Review of Micro- and Nanobubble Technologies: Advancements in Theory and Applications and Perspectives on Adsorption Cooling and Desalination Systems
Abstract
1. Introduction
2. Nanobubbles: Explanation and Properties
2.1. The Issue of Micropankes
2.2. Zeta Potential
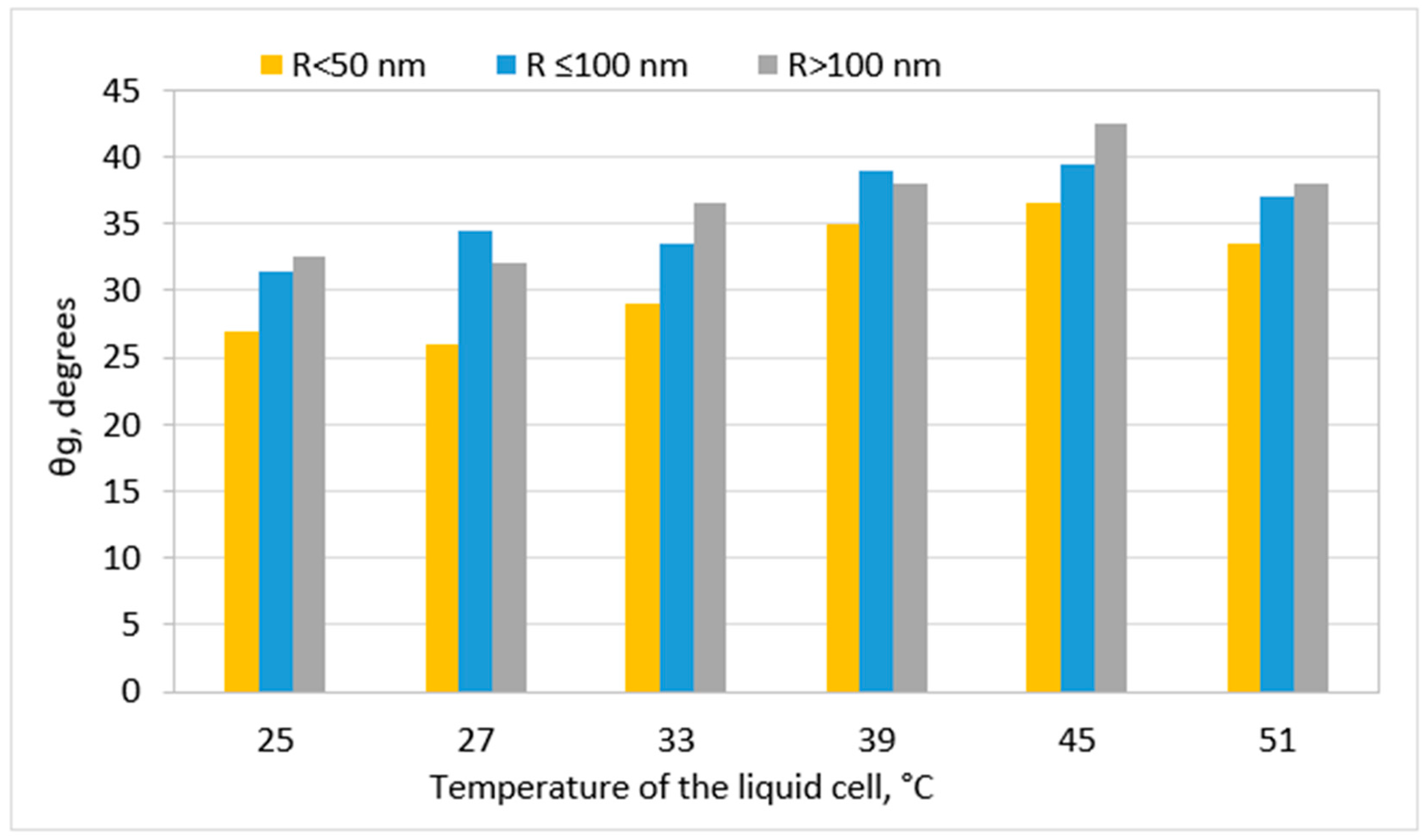
2.3. Stability of Nanobubbles
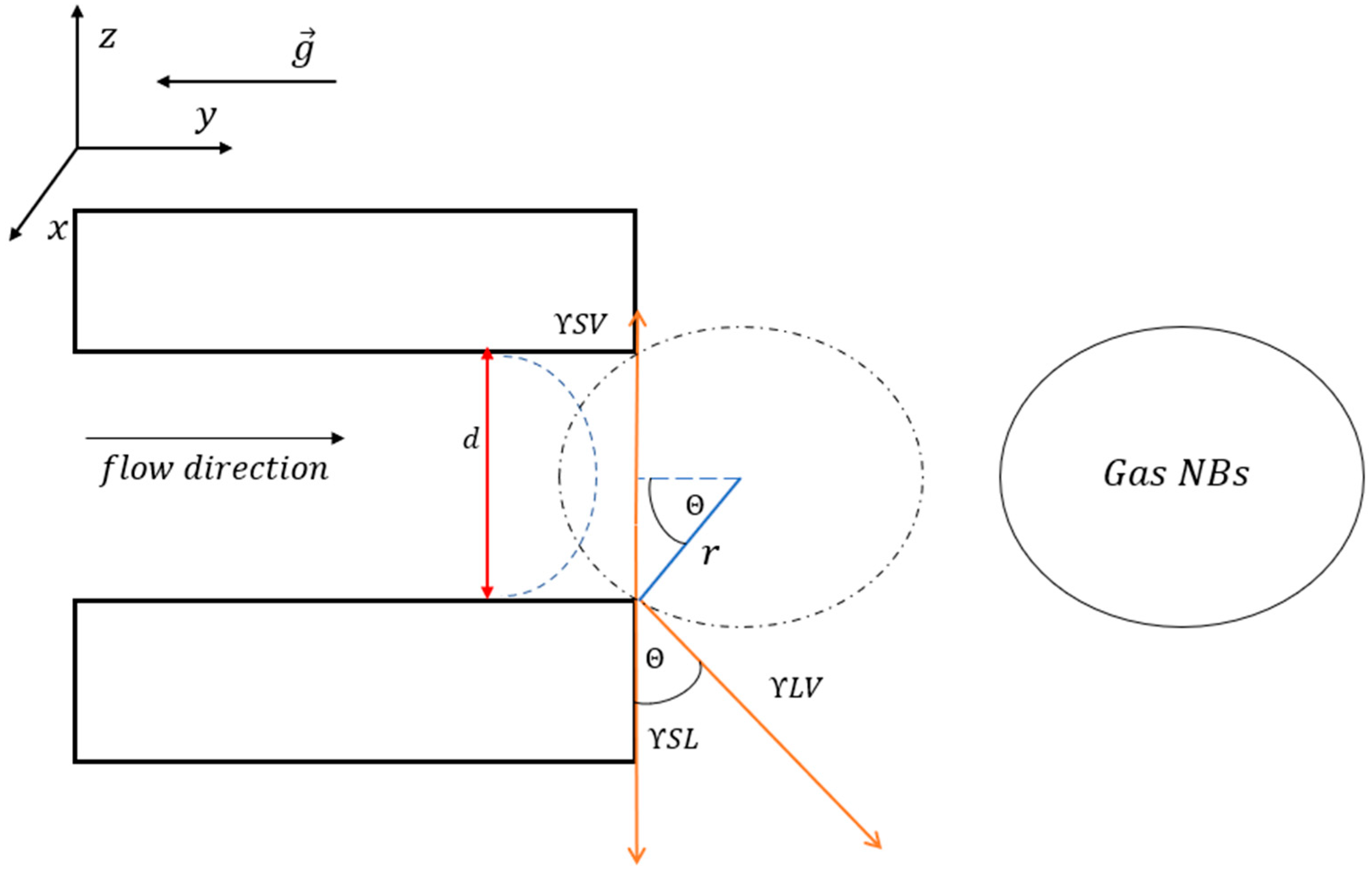
Surface Nanobubbles
3. Nanobubbles for the Surfaces Cleaning
4. Technological Advancements in Adsorption Refrigeration: Improving Performance and Efficiency
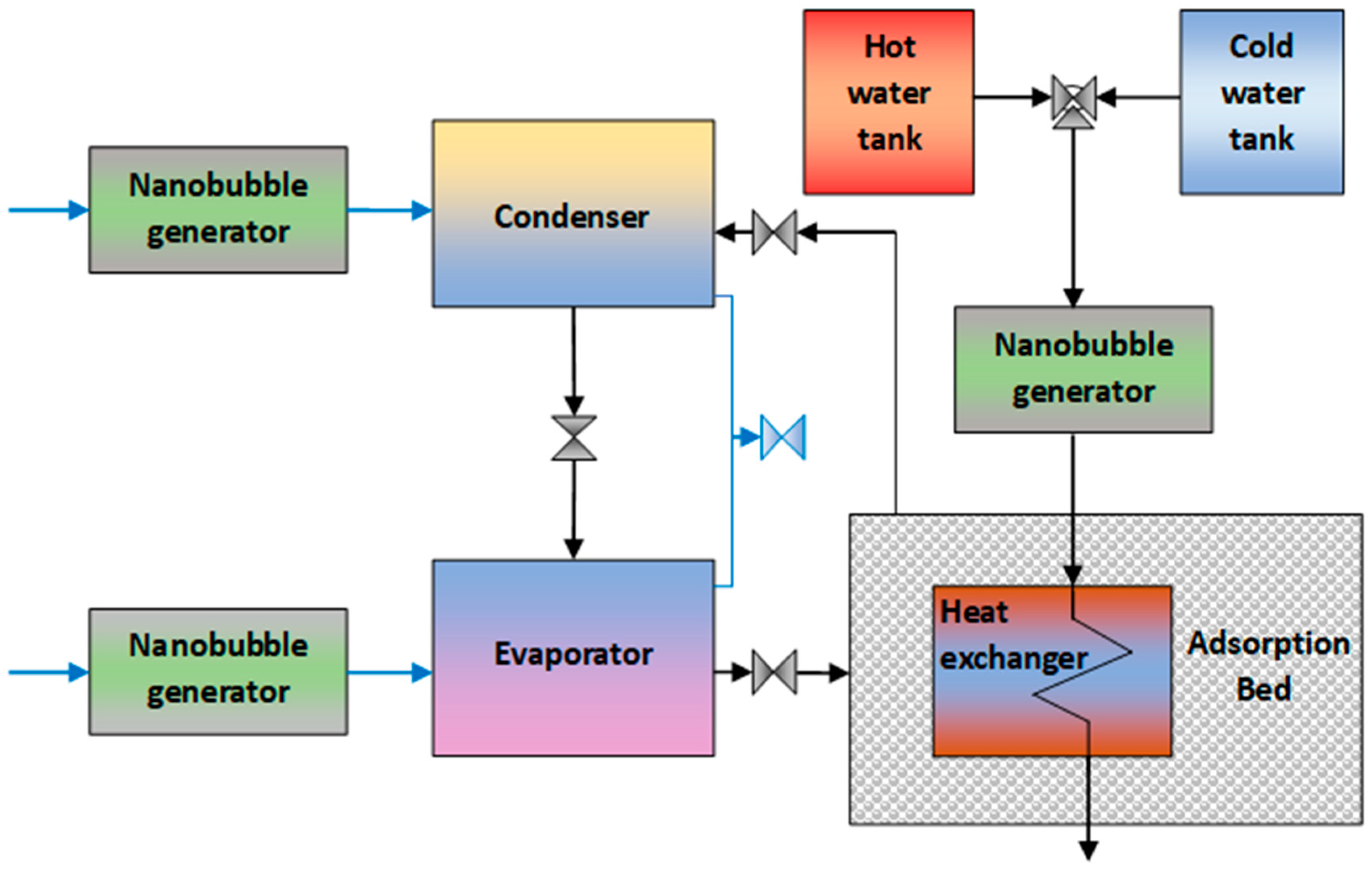
5. Advanced Adsorption and Desalination Systems Using Ultrafine Bubbles
6. Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Nomenclature
| NB | Nanobubble |
| MB | Microbubble |
| mNB | micro/nanobubble |
| SNB | surface nanobubble |
| BNB | bulk nanobubble |
| PDT | photodynamic therapy |
| PTT | photothermal therapy |
| ΥSV | solid–vapor interfacial energy |
| ΥSL | liquid–vapor interfacial energy |
| P | pressure [Pa] |
| r | bubble radius [m] |
| γ | surface tension [Pa] |
| Δ | change |
| θ | contact angle [°] |
| D | indicates pore size [m] |
References
- Gao, M.; Xiao, Y.; Chen, Z.; Ding, L.; Gao, Y.; Dai, Z.; Yu, G.; Krzywanski, J.; Wang, F. Comparison of Physicochemical Properties and Gasification Reactivity of Soot from Entrained Flow Gasification Processes. Chem. Eng. J. 2022, 450, 136660. [Google Scholar] [CrossRef]
- Muskała, W.; Krzywanski, J.; Rajczyk, R.; Cecerko, M.; Kierzkowski, B.; Nowak, W.; Gajewski, W. Investigation of Erosion in CFB Boilers. Rynek Energii 2010, 2, 97–102. [Google Scholar]
- Nowak, W.; Muskala, W.; Krzywanski, J.; Czakiert, T. The Research of CFB Boiler Operation for Oxygen Enhanced Dried Lignite Combustion. Rynek Energii 2011, 92, 172–176. [Google Scholar]
- Iheonye, A.C.; Raghavan, V.; Ferrie, F.P.; Orsat, V.; Gariepy, Y. Monitoring Visual Properties of Food in Real Time During Food Drying. Food Eng. Rev. 2023, 15, 242–260. [Google Scholar] [CrossRef]
- Krzywanski, J. Heat Transfer Performance in a Superheater of an Industrial CFBC Using Fuzzy Logic-Based Methods. Entropy 2019, 21, 919. [Google Scholar] [CrossRef]
- Krzywanski, J.; Blaszczuk, A.; Czakiert, T.; Rajczyk, R.; Nowak, W. Artificial intelligence treatment of NOx emissions from CFBC in air and oxy-fuel conditions, (2014) CFB-11. In Proceedings of the 11th International Conference on Fluidized Bed Technology, Beijing, China, 1 May 2014; pp. 619–624. [Google Scholar]
- Xiao, W.; Ke, S.; Quan, N.; Zhou, L.; Wang, J.; Zhang, L.; Dong, Y.; Qin, W.; Qiu, G.; Hu, J. The Role of Nanobubbles in the Precipitation and Recovery of Organic-Phosphine-Containing Beneficiation Wastewater. Langmuir 2018, 34, 6217–6224. [Google Scholar] [CrossRef]
- Singh, B.; Shukla, N.; Cho, C.H.; Kim, B.S.; Park, M.H.; Kim, K. Effect and Application of Micro- and Nanobubbles in Water Purification. Toxicol. Environ. Health Sci. 2021, 13, 9–16. [Google Scholar] [CrossRef]
