Improved Electrical and Thermal Conductivities of Graphene–Carbon Nanotube Composite Film as an Advanced Thermal Interface Material
Abstract
:1. Introduction
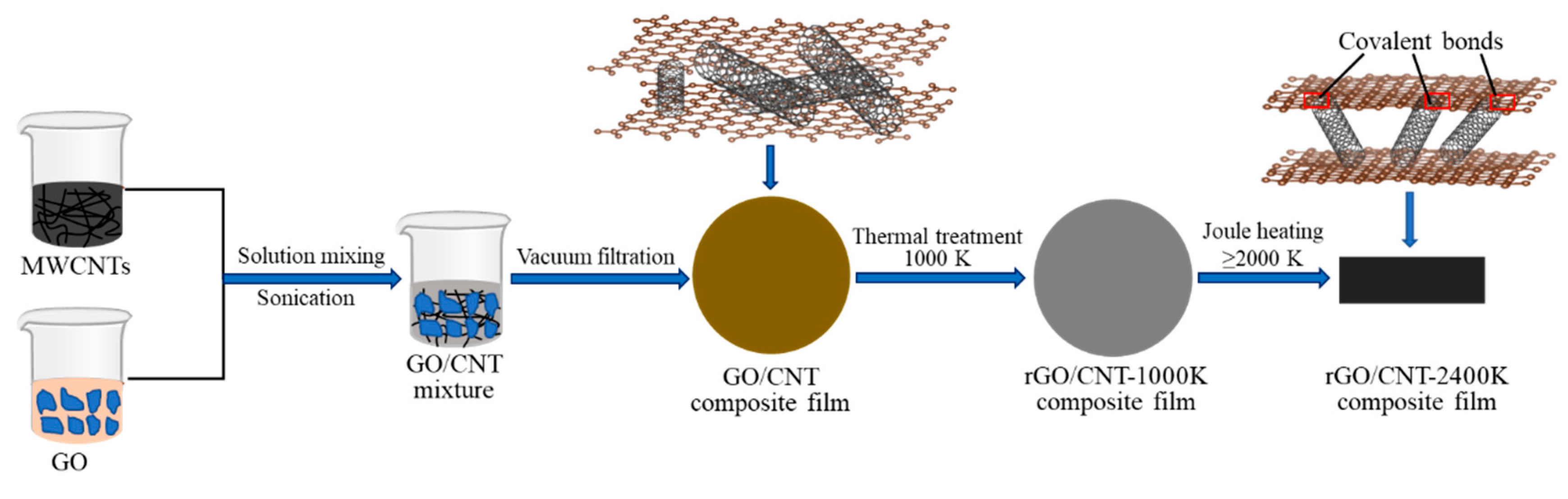
2. Experiments
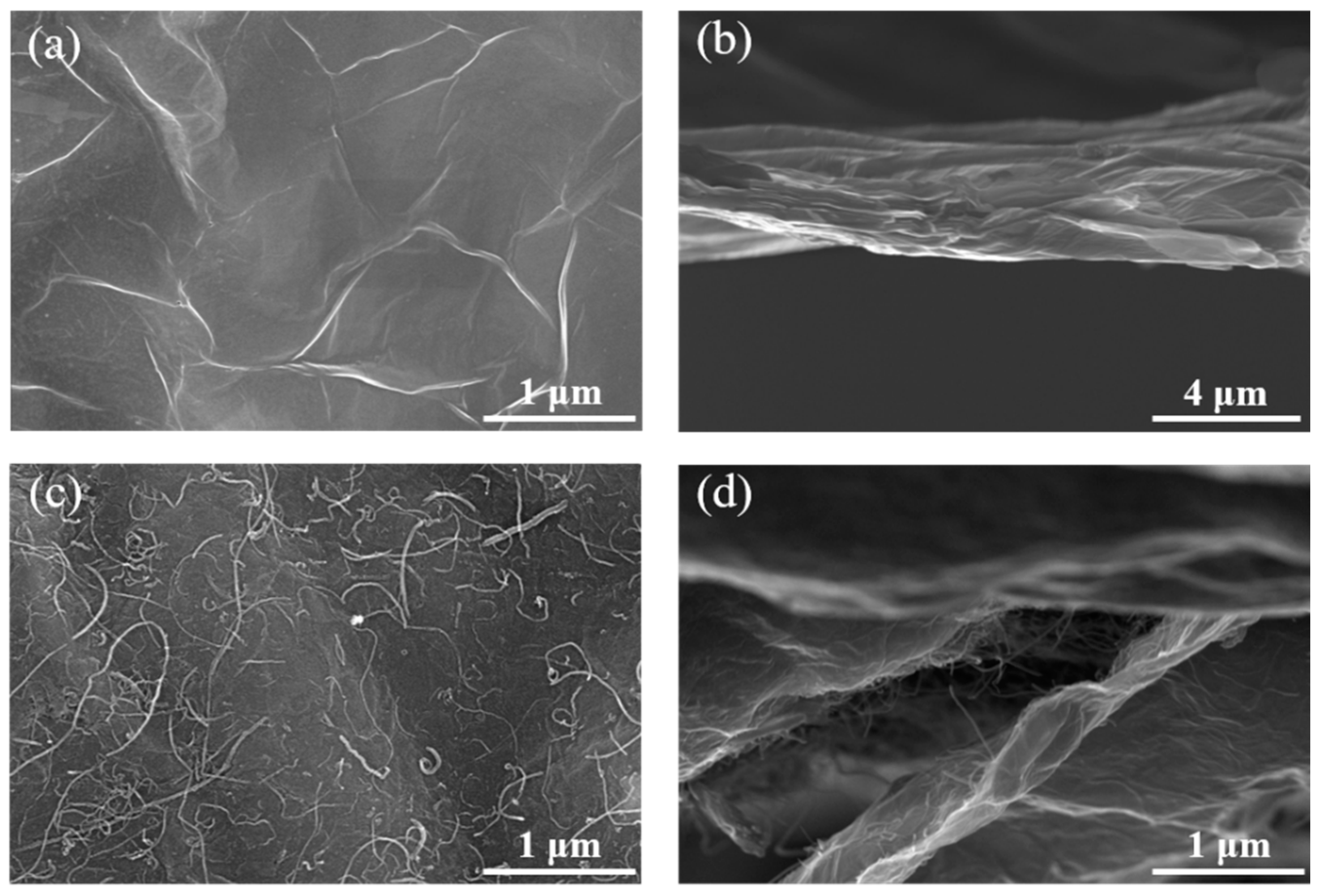
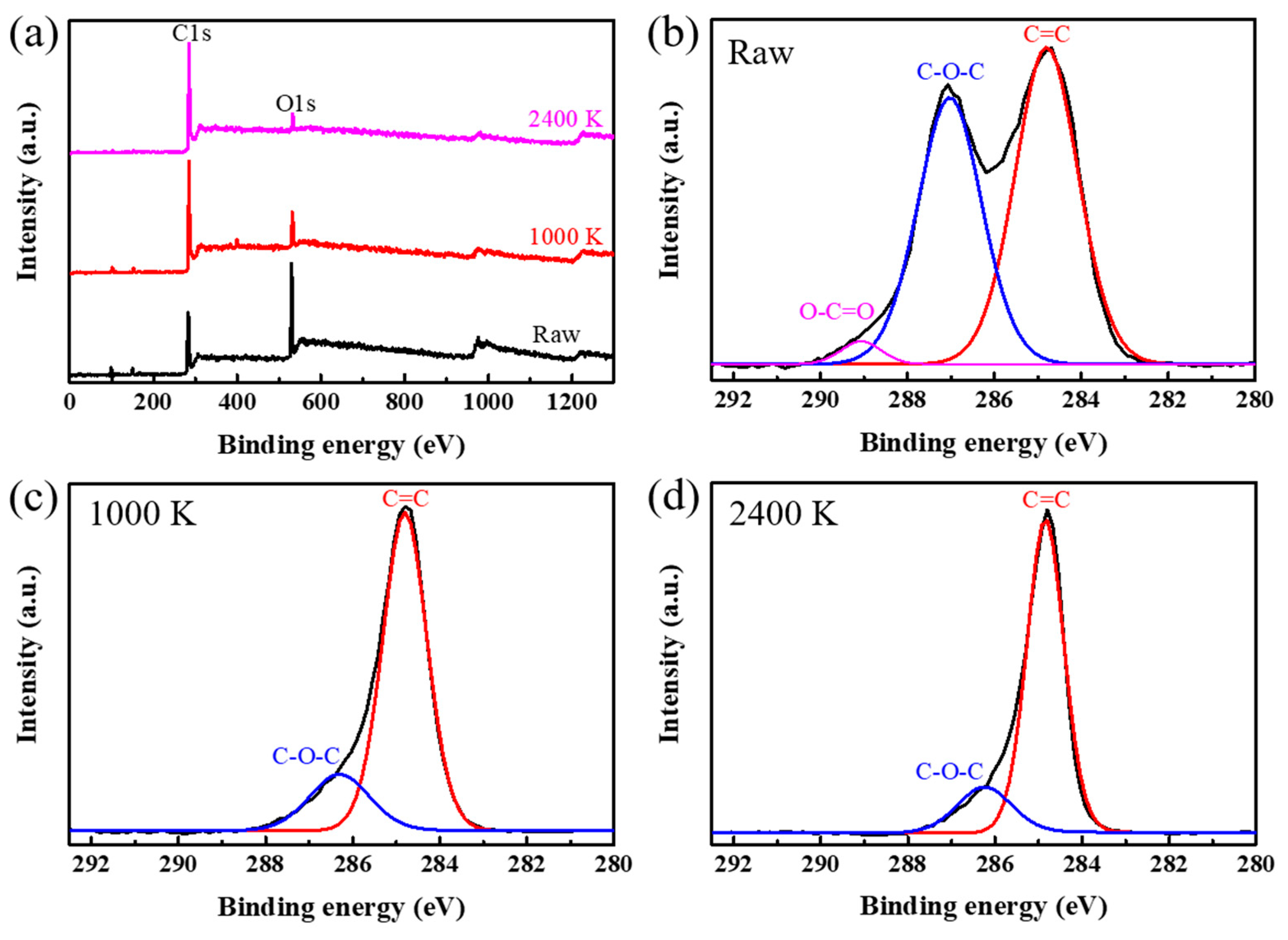
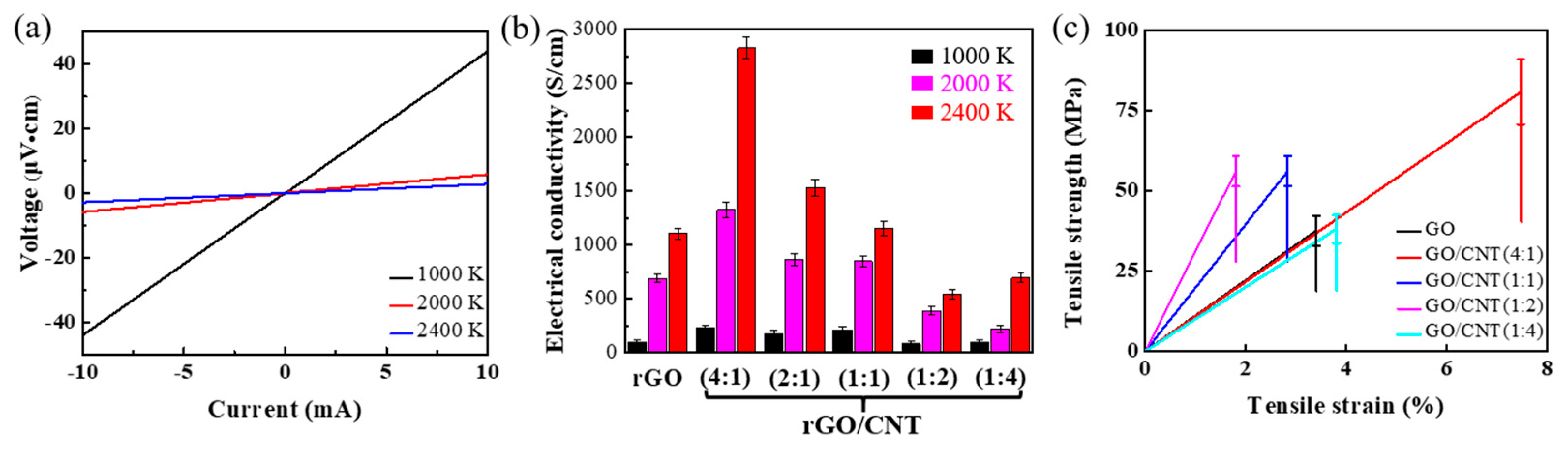
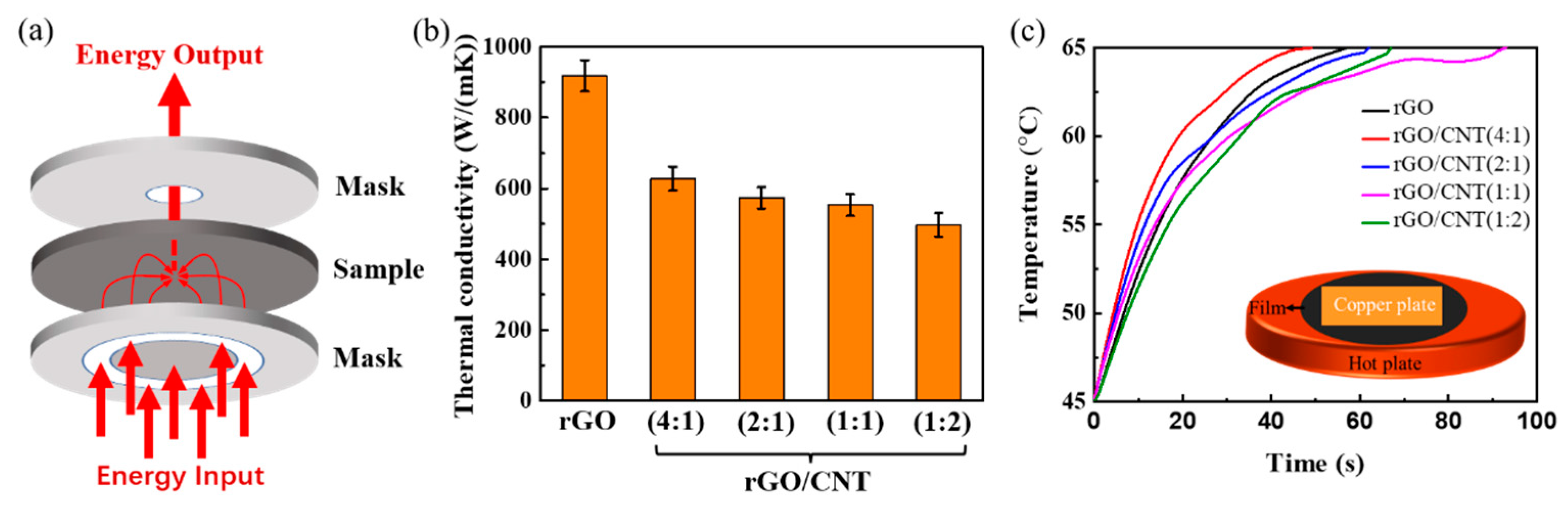
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Khan, J.; Momin, S.A.; Mariatti, M. A review on advanced carbon-based thermal interface materials for electronic devices. Carbon 2020, 168, 65–112. [Google Scholar] [CrossRef]
- Moore, A.L.; Shi, L. Emerging challenges and materials for thermal management of electronics. Mater. Today 2014, 17, 163–174. [Google Scholar] [CrossRef]
- Bulut, M.; Kandlikar, S.G.; Sozbir, N. A Review of Vapor Chambers. Heat Transf. Eng. 2019, 40, 1551–1573. [Google Scholar] [CrossRef]
- Velardo, J.; Singh, R.; Date, A.; Date, A. An investigation into the effective thermal conductivity of vapour chamber heat spreaders. Energy Procedia 2017, 110, 256–261. [Google Scholar] [CrossRef]
- Górecki, K.; Posobkiewicz, K. Cooling Systems of Power Semiconductor Devices—A Review. Energies 2022, 15, 4566. [Google Scholar] [CrossRef]
- Wu, X.; Wang, H.; Wang, Z.; Xu, J.; Wu, Y.; Xue, R.; Cui, H.; Tian, C.; Wang, Y.; Huang, X.; et al. Highly conductive thermal interface materials with vertically aligned graphite-nanoplatelet filler towards: High power density electronic device cooling. Carbon 2021, 182, 445–453. [Google Scholar] [CrossRef]
