1. Introduction
Concrete, which is made from a mixture of cement, coarse and fine aggregates, water, and, sometimes, other substances, is widely used in construction thanks to its high compression strength, rigidity, and low cost; however, its main disadvantage is its weak tensile strength, which can lead to the occurrence of cracks [
1].
Cracks in concrete form empty spaces in buildings, possibly causing less heat conduction, i.e., greater thermal resistance. The greater number of cracks and the larger their distribution, the higher the temperature gradient, thus affecting heat distribution. Although the air is a good thermal insulator (low heat conductor), under uncontrolled conditions, it can create hotspots in factories, making temperature conditions unhealthy for employees and reducing the energy efficiency of the structure. On the other hand, in bridges, these temperature differences can cause thermal dilation in the structure, which makes their periodic maintenance necessary [
2]. Studies have been conducted to develop self-healing processes that can be employed to seal cracks in concrete in an eco-sustainable and energy-efficient way.
Cement is the second concrete compound in terms of consumption. The cement industry is an important contributor to carbon dioxide (CO
2) emissions [
3]. Another major problem is the toxicity caused by particles containing silicon dioxide (SiO
2). Therefore, the duality between the high consumption and high toxicity of cement needs to be reconciled [
4].
The wetting and drying of cement mixed with coarse and fine aggregates in adequate proportions enable the formation of durable concrete [
5]. However, several environmental factors, such as physicochemical weathering, biological corrosion, subsidence of soil, and human activities, cause cracks to form in concrete structures. Synthetic materials used to fill the cracks and seal the concrete, such as epoxy or acrylic resins [
6], are harmful to human health and the environment; therefore, there is a need to explore novel cement materials. In this context, biomineralization could be a good option for filling cracks from the standpoint of preserving the environment [
7].
Biomineralization is a natural phenomenon involving the transformation of an organic substance into an inorganic one through chemical changes in the environment due to microbial activity. Different genera of bacteria are able to perform microbial production of calcium carbonate (CaCO
3) in a broad gamut of natural environments [
7].
Crystallization is another important aspect in bioconcrete systems for the control and filling of pores. This operation requires knowledge of the solubility curve of substances, the temperature for a microbial agent to be used under optimal crystallization conditions, and possible polymorphs and pseudo-polymorphs of substances [
4].
Several analysis methods are used to study the effects of crack width and measure self-healing. The assessment of self-healing involves a set of evaluations, such as the quantification of the cracking phenomenon, the recovery of one or more engineering characteristics of interest, and a qualitative characterization of precipitated crystals. Structural tests are performed on the macro-, micro-, and nanoscales to define a performance criterion for hardened concrete. Assessments of macrostructure, microstructures, and nanostructures are also performed [
8].
The present review article addresses the basic concepts and analysis methods related to self-healing concrete with the aim of obtaining stronger, more efficient, and less polluting materials capable of improving the energy efficiency of buildings, thus contributing to the building of a sustainable society.
2. Clinker, Silicon Dioxide, and Clay in Cements
The higher carbon emissions from cement production result from the combination of two of the three main sources of anthropogenic emissions of CO
2 to the atmosphere: i) carbonate decomposition; and ii) oxidation of fossil fuels. With roughly 60% of the raw material comprising of carbonates (mostly limestone, CaCO
3), the production of the main component of Portland cement (clinker) results in the release of large amounts of CO
2 to the atmosphere during the process of calcination (decomposition of CaCO
3 into CaO and CO
2 by the addition of heat). The energy required for crystallization (roughly 1450 °C) and all processes before and after the sintering process imply the combustion of significant amounts of fossil fuels, both directly (thermal energy generation) and indirectly (through electricity production). The emissions resulting from the thermal processing of the raw material to obtain the clinker, referred to as energy emissions, can add a further 60–90% of CO
2 to the process emissions, depending on the technology used [
9].
The generation of Portland cement accounts for 5 to 7% of CO
2 emissions throughout the world, with a tendency to increase by as much as 30% (reaching 6.5 to 9%) by 2050 if measures are not taken to intervene. Another important aspect that should be taken into consideration to minimize the impact on the environment and human health resides in the obtaining of Portland cement with a low content of clinker, as its replacement by other products with cement characteristics could assist in reducing CO
2 emissions during manufacturing [
10]. In addition to these factors, cement production is an energy-intensive industry, which consumes nearly 5% of global industrial energy. Since the energy cost of cement production accounts for 30–40% of the total production cost [
11], it is crucial to search for more eco-efficient concrete to diminish the discharge of CO
2 in the atmosphere and the energy cost of its production.
