Porous-Structured Three-Dimensional Iron Phosphides Nanosheets for Enhanced Oxygen Evolution Reaction
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
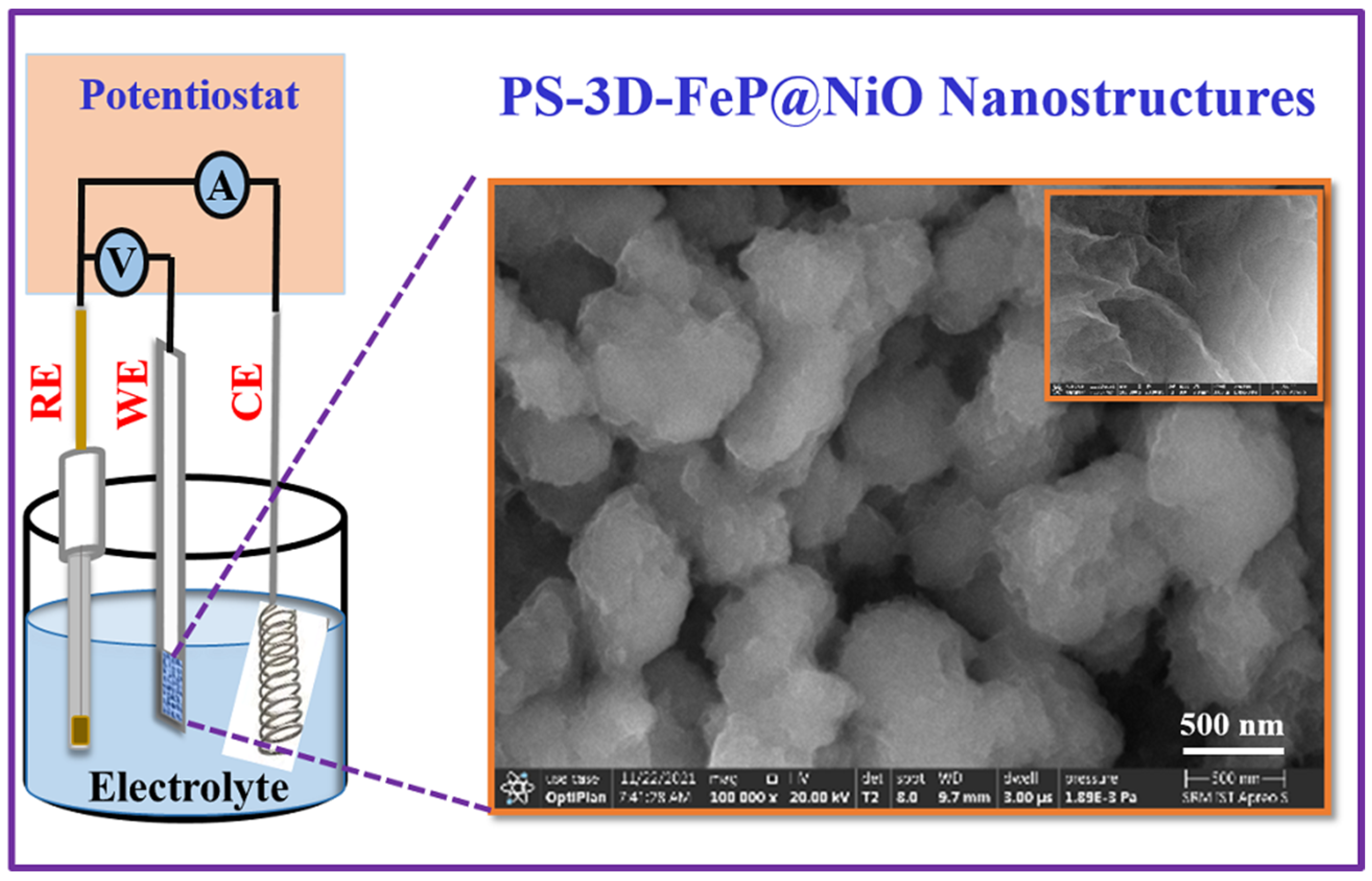
2.2. Fabrication of PS-3D-FeP@NiO Nanostructured Electrode
2.3. Physicochemical Characterization
2.4. Electrochemical Characterization
2.5. Calculation Method
3. Result and Discussion
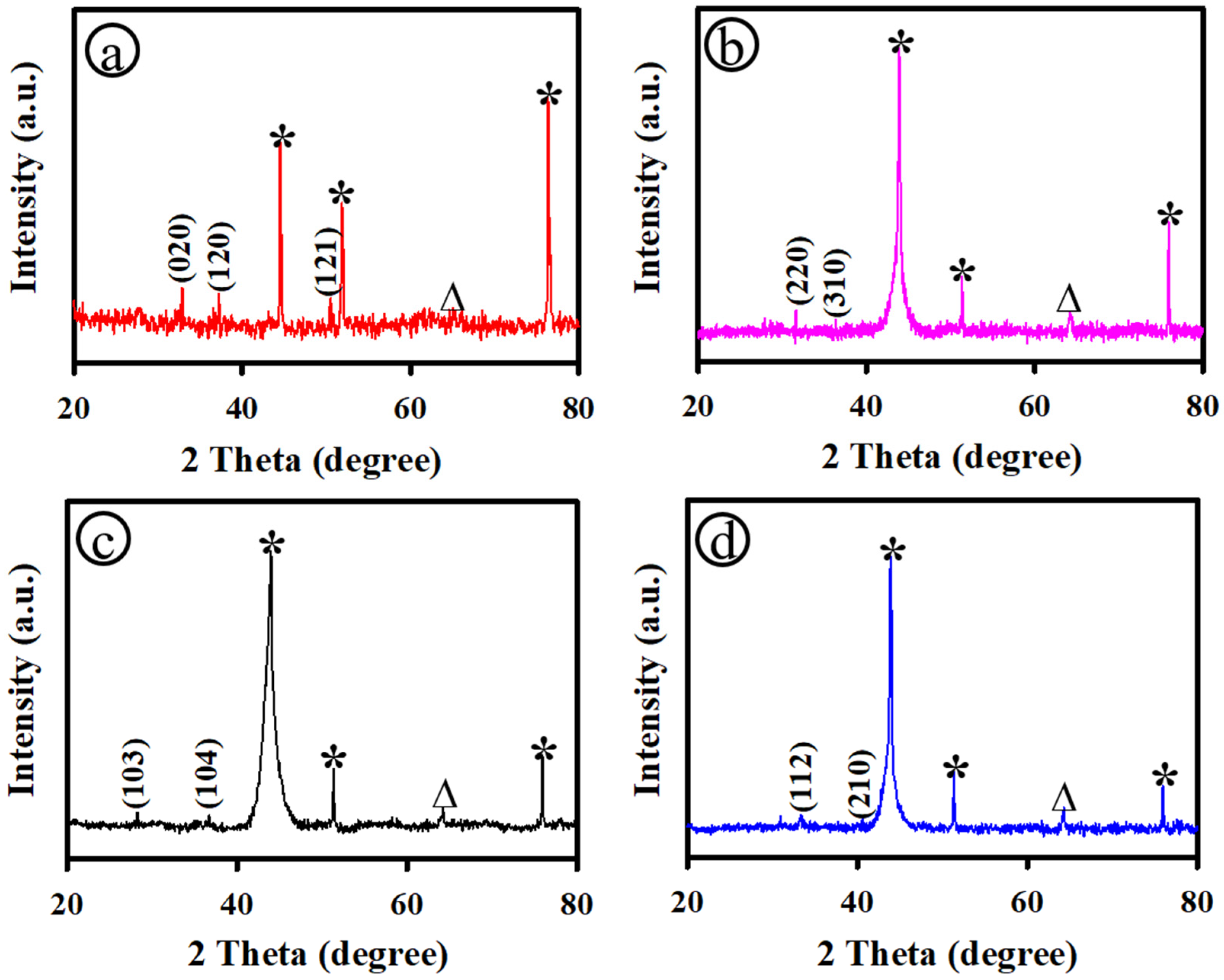
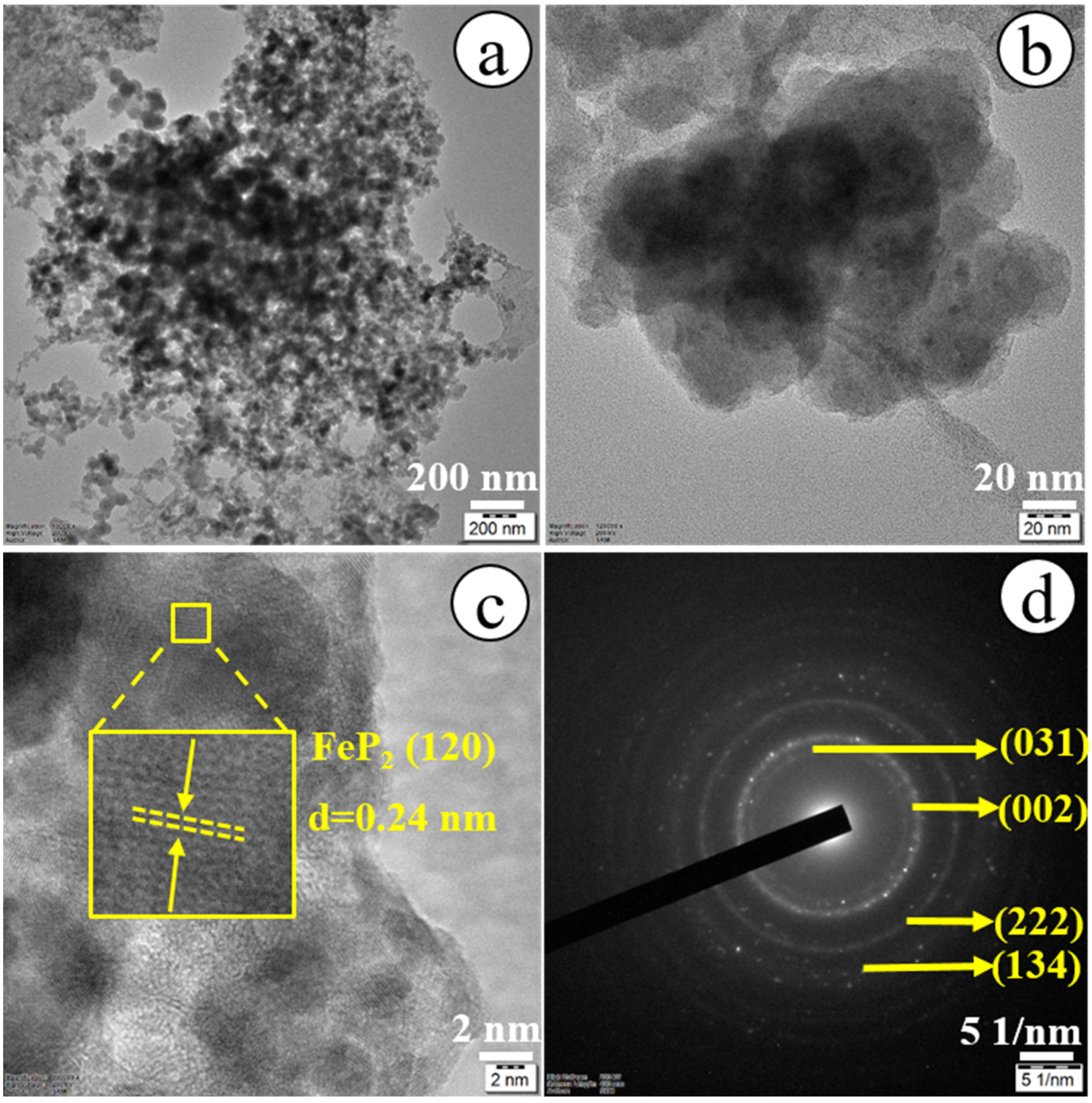
3.1. Physicochemical Characterization of PS-3D-FeP@NiO Nanostructures
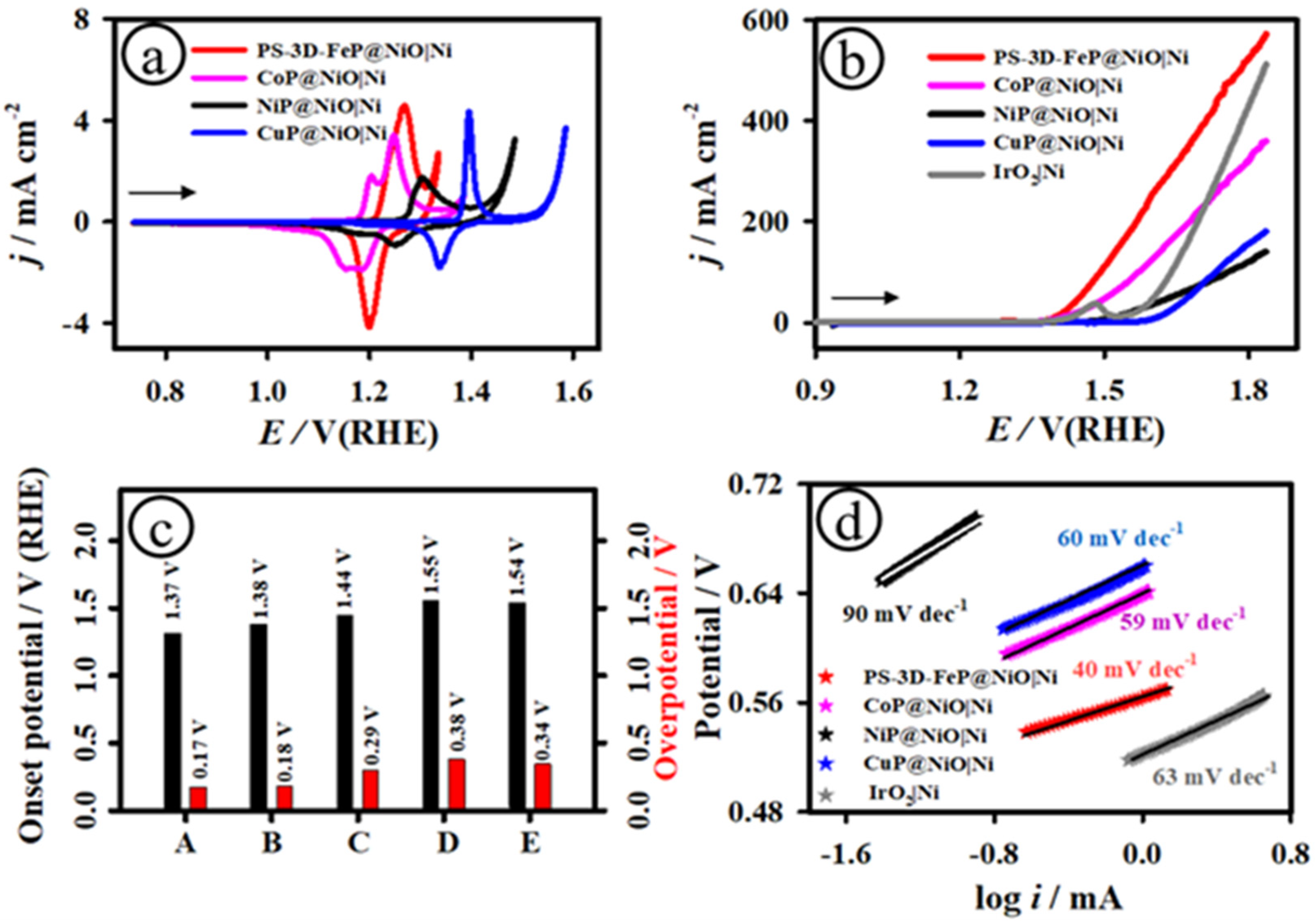
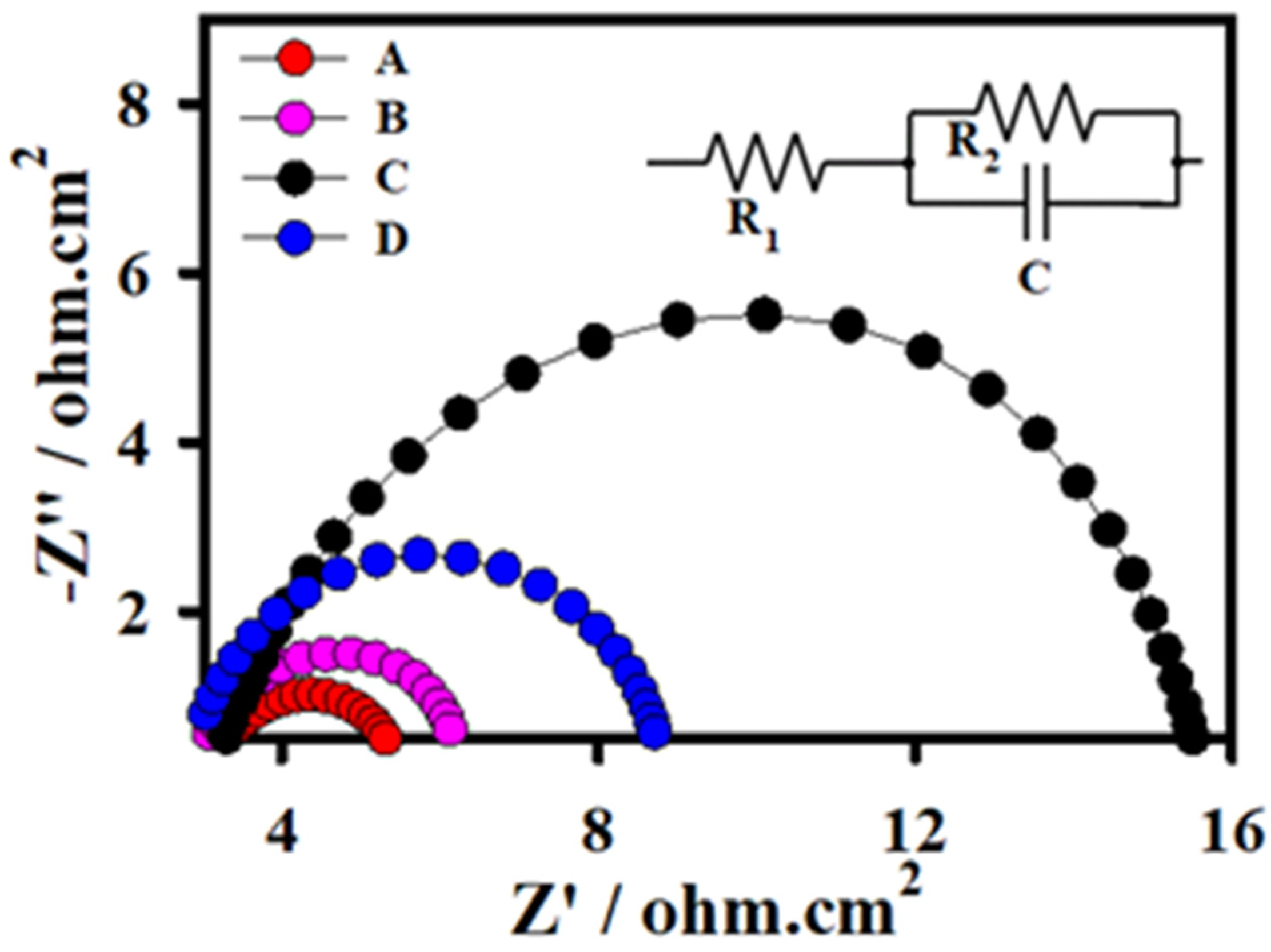
3.2. Electrochemical Characterization of PS-3D-FeP@NiO Nanostructures
3.3. Electrochemical Catalytic OER Activity of PS-3D-FeP@NiO Nanostructures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, H.; Yuan, Z. Design Strategies of Transition-Metal Phosphate and Phosphonate Electrocatalysts for Energy-Related Reactions. ChemSusChem 2021, 14, 130–149. [Google Scholar] [CrossRef] [PubMed]
- Alsabban, M.M.; Eswaran, M.K.; Peramaiah, K.; Wahyudi, W.; Yang, X.; Ramalingam, V.; Hedhili, M.N.; Miao, X.; Schwingenschlögl, U.; Li, L.-J.; et al. Unusual Activity of Rationally Designed Cobalt Phosphide/Oxide Heterostructure Composite for Hydrogen Production in Alkaline Medium. ACS Nano 2022, 16, 3906–3916. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.Y.; Duan, Y.; Feng, X.Y.; Yu, X.; Gao, M.R.; Yu, S.H. Clean and Affordable Hydrogen Fuel from Alkaline Water Splitting: Past, Recent Progress, and Future Prospects. Adv. Mater. 2021, 33, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, V.; Son, J.; Kim, J. CoP2 /Fe-CoP2 Yolk–Shell Nanoboxes as Efficient Electrocatalysts for the Oxygen Evolution Reaction. Nanoscale 2021, 13, 4569–4575. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhou, H.; Huang, Y.; Sun, J.; Qin, F.; Bao, J.; Goddard, W.A.; Chen, S.; Ren, Z. High-Performance Bifunctional Porous Non-Noble Metal Phosphide Catalyst for Overall Water Splitting. Nat. Commun. 2018, 9, 2551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Xiao, Y.; Cao, Y.; Fang, H.; Zhang, Y.; Das, P.; Zhang, H. Electrodeposition, Formation Mechanism, and Electrocatalytic Performance of Co-Ni-P Ternary Catalysts Coated on Carbon Fiber Paper. J. Solid State Electrochem. 2021, 25, 1503–1512. [Google Scholar] [CrossRef]
