 2014, 10, 174–184. [Google Scholar] [CrossRef]
- Liu, M.; Severino, J.; Bruno, F.; Majewski, P. Experimental investigation of specific heat capacity improvement of a binary nitrate salt by addition of nanoparticles/microparticles. J. Energy Storage 2019, 22, 137–143. [Google Scholar] [CrossRef]
- Banerjee, D. Enhanced specific heat capacity of molten salt-based nanomaterials: Effects of nanoparticle dispersion and solvent material. Acta Mater. 2014, 75, 80–91. [Google Scholar] [CrossRef]
- Muñoz-Sánchez, B.; Nieto-Maestre, J.; Iparraguirre-Torres, I.; Sánchez-García, J.; Julia, J.E.; García-Romero, A. The influence of mixing water on the thermophysical properties of nanofluids based on solar salt and silica nanoparticles. AIP Conf. Proc. 2016, 1734, 050031. [Google Scholar] [CrossRef]
- Jung, S. Numerical and Experimental Investigation of Inorganic Nanomaterials for Thermal Energy Storage (TES) and Concentrated Solar Power (CSP) Applications. Ph.D. Thesis, Texas A & M University, College Station, TX, USA, 2012. [Google Scholar]
- Iwadate, Y.; Okada, I.; Kawamura, K. Density and heat capacity of molten sodium nitrite-potassium nitrate mixtures. J. Chem. Eng. Data 1982, 27, 3–288. [Google Scholar] [CrossRef]
- Mondragón, R.; Juliá, J.E.; Cabedo, L.; Navarrete, N. On the relationship between the specific heat enhancement of salt-based nanofluids and the ionic exchange capacity of nanoparticles. Sci. Rep. 2018, 8, 7532. [Google Scholar] [CrossRef] [PubMed]
- Tiznobaik, H.; Banerjee, D.; Shin, D. Effect of formation of “long range” secondary dendritic nanostructures in molten salt nanofluids on the values of specific heat capacity. Int. J. Heat Mass Transf. 2015, 91, 342–346. [Google Scholar] [CrossRef]
- Sang, L.; Liu, T. The enhanced specific heat capacity of ternary carbonates nanofluids with different nanoparticles. Sol. Energy Mater. Sol. Cells 2017, 169, 297–303. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Z.; Sun, M.; Wu, Y.; Xu, P.; Qian, X.; Li, C.; Ding, Y.; Ma, C. Enhanced thermal energy storage of nitrate salts by silica nanoparticles for concentrating solar power. Int. J. Energy Res. 2021, 45, 5248–5262. [Google Scholar] [CrossRef]
- Jo, B.; Banerjee, D. Effect of solvent on specific heat capacity enhancement of binary molten salt-based carbon nanotube nanomaterials for thermal energy storage. Int. J. Therm. Sci. 2015, 98, 219–227. [Google Scholar] [CrossRef]
- Yaxuan, X.; Huixiang, W.; Zhenyu, W.; Yuting, W.; Qian, X.; Gang, W.; Chuan, L.; Yulong, D.; Chongfang, M. Insights into the Enhancement Mechanisms of Molten Salt Nanofluids. Int. J. Photoenergy 2022, 2022, 4912922. [Google Scholar] [CrossRef]
- Betts, M. The Effects of Nanoparticle Augmentation of Nitrate Thermal Storage Materials for Use in Concentrating Solar Power Applications. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2011. [Google Scholar]
- Lasfargues, M.; Stead, G.; Amjad, M.; Ding, Y.; Wen, D. In Situ Production of Copper Oxide Nanoparticles in a Binary Molten Salt for Concentrated Solar Power Applications. Materials 2017, 10, 537. [Google Scholar] [CrossRef]