To produce Portland cement, raw materials should be combined, in the consequence, an open mining method is used for mining operations. Quarrying is achieved by digging, blowing, and using heavy earth-moving machinery such as bulldozers and dump trucks. Enough calcium, silicon, aluminum, and iron are needed to form the typical clinker composition. Lime and silica are the primary qualities of cement while iron decreases the temperature of the reaction and gives the cement its characteristic grey color. In the cement manufacturing process, limestone, shale, and clay are dried, crushed, blended, and heated in cement ovens up to 1200–1450°C to produce the clinker. A high temperature is needed for chemical reactions to occur in the burning region. A nodular substance is formed and is then allowed to leave the oven. Clinker is then used as the main ingredient for cement. It is crushed and blended with other ingredients such as limestone and gypsum to produce ordinary Portland cement. During the process, a significant amount of fossil fuel and alternative fuel is burned to provide heat to cement ovens, dryers, and preheaters. In addition, a lot of energy is consumed in cement ovens and crushing machines. Thus, to manufacture a large volume of cement, a great amount of energy is consumed. The initial stages of the cement production process until the final stage contribute to pollution, especially air pollution. Studies have shown cement production releases roughly 0.8 tons of CO
2 per ton of cement into the atmosphere [
12].
Antoniazzi et al. [
13] evaluated the action of additives found in mortar and mixed into Portland cement paste. The compounds in the mortar were air-entraining additives and hydration stabilizers, the latter of which have a greater ability to stabilize Portland cement mixtures. These stabilized mortars and their chemical additives are widely used in civil construction and are mixed with cement, fine aggregates, and water to improve the process, increasing the workability time of the mixture. Nonetheless, considerable care is required with these techniques to avoid an increase in porosity [
14]. These additives delay the onset of chemical reactions in cement, for the benefit of its composition, such as the content of minerals in the clinker [
15].
Data reported in the literature show the influence of the type of cement and quantity of water employed in stabilized mortars as well as air-entraining additives and hydration stabilizers in Portland cement paste to understand the effect of these additives on cement particles as well as the combined effects among additives [
15]. Thus, it is possible to improve the characteristics and properties while avoiding environmental contamination and harm to human health due to CO
2 emissions.
3. Crystallization
When placed into a pure solvent, a salt dissociates into a cation and an anion due to intermolecular interactions between the solvent and salt (solute). As more salt crystals are added, more ions are formed until reaching a threshold beyond which the remaining solvent molecules do not have enough energy to break the salt ionic bonds, resulting in the precipitation of excess salt; therefore, every saline solution has the chemical potential necessary for dissociating salt. The solubility limits of the salt differ depending on variations in the solute, solvent, pressure, and temperature [
4].
The International Union of Pure and Applied Chemistry (IUPAC) defines solubility as the analytical composition of a saturated solution expressed in terms of the proportion of a given solute in a given solvent. Solubility can be expressed in molality, molar fraction, molar ratio, or other units of concentration [
16].
Solubility occurs in dynamic equilibrium, which results in simultaneous opposing processes of dissolution and aggregation (e.g., precipitation of solids). Solubility equilibrium occurs when the two processes proceed at a constant rate [
17]. The main factors that affect the solubility equilibrium [
18] are the following:
Temperature: The solubility of precipitates generally increases with the increase in temperature, with the exception of special cases such as calcium sulfate;
Composition of solvent: The interaction between a solute and a solvent determines how much solute can dissolve. Solutes with molecules that are strongly attracted to water molecules tend to dissolve more easily in this solvent;
Nature and concentration of other compounds: The solubility of a compound generally decreases in the presence of a common ion and increases in the presence of a foreign ion.
Solubility plays an important role in diverse fields of industry, including the chemical, environmental, food, pharmaceutical, and cosmetic industries [
19]. Solubility curves are diagrams that indicate the variation in the solubility coefficients of compounds as a function of temperature and illustrate the dependence of solubility on the substances present in solvents. Knowledge of the solubility curve of a solute in a solvent is fundamental to outlining an efficient crystallization strategy [
18].
The importance of solubility curves for crystallization is exemplified in
Figure 1. The region under the solubility curve characterizes unsaturated solutions in which the crystals of a solute will dissolve. A solution with a saturation concentration can coexist with a solid phase of solute (thermodynamic equilibrium). The region of supersaturation is above the solubility, and it is only in this region that crystallization can occur (i.e., the removal of the solute and/or solvent from the solution and its deposition in a solid phase). The supersaturation zone is subdivided into metastable and labile zones, which are separated by the metastable limit. If decreasing linearly or according to an exponential function, the solubility curve will completely change the metastable limit and, consequently, alter the mechanisms and methods for the occurrence of crystallization [
4,
18].
The degree of supersaturation of a solution represents its distance with respect to the equilibrium concentration (saturation). As crystallization can only occur if a solution is supersaturated, supersaturation is the driving force of crystallization processes [
18].
