Sorption–Dilatometric Properties of Coal from a High-Methane Mine in a CO2 and CH4 Atmosphere
Abstract
1. Introduction
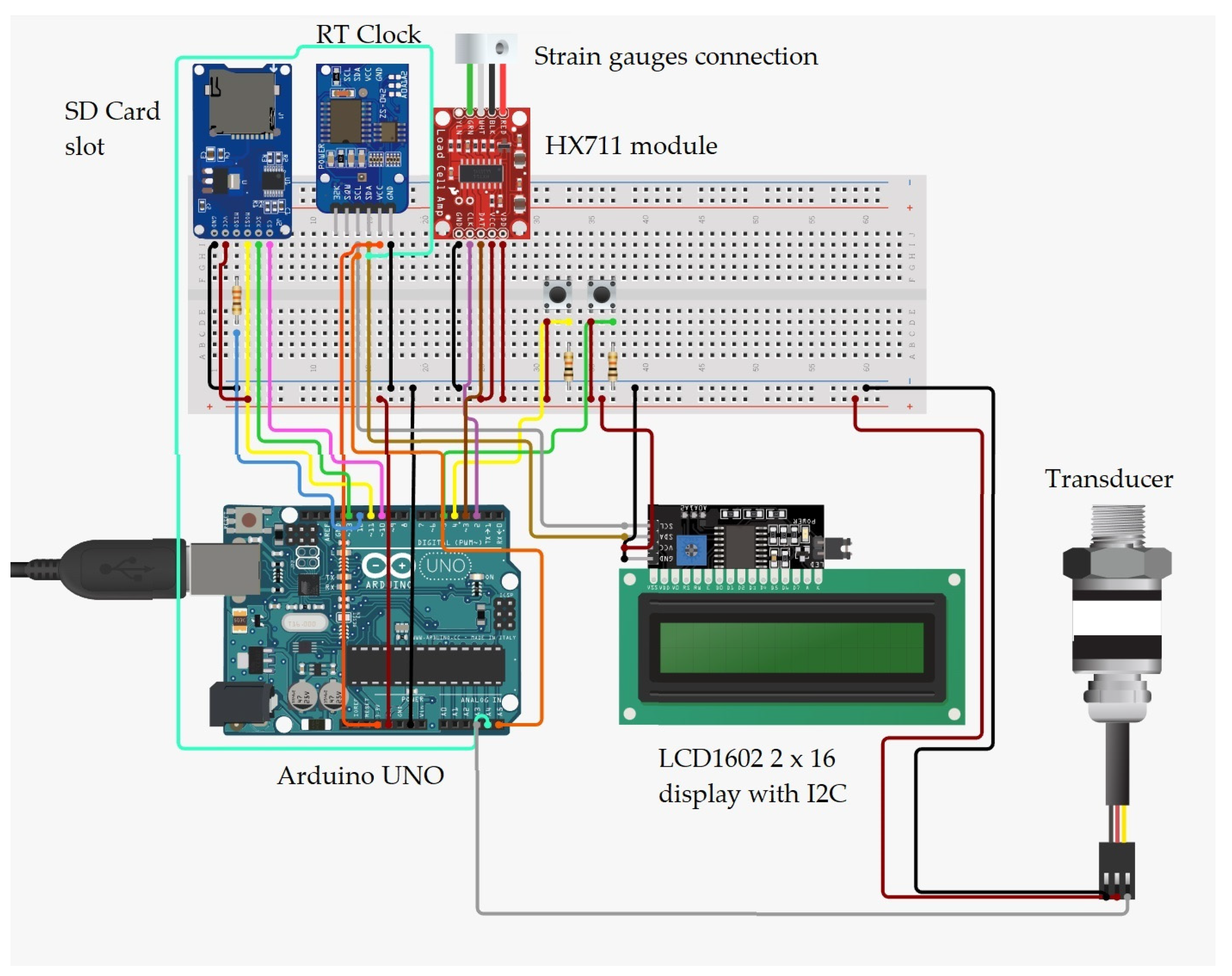
2. Materials and Methods
3. Test Procedure
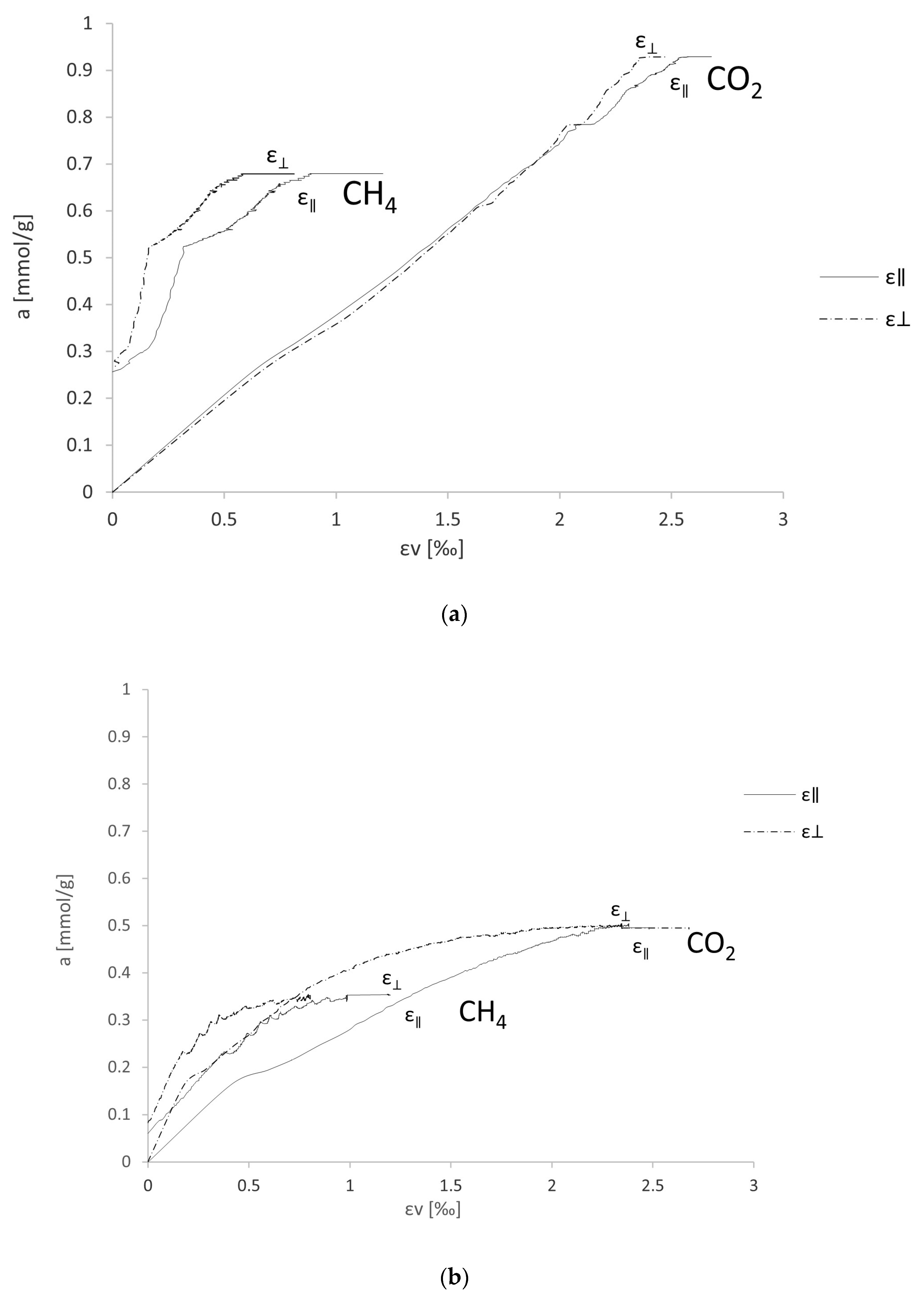
4. Results and Discussion
- εv—volumetric strain
- ε‖—deformations parallel to the layering
- ε⊥—deformations perpendicular to the layering
5. Conclusions
- Change in the linear dimensions of the sample in the isothermal process due to carbon dioxide sorption is 2.5× greater than for methane sorption, with a noticeable difference between the two samples due to the different vitrinite content.