- Li, D.; Zhou, C.; Yang, R.; Xing, Y.; Xu, S.; Jiang, D.; Tian, D.; Shi, W. Interfacial Engineering of the CoxP-Fe2P Heterostructure for Efficient and Robust Electrochemical Overall Water Splitting. ACS Sustain. Chem. Eng. 2021, 9, 7737–7748. [Google Scholar] [CrossRef]
- Lv, X.W.; Xu, W.S.; Tian, W.W.; Wang, H.Y.; Yuan, Z.Y. Activity Promotion of Core and Shell in Multifunctional Core–Shell Co2P@NC Electrocatalyst by Secondary Metal Doping for Water Electrolysis and Zn-Air Batteries. Small 2021, 17, 2101856. [Google Scholar] [CrossRef]
- Elakkiya, R.; Maduraiveeran, G. Iron Sulphide Rice Grain Nanostructures as Potential Electrocatalysts for an Improved Oxygen Evolution Reaction. Nanoscale 2021, 13, 14837–14846. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Liu, G.; Li, Z.; Liu, S.; Tiwari, S.K.; Ola, O.; Pang, B.; Wang, N.; Zhu, Y. Construction of CoP/Co2 P Coexisting Bifunctional Self-Supporting Electrocatalysts for High-Efficiency Oxygen Evolution and Hydrogen Evolution. ACS Omega 2022, 7, 12846–12855. [Google Scholar] [CrossRef]
- Guo, J.; Zhan, Z.; Lei, T.; Yin, P. Self-Supported FeNiP Nanosheet Arrays as a Robust Bifunctional Electrocatalyst for Water Splitting. ACS Appl. Energy Mater. 2022, 5, 5855–5866. [Google Scholar] [CrossRef]
- Chai, L.; Hu, Z.; Wang, X.; Xu, Y.; Zhang, L.; Li, T.; Hu, Y.; Qian, J.; Huang, S. Stringing Bimetallic Metal–Organic Framework-Derived Cobalt Phosphide Composite for High-Efficiency Overall Water Splitting. Adv. Sci. 2020, 7, 1903195. [Google Scholar] [CrossRef]
- Karmakar, A.; Karthick, K.; Kumaravel, S.; Sankar, S.S.; Kundu, S. Enabling and Inducing Oxygen Vacancies in Cobalt Iron Layer Double Hydroxide via Selenization as Precatalysts for Electrocatalytic Hydrogen and Oxygen Evolution Reactions. Inorg. Chem. 2021, 60, 2023–2036. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Montes, R.A.; Kawashima, K.; Son, Y.J.; Weeks, J.A.; Sun, H.H.; Celio, H.; Ramos-Sánchez, V.H.; Mullins, C.B. Mass Transport-Enhanced Electrodeposition of Ni–S–P–O Films on Nickel Foam for Electrochemical Water Splitting. J. Mater. Chem. A 2021, 9, 7736–7749. [Google Scholar] [CrossRef]