Crystallization is a natural process by which materials solidify from liquids or precipitate from liquid or gaseous solutions. This process consists of intermolecular bonds in highly ordered structures, denominated crystals, and can be triggered by changes in the physical or chemical environment, such as temperature. The crystallization process generally involves nucleation and growth of crystals [
20,
21].
Nucleation is the formation of crystalline bodies from a supersaturated solution, in whose supersaturation region a dynamic equilibrium of formation and rupture of quasi-liquids of the solute is established. An increase in the degree of supersaturation favors the formation of such clusters consisting of dozens of molecules linked by relatively weak intermolecular forces and organized in a regular manner. The transformation of larger aggregates to the form of nuclei occurs by both the arrangement of the constituents in the form of a crystalline structure and by a solid–liquid interface. This is accompanied by a change in the global Gibbs free energy of the system, which, in turn, is related to the size of the crystals. This change has a maximum point corresponding to a critical crystal size, denominated critical nucleus, and constitutes an energy barrier to be overcome so that nucleation can occur. Only clusters that reach the critical size stabilize, whereas those that do not redissolve [
18].
Nucleation can occur through different mechanisms and is divided into primary nucleation, when occurring in a solution completely free of solute crystals, or secondary nucleation, when solute crystals are already in the solution. Primary nucleation is associated with high levels of supersaturation and is classified as homogeneous or heterogeneous. Due to the high levels of supersaturation required, homogeneous nucleation does not occur in the metastable zone, but only at the limit between the labile and metastable zones [
18].
There are several crystallization techniques, which are identified based on the method by which supersaturation is achieved, such as cooling, evaporation, vacuum crystallization, addition of an anti-solvent (“drowning out” or “salting out”), or chemical reaction. The technique employed is generally the one providing the highest yield with the least energy consumption. When the solubility of a material diminishes considerably with the reduction in temperature, cooling is the most widely used technique to achieve supersaturation. Evaporation, the second most common technique, is especially preferred when solubility is not as dependent on temperature. A third technique, involving the use of a system under vacuum at a given temperature, solvent evaporation, and solution cooling, is known as adiabatic cooling. It is recommended for solutes whose solubility is not particularly dependent on temperature and are thermosensitive, i.e., the use of heat to evaporate the solvent would lead to degradation of the substance of interest. Lastly, precipitation, which is also known as reactive crystallization, is a chemical reaction that leads to the synthesis of a product with low solubility in concentrated solutions. As the product solubility is quickly exceeded, the solution becomes supersaturated, and the material is crystallized [
18].
In the case of the precipitation of calcium carbonate induced by microorganisms, the concrete or solidified cement has porous fractures, which are filled with solutions or gaseous systems containing calcium lactate, microorganisms, microbial spores, air, and/or O
2. Hence, these systems, which can be triphasic, involve two main phenomena, namely, biochemical reaction and crystallization, with the latter being performed in accordance with each crystallization model. When this complex adheres to the surface and crystalline nuclei are formed, calcium carbonate crystallization occurs through biomineralization [
22].
5. Concrete vs. Bioconcrete
Concrete is a basic mixture of cement, aggregates, and water that is widely used in civil construction. The high performance of concrete is attributed to the presence of chemical additives and mineral compounds in its composition, which are blended to obtain different characteristics and properties [
5,
33]. However, to fit into the definition of sustainable building material, some environmental burdens need to be minimized. One of the main reasons for this problem is the huge production of solid waste and frequently improper or clandestine disposal in dumps [
34,
35]. As a result, civil construction companies have established and mostly adopted the implementation of an environmental policy involving the definition of strategies, actions, and investments as well as institutional and juridical guidelines. The purpose is to ensure the quality of the environment, the conservation of biodiversity, and sustainable development [
35].
Discussion on environmental issues is required, whose main goal should be sustainability in an attempt to find adequate solutions to eliminate or to reduce impacts that affect the environment and human health. To meet the demands of society, the civil construction industry has invested in new technologies, such as recycling, exploitation of solid waste, improvement of chemical products, etc. [
36].
More than 1 m
3 of concrete is produced per person every year, with the emission of approximately 100 kg of CO
2 into the atmosphere during the production of one ton of concrete. This is mainly caused by the use of cement for the production of concrete to be used in most types of construction. Indeed, after water, cement is the most widely consumed material throughout the world. Cement production requires high energy consumption in the form of thermal energy through fuel burning to heat the rotary ovens to produce clinker, which is the basic material necessary for cement manufacture. Fuels that feed the ovens come mainly from non-renewable sources, such as oil, coal, coke, gasoline, and natural gas. During its life, concrete slowly absorbs CO
2 in the process of carbonation, by which the Ca(OH)
2 of concrete is converted into CaCO
3. However, the quantity of concrete produced remains a significant source of anthropogenic CO
2 emissions [
37,
38,
39].