- Due, J.; Robinson, A.J. Reliability of thermal interface materials: A review. Appl. Therm. Eng. 2013, 50, 455–463. [Google Scholar] [CrossRef]
- Gao, J.; Yan, Q.; Lv, L.; Tan, X.; Ying, J.; Yang, K.; Yu, J.; Du, S.; Wei, Q.; Xiang, R.; et al. Lightweight thermal interface materials based on hierarchically structured graphene paper with superior through-plane thermal conductivity. Chem. Eng. J. 2021, 419, 129609. [Google Scholar] [CrossRef]
- Shahil, K.M.F.; Balandin, A.A. Graphene–Multilayer Graphene Nanocomposites as Highly Efficient Thermal Interface Materials. Nano Lett. 2012, 12, 861–867. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Chen, S.; Moore, A.L.; Cai, W.; Suk, J.W.; An, J.; Mishra, C.; Amos, C.; Magnuson, C.W.; Kang, J.; Shi, L.; et al. Raman Measurements of Thermal Transport in Suspended Monolayer Graphene of Variable Sizes in Vacuum and Gaseous Environments. ACS Nano 2011, 5, 321–328. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Q.; Mishra, C.; Kang, J.; Zhang, H.; Cho, K.; Cai, W.; Balandin, A.A.; Ruoff, R.S. Thermal conductivity of isotopically modified graphene. Nat. Mater. 2012, 11, 203–207. [Google Scholar] [CrossRef]
- Gong, F.; Li, H.; Wang, W.; Xia, D.; Liu, Q.; Papavassiliou, D.; Xu, Z. Recent Advances in Graphene-Based Free-Standing Films for Thermal Management: Synthesis, Properties, and Applications. Coatings 2018, 8, 63. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Hansson, J.; Liu, Y.; Chen, S.; Zehri, A.; Samani, M.K.; Wang, N.; Ni, Y.; Zhang, Y.; Zhang, Z.-B.; et al. Graphene related materials for thermal management. 2D Mater. 2020, 7, 012001. [Google Scholar] [CrossRef]
