Dielectric Performance of Natural- and Synthetic-Ester-Based Nanofluids with Fullerene Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
3. Experimental Methods
3.1. AC Breakdown Voltage Measurement
3.2. Dielectric Performance Measurement
4. Experimental Results
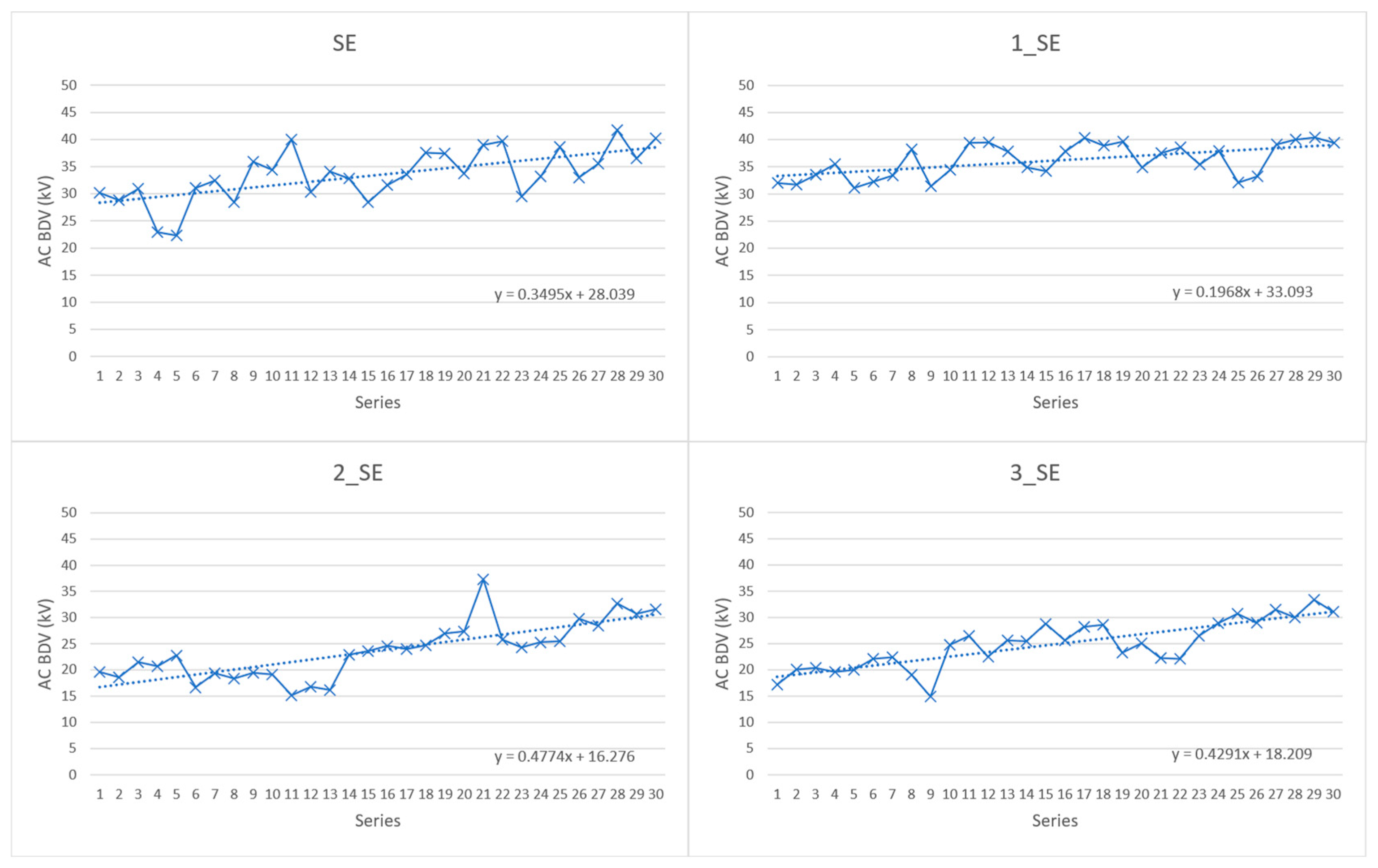
4.1. AC Breakdown Voltage Test Results
4.2. Weibull Probability Statistical Evaluation
4.3. Dissipation Factor Test Results
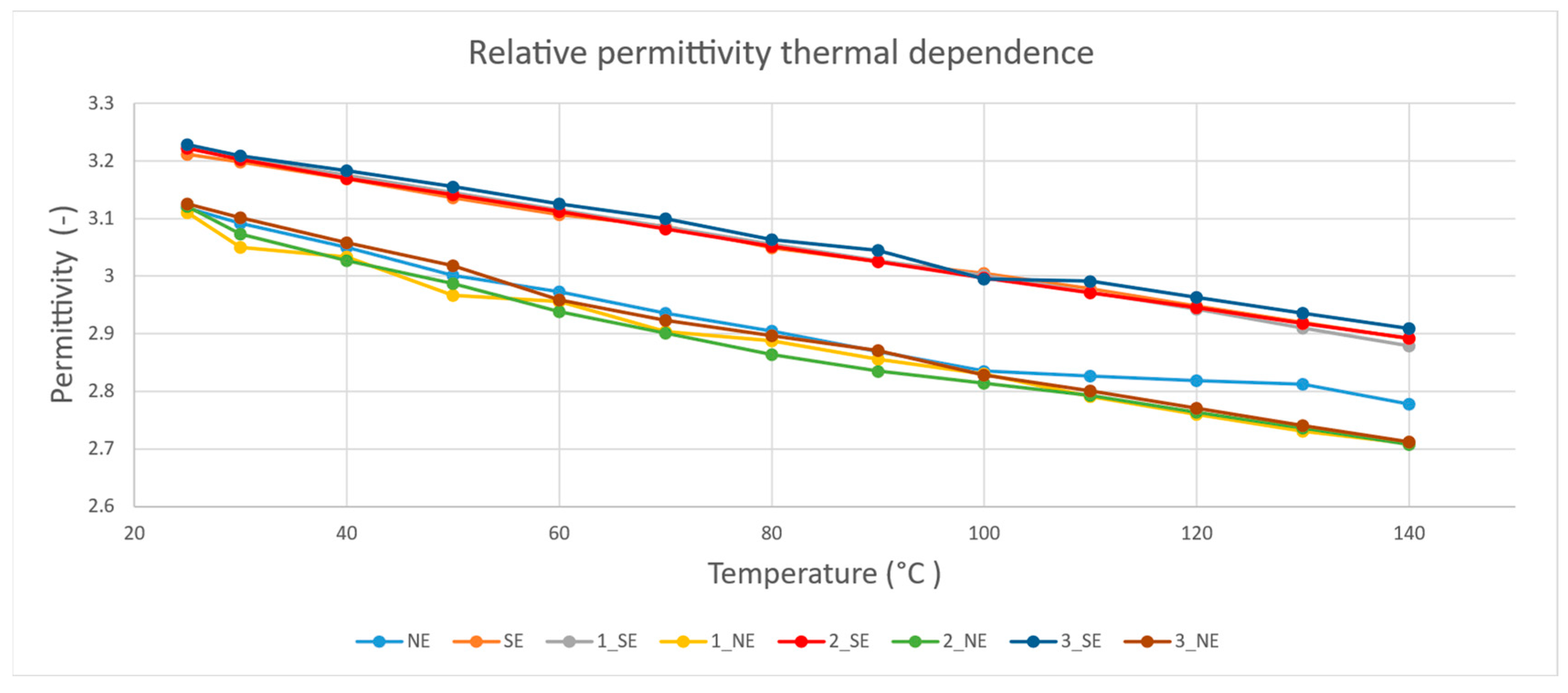
4.4. Relative Permittivity Test Results
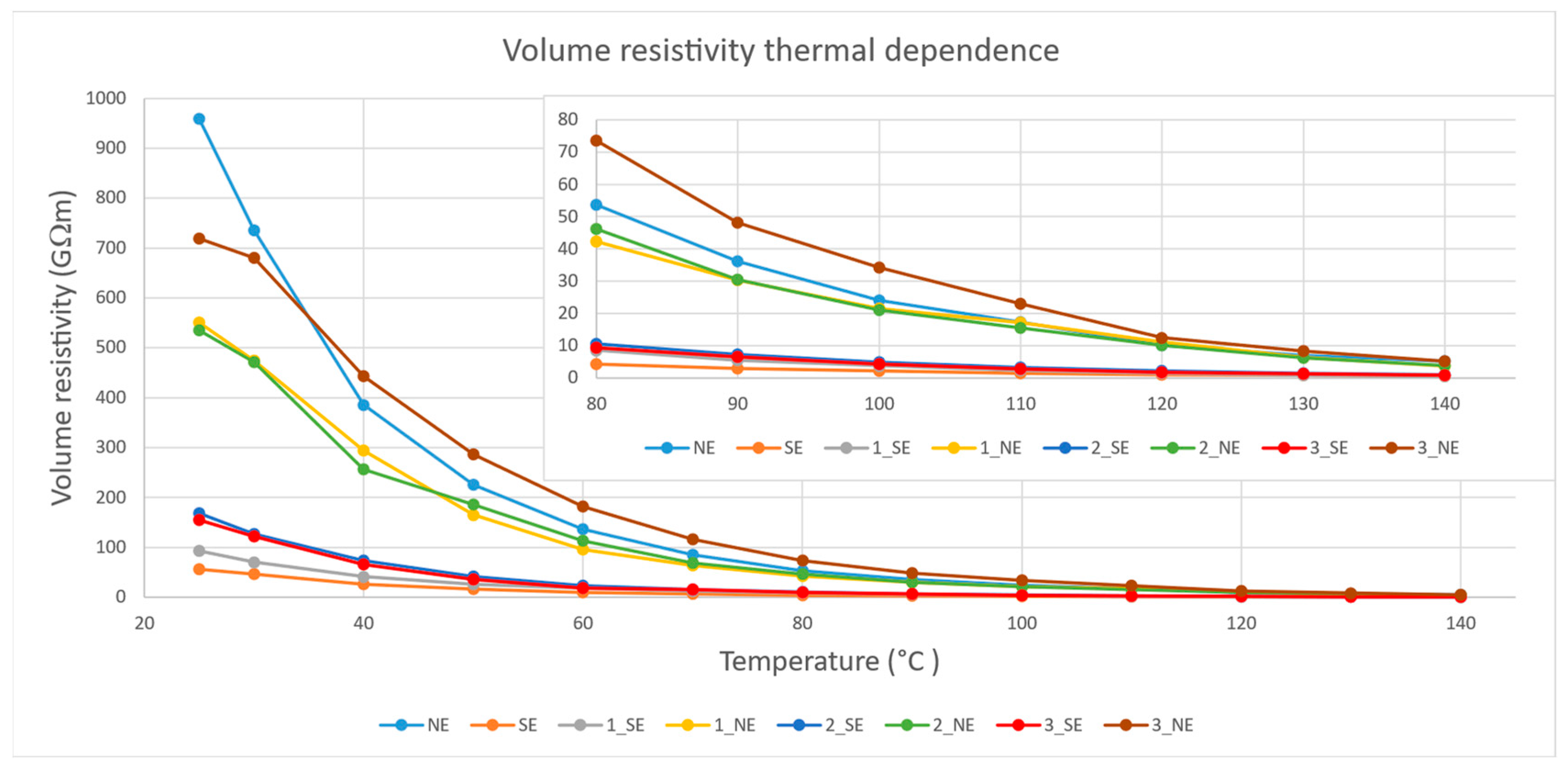
4.5. Volume Resistivity Test Results
5. Discussion
5.1. Experimental Data Integrity
5.2. Dielectric Performance
5.3. AC Breakdown Voltage
6. Conclusions
- According to the presented AC BDV test, the optimal concentration of fullerene nanoparticles in SE and NE nanofluids is 0.01% w/v;
- Only sample 1_SE showed enhancement in AC BDV (mean values). At a Weibull probability level of 1%, two samples (1_SE and 1_NE) reached increased values in comparison to their base oils of 32.93% and 18.75%, respectively;
- Comparison with previously published results indicates that the AC BDV performance of the studied nanofluids depends on the electrode separation and the related sample volume;
- According to the dielectric performance measurements, sample 3_NE returned the best and most complex results in comparison to the rest of the examined samples;
- Sample 2_SE returned better results in comparison to pure SE and other SE-based nanofluids from the dielectric performance point of view;