Cement is a ceramic material that produces an exothermic reaction when in contact with water and the crystallization of hydrated products, gaining mechanical strength. This favors the construction of dams, bridges, buildings, electricity posts, etc., which are subject to degradation due to atmospheric agents [
4,
32]. To repair cracks is more expensive than the manufacturing cost. Nowadays, some countries are investing a large amount of money in the maintenance and development of infrastructure. For example, the Netherlands spent one-third of the annual budget for major construction works on inspection, maintenance, and repair, while in the UK, this accounts for more than 45%. Therefore, all costs of production, as well as the repair of structures, need to be taken into consideration [
34].
A self-healing bioconcrete containing a mixture of a composite with additives and bacteria can be used to provide greater protection and a better appearance. Bioconcrete can be used in posts, dams, and other construction projects to make the material stronger and avoid subsequent microcracks [
40,
41]. A bioconcrete based on a composite, additives (sand, gravel, etc.), spheres, and bacterial cells is in fact able to assist in diminishing the occurrence of corrosion, making the structures stronger through the healing of reinforced concrete. In particular, additives give the bioconcrete special characteristics or improvements in its properties, with each of them playing a specific role in its manufacturing [
42,
43].
As a sustainable option, self-healing concrete should reduce greenhouse gas emissions to the atmosphere. This can be achieved indirectly with lower CO
2 emissions, since cement production alone is estimated to account for 7% of the total anthropogenic CO
2, particularly due to the high temperatures in the production process (around 1500 °C). In this set of ideas, if inevitable cracks could be sealed without needing reparation, concrete structures will serve a longer service life, making it sustainable and reducing CO
2 emissions on maintenance, repair, and production of new material. Since emissions should be avoided, reducing ammonia emissions by changing the hydrolysis of urea into calcium lactate should be also considered in order to increase the viability of the concrete [
34].
Furthermore, bacterial cells can play a vital role in particular properties and self-healing processes via the precipitation process of CaCO
3, which may help in accelerating the process of CO
2 sequestration into bioconcrete. The process in which bacterial cells accelerate the sequestrations of CO
2 could be explained as the result of the acceleration of the chemical hydration reaction used in the carbonation process. In this process, the CO
2 gas diffuses through the solution producing CO
2 (aq), HCO
3− (aq), and CO
32− (aq). The hydration reactions lead to the release of bicarbonate ions into the concrete mixture, which reacts with Ca in the cementation materials to form CaCO
3 [
44].
Alshalif et al. [
45] carried out a survey to show the CO
2 sequestration process in bio-foamed concrete bricks (B-FCB) using
Bacillus tequilensis 401 as an acceleration factor of natural carbonation. The results revealed that the
Bacillus tequilensis 401 accelerated CO
2 sequestration in B-FCB by 30% compared to foamed concrete bricks (FCB). The highest carbonation depths were 9.2 mm and 11.9 mm for FCB and B-FCB at the optimized conditions, respectively. The increase in carbonation depth resulted in increased formation of calcium carbonate (CaCO
3) in B-FCB compared to FCB, which was confirmed by microstructure analysis using a scanning electron microscope (SEM), energy dispersive X-ray (EDX), and X-ray diffraction (XRD).
6. Crack Repair and Healing Agents
Conventional reinforced concrete is subject to diverse physical and chemical factors that can exert a negative impact on its physical and mechanical characteristics over time, leading to the emergence of internal and external cracks [
46].
Seasonal changes in temperature favor the formation of cracks in concrete due to thermal expansion and retraction, which causes the loss of a greater quantity of heat on the surface compared to the center, thereby increasing the temperature in the interior of the concrete in summer. Recurrent freezing and thawing in winter also degrade concrete. Moreover, as concrete is porous, the lixiviation of water and gases further favors its degradation [
46,
47].
A complementary solution is to increase the effectiveness of concrete structures, thereby reducing the need for replacement materials. Recovery is currently achieved through the periodic inspection and repair of the structure, which is a slow and expensive process. Concrete degradation generally occurs with the emergence of microcracks, difficult to see with the naked eye, which slowly grow to the point of enabling water and salt to reach the steel of the reinforced concrete, thus leading to corrosion and, ultimately, structural failure. Thus, there has been increasing interest by researchers in innovative technologies and the search for better additives. Self-healing concrete, i.e., a material with the incorporation of microorganisms, is one such technology, as certain bacteria are capable of repairing cracks, thereby prolonging the useful life of concrete structures [
4,
32,
40].