- Lee, J.; Laoui, T.; Karnik, R. Nanofluidic Transport Governed by the Liquid/Vapour Interface. Nat. Nanotechnol. 2014, 9, 317–323. [Google Scholar] [CrossRef]
- Bai, M.; Liu, Z.; Zhang, J.; Lu, L. Prediction and Experimental Study of Mass Transfer Properties of Micronanobubbles. Ind. Eng. Chem. Res. 2021, 60, 8291–8300. [Google Scholar] [CrossRef]
- Bai, M.; Liu, Z.; Zhan, L.; Liu, Z.; Fan, Z. A Comparative Study of Removal Efficiency of Organic Contaminant in Landfill Leachate-Contaminated Groundwater under Micro-Nano-Bubble and Common Bubble Aeration. Environ. Sci. Pollut. Res. 2022, 29, 87534–87544. [Google Scholar] [CrossRef]
- Feng, Y.; Mu, H.; Liu, X.; Huang, Z.; Zhang, H.; Wang, J.; Yang, Y. Leveraging 3D Printing for the Design of High-Performance Venturi Microbubble Generators. Ind. Eng. Chem. Res. 2020, 59, 8447–8455. [Google Scholar] [CrossRef]
- Alheshibri, M.; Al Baroot, A.; Shui, L.; Zhang, M. Nanobubbles and Nanoparticles. Curr. Opin. Colloid Interface Sci. 2021, 55, 101470. [Google Scholar] [CrossRef]
- Xiao, W.; Xu, G. Mass Transfer of Nanobubble Aeration and Its Effect on Biofilm Growth: Microbial Activity and Structural Properties. Sci. Total Environ. 2020, 703, 134976. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, E.; Zhou, J.; Shen, C.; He, Z.; Xiao, C. Investigation on Enhanced Mechanism of Heat Transfer Assisted by Ultrasonic Vibration. Int. Commun. Heat Mass Transf. 2020, 115, 104523. [Google Scholar] [CrossRef]
- Amiri Delouei, A.; Sajjadi, H.; Ahmadi, G. Ultrasonic Vibration Technology to Improve the Thermal Performance of CPU Water-Cooling Systems: Experimental Investigation. Water 2022, 14, 4000. [Google Scholar] [CrossRef]
- Qii, P.; Wong, S.W.; Chon, W.Y. Effects of Ultrasonic Vibrations on Heat Transfer to Liquids by Natural Convection and by Boiling. AIChE J. 1969, 15, 281–288. [Google Scholar]
- Shen, G.; Ma, L.; Zhang, S.; Zhang, S.; An, L. Effect of Ultrasonic Waves on Heat Transfer in Al2O3 Nanofluid under Natural Convection and Pool Boiling. Int. J. Heat Mass Transf. 2019, 138, 516–523. [Google Scholar] [CrossRef]
- Zhang, D.; Guan, J.; He, Z.; Shen, C.; Cao, H. Experimental Investigation on Heat Transfer and Flow Patterns of Pulsating Heat Pipe Assisted by Ultrasonic Cavitation. Int. J. Heat Mass Transf. 2022, 183, 122187. [Google Scholar] [CrossRef]
- Tan, B.H.; An, H.; Ohl, C.D. Identifying Surface-Attached Nanobubbles. Curr. Opin. Colloid Interface Sci. 2021, 53, 101429. [Google Scholar] [CrossRef]
- Favvas, E.P.; Kyzas, G.Z.; Efthimiadou, E.K.; Mitropoulos, A.C. Bulk Nanobubbles, Generation Methods and Potential Applications. Curr. Opin. Colloid Interface Sci. 2021, 54, 101455. [Google Scholar] [CrossRef]
- An, H.; Liu, G.; Craig, V.S.J. Wetting of Nanophases: Nanobubbles, Nanodroplets and Micropancakes on Hydrophobic Surfaces. Adv. Colloid Interface Sci. 2015, 222, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Epstein, P.S.; Plesset, M.S. On the Stability of Gas Bubbles in Liquid-Gas Solutions. J. Chem. Phys. 1950, 18, 1505–1509. [Google Scholar] [CrossRef]