- NE and NE-based nanofluids showed better dielectric performance than SE and SE-based nanofluids; however, the AC BDV test showed opposite results, with higher values in SE and SE-based nanofluids.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rafiq, M.; Lv, Y.Z.; Zhou, Y.; Ma, K.B.; Wang, W.; Li, C.R.; Wang, Q. Use of Vegetable Oils as Transformer Oils—A Review. Renew. Sustain. Energy Rev. 2015, 52, 308–324. [Google Scholar] [CrossRef]
- Rozga, P.; Beroual, A.; Przybylek, P.; Jaroszewski, M.; Strzelecki, K. A Review on Synthetic Ester Liquids for Transformer Applications. Energies 2020, 13, 6429. [Google Scholar] [CrossRef]
- Šárpataky, M.; Kurimský, J.; Rajňák, M. Dielectric Fluids for Power Transformers with Special Emphasis on Biodegradable Nanofluids. Nanomaterials 2021, 11, 2885. [Google Scholar] [CrossRef] [PubMed]
- Havran, P.; Cimbala, R.; Kurimský, J.; Dolník, B.; Kolcunová, I.; Medveď, D.; Király, J.; Kohan, V.; Šárpataky, Ľ. Dielectric Properties of Electrical Insulating Liquids for High Voltage Electric Devices in a Time-Varying Electric Field. Energies 2022, 15, 391. [Google Scholar] [CrossRef]
- Khaled, U.; Beroual, A. AC Dielectric Strength of Synthetic Ester-Based Fe3O4, Al2O3 and SiO2 Nanofluids—Conformity with Normal and Weibull Distributions. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 625–633. [Google Scholar] [CrossRef]
- Charalampakos, V.P.; Peppas, G.D.; Pyrgioti, E.C.; Bakandritsos, A.; Polykrati, A.D.; Gonos, I.F. Dielectric Insulation Characteristics of Natural Ester Fluid Modified by Colloidal Iron Oxide Ions and Silica Nanoparticles. Energies 2019, 12, 3259. [Google Scholar] [CrossRef]
- Koutras, K.N.; Naxakis, I.A.; Pyrgioti, E.C.; Charalampakos, V.P.; Gonos, I.F.; Antonelou, A.E.; Yannopoulos, S.N. The Influence of Nanoparticles’ Conductivity and Charging on Dielectric Properties of Ester Oil Based Nanofluid. Energies 2020, 13, 6540. [Google Scholar] [CrossRef]