- The relationship between sorption capacity and volumetric expansion in the isothermal process for carbon dioxide for P1 is nearly linear, while for methane it is increasing. For P2, the graph has a curved shape. In the analysis of the later phase of the experiment, the rate of increase in volumetric strain is higher than the rate of increase in sorption capacity.
- Investigations carried out with increasing temperatures provided new data on the behaviour of the coal matrix. As the temperature increased, a slight increase in the sorption capacity of the sample was observed, with the sample shrinking in a carbon dioxide atmosphere and expanding in a methane atmosphere.
- The analysis of sorption capacity changes as a function of volume expansion under non-isothermal conditions provides relevant information about the carbon–gas system. As the temperature increases, no significant change in sorption capacity is observed, while a change in the volume dimensions of the sample is observed, indicating that the parameter related to the movement of vapours and gases within the carbon matrix has a greater influence on this process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- International Energy Agency. Coal 2019; Market Report Series: Coal; OECD: Paris, France, 2019; ISBN 9789264391604. [Google Scholar]
- Kowalska, N.; Brodawka, E.; Smoliński, A.; Zarębska, K. The European Education Initiative as a Mitigation Mechanism for Energy Transition. Energies 2022, 15, 6633. [Google Scholar] [CrossRef]
- Kelemen, P.; Benson, S.M.; Pilorgé, H.; Psarras, P.; Wilcox, J. An Overview of the Status and Challenges of CO2 Storage in Minerals and Geological Formations. Front. Clim. 2019, 1, 9. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Yang, J.; Tohidi, B.; Chuvilin, E.; Istomin, V.; Bukhanov, B.; Cheremisin, A. CO2 Capture by Injection of Flue Gas or CO2–N2 Mixtures into Hydrate Reservoirs: Dependence of CO2 Capture Efficiency on Gas Hydrate Reservoir Conditions. Environ. Sci. Technol. 2018, 52, 4324–4330. [Google Scholar] [CrossRef] [PubMed]
- Hassanpouryouzband, A.; Joonaki, E.; Edlmann, K.; Haszeldine, R.S. Offshore Geological Storage of Hydrogen: Is This Our Best Option to Achieve Net-Zero? ACS Energy Lett. 2021, 6, 2181–2186. [Google Scholar] [CrossRef]
- Markewitz, P.; Kuckshinrichs, W.; Leitner, W.; Linssen, J.; Zapp, P.; Bongartz, R.; Schreiber, A.; Müller, T.E. Worldwide Innovations in the Development of Carbon Capture Technologies and the Utilization of CO2. Energy Environ. Sci. 2012, 5, 7281–7305. [Google Scholar] [CrossRef]
- Zhang, D.; Song, J. Mechanisms for Geological Carbon Sequestration. Procedia IUTAM 2014, 10, 319–327. [Google Scholar] [CrossRef]
- Page, B.; Stern, N.; Hameister Oam, J. Global Status of CCS 2020; Global CCS Institute: Melbourne, Australia, 2020. [Google Scholar]
