Microalgal Carbon Dioxide (CO2) Capture and Utilization from the European Union Perspective
Abstract
1. Introduction
2. Global and EU Trends in CO2 Emissions
3. Current and Proposed EU Regulations
4. Microalgae—Potential Use to Reduce CO2 Emissions
5. Microalgal CO2 Sequestration—The Current State of Knowledge
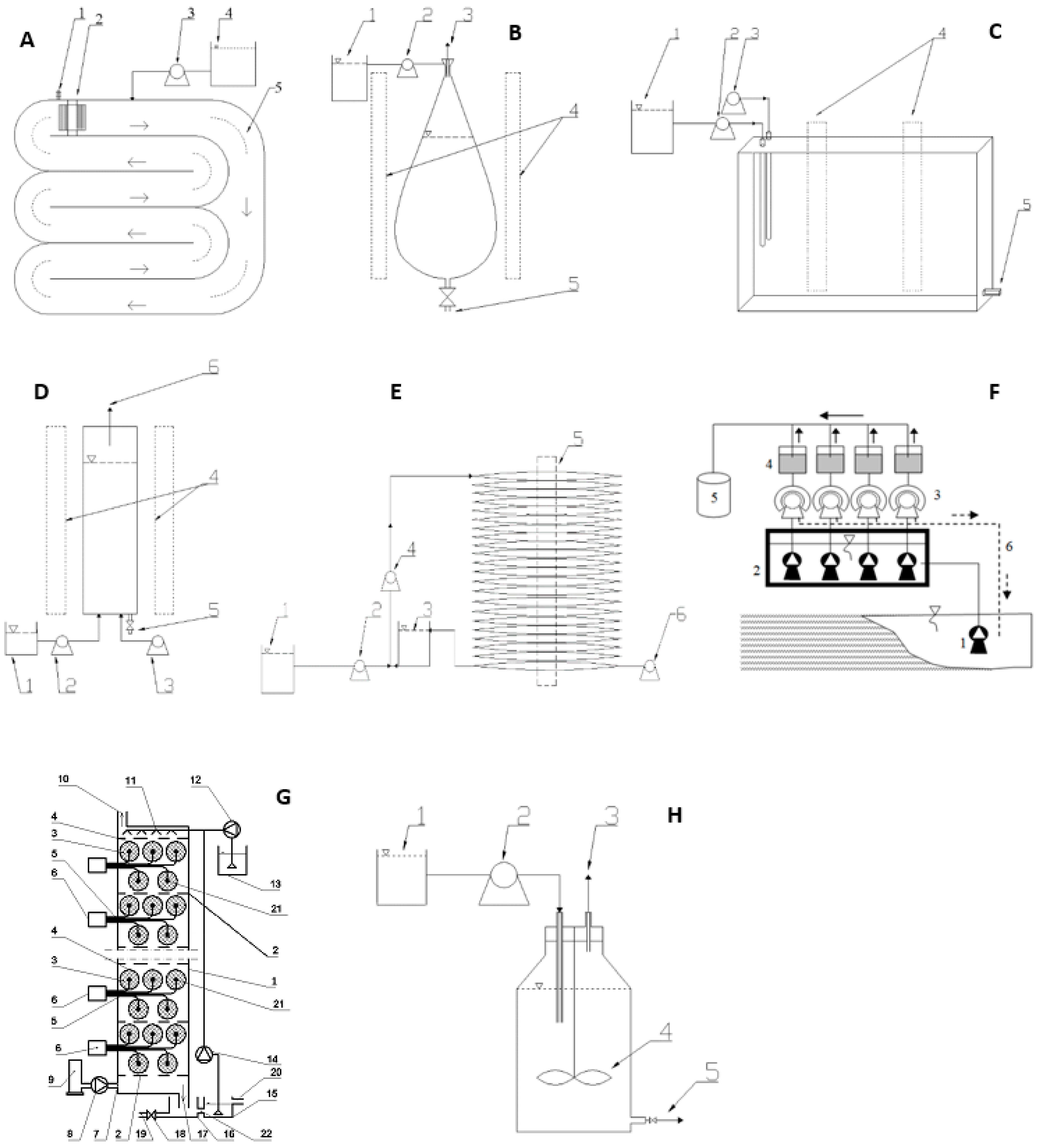
6. Photobioreactors Used for CO2 Biosequestration
7. Methods of Feeding CO2 to Microalgae Photobioreactors
8. Microalgae as a Way of Capture and Utilization of CO2 (CCU)
9. Blue Carbon and Phycoremediation
10. Future and Prospects
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CO2 | carbon dioxide, |
| GHG | greenhouse gas, |
| EU | European Union, |
| EC | European Commission, |
| EU ETS | European Union’s Emissions Trading Scheme, |
| LULUCF | Land Use, Land Use Change and Forestry, |
| ESR | Effort Sharing Regulation, |
| RED | Renewable Energy Directive, |
| EED | Energy Efficiency Directive, |
| AFID | Alternative Fuels Infrastructure Directive, |
| CBAM | Carbon Border Adjustment Mechanism, |
| RES | renewable energy sources, |
| ILUC | Indirect Land Use Change Impacts, |
| CCM | carbon-concentrating mechanism, |
| PBRs | photobioreactors, |
| TAP medium | Tris-Acetate-Phosphate medium, |
| SE medium | soil extract medium, |
| IMC-CO2PBR | Immobilized microalgae-based photobioreactor for CO2 capture, |
| PHAs | Polyhydroxyalkanoates |
References
- Iqbal, N.; Abbasi, K.R.; Shinwari, R.; Guangcai, W.; Ahmad, M.; Tang, K. Does Exports Diversification and Environmental Innovation Achieve Carbon Neutrality Target of OECD Economies? J. Environ. Manag. 2021, 291, 112648. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.J.R.; Dias, M.F. Perception of Climate Change Effects over Time and the Contribution of Different Areas of Knowledge to Its Understanding and Mitigation. Climate 2022, 10, 7. [Google Scholar] [CrossRef]
- Bouzarovski, S.; Thomson, H.; Cornelis, M. Confronting Energy Poverty in Europe: A Research and Policy Agenda. Energies 2021, 14, 858. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A. Renewable Energy and Climate Change. Renew. Sustain. Energy Rev. 2022, 158, 112111. [Google Scholar] [CrossRef]
- Rehman, A.; Ma, H.; Ozturk, I.; Ulucak, R. Sustainable Development and Pollution: The Effects of CO2 Emission on Population Growth, Food Production, Economic Development, and Energy Consumption in Pakistan. Environ. Sci. Pollut. Res. 2022, 29, 17319–17330. [Google Scholar] [CrossRef]
- Borowski, P.F. Management of Energy Enterprises in Zero-Emission Conditions: Bamboo as an Innovative Biomass for the Production of Green Energy by Power Plants. Energies 2022, 15, 1928. [Google Scholar] [CrossRef]
- Shakoor, A.; Shakoor, S.; Rehman, A.; Ashraf, F.; Abdullah, M.; Shahzad, S.M.; Farooq, T.H.; Ashraf, M.; Manzoor, M.A.; Altaf, M.M.; et al. Effect of Animal Manure, Crop Type, Climate Zone, and Soil Attributes on Greenhouse Gas Emissions from Agricultural Soils—A Global Meta-Analysis. J. Clean. Prod. 2021, 278, 124019. [Google Scholar] [CrossRef]
- Stokreef, S.; Sadri, F.; Stokreef, A.; Ghahreman, A. Mineral Carbonation of Ultramafic Tailings: A Review of Reaction Mechanisms and Kinetics, Industry Case Studies, and Modelling. Clean. Eng. Technol. 2022, 8, 100491. [Google Scholar] [CrossRef]
- Lee, B.J.; Il Lee, J.; Yun, S.Y.; Hwang, B.G.; Lim, C.S.; Park, Y.K. Methodology to Calculate the CO2 Emission Reduction at the Coal-Fired Power Plant: CO2 Capture and Utilization Applying Technology of Mineral Carbonation. Sustainability 2020, 12, 7402. [Google Scholar] [CrossRef]
- Middleton, R.S.; Ogland-Hand, J.D.; Chen, B.; Bielicki, J.M.; Ellett, K.M.; Harp, D.R.; Kammer, R.M. Identifying Geologic Characteristics and Operational Decisions to Meet Global Carbon Sequestration Goals. Energy Environ. Sci. 2020, 13, 5000–5016. [Google Scholar] [CrossRef]
- Chao, C.; Deng, Y.; Dewil, R.; Baeyens, J.; Fan, X. Post-Combustion Carbon Capture. Renew. Sustain. Energy Rev. 2021, 138, 110490. [Google Scholar] [CrossRef]
- Vale, M.A.; Ferreira, A.; Pires, J.C.M.; Gonçalves, G.A.L. CO2 Capture Using Microalgae. Adv. Carbon Capture Methods Technol. Appl. 2020, 381–405. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Z.; Xiao, Y.; Yuan, M.; Zhou, C.; Liu, G.; Fang, J.; Yang, B. Biochemical and Morphological Changes Triggered by Nitrogen Stress in the Oleaginous Microalga Chlorella vulgaris. Microorganisms 2022, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- de Kleijne, K.; Hanssen, S.V.; van Dinteren, L.; Huijbregts, M.A.J.; van Zelm, R.; de Coninck, H. Limits to Paris Compatibility of CO2 Capture and Utilization. One Earth 2022, 5, 168–185. [Google Scholar] [CrossRef]
- Schönfeld, K.C.; von Ferreira, A. Urban Planning and European Innovation Policy: Achieving Sustainability, Social Inclusion, and Economic Growth? Sustainability 2021, 13, 1137. [Google Scholar] [CrossRef]
- European Commission. European Climate Law. Available online: https://climate.ec.europa.eu/eu-action/european-green-deal/european-climate-law_en (accessed on 29 December 2022).
- Wang, Q.; Jiang, X.T.; Yang, X.; Ge, S. Comparative Analysis of Drivers of Energy Consumption in China, the USA and India—A Perspective from Stratified Heterogeneity. Sci. Total Environ. 2020, 698, 134117. [Google Scholar] [CrossRef] [PubMed]
- Aktar, M.A.; Alam, M.M.; Al-Amin, A.Q. Global Economic Crisis, Energy Use, CO2 Emissions, and Policy Roadmap amid COVID-19. Sustain. Prod. Consum. 2021, 26, 770–781. [Google Scholar] [CrossRef]
- Davis, S.J.; Liu, Z.; Deng, Z.; Zhu, B.; Ke, P.; Sun, T.; Guo, R.; Hong, C.; Zheng, B.; Wang, Y.; et al. Emissions Rebound from the COVID-19 Pandemic. Nat. Clim. Chang. 2022, 12, 412–414. [Google Scholar] [CrossRef]
- Global Carbon Project. Global Carbon Budget. 2021. Available online: https://www.globalcarbonproject.org/carbonbudget/ (accessed on 4 September 2022).
