Elevating the Practical Application of Sodium-Ion Batteries through Advanced Characterization Studies on Cathodes
Abstract
:1. Introduction
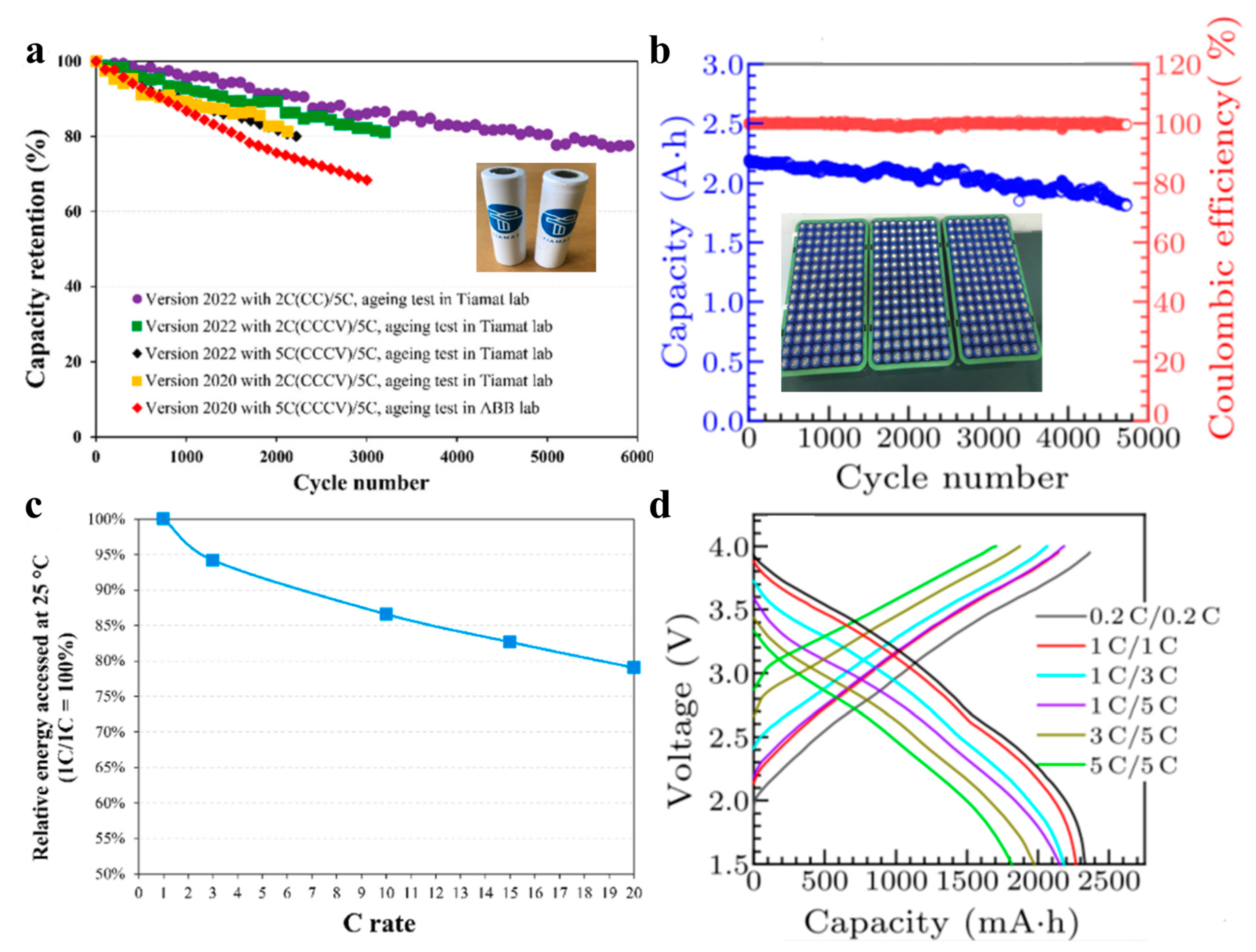
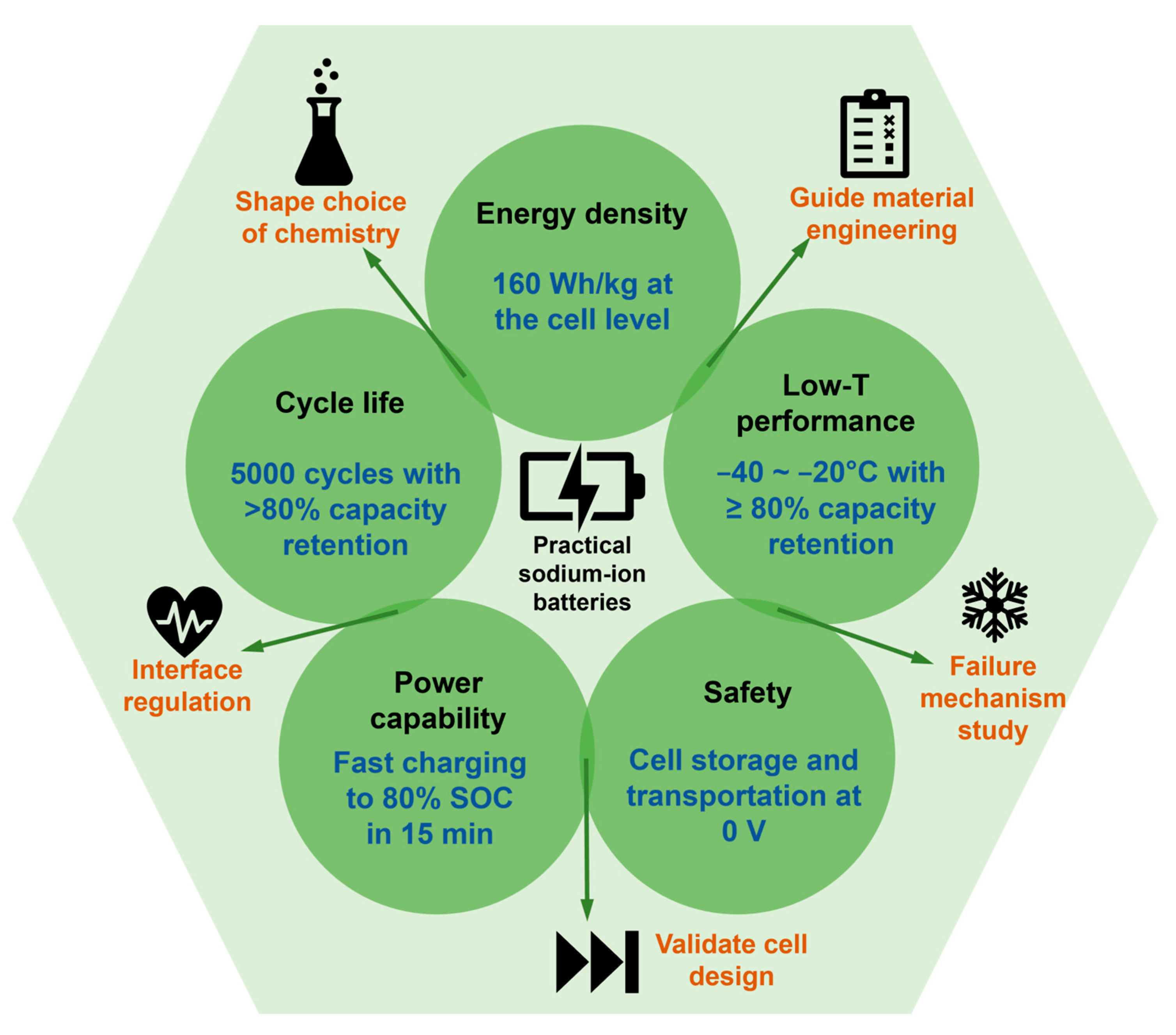
2. Metrics That Matter for Practical Sodium-Ion Batteries