- Olmo, C.; Méndez, C.; Ortiz, F.; Delgado, F.; Ortiz, A. Titania Nanofluids Based on Natural Ester: Cooling and Insulation Properties Assessment. Nanomaterials 2020, 10, 603. [Google Scholar] [CrossRef] [PubMed]
- Šárpataky, M.; Kurimský, J.; Rajňák, M.; Paulovičová, K.; Krbal, M.; Pelikán, L. Synthetic and Natural Ester-Based Nanofluids with Fullerene and Magnetite Nanoparticles—An Experimental AC Breakdown Voltage Study. J. Mol. Liq. 2022, 368, 120802. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, F.; Wang, Q.; Yao, W.; Sun, K.; Zhang, R.; Zhao, J.; Lou, Z.; Li, J. Significantly Enhanced Electrical Performances of Eco-Friendly Dielectric Liquids for Harsh Conditions with Fullerene. Nanomaterials 2019, 9, 989. [Google Scholar] [CrossRef] [PubMed]
- Mentlik, V.; Trnka, P.; Hornak, J.; Totzauer, P. Development of a Biodegradable Electro-Insulating Liquid and Its Subsequent Modification by Nanoparticles. Energies 2018, 11, 508. [Google Scholar] [CrossRef]
- Szcześniak, D.; Przybylek, P. Oxidation Stability of Natural Ester Modified by Means of Fullerene Nanoparticles. Energies 2021, 14, 490. [Google Scholar] [CrossRef]
- Beroual, A.; Duzkaya, H. AC and Lightning Impulse Breakdown Voltages of Natural Ester Based Fullerene Nanofluids. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 1996–2003. [Google Scholar] [CrossRef]