- de Palma, A.; Vosough, S.; Liao, F. An Overview of Effects of COVID-19 on Mobility and Lifestyle: 18 Months since the Outbreak. Transp. Res. Part A Policy Pract. 2022, 159, 372–397. [Google Scholar] [CrossRef]
- Priya, S.S.; Cuce, E.; Sudhakar, K. A Perspective of COVID-19 Impact on Global Economy, Energy and Environment. Int. J. Sustain. Eng. 2021, 14, 1290–1305. [Google Scholar] [CrossRef]
- Crippa, M.; Guizzardi, D.; Muntean, M.; Schaaf, E.; Solazzo, E.; Monforti-Ferrario, F.; Olivier, J.; Vignati, E. Fossil CO2 Emissions of All World Countries—2020 Report; EUR 30358 EN; JRC121460; Publications Office of the European Union: Luxembourg, 2020; pp. 1–239. [Google Scholar] [CrossRef]
- Eurostat. CO2 Emissions from Energy Use Clearly Decreased in the Eu in 2020. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/ddn-20210507-1 (accessed on 29 December 2022).
- Wang, X.; Si, C.; Gu, J.; Liu, G.; Liu, W.; Qiu, J.; Zhao, J. Electricity-Consumption Data Reveals the Economic Impact and Industry Recovery during the Pandemic. Sci. Rep. 2021, 11, 19960. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.A.; Kamal, M.A.; Tripathi, R. An Empirical Investigation of Nuclear Energy and Environmental Pollution Nexus in India: Fresh Evidence Using NARDL Approach. Environ. Sci. Pollut. Res. 2021, 28, 54744–54755. [Google Scholar] [CrossRef] [PubMed]
- Skjærseth, J.B. Towards a European Green Deal: The Evolution of EU Climate and Energy Policy Mixes. Int. Environ. Agreem. Polit. Law Econ. 2021, 21, 25–41. [Google Scholar] [CrossRef]
- Ciesielska-Maciągowska, D.; Klimczak, D.; Skrzek-Lubasińska, M. Central and Eastern European CO2 Market—Challenges of Emissions Trading for Energy Companies. Energies 2021, 14, 1051. [Google Scholar] [CrossRef]
- Kaveshnikov, N.Y. Analysis of the Influence of the European Parliament and the Council of the EU Exemplified by the EU Emissions Trading System Reform. Mirovaya Ekon. Mezhdunarodnye Otnos. 2021, 65, 21–32. [Google Scholar] [CrossRef]
- Gerbeti, A. Market Mechanisms for Reducing Emissions and the Introduction of a Flexible Consumption Tax. Glob. J. Flex. Syst. Manag. 2021, 22, 161–178. [Google Scholar] [CrossRef]
- Köhl, M.; Linser, S.; Prins, K.; Talarczyk, A. The EU Climate Package “Fit for 55”—A Double-Edged Sword for Europeans and Their Forests and Timber Industry. For. Policy Econ. 2021, 132, 102596. [Google Scholar] [CrossRef]
- Sztorc, M. The Implementation of the European Green Deal Strategy as a Challenge for Energy Management in the Face of the COVID-19 Pandemic. Energies 2022, 15, 2662. [Google Scholar] [CrossRef]
- Scheuing, H.; Kamm, J. The EU on the Road to Climate Neutrality—Is the ‘Fit for 55’ Package Fit for Purpose? Renew. Energy Law Policy Rev. 2022, 10, 4–18. [Google Scholar] [CrossRef]
- Hammond, G.P. The UK Industrial Decarbonisation Strategy Revisited. Proc. Inst. Civ. Eng.-Energy 2022, 175, 30–44. [Google Scholar] [CrossRef]
- Foster, W.; Azimov, U.; Gauthier-Maradei, P.; Molano, L.C.; Combrinck, M.; Munoz, J.; Esteves, J.J.; Patino, L. Waste-to-Energy Conversion Technologies in the UK: Processes and Barriers—A Review. Renew. Sustain. Energy Rev. 2021, 135, 110226. [Google Scholar] [CrossRef]
- Cadillo-Benalcazar, J.J.; Bukkens, S.G.F.; Ripa, M.; Giampietro, M. Why Does the European Union Produce Biofuels? Examining Consistency and Plausibility in Prevailing Narratives with Quantitative Storytelling. Energy Res. Soc. Sci. 2021, 71, 101810. [Google Scholar] [CrossRef]
- Procházková, L.; Řezanka, T.; Nedbalová, L.; Remias, D. Unicellular versus Filamentous: The Glacial Alga Ancylonema Alaskana Comb. et Stat. Nov. and Its Ecophysiological Relatedness to Ancylonema Nordenskioeldii (Zygnematophyceae, Streptophyta). Microorganisms 2021, 9, 1103. [Google Scholar] [CrossRef] [PubMed]
- Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Kujawska, N.; Talbierz, S. Microalgae Cultivation Technologies as an Opportunity for Bioenergetic System Development—Advantages and Limitations. Sustainability 2020, 12, 9980. [Google Scholar] [CrossRef]
- Prasad, R.; Gupta, S.K.; Shabnam, N.; Oliveira, C.Y.B.; Nema, A.K.; Ansari, F.A.; Bux, F. Role of Microalgae in Global CO2 Sequestration: Physiological Mechanism, Recent Development, Challenges, and Future Prospective. Sustainability 2021, 13, 13061. [Google Scholar] [CrossRef]
- Meyer, M.T.; Itakura, A.K.; Patena, W.; Wang, L.; He, S.; Emrich-Mills, T.; Lau, C.S.; Yates, G.; MacKinder, L.C.M.; Jonikas, M.C. Assembly of the Algal CO2-Fixing Organelle, the Pyrenoid, Is Guided by a Rubisco-Binding Motif. Sci. Adv. 2020, 6, eabd2408. [Google Scholar] [CrossRef]
- Burlacot, A.; Dao, O.; Auroy, P.; Cuiné, S.; Li-Beisson, Y.; Peltier, G. Alternative Photosynthesis Pathways Drive the Algal CO2-Concentrating Mechanism. Nature 2022, 605, 366–371. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kisielewska, M.; Kazimierowicz, J.; Dudek, M.; Świca, I.; Rudnicka, A. The Cultivation of Lipid-Rich Microalgae Biomass as Anaerobic Digestate Valorization Technology—A Pilot-Scale Study. Processes 2020, 8, 517. [Google Scholar] [CrossRef]
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Khan, T.M.Y.; Nghiem, L.D.; Ong, H.C.; et al. Microalgae Biomass as a Sustainable Source for Biofuel, Biochemical and Biobased Value-Added Products: An Integrated Biorefinery Concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- Grama, S.B.; Liu, Z.; Li, J. Emerging Trends in Genetic Engineering of Microalgae for Commercial Applications. Mar. Drugs 2022, 20, 285. [Google Scholar] [CrossRef]
- Dębowski, M.; Dudek, M.; Zieliński, M.; Nowicka, A.; Kazimierowicz, J. Microalgal Hydrogen Production in Relation to Other Biomass-Based Technologies—A Review. Energies 2021, 14, 6025. [Google Scholar] [CrossRef]
- Kazemi Shariat Panahi, H.; Dehhaghi, M.; Aghbashlo, M.; Karimi, K.; Tabatabaei, M. Shifting Fuel Feedstock from Oil Wells to Sea: Iran Outlook and Potential for Biofuel Production from Brown Macroalgae (Ochrophyta; Phaeophyceae). Renew. Sustain. Energy Rev. 2019, 112, 626–642. [Google Scholar] [CrossRef]
- Torzillo, G. Photosynthesis Basic Principles to Optimize Growth of Microalgae Cultures Outdoors. Environ. Biotechnol. 2021, 12, 30–34. [Google Scholar]
- Mehariya, S.; Goswami, R.K.; Verma, P.; Lavecchia, R.; Zuorro, A. Integrated Approach for Wastewater Treatment and Biofuel Production in Microalgae Biorefineries. Energies 2021, 14, 2282. [Google Scholar] [CrossRef]
- Sharma, P.; Gujjala, L.K.S.; Varjani, S.; Kumar, S. Emerging Microalgae-Based Technologies in Biorefinery and Risk Assessment Issues: Bioeconomy for Sustainable Development. Sci. Total Environ. 2022, 813, 152417. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Świca, I.; Kazimierowicz, J. Algae Biomass as a Potential Source of Liquid Fuels. Phycology 2021, 1, 105–118. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Verma, P. Phycoremediation of Nitrogen and Phosphate from Wastewater Using Picochlorum Sp.: A Tenable Approach. J. Basic Microbiol. 2022, 62, 279–295. [Google Scholar] [CrossRef]
- Tang, J.; Liang, Q.; Li, C.; Huang, X.; Xian, X.; Li, J.; Shang, Z.; Pang, C.; Liu, Y.; Zhang, R. Application of Marine Algae in Water Pollution Control. IOP Conf. Ser. Earth Environ. Sci. 2022, 966, 12001. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Freitas, B.C.B.; Cruz, C.G.; Silveira, J.; Morais, M.G. Potential of Microalgae as Biopesticides to Contribute to Sustainable Agriculture and Environmental Development. J. Environ. Sci. Health Part B 2019, 54, 366–375. [Google Scholar] [CrossRef]
- Peter, A.P.; Koyande, A.K.; Chew, K.W.; Ho, S.H.; Chen, W.H.; Chang, J.S.; Krishnamoorthy, R.; Banat, F.; Show, P.L. Continuous Cultivation of Microalgae in Photobioreactors as a Source of Renewable Energy: Current Status and Future Challenges. Renew. Sustain. Energy Rev. 2022, 154, 111852. [Google Scholar] [CrossRef]
- Chintagunta, A.D.; Zuccaro, G.; Kumar, M.; Kumar, S.P.J.; Garlapati, V.K.; Postemsky, P.D.; Kumar, N.S.S.; Chandel, A.K.; Simal-Gandara, J. Biodiesel Production From Lignocellulosic Biomass Using Oleaginous Microbes: Prospects for Integrated Biofuel Production. Front. Microbiol. 2021, 12, 2080. [Google Scholar] [CrossRef]
- Shokravi, H.; Shokravi, Z.; Heidarrezaei, M.; Ong, H.C.; Rahimian Koloor, S.S.; Petrů, M.; Lau, W.J.; Ismail, A.F. Fourth Generation Biofuel from Genetically Modified Algal Biomass: Challenges and Future Directions. Chemosphere 2021, 285, 131535. [Google Scholar] [CrossRef]
- Global Information. Global Microalgae Market—Industry Trends and Forecast to 2028. Available online: https://www.giiresearch.com/report/dbmr991581-global-microalgae-market-industry-trends-forecast.html (accessed on 29 December 2022).