- Karacan, C.Ö.; Ruiz, F.A.; Cotè, M.; Phipps, S. Coal Mine Methane: A Review of Capture and Utilization Practices with Benefits to Mining Safety and to Greenhouse Gas Reduction. Int. J. Coal Geol. 2011, 86, 121–156. [Google Scholar] [CrossRef]
- Mangi, H.N.; Detian, Y.; Hameed, N.; Ashraf, U.; Rajper, R.H. Pore Structure Characteristics and Fractal Dimension Analysis of Low Rank Coal in the Lower Indus Basin, SE Pakistan. J. Nat. Gas Sci. Eng. 2020, 77, 103231. [Google Scholar] [CrossRef]
- Moore, T.A. Coalbed Methane: A Review. Int. J. Coal Geol. 2012, 101, 36–81. [Google Scholar] [CrossRef]
- Gürdal, G.; Yalçın, M.N. Gas Adsorption Capacity of Carboniferous Coals in the Zonguldak Basin (NW Turkey) and Its Controlling Factors. Fuel 2000, 79, 1913–1924. [Google Scholar] [CrossRef]
- Corrente, N.J.; Zarȩbska, K.; Neimark, A. V Deformation of Nanoporous Materials in the Process of Binary Adsorption: Methane Displacement by Carbon Dioxide from Coal. J. Phys. Chem. C 2021, 125, 21310–21316. [Google Scholar] [CrossRef]
- Guo, D.; Guo, X. The Influence Factors for Gas Adsorption with Different Ranks of Coals. Adsorpt. Sci. Technol. 2017, 36, 904–918. [Google Scholar] [CrossRef]
- Zarebska, K.; Baran, P.; Cygankiewicz, J.; Dudzinska, A.; Zarçbska, K.; Baran, P.; Cygankiewicz, J.; Dudzińska, A. Sorption of Carbon Dioxide on Polish Coals in Low and Elevated Pressure. Fresenius Environ. Bull. 2012, 21, 4003–4008. [Google Scholar]
- Macuda, J.; Baran, P.; Wagner, M. Evaluation of the Presence of Methane in Złoczew Lignite: Comparison with Other Lignite Deposits in Poland. Nat. Resour. Res. 2020, 29, 3841–3856. [Google Scholar] [CrossRef]
- Jian, X.; Guan, P.; Zhang, W. Carbon Dioxide Sorption and Diffusion in Coals: Experimental Investigation and Modeling. Sci. China Earth Sci. 2012, 55, 633–643. [Google Scholar] [CrossRef]
- Hildenbrand, A.; Krooss, B.M.; Busch, A.; Gaschnitz, R. Evolution of Methane Sorption Capacity of Coal Seams as a Function of Burial History—A Case Study from the Campine Basin, NE Belgium. Int. J. Coal Geol. 2006, 66, 179–203. [Google Scholar] [CrossRef]
- Wojtacha-Rychter, K.; Howaniec, N.; Smoliński, A. Effect of Porous Structure of Coal on Propylene Adsorption from Gas Mixtures. Sci. Rep. 2020, 10, 11277. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Marc Bustin, R. Variation in Micropore Capacity and Size Distribution with Composition in Bituminous Coal of the Western Canadian Sedimentary Basin: Implications for Coalbed Methane Potential. Fuel 1996, 75, 1483–1498. [Google Scholar] [CrossRef]
- Laxminarayana, C.; Crosdale, P.J. Role of Coal Type and Rank on Methane Sorption Characteristics of Bowen Basin, Australia Coals. Int. J. Coal Geol. 1999, 40, 309–325. [Google Scholar] [CrossRef]
