Performance Characteristic Analysis of Metallic and Non-Metallic Oxide Nanofluids for a Compound Parabolic Collector: Improvement of Renewable Energy Technologies in Buildings
Abstract
1. Introduction
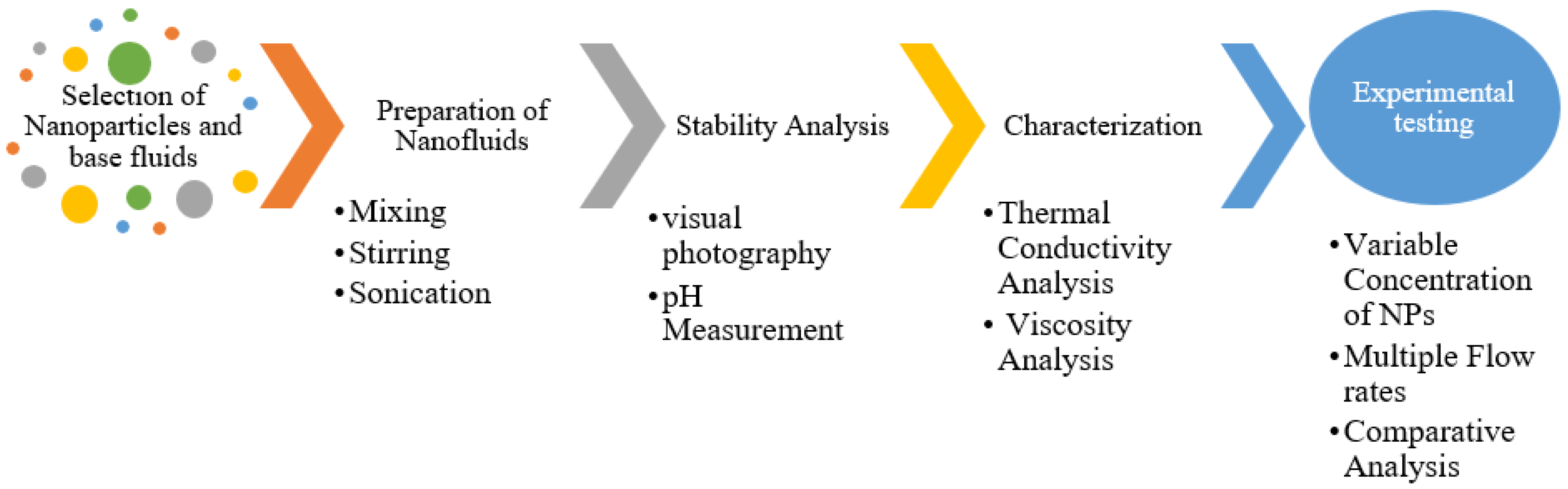
2. Materials and Methods: Selection and Preparation of Nanofluids
2.1. Selection of Nanoparticles
2.2. Preparation of Nanofluids
2.3. pH Measurement
2.4. Thermal Conductivity Measurement
2.5. Viscosity Measurement
2.6. Key Performance Indicators
2.7. Experimental Setup and Measurement Procedure
2.8. Uncertainty Analysis
3. Results and Discussion
3.1. Stability and Characterisation Analysis of Nanofluids
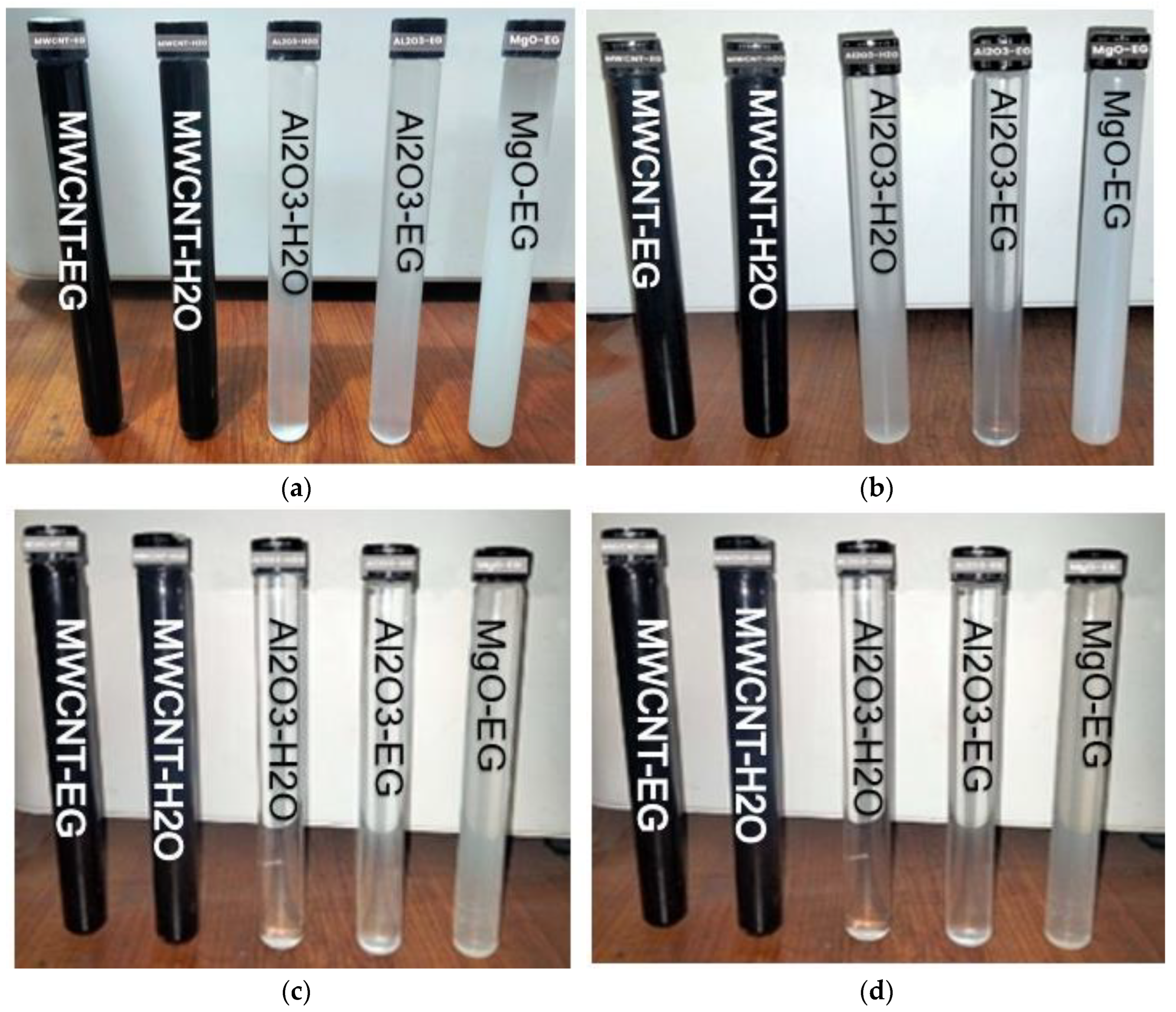
3.1.1. Visual Stability
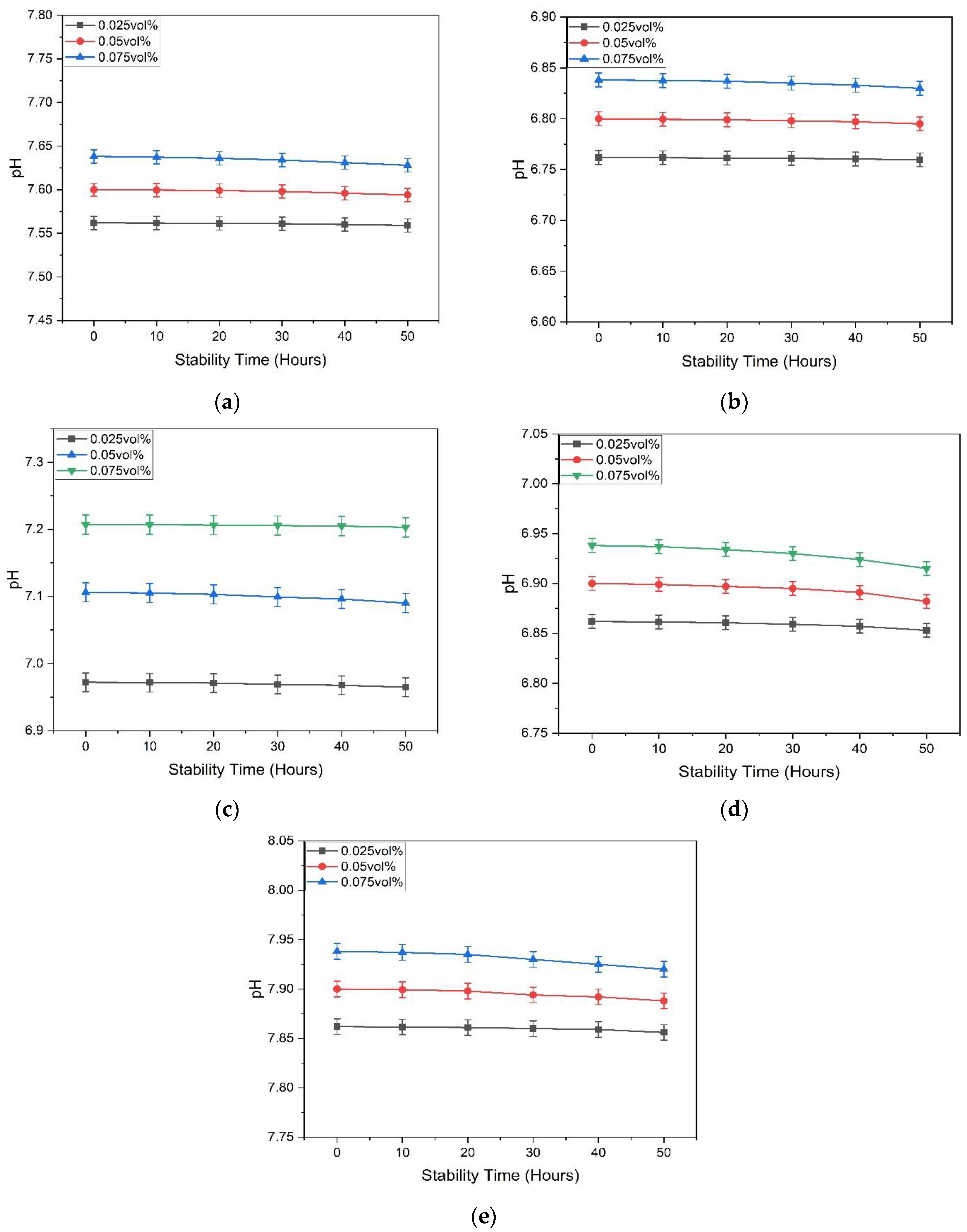
3.1.2. pH Measurement
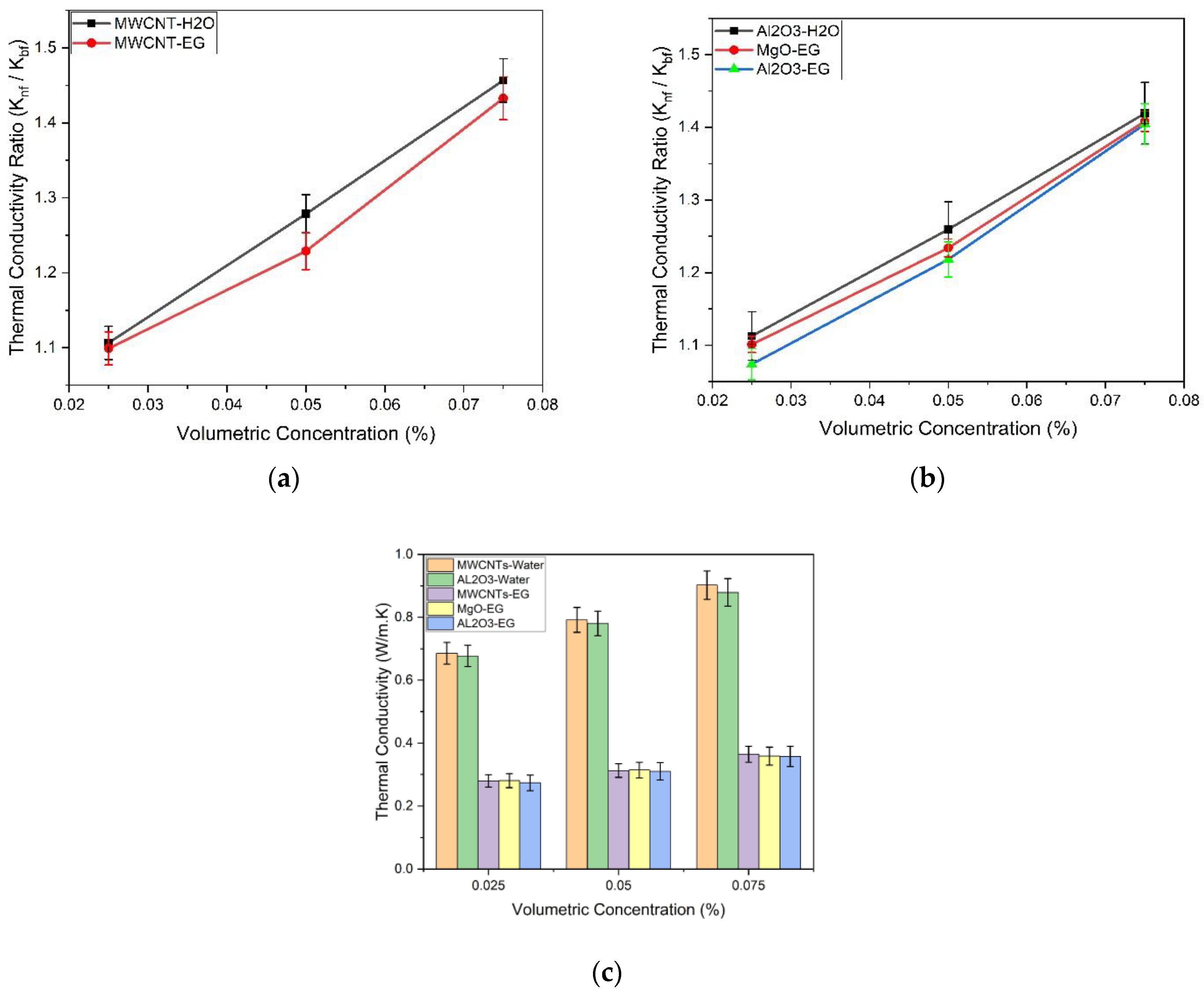
3.1.3. Thermal Conductivity Analysis
3.1.4. Viscosity Analysis
Effect of Temperature at Constant Shear Rate
Effect of Temperature at Constant Shear Rate
3.2. Experimental Analysis in CPC System
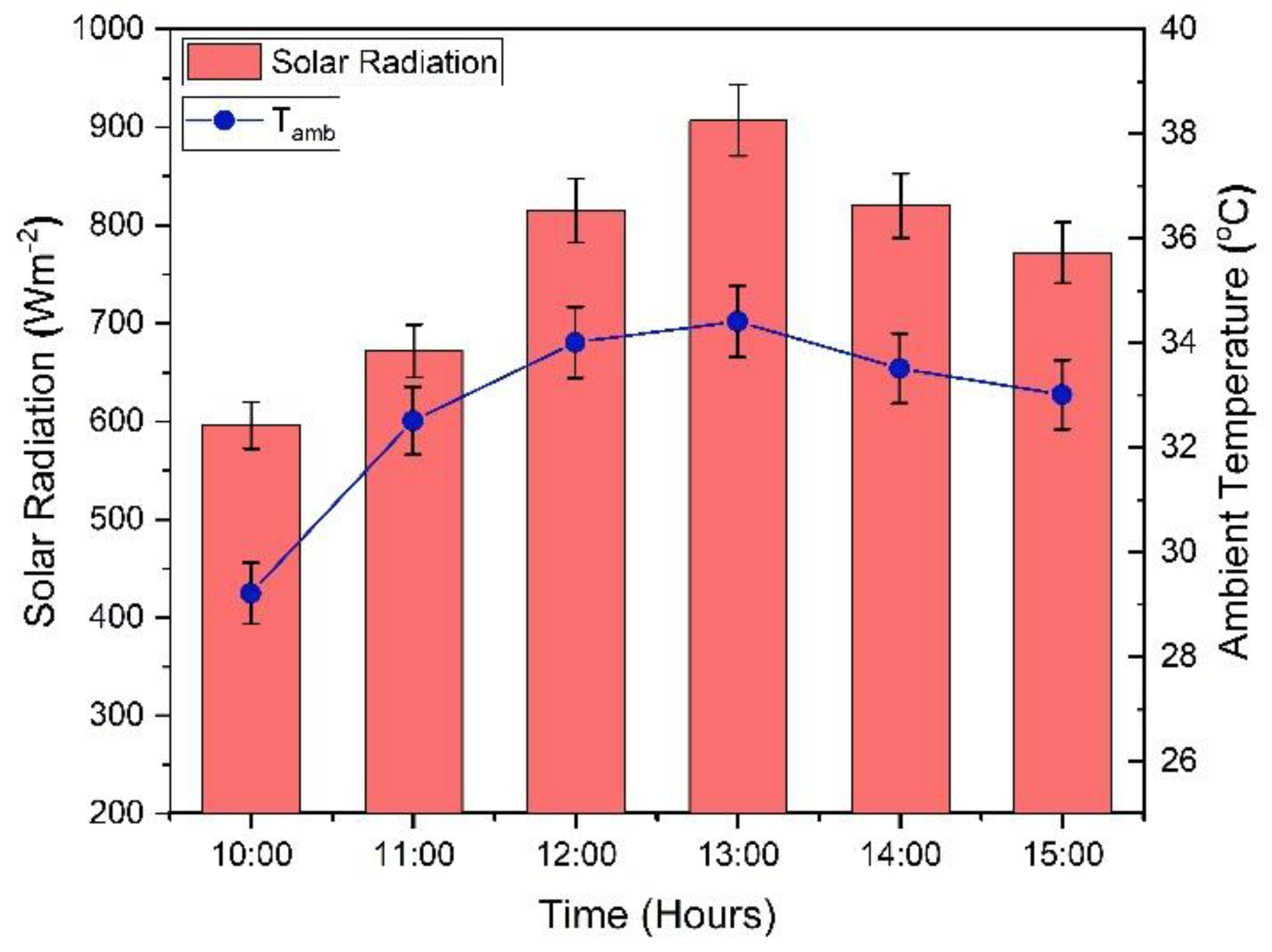
3.2.1. Variation in Climatic Conditions during Testing
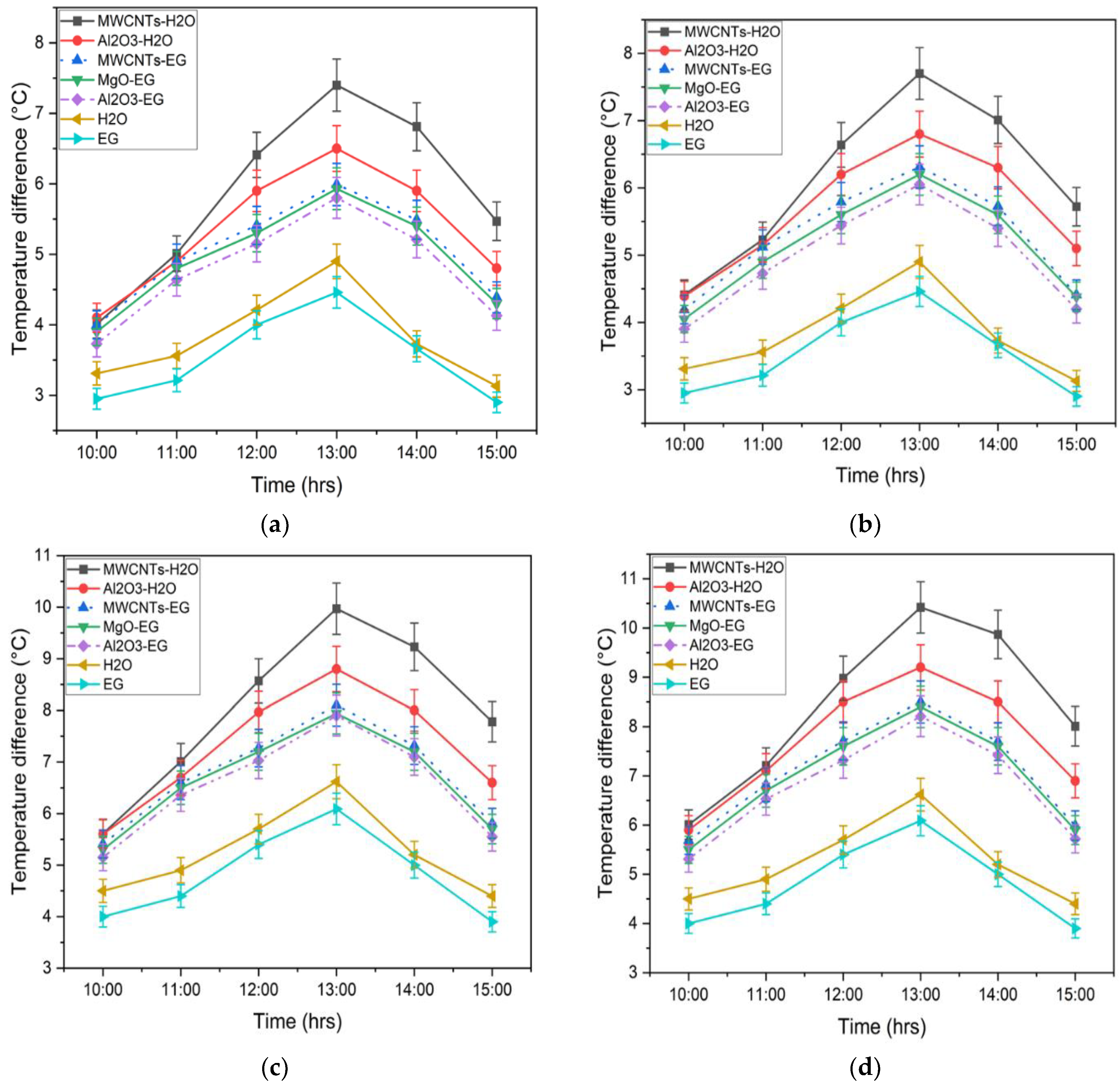
3.2.2. Comparison of Temperature Difference
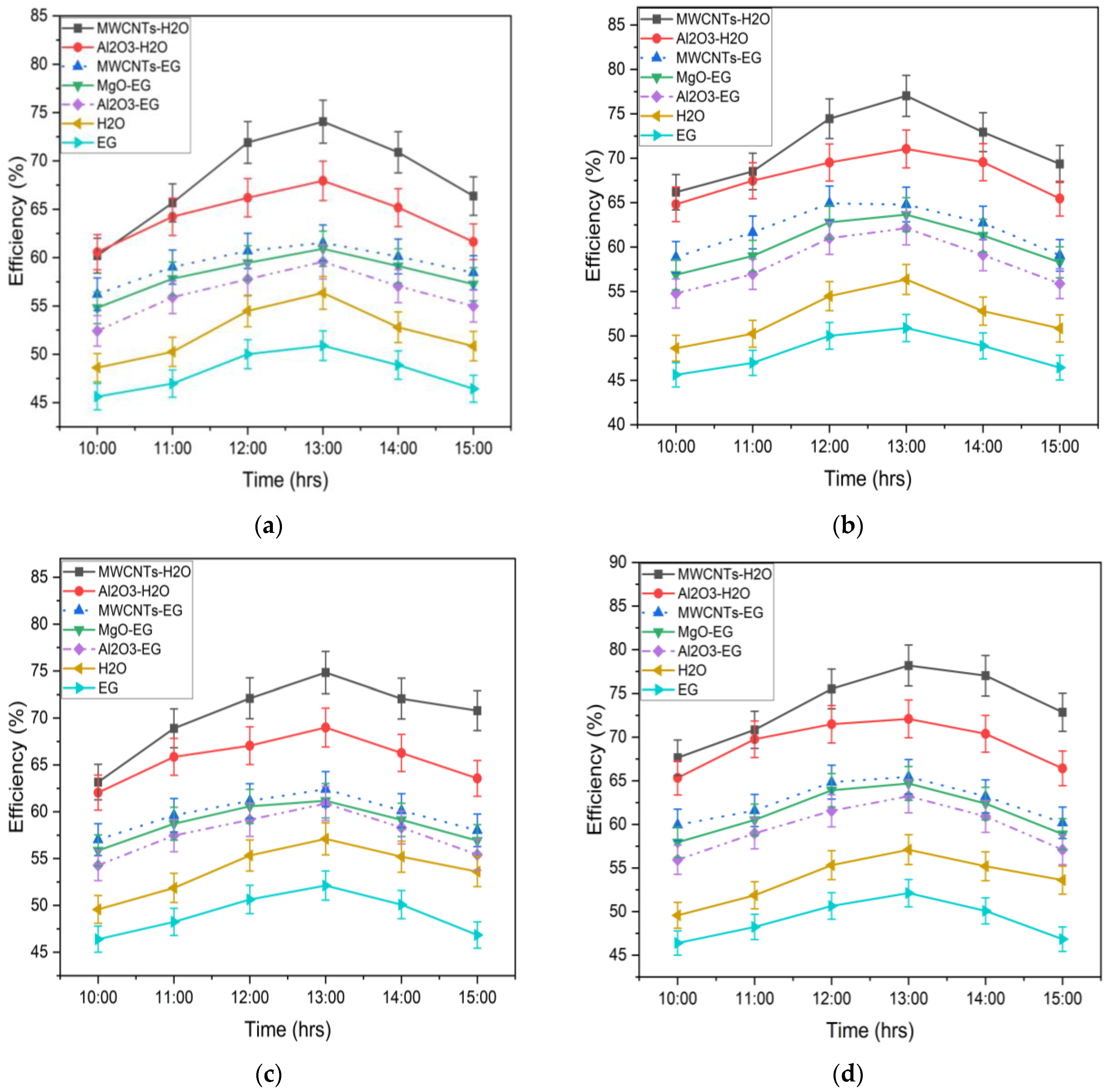
3.2.3. Comparison of Thermal Efficiency
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | Area (m2) |
| Aa | Aperture Area (m2) |
| Al2O3 | Aluminium oxide |
| Au | Gold |
| Ag | Silver |
| CeO2 | Ceric oxide |
| CNTs | Carbon nanotubes |
| CuO | Cupric oxide |
| CPC | Compound parabolic collector |
| Cp | Specific heat capacity (J kg−1 K−1) |
| EG | Ethylene glycol |
| Fe3O4 | Iron oxide |
| FPSC | Flat-plate solar collector |
| GNPs | Graphene nanoplatelets |
| Ge | Total effective solar irradiance on collector’s aperture (Wm−2) |
| MgO | Magnesium oxide |
| MWCNTs | Multi-walled carbon nanotubes |
| Mass flow rate (kg s−1) | |
| Useful solar heat gain (W) | |
| Qs | Solar power entering into collector’s aperture (W) |
| SiO2 | Silicon dioxide |
| SiC | Silicon carbide |
| SDBS | Sodium dodecyl benzene sulphonate |
| SFT | Single flow through |
| TiO2 | Titanium oxide |
| T | Temperature (°C) |
| To | Outlet temperature (°C) |
| Ti | Inlet temperature (°C) |
| Temperature difference | |
| ZnO | Zinc oxide |
| Greek Symbols | |
| Density (kg·m−3) | |
| Volumetric concentration (%) | |
| Thermal efficiency (%) | |
| Subscripts | |
| a | Aperture |
| bf | Base fluid |
| i | Inlet |
| nf | Nanofluid |
| np | Nanoparticle |
| o | Outlet |
References
- Mittelman, G.; Epstein, M. A Novel Power Block for CSP Systems. Sol. Energy 2010, 84, 1761–1771. [Google Scholar] [CrossRef]
- Bhutto, A.W.; Bazmi, A.A.; Zahedi, G. Greener Energy: Issues and Challenges for Pakistan-Solar Energy Prospective. Renew. Sustain. Energy Rev. 2012, 16, 2762–2780. [Google Scholar] [CrossRef]