- Ducker, W.A. Contact Angle and Stability of Interfacial Nanobubbles. Langmuir 2009, 25, 8907–8910. [Google Scholar] [CrossRef]
- Berge, L.I. Dissolution of Air Bubbles by the Resistive Pulse and the Pressure Reversal Technique. J. Colloid Interface Sci. 1990, 134, 548–562. [Google Scholar] [CrossRef]
- Batchelor, D.V.B.; Armistead, F.J.; Ingram, N.; Peyman, S.A.; Mclaughlan, J.R.; Coletta, P.L.; Evans, S.D. Nanobubbles for Therapeutic Delivery: Production, Stability and Current Prospects. Curr. Opin. Colloid Interface Sci. 2021, 54, 101456. [Google Scholar] [CrossRef]
- Der, O.; Alqahtani, A.A.; Marengo, M.; Bertola, V. Characterization of Polypropylene Pulsating Heat Stripes: Effects of Orientation, Heat Transfer Fluid, and Loop Geometry. Appl. Therm. Eng. 2021, 184, 116304. [Google Scholar] [CrossRef]
- Alheshibri, M.; Qian, J.; Jehannin, M.; Craig, V.S.J. A History of Nanobubbles. Langmuir 2016, 32, 11086–11100. [Google Scholar] [CrossRef]
- Montero De Hijes, P.; Shi, K.; Noya, E.G.; Santiso, E.E.; Gubbins, K.E.; Sanz, E.; Vega, C. The Young-Laplace Equation for a Solid-Liquid Interface. J. Chem. Phys. 2020, 153, 191102. [Google Scholar] [CrossRef]
- Kwan, J.J.; Borden, M.A. Lipid Monolayer Collapse and Microbubble Stability. Adv. Colloid Interface Sci. 2012, 183–184, 82–99. [Google Scholar] [CrossRef]
- Nirmalkar, N.; Pacek, A.W.; Barigou, M. On the Existence and Stability of Bulk Nanobubbles. Langmuir 2018, 34, 10964–10973. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.L.; Claesson, P.M.; Attard, P. Bubbles, Cavities, and the Long-Ranged Attraction between Hydrophobic Surfaces. J. Phys. Chem. 1994, 98, 8468–8480. [Google Scholar] [CrossRef]
- Berkelaar, R.P.; Dietrich, E.; Kip, G.A.M.; Kooij, E.S.; Zandvliet, H.J.W.; Lohse, D. Exposing Nanobubble-like Objects to a Degassed Environment. Soft Matter 2014, 10, 4947–4955. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Nguyen, D.C.; Han, M. Average Size and Zeta Potential of Nanobubbles in Different Reagent Solutions. J. Nanoparticle Res. 2019, 21, 173. [Google Scholar] [CrossRef]
- Han, Z.; Kurokawa, H.; Matsui, H.; He, C.; Wang, K.; Wei, Y.; Dodbiba, G.; Otsuki, A.; Fujita, T. Stability and Free Radical Production for CO2 and H2 in Air Nanobubbles in Ethanol Aqueous Solution. Nanomaterials 2022, 12, 237. [Google Scholar] [CrossRef]
- Alam, H.S.; Sutikno, P.; Soelaiman, T.A.F.; Sugiarto, A.T. Bulk Nanobubbles: Generation Using a Two-Chamber Swirling Flow Nozzle and Long-Term Stability in Water. J. Flow. Chem. 2022, 12, 161–173. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Aluthgun Hewage, S.; Batagoda, J.H. Stability of Nanobubbles. Environ. Eng. Sci. 2018, 35, 1216–1227. [Google Scholar] [CrossRef]
- Takahashi, M. ζ Potential of Microbubbles in Aqueous Solutions: Electrical Properties of the Gas-Water Interface. J. Phys. Chem. B 2005, 109, 21858–21864. [Google Scholar] [CrossRef]
