Recent Approaches to Achieve High Temperature Operation of Nafion Membranes
Abstract
1. Introduction
2. Proton Conduction Mechanism and Structural Characteristics of Nafion Membranes
3. Modifying Agent
3.1. Hygroscopic Materials
3.2. High-Temperature Proton Conductor Materials
3.3. Functional-Group-Modified Materials
3.4. Promote Proton Conduction Materials
4. Modifying Methods Applied to Nafion Membranes
4.1. The Solution-Casting Method
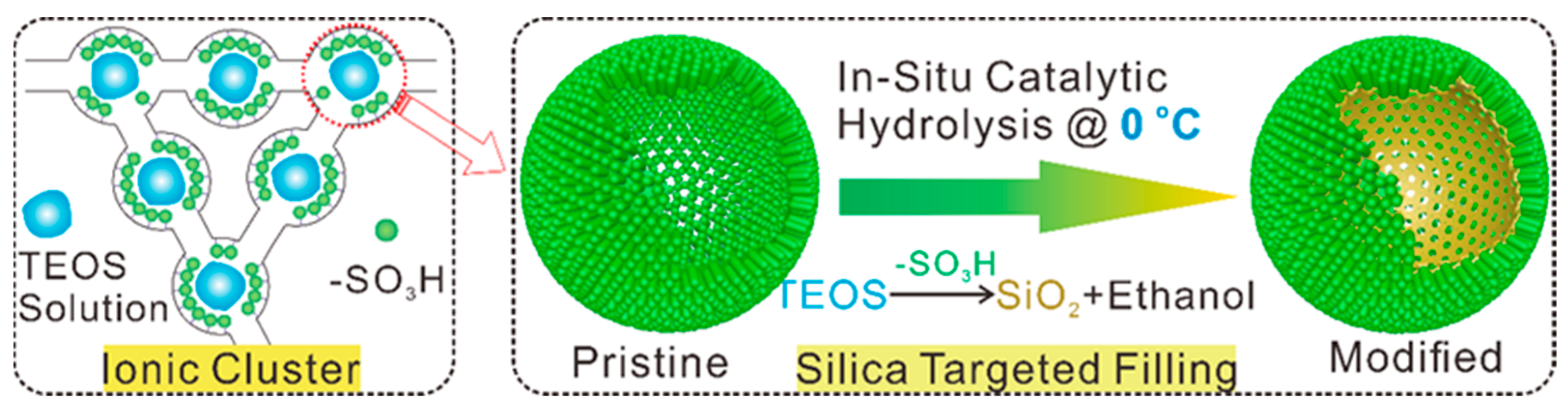
4.2. The Swelling-Filling Method
5. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karanfil, G. Importance and applications of DOE/optimization methods in PEM fuel cells: A review. Int. J. Energy Res. 2019, 44, 4–25. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Prospects of Fuel Cell Combined Heat and Power Systems. Energies 2020, 13, 4104. [Google Scholar] [CrossRef]
- Asim, A.Y.; Nabil, A.; Amira, S.Y.; Khalid, U. Potato waste as an effective source of electron generation and bioremediation of pollutant through benthic microbial fuel cell. Sustain. Energy Technol. Assess. 2022, 53, 102560. [Google Scholar]
- Asim, A.Y.; Claudia, G.B.; Mohamad, N.M.I.; Khalid, U.A.; Suriaty, Y. Local fruit wastes driven benthic microbial fuel cell: A sustainable approach to toxic metal removal and bioelectricity generation. Environ. Sci. Pollut. Res. 2022, 29, 32913–32928. [Google Scholar]
- Xiao, X. The direct use of enzymatic biofuel cells as functional bioelectronics. eScience 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H. Review of the Development of First-Generation Redox Flow Batteries: Iron-Chromium System. ChemSusChem 2022, 15, e202101798. [Google Scholar] [CrossRef]
- Andújar, J.M.; Segura, F. Fuel cells: History and updating. A walk along two centuries. Renew. Sustain. Energy Rev. 2009, 13, 2309–2322. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T.S. High-performance alkaline ionomer for alkaline exchange membrane fuel cells. Electrochem. Commun. 2013, 34, 278–281. [Google Scholar] [CrossRef]
- Antolini, E.; Gonzalez, E.R. Alkaline direct alcohol fuel cells. J. Power Sources 2010, 195, 3431–3450. [Google Scholar] [CrossRef]
- Adamiec, M. Research methodology of fuel cells. Prz. Elektrotechniczny 2010, 86, 334–337. [Google Scholar]
- Wang, S.; He, Z.; Wang, X.; Wang, C.; Li, X.; Zhao, Y. Ultrathin Semi-Interpenetrating Network Membranes Based on Perfluorinated Sulfonic Acid Resin and Polydivinylbenzene with Declined Hydrogen Crossover for Proton Exchange Membrane Fuel Cell. J. Electrochem. Soc. 2021, 168, 084508–084513. [Google Scholar] [CrossRef]
- He, H.; Zhu, Y.; Li, T.; Song, S.; Zhai, L.; Li, X.; Wu, L.; Li, H. Supramolecular Anchoring of Polyoxometalate Amphiphiles into Nafion Nanophases for Enhanced Proton Conduction. ACS Nano 2022, 16, 19240–19252. [Google Scholar] [CrossRef] [PubMed]
- Neelakandan, S.; Wang, L.; Zhang, B.; Ni, J.; Hu, M.; Gao, C.; Wong, W.-Y.; Wang, L. Branched Polymer Materials as Proton Exchange Membranes for Fuel Cell Applications. Polym. Rev. 2022, 62, 261–295. [Google Scholar] [CrossRef]
- Arslan, F.; Bohm, T.; Kerres, J.; Thiele, S. Spatially and temporally resolved monitoring of doping polybenzimidazole membranes with phosphoric acid. J. Membr. Sci. 2021, 625, 119145. [Google Scholar] [CrossRef]
- Oono, Y.; Sounai, A.; Hori, M. Influence of the phosphoric acid-doping level in a polybenzimidazole membrane on the cell performance of high-temperature proton exchange membrane fuel cells. J. Power Sources 2009, 189, 943–949. [Google Scholar] [CrossRef]
- Lu, C.-L.; Chang, C.-P.; Guo, Y.-H.; Yeh, T.-K.; Su, Y.-C.; Wang, P.-C.; Hsueh, K.-L.; Tseng, F.-G. High-performance and low-leakage phosphoric acid fuel cell with synergic composite membrane stacking of micro glass microfiber and nano PTFE. Renew. Energy 2019, 134, 982–988. [Google Scholar] [CrossRef]
- Wu, J.; Chen, L. Energy Efficiency Management of Coupling System for Molten Carbonate Fuel Cells. Int. J. Electrochem. Sci. 2022, 17, 220945. [Google Scholar] [CrossRef]
- Cassir, M.; McPhail, S.J.; Moreno, A. Strategies and new developments in the field of molten carbonates and high-temperature fuel cells in the carbon cycle. Int. J. Hydrogen Energy 2012, 37, 19345–19350. [Google Scholar] [CrossRef]
- Kulkarni, A.; Giddey, S. Materials issues and recent developments in molten carbonate fuel cells. J. Solid State Electrochem. 2012, 16, 3123–3146. [Google Scholar] [CrossRef]