- Van Noorden, R. Moving towards a graphene world. Nature 2006, 442, 228–229. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, L.; Zhou, C. Review of Chemical Vapor Deposition of Graphene and Related Applications. Acc. Chem. Res. 2013, 46, 2329–2339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Peng, L.; Xu, Z.; Liu, Z.; Guo, Y.; Li, P.; Gao, C. Ultrahigh Thermal Conductive yet Superflexible Graphene Films. Adv. Mater. 2017, 29, 1700589. [Google Scholar] [CrossRef]
- Kumar, P.; Shahzad, F.; Yu, S.; Hong, S.M.; Kim, Y.-H.; Koo, C.M. Large-area reduced graphene oxide thin film with excellent thermal conductivity and electromagnetic interference shielding effectiveness. Carbon 2015, 94, 494–500. [Google Scholar] [CrossRef]
- Jin, S.; Gao, Q.; Zeng, X.; Zhang, R.; Liu, K.; Shao, X.; Jin, M. Effects of reduction methods on the structure and thermal conductivity of free-standing reduced graphene oxide films. Diam. Relat. Mater. 2015, 58, 54–61. [Google Scholar] [CrossRef]
- Gómez-Navarro, C.; Meyer, J.C.; Sundaram, R.S.; Chuvilin, A.; Kurasch, S.; Burghard, M.; Kern, K.; Kaiser, U. Atomic structure of reduced graphene oxide. Nano Lett. 2010, 10, 1144–1148. [Google Scholar] [CrossRef] [Green Version]
- Maldovan, M. Sound and heat revolutions in phononics. Nature 2013, 503, 209–217. [Google Scholar] [CrossRef]