Concrete is highly alkaline and a hostile environment for most microorganisms, but some bacteria are capable of growing in alkaline media with a pH of around 10–11 and can fill cracks in concrete through the production of CaCO
3. However, such bacteria have been applied manually after the emergence of a crack, which means that regular inspection of the concrete structure is still necessary. Bacteria can be incorporated into the concrete along with calcium lactate and remain viable in a dormant state as spores. When a crack is formed, water infiltrates the concrete, activates the bacteria, and oxidizes calcium lactate, and the resulting insoluble calcium carbonate, precipitating, fills and repairs the crack. Thus, the combination of a high pH and CO
2 helps bacteria further in the formation of carbonate [
4,
32,
37,
39].
Since bacterial spores can survive for decades without water and nutrients, concrete containing spores would ideally maintain its self-healing properties. However, Knoben [
37] found that bioconcrete was unable to continue healing itself after only four weeks; nonetheless, the incorporation of bacteria and nutrients in clay capsules prior to mixing them with concrete greatly prolonged the useful life of structures.
The literature reports that the bacteria
Bacillus pseudofirmus and
Sporosarcina pasteurii have great efficiency in the production of calcium carbonate. They were found in alkaline lakes in the proximity of volcanoes and can remain dormant for up to 200 years. When cracks emerge in concrete, the bacteria are activated due to contact with air and moisture, and the calcium lactate incorporated into the concrete is transformed into CaCO
3 sealing the cracks [
46].
Biomineralization for the reconstitution of the concrete matrix has been studied mainly with regard to crack repair. Feurgard et al. [
48], who studied the application of colloidal thickeners for the retention and cultivation of
B. pseudofirmus, reported the repair of 150 to 800 µm cracks, while Su et al. [
49] repaired even 4000 µm cracks using encapsulated
Bacillus megaterium and calcium lactate.
7. Microencapsulation
Surface deterioration revealed by cracks can be repaired with materials such as silicates or mortar. Since such repair methods are time-consuming and expensive, the focus has recently been changed to the use of intelligent materials and techniques, such as microencapsulation, to prevent damage and minimize the deterioration of concrete structures [
50].
Microencapsulation consists of the use of small solid particles, liquid droplets, or gases in a lining that can be used to protect, transport, and control the release of active compounds [
51]. Microcapsules containing healing agents are incorporated into the concrete, and, when a crack emerges, it breaks the microcapsule shell, releasing the healing agents, whose interaction with a catalyst leads to the formation of a hydrated calcium silicate gel that fills the crack and avoids its propagation [
50].
The advantage of microencapsulating microorganisms together with nutrients is the preservation of their metabolic activity by the creation of a microenvironment suitable for their development [
52]. Microencapsulation also protects the cells from harm during the mechanical mixing of concrete [
53].
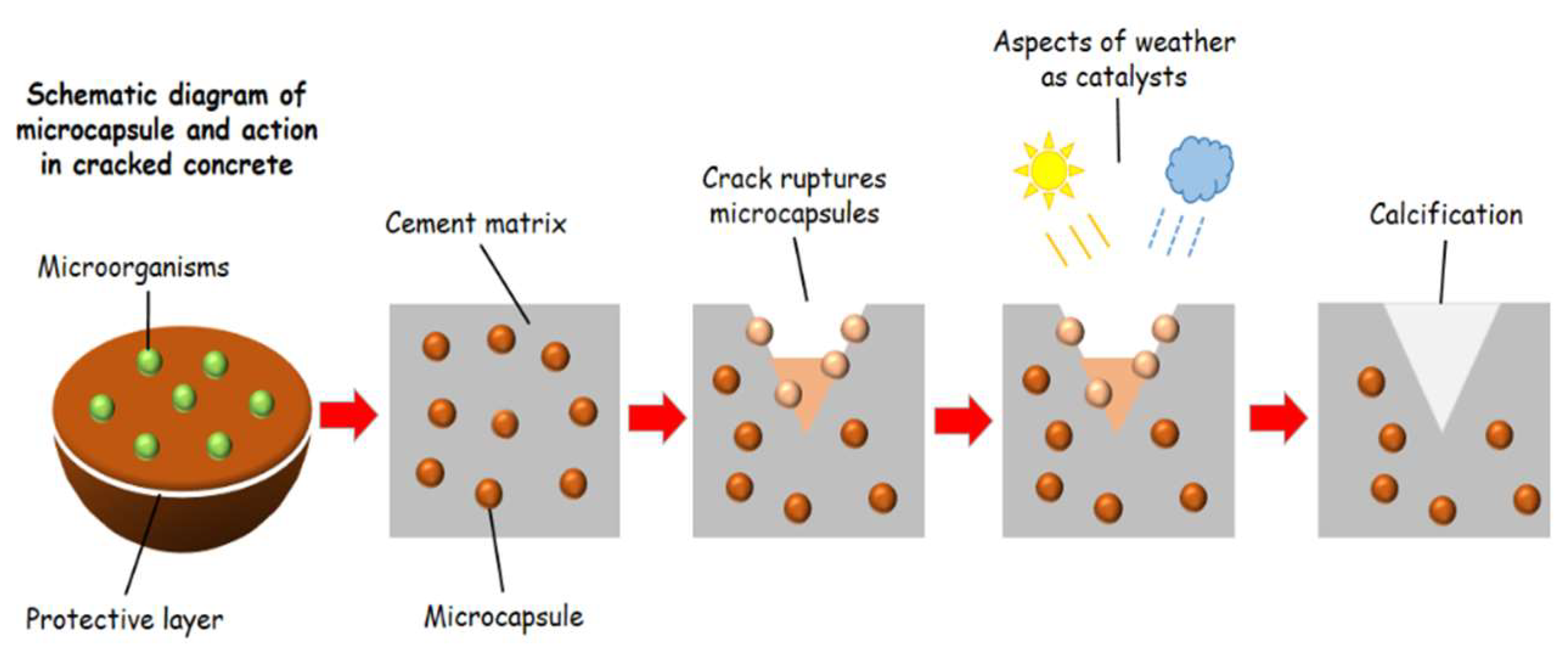
Figure 3 displays a schematic model of a microcapsule and how it contributes to the healing of cracks in concrete.
Immobilization with polyurethane is widely used to prepare microcapsules containing bacteria and nutrients to be incorporated into bioconcrete, as this polymer is chemically stable, strong, and inert. Silica gel and expanded clay are other options [