- Sadasivam, S.; Masum, S.; Chen, M.; Stańczyk, K.; Thomas, H. Kinetics of Gas Phase CO2 Adsorption on Bituminous Coal from a Shallow Coal Seam. Energy Fuels 2022, 36, 8360–8370. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, S.; Wang, K.; Wang, L.; Xu, Y.; Wu, Z.; Shao, H.; Wang, Y.; Miao, M. Thermogravimetric Dynamics and FTIR Analysis on Oxidation Properties of Low-Rank Coal at Low and Moderate Temperatures. Int. J. Coal Prep. Util. 2015, 35, 39–50. [Google Scholar] [CrossRef]
- Talapatra, A. A Study on the Carbon Dioxide Injection into Coal Seam Aiming at Enhancing Coal Bed Methane (ECBM) Recovery. J. Pet. Explor. Prod. Technol. 2020, 10, 1965–1981. [Google Scholar] [CrossRef]
- Talapatra, A.; Karim, M.M. The Influence of Moisture Content on Coal Deformation and Coal Permeability during Coalbed Methane (CBM) Production in Wet Reservoirs. J. Pet. Explor. Prod. Technol. 2020, 10, 1907–1920. [Google Scholar] [CrossRef]
- Czerw, K.; Baran, P.; Zarębska, K. Application of the Stretched Exponential Equation to Sorption of Mine Gases and Sorption Induced Swelling of Bituminous Coal. Int. J. Coal Geol. 2017, 173, 76–83. [Google Scholar] [CrossRef]
- Ranathunga, A.S.; Perera, M.S.A.; Ranjith, P.G.; Rathnaweera, T.D.; Zhang, X.G. Effect of Coal Rank on CO2 Adsorption Induced Coal Matrix Swelling with Different CO2 Properties and Reservoir Depths. Energy Fuels 2017, 31, 5297–5305. [Google Scholar] [CrossRef]
- Czerw, K. Methane and Carbon Dioxide Sorption/Desorption on Bituminous Coal—Experiments on Cubicoid Sample Cut from the Primal Coal Lump. Int. J. Coal Geol. 2011, 85, 72–77. [Google Scholar] [CrossRef]
- Espinoza, D.N.; Vandamme, M.; Pereira, J.M.; Dangla, P.; Vidal-Gilbert, S. Measurement and Modeling of Adsorptive-Poromechanical Properties of Bituminous Coal Cores Exposed to CO2: Adsorption, Swelling Strains, Swelling Stresses and Impact on Fracture Permeability. Int. J. Coal Geol. 2014, 134, 80–95. [Google Scholar] [CrossRef]
- Gaowei, Y.; Chunlin, Z.; Liupeng, H.; Xinjun, Z. Measurement and Modeling of Temperature Evolution during Methane Desorption in Coal. Sci. Rep. 2020, 10, 3146. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, X.; Yue, G.; Kang, B.; Xie, C.; Li, X. Physical Simulation of Temperature Influence on Methane Sorption and Kinetics in Coal: Benefits of Temperature under 273.15 K. Fuel 2015, 158, 207–216. [Google Scholar] [CrossRef]
- Jakubów, A.; Tor, A.; Tobiczyk, S. Wyrzut Metanu i Skał w Drążonej Lunecie Rurowej Do Szybu II Na Poziomie 1000m w KRK Pniówek- Okoliczności, Przyczyny i Skutki. In Proceedings of the Szkoła Ekspoloatacji Podziemnej, Szczyrk, Poland, 7–21 February 2003. [Google Scholar]
- Ocena Stanu Bezpieczeństwa Pracy, Ratownictwa Górniczego Oraz Bezpieczeństwa Powszechnego w Związku z Działalnością Górniczo-Geologiczną w 2015r; WUG: Katowice, Poland, 2016.