- Gao, H.; Sun, W.; Tian, X.; Liao, J.; Ma, C.; Hu, Y.; Du, G.; Yang, J.; Ge, C. Amorphous–Amorphous Coupling Enhancing the Oxygen Evolution Reaction Activity and Stability of the NiFe-Based Catalyst. ACS Appl. Mater. Interfaces 2022, 14, 15205–15213. [Google Scholar] [CrossRef]
- Hafezi Kahnamouei, M.; Shahrokhian, S. Coupling NiCoS and CoFeS Frame/Cagelike Hybrid as an Efficient Electrocatalyst for Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2022, 5, 5199–5211. [Google Scholar] [CrossRef]
- Asghar, M.A.; Ali, A.; Haider, A.; Zaheer, M.; Nisar, T.; Wagner, V.; Akhter, Z. Electrochemically Deposited Amorphous Cobalt-Nickel-Doped Copper Oxide as an Efficient Electrocatalyst toward Water Oxidation Reaction. ACS Omega 2021, 6, 19419–19426. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, C.; Cai, W.; Chen, H.M.; Liu, W.; Hung, S.; Yang, H.B.; Li, J.; Liu, B. Coordination Engineering of Iridium Nanocluster Bifunctional Electrocatalyst for Highly Efficient and PH-Universal Overall Water Splitting. Nat. Commun. 2020, 11, 4246. [Google Scholar] [CrossRef]
- Li, M.; Zheng, K.; Zhang, J.; Li, X.; Xu, C. Design and Construction of 2D/2D Sheet-on-Sheet Transition Metal Sulfide/Phosphide Heterostructure for Efficient Oxygen Evolution Reaction. Appl. Surf. Sci. 2021, 565, 150510. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Yang, P.; Wang, D.; Xu, H.; Wang, B.; Meng, F.; Zhang, J.; An, M. Electrodeposition: Synthesis of Advanced Transition Metal-Based Catalyst for Hydrogen Production via Electrolysis of Water. J. Energy Chem. 2021, 57, 547–566. [Google Scholar] [CrossRef]
- Díaz-Sainz, G.; Fernández-Caso, K.; Lagarteira, T.; Delgado, S.; Alvarez-Guerra, M.; Mendes, A.; Irabien, A. Coupling Continuous CO2 Electroreduction to Formate with Efficient Ni-Based Anodes. J. Environ. Chem. Eng. 2023, 11, 109171. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.; Li, Z.; Bu, X. Recent Progress on NiFe-Based Electrocatalysts for the Oxygen Evolution Reaction. Small 2020, 16, 2003916. [Google Scholar] [CrossRef] [PubMed]
- Sayed, D.M.; El-Nagar, G.A.; Sayed, S.Y.; El-Anadouli, B.E.; El-Deab, M.S. Activation/Deactivation Behavior of Nano-NiOx Based Anodes towards the OER: Influence of Temperature. Electrochim. Acta 2018, 276, 176–183. [Google Scholar] [CrossRef]