54]. Studies report that the addition of bacteria capable of inducing the biological production of calcium carbonate can significantly enhance the mechanical properties of mortar and concrete thanks to the deposition of a new layer of calcium carbonate among the particles, which serves as a ligand, diminishing the empty spaces between the sand and cement matrix [
55].
8. pH and Aeration
Bacterial production of CaCO
3 depends on several variables that affect self-regeneration performance, such as the concentration of dissolved organic carbon, calcium, pH, and location of nucleation [
56]. Concrete has a pH of approximately 12 and is exposed to environmental conditions that can inhibit bacterial metabolism; therefore, a selection of bacteria that can tolerate high pH and also precipitate CaCO
3 under such conditions is a major aspect of designing sustainable bioconcrete [
57].
Studies indicate that the increase in pH accelerates CaCO
3 precipitation. As the bacterial production of CaCO
3 occurs under different environmental conditions, it is important to investigate the effect of pH on the efficiency of the biomineralization process. Thus, the bacteria selected for the process need to be well adapted to grow under alkaline conditions and induce CaCO
3 precipitation [
56].
Oxygen availability is another significant factor influencing the biomineralization of CaCO
3. Aerobic microorganisms use oxygen for their growth, leading to the production of bioproducts under certain conditions; therefore, their production of CaCO
3 can be enhanced if a sufficient quantity of oxygen is available in the medium. The actions of the O
2 and pH increase in bioconcrete can be seen in
Figure 4. However, toxins with an inhibitory effect on their metabolism can be produced if an oxygen level beyond the critical one is present in the medium [
57].
Since metabolic pathways and fluxes can be affected by the oxygen transfer rate, the effect of oxygen on microbial growth and bioproducts has been widely documented. Zhang et al. [
58] observed that the supply of oxygen can increase bacterial CaCO
3 production. Some bioprocesses require a high oxygen transfer rate, whereas others, such as the calcium lactate pathway mentioned above, require a lower one to regulate oxygen uptake [
56].
9. Biodeterioration of Concrete
Corrosion of steel reinforcement is a major durability issue of reinforced concrete structures using Portland cement concrete in many parts of the world. Concrete cracks are inevitable due to cement hydration shrinkage, hydration heat, and mechanical loading. The presence of cracks in concrete, regardless of their source and form, can have a considerable influence on the mechanism and dynamics of reinforcement corrosion of reinforced structures. Reinforcement corrosion in concrete is affected by moisture, chloride ions, and oxygen concentration and is closely related to the exposed area of reinforcement [
59,
60].
Corrosion is defined as an irreversible interfacial reaction between a metal, ceramic, or polymer material and the environment, which results in the consumption of the material or dissolution of a component of the environment in the material [
61]. Corrosion of reinforcing steel, which leads to loss of its cross-sectional area, is considered the dominant type of deterioration of concrete infrastructure and is generally initiated due to the degradation of the protective barrier around the steel rod. Microorganisms can produce acids in concrete, lowering its pH, which enables the penetration of corrosion-causing agents, such as chloride ions, carbon dioxide, and moisture, into the concrete/steel interface [
62].