- Muñoz-Sánchez, B.; Nieto-Maestre, J.; Guerreiro, L.; Julia, J.E.; Collares-Pereira, M.; García-Romero, A. Molten salt based Nanofluids based on solar salt and alumina nanoparticles: An industrial approach. AIP Conf. Proc. 2017, 1850, 080016. [Google Scholar] [CrossRef]
- Navarrete, N.; Gimeno-Furió, A.; Forner-Escrig, J.; Juliá, J.E.; Mondragón, R. Colloidal stability of molten salt-based nanofluids: Dynamic Light Scattering tests at high temperature conditions. Powder Technol. 2019, 352, 1–10. [Google Scholar] [CrossRef]
- Gil, A.; Medrano, M.; Martorell, I.; Lázaro, A.; Dolado, P.; Zalba, B.; Cabeza, L.F. State of the art on high temperature thermal energy storage for power generation. Part 1-Concepts, materials and modelization. Renew. Sustain. Energy Rev. 2010, 14, 31–55. [Google Scholar] [CrossRef]
- Bonk, A.; Sau, S.; Uranga, N.; Hernaiz, M.; Bauer, T. Advanced heat transfer fluids for direct molten salt line-focusing CSP plants. Prog. Energy Combust. Sci. 2018, 67, 69–87. [Google Scholar] [CrossRef]
- Dunn, R.I.; Hearps, P.J.; Wright, M.N. Molten-salt power towers: Newly commercial concentrating solar storage. Proc. IEEE 2012, 100, 504–515. [Google Scholar] [CrossRef]
- González-Roubaud, E.; Pérez-Osorio, D.; Prieto, C. Review of commercial thermal energy storage in concentrated solar power plants: Steam vs. molten salts. molten salts. Renew. Sustain. Energy Rev. 2017, 80, 133–148. [Google Scholar] [CrossRef]
- Caraballo, A.; Galan-Casado, S.; Caballero, A.; Serena, S. Molten salts for sensible thermal energy storage: A review and an energy performance analysis. Energies 2021, 14, 1197. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Li, B.; Sunde, B. A review on molten-salt-based and ionic-liquid-based nanofluids for medium-to-high temperature heat transfer. J. Therm. Anal. Calorim. 2019, 136, 1037–1051. [Google Scholar] [CrossRef]
- Alnaimat, F.; Rashid, Y. Thermal Energy Storage in Solar Power Plants: A Review of the Materials, Associated Limitations, and Proposed Solutions. Energies 2019, 12, 4164. [Google Scholar] [CrossRef]
- Corradini, D.; Coudert, F.X.; Vuilleumier, R. Insight into the Li2CO3-K2CO3 eutectic mixture from classical molecular dynamics: Thermodynamics, structure, and dynamics. J. Chem. Phys. 2016, 144, 104507. [Google Scholar] [CrossRef]
- Pan, G.; Wei, X.; Yu, C.; Lu, Y.; Li, J.; Ding, J.; Wang, W.; Yan, J. Thermal performance of a binary carbonate molten eutectic salt for high-temperature energy storage applications. Appl. Energy 2020, 262, 114418. [Google Scholar] [CrossRef]
- Aljaerani, H.A.; Samykano, M.; Saidur, R.; Pandey, A.K.; Kadirgama, K. Nanoparticles as molten salts thermophysical properties enhancer for concentrated solar power: A critical review. J. Energy Storage 2021, 44, 103280. [Google Scholar] [CrossRef]
- Ibrahim, A.; Peng, H.; Riaz, A.; Basit, M.A.; Rashid, U.; Basit, A. Molten salts in the light of corrosion mitigation strategies and embedded with nanoparticles to enhance the thermophysical properties for CSP plants. Sol. Energy Mater. Sol. Cells 2021, 219, 110768. [Google Scholar] [CrossRef]