- Ahmed, A.K.A.; Sun, C.; Hua, L.; Zhang, Z.; Zhang, Y.; Zhang, W.; Marhaba, T. Generation of Nanobubbles by Ceramic Membrane Filters: The Dependence of Bubble Size and Zeta Potential on Surface Coating, Pore Size and Injected Gas Pressure. Chemosphere 2018, 203, 327–335. [Google Scholar] [CrossRef]
- Tan, B.H.; An, H.; Ohl, C.D. How Bulk Nanobubbles Might Survive. Phys. Rev. Lett. 2020, 124, 134503. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, X.; Zhou, L.; Zhou, W.; Wang, J.; Qin, W.; Qiu, G.; Hu, J.; Zhang, L. Influence of Mixing and Nanosolids on the Formation of Nanobubbles. J. Phys. Chem. B 2019, 123, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Olszok, V.; Rivas-Botero, J.; Wollmann, A.; Benker, B.; Weber, A.P. Particle-Induced Nanobubble Generation for Material-Selective Nanoparticle Flotation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 592, 124576. [Google Scholar] [CrossRef]
- Berkelaar, R.P.; Seddon, J.R.T.; Zandvliet, H.J.W.; Lohse, D. Temperature Dependence of Surface Nanobubbles. ChemPhysChem 2012, 13, 2213–2217. [Google Scholar] [CrossRef] [PubMed]
- Borkent, B.M.; De Beer, S.; Mugele, F.; Lohse, D. On the Shape of Surface Nanobubbles. Langmuir 2010, 26, 260–268. [Google Scholar] [CrossRef]
- Sun, Y.; Xie, G.; Peng, Y.; Xia, W.; Sha, J. Stability Theories of Nanobubbles at Solid-Liquid Interface: A Review. Colloids Surf. A Physicochem. Eng. Asp. 2016, 495, 176–186. [Google Scholar] [CrossRef]
- Das, S.; Snoeijer, J.H.; Lohse, D. Effect of Impurities in Description of Surface Nanobubbles. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2010, 82, 056310. [Google Scholar] [CrossRef]
- Weijs, J.H.; Lohse, D. Why Surface Nanobubbles Live for Hours. Phys. Rev. Lett. 2013, 110, 054501. [Google Scholar] [CrossRef]
- Brenner, M.P.; Lohse, D. Dynamic Equilibrium Mechanism for Surface Nanobubble Stabilization. Phys. Rev. Lett. 2008, 101, 214505. [Google Scholar] [CrossRef]
- Das, S. Effect of Added Salt on Preformed Surface Nanobubbles: A Scaling Estimate. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2011, 84, 036303. [Google Scholar] [CrossRef]
- Zhang, X.; Uddin, M.H.; Yang, H.; Toikka, G.; Ducker, W.; Maeda, N. Effects of Surfactants on the Formation and the Stability of Interfacial Nanobubbles. Langmuir 2012, 28, 10471–10477. [Google Scholar] [CrossRef]
- Zhang, X.H.; Zhang, X.D.; Lou, S.T.; Zhang, Z.X.; Sun, J.L.; Hu, J. Degassing and Temperature Effects on the Formation of Nanobubbles at the Mica/Water Interface. Langmuir 2004, 20, 3813–3815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Seddon, J. Nanobubble-Nanoparticle Interactions in Bulk Solutions. Langmuir 2016, 32, 11280–11286. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, A.; Kioka, A.; Nakagawa, M.; Anzai, S. Nanobubbles as Corrosion Inhibitor in Acidic Geothermal Fluid. Geothermics 2021, 89, 101962. [Google Scholar] [CrossRef]