The corrosion process is a complicated process related to external factors (the concentration of chloride and oxygen) and internal factors (resistance of mortar substrate and steel) that can be summarized as passivation–de-passivation–initial corrosion and corrosion propagation. Chloride, which participates in the formation of intermediate iron–chloride complexes and, ultimately, is not consumed, plays a vital role in the whole process of corrosion. The uncracked mortar can provide effective protection for steel from the invasion of corrosion agents. Although microcracks have a certain self-healing ability, microcracks can facilitate the initial corrosion of steel, and wider cracks seriously enhance corrosion [
60].
The following oxidizing dissolution reactions occur in the anodic zone (Equations (12) and (13)) on the metal surface [
63]:
On the other hand, as in the case of reinforced concrete, oxygen reduction reactions occur in the cathodic zone (Equations (14) and (15)) at the interface between metal and electrolyte, which depend on both the availability of dissolved oxygen and the pH [
63]:
Besides chemical processes, the durability of concrete is also influenced by biological agents including microorganisms. Hydrolytic enzymes and corrosive metabolites secreted by microorganisms such as acids can react with the bonding material on the concrete surface [
64]. The production of sulfuric acid as a result of microbial activity and its subsequent reactions with concrete components that form expansive low-strength products is believed to be the mechanism underlying the deterioration of concrete, weakening its structural integrity, and diminishing its useful life. Favorable conditions, such as high relative humidity, a high concentration of carbon dioxide, chloride ions, other salts, sulfates, and small quantities of acids (low pH), stimulate microbial growth on the surface of concrete and increase the biodeterioration rate [
62].
Biodeterioration occurs when biological activity results in the degradation of a desirable property of a structure [
65]. This process can occur in concrete structures and other materials located in marine environments, including bridges and piers, or in conventional buildings and is often found in sanitation structures [
66,
67]. In recent decades, biodeterioration has become the focus of attention due to its significant economic impact [
62].
Concrete structures are prone to deterioration by microorganisms that starts at the aggregate–cement paste interface [
68]. These microorganisms may be algae, bacteria, fungi, or lichens [
65,
69,
70], whose role in the biodeterioration of concrete can be classified as follows [
62].
- (1)
Physical deterioration: the material structure is affected by microbial growth or movement (physical or mechanical breaking) [
62];
- (2)
Esthetic deterioration: fouling, i.e., formation of a biofilm, alters the surface conditions, influencing the absorption of solar energy, moisture content, and temperature [
62,
71];
- (3)
Chemical deterioration: excretion of metabolites or other compounds, such as hydrogen sulfide and acids, adversely affects the concrete structural properties, e.g., increasing porosity, weakening mineral matrix, etc. [
62].
Biological activity is often associated with the emission of acids that can dissolve minerals such as calcite, dolomite, and hydrated calcium silicate, which is the main component of cement. Moreover, even if the resulting salt is solid and insoluble (i.e., calcite or calcium oxalate), the reduction in pH of the solution in concrete pores causes the de-passivation of reinforcing steel, which initiates a process of concrete structure deterioration due to steel corrosion [
66,
72].
A sulfate attack is a deterioration process exclusive to concrete. Hydrated calcium aluminate and calcium aluminate monosulfate react with sulfur to form ettringite (hydrated calcium aluminum hydroxide sulfate), while hydrated calcium silicate reacts to form thaumasite. Both minerals have a greater volume than reagents and cause the disintegration of concrete [
73].
10. Mechanical Properties, Durability, and Analysis Methods
The addition of bacteria in concrete is commonly reported to improve the mechanical (compressive and flexural strengths, modulus of elasticity, and toughness) and durability properties of concrete. This can be due to the reduction in the number of pores and the increase in compaction in the microstructure of the concrete, decreasing the potential entrance of chemicals and acids into the concrete [
25].
Studies carried out by Nasser et al. [
74] were capable to produce calcite crystals to block the micro-cracks in mortar matrix using
Bacillus pasteurii and
Bacillus sphaericus. The
Bacillus sphaericus and
Bacillus pasteurii improved the physico-mechanical properties with high restoration for load-deflection of bioconcrete. Scanning electron microscopy (SEM), energy Dispersive X-ray Spectrometer (EDAX), and differential thermal analysis (DTA) analyses verified that both strains induced bio-precipitation of calcite, which filled up the matrix pores, and decreased water absorption, capillary permeability, and volume of permeable voids, thus enhancing the physico-mechanical properties of bioconcrete. The bacterial bioactivity induced stiffer behavior when under the same load. Treated samples had less deformation as compared with the control. Both bacterial strains were considered promising for bio-application, and treated samples revealed better physical properties, mechanical performance, and self-healing of bioconcrete.