- Grosu, Y.; Anagnostopoulos, A.; Navarro, M.E.; Ding, Y.; Faik, A. Inhibiting hot corrosion of molten Li2CO3-Na2CO3-K2CO3 salt through graphitization of construction materials for concentrated solar power. Sol. Energy Mater. Sol. Cells 2020, 215, 110650. [Google Scholar] [CrossRef]
- Gonzalez, M.; Nithiyanantham, U.; Carbó-Argibay, E.; Bondarchuk, O.; Grosu, Y.; Faik, A. Graphitization as efficient inhibitor of the carbon steel corrosion by molten binary nitrate salt for thermal energy storage at concentrated solar power. Sol. Energy Mater. Sol. Cells 2019, 203, 110172. [Google Scholar] [CrossRef]
- Ding, W.; Shi, H.; Jianu, A.; Xiu, Y.; Bonk, A.; Weisenburger, A.; Bauer, T. Molten chloride salts for next generation concentrated solar power plants: Mitigation strategies against corrosion of structural materials. Sol. Energy Mater. Sol. Cells 2019, 193, 298–313. [Google Scholar] [CrossRef]
- Ding, W.; Gomez-Vidal, J.; Bonk, A.; Bauer, T. Molten chloride salt for next generation CSP plants: Electrolytical salt purification for reducing corrosive impurity level. Sol. Energy Mater. Sol. Cells 2019, 199, 8–15. [Google Scholar] [CrossRef]
- Gomes, A.; Navas, M.; Uranga, N.; Paiva, T.; Figueira, I.; Diamantino, T.C. High-temperature corrosion performance of austenitic stainless-steels type AISI 316L and AISI 321H, in molten solar salt. Sol. Energy 2019, 177, 408–419. [Google Scholar] [CrossRef]
- Liu, T.; Xu, X.; Liu, W.; Zhuang, X. Corrosion of alloys in high temperature molten-salt heat transfer fluids with air as the cover gas. Sol. Energy 2019, 191, 435–448. [Google Scholar] [CrossRef]
- Fernández, A.G.; Pineda, F.; Walczak, M.; Cabeza, L.F. Corrosion evaluation of alumina-forming alloys in carbonate molten salt for CSP plants. Renew. Energy 2019, 140, 227–233. [Google Scholar] [CrossRef]
- Frangini, S.; Seta, L.D.; Paoletti, C.; Felici, C.; Turchetti, L.; Bellucci, A. Corrosion behavior of aluminide diffusion coatings in low temperature molten carbonate electrolysis environments. Mater. Corros. 2018, 69, 1837–1846. [Google Scholar] [CrossRef]
- Zhu, H.; Li, B.; Chen, M.; Liu, Z.; Tang, Z.; Qiu, C. AlN coatings on Hastelloy-N alloy offering superior corrosion resistance in LiF-KF-NaF molten salt. J. Fluor. Chem. 2018, 213, 80–86. [Google Scholar] [CrossRef]
- Karim, M.A.; Arthur, O.; Yarlagadda, P.K.D.V.; Islam, M.; Mahiuddin, M. Performance Investigation of High Temperature Application of Molten Solar Salt Nanofluid in a Direct Absorption Solar Collector. Molecules 2019, 24, 285. [Google Scholar] [CrossRef]
- Karim, M.A.; Islam, M.; Arhur, O.; Yarlagadda, P.K.D.V. Performance of Graphite-Dispersed Li2CO3-K2CO3 Molten Salt Nanofluid for a Direct Absorption Solar Collector System. Molecules 2020, 25, 375. [Google Scholar] [CrossRef]
- Liaqat, K.; Ordonez, J.C. Molten Salt Based Nanofluids for Solar Thermal Power Plant: A Case Study. In Proceedings of the IEEE Conference on Technologies for Sustainability (SusTech), Irvine, CA, USA, 22–24 April 2021. [Google Scholar]
- ISO 14044:2006; Environmental Management–LCA—Requirements and Guidelines. International Organization for Standardization (IOS): Geneve, Switzerland, 2006.