- Wasilewski, S.; Jamróz, P. Wybrane Katastrofy i Wypadki w Górnictwie Polskim—Zebranie Danych. Pr. Inst. Mech. Górotworu PAN 2018, 20, 197–206. [Google Scholar]
- Majewska, Z.; Ceglarska-Stefańska, G.; Majewski, S.; Ziętek, J. Binary Gas Sorption/Desorption Experiments on a Bituminous Coal: Simultaneous Measurements on Sorption Kinetics, Volumetric Strain and Acoustic Emission. Int. J. Coal Geol. 2009, 77, 90–102. [Google Scholar] [CrossRef]
- Zarębska, K.; Ceglarska-Stefańska, G. The Change in Effective Stress Associated with Swelling during Carbon Dioxide Sequestration on Natural Gas Recovery. Int. J. Coal Geol. 2008, 74, 167–174. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Cheng, Q.; Gao, Z. Characterization of Anisotropic Coal Permeability with the Effect of Sorption-Induced Deformation and Stress. Fuel 2022, 309, 122089. [Google Scholar] [CrossRef]
- Nikoosokhan, S.; Vandamme, M.; Dangla, P. A Poromechanical Model for Coal Seams Saturated with Binary Mixtures of CH4 and CO2. J. Mech. Phys. Solids 2014, 71, 97–111. [Google Scholar] [CrossRef]
- Guo, P. A Theoretical Model for Coal Swelling Induced by Gas Adsorption in the Full Pressure Range. Adsorpt. Sci. Technol. 2020, 38, 94–112. [Google Scholar] [CrossRef]
- Ceglarska-Stefanska, G.; Zarebska, K. Expansion and Contraction of Variable Rank Coals During the Exchange Sorption of CO2 and CH4. Adsorpt. Sci. Technol. 2002, 20, 49–62. [Google Scholar] [CrossRef]
- Ceglarska-Stefańska, G.; Zarębska, K. The Competitive Sorption of CO2 and CH4 with Regard to the Release of Methane from Coal. Fuel Process. Technol. 2002, 77–78, 423–429. [Google Scholar] [CrossRef]
- Wierzbicki, M. Changes in the Sorption/Diffusion Kinetics of a Coal-Methane System Caused by Different Temperatures and Pressures. Gospod. Surowcami Miner.—Miner. Resour. Manag. 2013, 29, 155–168. [Google Scholar] [CrossRef]
- Dutka, B.; Kudasik, M.; Pokryszka, Z.; Skoczylas, N.; Topolnicki, J.; Wierzbicki, M. Balance of CO2/CH4 Exchange Sorption in a Coal Briquette. Fuel Process. Technol. 2013, 106, 95–101. [Google Scholar] [CrossRef]