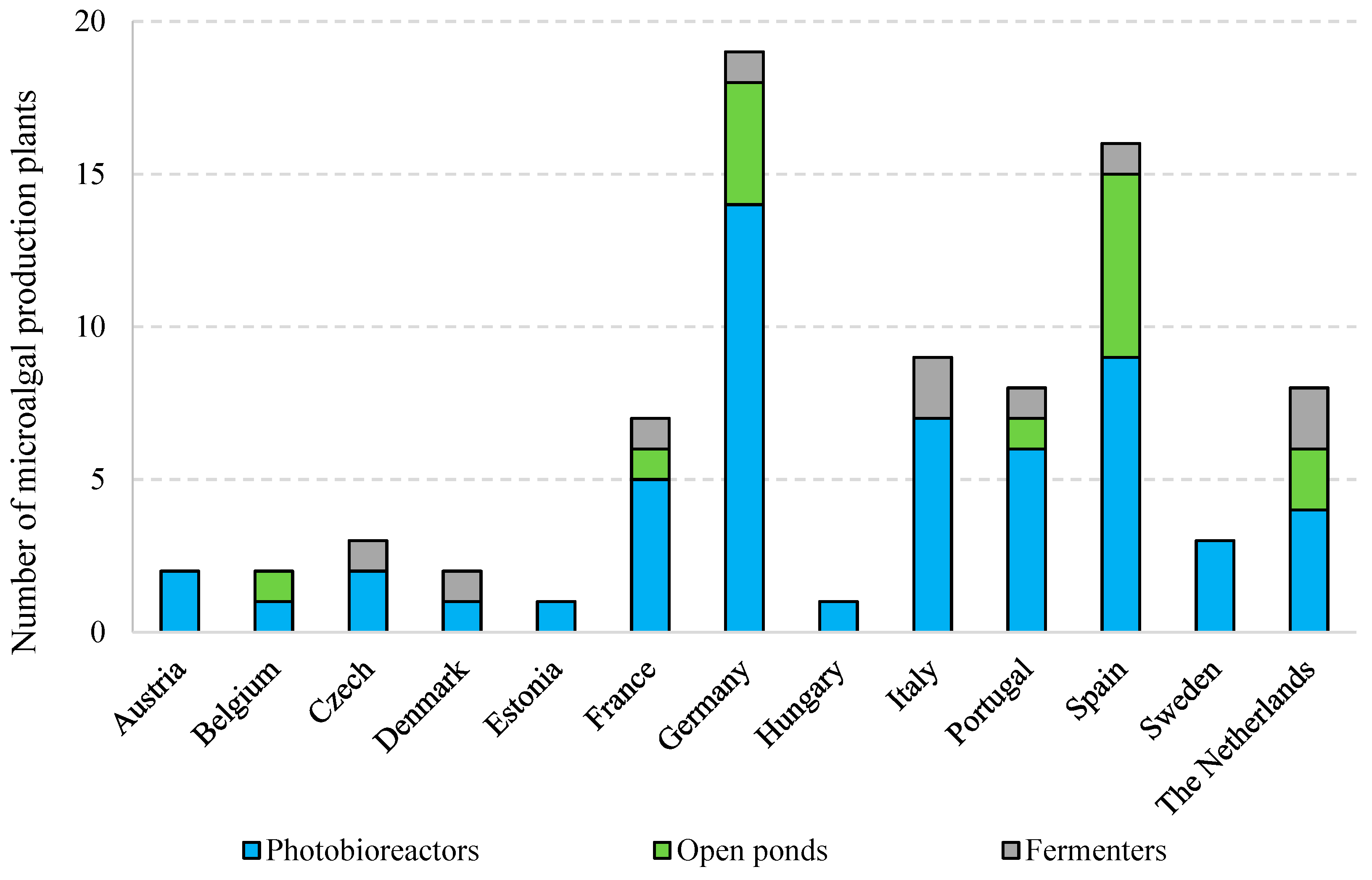
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 1247. [Google Scholar] [CrossRef]
- Roles, J.; Yarnold, J.; Hussey, K.; Hankamer, B. Techno-Economic Evaluation of Microalgae High-Density Liquid Fuel Production at 12 International Locations. Biotechnol. Biofuels 2021, 14, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pan, K.; Tang, X.; Li, Y.; Zhu, B.; Zhao, Y. The Molecular Mechanisms of Chlorella Sp. Responding to High CO2: A Study Based on Comparative Transcriptome Analysis between Strains with High- and Low-CO2 Tolerance. Sci. Total Environ. 2021, 763, 144185. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Bhatti, H.N.; Maqbool, M.; Iqbal, M. Microalgae Biosorption, Bioaccumulation and Biodegradation Efficiency for the Remediation of Wastewater and Carbon Dioxide Mitigation: Prospects, Challenges and Opportunities. J. Water Process Eng. 2021, 41, 102009. [Google Scholar] [CrossRef]
- Stewart, C.; Hessami, M.A. A Study of Methods of Carbon Dioxide Capture and Sequestration—The Sustainability of a Photosynthetic Bioreactor Approach. Energy Convers. Manag. 2005, 46, 403–420. [Google Scholar] [CrossRef]
- Van Den Hende, S.; Vervaeren, H.; Boon, N. Flue Gas Compounds and Microalgae: (Bio-)Chemical Interactions Leading to Biotechnological Opportunities. Biotechnol. Adv. 2012, 30, 1405–1424. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Kao, C.Y.; Huang, T.T.; Lin, C.J.; Ong, S.C.; Chen, C.D.; Chang, J.S.; Lin, C.S. Microalgal Biomass Production and On-Site Bioremediation of Carbon Dioxide, Nitrogen Oxide and Sulfur Dioxide from Flue Gas Using Chlorella Sp. Cultures. Bioresour. Technol. 2011, 102, 9135–9142. [Google Scholar] [CrossRef]
- Singh Chauhan, D.; Sahoo, L.; Mohanty, K. Maximize Microalgal Carbon Dioxide Utilization and Lipid Productivity by Using Toxic Flue Gas Compounds as Nutrient Source. Bioresour. Technol. 2022, 348, 126784. [Google Scholar] [CrossRef]
- Nagappan, S.; Tsai, P.C.; Devendran, S.; Alagarsamy, V.; Ponnusamy, V.K. Enhancement of Biofuel Production by Microalgae Using Cement Flue Gas as Substrate. Environ. Sci. Pollut. Res. 2020, 27, 17571–17586. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Banerjee, D.; Das, D. Carbon Dioxide Sequestration from Industrial Flue Gas by Chlorella Sorokiniana. Bioresour. Technol. 2014, 152, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, S.; Mallick, N. Carbon Dioxide Mitigation and Biodiesel Production by a Marine Microalga under Mixotrophic Mode by Using Transesterification By-Product Crude Glycerol: A Synergy of Biofuels and Waste Valorization. Environ. Technol. Innov. 2022, 27, 102441. [Google Scholar] [CrossRef]
- de Morais, M.G.; Costa, J.A.V. Isolation and Selection of Microalgae from Coal Fired Thermoelectric Power Plant for Biofixation of Carbon Dioxide. Energy Convers. Manag. 2007, 48, 2169–2173. [Google Scholar] [CrossRef]
- Sung, K.D.; Lee, J.S.; Shin, C.S.; Park, S.C.; Choi, M.J. CO2 Fixation by Chlorella Sp. KR-1 and Its Cultural Characteristics. Bioresour. Technol. 1999, 68, 269–273. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Kao, C.Y.; Chen, C.H.; Kuan, T.C.; Ong, S.C.; Lin, C.S. Reduction of CO2 by a High-Density Culture of Chlorella Sp. in a Semicontinuous Photobioreactor. Bioresour. Technol. 2008, 99, 3389–3396. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, L.; Chen, H.; Gao, C. Carbon Dioxide Removal from Air by Microalgae Cultured in a Membrane-Photobioreactor. Sep. Purif. Technol. 2006, 50, 324–329. [Google Scholar] [CrossRef]
- Dębowski, M.; Krzemieniewski, M.; Zieliński, M.; Kazimierowicz, J. Immobilized Microalgae-Based Photobioreactor for CO2 Capture (IMC-CO2PBR): Efficiency Estimation, Technological Parameters, and Prototype Concept. Atmosphere 2021, 12, 1031. [Google Scholar] [CrossRef]
- Li, F.F.; Yang, Z.H.; Zeng, R.; Yang, G.; Chang, X.; Yan, J.B.; Hou, Y.L. Microalgae Capture of CO2 from Actual Flue Gas Discharged from a Combustion Chamber. Ind. Eng. Chem. Res. 2011, 50, 6496–6502. [Google Scholar] [CrossRef]
- Vunjak-Novakovic, G.; Kim, Y.; Wu, X.; Berzin, I.; Merchuk, J.C. Air-Lift Bioreactors for Algal Growth on Flue Gas: Mathematical Modeling and Pilot-Plant Studies. Ind. Eng. Chem. Res. 2005, 44, 6154–6163. [Google Scholar] [CrossRef]
- Aslam, A.; Thomas-Hall, S.R.; Mughal, T.A.; Schenk, P.M. Selection and Adaptation of Microalgae to Growth in 100% Unfiltered Coal-Fired Flue Gas. Bioresour. Technol. 2017, 233, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Doucha, J.; Straka, F.; Lívanský, K. Utilization of Flue Gas for Cultivation of Microalgae Chlorella Sp. in an Outdoor Open Thin-Layer Photobioreactor. J. Appl. Phycol. 2005, 17, 403–412. [Google Scholar] [CrossRef]
- Yadav, G.; Dubey, B.K.; Sen, R. A Comparative Life Cycle Assessment of Microalgae Production by CO2 Sequestration from Flue Gas in Outdoor Raceway Ponds under Batch and Semi-Continuous Regime. J. Clean. Prod. 2020, 258, 120703. [Google Scholar] [CrossRef]
- Dębowski, M.; Świca, I.; Kazimierowicz, J.; Zieliński, M. Large Scale Microalgae Biofuel Technology—Development Perspectives in Light of the Barriers and Limitations. Energies 2022, 16, 81. [Google Scholar] [CrossRef]
- Tejada Carbajal, E.M.; Martínez Hernández, E.; Fernández Linares, L.; Novelo Maldonado, E.; Limas Ballesteros, R. Techno-Economic Analysis of Scenedesmus Dimorphus Microalgae Biorefinery Scenarios for Biodiesel Production and Glycerol Valorization. Bioresour. Technol. Rep. 2020, 12, 100605. [Google Scholar] [CrossRef]
- Gifuni, I.; Pollio, A.; Safi, C.; Marzocchella, A.; Olivieri, G. Current Bottlenecks and Challenges of the Microalgal Biorefinery. Trends Biotechnol. 2019, 37, 242–252. [Google Scholar] [CrossRef]
- Feng, P.; Xu, Z.; Qin, L.; Asraful Alam, M.; Wang, Z.; Zhu, S. Effects of Different Nitrogen Sources and Light Paths of Flat Plate Photobioreactors on the Growth and Lipid Accumulation of Chlorella Sp. GN1 Outdoors. Bioresour. Technol. 2020, 301, 122762. [Google Scholar] [CrossRef]
- Shekh, A.; Sharma, A.; Schenk, P.M.; Kumar, G.; Mudliar, S. Microalgae Cultivation: Photobioreactors, CO2 Utilization, and Value-Added Products of Industrial Importance. J. Chem. Technol. Biotechnol. 2022, 97, 1064–1085. [Google Scholar] [CrossRef]
- Talaei, M.; Mahdavinejad, M.; Azari, R. Thermal and Energy Performance of Algae Bioreactive Façades: A Review. J. Build. Eng. 2020, 28, 101011. [Google Scholar] [CrossRef]
- Sirohi, R.; Kumar Pandey, A.; Ranganathan, P.; Singh, S.; Udayan, A.; Kumar Awasthi, M.; Hoang, A.T.; Chilakamarry, C.R.; Kim, S.H.; Sim, S.J. Design and Applications of Photobioreactors—A Review. Bioresour. Technol. 2022, 349, 126858. [Google Scholar] [CrossRef]
- Raeisossadati, M.; Vadiveloo, A.; Bahri, P.A.; Parlevliet, D.; Moheimani, N.R. Treating Anaerobically Digested Piggery Effluent (ADPE) Using Microalgae in Thin Layer Reactor and Raceway Pond. J. Appl. Phycol. 2019, 31, 2311–2319. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Sen, R. A Sustainable Perspective of Microalgal Biorefinery for Co-Production and Recovery of High-Value Carotenoid and Biofuel with CO2 Valorization. Biofuels Bioprod. Biorefin. 2020, 14, 879–897. [Google Scholar] [CrossRef]
- Onyeaka, H.; Miri, T.; Obileke, K.; Hart, A.; Anumudu, C.; Al-Sharify, Z.T. Minimizing Carbon Footprint via Microalgae as a Biological Capture. Carbon Capture Sci. Technol. 2021, 1, 100007. [Google Scholar] [CrossRef]
- Paul, S.; Bera, S.; Dasgupta, R.; Mondal, S.; Roy, S. Review on the Recent Structural Advances in Open and Closed Systems for Carbon Capture through Algae. Energy Nexus 2021, 4, 100032. [Google Scholar] [CrossRef]