- Parrott, J.E.; Stuckes, A.D.; Klemens, P.G. Thermal Conductivity of Solids. Phys. Today 1977, 30, 60–61. [Google Scholar] [CrossRef] [Green Version]
- Akbari, A.; Cunning, B.V.; Joshi, S.R.; Wang, C.; Camacho-Mojica, D.C.; Chatterjee, S.; Modepalli, V.; Cahoon, C.; Bielawski, C.W.; Bakharev, P.; et al. Highly Ordered and Dense Thermally Conductive Graphitic Films from a Graphene Oxide/Reduced Graphene Oxide Mixture. Matter 2020, 2, 1198–1206. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, H.; Wang, N.; Zhang, P.; Fu, Y.; Murugesan, M.; Edwards, M.; Jeppson, K.; Volz, S.; Liu, J. Improved heat spreading performance of functionalized graphene in microelectronic device application. Adv. Funct. Mater. 2015, 25, 4430–4435. [Google Scholar] [CrossRef]
- Renteria, J.D.; Ramirez, S.; Malekpour, H.; Alonso, B.; Centeno, A.; Zurutuza, A.; Cocemasov, A.I.; Nika, D.L.; Balandin, A.A. Strongly Anisotropic Thermal Conductivity of Free-Standing Reduced Graphene Oxide Films Annealed at High Temperature. Adv. Funct. Mater. 2015, 25, 4664–4672. [Google Scholar] [CrossRef]
- Jia, H.; Kong, Q.-Q.; Liu, Z.; Wei, X.-X.; Li, X.-M.; Chen, J.-P.; Li, F.; Yang, X.; Sun, G.-H.; Chen, C.-M. 3D graphene/ carbon nanotubes/ polydimethylsiloxane composites as high-performance electromagnetic shielding material in X-band. Compos. Part A Appl. Sci. Manufac. 2020, 129, 105712. [Google Scholar] [CrossRef]