- Cai, Z.; Bu, X.; Wang, P.; Ho, J.C.; Yang, J.; Wang, X. Recent Advances in Layered Double Hydroxide Electrocatalysts for the Oxygen Evolution Reaction. J. Mater. Chem. A 2019, 7, 5069–5089. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Fei, T.; Mao, C.; Song, Y.; Zhou, Y.; Dong, G. NiCoP/NF 1D/2D Biomimetic Architecture for Markedly Enhanced Overall Water Splitting. ChemElectroChem 2021, 8, 3064–3072. [Google Scholar] [CrossRef]
- Pu, Z.; Liu, T.; Amiinu, I.S.; Cheng, R.; Wang, P.; Zhang, C.; Ji, P.; Hu, W.; Liu, J.; Mu, S. Transition-Metal Phosphides: Activity Origin, Energy-Related Electrocatalysis Applications, and Synthetic Strategies. Adv. Funct. Mater. 2020, 30, 2004009. [Google Scholar] [CrossRef]
- Yaqoob, L.; Noor, T.; Iqbal, N.; Nasir, H.; Zaman, N.; Talha, K. Electrochemical Synergies of Fe–Ni Bimetallic MOF CNTs Catalyst for OER in Water Splitting. J. Alloys Compd. 2021, 850, 156583. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; He, Y.; Zhu, H. Recent Advances in Transition-Metal-Sulfide-Based Bifunctional Electrocatalysts for Overall Water Splitting. J. Mater. Chem. A 2021, 9, 5320–5363. [Google Scholar] [CrossRef]
- Ibn Shamsah, S.M. Earth-Abundant Electrocatalysts for Water Splitting: Current and Future Directions. Catalysts 2021, 11, 429. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, Y. Designing Transition-Metal-Boride-Based Electrocatalysts for Applications in Electrochemical Water Splitting. Nanoscale 2020, 12, 9327–9351. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Wei, Y.; Chen, X.; Hao, S.; Ding, X.; Pan, L. A Highly Efficient FeP/CeO2 –NF Hybrid Electrode for the Oxygen Evolution Reaction. Chem. Commun. 2020, 56, 4228–4231. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zou, Y.; Ye, Y.-Q.; Ouyang, T.; Xiao, K.; Liu, Z.-Q. Unveiling the Active Sites of Ni–Fe Phosphide/Metaphosphate for Efficient Oxygen Evolution under Alkaline Conditions. Chem. Commun. 2019, 55, 7687–7690. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yu, L.; Zhang, F.; McElhenny, B.; Luo, D.; Karim, A.; Chen, S.; Ren, Z. Heterogeneous Bimetallic Phosphide Ni2P-Fe2P as an Efficient Bifunctional Catalyst for Water/Seawater Splitting. Adv. Funct. Mater. 2021, 31, 1–12. [Google Scholar]