- Kusnezoff, M.; Michaelis, A.; Schiel, J. Solid Oxide Fuel Cell Industry and Technology in Europe, Japan and the USA. Cfi-Ceram. Forum Int. 2013, 90, E27–E33. [Google Scholar]
- Wu, J.; Myung, J.-h.; Ding, D.; Zhu, T. Editorial: High Temperature Solid Oxide Cells. Front. Chem. 2021, 9, 719826. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, Y.S.; Lee, Y.D.; Kim, M.; Kim, D.K. Study on Internal Phenomena of Solid Oxide Fuel Cells Using Liquefied Natural Gas as Fuel. J. Electrochem. Soc. 2021, 168, 124513. [Google Scholar] [CrossRef]
- Haider, R.; Wen, Y.; Ma, Z.F.; Wilkinson, D.P.; Zhang, L.; Yuan, X.; Song, S.; Zhang, J. High temperature proton exchange membrane fuel cells: Progress in advanced materials and key technologies. Chem. Soc. Rev. 2021, 50, 1138–1187. [Google Scholar] [CrossRef]
- Qu, E.; Hao, X.; Xiao, M.; Han, D.; Huang, S.; Huang, Z.; Wang, S.; Meng, Y. Proton exchange membranes for high temperature proton exchange membrane fuel cells: Challenges and perspectives. J. Power Sources 2022, 533, 231386. [Google Scholar] [CrossRef]
- Jamil, A.; Rafiq, S.; Iqbal, T.; Khan, H.A.A.; Khan, H.M.; Azeem, B.; Mustafa, M.Z.; Hanbazazah, A.S. Current status and future perspectives of proton exchange membranes for hydrogen fuel cells. Chemosphere 2022, 303, 135204. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Xing, L.; Tu, Z. Simulations and analysis of high-temperature proton exchange membrane fuel cell and its cooling system to power an automotive vehicle. Energy Convers. Manag. 2022, 253, 115182. [Google Scholar] [CrossRef]
- Chakraborty, U. Fuel crossover and internal current in proton exchange membrane fuel cell modeling. Appl. Energ. 2016, 163, 60–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Ma, L.; Cai, W.; Cheng, H. Recent Developments on Alternative Proton Exchange Membranes: Strategies for Systematic Performance Improvement. Energy Technol. 2015, 3, 675–691. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Huo, J.; Meng, X.; Cui, L.; Zhou, Q. Effects of Conducting Channels Microstructure in Proton Exchange Membrane on the Performance of Fuel Cells. Prog. Chem. 2015, 27, 395–403. [Google Scholar]
- Shimizu, G.K.H. Shrink-wrapping water to conduct protons. Nat. Energy 2017, 2, 842–843. [Google Scholar] [CrossRef]
- Lin, B.; Yuan, W.; Xu, F.; Chen, Q.; Zhu, H.; Li, X.; Yuan, N.; Chu, F.; Ding, J. Protic ionic liquid/functionalized graphene oxide hybrid membranes for high temperature proton exchange membrane fuel cell applications. Appl. Surf. Sci. 2018, 455, 295–301. [Google Scholar] [CrossRef]
- Hasheminasab, M.; Kermani, M.J.; Nourazar, S.S.; Khodsiani, M.H. A novel experimental based statistical study for water management in proton exchange membrane fuel cells. Appl. Energy 2020, 264, 114713. [Google Scholar] [CrossRef]
- Janicka, E.; Mielniczek, M.; Gawel, L.; Darowicki, K.; Landowska, P. The impact of air humidity on the operation of proton exchange membrane fuel cells determined using dynamic electrochemical impedance spectroscopy. Electrochim. Acta 2020, 341, 136036. [Google Scholar] [CrossRef]
- Esmaeili, N.; Gray, E.M.; Webb, C.J. Non-Fluorinated Polymer Composite Proton Exchange Membranes for Fuel Cell Applications-A Review. Chemphyschem 2019, 20, 2016–2053. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Lee, B.; Kim, J.-H.; Pak, C.; Moon, S.-H. High-temperature operation of PEMFC using pore-filling PTFE/Nafion composite membrane treated with electric field. Int. J. Energy Res. 2021, 45, 19136–19146. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Ni, J.; Xu, M.; Pan, C.; Wang, D.; Liu, D.; Wang, L. Preparation and investigation of block polybenzimidazole membranes with high battery performance and low phosphoric acid doping for use in high-temperature fuel cells. J. Membr. Sci. 2019, 572, 350–357. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Fang, M.; Chen, J.; Liu, X.; Yin, B.; Wang, L. Synthesis and preparation of branched block polybenzimidazole membranes with high proton conductivity and single-cell performance for use in high temperature proton exchange membrane fuel cells. J. Membr. Sci. 2020, 602, 117981. [Google Scholar] [CrossRef]
- Escorihuela, J.; Olvera-Mancilla, J.; Alexandrova, L.; del Castillo, L.F.; Compan, V. Recent Progress in the Development of Composite Membranes Based on Polybenzimidazole for High Temperature Proton Exchange Membrane (PEM) Fuel Cell Applications. Polymers 2020, 12, 1861. [Google Scholar] [CrossRef]