- Chen, Y.; Hou, X.; Liao, M.; Dai, W.; Wang, Z.; Yan, C.; Li, H.; Lin, C.-T.; Jiang, N.; Yu, J. Constructing a “pea-pod-like” alumina-graphene binary architecture for enhancing thermal conductivity of epoxy composite. Chem. Eng. J. 2020, 381, 122690. [Google Scholar] [CrossRef]
- Pan, T.-W.; Kuo, W.-S.; Tai, N.-H. Tailoring anisotropic thermal properties of reduced graphene oxide/multi-walled carbon nanotube hybrid composite films. Compos. Sci. Technol. 2017, 151, 44–51. [Google Scholar] [CrossRef]
- Yuan, H.-C.; Lee, C.-Y.; Tai, N.-H. Extremely high thermal conductivity of nanodiamond-polydopamine/thin-layer graphene composite films. Compos. Sci. Technol. 2018, 167, 313–322. [Google Scholar] [CrossRef]
- Rathinavel, S.; Priyadharshini, K.; Panda, D. A review on carbon nanotube: An overview of synthesis, properties, functionalization, characterization, and the application. Mater. Sci. Eng. B 2021, 268, 115095. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Lacey, S.D.; Xu, L.; Xie, H.; Li, T.; Danner, V.A.; Hu, L. Reduced graphene oxide film with record-high conductivity and mobility. Mater. Today 2018, 21, 186–192. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 02, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-M.; Huang, J.-Q.; Zhang, Q.; Gong, W.-Z.; Yang, Q.-H.; Wang, M.-Z.; Yang, Y.-G. Annealing a graphene oxide film to produce a free standing high conductive graphene film. Carbon 2012, 50, 659–667. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, H.-B.; Zhang, H.; Ren, W.; Dasari, A.; Tang, G.-S.; Yu, Z.-Z. Structural evolution of functionalized graphene sheets during solvothermal reduction. Carbon 2013, 56, 132–138. [Google Scholar] [CrossRef]