- Sarat Chandra, T.; Maneesh Kumar, M.; Mukherji, S.; Chauhan, V.S.; Sarada, R.; Mudliar, S.N. Comparative Life Cycle Assessment of Microalgae-Mediated CO2 Capture in Open Raceway Pond and Airlift Photobioreactor System. Clean Technol. Environ. Policy 2018, 20, 2357–2364. [Google Scholar] [CrossRef]
- Xu, L.; Weathers, P.J.; Xiong, X.-R.; Liu, C.-Z. Microalgal Bioreactors: Challenges and Opportunities. Eng. Life Sci. 2009, 9, 178–189. [Google Scholar] [CrossRef]
- Divya Kuravi, S.; Venkata Mohan, S. Mixotrophic Cultivation of Monoraphidium Sp. In Dairy Wastewater Using Flat-Panel Photobioreactor and Photosynthetic Performance. Bioresour. Technol. 2022, 348, 126671. [Google Scholar] [CrossRef]
- Legrand, J.; Artu, A.; Pruvost, J. A Review on Photobioreactor Design and Modelling for Microalgae Production. React. Chem. Eng. 2021, 6, 1134–1151. [Google Scholar] [CrossRef]
- da Silva, P.A.S.; Vargas, J.V.C.; Mariano, A.B.; Severo, I.A. Phycoremediation: Role of Microalgae in Waste Management and Energy Production. Waste-Energy 2022, 511–537. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, J.; Chen, P.; Ji, C.; Kang, Q.; Lu, B.; Li, K.; Liu, J.; Ruan, R. Bio-Mitigation of Carbon Dioxide Using Microalgal Systems: Advances and Perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1163–1175. [Google Scholar] [CrossRef]
- Wang, B.; Lan, C.Q.; Horsman, M. Closed Photobioreactors for Production of Microalgal Biomasses. Biotechnol. Adv. 2012, 30, 904–912. [Google Scholar] [CrossRef]
- Singh, D.; Goswami, R.K.; Agrawal, K.; Chaturvedi, V.; Verma, P. Bio-Inspired Remediation of Wastewater: A Contemporary Approach for Environmental Clean-Up. Curr. Res. Green Sustain. Chem. 2022, 5, 100261. [Google Scholar] [CrossRef]
- Prabha, S.; Vijay, A.K.; Paul, R.R.; George, B. Cyanobacterial Biorefinery: Towards Economic Feasibility through the Maximum Valorization of Biomass. Sci. Total Environ. 2022, 814, 152795. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Yang, J.; Cui, M.; Zhang, W.; Zhao, J. Comparative Experiments of Two Novel Tubular Photobioreactors with an Inner Aerated Tube for Microalgal Cultivation: Enhanced Mass Transfer and Improved Biomass Yield. Algal Res. 2021, 58, 102364. [Google Scholar] [CrossRef]
- Alami, A.H.; Alasad, S.; Ali, M.; Alshamsi, M. Investigating Algae for CO2 Capture and Accumulation and Simultaneous Production of Biomass for Biodiesel Production. Sci. Total Environ. 2021, 759, 143529. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.A.; Ilankoon, I.M.S.K.; Chong, M.N.; Foo, S.C. Improving Microalgae Growth and Carbon Capture through Micro-Size Bubbles Generation in Flat-Panel Photobioreactors: Impacts of Different Gas Sparger Designs on Mixing Performance. Renew. Sustain. Energy Rev. 2023, 171, 113001. [Google Scholar] [CrossRef]
- Meena, M.; Yadav, G.; Sonigra, P.; Shah, M.P. A Comprehensive Review on Application of Bioreactor for Industrial Wastewater Treatment. Lett. Appl. Microbiol. 2022, 74, 131–158. [Google Scholar] [CrossRef]
- Xia, A.; Hu, Z.; Liao, Q.; Huang, Y.; Zhu, X.; Ye, W.; Sun, Y. Enhancement of CO2 Transfer and Microalgae Growth by Perforated Inverted Arc Trough Internals in a Flat-Plate Photobioreactor. Bioresour. Technol. 2018, 269, 292–299. [Google Scholar] [CrossRef]
- Zhao, Z.; Muylaert, K.; Szymczyk, A.; Vankelecom, I.F.J. Enhanced Microalgal Biofilm Formation and Facilitated Microalgae Harvesting Using a Novel PH-Responsive, Crosslinked Patterned and Vibrating Membrane. Chem. Eng. J. 2021, 410, 127390. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z. Advances in the Biological Fixation of Carbon Dioxide by Microalgae. J. Chem. Technol. Biotechnol. 2021, 96, 1475–1495. [Google Scholar] [CrossRef]
- Duan, Y.; Guo, X.; Yang, J.; Zhang, M.; Li, Y. Nutrients Recycle and the Growth of Scenedesmus Obliquus in Synthetic Wastewater under Different Sodium Carbonate Concentrations. R. Soc. Open Sci. 2020, 7, 191214. [Google Scholar] [CrossRef]
- Gulzar, A.; Gulzar, A.; Ansari, M.B.; He, F.; Gai, S.; Yang, P. Carbon Dioxide Utilization: A Paradigm Shift with CO2 Economy. Chem. Eng. J. Adv. 2020, 3, 100013. [Google Scholar] [CrossRef]
- Rodas-Zuluaga, L.I.; Castañeda-Hernández, L.; Castillo-Vacas, E.I.; Gradiz-Menjivar, A.; López-Pacheco, I.Y.; Castillo-Zacarías, C.; Iqbal, H.M.N.; Parra-Saldívar, R. Bio-Capture and Influence of CO2 on the Growth Rate and Biomass Composition of the Microalgae Botryococcus Braunii and Scenedesmus sp. J. CO2 Util. 2021, 43, 101371. [Google Scholar] [CrossRef]
- Ding, G.T.; Mohd Yasin, N.H.; Takriff, M.S.; Kamarudin, K.F.; Salihon, J.; Yaakob, Z.; Mohd Hakimi, N.I.N. Phycoremediation of Palm Oil Mill Effluent (POME) and CO2 Fixation by Locally Isolated Microalgae: Chlorella Sorokiniana UKM2, Coelastrella Sp. UKM4 and Chlorella Pyrenoidosa UKM7. J. Water Process Eng. 2020, 35, 101202. [Google Scholar] [CrossRef]
- Liu, X.; Chen, G.; Tao, Y.; Wang, J. Application of Effluent from WWTP in Cultivation of Four Microalgae for Nutrients Removal and Lipid Production under the Supply of CO2. Renew. Energy 2020, 149, 708–715. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Truong, M.V.; Nguyen, A.Q.; Johir, M.A.H.; Commault, A.S.; Ralph, P.J.; Semblante, G.U.; Nghiem, L.D. A Sequential Membrane Bioreactor Followed by a Membrane Microalgal Reactor for Nutrient Removal and Algal Biomass Production. Environ. Sci. Water Res. Technol. 2020, 6, 189–196. [Google Scholar] [CrossRef]
- Zimmermann, A.W.; Buchner, G.A.; Schomäcker, R. Apples and Apples: A Shortcut Assessment Framework for Early-Stage Carbon Capture and Utilization Technologies Based on Efficiency, Feasibility, and Risk. Energy Technol. 2021, 9, 2000691. [Google Scholar] [CrossRef]
- In-na, P.; Byrne, F.; Caldwell, G.S.; Lee, J.G.M. Techno-Economic Analysis of Living Biocomposites for Carbon Capture from Breweries. Algal Res. 2022, 66, 102781. [Google Scholar] [CrossRef]
- Shahbaz, M.; Al-Ansari, T.; Aslam, M.; Khan, Z.; Inayat, A.; Athar, M.; Naqvi, S.R.; Ahmed, M.A.; McKay, G. A State of the Art Review on Biomass Processing and Conversion Technologies to Produce Hydrogen and Its Recovery via Membrane Separation. Int. J. Hydrogen Energy 2020, 45, 15166–15195. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae Biorefinery: High Value Products Perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Zhang, X.; Han, F.; Tu, W.; Yang, W. Microalgal Hydrogen Production. Small Methods 2020, 4, 1900514. [Google Scholar] [CrossRef]
- Tsolcha, O.N.; Patrinou, V.; Economou, C.N.; Dourou, M.; Aggelis, G.; Tekerlekopoulou, A.G. Utilization of Biomass Derived from Cyanobacteria-Based Agro-Industrial Wastewater Treatment and Raisin Residue Extract for Bioethanol Production. Water 2021, 13, 486. [Google Scholar] [CrossRef]
- Al-Dailami, A.; Ahmad, I.; Abdullah, N.; Koji, I.; Yuzir, A. Feasibility and Viability of Procuring Biohydrogen from Microalgae: An Emerging and Sustainable Energy Resource Technology. J. Phys. Conf. Ser. 2022, 2259, 12014. [Google Scholar] [CrossRef]
- Dudek, M.; Dębowski, M.; Nowicka, A.; Kazimierowicz, J.; Zieliński, M. The Effect of Autotrophic Cultivation of Platymonas Subcordiformis in Waters from the Natural Aquatic Reservoir on Hydrogen Yield. Resources 2022, 11, 31. [Google Scholar] [CrossRef]
- Kujawska, N.; Talbierz, S.; Dębowski, M.; Kazimierowicz, J.; Zieliński, M. Optimizing Docosahexaenoic Acid (DHA) Production by Schizochytrium Sp. Grown on Waste Glycerol. Energies 2021, 14, 1685. [Google Scholar] [CrossRef]
- Ong, H.C.; Chen, W.-H.; Farooq, A.; Gan, Y.Y.; Lee, K.T.; Ashokkumar, V. Catalytic Thermochemical Conversion of Biomass for Biofuel Production: A Comprehensive Review. Renew. Sustain. Energy Rev. 2019, 113, 109266. [Google Scholar] [CrossRef]
- Sekar, M.; Mathimani, T.; Alagumalai, A.; Chi, N.T.L.; Duc, P.A.; Bhatia, S.K.; Brindhadevi, K.; Pugazhendhi, A. A Review on the Pyrolysis of Algal Biomass for Biochar and Bio-Oil—Bottlenecks and Scope. Fuel 2021, 283, 119190. [Google Scholar] [CrossRef]