- Ren, P.; Pei, P.; Li, Y.; Wu, Z.; Chen, D.; Huang, S. Degradation mechanisms of proton exchange membrane fuel cell under typical automotive operating conditions. Prog. Energy Combust. Sci. 2020, 81, 100859. [Google Scholar] [CrossRef]
- Qiu, D.; Peng, L.; Lai, X.; Ni, M.; Lehnert, W. Mechanical failure and mitigation strategies for the membrane in a proton exchange membrane fuel cell. Renew. Sustain. Energy Rev. 2019, 113, 109289. [Google Scholar] [CrossRef]
- Kim, J.; Yoo, H.; Kim, H.; Kim, D.; Kim, G.H.; Kwon, O.; Choi, H.; Cha, H.; Park, T. Non-destructive platinum catalyst-replenishment method for deteriorated polymer electrolyte membrane fuel cells. Energy Sci. Eng. 2022, 10, 414–422. [Google Scholar] [CrossRef]
- Friedman, A.; Mizrahi, M.; Levy, N.; Zion, N.; Zachman, M.; Elbaz, L. Application of Molecular Catalysts for the Oxygen Reduction Reaction in Alkaline Fuel Cells. ACS Appl. Mater. Interfaces 2021, 13, 58532–58538. [Google Scholar] [CrossRef]
- Zhang, Y.; Wilkinson, D.P.; Taghipour, F. Performance Analysis of an Air-Breathing Micro-Direct Methanol Fuel Cell with an Extended Anode Region. Fuel Cells 2020, 20, 634–642. [Google Scholar] [CrossRef]
- El-Hay, E.A.; El-Hameed, M.A.; El-Fergany, A.A. Performance enhancement of autonomous system comprising proton exchange membrane fuel cells and switched reluctance motor. Energy 2018, 163, 699–711. [Google Scholar] [CrossRef]
- Kuan, Y.-D.; Chang, J.-Y.; Ku, H.-T. Proton exchange membrane fuel cell purge and fan control using a microcontroller. Int. J. Green Energy 2017, 14, 86–91. [Google Scholar] [CrossRef]
- Sun, H.; Sun, Z.; Wu, Y. Proton transfer mechanism in perfluorinated sulfonic acid polytetrafluoroethylene. Int. J. Hydrogen Energy 2012, 37, 12821–12826. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Bang, Y.H.; Noto, V.D. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Oh, K.-H.; Kang, H.S.; Choo, M.-J.; Jang, D.-H.; Lee, D.; Lee, D.G.; Kim, T.-H.; Hong, Y.T.; Park, J.-K.; Kim, H.-T. Interlocking Membrane/Catalyst Layer Interface for High Mechanical Robustness of Hydrocarbon-Membrane-Based Polymer Electrolyte Membrane Fuel Cells. Adv. Mater. 2015, 27, 2974–2980. [Google Scholar] [CrossRef]
- Raja, P.M.; Gayathri, R.; Cao, G.; Ramesh, P.M. Study of amine customized exfoliated BN sheets in SPEEK-PES based blend membrane for acid-base cation exchange membrane fuel cells. J. Environ. Chem. Eng. 2019, 10, 107025. [Google Scholar]
- Chandan, A.; Hattenberger, M.; El-Kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)–A review. J. Power Sources 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Chen, H.; Wang, S.; Liu, F.; Wang, D.; Li, J.; Mao, T.; Liu, G.; Wang, X.; Xu, J.; Wang, Z. Base-acid doped polybenzimidazole with high phosphoric acid retention for HT-PEMFC applications. J. Membr. Sci. 2019, 596, 117722. [Google Scholar] [CrossRef]
- Li, Q.; He, R.; Jensen, J.; Bjerrum, N. PBI-based polymer membranes for high temperature fuel cells–preparation, characterization and fuel cell demonstration. Fuel Cells 2004, 4, 147–159. [Google Scholar] [CrossRef]
- Zhou, Z.; Zholobko, O.; Wu, X.-F.; Aulich, T.; Thakare, J.; Hurley, J. Polybenzimidazole-Based Polymer Electrolyte Membranes for High-Temperature Fuel Cells: Current Status and Prospects. Energies 2021, 14, 135. [Google Scholar] [CrossRef]
- Qu, T.; Hu, J.; Tan, Q.; Liu, Y.; Chen, Y.; Sun, J.; Liu, Y. Effect of phosphoric acid-doped polybenzimidazole membranes on the performance of H+-ion concentration cell. Int. J. Hydrogen Energy 2021, 46, 4354–4364. [Google Scholar] [CrossRef]
- Fencl, V.; Leith, D.E. Stewart’s quantitative acid-base chemistry: Applications in biology and medicine. Respir. Physiol. 1993, 91, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, L.; Mu, D.; Yu, L.; Wang, L.; Xi, J. Acid-base membranes of imidazole-based sulfonated polyimides for vanadium flow batteries. J. Membr. Sci. 2018, 552, 167–176. [Google Scholar] [CrossRef]
- Eldin, M.S.M.; Abu-Saied, M.A.; Elzatahry, A.A.; El-Khatib, K.M.; Hassan, E.A.; El-Sabbah, M.M. Novel Acid-Base Poly vinyl chloride-Doped Ortho-Phosphoric Acid Membranes for Fuel Cell Applications. Int. J. Electrochem. Sci. 2011, 6, 5417–5429. [Google Scholar]
- Yan, H.; Peng, K.; Yan, J.; Jiang, C.; Wang, Y.; Feng, H.; Yang, Z.; Wu, L.; Xu, T. Bipolar membrane-assisted reverse electrodialysis for high power density energy conversion via acid-base neutralization. J. Membr. Sci. 2022, 647, 120288. [Google Scholar] [CrossRef]