- Burgaleta, J.I.; Arias, S.; Ramirez, D. Gemasolar, the First Tower Thermosolar Commercial Plant with Molten Salt Storage; SolarPACES: Granada, Spain, 2011; pp. 20–23. [Google Scholar]
- Relloso, S.; Delgado, E.B. Experience with molten salt thermal storage in a commercial parabolic trough plant. Andasol-1 commissioning and operation. In Proceedings of the 15th SolarPACES Symposium, Berlin, Germany, 15–18 September 2009; pp. 14–18. [Google Scholar]
- Barberio, G.; Scalbi, S.; Buttol, P.; Masoni, P.; Righi, S. Combining life cycle assessment and qualitative risk assessment: The case study of alumina nanofluid production. Sci. Total Environ. 2014, 496, 122–131. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J. Factors affecting the lower limit of the safe mud weight window for drilling operation in hydrate bearing sediments in the Northern South China Sea. Geomech. Geophys. Geo-Energy Geo-Resour. 2022, 8, 82. [Google Scholar] [CrossRef]
- Li, Q.; Wang, F.; Forson, K.; Zhang, J.; Zhang, C.; Chen, J.; Xu, N.; Wang, Y. Affecting analysis of the rheological characteristic and reservoir damage of CO2 fracturing fluid in low permeability shale reservoir. Environ. Sci. Pollut. Res. 2022, 29, 37815–37826. [Google Scholar] [CrossRef]
- Ye, H.; Wu, X.; Li, D.; Jiang, Y.; Gong, B. A novel thermal stimulation approach for natural gas hydrate exploitation—The application of the self-entry energy compensation device in the Shenhu sea. J. Gas Sci. Eng. 2022, 105, 104723. [Google Scholar] [CrossRef]
- Liu, J.; Liang, J.; Xue, Y.; Yao, K.; Fu, Y. Numerical evaluation on multiphase flow and heat transfer during thermal stimulation enhanced shale gas recovery. Appl. Therm. Eng. 2020, 178, 115554. [Google Scholar] [CrossRef]
- Sun, X.-H.; Yan, H.; Massoudi, M.; Chen, Z.-H.; Wu, W.-T. Numerical Simulation of Nanofluid Suspensions in A geothermal Heat Exchanger. Energies 2018, 11, 919. [Google Scholar] [CrossRef]
- Harish, R.; Nimmagada, R.; Reddy, S.R. Turbulent melting characteristics of hybrid nano-enhanced molten salt phase change material in rectangular enclosure. J. Energy Storage 2022, 54, 105328. [Google Scholar] [CrossRef]
- Harish, R.; Sivakumar, R. Turbulent thermal convection of nanofluids in cubical enclosure using two-phase mixture model. Int. J. Mech. Sci. 2021, 190, 106033. [Google Scholar] [CrossRef]
- Harish, R.; Sivakumar, R. Effects of nanoparticle dispersion on turbulent mixed convection flows in cubical enclosure considering Brownian motion and thermophoresis. Powder Technol. 2021, 378, 303–316. [Google Scholar] [CrossRef]
- Janjanam, N.; Nimmagadda, R.; Asirvatham, L.G.; Harish, R.; Wongwises, S. Conjugate heat transfer performance of stepped lid-driven cavity with Al2O3/water nanofluid under forced and mixed convection. SN Appl. Sci. 2021, 3, 605. [Google Scholar] [CrossRef]





| Class | Melting Point Range | Molten Salts | Applications | Benefits | Limitations | Authors/ Reference |
|---|---|---|---|---|---|---|
| Low Melting Point | 70–200 °C | Hitec Salt | Thermal Energy Storage | Avoid Freezing in the Circuits Improved Thermal Energy Storage Smaller Dimensions of the Storage Tanks | Low Thermal Stability Upper Limit of the Nitrites Reduced Long-term Stability and Increased Viscosity with Calcium Nitrates | Isaza-Ruiz et al. [5] |
| Hitec XL Salt | Chen et al. [6] | |||||
| LiNaKCaNO3 Salt | Peng et al. [7] | |||||
| Medium Melting Point | 200–350 °C | Solar Salt KNO3-NaNO3 | Concentrated Solar Power | Improved Thermal Energy Storage Relative Low Cost Chemical Safety Relative Low Corrosivity | Temperature Range Restricted by Crystallization at 240 °C Maximum Operating Temperature of 565 °C | Bauer et al. [8] |