- Chen, J.; Sun, P.; Sima, W.; Shao, Q.; Ye, L.; Li, C. A Promising Nano-Insulating-Oil for Industrial Application: Electrical Properties and Modification Mechanism. Nanomaterials 2019, 9, 788. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, F.; Li, J.; Liang, S.; Zhou, J. Electronic Properties of Typical Molecules and the Discharge Mechanism of Vegetable and Mineral Insulating Oils. Energies 2018, 11, 523. [Google Scholar] [CrossRef]
- Duzkaya, H.; Beroual, A. Statistical Analysis of AC Dielectric Strength of Natural Ester-Based ZnO Nanofluids. Energies 2020, 14, 99. [Google Scholar] [CrossRef]
- Timoshkin, I.V.; Fouracre, R.A.; Given, M.J.; MacGregor, S.J.; Mason, P.; Clephan, R. Dielectric Properties of Diala D, MIDEL 7131 and THESO Insulating Liquids. In Proceedings of the 2008 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Quebec City, QC, Canada, 26–29 October 2008; pp. 622–625. [Google Scholar]
- Galar, D.; Kumar, U. Chapter 6—Prognosis. In eMaintenance; Galar, D., Kumar, U., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 311–370. ISBN 978-0-12-811153-6. [Google Scholar]
- Volume Resistivity: Electrical Resistivity of Plastic. Available online: https://omnexus.specialchem.com/polymer-properties/properties/volume-resistivity (accessed on 21 March 2022).
- Fasehullah, M.; Wang, F.; Jamil, S.; Bhutta, M.S. Influence of Emerging Semiconductive Nanoparticles on AC Dielectric Strength of Synthetic Ester Midel-7131 Insulating Oil. Materials 2022, 15, 4689. [Google Scholar] [CrossRef]
- Beroual, A.; Khaled, U. Effect of Nanoparticles’ Mixtures on AC Breakdown Voltage of Mineral Oil. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 1216–1222. [Google Scholar] [CrossRef]
- Khelifa, H.; Beroual, A.; Vagnon, E. Effect of Conducting, Semi-Conducting and Insulating Nanoparticles on AC Breakdown Voltage and Partial Discharge Activity of Synthetic Ester: A Statistical Analysis. Nanomaterials 2022, 12, 2105. [Google Scholar] [CrossRef] [PubMed]