- Baran, P.; Czerw, K.; Samojeden, B.; Czuma, N.; Zarębska, K.; Baran, P.; Czerw, K.; Samojeden, B.; Czuma, N.; Zarębska, K. The Influence of Temperature on the Expansion of a Hard Coal-Gas System. Energies 2018, 11, 2735. [Google Scholar] [CrossRef]
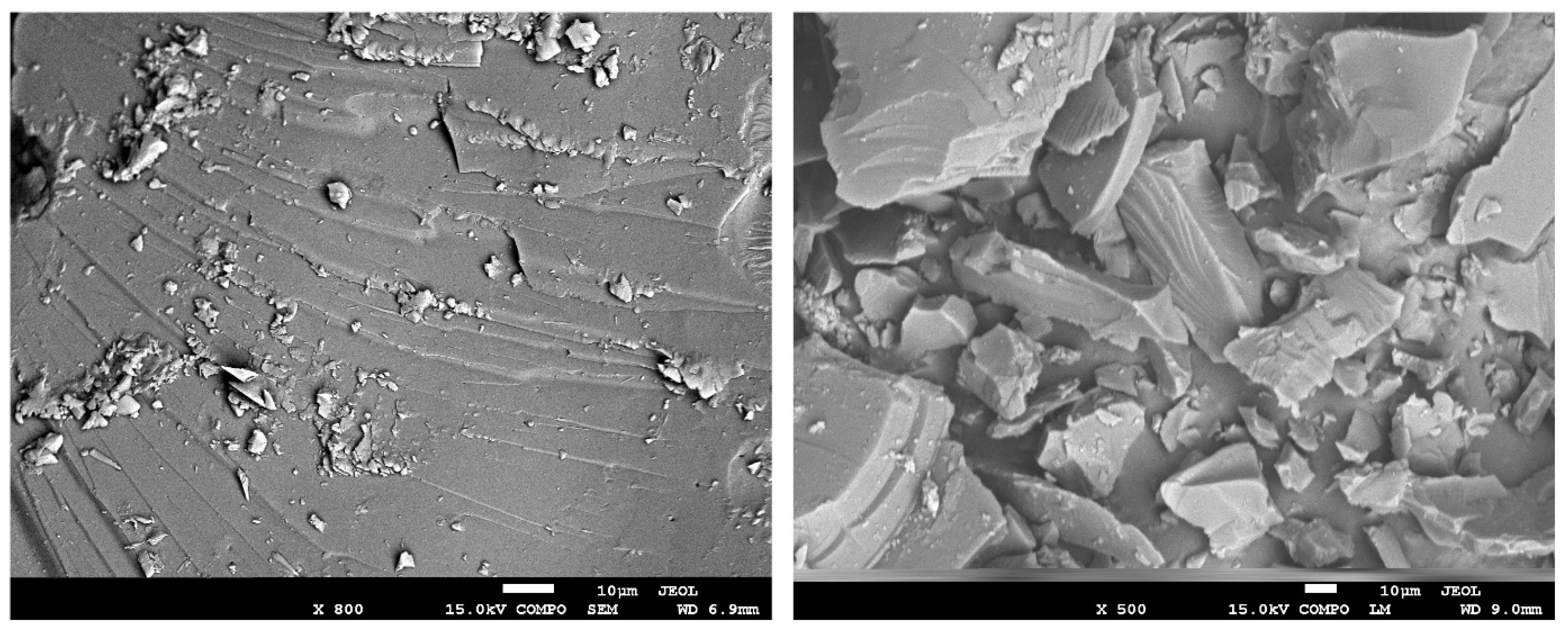




| Sample | C daf | S daf | H daf | N daf | O daf | W a | A a | V daf | Vitrinite | Liptinite | Inertinite |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | |
| P1 | 84.24 | 0.39 | 4.58 | 1.52 | 4.58 | 1.75 | 3.01 | 27.12 | 73 | 7 | 20 |
| P2 | 84.96 | 0.58 | 4.60 | 1.70 | 3.76 | 0.68 | 3.78 | 25.50 | 53 | 8 | 39 |
| Sorbate | Melting Point | Boiling Point | Density at 1 atm, 273 K | Hybridization | Kinetic Diameter |
|---|---|---|---|---|---|
| (K) | (K) | (kg/m3) | (-) | (A) | |
| CO2 | 84.24 | 194.7 (sublimes) | 1.977 | sp | 3.3 |
| CH4 | 90.694 | 111.6 | 0.717 | sp3 | 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baran, P.; Kozioł, S.; Czerw, K.; Smoliński, A.; Zarębska, K. Sorption–Dilatometric Properties of Coal from a High-Methane Mine in a CO2 and CH4 Atmosphere. Energies 2023, 16, 1785. https://doi.org/10.3390/en16041785
Baran P, Kozioł S, Czerw K, Smoliński A, Zarębska K. Sorption–Dilatometric Properties of Coal from a High-Methane Mine in a CO2 and CH4 Atmosphere. Energies. 2023; 16(4):1785. https://doi.org/10.3390/en16041785
Chicago/Turabian StyleBaran, Paweł, Stanisław Kozioł, Katarzyna Czerw, Adam Smoliński, and Katarzyna Zarębska. 2023. "Sorption–Dilatometric Properties of Coal from a High-Methane Mine in a CO2 and CH4 Atmosphere" Energies 16, no. 4: 1785. https://doi.org/10.3390/en16041785
APA StyleBaran, P., Kozioł, S., Czerw, K., Smoliński, A., & Zarębska, K. (2023). Sorption–Dilatometric Properties of Coal from a High-Methane Mine in a CO2 and CH4 Atmosphere. Energies, 16(4), 1785. https://doi.org/10.3390/en16041785