| High Melting Point | >350 °C | Chloride Mixtures | Nuclear Power Coolants | High Operating Temperatures Low Vapor Pressure Good Thermal Stability | High Cost of Lithium and Zinc Chlorides Very High Corrosivity | Du et al. [9] |
| Fluoride Mixtures | Nuclear Power Coolants and Fuel Cells | Very Low Vapor Pressure Improved Heat Transfer Capability | Relative High Cost | Forsberg et al. [10] | ||
| Carbonate Mixtures | Thermal Energy Storage | Improved Thermal Energy Storage | High Corrosivity | An et al. [11] |
| Method | Liquid Dispersion | Mechanical Dispersion | In-Situ Production |
|---|---|---|---|
| Involved Techniques | Solid Mixing of the Salt and Nanoparticles Dissolution Ultrasonication Drying by Evaporation | Dry Mixing and Milling with Mechanical Aid Mechanical Stirring Ultrasonication | Physical Route: Vapor Condensation, Arc Spray Analysis, Laser Ablation Chemical Route: Precipitation, Thermal Decomposition of Precursors Wet Mixing Dry Mixing |
| Benefits | Use of Commercial Nanopowders | Scalability to Produce Large Amounts of Molten Salt Nanofluids | Mitigation of the Agglomeration and Sedimentation Effects of the Nanoparticles Improved Stability |
| Limitations | Possible Agglomeration of the Nanoparticles | Eventual Heterogeneous Dispersion of the Nanoparticles Possible Contamination from the Milling Equipment | Requires Base Fluids with Low Vapor Pressure and Expensive Equipment Possible Residual Reactants Hinder the Positive Effect of the Nanoparticles Health Risks Arising from the High-Temperature Molten Salt Manipulation Poor Scalability to Industrial Scale |
| Main Findings | 30.6% Specific Heat Increase | 38.5% Specific Heat Increase 50% Thermal Conductivity Increase | 7.5% Specific Heat Increase |
| Authors/Reference | Schuller et al. [31] | Sang et al. [25] | Lasfargues et al. [30] |
| Molten Salt | Nanoparticles | Best Concentration % wt. | Thermal Conductivity/Diffusivity Enhancement (%) | Authors/Reference |
|---|---|---|---|---|
| NaNO3-KNO3 | Silica | 1.0 | 50.0 (Diffusivity) | Li et al. [43] |
| NaNO3-KNO3 | Silica | 1.0 | 60.9 | Yu et al. [44] |
| NaNO3-KNO3 | SiO2@Al2O3 Core-shell | 1.0 | 19.0 | Nithiyanantham et al. [45] |
| NaNO3-KNO3 | Alumina NPs/Alumina NRs | 1.0 | 16.0/12.0 | Nithiyanantham et al. [46] |
| NaNO3-KNO3 | Multi-Walled Carbon Nanotubes | 0.3 | 293.0 | Wu et al. [47] |
| MgCl2-KCl-NaCl | Alumina | 0.7 | 48.0 | Han et al. [48] |
| NaCl-CaCl2-MgCl2 | Expanded Graphite | 1.0 | 78.0 | Tian et al. [49] |
| Li2CO3-K2CO3 | Single-Walled Carbon Nanotubes | 1.5 | 57.0 | Tao et al. [50] |
| Li2CO3-K2CO3-Na2CO3 | Carbon Nanotubes | 1.0 | 149.2 | Sang et al. [51] |
| Li2CO3-K2CO3-Na2CO3 | Magnesium Oxide | 10.0 | 155.9 | Wei et al. [52] |
| Molten Salt | Nanoparticles | Concentration % wt. | Viscosity Findings | Authors/Reference |
|---|---|---|---|---|
| NaNO3-KNO3 | Magnesium Oxide | 2.5, 3.5, 4.5, 5.0 and 10.0 | 5.1 cp-2.4 cp Nearly the Same Viscosity of the Molten Salt | Wei et al. [39] |
| NaNO3-KNO3 | Silica and Alumina | 0.5–1.5 | 4.94 mPa.s at 300 °C | Munoz-Sanchez et al. [58] |
| NaNO3-KNO3 and LiNO3-NaNO3-KNO3 and LiNO3-NaNO3-KNO3-Ca(NO3)2 | Silica | 0.5 and 1.0 | 100.89–188.85% Increase for the Binary Salt, 18.75–71.12% Increase for the Ternary Salt, and 29.02–53.61% Increase for the Quaternary Salt | Jiang et al. [59] |
| NaNO2-NaO3-KNO3 and NaNO3-KNO3 | Alumina and Graphene | 1.0 Alumina and 2.0 Graphene | 35.4~8.1% Increase for the Ternary Salt and −9.2~68.1% Increase or the Binary Salt with Alumina; Remarkable Increase with Graphene (e.g., 987.3%) | Xiao et al. [57] |