- Ashraf Chaudhry, M.; Raza, R.; Hayat, S.A. Renewable Energy Technologies in Pakistan: Prospects and Challenges. Renew. Sustain. Energy Rev. 2009, 13, 1657–1662. [Google Scholar] [CrossRef]
- Grabowska, K.; Zylka, A.; Kulakowska, A.; Skrobek, D.; Krzywanski, J.; Sosnowski, M.; Ciesielska, K.; Nowak, W. Experimental Investigation of an Intensified Heat Transfer Adsorption Bed (IHTAB) Reactor Prototype. Materials 2021, 14, 3520. [Google Scholar] [CrossRef]
- Skrobek, D.; Krzywanski, J.; Sosnowski, M.; Kulakowska, A.; Zylka, A.; Grabowska, K.; Ciesielsak, K.; Nowak, W. Prediction of sorption processes using the deep learning methods (long short-term memory). Energies 2020, 13, 6601. [Google Scholar] [CrossRef]
- Kulakowska, A.; Pajdak, A.; Krzywanski, J.; Grabowska, K.; Zylka, A.; Sosnowski, M.; Wesolowka, M.; Sztekler, K.; Nowak, W. Effect of Metal and Carbon Nanotube Additives on the Thermal Diffusivity of a Silica Gel-Based Adsorption Bed. Energies 2020, 13, 1391. [Google Scholar] [CrossRef]
- Hachicha, A.A.; Rodríguez, I.; Capdevila, R.; Oliva, A. Heat Transfer Analysis and Numerical Simulation of a Parabolic Trough Solar Collector. Appl. Energy 2013, 111, 581–592. [Google Scholar] [CrossRef]
- Krzywanski, J.; Grabowska, K.; Sosnowski, M.; Zylka, A.; Kulakowska, A.; Czakiert, T.; Sztekler, K.; Wesolowska, M.; Nowak, W. Heat transfer in adsorption chillers with fluidized beds of silica gel, zeolite, and carbon nanotubes. Heat Transf. Eng. 2021, 43, 172–182. [Google Scholar] [CrossRef]
- Skrobek, D.; Krzywanski, J.; Sosnowski, M.; Kulakowska, A.; Zylka, A.; Grabowska, K.; Ciesielska, K.; Nowak, W. Implementation of deep learning methods in prediction of adsorption processes. Adv. Eng. Softw. 2022, 173, 103190. [Google Scholar] [CrossRef]
- Pajdak, A.; Kulakowska, A.; Liu, J.; Berent, K.; Kudasik, M.; Krzywanski, J.; Kalawa, W.; Sztekler, K.; Skoczylas, N. Accumulation and Emission of Water Vapor by Silica Gel Enriched with Carbon Nanotubes CNT-Potential Applications in Adsorption Cooling and Desalination Technology. Appl. Sci. 2022, 12, 5644. [Google Scholar] [CrossRef]
- Kasaeian, A.B.; Bakhsh, A. Convection Heat Transfer Modeling of Ag Nanofluid Using Different Viscosity Theories. IIUM Eng. J. 2012, 13, 1–11. [Google Scholar] [CrossRef]
- Akhtar, F.; Ali, M.; Sheikh, N.A.; Shehryar, M. Experimental Investigation of Solar Compound Parabolic Collector Using Al2O3/H2O Nanofluid in A Subtropical Climate. Therm. Sci. 2021, 25, 3453–3465. [Google Scholar] [CrossRef]
- Mesgari, S.; Coulombe, S.; Hordy, N.; Taylor, R.A. Thermal Stability of Carbon Nanotube-Based Nanofluids for Solar Thermal Collectors. Mater. Res. Innov. 2015, 19, S5650–S5653. [Google Scholar] [CrossRef]