- Silambarasan, P.; Ramu, A.G.; Govarthanan, M.; Kim, W.; Moon, I.S. Cerium-polysulfide redox flow battery with possible high energy density enabled by MFI-Zeolite membrane working with acid-base electrolytes. Chemosphere 2021, 291, 132680. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Dang, J.; Zhou, G.; Wang, Y.; Zhang, Y.; Qu, L.; Wu, W. Lamellar composite membrane with acid-base pair anchored layer-by-layer structure towards highly enhanced conductivity and stability. J. Membr. Sci. 2020, 602, 117978. [Google Scholar] [CrossRef]
- Ru, C.; Gu, Y.; Duan, Y.; Zhao, C.; Na, H. Enhancement in proton conductivity and methanol resistance of Nafion membrane induced by blending sulfonated poly(arylene ether ketones) for direct methanol fuel cells. J. Membr. Sci. 2019, 573, 439–447. [Google Scholar] [CrossRef]
- Liu, L.; Li, Z.; Che, Q. Multilayered Membrane Electrolytes Based on Aramid Nanofibers for High-Temperature Proton Exchange Membrane Fuel Cells. ACS Appl. Nano Mater. 2019, 2, 2160–2168. [Google Scholar] [CrossRef]
- Lee, H.-F.; Wang, P.-C.; Chen-Yang, Y.W. An electrospun hygroscopic and electron-conductive core-shell silica@carbon nanofiber for microporous layer in proton-exchange membrane fuel cell. J. Solid State Electrochem. 2019, 23, 971–984. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, H.; Wu, H.; Jiang, Z. Enhanced proton conductivity of the hybrid membranes by regulating the proton conducting groups anchored on the mesoporous silica. J. Power Sources 2014, 270, 292–303. [Google Scholar] [CrossRef]
- Matos, B.R.; Santiago, E.I.; Rey, J.F.Q.; Scuracchio, C.H.; Mantovani, G.L.; Hirano, L.A.; Fonseca, F.C. dcProton conductivity at low-frequency in Nafion conductivity spectrum probed by time-resolved SAXS measurements and impedance spectroscopy. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 822–828. [Google Scholar] [CrossRef]
- Dresch, M.A.; Matos, B.R.; Fonseca, F.C.; Santiago, E.I.; Carmo, M.; Lanfredi, A.J.C.; Balog, S. Small-angle X-ray and neutron scattering study of Nafion-SiO2 hybrid membranes prepared in different solvent media. J. Power Sources 2015, 274, 560–567. [Google Scholar] [CrossRef]
- Haubold, H.G.; Vad, T.; Jungbluth, H.; Hiller, P. Nano structure of NAFION: A SAXS study. Electrochim. Acta 2001, 46, 1559–1563. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.; Deng, N.; Wang, X.; Xiang, H.; Cheng, B.; Kang, W. Electrospun multi-scale nanofiber network: Hierarchical proton-conducting channels in Nafion composite proton exchange membranes. Cellu 2021, 28, 6567–6585. [Google Scholar] [CrossRef]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Non-humidified fuel cells using a deep eutectic solvent (DES) as the electrolyte within a polymer electrolyte membrane (PEM): The effect of water and counterions. Phys. Chem. Chem. Phys. 2020, 22, 2917–2929. [Google Scholar] [CrossRef]
- Han, A.; Fu, C.; Yan, X.; Chen, J.; Cheng, X.; Ke, C.; Hou, J.; Shen, S.; Zhang, J. Effect of cobalt ion contamination on proton conduction of ultrathin Nafion film. Int. J. Hydrogen Energy 2020, 45, 25276–25285. [Google Scholar] [CrossRef]
- Teixeira, F.C.; de Sa, A.I.; Teixeira, A.P.S.; Rangel, C.M. Nafion phosphonic acid composite membranes for proton exchange membranes fuel cells. Appl. Surf. Sci. 2019, 487, 889–897. [Google Scholar] [CrossRef]
- Ressam, I.; Krins, N.; Laberty-Robert, C.; Selmane, M.; Lahcini, M.; Raihane, M.; El Kadib, A.; Perrot, H.; Sel, O. Sulfonic Acid Functionalized Chitosan as a Sustainable Component for Proton Conductivity Management in PEMs. Chemistryselect 2017, 2, 2503–2511. [Google Scholar] [CrossRef]
- Sapurina, I.Y.; Kompan, M.E.; Malyshkin, V.V.; Rosanov, V.V.; Stejskal, J. Properties of proton-conducting Nafion-type membranes with Nanometer-thick polyaniline surface layers. Russ. J. Electrochem. 2009, 45, 697–706. [Google Scholar]
- Zhou, D.; Ge, A.; Kogina, T.; Inoue, K.-i.; Chen, Y.-X.; Ye, S. Molecular Structures at Nafion/Graphene Interfaces Investigated by Sum-Frequency Generation Spectroscopy. J. Phys. Chem. C 2022, 126, 6523–6530. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Xu, W.; Long, J.; Huang, W.; He, Z.; Liu, S.; Zhang, Y. A Sulfonated Polyimide/Nafion Blend Membrane with High Proton Selectivity and Remarkable Stability for Vanadium Redox Flow Battery. Membranes 2021, 11, 946. [Google Scholar] [CrossRef]