- Kumar, M.; Oyedun, A.O.; Kumar, A. A Comparative Analysis of Hydrogen Production from the Thermochemical Conversion of Algal Biomass. Int. J. Hydrogen Energy 2019, 44, 10384–10397. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Suresh, S.; Kanwal, S.; Ramadoss, G.; Ramprakash, B.; Incharoensakdi, A. Microalgal Biorefinery Concepts’ Developments for Biofuel and Bioproducts: Current Perspective and Bottlenecks. Int. J. Mol. Sci. 2022, 23, 2623. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Monlau, F. Nutrient Recycling for Sustainable Production of Algal Biofuels. In Biofuels from Algae; Elsevier: Amsterdam, The Netherlands, 2019; pp. 109–133. [Google Scholar] [CrossRef]
- Kato, N. Production of Crude Bioplastic-Beads with Microalgae: Proof-of-Concept. Bioresour. Technol. Rep. 2019, 6, 81–84. [Google Scholar] [CrossRef]
- Yong, J.J.J.Y.; Chew, K.W.; Khoo, K.S.; Show, P.L.; Chang, J.S. Prospects and Development of Algal-Bacterial Biotechnology in Environmental Management and Protection. Biotechnol. Adv. 2021, 47, 107684. [Google Scholar] [CrossRef]
- Yang, W.; Dong, Y.; Li, J.; Fu, Q.; Zhang, L. Templating synthesis of hierarchically meso/macroporous N-doped microalgae derived biocarbon as oxygen reduction reaction catalyst for microbial fuel cells. Int. J. Hydrogen Energy 2021, 46, 2530–2542. [Google Scholar] [CrossRef]
- Lan, L.; Li, J.; Feng, Q.; Zhang, L.; Fu, Q.; Zhu, X.; Liao, Q. Enhanced Current Production of the Anode Modified by Microalgae Derived Nitrogen-Rich Biocarbon for Microbial Fuel Cells. Int. J. Hydrogen Energy 2020, 45, 3833–3839. [Google Scholar] [CrossRef]
- Thakkar, A.; Pienkos, P.T.; Nagle, N.; Dong, T.; Kruger, J.; Kumar, S. Comparative Study of Flash and Acid Hydrolysis of Microalgae (Scenedesmus Sp.) for the Recovery of Biochemicals and Production of Porous Biocarbon Nanosheets. Biomass Convers. Biorefin. 2022, 1, 1–10. [Google Scholar] [CrossRef]
- Wen, X.; Du, K.; Wang, Z.; Peng, X.; Luo, L.; Tao, H.; Xu, Y.; Zhang, D.; Geng, Y.; Li, Y. Effective Cultivation of Microalgae for Biofuel Production: A Pilot-Scale Evaluation of a Novel Oleaginous Microalga Graesiella Sp. WBG-1. Biotechnol. Biofuels 2016, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Supraja, K.V.; Behera, B.; Balasubramanian, P. Efficacy of Microalgal Extracts as Biostimulants through Seed Treatment and Foliar Spray for Tomato Cultivation. Ind. Crops Prod. 2020, 151, 112453. [Google Scholar] [CrossRef]
- Ferreira, A.; Melkonyan, L.; Carapinha, S.; Ribeiro, B.; Figueiredo, D.; Avetisova, G.; Gouveia, L. Biostimulant and Biopesticide Potential of Microalgae Growing in Piggery Wastewater. Environ. Adv. 2021, 4, 100062. [Google Scholar] [CrossRef]
- Martini, F.; Beghini, G.; Zanin, L.; Varanini, Z.; Zamboni, A.; Ballottari, M. The Potential Use of Chlamydomonas Reinhardtii and Chlorella Sorokiniana as Biostimulants on Maize Plants. Algal Res. 2021, 60, 102515. [Google Scholar] [CrossRef] [PubMed]
- García, G.; Sosa-Hernández, J.E.; Rodas-Zuluaga, L.I.; Castillo-Zacarías, C.; Iqbal, H.; Parra-Saldívar, R. Accumulation of PHA in the Microalgae Scenedesmus Sp. under Nutrient-Deficient Conditions. Polymers 2020, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Mandal, A.; Ayton, E.; Hunt, R.; Zeller, M.A.; Sharma, S. Modification of Protein Rich Algal-Biomass to Form Bioplastics and Odor Removal. In Protein Byproducts; Academic Press: New York, NY, USA, 2016; pp. 107–117. [Google Scholar] [CrossRef]
- Zeller, M.A.; Hunt, R.; Jones, A.; Sharma, S. Bioplastics and Their Thermoplastic Blends from Spirulina and Chlorella Microalgae. J. Appl. Polym. Sci. 2013, 130, 3263–3275. [Google Scholar] [CrossRef]
- Mathiot, C.; Ponge, P.; Gallard, B.; Sassi, J.F.; Delrue, F.; Le Moigne, N. Microalgae Starch-Based Bioplastics: Screening of Ten Strains and Plasticization of Unfractionated Microalgae by Extrusion. Carbohydr. Polym. 2019, 208, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.F.; Hossain, S.M.Z.; Khalid, H.; Sadaf, F.; Al-Thawadi, S.; Alshater, A.; Hossain, M.M.; Razzak, S.A. Optimization of Bio-Cement Production from Cement Kiln Dust Using Microalgae. Biotechnol. Rep. 2019, 23, e00356. [Google Scholar] [CrossRef] [PubMed]
- Arabian, D. Investigation of Effective Parameters on the Productivity of Biomass and Bio-Cement as a Soil Improver from Chlorella vulgaris. Geomicrobiol. J. 2022, 39, 781–790. [Google Scholar] [CrossRef]
- Srinivas, M.K.; Alengaram, U.J.; Ibrahim, S.; Phang, S.M.; Vello, V.; Jun, H.K.; Alnahhal, A.M. Evaluation of Crack Healing Potential of Cement Mortar Incorporated with Blue-Green Microalgae. J. Build. Eng. 2021, 44, 102958. [Google Scholar] [CrossRef]
- Biswas, J.K.; Kumar, A.; Biswas, S.; Shah, M.P.; Rodríguez-Couto, S. Win-Win Wastewater Phycoremediation: Coupled Carbon Sequestration and Heavy Metal Removal. In An Integration of Phycoremediation Processes in Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2022; pp. 529–548. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, C. Exploring New Blue Carbon Plants for Sustainable Ecosystems. Trends Plant Sci. 2020, 25, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Wedding, L.M.; Moritsch, M.; Verutes, G.; Arkema, K.; Hartge, E.; Reiblich, J.; Douglass, J.; Taylor, S.; Strong, A.L. Incorporating Blue Carbon Sequestration Benefits into Sub-National Climate Policies. Glob. Environ. Chang. 2021, 69, 102206. [Google Scholar] [CrossRef]
- Babich, O.; Sukhikh, S.; Larina, V.; Kalashnikova, O.; Kashirskikh, E.; Prosekov, A.; Noskova, S.; Ivanova, S.; Fendri, I.; Smaoui, S.; et al. Algae: Study of Edible and Biologically Active Fractions, Their Properties and Applications. Plants 2022, 11, 780. [Google Scholar] [CrossRef]
- Beerling, D.J.; Kantzas, E.P.; Lomas, M.R.; Wade, P.; Eufrasio, R.M.; Renforth, P.; Sarkar, B.; Andrews, M.G.; James, R.H.; Pearce, C.R.; et al. Potential for Large-Scale CO2 Removal via Enhanced Rock Weathering with Croplands. Nature 2020, 583, 242–248. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Rokicka, M.; Kupczyk, K. The Possibility of Using Macroalgae Biomass from Natural Reservoirs as a Substrate in the Methane Fermentation Process. Int. J. Green Energy 2015, 12, 970–977. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, Z.; Luo, Y.; Qiao, W.; Xiao, M.; Zhang, M. Biogas Productivity by Co-Digesting Taihu Blue Algae with Corn Straw as an External Carbon Source. Bioresour. Technol. 2012, 114, 281–286. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Li, N.; Wang, Y.; Yu, R.; Zhu, G.; Zeng, R.J. Mixotrophic Cultivation of Microalgae Using Biogas as the Substrate. Environ. Sci. Technol. 2022, 56, 3669–3677. [Google Scholar] [CrossRef] [PubMed]
- Llamas, M.; Magdalena, J.A.; Tomás-Pejó, E.; González-Fernández, C. Microalgae-Based Anaerobic Fermentation as a Promising Technology for Producing Biogas and Microbial Oils. Energy 2020, 206, 118184. [Google Scholar] [CrossRef]
- Bourgougnon, N.; Burlot, A.S.; Jacquin, A.G. Algae for Global Sustainability? Adv. Bot. Res. 2021, 100, 145–212. [Google Scholar] [CrossRef]
- Wicker, R.J.; Kumar, G.; Khan, E.; Bhatnagar, A. Emergent Green Technologies for Cost-Effective Valorization of Microalgal Biomass to Renewable Fuel Products under a Biorefinery Scheme. Chem. Eng. J. 2021, 415, 128932. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Q.; Gu, D.; Yu, L.; Yu, X. The Synergistic Effects of Gamma-Aminobutyric Acid and Salinity during the Enhancement of Microalgal Lipid Production in Photobioreactors. Energy Convers. Manag. 2022, 267, 115928. [Google Scholar] [CrossRef]
- European Commission. The EU Blue Economy Report 2020; European Commission: Luxembourg, 2020; Available online: https://blueindicators.ec.europa.eu/ (accessed on 5 September 2022).