3. Guidance from Cutting-Edge Advanced Characterization Results
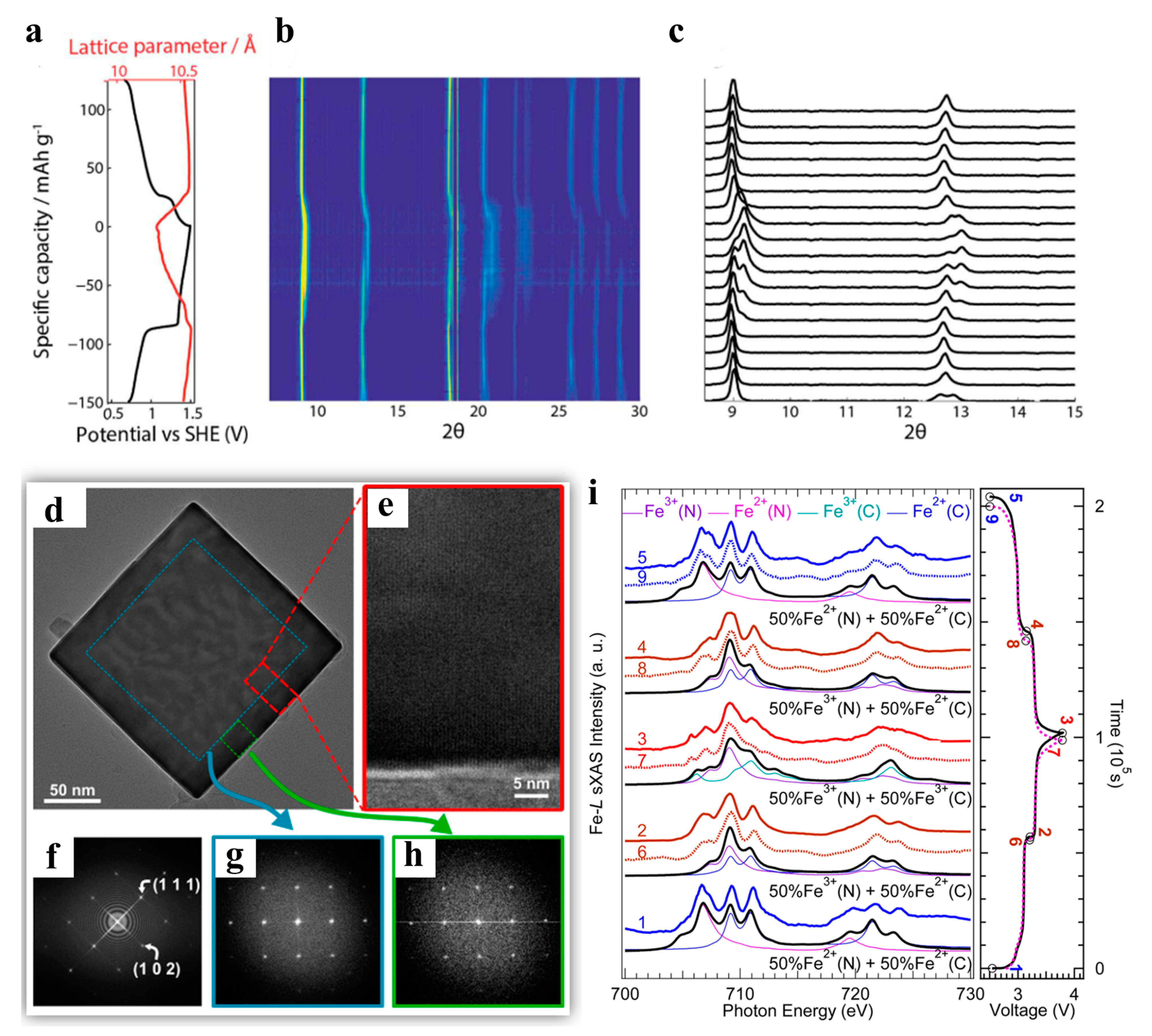
3.1. Sodium-Layered Oxide
3.2. Prussian Blue and Its Analogues
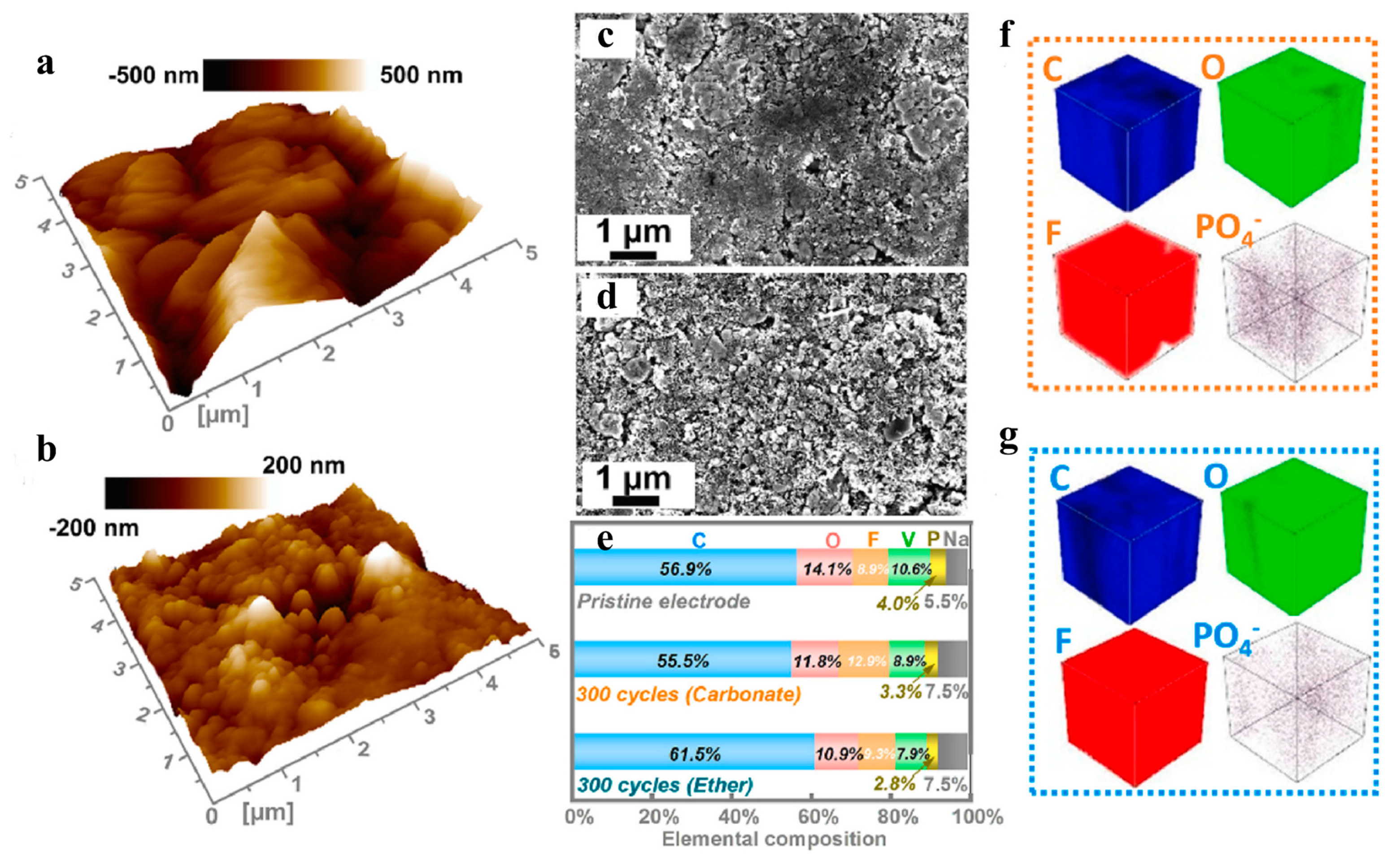
3.3. Phosphates (or Polyanions)
- Unraveling the redox mechanism within SIBs to shape the choice of chemistry tailored for diverse applications;
- Assessing the interface stability and validating the cell designs for the development of long-life-span SIBs;
- Understanding the failure mechanism across different materials, and offering insights for future material engineering pathways (Figure 5).