- Asadchikov, V.E.; Bunkin, N.F.; Volkov, V.V.; Volkov, Y.O.; Nuzhdin, A.D.; Stepina, N.D.; Roshchin, B.S.; Tikhonov, A.M. On the Influence of the Alkaline Composition of Liquid Subphase on the Nafion Film Morphology. Phys. Wave Phenom. 2021, 29, 131–135. [Google Scholar] [CrossRef]
- Loccufier, E.; Geltmeyer, J.; Daelemans, L.; D’Hooge, D.R.; De Buysser, K.; De Clerck, K. Silica Nanofibrous Membranes for the Separation of Heterogeneous Azeotropes. Adv. Funct. Mater. 2018, 28, 1804138. [Google Scholar] [CrossRef]
- Feng, G.; Wang, J.; Boronat, M.; Li, Y.; Su, J.H.; Huang, J.; Ma, Y.; Yu, J. Radical-Facilitated Green Synthesis of Highly Ordered Mesoporous Silica Materials. J. Am. Chem. Soc. 2018, 140, 4770–4773. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Hung, Y.; Mou, C.Y. Mesoporous silica nanoparticles as nanocarriers. Chem. Commun. 2011, 47, 9972–9985. [Google Scholar] [CrossRef] [PubMed]
- Patil, Y.; Kulkarni, S.; Mauritz, K.A. In situ grown titania composition for optimal performance and durability of Nafion® fuel cell membranes. J. Appl. Polym. Sci. 2011, 121, 2344–2353. [Google Scholar] [CrossRef]
- Santiago, E.I.; Isidoro, R.A.; Dresch, M.A.; Matos, B.R.; Linardi, M.; Fonseca, F.C. Nafion TiO2 hybrid electrolytes for stable operation of PEM fuel cells at high temperature. Electrochim. Acta 2009, 54, 4111–4117. [Google Scholar] [CrossRef]
- Divona, M.; Ahmed, Z.; Bellitto, S.; Lenci, A.; Traversa, E.; Licoccia, S. SPEEK-TiO2 nanocomposite hybrid proton conductive membranes via in situ mixed sol–gel process. J. Membr. Sci. 2007, 296, 156–161. [Google Scholar] [CrossRef]
- Taghizadeh, M.T.; Vatanparast, M. Ultrasonic-assisted synthesis of ZrO2 nanoparticles and their application to improve the chemical stability of Nafion membrane in proton exchange membrane (PEM) fuel cells. J. Colloid Interface Sci. 2016, 483, 1–10. [Google Scholar] [CrossRef]
- Guzman, C.; Alvarez, A.; Godinez, L.A.; Ledesma-Garcia, J.; Arriaga, L.G. Evaluation of ZrO2 Composite Membrane Operating at High Temperature (100 degrees C) in Direct Methanol Fuel Cells. Int. J. Electrochem. Sci. 2012, 7, 6106–6117. [Google Scholar]
- Gandhi, K.; Dixit, B.K.; Dixit, D.K. Effect of addition of zirconium tungstate, lead tungstate and titanium dioxide on the proton conductivity of polystyrene porous membrane. Int. J. Hydrogen Energy 2012, 37, 3922–3930. [Google Scholar] [CrossRef]
- Ke, C.; Li, X.; Qu, S.; Shao, Z.; Yi, B. Preparation and properties of Nafion SiO2 composite membrane derived via in situ sol gel reaction size controlling and size effects of SiO2 nano particles. Polym. Adv. Technol. 2012, 23, 92–98. [Google Scholar] [CrossRef]
- Saccà, A.; Carbone, A.; Passalacqua, E.; D’Epifanio, A.; Licoccia, S.; Traversa, E.; Sala, E.; Traini, F.; Ornelas, R. Nafion–TiO2 hybrid membranes for medium temperature polymer electrolyte fuel cells (PEFCs). J. Power Sources 2005, 152, 16–21. [Google Scholar] [CrossRef]
- Baglio, V.; Di Blasi, A.; Aricò, A.S.; Antonucci, V.; Antonucci, P.L.; Trakanprapai, C.; Esposito, V.; Licoccia, S.; Traversa, E. Composite Mesoporous Titania Nafion Based Membranes for Direct Methanol Fuel Cell Operation at High Temperature. J. Electrochem. Soc. 2005, 152, A1373. [Google Scholar] [CrossRef]
- Yana, J.; Nimmanpipug, P.; Chirachanchai, S.; Gosalawit, R.; Dokmaisrijan, S.; Vannarat, S.; Vilaithong, T.; Lee, V.S. Molecular dynamics simulations of Krytox-Silica–Nafion composite for high temperature fuel cell electrolyte membranes. Polymer 2010, 51, 4632–4638. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhuang, X.; Cheng, B.; Wang, W.; Kang, W.; Shi, L.; Li, H. Modification of Nafion membrane with biofunctional SiO2 nanofiber for proton exchange membrane fuel cells. J. Power Sources 2017, 340, 201–209. [Google Scholar] [CrossRef]
- Xu, G.; Zou, J.; Guo, Z.; Li, J.; Ma, L.; Li, Y.; Cai, W. Bi-Functional Composting the Sulfonic Acid Based Proton Exchange Membrane for High Temperature Fuel Cell Application. Polymers 2020, 12, 1000. [Google Scholar] [CrossRef]
- Ko, E.H.; Yoon, Y.; Park, J.H.; Yang, S.H.; Hong, D.; Lee, K.B.; Shon, H.K.; Lee, T.G.; Choi, I.S. Bioinspired, cytocompatible mineralization of silica-titania composites: Thermoprotective nanoshell formation for individual chlorella cells. Angew. Chem. 2013, 52, 12279–12282. [Google Scholar] [CrossRef] [PubMed]