- Li, S.; Li, X.; Ho, S.H. How to Enhance Carbon Capture by Evolution of Microalgal Photosynthesis? Sep. Purif. Technol. 2022, 291, 120951. [Google Scholar] [CrossRef]
- Cardoso, L.G.; Lemos, P.V.F.; de Souza, C.O.; Oliveira, M.B.P.P.; Chinalia, F.A. Current Advances in Phytoremediation and Biochemical Composition of Arthrospira (Spirulina) Grown in Aquaculture Wastewater. Aquac. Res. 2022, 53, 4931–4943. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kazimierowicz, J. Outflow from a Biogas Plant as a Medium for Microalgae Biomass Cultivation—Pilot Scale Study and Technical Concept of a Large-Scale Installation. Energies 2022, 15, 2912. [Google Scholar] [CrossRef]
- Al Jabri, H.; Taleb, A.; Touchard, R.; Saadaoui, I.; Goetz, V.; Pruvost, J. Cultivating Microalgae in Desert Conditions: Evaluation of the Effect of Light-Temperature Summer Conditions on the Growth and Metabolism of Nannochloropsis QU130. Appl. Sci. 2021, 11, 3799. [Google Scholar] [CrossRef]
- Garbowski, T.; Pietryka, M.; Pulikowski, K.; Richter, D. The Use of a Natural Substrate for Immobilization of Microalgae Cultivated in Wastewater. Sci. Rep. 2020, 10, 7915. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, L.; Liao, Q.; Gao, M.; Zhao, Y.; Jin, C.; She, Z.; Wang, G. Bacterial-Algal Coupling System for High Strength Mariculture Wastewater Treatment: Effect of Temperature on Nutrient Recovery and Microalgae Cultivation. Bioresour. Technol. 2021, 338, 125574. [Google Scholar] [CrossRef]
- Piwowar, A.; Dzikuć, M. Bioethanol Production in Poland in the Context of Sustainable Development-Current Status and Future Prospects. Energies 2022, 15, 2582. [Google Scholar] [CrossRef]
- Lin, Y.; Song, G.; Ling, H.; Ge, J.; Ping, W. Isolation of a High-Ammonium-Tolerant Monoraphidium Sp. and Evaluation of Its Potential for Biodiesel Production. Process Biochem. 2021, 111, 297–304. [Google Scholar] [CrossRef]
- Yadav, G.; Mathimani, T.; Sekar, M.; Sindhu, R.; Pugazhendhi, A. Strategic Evaluation of Limiting Factors Affecting Algal Growth—An Approach to Waste Mitigation and Carbon Dioxide Sequestration. Sci. Total Environ. 2021, 796, 149049. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, N.; Talbierz, S.; Dębowski, M.; Kazimierowicz, J.; Zieliński, M. Cultivation Method Effect on Schizochytrium Sp. Biomass Growth and Docosahexaenoic Acid (DHA) Production with the Use of Waste Glycerol as a Source of Organic Carbon. Energies 2021, 14, 2952. [Google Scholar] [CrossRef]
- Brzychczyk, B.; Hebda, T.; Fitas, J.; Giełżecki, J. The Follow-up Photobioreactor Illumination System for the Cultivation of Photosynthetic Microorganisms. Energies 2020, 13, 1143. [Google Scholar] [CrossRef]
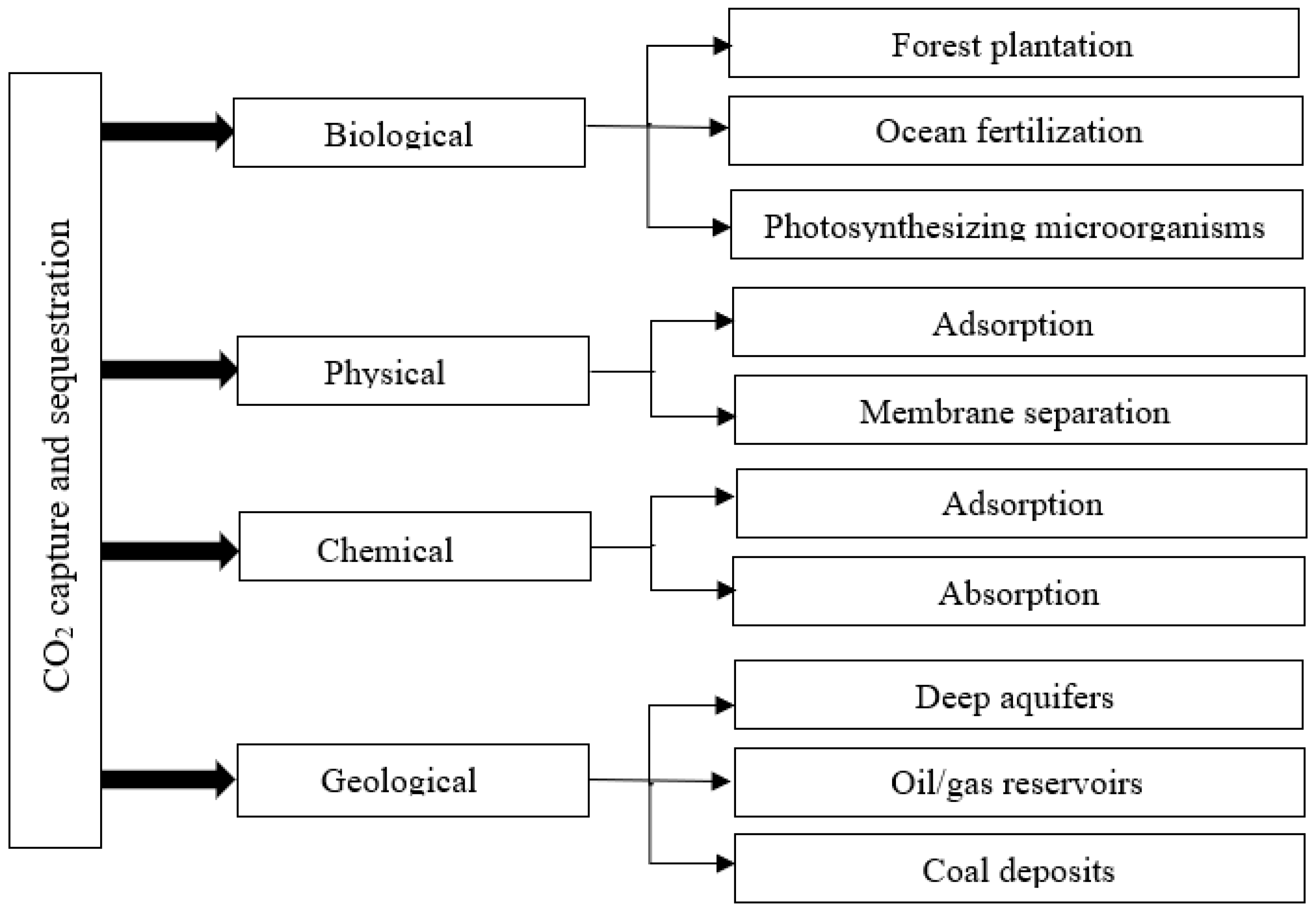
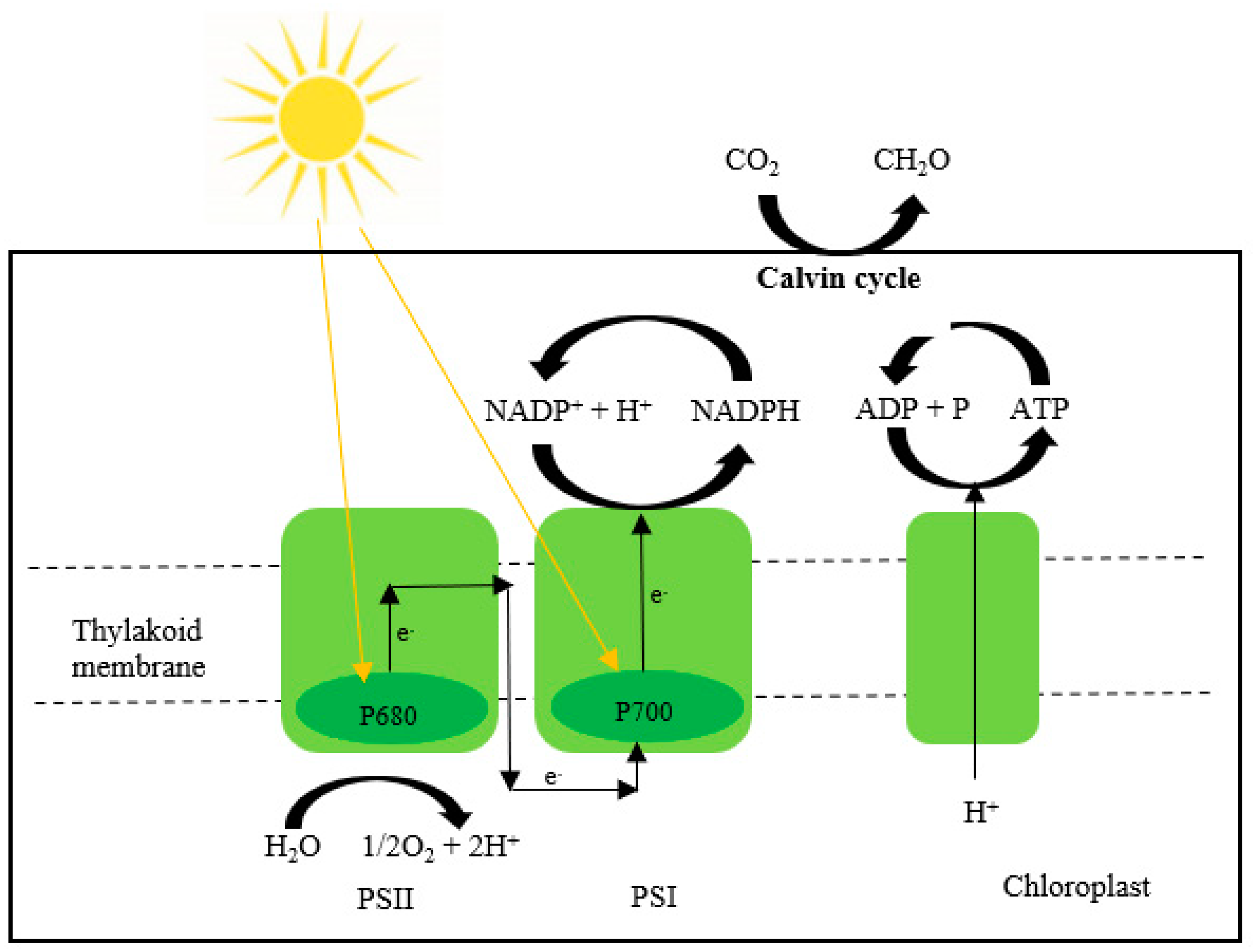

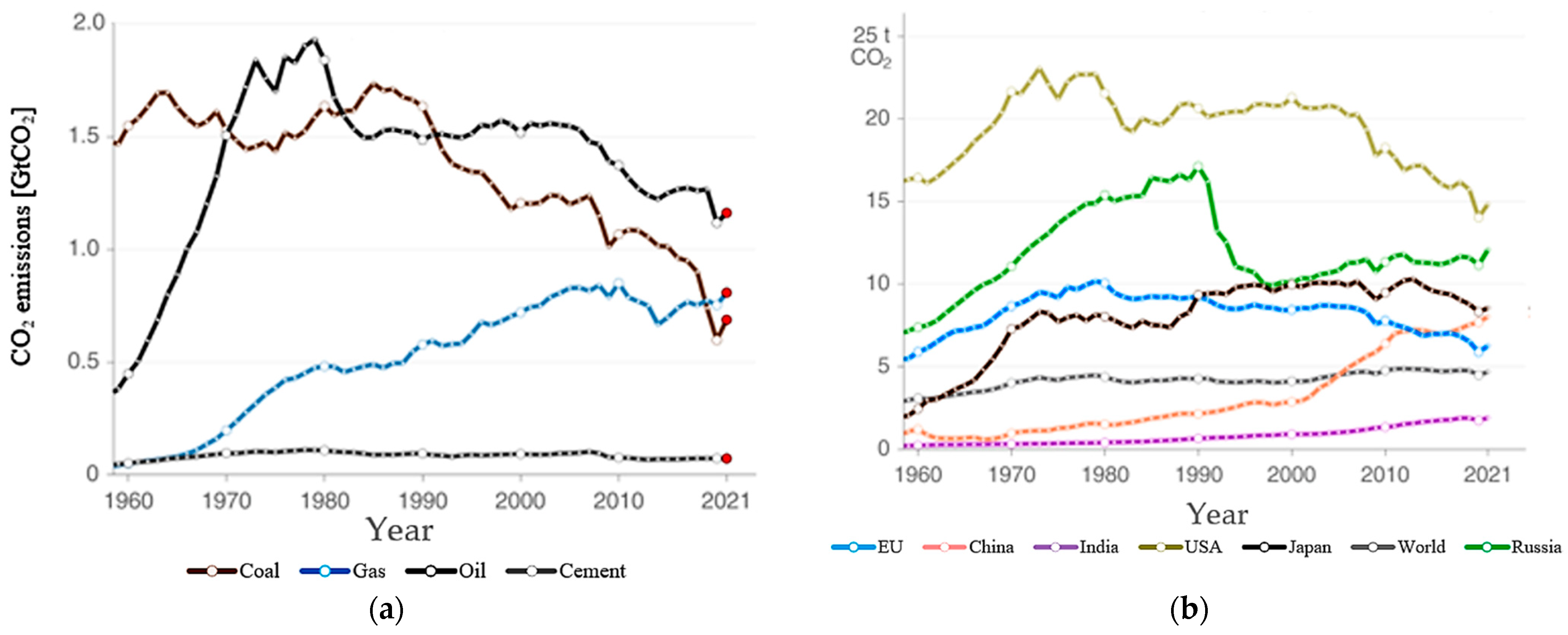
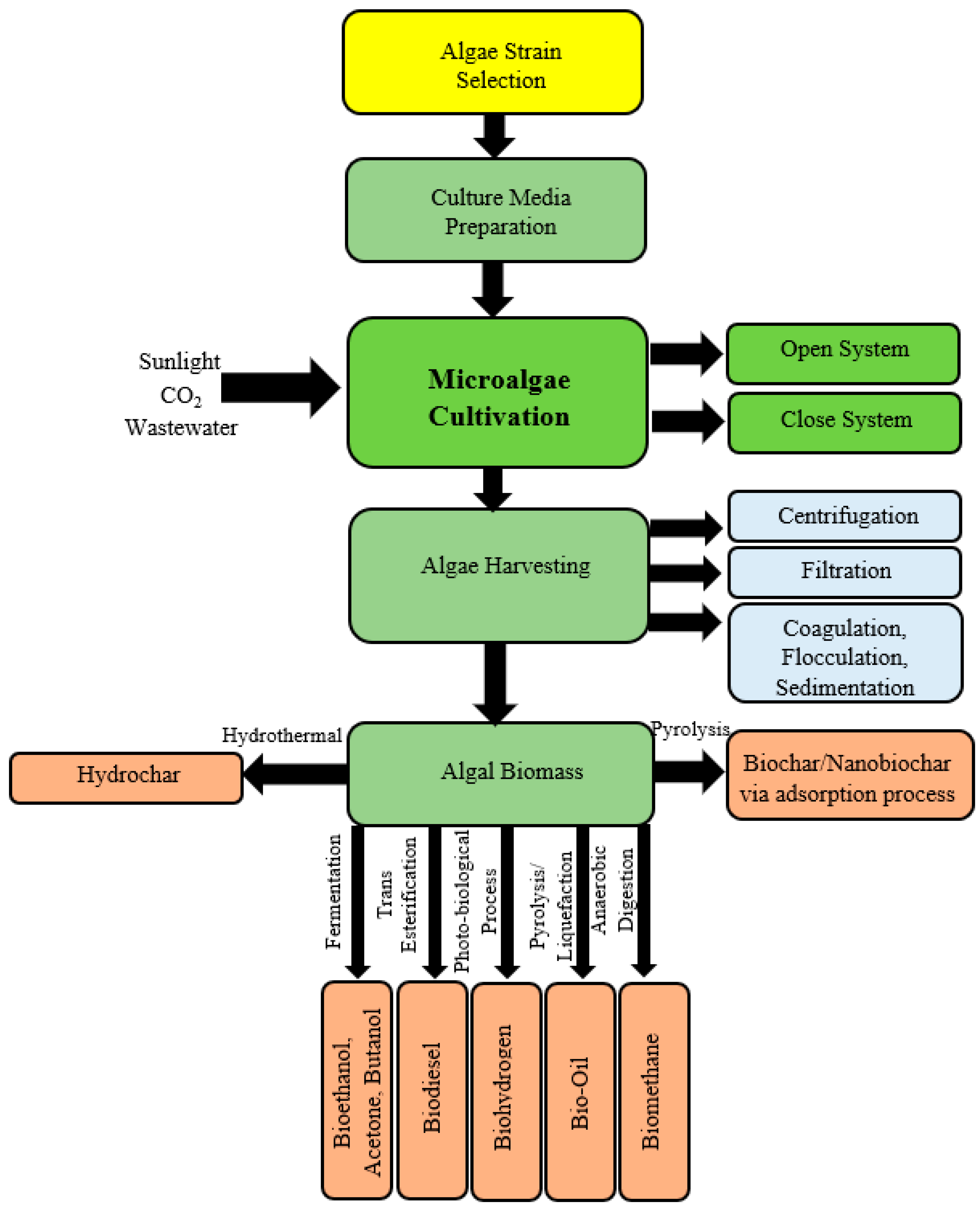
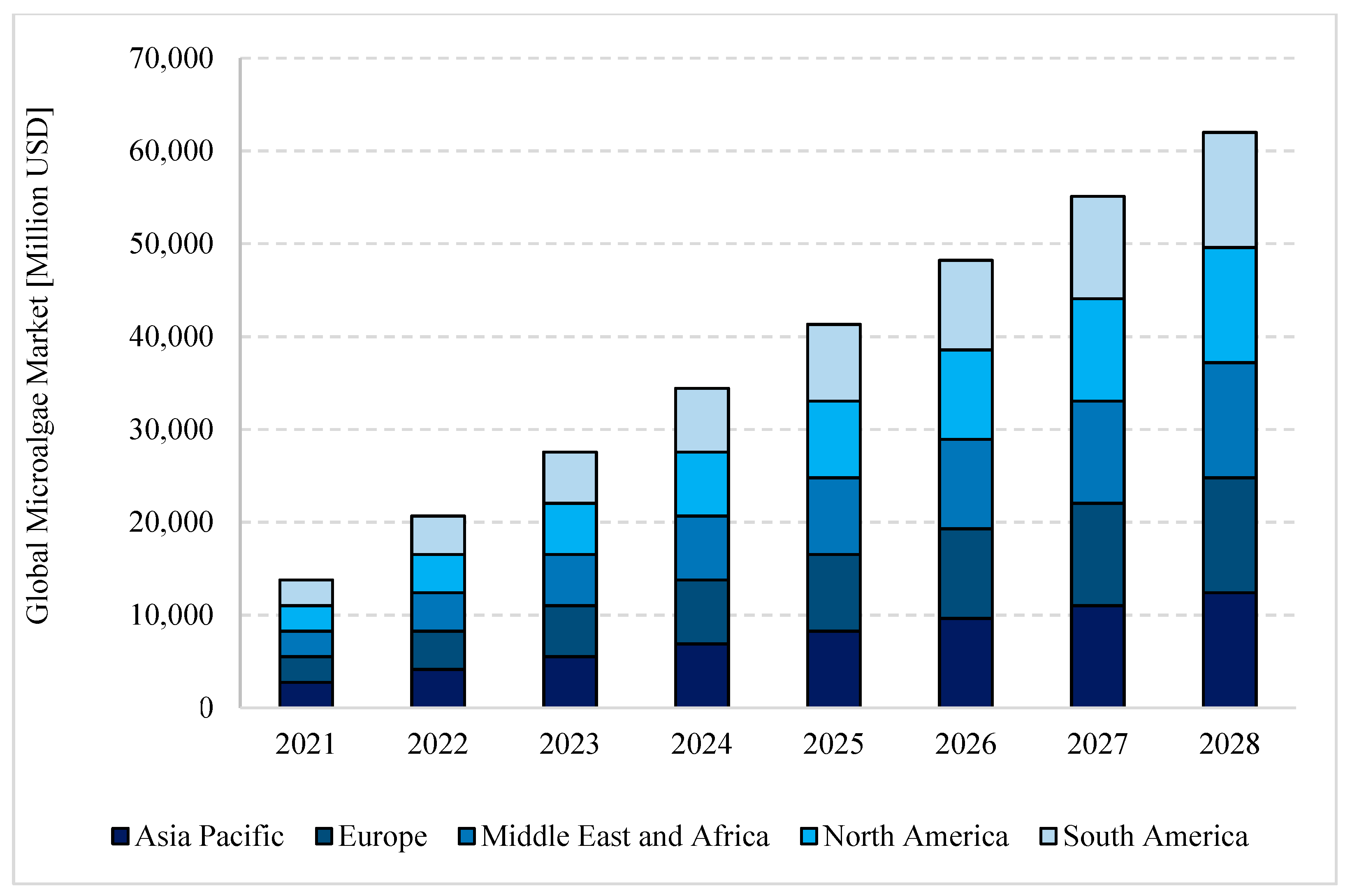
| Pricing | Targets | Rules |
|---|---|---|
| New carbon border adjustment mechanism (CBAM) Updated Energy Taxation Directive Stronger Emissions Trading System including aviation, extending emissions trading to maritime, road transport, and buildings | Updated Energy Efficiency Directive Updated Renewable Energy Directive Updated Land Use, Land Use Change and Forestry Regulation Updated Effort Sharing Regulation | New infrastructure for alternative fuels Stricter CO2 performance for cars and vans FuelEU: cleaner maritime fuels ReFuelEU: more sustainable aviation fuels |
| Support measures | ||
| Combating climate change through the use of funds and regulations to foster innovation, enhance utility, and alleviate the effects on vulnerable communities through the establishment of the Social Climate Fund and improved Modernisation and Innovation Fund. | ||