Smitha et al. [
75] investigated the influence of
Bacillus megaterium on the mechanical and durability properties of concrete. Based on the outcome of the study, it was found that the induction of
B. megaterium into concrete mixtures can be used to improve the mechanical and durability properties of concrete. The optimal concentration of
B. megaterium was found 10
5 cells/mL. Concrete made with 10
5 cells/mL
B. megaterium exhibited compressive strength, split tensile strength, and flexural strength at 11.3%, 97.5%, and 8.6%, respectively, higher than that of the control at 28 days. Similarly, the chloride ion penetration and coefficient of water permeability of concrete incorporating the optimum cell concentration of
B. megaterium was 26.8% and 98.7% lower than that of the control concrete. The enhancement in the properties of the concrete incorporating bacteria can be ascribed to the precipitation of the calcite and the presence of bacteria biomass within the matrix of the concrete, which results in the refinement of the microstructure. Scanning electron microscopy (SEM) images of the concrete mixtures showed that more calcite was produced in concrete made with
B. megaterium at a concentration of 10
5 cells/mL. Energy Dispersive X-ray Analysis (EDX) results also confirmed the formation of calcite in concrete incorporating
B. megaterium as it contains a higher amount of calcium compared to that of the control.
10.1. X-ray Microtomography
X-ray microtomography is a nondestructive method for the percentage determination of the volume of a self-regenerating agent in a cement matrix. The differentiation of phases is more difficult in more complex matrices. With this method, the volume is analyzed three-dimensionally and is separated into multiple regions. Image analysis is applied to classify and quantify each region of interest, which is analyzed to determine the characteristics of the control volume, such as the percentage of self-regenerating agents and distribution of particle size [
76].
10.2. X-ray Diffractometry
X-ray diffractometry (XRD) is used for the determination of the purity of CaCO
3 in concrete self-regeneration. This method enables the comparison of crystallographic profiles of minerals found in nature and the microbially produced CaCO
3. An X-ray diffractometer basically has three main components: an X-ray source, an X-ray detector, and a plate with a crystal sample. The source and detectors have an angular range of 0 to 80°. The X-rays fired by the source diffract in the crystalline arrangement of the sample at a given angle and proceed to the detector under two forms of interference: constructive or destructive. If the rays are constructive, the amplitudes of the X-ray waves are summed, resulting in high wave intensities. Otherwise, the amplitudes of the waves are subtracted, resulting in low or null wave intensities [
77].
When a sample with CaCO
3 crystals is submitted to XRD analysis, three polymorphs are possible in the diffractograms: calcite, aragonite, and vaterite [
78]. The diffractometer furnishes the diffractogram but does not analyze the crystal. Hence, an option for crystallographic analysis is to use software programs that are able to compare the diffractograms of pure components to those generated by the diffractometer. For instance, the HighScore Plus program has a vast crystallographic library and also enables the treatment of noise in the diffractograms, as well as the percentage analysis of intensity peaks, to determine the sample crystallographic distribution.
10.3. Scanning Electron Microscopy
Scanning electron microscopy (SEM) involves the use of an electron beam through the lenses of the microscope, which direct and focus the beam on the sample. This method enables obtaining a three-dimensional topography of chemical compounds and even microorganisms [
79]. After XRD, SEM constitutes a confirmatory analysis for the elucidation of the ordered arrangement of atoms corresponding to CaCO
3 [
80]. With SEM, it is also possible to investigate the porosity of the material, possible microorganisms adhered to the surface of the concrete, and impurities that may be synthesized or crystallized after the biomineralization process [
80].
10.4. Compression, Tensile, and Flexion Strength
The most practical applications that determine whether a concrete sample can support high loads are tests of compression, tensile, and flexion strength. To determine compression and tensile strength, the sample is submitted to two opposing axial forces at the extremities. The difference is that the sample is forced to diminish in volume during the compression test and forced to increase in volume during the tensile test. To determine flexion strength, the sample is submitted to three radial forces, two in one direction at the extremities and one in the opposite direction in the center [
5].
10.5. Water Permeability
Pores in concrete have different size scales and, depending on their quantity, can enable water and other dissolved substances in the universal solvent to percolate through the concrete, carrying undesirable substances, such as chloride ions, which accelerate the corrosion process in reinforced concrete. Another disadvantage of excessive porosity is the formation of water paths in the concrete structure, reducing the strength of the material and causing accelerated wear [
81].
Experimentally, concrete blocks are immersed in water for the saturation of pores and the application of a flux. Equation (16) enables calculating the permeability (
K) in a block of concrete of a given length (
L) [
81]:
where
Q is the flux applied over an area
A,
η is the liquid dynamic viscosity, and Δ
P is the difference in pressure.
Figure 5 displays the methods for bioconcrete analysis.