- Zhang, H.; Maijenburg, A.W.; Li, X.; Schweizer, S.L.; Wehrspohn, R.B. Bifunctional Heterostructured Transition Metal Phosphides for Efficient Electrochemical Water Splitting. Adv. Funct. Mater. 2020, 30, 2003261. [Google Scholar] [CrossRef]
- Dondapati, J.S.; Govindhan, M.; Chen, A. Direct Growth of Three-Dimensional Nanoflower-like Structures from Flat Metal Surfaces. Chem. Commun. 2022, 58, 11127–11130. [Google Scholar] [CrossRef]
- Zhang, J.; Shewale, P.S.; Yun, K.S. Fiber-Shaped Supercapacitors Fabricated Using Hierarchical Nanostructures of NiCo2O4 Nanoneedles and MnO2 Nanoflakes on Roughened Ni Wire. Energies 2019, 12, 3127. [Google Scholar] [CrossRef]
- Arivazhagan, M.; Maduraiveeran, G. Hierarchical Gold Dispersed Nickel Oxide Nanodendrites Microarrays as a Potential Platform for the Sensitive Electrochemical Detection of Glucose and Lactate in Human Serum and Urine. Mater. Chem. Phys. 2023, 295, 127084. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, W.-M.; Li, J.; Zhou, Y.; Li, Z.-Q.; Yin, Y.-C.; Xia, X.-H. Site-Specific Electrodeposition Enables Self-Terminating Growth of Atomically Dispersed Metal Catalysts. Nat. Commun. 2020, 11, 4558. [Google Scholar] [CrossRef]
- Shankar, A.; Maduraiveeran, G. Hierarchical Bimetallic Iron-Cobalt Phosphides Nano-Island Nanostructures for Improved Oxygen Evolution Reaction. J. Electroanal. Chem. 2022, 923, 116806. [Google Scholar] [CrossRef]
- Jadhav, R.G.; Singh, D.; Krivoshapkin, P.V.; Das, A.K. Electrodeposited Organic–Inorganic Nanohybrid as Robust Bifunctional Electrocatalyst for Water Splitting. Inorg. Chem. 2020, 59, 7469–7478. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, G.; Chen, Y.; Rui, K.; Mao, H.; Dou, S.X.; Sun, W. Iron-Doped Nickel Molybdate with Enhanced Oxygen Evolution Kinetics. Chem.-A Eur. J. 2019, 25, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, C.; Zhang, J.; Wang, W.; Zhou, X.; Pan, B.; Tang, K.; Zuo, J.; Yang, Q. Synthesis of FeP2 /C Nanohybrids and Their Performance for Hydrogen Evolution Reaction. J. Mater. Chem. A 2015, 3, 499–503. [Google Scholar] [CrossRef]