- Zhang, M.; Lemay, S.G. Interaction of Anionic Bulk Nanobubbles with Cationic Liposomes: Evidence for Reentrant Condensation. Langmuir 2019, 35, 4146–4151. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; An, H.; Alheshibri, M.; Liu, L.; Terpstra, P.M.J.; Liu, G.; Craig, V.S.J. Cleaning with Bulk Nanobubbles. Langmuir 2016, 32, 11203–11211. [Google Scholar] [CrossRef] [PubMed]
- Dayarathne, H.N.P.; Jeong, S.; Jang, A. Chemical-Free Scale Inhibition Method for Seawater Reverse Osmosis Membrane Process: Air Micro-Nano Bubbles. Desalination 2019, 461, 1–9. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, W. Microwave-Enhanced Membrane Filtration for Water Treatment. J. Membr. Sci. 2018, 568, 97–104. [Google Scholar] [CrossRef]
- Dehariya, D.; Eswar, K.; Tarafdar, A.; Balusamy, S.; Rengan, A.K. Recent Advances of Nanobubble-Based Systems in Cancer Therapeutics: A Review. Biomed. Eng. Adv. 2023, 5, 100080. [Google Scholar] [CrossRef]
- Gao, Y.; Hernandez, C.; Yuan, H.X.; Lilly, J.; Kota, P.; Zhou, H.; Wu, H.; Exner, A.A. Ultrasound Molecular Imaging of Ovarian Cancer with CA-125 Targeted Nanobubble Contrast Agents. Nanomedicine 2017, 13, 2159–2168. [Google Scholar] [CrossRef]
- Yu, Z.; Hu, M.; Li, Z.; Xu, D.; Zhu, L.; Guo, Y.; Liu, Q.; Lan, W.; Jiang, J.; Wang, L. Anti-G250 Nanobody-Functionalized Nanobubbles Targeting Renal Cell Carcinoma Cells for Ultrasound Molecular Imaging. Nanotechnology 2020, 31, 205101. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X. A Unified Mechanism for the Stability of Surface Nanobubbles: Contact Line Pinning and Supersaturation. J. Chem. Phys. 2014, 141, 134702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, M.; Zhang, Y.; Zhang, J.; Su, J.; Yang, C. Molecular Imaging of Atherosclerotic Plaque with Lipid Nanobubbles as Targeted Ultrasound Contrast Agents. Colloids Surf. B Biointerfaces 2020, 189, 110861. [Google Scholar] [CrossRef] [PubMed]
- de Leon, A.; Perera, R.; Nittayacharn, P.; Cooley, M.; Jung, O.; Exner, A.A. Chapter Three—Ultrasound Contrast Agents and Delivery Systems in Cancer Detection and Therapy. Adv. Cancer Res. 2018, 139, 57–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhao, H.; Guo, L.; Wang, Y.; Song, J.; Zhao, X.; Li, C.; Hao, L.; Wang, D.; Tang, J. Ultrasound-Mediated Nanobubble Destruction (UMND) Facilitates the Delivery of A10-3.2 Aptamer Targeted and SiRNA-Loaded Cationic Nanobubbles for Therapy of Prostate Cancer. Drug Deliv. 2018, 25, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Bessone, F.; Argenziano, M.; Grillo, G.; Ferrara, B.; Pizzimenti, S.; Barrera, G.; Cravotto, G.; Guiot, C.; Stura, I.; Cavalli, R.; et al. Low-Dose Curcuminoid-Loaded in Dextran Nanobubbles Can Prevent Metastatic Spreading in Prostate Cancer Cells. Nanotechnology 2019, 30, 214004. [Google Scholar] [CrossRef] [PubMed]