- López, G.P.; López, R.R.; Viveros, T. Dehydrocyclization of n-heptane over Pt catalysts supported on Al-and Si-promoted TiO2. Catal. Today 2014, 220, 61–65. [Google Scholar] [CrossRef]
- Byrne, H.E.; Mazyck, D.W. Removal of trace level aqueous mercury by adsorption and photocatalysis on silica titania composites. J. Hazard. Mater. 2009, 170, 915–919. [Google Scholar] [CrossRef]
- Li, W.; Zhao, D. Extension of the Stöber method to construct mesoporous SiO2 and TiO2 shells for uniform multifunctional core shell structures. Adv. Mater. 2013, 25, 142–149. [Google Scholar] [CrossRef]
- Sayeed, M.D.A.; Kim, H.J.; Park, Y.; Gopalan, A.I.; Kim, Y.H.; Lee, K.-P.; Choi, S.-J. Sulfated Titania Silica Reinforced Nafion Nanocomposite Membranes for Proton Exchange Membrane Fuel Cells. J. Nanosci. Nanotechnol. 2015, 15, 7054–7059. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Liu, Y.; Wu, H.; Cao, L.; He, X.; Zhang, B.; Wang, C.; Jiang, Z. One pot synthesis of silica titania binary nanoparticles with acid base pairs via biomimetic mineralization to fabricate highly proton conductive membranes. J. Mater. Chem. A 2017, 5, 18585–18593. [Google Scholar] [CrossRef]
- Lee, C.; Park, J.; Jeon, Y.; Park, J.-I.; Einaga, H.; Truong, Y.B.; Kyratzis, I.L.; Mochida, I.; Choi, J.; Shul, Y.-G. Phosphate-Modified TiO2/ZrO2 Nanofibrous Web Composite Membrane for Enhanced Performance and Durability of High-Temperature Proton Exchange Membrane Fuel Cells. Energy Fuels 2017, 31, 7645–7652. [Google Scholar] [CrossRef]
- Cavani, F. Heteropolycompound-based catalysts: A blend of acid and oxidizing properties. Catal. Today 1998, 41, 73–86. [Google Scholar] [CrossRef]
- Mioč, U.; Davidović, M.; Tjapkin, N.; Colomban, P.; Novak, A. Equilibrium of the protonic species in hydrates of some heteropolyacids at elevated temperatures. Solid State Ion. 1991, 46, 103–109. [Google Scholar] [CrossRef]
- Wu, H.; Shen, X.; Cao, Y.; Li, Z.; Jiang, Z. Composite proton conductive membranes composed of sulfonated poly (ether ether ketone) and phosphotungstic acid-loaded imidazole microcapsules as acid reservoirs. J. Membr. Sci. 2014, 451, 74–84. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, M.; Zhang, J.; Lan, F.; Lu, S. Phosphotungstic acid (HPW) molecules anchored in the bulk of Nafion as methanol-blocking membrane for direct methanol fuel cells. J. Membr. Sci. 2011, 368, 241–245. [Google Scholar] [CrossRef]
- Bose, A.B.; Gopu, S.; Li, W. Enhancement of proton exchange membrane fuel cells performance at elevated temperatures and lower humidities by incorporating immobilized phosphotungstic acid in electrodes. J. Power Sources 2014, 263, 217–222. [Google Scholar] [CrossRef]
- Abouzari-lotf, E.; Nasef, M.M.; Ghassemi, H.; Zakeri, M.; Ahmad, A.; Abdollahi, Y. Improved Methanol Barrier Property of Nafion Hybrid Membrane by Incorporating Nanofibrous Interlayer Self-Immobilized with High Level of Phosphotungstic Acid. ACS Appl. Mater. Interfaces 2015, 7, 17008–17015. [Google Scholar] [CrossRef]
- Lu, S.; Wang, D.; Jiang, S.P.; Xiang, Y.; Lu, J.; Zeng, J. HPW/MCM-41 phosphotungstic acid/mesoporous silica composites as novel proton-exchange membranes for elevated-temperature fuel cells. Adv. Mater. 2010, 22, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Joghee, P.; Hsing, I.M. Preparation and characterization of hybrid Nafion silica membrane doped with phosphotungstic acid for high temperature operation of proton exchange membrane fuel cells. J. Membr. Sci. 2004, 229, 43–51. [Google Scholar] [CrossRef]
- Xu, W.; Lu, T.; Liu, C.; Xing, W. Low methanol permeable composite Nafion/silica/PWA membranes for low temperature direct methanol fuel cells. Electrochim. Acta 2005, 50, 3280–3285. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Majedi, F.S.; VanDersarl, J.J.; Bertsch, A.; Renaud, P.; Jacob, K.I. Ionic nanopeapods: Next-generation proton conducting membranes based on phosphotungstic acid filled carbon nanotube. Nano Energy 2016, 23, 114–121. [Google Scholar] [CrossRef]
- Yang, X.-B.; Meng, L.-H.; Sui, X.-L.; Wang, Z.-B. High proton conductivity polybenzimidazole proton exchange membrane based on phosphotungstic acid-anchored nano-Kevlar fibers. J. Mater. Sci. 2018, 54, 1640–1653. [Google Scholar] [CrossRef]
- Yang, X.B.; Zhao, L.; Sui, X.L.; Meng, L.H.; Wang, Z.B. Phosphotungstic acid immobilized nanofibers-Nafion composite membrane with low vanadium permeability and high selectivity for vanadium redox flow battery. J Colloid Interface Sci 2019, 542, 177–186. [Google Scholar] [CrossRef]