- Singh, T.; Almanassra, I.W.; Ghani Olabi, A.; Al-Ansari, T.; McKay, G.; Ali Atieh, M. Performance Investigation of Multiwall Carbon Nanotubes Based Water/Oil Nanofluids for High Pressure and High Temperature Solar Thermal Technologies for Sustainable Energy Systems. Energy Convers. Manag. 2020, 225, 113453. [Google Scholar] [CrossRef]
- Eltaweel, M.; Abdel-Rehim, A.A.; Attia, A.A.A. Energetic and Exergetic Analysis of a Heat Pipe Evacuated Tube Solar Collector Using MWCNT/Water Nanofluid. Case Stud. Therm. Eng. 2020, 22, 100743. [Google Scholar] [CrossRef]
- Ganesh Kumar, P.; Sakthivadivel, D.; Thangapandian, N.; Salman, M.; Kumar Thakur, A.; Sathyamurthy, R.; Chul Kim, S. Effects of Ultasonication and Surfactant on the Thermal and Electrical Conductivity of Water—Solar Glycol Mixture Based Al2O3 Nanofluids for Solar-Thermal Applications. Sustain. Energy Technol. Assess. 2021, 47, 101371. [Google Scholar] [CrossRef]
- Sathishkumar, A.; Cheralathan, M. Charging and Discharging Processes of Low Capacity Nano-PCM Based Cool Thermal Energy Storage System: An Experimental Study. Energy 2023, 263, 125700. [Google Scholar] [CrossRef]
- Kumar, P.G.; Prabakaran, R.; Sakthivadivel, D.; Somasundaram, P.; Vigneswaran, V.S.; Kim, S.C. Ultrasonication Time Optimization for Multi-Walled Carbon Nanotube Based Therminol-55 Nanofluid: An Experimental Investigation. J. Therm. Anal. Calorim. 2022, 147, 10329–10336. [Google Scholar] [CrossRef]
- Mahbubul, I.M.; Khan, M.M.A.; Ibrahim, N.I.; Ali, H.M.; Al-Sulaiman, F.A.; Saidur, R. Carbon Nanotube Nanofluid in Enhancing the Efficiency of Evacuated Tube Solar Collector. Renew. Energy 2018, 121, 36–44. [Google Scholar] [CrossRef]
- Eltaweel, M.; Abdel-Rehim, A.A. Energy and Exergy Analysis of a Thermosiphon and Forced-Circulation Flat-Plate Solar Collector Using MWCNT/Water Nanofluid. Case Stud. Therm. Eng. 2019, 14, 100416. [Google Scholar] [CrossRef]
- ANSI Standard B198.1; Methods of Testing to Determine the Thermal Performance of Solar Collectors. ASHARE: Peachtree Corners, GA, USA, 1977. Available online: https://webstore.ansi.org/preview-pages/ASHRAE/preview_ANSI+ASHRAE+93-2003.pdf (accessed on 1 August 2022).
- Sabiha, M.A.; Saidur, R.; Hassani, S.; Said, Z.; Mekhilef, S. Energy Performance of an Evacuated Tube Solar Collector Using Single Walled Carbon Nanotubes Nanofluids. Energy Convers. Manag. 2015, 105, 1377–1388. [Google Scholar] [CrossRef]
- Said, Z.; Saidur, R.; Sabiha, M.A.; Rahim, N.A.; Anisur, M.R. Thermophysical Properties of Single Wall Carbon Nanotubes and Its Effect on Exergy Efficiency of a Flat Plate Solar Collector. Sol. Energy 2015, 115, 757–769. [Google Scholar] [CrossRef]
- Verma, S.K.; Tiwari, A.K.; Chauhan, D.S. Experimental Evaluation of Flat Plate Solar Collector Using Nanofluids. Energy Convers. Energy Convers. Manag. 2017, 134, 103–115. [Google Scholar] [CrossRef]