- Wu, T.; Pi, M.; Wang, X.; Zhang, D.; Chen, S. Three-Dimensional Metal–Organic Framework Derived Porous CoP3 Concave Polyhedrons as Superior Bifunctional Electrocatalysts for the Evolution of Hydrogen and Oxygen. Phys. Chem. Chem. Phys. 2017, 19, 2104–2110. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lu, S.; Sun, J.; Pei, J.; Liu, D.; Xue, Y.; Mao, J.; Zhu, W.; Zhuang, Z. Phase-Controlled Synthesis of Nickel Phosphide Nanocrystals and Their Electrocatalytic Performance for the Hydrogen Evolution Reaction. Chem.-A Eur. J. 2018, 24, 11748–11754. [Google Scholar] [CrossRef]
- Chandrasekar, M.S.; Mitra, S. Thin Copper Phosphide Films as Conversion Anode for Lithium-Ion Battery Applications. Electrochim. Acta 2013, 92, 47–54. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, B.; Chen, Y. Iron Phosphides Supported on Three-Dimensional Iron Foam as an Efficient Electrocatalyst for Water Splitting Reactions. J. Mater. Sci. 2019, 54, 14872–14883. [Google Scholar] [CrossRef]
- Yan, P.; Hu, Y.; Shoko, E.; Isimjan, T.T.; Tian, J.; Yang, X. Hierarchical Core-Shell N-Doped Carbon@FeP4 -CoP Arrays as Robust Bifunctional Electrocatalysts for Overall Water Splitting at High Current Density. Adv. Mater. Interfaces 2021, 8, 2100065. [Google Scholar] [CrossRef]
- Ju, Y.; Feng, S.; Wang, X.; Li, M.; Wang, L.; Xu, R.; Wang, J. Facile Preparation of a Porous Nanosheet P X -Doped Fe Bi-Functional Catalyst with Excellent OER and HER Electrocatalytic Activity. ChemistrySelect 2021, 6, 4979–4990. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, W.; Jia, D.; Liu, Y.; Zhang, A.; Wen, T.; Liu, J.; Ai, Y.; Song, W.; Wang, X. N, P, and S Codoped Graphene-Like Carbon Nanosheets for Ultrafast Uranium (VI) Capture with High Capacity. Adv. Sci. 2018, 5, 1800235. [Google Scholar] [CrossRef]
- Li, R.; Qin, Y.; Tong, X. Synthesis of Iron Phosphide Nanoclusters by an Electroless Plating Method for Enhanced Oxygen Evolution Reaction. J. Electron. Mater. 2021, 50, 3071–3077. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Lv, H.; Xie, D.; Wang, W.; Zhi, C.; Li, H. Stabilized Co3+/Co4+ Redox Pair in In Situ Produced CoSe2− x -Derived Cobalt Oxides for Alkaline Zn Batteries with 10 000-Cycle Lifespan and 1.9-V Voltage Plateau. Adv. Energy Mater. 2020, 10, 2000892. [Google Scholar] [CrossRef]