- Bujok, T.; Boruta, P.; Mika, Ł.; Sztekler, K. Analysis of Designs of Heat Exchangers Used in Adsorption Chillers. Energies 2021, 14, 8038. [Google Scholar] [CrossRef]
- Sztekler, K.; Kalawa, W.; Nowak, W.; Mika, L.; Krzywanski, J.; Grabowska, K.; Sosnowski, M.; Alharbi, A. Performance Evaluation of a Single-Stage Two-Bed Adsorption Chiller with Desalination Function. J. Energy Resour. Technol. Trans. ASME 2021, 143, 082101. [Google Scholar] [CrossRef]
- Chen, C.J.; Wang, R.Z.; Wang, L.W.; Lu, Z.S. Studies on Cycle Characteristics and Application of Split Heat Pipe Adsorption Ice Maker. Energy Convers. Manag. 2007, 48, 1106–1112. [Google Scholar] [CrossRef]
- Doubek, M.; Vacek, V. Universal Heat Exchanger for Air and Evaporative Cooling of Electronics. Therm. Sci. Eng. Prog. 2021, 23, 100865. [Google Scholar] [CrossRef]
- Grabowska, K.; Sztekler, K.; Krzywanski, J.; Sosnowski, M.; Stefanski, S.; Nowak, W. Construction of an Innovative Adsorbent Bed Configuration in the Adsorption Chiller Part 2. Experimental Research of Coated Bed Samples. Energy 2021, 215, 119123. [Google Scholar] [CrossRef]
- Krzywanski, J.; Sztekler, K.; Bugaj, M.; Kalawa, W.; Grabowska, K.; Chaja, P.R.; Sosnowski, M.; Nowak, W.; Mika, L.; Bykuc, S. Adsorption Chiller in a Combined Heating and Cooling System: Simulation and Optimization by Neural Networks. Bull. Pol. Acad. Sci. Tech. Sci. 2021, 69, e137054. [Google Scholar] [CrossRef]
- Nikbakhti, R.; Wang, X.; Chan, A. Performance Optimization of an Integrated Adsorption-Absorption Cooling System Driven by Low-Grade Thermal Energy. Appl. Therm. Eng. 2021, 193, 117035. [Google Scholar] [CrossRef]
- Nikbakhti, R.; Wang, X.; Chan, A. Performance Analysis of an Integrated Adsorption and Absorption Refrigeration System. Int. J. Refrig. 2020, 117, 269–283. [Google Scholar] [CrossRef]
- Nikbakhti, R.; Iranmanesh, A. Potential Application of a Novel Integrated Adsorption–Absorption Refrigeration System Powered with Solar Energy in Australia. Appl. Therm. Eng. 2021, 194, 117114. [Google Scholar] [CrossRef]
- Khan, M.Z.I.; Alam, K.C.A.; Saha, B.B.; Akisawa, A.; Kashiwagi, T. Study on a Re-Heat Two-Stage Adsorption Chiller—The Influence of Thermal Capacitance Ratio, Overall Thermal Conductance Ratio and Adsorbent Mass on System Performance. Appl. Therm. Eng. 2007, 27, 1677–1685. [Google Scholar] [CrossRef]
- Krzywanski, J.; Grabowska, K.; Sosnowski, M.; Zyłka, A.; Sztekler, K.; Kalawa, W.; Wójcik, T.; Nowak, W. Modeling of a Re-Heat Two-Stage Adsorption Chiller by AI Approach. Proc. MATEC Web Conf. 2018, 240, 05014. [Google Scholar] [CrossRef]
- Kulakowska, A.; Pajdak, A.; Krzywanski, J.; Grabowska, K.; Zylka, A.; Sosnowski, M.; Wesolowska, M.; Sztekler, K.; Nowak, W. Effect of Metal and Carbon Nanotube Additives on the Thermal Diffusivity of a Silica-Gel-Based Adsorption Bed. Energies 2020, 16, 1391. [Google Scholar] [CrossRef]