- Bernard, S.S.; Suresh, G.; Ahmed, M.D.J.; Mageshwaran, G.; Madanagopal, V.; Karthikeyan, J. Performance Analysis of MWCNT Fluid Parabolic Trough Collector for Whole Year. Mater. Today Proc. 2021, 45, 1308–1311. [Google Scholar] [CrossRef]
- Kumar, L.H.; Kazi, S.N.; Masjuki, H.H.; Zubir, M.N.M.; Jahan, A.; Bhinitha, C. Energy, Exergy and Economic Analysis of Liquid Flat-Plate Solar Collector Using Green Covalent Functionalized Graphene Nanoplatelets. Appl. Therm. Eng. 2021, 192, 116916. [Google Scholar] [CrossRef]
- Eltaweel, M.; Abdel-Rehim, A.A.; Attia, A.A.A. A Comparison between Flat-Plate and Evacuated Tube Solar Collectors in Terms of Energy and Exergy Analysis by Using Nanofluid. Appl. Therm. Eng. 2021, 186, 116516. [Google Scholar] [CrossRef]
- Mwesigye, A.; Yılmaz, İ.H.; Meyer, J.P. Numerical Analysis of the Thermal and Thermodynamic Performance of a Parabolic Trough Solar Collector Using SWCNTs-Therminol®VP-1 Nanofluid. Renew. Energy 2018, 119, 844–862. [Google Scholar] [CrossRef]
- Liu, S.; Afan, H.A.; Aldlemy, M.S.; Al-Ansari, N.; Yaseen, Z.M. Energy Analysis Using Carbon and Metallic Oxides-Based Nanomaterials inside a Solar Collector. Energy Rep. 2020, 6, 1373–1381. [Google Scholar] [CrossRef]
- Naphon, P.; Assadamongkol, P.; Borirak, T. Experimental Investigation of Titanium Nanofluids on the Heat Pipe Thermal Efficiency. Int. Commun. Heat Mass Transf. 2008, 35, 1316–1319. [Google Scholar] [CrossRef]
- Sundar, L.S.; Singh, M.K.; Sousa, A.C.M. Enhanced Heat Transfer and Friction Factor of MWCNT-Fe3O4/Water Hybrid Nanofluids. Int. Commun. Heat Mass Transf. 2014, 52, 73–83. [Google Scholar] [CrossRef]
- Naphon, P.; Thongkum, D.; Assadamongkol, P. Heat Pipe Efficiency Enhancement with Refrigerant-Nanoparticles Mixtures. Energy Convers. Manag. 2009, 50, 772–776. [Google Scholar] [CrossRef]
- Farajzadeh, E.; Movahed, S.; Hosseini, R. Experimental and Numerical Investigations on the Effect of Al2O3/TiO2[Sbnd]H2O Nanofluids on Thermal Efficiency of the Flat Plate Solar Collector. Renew. Energy 2018, 118, 122–130. [Google Scholar] [CrossRef]
- Noie, S.H.; Heris, S.Z.; Kahani, M.; Nowee, S.M. Heat Transfer Enhancement Using Al2O3/Water Nanofluid in a Two-Phase Closed Thermosyphon. Int. J. Heat Fluid Flow 2009, 30, 700–705. [Google Scholar] [CrossRef]
- Menbari, A.; Alemrajabi, A.A.; Rezaei, A. Experimental Investigation of Thermal Performance for Direct Absorption Solar Parabolic Trough Collector (DASPTC) Based on Binary Nanofluids. Exp. Therm. Fluid Sci. 2017, 80, 218–227. [Google Scholar] [CrossRef]
- Choudhary, S.; Sachdeva, A.; Kumar, P. Time-Based Analysis of Stability and Thermal Efficiency of Flat Plate Solar Collector Using Iron Oxide Nanofluid. Appl. Therm. Eng. 2021, 183, 115931. [Google Scholar] [CrossRef]