| Li2CO3-K2CO3 | Silica | 1.0 | 34.0% to 94.4% Increase | El Far et al. [60] |
| Ca(NO3)2-KNO3-NaNO3-LiNO3 | Silica | 0.5 | 0.72–2.20 mPa·s in the Temperature Range 150–450 °C | Chen et al. [61] |
| K2CO3-Na2CO3-Li2CO3 | Alumina | 1.0 | 35% Increase | Grosu et al. [62] |
| Molten Salt | Nanoparticles | Concentration % wt. | Thermal Stability Findings | Authors/Reference |
|---|---|---|---|---|
| NaNO3-KNO3 | Hexagonal Boron Nitride | 0.5, 1.0 and 1.5 | 16% Increase at 1.5% wt. | Aslfattahi et al. [70] |
| NaNO3-KNO3 | Silica | 1.0 | The heat treatments, both exposure to constant high temperature and low-high temperature circulation, can decrease the thermophysical properties of samples significantly. With equal operation time, the decrease rate of the cycled sample is lower than that of the sample exposed to constant high temperature | Li et al. [43] |
| Ca(NO3)2-KNO3-NaNO3-LiNO3 | Silica | 1.0 | Good thermal stability under the same conditions. Moreover, the change in specific heat was minimal after 2000 h, which was less than 5%. | Chen et al. [61] |
| LiNO3-KNO3-Ca(NO3)2 | Molybdenum Disulfide and Copper Oxide | 0.5, 1.0, and 2.0 | Good thermal stability up to 400 °C The thermal stability was lower with the molybdenum disulfide and increased with the copper oxide, since the pure MoS2 decomposes around 400 °C | Madathil et al. [71] |
| K2CO3-Li2CO3-Na2CO3 | Silica | 1.0 | Good thermal stability and no considerable deterioration in the specific heat after the thermostatic at 600 °C for 150 h and thermal shock of 50 cycles | Sang et al. [51] |
| Li2CO3Na2CO3, Li2CO3-K2CO3, and Li2CO3-Na2CO3 | Alumina | 1.0 | Good thermal stability up to 600 °C. However, the Li2CO3-Na2CO3 exhibited poor thermal stability since its nanofluids decomposed after 470 °C | Rizvi and Shin [67] |
| Procedure | Techniques/Examples | Benefits | Limitations | Authors/Reference |
|---|---|---|---|---|
| Graphitization of Steel | Spray Graphitization of Steel | Corrosion Rate Reduction for Nitrate Molten Salts | Reduced Chloride Content in the Molten Salt Mixtures Humid Conditions May Still Provoke Severe Consequences The Addition of Nanoparticles May Entail Adverse Impacts on the Effectiveness of the Graphitization | Grosu et al. [119] |
| Addition of Graphite Nanoparticles | Addition of Graphite Nanoparticles in the Molten Salts | Mitigation of the Corrosive Behavior of the Nitrate Molten Salts | Eventual Formation of Microbubbles That Increase the Corrosion Layer Thickness Owing to the Higher Amount of Oxygen in the System Severe Corrosion Rates in Stainless-Steels Against Fluoride Molten Salts | Gonzalez et al. [120] |
| Addition of Magnesium Nanoparticles | Addition of Magnesium Nanoparticles into the Oxidation Layer of Chloride Molten Salts | Corrosion Inhibition by Reducing the Redox Potential of the Molten Salts Reduction of the Corrosion Rate by More Than 90% | Eventual Formation of Microbubbles That Increase the Corrosion Layer Thickness Due to the Higher Oxygen Amount in the System | Ding et al. [121] |
| Salt Purification | Physical, Chemical, and Electrochemical Purification of the Molten Salts | Strongly Minimizes the Corrosive Impurities (e.g., MgOH+) in the Molten Salt Cost-Effective Operation in CSP Plants | Pre-Implementation Process Designed Primarily for Laboratory Environment Lack of In-Situ Purification Methods High Flammability and Toxicity of Purge Gases | Ding et al. [122] |