- Khelifa, H.; Vagnon, E.; Beroual, A. AC Breakdown Voltage and Partial Discharge Activity in Synthetic Ester-Based Fullerene and Graphene Nanofluids. IEEE Access 2022, 10, 5620–5634. [Google Scholar] [CrossRef]





| Properties | MIDEL 7131 | MIDEL eN 1204 |
|---|---|---|
| Density at 20 °C (g/cm3) | 0.97 | 0.92 |
| Kinematic viscosity at 40 °C (mm2/s) | 29 | 8 |
| Pour temperature (°C) | −56 | −31 |
| Flash point (°C) | 260 | >315 |
| Fire point (°C) | 316 | >350 |
| Total acid number (mg KOH/g) | <0.03 mg | 0.04 |
| Base Oil | Concentration of C60 Nanoparticles | Sample Name |
|---|---|---|
| NE | - | NE |
| NE | 0.01% w/v | 1_NE |
| NE | 0.02% w/v | 2_NE |
| NE | 0.03% w/v | 3_NE |
| SE | - | SE |
| SE | 0.01% w/v | 1_SE |
| SE | 0.02% w/v | 2_SE |
| SE | 0.03% w/v | 3_SE |
| Sample | Mean Values of AC BDV (kV) | St.Dev (kV) | Change (%) |
|---|---|---|---|
| SE | 33.46 | 4,80 | - |
| NE | 32.52 | 4.38 | - |
| 1_SE | 36.14 | 3.12 | 8.01 |
| 2_SE | 23.68 | 5.39 | −41.30 |
| 3_SE | 24.86 | 4.62 | −34.59 |
| 1_NE | 32.23 | 2.94 | −9.00 |
| 2_NE | 26.81 | 4.12 | −21.30 |
| 3_NE | 27.26 | 4.64 | −19.30 |
| Sample | Shape | Scale | p-Value | Conformity to Weibull Distribution |
|---|---|---|---|---|
| SE | 8.318 | 35.463 | >0.25 | Accepted |
| NE | 8.352 | 34.37 | 0.248 | Accepted |
| 1_SE | 14.147 | 37.539 | <0.01 | Not accepted |
| 2_SE | 4.689 | 25.817 | >0.25 | Accepted |
| 3_SE | 6.294 | 26.743 | >0.25 | Accepted |
| 1_NE | 13.018 | 33.519 | >0.25 | Accepted |
| 2_NE | 7.305 | 28.565 | >0.25 | Accepted |
| 3_NE | 6.681 | 29.191 | 0.236 | Accepted |