- Sharafeldin, M.A.; Gróf, G. Experimental Investigation of Flat Plate Solar Collector Using CeO2-Water Nanofluid. Energy Convers. Energy Convers. Manag. 2018, 155, 32–41. [Google Scholar] [CrossRef]
- Hawwash, A.A.; Abdel Rahman, A.K.; Nada, S.A.; Ookawara, S. Numerical Investigation and Experimental Verification of Performance Enhancement of Flat Plate Solar Collector Using Nanofluids. Appl. Therm. Eng. 2018, 130, 363–374. [Google Scholar] [CrossRef]
- Verma, S.K.; Tiwari, A.K.; Chauhan, D.S. Performance Augmentation in Flat Plate Solar Collector Using MgO/Water Nanofluid. Energy Convers. Energy Convers. Manag. 2016, 124, 607–617. [Google Scholar] [CrossRef]
- Gupta, H.K.; Das Agrawal, G.; Mathur, J. An Experimental Investigation of a Low Temperature Al2O3-H2O Nanofluid Based Direct Absorption Solar Collector. Sol. Energy 2015, 118, 390–396. [Google Scholar] [CrossRef]
- Faizal, M.; Saidur, R.; Mekhilef, S.; Alim, M.A. Energy, Economic and Environmental Analysis of Metal Oxides Nanofluid for Flat-Plate Solar Collector. Energy Convers. Energy Convers. Manag. 2013, 76, 162–168. [Google Scholar] [CrossRef]
- Eastman, J.A.; Choi, S.U.S.; Li, S.; Yu, W.; Thompson, L.J. Anomalously Increased Effective Thermal Conductivities of Ethylene Glycol-Based Nanofluids Containing Copper Nanoparticles. Appl. Phys. Lett. 2001, 78, 718–720. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Li, S.; Eastman, J.A. Measuring Thermal Conductivity of Fluids Containing Oxide Nanoparticles. J. Heat Transf. 1999, 121, 280–289. [Google Scholar] [CrossRef]
- Hong, T.K.; Yang, H.S.; Choi, C.J. Study of the Enhanced Thermal Conductivity of Fe Nanofluids. J. Appl. Phys. 2005, 97, 064311. [Google Scholar] [CrossRef]
- Xie, H.; Wang, J.; Xi, T.; Liu, Y. Thermal Conductivity of Suspensions Containing Nanosized SiC Particles. Int. J. Thermophys. 2002, 23, 571–580. [Google Scholar] [CrossRef]
- Patel, H.E.; Das, S.K.; Sundararajan, T.; Sreekumaran Nair, A.; George, B.; Pradeep, T. Thermal Conductivities of Naked and Monolayer Protected Metal Nanoparticle Based Nanofluids: Manifestation of Anomalous Enhancement and Chemical Effects. Appl. Phys. Lett. 2003, 83, 2931–2933. [Google Scholar] [CrossRef]
- Xie, H.; Lee, H.; Youn, W.; Choi, M. Nanofluids Containing Multiwalled Carbon Nanotubes and Their Enhanced Thermal Conductivities. J. Appl. Phys. 2003, 94, 4967–4971. [Google Scholar] [CrossRef]
- Assael, M.J.; Chen, C.F.; Metaxa, I.; Wakeham, W.A. Thermal Conductivity of Suspensions of Carbon Nanotubes in Water. Int. J. Thermophys. 2004, 25, 971–985. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Zhang, Z.G.; Yu, W.; Lockwood, F.E.; Grulke, E.A. Anomalous Thermal Conductivity Enhancement in Nanotube Suspensions. Appl. Phys. Lett. 2001, 79, 2252–2254. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Enhanced Thermal Conductivity of TiO2—Water Based Nanofluids. Int. J. Therm. Sci. 2005, 44, 367–373. [Google Scholar] [CrossRef]