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Whittingham, M.S.; Huggins, R.A. Measurement of sodium ion transport in beta alumina using reversible solid electrodes. J. Chem. Phys. 1971, 54, 414–416. [Google Scholar] [CrossRef]
- Tarascon, J.; Hull, G. Sodium intercalation into the layer oxides NaxMo2O4. Solid State Ion. 1986, 22, 85–96. [Google Scholar] [CrossRef]
- Doeff, M.M.; Peng, M.Y.; Ma, Y.; De Jonghe, L. Orthorhombic NaxMnO2 as a cathode material for secondary sodium and lithium polymer batteries. J. Electrochem. Soc. 1994, 141, L145. [Google Scholar] [CrossRef]
- Delmas, C.; Braconnier, J.-J.; Fouassier, C.; Hagenmuller, P. Electrochemical intercalation of sodium in NaxCoO2 bronzes. Solid State Ion. 1981, 3, 165–169. [Google Scholar] [CrossRef]
- Delmas, C.; Cherkaoui, F.; Nadiri, A.; Hagenmuller, P. A nasicon-type phase as intercalation electrode: NaTi2(PO4)3. Mater. Res. Bull. 1987, 22, 631–639. [Google Scholar] [CrossRef]
- Kezuka, K.; Hatazawa, T.; Nakajima, K. The status of Sony Li-ion polymer battery. J. Power Source 2001, 97, 755–757. [Google Scholar] [CrossRef]
- Buiel, E.; Dahn, J. Li-insertion in hard carbon anode materials for Li-ion batteries. Electrochim. Acta 1999, 45, 121–130. [Google Scholar] [CrossRef]
- Buiel, E.; George, A.; Dahn, J. Model of micropore closure in hard carbon prepared from sucrose. Carbon 1999, 37, 1399–1407. [Google Scholar] [CrossRef]
- Buiel, E.; Dahn, J. Reduction of the Irreversible Capacity in Hard-Carbon Anode Materials Prepared from Sucrose for Li-Ion Batteries. J. Electrochem. Soc. 1998, 145, 1977. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Cheng, J.; Goodenough, J.B. Prussian blue: A new framework of electrode materials for sodium batteries. Chem. Commun. 2012, 48, 6544–6546. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.-I.; Park, J.-K.; Choi, J.W. Sodium zinc hexacyanoferrate with a well-defined open framework as a positive electrode for sodium ion batteries. Chem. Commun. 2012, 48, 8416–8418. [Google Scholar] [CrossRef]
- Wu, X.; Deng, W.; Qian, J.; Cao, Y.; Ai, X.; Yang, H. Single-crystal FeFe (CN) 6 nanoparticles: A high capacity and high rate cathode for Na-ion batteries. J. Mater. Chem. A 2013, 1, 10130–10134. [Google Scholar] [CrossRef]
- Wessells, C.D.; Peddada, S.V.; Huggins, R.A.; Cui, Y. Nickel hexacyanoferrate nanoparticle electrodes for aqueous sodium and potassium ion batteries. Nano Lett. 2011, 11, 5421–5425. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Häringer, M.; Schmidt, M.; Schneider, L.; Peters, J.F.; Bauer, W.; Binder, J.R.; Weil, M. Prospective Sustainability Screening of Sodium-Ion Battery Cathode Materials. Adv. Energy Mater. 2022, 12, 2202636. [Google Scholar] [CrossRef]
- Available online: https://www.catl.com/en/news/685.html (accessed on 29 July 2021).