| Sample | AC BDV Probability 1% (kV) | AC BDV Probability 10% (kV) | AC BDV Probability 50% (kV) |
|---|---|---|---|
| Increase/Decrease (%) | Increase/Decrease (%) | Increase/Decrease (%) | |
| SE | 20.5 | 27.12 | 34 |
| - | - | - | |
| NE | 19.89 | 26.25 | 32.86 |
| - | - | - | |
| 1_SE | 27.25 | 32.64 | 36.6 |
| +32.93 | +20.35 | +7.65 | |
| 2_SE | 9.73 | 16.04 | 24.53 |
| −52.54 | −40.86 | −27.85 | |
| 3_SE | 13.13 | 18.71 | 25.89 |
| −35.95 | −31.01 | −23.85 | |
| 1_NE | 23.62 | 28.27 | 32.73 |
| +18.75 | +7.7 | −0.4 | |
| 2_NE | 15.32 | 21 | 27.14 |
| −22.98 | −20 | −17.41 | |
| 3_NE | 14.68 | 20.98 | 27.94 |
| −26.19 | −20.08 | −14.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šárpataky, M.; Kurimský, J.; Rajňák, M.; Krbal, M.; Adamčák, M. Dielectric Performance of Natural- and Synthetic-Ester-Based Nanofluids with Fullerene Nanoparticles. Energies 2023, 16, 343. https://doi.org/10.3390/en16010343
Šárpataky M, Kurimský J, Rajňák M, Krbal M, Adamčák M. Dielectric Performance of Natural- and Synthetic-Ester-Based Nanofluids with Fullerene Nanoparticles. Energies. 2023; 16(1):343. https://doi.org/10.3390/en16010343
Chicago/Turabian StyleŠárpataky, Miloš, Juraj Kurimský, Michal Rajňák, Michal Krbal, and Marek Adamčák. 2023. "Dielectric Performance of Natural- and Synthetic-Ester-Based Nanofluids with Fullerene Nanoparticles" Energies 16, no. 1: 343. https://doi.org/10.3390/en16010343
APA StyleŠárpataky, M., Kurimský, J., Rajňák, M., Krbal, M., & Adamčák, M. (2023). Dielectric Performance of Natural- and Synthetic-Ester-Based Nanofluids with Fullerene Nanoparticles. Energies, 16(1), 343. https://doi.org/10.3390/en16010343