| Species | Scale | photobioreactor Type | Growth Medium | Dry Matter Levels | Gas Type | CO2 (%) | CO2 Removal (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Chlorella sp. | Laboratory | PBR | Modified f/2 medium in artificial sea water | 8 × 105 cells mL−1 (low-density) or 8 × 106 cells mL−1 (high-density) | Air supplemented with CO2 | 2–15 | 16–58 | [71] |
| Chlorella sorokiniana | Laboratory | Air-lift PBR | Modified TAP (-acetate) medium | - | Flue gas | 15.6 | 4.1 | [67] |
| Chlorella sp. MTF-7 | Laboratory | PBR | Modified f/2 medium in artificial sea water | 0.1 g/L | Flue gas | 25 | 60 | [71] |
| Chlorella kessleri | Laboratory | PBR | Bristol medium | 0.15 g/L (dry biomass basis) | Air supplemented with CO2 from a cylinder | 6 | - | [69] |
| Scenedesmus obliquus | 12 | - | ||||||
| Chlorella sp. KR-1 | Laboratory | Conical flasks | Modified M4N medium | 0.1 g/L | Air supplemented with CO2 | 10–70 | - | [70] |
| Chlorella vulgaris | Laboratory | Membrane-PBR | Mineral medium | 2 × 107 cells mL−1 | Air supplemented with CO2 | 1 | 70 | [72] |
| Chlorella vulgaris | Laboratory and large-scale | IMC-CO2PBR | Synthetic medium | 100 g DM/L | Flue gas | 25 | 45 | [73] |
| Scenedesmus obliquus WUST4 | Pilot scale | Air-lift PBR | Modified soil extract medium (SE medium) | OD685 = 2.0 (OD685 is the optical density at 685 nm, which is used to indicate the algal biomass density based on the turbidimetry) | Flue gas | 18 | 64 | [74] |
| Dunaliella sp. | Pilot scale | Air-lift PBR | - | - | Flue gas | - | 82.3 ± 12.5 (sunny days) 50.1 ± 6.5 (cloudy days) | [75] |
| Mixed biodiverse microalgal (Desmodesmus spp. were identified as dominant microalgae) | Pilot scale | PBR | Bold Basal Medium and f/2 medium | 0.1–0.4 g/L | Flue gas | 11 | - | [76] |
| Chlorella sp. | Pilot scale | PBR | Macronutrients (technical grade)–urea | - | Flue gas | 6–8 | 10–50 | [77] |
| Production System | Prospects | Limitation | Ref. |
|---|---|---|---|
| Open systems | Good for mass cultivation. Relatively economical after cultivation. Easier to construct, operate and clean up. | Requirement of large areas of land. Easily contaminated. Poor light utilization. Diffusion of CO2 to the atmosphere. Evaporative losses. Limited to few strains of algae, cultures. | [88,89,90] |
| Flat panel photobioreactor | Low accumulation of dissolved oxygen and high concentration of sunlight per cm2. Modular design makes it easy to scale up production. | Algal biofilm formation. Strain-specific hydrodynamic stress issues. Temperature control issues. | [83,91,92] |
| Tubular photobioreactor | Good biomass productivities. Relatively inexpensive Suitable for outdoor. | Adverse pH and CO2 gradients. High levels of dissolved oxygen. Fouling. Photoinhibition is very common (outdoor). | [93,94,95] |
| Tic bag photobioreactor | Good sterility. Low cost. | Disposal of used plastic bags may present a significant challenge at large scale operation. | [96,97,98] |
| Species | Supply Method | CO2 Supply (%, v/v) | Scale | Biomass Produced (g/Ld) | CO2 Removal Efficiency (%) | CO2 Fixation Rate (g/Ld) | Ref. |
|---|---|---|---|---|---|---|---|
| Botryococcus braunii | Sparging | 0.03 | 1 L glass bottle | 0.04 | - | 0.08 | [108] |
| Botryococcus braunii | 10 | 1 L glass bottle | 0.02 | 6.78 ± 3.58 | 0.03 | [108] | |
| Botryococcus braunii | 20 | 1 L glass bottle | 0.03 | 3.73 ± 0.74 | 0.05 | [108] | |
| Chlorella pyrenoidosa | 1 | 2 L flask | 0.24 | - | 0.49 | [109] | |
| Chlorella pyrenoidosa | 10 | 1.8 L bubble column | 0.14 | 95.1 | 0.25 | [110] | |