- Song, L.; Khoerunnisa, F.; Gao, W.; Dou, W.; Hayashi, T.; Kaneko, K.; Endo, M.; Ajayan, P.M. Effect of high-temperature thermal treatment on the structure and adsorption properties of reduced graphene oxide. Carbon 2013, 52, 608–612. [Google Scholar] [CrossRef]
- Hu, C.; Sedghi, S.; Silvestre-Albero, A.; Andersson, G.G.; Sharma, A.; Pendleton, P.; Rodríguez-Reinoso, F.; Kaneko, K.; Biggs, M.J. Raman spectroscopy study of the transformation of the carbonaceous skeleton of a polymer-based nanoporous carbon along the thermal annealing pathway. Carbon 2015, 85, 147–158. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, J.; Luo, J.; Gong, W.; Li, C.; Li, Q.; Zhang, K.; Hu, M.; Yao, Y. Enhanced thermal conductivity of free-standing 3D hierarchical carbon nanotube-graphene hybrid paper. Compos. Part A Appl. Sci. Manufac. 2017, 102, 1–8. [Google Scholar] [CrossRef]
- Cançado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Jorio, A.; Coelho, L.N.; Magalhães-Paniago, R.; Pimenta, M.A. General equation for the determination of the crystallite size La of nanographite by Raman spectroscopy. Appl. Phys. Lett. 2006, 88, 163106. [Google Scholar] [CrossRef]
- Xue, Y.; Li, X.; Wang, H.; Zhao, F.; Zhang, D.; Chen, Y. Improvement in thermal conductivity of through-plane aligned boron nitride/silicone rubber composites. Mater Des. 2019, 165, 107580. [Google Scholar] [CrossRef]