- He, M.; Mejdoubi, A.E.; Chartouni, D.; Morcrette, M.; Troendle, P.; Castiglioni, R. High power NVPF/HC-based sodium-ion batteries. J. Power Source 2023, 588, 233741. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, Y.; Tang, F.; Li, K.; Rong, X.; Lu, Y.; Chen, L.; Hu, Y.-S. Thermal stability of high power 26650-type cylindrical Na-ion batteries. Chin. Phys. Lett. 2021, 38, 076501. [Google Scholar] [CrossRef]
- Available online: https://news.metal.com/newscontent/101860656/natrium-energy-signed-a-contract-of-80000-mt-of-sodium-ion-battery-cathode-material-project-further-accelerating-the-industrialisation/ (accessed on 14 June 2022).
- Lu, J.; Wu, T.; Amine, K. State-of-the-art characterization techniques for advanced lithium-ion batteries. Nat. Energy 2017, 2, 17011. [Google Scholar] [CrossRef]
- Shen, Q.; Liu, Y.; Jiao, L.; Qu, X.; Chen, J. Current state-of-the-art characterization techniques for probing the layered oxide cathode materials of sodium-ion batteries. Energy Storage Mater. 2021, 35, 400–430. [Google Scholar] [CrossRef]
- Wang, L.; Liu, T.; Wu, T.; Lu, J. Exploring new battery knowledge by advanced characterizing technologies. In Exploration; Wiley Online Library: Hoboken, NJ, USA, 2021; p. 20210130. [Google Scholar]
- Li, Q.; Liu, Z.; Zheng, F.; Liu, R.; Lee, J.; Xu, G.L.; Zhong, G.; Hou, X.; Fu, R.; Chen, Z. Identifying the structural evolution of the sodium ion battery Na2FePO4F cathode. Angew. Chem. 2018, 130, 12094–12099. [Google Scholar] [CrossRef]
- Wang, W.; Gang, Y.; Hu, Z.; Yan, Z.; Li, W.; Li, Y.; Gu, Q.-F.; Wang, Z.; Chou, S.-L.; Liu, H.-K. Reversible structural evolution of sodium-rich rhombohedral Prussian blue for sodium-ion batteries. Nat. Commun. 2020, 11, 980. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Xiao, Y.; Hua, W.B.; Indris, S.; Dou, S.X.; Guo, Y.G.; Chou, S.L. Manipulating layered P2@ P3 integrated spinel structure evolution for high-performance sodium-ion batteries. Angew. Chem. Int. Ed. 2020, 59, 9299–9304. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, S.; Wang, B.; Li, Y.; Sun, W.; Lu, Y.; Yan, M.; Song, B.; Dou, S. Prussian blue@ C composite as an ultrahigh-rate and long-life sodium-ion battery cathode. Adv. Funct. Mater. 2016, 26, 5315–5321. [Google Scholar] [CrossRef]
- Song, T.; Yao, W.; Kiadkhunthod, P.; Zheng, Y.; Wu, N.; Zhou, X.; Tunmee, S.; Sattayaporn, S.; Tang, Y. A low-cost and environmentally friendly mixed polyanionic cathode for sodium-ion storage. Angew. Chem. 2020, 132, 750–755. [Google Scholar] [CrossRef]
- Cao, X.; Li, H.; Qiao, Y.; Li, X.; Jia, M.; Cabana, J.; Zhou, H. Stabilizing reversible oxygen redox chemistry in layered oxides for sodium-ion batteries. Adv. Energy Mater. 2020, 10, 1903785. [Google Scholar] [CrossRef]
- Wang, P.-F.; Xiao, Y.; Piao, N.; Wang, Q.-C.; Ji, X.; Jin, T.; Guo, Y.-J.; Liu, S.; Deng, T.; Cui, C. Both cationic and anionic redox chemistry in a P2-type sodium layered oxide. Nano Energy 2020, 69, 104474. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, W.; Li, Y.-t.; Wu, N.; Guo, Y.-d.; Cheng, W.-j.; Sun, W.-w.; Li, J.-z.; Zhou, A.-j. Ion-exchange surface modification enhances cycling stability and kinetics of sodium manganese hexacyanoferrate cathode in sodium-ion batteries. Electrochim. Acta 2021, 390, 138842. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Q.; Wan, M.; Peng, J.; Luo, Y.; Zhang, H.; Ren, J.; Xue, L.; Zhang, W. Surface passivation of NaxFe[Fe(CN)6] cathode to improve its electrochemical kinetics and stability in sodium-ion batteries. J. Power Source 2020, 448, 227421. [Google Scholar] [CrossRef]
- Li, J.; Hu, H.; Wang, J.; Xiao, Y. Surface chemistry engineering of layered oxide cathodes for sodium-ion batteries. Carbon Neutralization 2022, 1, 96–116. [Google Scholar] [CrossRef]
- Nelson, P.A.; Ahmed, S.; Gallagher, K.G.; Dees, D.W. Modeling the Performance and Cost of Lithium-Ion Batteries for Electric-Drive Vehicles; Argonne National Lab (ANL): Argonne, IL, USA, 2019.