- Pajdak, A.; Kulakowska, A.; Liu, J.; Berent, K.; Kudasik, M.; Krzywanski, J.; Kalawa, W.; Sztekler, K.; Skoczylas, N. Accumulation and Emission of Water Vapor by Silica Gel Enriched with Carbon Nanotubes CNT-Potential Applications in Adsorption Cooling and Desalination Technology. Appl. Sci. 2022, 12, 5644. [Google Scholar] [CrossRef]
- Lasek, L.; Zylka, A.; Krzywanski, J.; Skrobek, D.; Sztekler, K.; Nowak, W. Review of Fluidized Bed Technology Application for Adsorption Cooling and Desalination Systems. Energies 2023, 16, 7311. [Google Scholar] [CrossRef]
- Rogala, Z.; Kolasinski, P.; Błasiak, P. The Influence of Operating Parameters on Adsorption/Desorption Characteristics and Performance of the Fluidised Desiccant Cooler. Energies 2018, 11, 1597. [Google Scholar] [CrossRef]
- Rogala, Z.; Kolasiński, P.; Gnutek, Z. Modelling and Experimental Analyzes on Air-Fluidised Silica Gel-Water Adsorption and Desorption. Appl. Therm. Eng. 2017, 127, 950–962. [Google Scholar] [CrossRef]
- Skrobek, D.; Krzywanski, J.; Sosnowski, M.; Kulakowska, A.; Zylka, A.; Grabowska, K.; Ciesielska, K.; Nowak, W. Prediction of Sorption Processes Using the Deep Learning Methods (Long Short-Term Memory). Energies 2020, 13, 6601. [Google Scholar] [CrossRef]
- Krzywanski, J.; Skrobek, D.; Zylka, A.; Grabowska, K.; Kulakowska, A.; Sosnowski, M.; Nowak, W.; Blanco-Marigorta, A.M. Heat and Mass Transfer Prediction in Fluidized Beds of Cooling and Desalination Systems by AI Approach. Appl. Therm. Eng. 2023, 225, 120200. [Google Scholar] [CrossRef]
- Krzywanski, J.; Grabowska, K.; Sosnowski, M.; Zylka, A.; Kulakowska, A.; Czakiert, T.; Sztekler, K.; Wesolowska, M.; Nowak, W. Heat Transfer in Adsorption Chillers with Fluidized Beds of Silica Gel, Zeolite, and Carbon Nanotubes. Heat Transf. Eng. 2021, 43, 172–182. [Google Scholar] [CrossRef]
- Skrobek, D.; Krzywanski, J.; Sosnowski, M.; Kulakowska, A.; Zylka, A.; Grabowska, K.; Ciesielska, K.; Nowak, W. Implementation of Deep Learning Methods in Prediction of Adsorption Processes. Adv. Eng. Softw. 2022, 173, 103190. [Google Scholar] [CrossRef]
- Bai, M.; Liu, Z.; Zhan, L.; Yuan, M.; Yu, H. Effect of Pore Size Distribution and Colloidal Fines of Porous Media on the Transport Behavior of Micro-Nano-Bubbles. Colloids Surf. A Physicochem. Eng. Asp. 2023, 660, 130851. [Google Scholar] [CrossRef]
- Senthilkumar, G.; Rameshkumar, C.; Nikhil, M.N.V.S.; Kumar, J.N.R. An Investigation of Nanobubbles in Aqueous Solutions for Various Applications. Appl. Nanosci. 2018, 8, 1557–1567. [Google Scholar] [CrossRef]
- Sakr, M.; Mohamed, M.M.; Maraqa, M.A.; Hamouda, M.A.; Aly Hassan, A.; Ali, J.; Jung, J. A Critical Review of the Recent Developments in Micro–Nano Bubbles Applications for Domestic and Industrial Wastewater Treatment. Alex. Eng. J. 2022, 61, 6591–6612. [Google Scholar] [CrossRef]
- Amburi, P.K.; Senthilkumar, G.; Neme Mogose, I. Heat Transfer Augmentation: Experimental Study with Nanobubbles Technology. Adv. Mater. Sci. Eng. 2022, 2022, 5885280. [Google Scholar] [CrossRef]