| Samples | GO | rGO−1000 K | rGO−2400 K | GO/CNT(4:1) | rGO/CNT(4:1)−1000 K | rGO/CNT(4:1)−2400 K |
|---|---|---|---|---|---|---|
| d/nm | 0.8975 | 0.3446 | 0.3436 | 0.3437 | 0.3432 | 0.3422 |
| Lc/nm | 0.1669 | 0.0797 | 0.4177 | 0.0730 | 0.0578 | 0.2653 |
| ID/IG | 0.84 | 1.16 | 0.13 | 0.96 | 1.14 | 0.08 |
| La/nm | 22.89 | 16.57 | 147.88 | 20.02 | 16.86 | 240.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Song, S.; Mi, M.; Yu, L.; Xu, L.; Jiang, P.; Wang, Y. Improved Electrical and Thermal Conductivities of Graphene–Carbon Nanotube Composite Film as an Advanced Thermal Interface Material. Energies 2023, 16, 1378. https://doi.org/10.3390/en16031378
Jiang Y, Song S, Mi M, Yu L, Xu L, Jiang P, Wang Y. Improved Electrical and Thermal Conductivities of Graphene–Carbon Nanotube Composite Film as an Advanced Thermal Interface Material. Energies. 2023; 16(3):1378. https://doi.org/10.3390/en16031378
Chicago/Turabian StyleJiang, Youcheng, Shangzhi Song, Mengjuan Mi, Lixuan Yu, Lisha Xu, Puqing Jiang, and Yilin Wang. 2023. "Improved Electrical and Thermal Conductivities of Graphene–Carbon Nanotube Composite Film as an Advanced Thermal Interface Material" Energies 16, no. 3: 1378. https://doi.org/10.3390/en16031378
APA StyleJiang, Y., Song, S., Mi, M., Yu, L., Xu, L., Jiang, P., & Wang, Y. (2023). Improved Electrical and Thermal Conductivities of Graphene–Carbon Nanotube Composite Film as an Advanced Thermal Interface Material. Energies, 16(3), 1378. https://doi.org/10.3390/en16031378