- Li, M.Y.; Du, Z.J.; Khaleel, M.A.; Belharouak, I. Materials and engineering endeavors towards practical sodium-ion batteries. Energy Storage Mater. 2020, 25, 520–536. [Google Scholar] [CrossRef]
- Vaalma, C.; Buchholz, D.; Weil, M.; Passerini, S. A cost and resource analysis of sodium-ion batteries. Nat. Rev. Mater. 2018, 3, 18013. [Google Scholar] [CrossRef]
- Muruganantham, R.; Chiu, Y.-T.; Yang, C.-C.; Wang, C.-W.; Liu, W.-R. An efficient evaluation of F-doped polyanion cathode materials with long cycle life for Na-ion batteries applications. Sci. Rep. 2017, 7, 14808. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Liu, J.; Xiong, S.; Ma, X.; Liu, P.; Bai, J.; Xu, W.; Tang, Y.; Hu, Y.-Y. Lithium-doping stabilized high-performance P2–Na0.66Li0.18Fe0.12Mn0.7O2 cathode for sodium ion batteries. J. Am. Chem. Soc. 2019, 141, 6680–6689. [Google Scholar] [CrossRef]
- Wang, J.; Mi, C.; Nie, P.; Dong, S.; Tang, S.; Zhang, X. Sodium-rich iron hexacyanoferrate with nickel doping as a high performance cathode for aqueous sodium ion batteries. J. Electroanal. Chem. 2018, 818, 10–18. [Google Scholar] [CrossRef]
- Lamb, J.; Manthiram, A. Surface-modified Na (Ni0.3Fe0.4Mn0.3)O2 cathodes with enhanced cycle life and air stability for sodium-ion batteries. ACS Appl. Energy Mater. 2021, 4, 11735–11742. [Google Scholar] [CrossRef]
- Chen, M.; Chen, L.; Hu, Z.; Liu, Q.; Zhang, B.; Hu, Y.; Gu, Q.; Wang, J.L.; Wang, L.Z.; Guo, X. Carbon-Coated Na3.32Fe2.34(P2O7)2 Cathode Material for High-Rate and Long-Life Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1605535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fu, L.; Luan, J.; Huang, X.; Tang, Y.; Xie, H.; Wang, H. Surface engineering induced core-shell Prussian blue@ polyaniline nanocubes as a high-rate and long-life sodium-ion battery cathode. J. Power Source 2018, 395, 305–313. [Google Scholar] [CrossRef]
- Available online: https://natron.energy/product/ (accessed on 6 October 2023).
- Li, Y.; Zhao, Y.; Feng, X.; Wang, X.; Shi, Q.; Wang, J.; Wang, J.; Zhang, J.; Hou, Y. A durable P2-type layered oxide cathode with superior low-temperature performance for sodium-ion batteries. Sci. China -Mater. 2022, 65, 328–336. [Google Scholar] [CrossRef]
- You, Y.; Yao, H.R.; Xin, S.; Yin, Y.X.; Zuo, T.T.; Yang, C.P.; Guo, Y.G.; Cui, Y.; Wan, L.J.; Goodenough, J.B. Subzero-temperature cathode for a sodium-ion battery. Adv. Mater. 2016, 28, 7243–7248. [Google Scholar] [CrossRef]
- Gu, Z.Y.; Guo, J.Z.; Sun, Z.H.; Zhao, X.X.; Wang, X.T.; Liang, H.J.; Zhao, B.; Li, W.H.; Pan, X.M.; Wu, X.L. Aliovalent-ion-induced lattice regulation based on charge balance theory: Advanced fluorophosphate cathode for sodium-ion full batteries. Small 2021, 17, 2102010. [Google Scholar] [CrossRef]
- Available online: https://natron.energy/battery-safety/ (accessed on 28 September 2022).