- Yiamsawas, T.; Mahian, O.; Dalkilic, A.S.; Kaewnai, S.; Wongwises, S. Experimental Studies on the Viscosity of TiO2 and Al2O3 Nanoparticles Suspended in a Mixture of Ethylene Glycol and Water for High Temperature Applications. Appl. Energy 2013, 111, 40–45. [Google Scholar] [CrossRef]
- Ouikhalfan, M.; Labihi, A.; Belaqziz, M.; Chehouani, H.; Benhamou, B.; Sarı, A.; Belfkira, A. Stability and Thermal Conductivity Enhancement of Aqueous Nanofluid Based on Surfactant-Modified TiO2. J. Dispers. Sci. Technol. 2020, 41, 374–382. [Google Scholar] [CrossRef]
- Teng, T.P.; Fang, Y.B.; Hsu, Y.C.; Lin, L. Evaluating Stability of Aqueous Multiwalled Carbon Nanotube Nanofluids by Using Different Stabilizers. J. Nanomater. 2014, 2014, 693459. [Google Scholar] [CrossRef]
- Nikkam, N.; Ghanbarpour, M.; Saleemi, M.; Haghighi, E.B.; Khodabandeh, R.; Muhammed, M.; Palm, B.; Toprak, M.S. Experimental Investigation on Thermo-Physical Properties of Copper/Diethylene Glycol Nanofluids Fabricated via Microwave-Assisted Route. Appl. Therm. Eng. 2014, 65, 158–165. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Synthesis, Characterization and Dispersion Stability of Water-Based Cu–CNT Hybrid Nanofluid without Surfactant. Microfluid. Nanofluid. 2021, 25, 14. [Google Scholar] [CrossRef]
- Sundar, L.S.; Singh, M.K.; Sousa, A.C.M. Investigation of Thermal Conductivity and Viscosity of Fe3O4 Nanofluid for Heat Transfer Applications. Int. Commun. Heat Mass Transf. 2013, 44, 7–14. [Google Scholar] [CrossRef]
- Shahsavar, A.; Salimpour, M.R.; Saghafian, M.; Shafii, M.B. Effect of Magnetic Field on Thermal Conductivity and Viscosity of a Magnetic Nanofluid Loaded with Carbon Nanotubes. J. Mech. Sci. Technol. 2016, 30, 809–815. [Google Scholar] [CrossRef]
- Gallego, A.; Cacua, K.; Herrera, B.; Cabaleiro, D.; Piñeiro, M.M.; Lugo, L. Experimental Evaluation of the Effect in the Stability and Thermophysical Properties of Water-Al2O3 Based Nanofluids Using SDBS as Dispersant Agent. Adv. Powder Technol. 2020, 31, 560–570. [Google Scholar] [CrossRef]
- Kumar, P.C.M.; Muruganandam, M. Stability Analysis of Heat Transfer MWCNT with Different Base Fluids. J. Appl. Fluid Mech. 2017, 10, 51–59. [Google Scholar] [CrossRef]
- Bin-Abdun, N.A.; Razlan, Z.M.; Bakar, S.A.; Voon, C.H.; Ibrahim, Z.; Wan, W.K.; Ridzuan, M.J.M. Heat Transfer Improvement in Simulated Small Battery Compartment Using Metal Oxide (CuO)/Deionized Water Nanofluid. Heat Mass Transf. 2020, 56, 399–406. [Google Scholar] [CrossRef]
- US Research Nanomaterials, Inc. Available online: https://www.us-nano.com/ (accessed on 1 August 2022).