- Msp, S.; Gnanasekaran, G.; Pazhamalai, P.; Sahoo, S.; Hossain, M.M.; Bhattarai, R.M.; Kim, S.-J.; Mok, Y.S. Hierarchically Porous Nanostructured Nickel Phosphide with Carbon Particles Embedded by Dielectric Barrier Discharge Plasma Deposition as a Binder-Free Electrode for Hybrid Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 14805–14814. [Google Scholar]
- Prakash, V.; Srivastava, K.; Prasad, J. Cyclic Voltammetric Behavior of Binary and Mixed Ligand Copper Complexes with Picolinic Acid, Nicotinic Acid and Diethyldithiocarbamate in Acetone Medium. J. Indian Chem. Soc. 2019, 96, 585–591. [Google Scholar]
- Shankar, A.; Elakkiya, R.; Maduraiveeran, G. Self-Supported Fabrication and Electrochemical Water Splitting Study of Transition-Metal Sulphide Nanostructured Electrodes. New J. Chem. 2020, 44, 5071–5078. [Google Scholar] [CrossRef]
- Duan, D.; Guo, D.; Gao, J.; Liu, S.; Wang, Y. Electrodeposition of Cobalt-Iron Bimetal Phosphide on Ni Foam as a Bifunctional Electrocatalyst for Efficient Overall Water Splitting. J. Colloid Interface Sci. 2022, 622, 250–260. [Google Scholar] [CrossRef]
- Ganesan, P.; Sivanantham, A.; Shanmugam, S. Inexpensive Electrochemical Synthesis of Nickel Iron Sulphides on Nickel Foam: Super Active and Ultra-Durable Electrocatalysts for Alkaline Electrolyte Membrane Water Electrolysis. J. Mater. Chem. A 2016, 4, 16394–16402. [Google Scholar] [CrossRef]
- Man, H.-W.; Tsang, C.-S.; Li, M.M.-J.; Mo, J.; Huang, B.; Lee, L.Y.S.; Leung, Y.; Wong, K.-Y.; Tsang, S.C.E. Tailored Transition Metal-Doped Nickel Phosphide Nanoparticles for the Electrochemical Oxygen Evolution Reaction (OER). Chem. Commun. 2018, 54, 8630–8633. [Google Scholar] [CrossRef]
- Kale, S.B.; Babar, P.T.; Kim, J.H.; Lokhande, C.D. Synthesis of One Dimensional Cu2S Nanorods Using a Self-Grown Sacrificial Template for the Electrocatalytic Oxygen Evolution Reaction (OER). New J. Chem. 2020, 44, 8771–8777. [Google Scholar] [CrossRef]
- Li, G.; Yang, Q.; Rao, J.; Fu, C.; Liou, S.; Auffermann, G.; Sun, Y.; Felser, C. In Situ Induction of Strain in Iron Phosphide (FeP2 ) Catalyst for Enhanced Hydroxide Adsorption and Water Oxidation. Adv. Funct. Mater. 2020, 30, 1907791. [Google Scholar] [CrossRef]