- Zhao, E.Y.; Wang, H.W.; Yin, W.; He, L.H.; Ke, Y.B.; Wang, F.W.; Zhao, J.K. Spatiotemporal-scale neutron studies on lithium-ion batteries and beyond. Appl. Phys. Lett. 2022, 121, 110501. [Google Scholar] [CrossRef]
- Beck, F.R.; Cheng, Y.; Bi, Z.; Feygenson, M.; Bridges, C.A.; Moorhead-Rosenberg, Z.; Manthiram, A.; Goodenough, J.B.; Paranthaman, M.P.; Manivannan, A. Neutron diffraction and electrochemical studies of Na0.79CoO2 and Na0.79Co0.7Mn0.3O2 cathodes for sodium-ion batteries. J. Electrochem. Soc. 2014, 161, A961. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Ma, X.; Sun, K.; He, L.; Li, Y.; Chen, D. Na2/3Li1/9 [Ni2/9Li1/9Mn2/3]O2: A high-performance solid-solution reaction layered oxide cathode material for sodium-ion batteries. ACS Appl. Energy Mater. 2021, 5, 1126–1135. [Google Scholar] [CrossRef]
- Nielsen, I.; Dzodan, D.; Ojwang, D.O.; Henry, P.F.; Ulander, A.; Ek, G.; Häggström, L.; Ericsson, T.; Boström, H.; Brant, W. Water driven phase transitions in Prussian white cathode materials. J. Phys. Energy 2022, 4, 044012. [Google Scholar] [CrossRef]
- Barpanda, P.; Avdeev, M.; Ling, C.D.; Lu, J.; Yamada, A. Magnetic structure and properties of the Na2CoP2O7 pyrophosphate cathode for sodium-ion batteries: A supersuperexchange-driven non-collinear antiferromagnet. Inorg. Chem. 2013, 52, 395–401. [Google Scholar] [CrossRef]
- Ma, T.; Xu, G.-L.; Zeng, X.; Li, Y.; Ren, Y.; Sun, C.; Heald, S.M.; Jorne, J.; Amine, K.; Chen, Z. Solid state synthesis of layered sodium manganese oxide for sodium-ion battery by in-situ high energy X-ray diffraction and X-ray absorption near edge spectroscopy. J. Power Source 2017, 341, 114–121. [Google Scholar] [CrossRef]
- Bai, X.; Sathiya, M.; Mendoza-Sánchez, B.; Iadecola, A.; Vergnet, J.; Dedryvère, R.; Saubanère, M.; Abakumov, A.M.; Rozier, P.; Tarascon, J.M. Anionic redox activity in a newly Zn-doped sodium layered oxide P2-Na2/3Mn1−yZnyO2 (0 < y < 0.23). Adv. Energy Mater. 2018, 8, 1802379. [Google Scholar]
- Tripathi, A.; Rudola, A.; Gajjela, S.R.; Xi, S.; Balaya, P. Developing an O3 type layered oxide cathode and its application in 18650 commercial type Na-ion batteries. J. Mater. Chem. A 2019, 7, 25944–25960. [Google Scholar] [CrossRef]
- Sottmann, J.; Bernal, F.L.; Yusenko, K.V.; Herrmann, M.; Emerich, H.; Wragg, D.S.; Margadonna, S. In operando synchrotron XRD/XAS investigation of sodium insertion into the Prussian Blue Analogue Cathode material Na1.32Mn[Fe(CN)6]0.83·z H2O. Electrochim. Acta 2016, 200, 305–313. [Google Scholar] [CrossRef]
- Xiao, B.; Liu, X.; Chen, X.; Lee, G.H.; Song, M.; Yang, X.; Omenya, F.; Reed, D.M.; Sprenkle, V.; Ren, Y. Uncommon behavior of Li doping suppresses oxygen redox in P2-type manganese-rich sodium cathodes. Adv. Mater. 2021, 33, 2107141. [Google Scholar] [CrossRef]
- Hakim, C.; Sabi, N.; Ma, L.A.; Dahbi, M.; Brandell, D.; Edström, K.; Duda, L.C.; Saadoune, I.; Younesi, R. Understanding the redox process upon electrochemical cycling of the P2-Na0.78Co1/2Mn1/3Ni1/6O2 electrode material for sodium-ion batteries. Commun. Chem. 2020, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- House, R.A.; Maitra, U.; Pérez-Osorio, M.A.; Lozano, J.G.; Jin, L.; Somerville, J.W.; Duda, L.C.; Nag, A.; Walters, A.; Zhou, K.-J. Superstructure control of first-cycle voltage hysteresis in oxygen-redox cathodes. Nature 2020, 577, 502–508. [Google Scholar] [CrossRef]
- Lombardo, T.; Walther, F.; Kern, C.; Moryson, Y.; Weintraut, T.; Henss, A.; Rohnke, M. ToF-SIMS in battery research: Advantages, limitations, and best practices. J. Vac. Sci. Technol. A 2023, 41, 053207. [Google Scholar] [CrossRef]
- Ma, X.H.; Chen, H.L.; Ceder, G. Electrochemical Properties of Monoclinic NaMnO2. J. Electrochem. Soc. 2011, 158, A1307–A1312. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Yoshida, H.; Komaba, S. Crystal Structures and Electrode Performance of Alpha-NaFeO2 for Rechargeable Sodium Batteries. Electrochemistry 2012, 80, 716–719. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Nie, W.Y.; Chen, P.P.; Zhou, Y.F.; Xu, Y. Li-doping stabilized P2-Li0.2Na1.0Mn0.8O2 sodium ion cathode with oxygen redox activity. Int. J. Energ. Res. 2020, 44, 3253–3259. [Google Scholar] [CrossRef]