- Zhou, D.W. Heat Transfer Enhancement of Copper Nanofluid with Acoustic Cavitation. Int. J. Heat Mass Transf. 2004, 47, 3109–3117. [Google Scholar] [CrossRef]
- Aluthgun Hewage, S.; Meegoda, J.N. Molecular Dynamics Simulation of Bulk Nanobubbles. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129565. [Google Scholar] [CrossRef]
- Fan, M.; Tan, D.; Honaker, R.; Luo, Z. Nanobubble Generation and Its Application in Froth Flotation (Part I): Nanobubble Generation and Its Effects on Properties of Microbubble and Millimeter Scale Bubble Solutions. Min. Sci. Technol. 2010, 20, 1–19. [Google Scholar] [CrossRef]
- Hampton, M.A.; Nguyen, A.V. Accumulation of Dissolved Gases at Hydrophobic Surfaces in Water and Sodium Chloride Solutions: Implications for Coal Flotation. Miner. Eng. 2009, 22, 786–792. [Google Scholar] [CrossRef]
- Zhang, X.H.; Quinn, A.; Ducker, W.A. Nanobubbles at the Interface between Water and a Hydrophobic Solid. Langmuir 2008, 24, 4756–4764. [Google Scholar] [CrossRef]
- Lienhard, J.H. A Heat Transfer Textbook Third Edition; Phlogiston Press: Cambridge, UK, 2008. [Google Scholar]
- Han, S.; Lee, S.; Joung, Y.S. Long-Term Effect of Nanobubbles Generated by Turbulent Flow through Diamond-Pattern Notches on Liquid Properties. Results Eng. 2022, 14, 100375. [Google Scholar] [CrossRef]
- Krzywanski, J.; Skoczylas, N.; Sosnowski, M. Adsorption Desalination and Cooling Systems Advances in Design, Modeling and Performance. Energies 2022, 15, 4036. [Google Scholar]
- Craig, V.S.J. Very Small Bubbles at Surfaces—The Nanobubble Puzzle. Soft Matter 2011, 7, 40–48. [Google Scholar] [CrossRef]
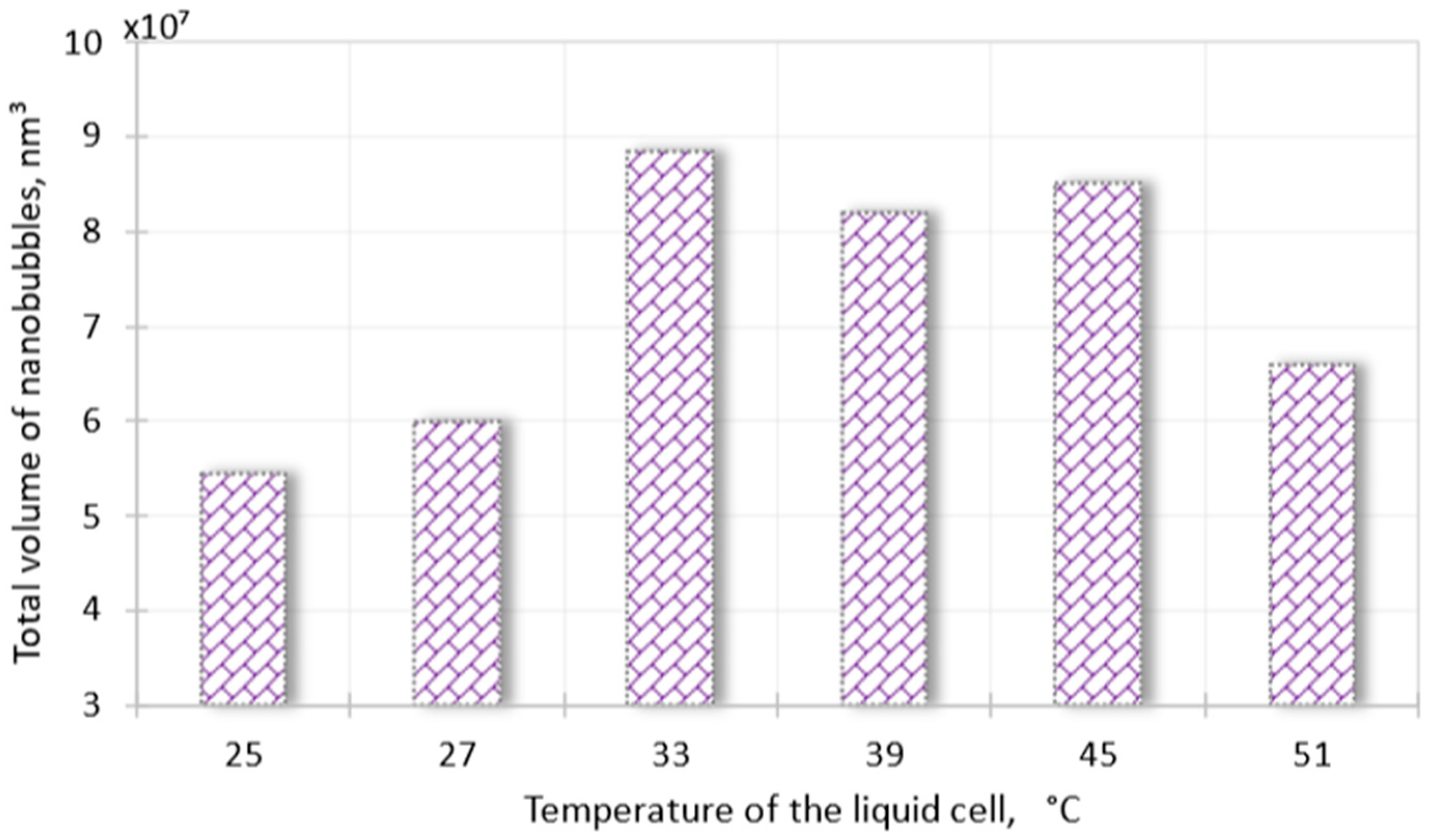
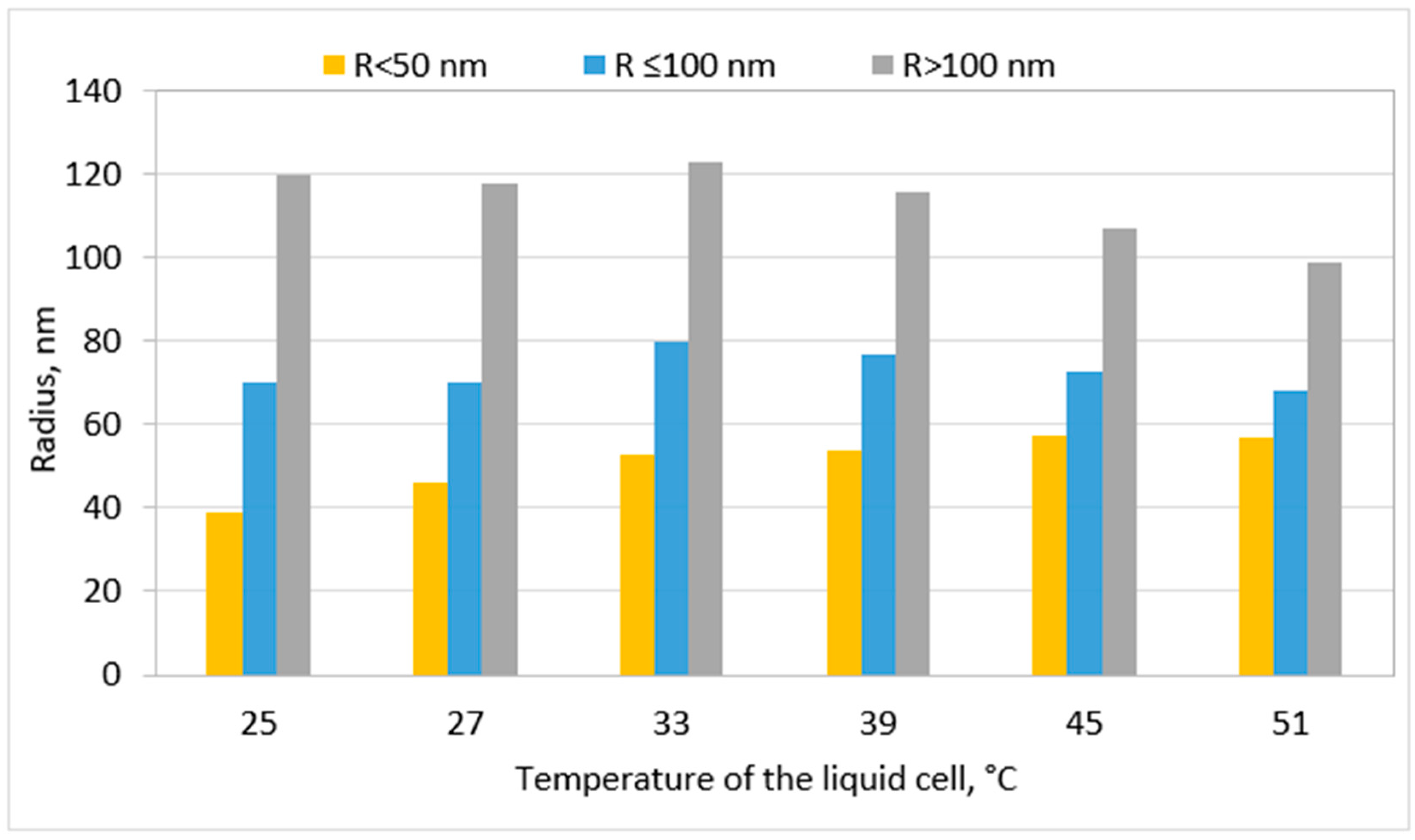
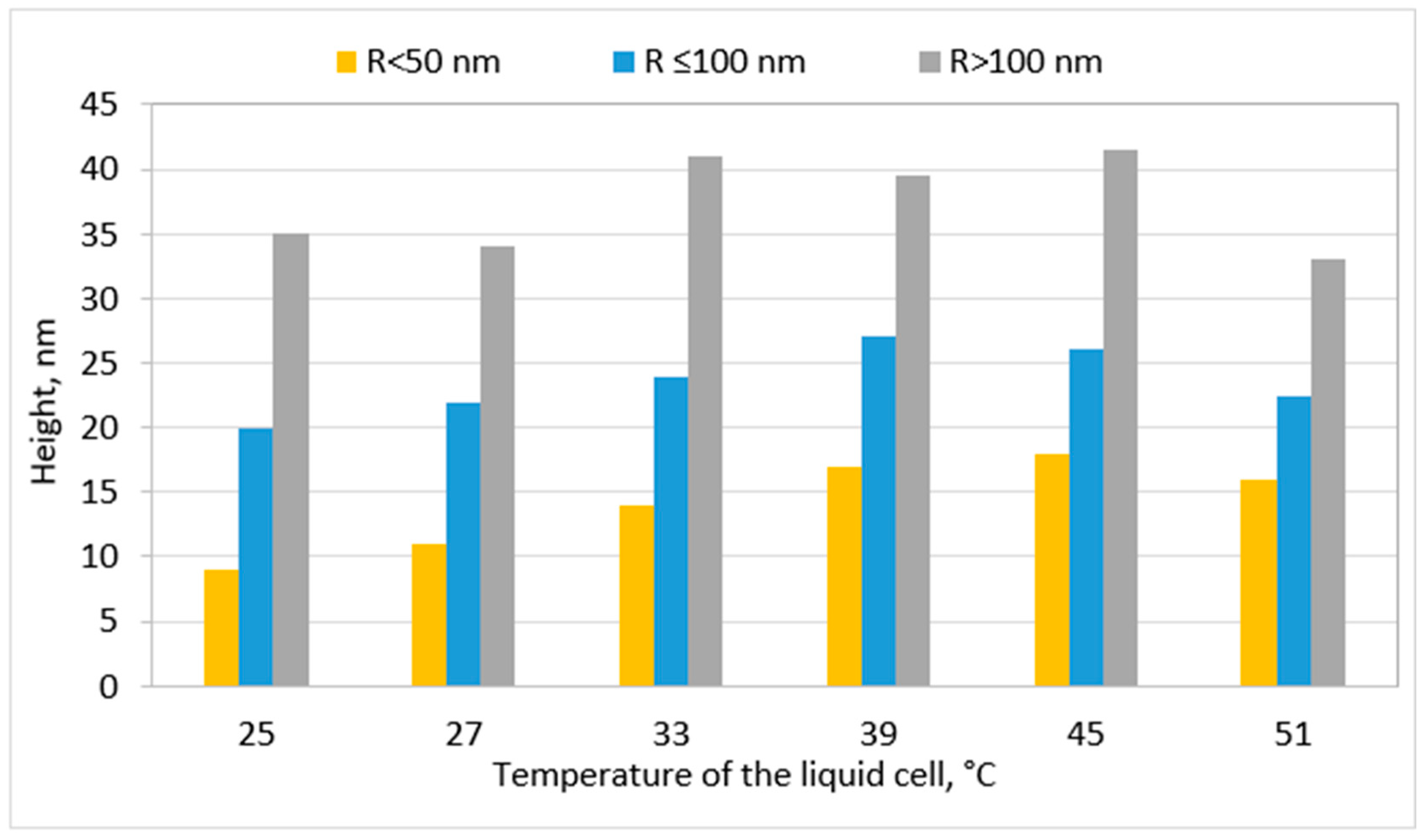
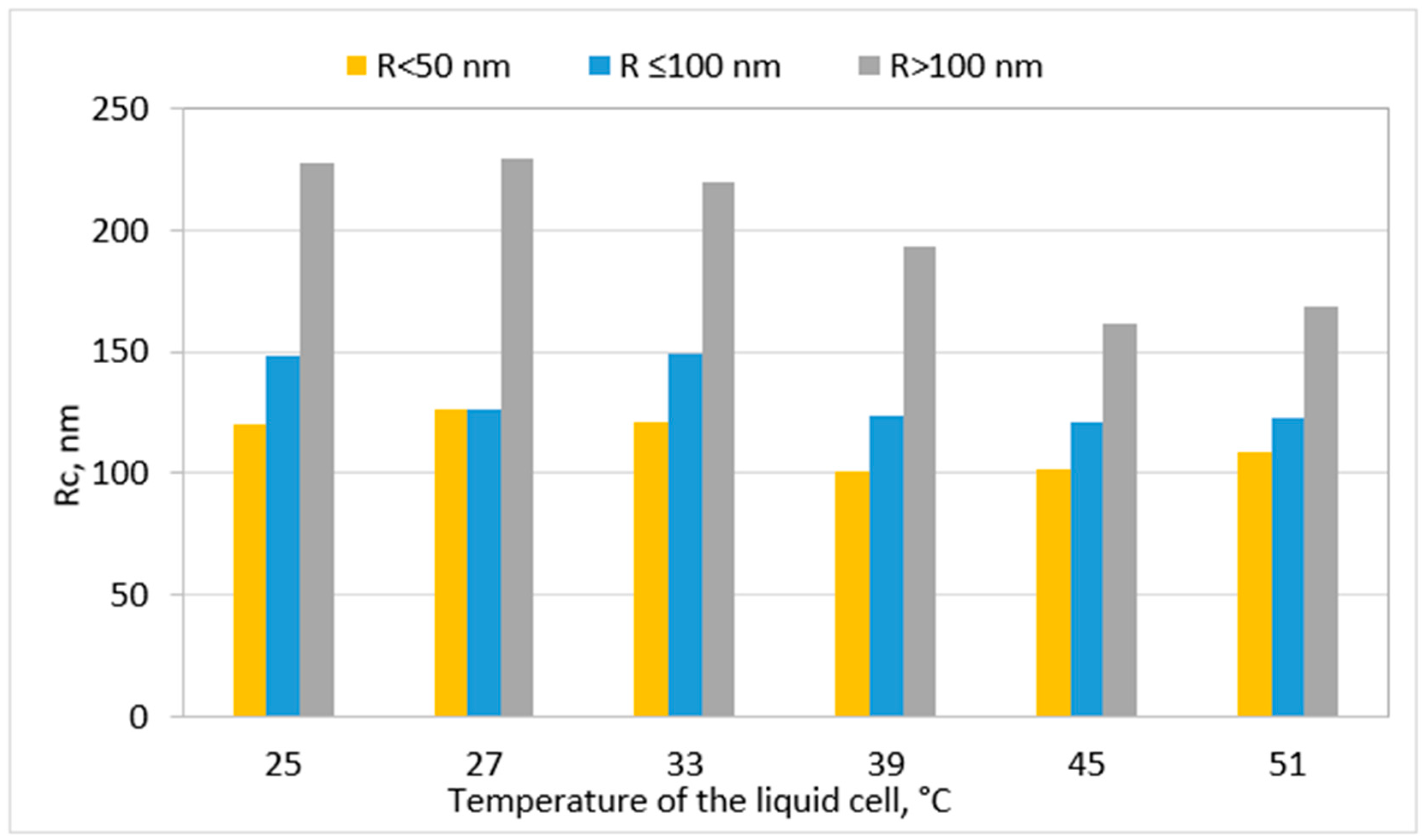
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasek, L.; Krzywanski, J.; Skrobek, D.; Zylka, A.; Nowak, W. Review of Micro- and Nanobubble Technologies: Advancements in Theory and Applications and Perspectives on Adsorption Cooling and Desalination Systems. Energies 2023, 16, 8078. https://doi.org/10.3390/en16248078
Lasek L, Krzywanski J, Skrobek D, Zylka A, Nowak W. Review of Micro- and Nanobubble Technologies: Advancements in Theory and Applications and Perspectives on Adsorption Cooling and Desalination Systems. Energies. 2023; 16(24):8078. https://doi.org/10.3390/en16248078
Chicago/Turabian StyleLasek, Lukasz, Jaroslaw Krzywanski, Dorian Skrobek, Anna Zylka, and Wojciech Nowak. 2023. "Review of Micro- and Nanobubble Technologies: Advancements in Theory and Applications and Perspectives on Adsorption Cooling and Desalination Systems" Energies 16, no. 24: 8078. https://doi.org/10.3390/en16248078
APA StyleLasek, L., Krzywanski, J., Skrobek, D., Zylka, A., & Nowak, W. (2023). Review of Micro- and Nanobubble Technologies: Advancements in Theory and Applications and Perspectives on Adsorption Cooling and Desalination Systems. Energies, 16(24), 8078. https://doi.org/10.3390/en16248078