- Elakkiya, R.; Maduraiveeran, G. Two-Dimensional Earth-Abundant Transition Metal Oxides Nanomaterials: Synthesis and Application in Electrochemical Oxygen Evolution Reaction. Langmuir 2020, 36, 4728–4736. [Google Scholar] [CrossRef]
- Kumar, L.; Antil, B.; Kumar, A.; Das, M.R.; Deka, S. A Superior and Stable Electrocatalytic Oxygen Evolution Reaction by One-Dimensional FeCoP Colloidal Nanostructures. ACS Appl. Mater. Interfaces 2022, 14, 5468–5477. [Google Scholar] [CrossRef] [PubMed]






| Electrodes | Overpotential Ƞ (V) | Current Density (mA cm−2 ) | Tafel Slope (mV dec−1) | Mass Activity (A g−1) | TOF (s−1) | Electrolyte | Ref. |
|---|---|---|---|---|---|---|---|
| FeS | 0.32 | 10 | 69 | 79.9 | - | 1.0 M KOH | [54] |
| CoFe-P/NF | 0.28 | 10 | 43.2 | - | - | 1.0 M KOH | [55] |
| NiFeS-1/NF | 0.23 | 100 | 55 | 276 | 0.520 | 1.0 M KOH | [56] |
| NiFeP | 0.33 | 20 | 39 | - | - | 1.0 M KOH | [57] |
| Cu2S/Cu | 0.27 | 10 | 128 | - | - | 1.0 M KOH | [58] |
| strained FeP2 | 0.24 | 10 | 56 | - | - | 1.0 M KOH | [59] |
| Co3O4 nanosheet | 0.38 | 10 | 52 | 112.3 | 0.099 | 1.0 M KOH | [60] |
| FeCoP | 0.26 | 100 | 54 | - | - | 1.0 M KOH | [61] |
| PS-3D-FeP@NiO|Ni | 0.17 | 10 | 40 | 100.0 | 0.435 | 1.0 M KOH | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marimuthu, S.; Shankar, A.; Maduraiveeran, G. Porous-Structured Three-Dimensional Iron Phosphides Nanosheets for Enhanced Oxygen Evolution Reaction. Energies 2023, 16, 1124. https://doi.org/10.3390/en16031124
Marimuthu S, Shankar A, Maduraiveeran G. Porous-Structured Three-Dimensional Iron Phosphides Nanosheets for Enhanced Oxygen Evolution Reaction. Energies. 2023; 16(3):1124. https://doi.org/10.3390/en16031124
Chicago/Turabian StyleMarimuthu, Sundaramoorthy, Ayyavu Shankar, and Govindhan Maduraiveeran. 2023. "Porous-Structured Three-Dimensional Iron Phosphides Nanosheets for Enhanced Oxygen Evolution Reaction" Energies 16, no. 3: 1124. https://doi.org/10.3390/en16031124
APA StyleMarimuthu, S., Shankar, A., & Maduraiveeran, G. (2023). Porous-Structured Three-Dimensional Iron Phosphides Nanosheets for Enhanced Oxygen Evolution Reaction. Energies, 16(3), 1124. https://doi.org/10.3390/en16031124