- Zhang, B.; Cao, Y.; Jiang, S.; Li, Z.; He, G.; Wu, H. Enhanced proton conductivity of Nafion nanohybrid membrane incorporated with phosphonic acid functionalized graphene oxide at elevated temperature and low humidity. J. Membr. Sci. 2016, 518, 243–253. [Google Scholar] [CrossRef]
- Ibrahim, A.; Hossain, O.; Chaggar, J.; Steinberger-Wilckens, R.; El-Kharouf, A. GO-nafion composite membrane development for enabling intermediate temperature operation of polymer electrolyte fuel cell. Int. J. Hydrogen Energy 2020, 45, 5526–5534. [Google Scholar] [CrossRef]
- Yin, C.; Li, J.; Zhou, Y.; Zhang, H.; Fang, P.; He, C. Enhancement in Proton Conductivity and Thermal Stability in Nafion Membranes Induced by Incorporation of Sulfonated Carbon Nanotubes. ACS Appl. Mater. Interfaces 2018, 10, 14026–14035. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, Y.; Liu, J.; Wang, J.; He, Y.; Davey, K.; Qiao, S.Z. Molecular-Level Hybridization of Nafion with Quantum Dots for Highly Enhanced Proton Conduction. Adv. Mater. 2018, 30, e1707516. [Google Scholar] [CrossRef]
- Firouz Tadavani, K.; Abdolmaleki, A.; Molavian, M.R.; Borandeh, S.; Sorvand, E.; Zhiani, M. Synergistic Behavior of Phosphonated and Sulfonated Groups on Proton Conductivity and Their Performance for High-Temperature Proton Exchange Membrane Fuel Cells (PEMFCs). Energy Fuels 2017, 31, 11460–11470. [Google Scholar] [CrossRef]
- Maiti, J.; Kakati, N.; Woo, S.P.; Yoon, Y.S. Nafion based hybrid composite membrane containing GO and dihydrogen phosphate functionalized ionic liquid for high temperature polymer electrolyte membrane fuel cell. Compos. Sci. Technol. 2018, 155, 189–196. [Google Scholar] [CrossRef]
- Klose, C.; Breitwieser, M.; Vierrath, S.; Klingele, M.; Cho, H.; Büchler, A.; Kerres, J.; Thiele, S. Electrospun sulfonated poly(ether ketone) nanofibers as proton conductive reinforcement for durable Nafion composite membranes. J. Power Sources 2017, 361, 237–242. [Google Scholar] [CrossRef]
- Yan, L.M.; Zhu, S.H.; Ji, X.B.; Lu, W.C. Proton hopping in phosphoric acid solvated nafion membrane: A molecular simulation study. J. Phys. Chem. B 2007, 111, 6357–6363. [Google Scholar] [CrossRef]
- Aili, D.; Hansen, M.K.; Pan, C.; Li, Q.; Christensen, E.; Jensen, J.O.; Bjerrum, N.J. Phosphoric acid doped membranes based on Nafion, PBI and their blends Membrane preparation, characterization and steam electrolysis testing. Int. J. Hydrogen Energy 2011, 36, 6985–6993. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhang, H.; Zhang, Y.; Xing, D. A novel H3PO4/Nafion PBI composite membrane for enhanced durability of high temperature PEM fuel cells. J. Power Sources 2007, 169, 259–264. [Google Scholar] [CrossRef]
- Kim, J.D.; Suzuki, A.; Jun, M.S. Nafion-Azole-H3PO4 Composite Membranes using Solution Processing for High Temperature PEMFCs. In Polymer Electrolyte Fuel Cells 2013, 58, 1185–1194. [Google Scholar]
- Yin, Y.; Li, Z.; Yang, X.; Cao, L.; Wang, C.; Zhang, B.; Wu, H.; Jiang, Z. Enhanced proton conductivity of Nafion composite membrane by incorporating phosphoric acid-loaded covalent organic framework. J. Power Sources 2016, 332, 265–273. [Google Scholar] [CrossRef]
- Yang, J.; Che, Q.; Zhou, L.; He, R.; Savinell, R.F. Studies of a high temperature proton exchange membrane based on incorporating an ionic liquid cation 1-butyl-3-methylimidazolium into a Nafion matrix. Electrochim. Acta 2011, 56, 5940–5946. [Google Scholar] [CrossRef]
- Lu, F.; Gao, X.; Yan, X.; Gao, H.; Shi, L.; Jia, H.; Zheng, L. Preparation and characterization of nonaqueous proton-conducting membranes with protic ionic liquids. ACS Appl. Mater. Interfaces 2013, 5, 7626–7632. [Google Scholar] [CrossRef]
- Sunda, A.P. Ammonium-based protic ionic liquid doped Nafion membranes as anhydrous fuel cell electrolytes. J. Mater. Chem. A 2015, 3, 12905–12912. [Google Scholar] [CrossRef]
- Lee, J.-W.; Yi, C.-W.; Kim, K. Phosphoric Acid-Functionalized Mesoporous Silica/Nafion Composite Membrane for High Temperature PEMFCs. Bull. Korean Chem. Soc. 2012, 33, 1397–1400. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Lu, S.; Zhu, H.; Aili, D.; De Marco, R.; Xiang, Y.; Forsyth, M.; Li, Q.; Jiang, S. Ion-Exchange-Induced Selective Etching for the Synthesis of Amino-Functionalized Hollow Mesoporous Silica for Elevated-High-Temperature Fuel Cells. ACS Appl. Mater. Interfaces 2017, 9, 31922–31930. [Google Scholar] [CrossRef]