- Bellos, E.; Korres, D.; Tzivanidis, C.; Antonopoulos, K.A. Design, Simulation and Optimization of a Compound Parabolic Collector. Sustain. Energy Technol. Assess. 2016, 16, 53–63. [Google Scholar] [CrossRef]
- Ali, A.; Ilyas, S.U.; Garg, S.; Alsaady, M.; Maqsood, K.; Nasir, R.; Abdulrahman, A.; Zulfiqar, M.; Mahfouz, A.B.; Ahmed, A.; et al. Dynamic Viscosity of Titania Nanotubes Dispersions in Ethylene Glycol/Water-Based Nanofluids: Experimental Evaluation and Predictions from Empirical Correlation and Artificial Neural Network. Int. Commun. Heat Mass Transf. 2020, 118, 104882. [Google Scholar] [CrossRef]
- Khairul, M.A.; Shah, K.; Doroodchi, E.; Azizian, R.; Moghtaderi, B. Effects of Surfactant on Stability and Thermo-Physical Properties of Metal Oxide Nanofluids. Int. J. Heat Mass Transf. 2016, 98, 778–787. [Google Scholar] [CrossRef]
- Qamar, A.; Anwar, Z.; Ali, H.; Shaukat, R.; Imran, S.; Arshad, A.; Ali, H.M.; Korakianitis, T. Preparation and Dispersion Stability of Aqueous Metal Oxide Nanofluids for Potential Heat Transfer Applications: A Review of Experimental Studies. J. Therm. Anal. Calorim. 2022, 147, 23–46. [Google Scholar] [CrossRef]
- Qamar, A.; Anwar, Z.; Ali, H.; Imran, S.; Shaukat, R.; Mujtaba Abbas, M. Experimental Investigation of Dispersion Stability and Thermophysical Properties of ZnO/DIW Nanofluids for Heat Transfer Applications. Alex. Eng. J. 2022, 61, 4011–4026. [Google Scholar] [CrossRef]
- Impact Test Equipment. Available online: https://www.impact-test.co.uk/products/5420-kd2-pro-thermal-properties-meter/ (accessed on 1 August 2022).
- RHEOTEST® RN 5.1. Available online: https://www.rheotest.de/dsr-rheometer-rheotest-rn-5-1/ (accessed on 1 August 2022).
- Tariq, R.; Ali, M.; Sheikh, N.A.; Shahzad, M.W.; Bin Xu, B. Deep learning artificial intelligence framework for sustainable desiccant air conditioning system: Optimization towards reduction in water footprints. Int. Commun. Heat Mass Transf. 2023, 140, 106538. [Google Scholar] [CrossRef]
- Tariq, R.; Abatal, M.; Bassam, A. Computational intelligence for empirical modeling and optimization of methylene blue adsorption phenomena using available local zeolites and clay of Morocco. J. Clean. Prod. 2022, 370, 133517. [Google Scholar] [CrossRef]
- Tariq, R.; Torres-Aguilar, C.; Sheikh, N.A.; Ahmad, T.; Xamán, J.; Bassam, A. Data engineering for digital twining and optimization of naturally ventilated solar façade with phase changing material under global projection scenarios. Elsevier Renew. Energy 2022, 187, 1184–1203. [Google Scholar] [CrossRef]
- Tariq, R.; Torres-Aguilar, C.; Xamán, J.; Zavala-Guillén, I.; Bassam, A.; Ricalde, L.J.; Carvente, O. Digital twin models for optimization and global projection of building-integrated solar chimney. Elsevier Build. Environ. 2022, 213, 108807. [Google Scholar] [CrossRef]
- Tariq, R.; Cetina-Quiñones, A.; Cardoso-Fernández, V.; Daniela-Abigail, H.-L.; Soberanis, M.A.E.; Bassam, A.; De Lille, M.V. Artificial intelligence assisted technoeconomic optimization scenarios of hybrid energy systems for water management of an isolated community. Sustain. Energy Technol. Assess. 2021, 48, 101561. [Google Scholar] [CrossRef]




| Reference | Base Fluid | Nanoparticles | Average Particle Size | Volumetric Concentration | Thermal Conductivity Enhancement |
|---|---|---|---|---|---|
| Xuan et al. [42] | Water | Cu | 100 nm | 7.5% | 78% |
| Lee et al. [43] | Water | Al2O3 | 33 nm | 4.3% | 15% |
| Hong et al. [44] | EG | Fe | 10 nm | 0.55% | 18% |
| Xie et al. [45] | Water | SiC | 26 nm | 4.2% | 16% |
| Patel et al. [46] | Water | Ag | 60–80 nm | 0.001% | 17% |
| Choi et al. [43] | EG | Cu | 10 nm | 0.3% | 40% |
| Patel et al. [46] | Water | Au | 10–20 nm | 0.026% | 21% |
| Lee et al. [43] | Water | CuO | 36 nm | 3.4% | 12% |
| Xie et al. [47] | EG–Water | MWCNT | 15 nm | 1% | 20% |
| Assael et al. [48] | Water | MWCNT | 100 nm | 0.6% | 38% |
| Choi et al. [49] | Synthetic oil | MWCNT | 25 nm | 1% | 150% |
| Murshed et al. [50] | Water | TiO2 | 15 nm | 5% | 30% |
| References | Nanoparticles | Base Fluid | Stirring Time (min) | Homogeniser Time/min | Sonication Time/H | Stability Study Method | Stability Duration/Day |
|---|---|---|---|---|---|---|---|
| Gupta et al. [51] | CU–CNTs | DI Water | 15 | 30 | 1 | Zeta potential | 7 |
| Choudhary et al. [52] | Al2O3 | DI Water | - | - | 3 | pH | 16 |
| Kakati et al. [53] | Al2O3 | DI Water | 10 | - | - | pH | 4–5 |
| Li et al. [54] | Ag, Au | Water | 60 | - | - | Zeta potential | 60 |
| Chen et al. [55] | Al2O3 | Paraffin | 30–45 | - | 2.75–3.5 | Zeta potential | 60 |
| Esmaeili et al. [53] | AlN–C | EG | 60 | - | 0.5 | Zeta potential | 90 |
| Chen et al. [56] | MWCNT | DI Water | 45 | - | 1 | Zeta potential, UV–Vis, | 60 |
| Mukesh Kumar et al. [57] | MWCNT | Engine oil | 30 | - | 1 | Visual observation | 30 |