| Chlorella sorokiniana | 1 | 2 L flask | 0.29 | - | 0.58 | [109] | |
| Chlorella vulgaris | 0.03 | 1.5 L membrane bioreactor | 0.05 | - | 0.09 | [111] | |
| Chlorella vulgaris | 10 | 1.8 L bubble column | 0.07 | 95.3 | 0.13 | [110] | |
| Scenedesmus dimorphus | 10 | 1.8 L bubble column | 0.12 | 94.6 | 0.22 | [110] | |
| Scenedesmus obliquus | 10 | 1.8 L bubble column | 0.15 | 94.7 | 0.27 | [110] | |
| Scenedesmus sp. | 20 | 1 L glass bottle | 0.13 | 3.82 ± 1.71 | 0.23 | [108] |
| Species | Product | Characteristics | Ref. |
|---|---|---|---|
| Chlorella pyrenoidosa | Biocarbon | Highly microporous design and heteroatom-containing composition of the sustainable biocarbon will make it an attractive material for a lot of applications, including supercapacitors, CO2 capture, and catalysts for oxygen reduction reaction. | [128] |
| Chlorella pyrenoidosa | [129] | ||
| Scenedesmus sp. | [130] | ||
| Graesiella sp. WBG-1 | [131] | ||
| Chlorella sp., Scenedesmus sp., Spirulina sp., Synechocystis sp. | Biostimulants | Sustainable and economical substitute to synthetic liquid fertilizer for promotion of eco-agriculture. | [132] |
| Tetradesmus obliquus, Chlorella protothecoides, Chlorella vulgaris | [133] | ||
| Chlamydomonas reinhardtii, Chlorella sorokiniana | [134] | ||
| Scenedesmus sp. UTEX 1589 | Bioplastics | They have great biodegradability, biocompatibility, and properties comparable to regular thermoplastics. Being derived from sustainable natural materials, these biopolymers establish to solve the environmental issues caused by petrochemical plastics. Thanks to the combination of their unique characteristics, PHAs have the ability to be used in a wide variety of applications. Their biodegradability and biocompatibility allow their use in medical utilization, e.g., in the manufacture of implants, wound dressings, or drug delivery carriers. | [135] |
| Nannochloropsis | [136] | ||
| Spirulina | [137] | ||
| Chlamydomonas reinhardtii 11-32A | [138] | ||
| Chlorella kessleri | Cement | Biocement is a competitive material compared to conventional products. Its manufacturing is economically and environmentally justified as high temperatures are not required. Biocement is used to repair damaged structures and to strengthen eroding rock formations. | [139] |
| Chlorella vulgaris | [140] | ||
| Synechococcus elongatus and Spirulina platensis | [141] |
| Blue Carbon Warriors | CO2 Sequestration Intensity [MgCO2/km2·year] | Total Carbon Sequestration [TgC/year] |
|---|---|---|
| Mangroves | 829 | 31.45 |
| Salt marshes | 799 | 11.12 |
| Seagrasses | 506 | 44.02 |
| Cultured macroalgae | 1500 | 0.68 |
| Coral reefs | 543 | 16.5 |
| Wild macroalgae | 150 | 173 |
| Microalgae | 11,280 | Not available |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zieliński, M.; Dębowski, M.; Kazimierowicz, J.; Świca, I. Microalgal Carbon Dioxide (CO2) Capture and Utilization from the European Union Perspective. Energies 2023, 16, 1446. https://doi.org/10.3390/en16031446
Zieliński M, Dębowski M, Kazimierowicz J, Świca I. Microalgal Carbon Dioxide (CO2) Capture and Utilization from the European Union Perspective. Energies. 2023; 16(3):1446. https://doi.org/10.3390/en16031446
Chicago/Turabian StyleZieliński, Marcin, Marcin Dębowski, Joanna Kazimierowicz, and Izabela Świca. 2023. "Microalgal Carbon Dioxide (CO2) Capture and Utilization from the European Union Perspective" Energies 16, no. 3: 1446. https://doi.org/10.3390/en16031446
APA StyleZieliński, M., Dębowski, M., Kazimierowicz, J., & Świca, I. (2023). Microalgal Carbon Dioxide (CO2) Capture and Utilization from the European Union Perspective. Energies, 16(3), 1446. https://doi.org/10.3390/en16031446