| Use of Stainless-Steel | SS AISI 316 L, SS AISI 430, SS AISI 347, SS AISI 321H, Among Others | Reduced Corrosion Rates of Circuits and Containers Especially Against Nitrate Molten Salts | Relative High Cost in CSP Environment Some Stainless-Steels (e.g., SS AISI 316L) Exhibit Excessive Corrosion Rates for Industrial Applications | Gomes et al. [123] |
| Use of Special Alloys | Hastelloy C-276, Inconel 718, and Inconel 625 | Reduced Corrosion Rates of Pipping and Vessels The Hastelloy Alloy Achieved the Corrosion Rate up To Industrial Implementations | High Cost in CSP Environment Short-Term Corrosion Protection Approach for Certain Alloys The Fluoride Molten Salts Together with an Increased Moisture level Provokes Intergranular Corrosion and Pitting | Liu et al. [124] |
| Use of Alumina Forming Austenitic Alloys | OC4 and HR224 Alloys | Reduced Corrosion Rates of Pipping and Vessels | Need to Carefully Control the Thickness, Uniformity, and Stability of the Layers Eventual Cracks in the Layers May Initiate Further Localized Corrosion | Fernandez et al. [125] |
| Use of Pre-Oxidized Alloys | Pre-oxidizing the Fe-Cr-Al Alloys Conducts to the Formation of an Alumina Scale | Improved Corrosion Resistance of Commercial Alloys Against Chloride Molten Salts | Present Better Corrosion Resistances in CO2 Atmosphere | Frangini et al. [126] |
| Metal and Metal Oxide Coatings | Nickel, Cobalt, Aluminum Nitride, and Alumina | Drastic Reduction of the Corrosive Rates of Fluoride and Chloride Molten Salts Remarkable Thermal Stability at High Temperatures | Complexity of the Coating Procedures Long-Term Material Compatibility | Zhu et al. [127] |
| Molten Salts | Nitrate | Chloride | Fluoride | Carbonate |
|---|---|---|---|---|
| Specific Heat Capacity | High Specific Heat Capacity | Relatively High but Lower Than That of the Other Molten Salts | Very High Specific Heat Capacity | High Specific Heat Capacity |
| Thermal Stability | Up to 500–600 °C | High Thermal Stability at T > 800 °C | High Thermal Stability at T > 700 °C | High Thermal Stability at 650–850 °C |
| Melting point | Low Melting Point LiNO3 and Ca(NO3)2 Reduce the Melting Point Near 100 °C | Moderate Melting Point at around 400 °C | Relatively High Melting Point | Moderate Melting Point at around 400 °C |
| Effect of Impurities | Affect the Thermal Stability Range | Aggravate the High Corrosivity of the Molten Salts | Aggravate the Corrosion Behavior | Do Not Require Salt Purification Procedures |
| Counter Corrosion Strategies | Graphitization, Use of Stainless-Steels, and Alumina Forming Austenitic Alloys | Anaerobic Atmosphere, Salt Electrochemical Purification, and Addition of Mg Inhibitor | Metal and Metal Oxide Coatings | CO2 Inert Atmosphere, Use of Alumina Forming Alloys, and Pre-Oxidized Alloys |
| Economic Feasibility | Should be Carefully Considered | Relative Low Cost | High Cost | High Cost due to Li2CO3 Addition |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, J.; Moita, A.; Moreira, A. An Overview of the Molten Salt Nanofluids as Thermal Energy Storage Media. Energies 2023, 16, 1825. https://doi.org/10.3390/en16041825
Pereira J, Moita A, Moreira A. An Overview of the Molten Salt Nanofluids as Thermal Energy Storage Media. Energies. 2023; 16(4):1825. https://doi.org/10.3390/en16041825
Chicago/Turabian StylePereira, José, Ana Moita, and António Moreira. 2023. "An Overview of the Molten Salt Nanofluids as Thermal Energy Storage Media" Energies 16, no. 4: 1825. https://doi.org/10.3390/en16041825
APA StylePereira, J., Moita, A., & Moreira, A. (2023). An Overview of the Molten Salt Nanofluids as Thermal Energy Storage Media. Energies, 16(4), 1825. https://doi.org/10.3390/en16041825