| Afzal et al. [58] | ZnO | DI Water | 35 | - | 2–8 | Zeta potential | 74 |
| Ilyas et al. [52] | MWCNT | Thermal oil | - | 30 | 1 | Visual observation | 30 |
| Hamid et al. [59] | TiO2–SiO2 | EG–Water | 180 | - | 2 | pH | 14 |
| Teng et al. [53] | MWCNTs | Water | 110 | 30 | 1 | Zeta potential | 30 |
| Yousefi et al. [60] | MWCNTs | DI Water | - | - | 0.5 | pH | 10 |
| Properties | Al2O3 | MgO | MWCNT |
|---|---|---|---|
| Morphology (γ) | Spherical | Spherical | Aligned shape |
| Average diameter (nm) | 20 | 20 | 10–20 |
| Particle colour | White | White | Black |
| Particle density (kg/m3) | 3890 | 3580 | 2100 |
| Particle-specific heat (J/kg.K) | 880 | 1030 | 796 |
| Thermal conductivity (W/m.k) | 36 | 42 | 3000 |
| Name | Model | Specifications |
|---|---|---|
| Analytical balance | AWU-220D Shimadzu | Minimum display: 0.01 mg; standard deviation: 0.1 mg; response time: 2–14 s; measuring capacity: 0–83 g. |
| Hot plate magnetic stirrer | SCILOGE MS-H380-Pro | Temperature range: 20–380 °C; stirring capacity: 2 dm3; temperature range accuracy: ±1 °C; maximum speed: 1400 rpm. |
| Shear homogeniser | ULTRA-TURRAX T25 | Permissible ambient temperature: 5–40 °C; speed range: 3000–25,000 rpm; volume range: 1–2000 dm3. |
| Ultrasonication bath | FSF-020S | Ultrasonic frequency: 50 Hz; effective ultrasonic power: 100 W; heat output: 400 W; temperature range: 30–80 °C. |
| pH meter | edge® pH HI2002 | pH range: −2.0–16.0 pH; temperature Range: −20.0–120.0 °C. |
| Rotary rheometer | RHEOTEST RN 5.1 | Torque resolution: 0.002 mNm; viscosity range: 1–3 × 109 mPa·s; temperature range: −60–200 °C. |
| Cylinder measuringsystem | RHEOTEST RN 5.1 | Measuring cup diameter: 38 mm; rotor length: 52.56 mm, viscosity range: 20–105 mPa·s, rotor diameter: 35.04 mm, shear rate range: 0–1300 s−1. |
| Thermal conductivity analyser | TEMPOS | Sensor operating range: −50–150 °C; accuracy: ±10%; |
| thermal conductivity range: 0.02–2 W/mK. |
| Nanofluids | Particle Concentration | Surfactant | Equipment Used | Operating Time (Hours) | Operating Speed and Temperature |
|---|---|---|---|---|---|
| Al2O3–H20 | 0.025–0.075% | SDBS | Magnetic Stirrer | 1 | 800 rpm, 60 °C |
| Ultra-Turrax T25 | 0.5 | 9000 rpm | |||
| Ultrasonic Bath | 2 | Up to 60 °C | |||
| Al2O3–EG | 0.025–0.075% | SDBS | Magnetic Stirrer | 1 | 800 rpm, 60 °C |
| Ultra-Turrax T25 | 0.5 | 9000 rpm | |||
| Ultrasonic Bath | 2 | Up to 60 °C | |||
| MgO–EG | 0.025–0.075% | SDBS | Magnetic Stirrer | 1 | 800 rpm, |
| Ultra-Turrax T25 | 0.5 | 9000 rpm | |||
| Ultrasonic Bath | 2.5 | Up to 60 °C | |||
| MWCNT–H2O | 0.025–0.075% | SDBS | Magnetic Stirrer | 2 | 900 rpm, 70 °C |
| Ultra-Turrax T25 | 0.5 | 15,000 rpm | |||
| Ultrasonic Bath | 3 | Up to 70 °C | |||
| MWCNT–EG | 0.025–0.075% | SDBS | Magnetic Stirrer | 2 | 900 rpm, 40 °C |
| Ultra-Turrax T25 | 0.5 | 15,000 rpm | |||
| Ultrasonic Bath | 3 | Up to 70 °C |
| Parameter | Input Value |
|---|---|
| Collector area | 0.828 m2 |
| Aperture width | 0.4456 m |
| Aperture length | 1.858 m |
| Collector fin efficiency | 0.7 |
| Half acceptance angle | 24° |
| Truncation ratio | 0.2 |
| Concentration ratio | 4.17 |
| Absorber length | 1.858 m |
| Incident radiations | 945 W/m2 |
| Glass outer diameter | 60 mm |
| Thickness | 2.5 mm |
| Absorber outer diameter | 34 mm |
| Parameter | Measuring Instrument | Uncertainty |
|---|---|---|
| Solar radiations | Pyranometer | ±2% |
| Inlet, outlet, and ambient temperatures | K-Type thermocouple | ±0.1 °C |
| Flow rate | Water flow meter | ±2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaleem, M.; Ali, M.; Sheikh, N.A.; Akhtar, J.; Tariq, R.; Krzywanski, J. Performance Characteristic Analysis of Metallic and Non-Metallic Oxide Nanofluids for a Compound Parabolic Collector: Improvement of Renewable Energy Technologies in Buildings. Energies 2023, 16, 1298. https://doi.org/10.3390/en16031298
Kaleem M, Ali M, Sheikh NA, Akhtar J, Tariq R, Krzywanski J. Performance Characteristic Analysis of Metallic and Non-Metallic Oxide Nanofluids for a Compound Parabolic Collector: Improvement of Renewable Energy Technologies in Buildings. Energies. 2023; 16(3):1298. https://doi.org/10.3390/en16031298
Chicago/Turabian StyleKaleem, Muhammad, Muzaffar Ali, Nadeem Ahmed Sheikh, Javed Akhtar, Rasikh Tariq, and Jaroslaw Krzywanski. 2023. "Performance Characteristic Analysis of Metallic and Non-Metallic Oxide Nanofluids for a Compound Parabolic Collector: Improvement of Renewable Energy Technologies in Buildings" Energies 16, no. 3: 1298. https://doi.org/10.3390/en16031298
APA StyleKaleem, M., Ali, M., Sheikh, N. A., Akhtar, J., Tariq, R., & Krzywanski, J. (2023). Performance Characteristic Analysis of Metallic and Non-Metallic Oxide Nanofluids for a Compound Parabolic Collector: Improvement of Renewable Energy Technologies in Buildings. Energies, 16(3), 1298. https://doi.org/10.3390/en16031298