- Ketpang, K.; Son, B.; Lee, D.; Shanmugam, S. Porous zirconium oxide nanotube modified Nafion composite membrane for polymer electrolyte membrane fuel cells operated under dry conditions. J. Membr. Sci. 2015, 488, 154–165. [Google Scholar] [CrossRef]
- Li, J.; Fan, K.; Cai, W.; Ma, L.; Xu, G.; Xu, S.; Ma, L.; Cheng, H. An in-situ nano-scale swelling-filling strategy to improve overall performance of Nafion membrane for direct methanol fuel cell application. J. Power Sources 2016, 332, 37–41. [Google Scholar] [CrossRef]
- Li, J.; Xu, G.; Cai, W.; Xiong, J.; Ma, L.; Yang, Z.; Huang, Y.; Cheng, H. Non-destructive modification on Nafion membrane via in-situ inserting of sheared graphene oxide for direct methanol fuel cell applications. Electrochim. Acta 2018, 282, 362–368. [Google Scholar] [CrossRef]
- Li, J.; Xu, G.; Luo, X.; Xiong, J.; Liu, Z.; Cai, W. Effect of nano-size of functionalized silica on overall performance of swelling-filling modified Nafion membrane for direct methanol fuel cell application. Appl. Energy 2018, 213, 408–414. [Google Scholar] [CrossRef]
- Xu, G.; Li, J.; Ma, L.; Xiong, J.; Mansoor, M.; Cai, W.; Cheng, H. Performance dependence of swelling-filling treated Nafion membrane on nano-structure of macromolecular filler. J. Membr. Sci. 2017, 534, 68–72. [Google Scholar] [CrossRef]
- Xu, G.; Wu, Z.; Wei, Z.; Zhang, W.; Wu, J.; Li, Y.; Li, J.; Qu, K.; Cai, W. Non-destructive fabrication of Nafion/silica composite membrane via swelling-filling modification strategy for high temperature and low humidity PEM fuel cell. Renew. Energy 2020, 153, 935–939. [Google Scholar] [CrossRef]
- Xu, G.; Li, S.; Li, J.; Liu, Z.; Li, Y.; Xiong, J.; Cai, W.; Qu, K.; Cheng, H. Targeted filling of silica in Nafion by a modified in situ sol-gel method for enhanced fuel cell performance at elevated temperatures and low humidity. Chem. Commun. 2019, 55, 5499–5502. [Google Scholar] [CrossRef] [PubMed]
- Stefanithis, I.D.; Mauritz, K.A. Microstructural Evolution of a Silicon Oxide Phase in a Perfluorosulfonic Acid Ionomer by an in Situ Sol-Gel Reaction. Macromolecules 1990, 23, 1380–1388. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Stefanithis, I.D.; Davis, V.; Scheetz, R.W.; Pope, R.K.; Wilkes, G.L.; Huanc, H. Microstructural Evolution of a Silicon Oxide Phase in a Perfluorosulfonic Acid lonomer by an In Situ Sol-Gel Reaction. J. Appl. Polym. Sci. 1995, 55, 181–190. [Google Scholar] [CrossRef]
- Xi, J.; Wu, Z.; Qiu, X.; Chen, L. Nafion/SiO2 hybrid membrane for vanadium redox flow battery. J. Power Sources 2007, 166, 531–536. [Google Scholar] [CrossRef]
- Chen, Z.; Holmberg, B.; Li, W.; Wang, X.; Deng, W.; Munoz, R.; Yan, Y. Nafion/zeolite nanocomposite membrane by in situ crystallization for a direct methanol fuel cell. Chem. Mater. 2006, 18, 5669–5675. [Google Scholar] [CrossRef]
- Xu, G.; Xue, S.; Wei, Z.; Li, J.; Qu, K.; Li, Y.; Cai, W. Stabilizing phosphotungstic acid in Nafion membrane via targeted silica fixation for high-temperature fuel cell application. Int. J. Hydrogen Energy 2021, 46, 4301–4308. [Google Scholar] [CrossRef]
- Xu, G.; Wu, Z.; Xie, Z.; Wei, Z.; Li, J.; Qu, K.; Li, Y.; Cai, W. Graphene quantum dot reinforced hyperbranched polyamide proton exchange membrane for direct methanol fuel cell. Int. J. Hydrogen Energy 2021, 46, 9782–9789. [Google Scholar] [CrossRef]
- Xu, G.X.; Wei, Z.L.; Li, S.; Li, J.; Yang, Z.H.; Grigoriev, S.A. In-situ sulfonation of targeted silica-filled Nafion for high-temperature PEM fuel cell application. Int. J. Hydrogen Energy 2019, 44, 29711–29716. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Dong, X.; Xue, B.; Huang, J.; Wu, J.; Cai, W. Recent Approaches to Achieve High Temperature Operation of Nafion Membranes. Energies 2023, 16, 1565. https://doi.org/10.3390/en16041565
Xu G, Dong X, Xue B, Huang J, Wu J, Cai W. Recent Approaches to Achieve High Temperature Operation of Nafion Membranes. Energies. 2023; 16(4):1565. https://doi.org/10.3390/en16041565
Chicago/Turabian StyleXu, Guoxiao, Xinwei Dong, Bin Xue, Jianyou Huang, Junli Wu, and Weiwei Cai. 2023. "Recent Approaches to Achieve High Temperature Operation of Nafion Membranes" Energies 16, no. 4: 1565. https://doi.org/10.3390/en16041565
APA StyleXu, G., Dong, X., Xue, B., Huang, J., Wu, J., & Cai, W. (2023). Recent Approaches to Achieve High Temperature Operation of Nafion Membranes. Energies, 16(4), 1565. https://doi.org/10.3390/en16041565