- Velikokhatnyi, O.I.; Choi, D.; Kumta, P.N. Effect of boron on the stability of monoclinic NaMnO2: Theoretical and experimental studies. Mater. Sci. Eng. B-Solid 2006, 128, 115–124. [Google Scholar] [CrossRef]
- Clément, R.J.; Billaud, J.; Armstrong, A.R.; Singh, G.; Rojo, T.; Bruce, P.G.; Grey, C.P. Structurally stable Mg-doped P2-Na2/3Mn1−yMgyO2 sodium-ion battery cathodes with high rate performance: Insights from electrochemical, NMR and diffraction studies. Energy Environ. Sci. 2016, 9, 3240–3251. [Google Scholar] [CrossRef]
- Nayak, D.; Jha, P.K.; Ghosh, S.; Adyam, V. Aluminium substituted β–type NaMn1-xAlxO2: A stable and enhanced electrochemical kinetic sodium-ion battery cathode. J. Power Source 2019, 438, 227025. [Google Scholar] [CrossRef]
- Sato, T.; Yoshikawa, K.; Zhao, W.; Kobayashi, T.; Rajendra, H.B.; Yonemura, M.; Yabuuchi, N. Efficient stabilization of Na storage reversibility by Ti integration into O′ 3-type NaMnO2. Energy Mater. Adv. 2021, 2021, 9857563. [Google Scholar]
- Choi, J.U.; Park, Y.J.; Jo, J.H.; Kuo, L.Y.; Kaghazchi, P.; Myung, S.T. Unraveling the Role of Earth-Abundant Fe in the Suppression of Jahn-Teller Distortion of P′2-Type Na2/3MnO2: Experimental and Theoretical Studies. Acs Appl. Mater. Interfaces 2018, 10, 40978–40984. [Google Scholar] [CrossRef]
- Hemalatha, K.; Jayakumar, M.; Prakash, A. Influence of the manganese and cobalt content on the electrochemical performance of P2-Na0.67MnxCo1−xO2 cathodes for sodium-ion batteries. Dalton Trans. 2018, 47, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Kalapsazova, M.; Stoyanova, R.; Zhecheva, E.; Tyuliev, G.; Nihtianova, D. Sodium deficient nickel–manganese oxides as intercalation electrodes in lithium ion batteries. J. Mater. Chem. A 2014, 2, 19383–19395. [Google Scholar] [CrossRef]
- Ling, Y.X.; Zhou, J.; Guo, S.; Fu, H.W.; Zhou, Y.F.; Fang, G.Z.; Wang, L.B.; Lu, B.A.; Cao, X.X.; Liang, S.Q. Copper-Stabilized P′2-Type Layered Manganese Oxide Cathodes for High-Performance Sodium-Ion Batteries. Acs Appl. Mater. Interfaces 2021, 13, 58665–58673. [Google Scholar] [CrossRef]
- Wu, X.H.; Xu, G.L.; Zhong, G.M.; Gong, Z.L.; McDonald, M.J.; Zheng, S.Y.; Fu, R.Q.; Chen, Z.H.; Amine, K.; Yang, Y. Insights into the Effects of Zinc Doping on Structural Phase Transition of P2-Type Sodium Nickel Manganese Oxide Cathodes for High-Energy Sodium Ion Batteries. Acs Appl. Mater. Interfaces 2016, 8, 22227–22237. [Google Scholar] [CrossRef] [PubMed]
- Song, B.H.; Hu, E.Y.; Liu, J.; Zhang, Y.M.; Yang, X.Q.; Nanda, J.; Huq, A.; Page, K. A novel P3-type Na2/3Mg1/3Mn2/3O2 as high capacity sodium-ion cathode using reversible oxygen redox. J. Mater. Chem. A 2019, 7, 1491–1498. [Google Scholar] [CrossRef]
- Rong, X.H.; Liu, J.; Hu, E.Y.; Liu, Y.J.; Wang, Y.; Wu, J.P.; Yu, X.Q.; Page, K.; Hu, Y.S.; Yang, W.L.; et al. Structure-Induced Reversible Anionic Redox Activity in Na Layered Oxide Cathode. Joule 2018, 2, 125–140. [Google Scholar] [CrossRef]
- Jin, Y.; Xu, Y.B.; Le, P.M.L.; Vo, T.D.; Zhou, Q.; Qi, X.G.; Engelhard, M.H.; Matthews, B.E.; Jia, H.; Nie, Z.M.; et al. Highly Reversible Sodium Ion Batteries Enabled by Stable Electrolyte-Electrode Interphases. Acs. Energy Lett. 2020, 5, 3212–3220. [Google Scholar] [CrossRef]
- Wang, H.; Liao, X.-Z.; Yang, Y.; Yan, X.; He, Y.-S.; Ma, Z.-F. Large-scale synthesis of NaNi1/3Fe1/3Mn1/3O2 as high performance cathode materials for sodium ion batteries. J. Electrochem. Soc. 2016, 163, A565. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, H.; Harder, R.; Li, L.; Gim, J.; Che, H.; Wang, H.; Ren, Y.; Zhang, X.; Li, L. Revealing the Structural Evolution and Phase Transformation of O3-Type NaNi1/3Fe1/3Mn1/3O2 Cathode Material on Sintering and Cycling Processes. ACS Appl. Energy Mater. 2020, 3, 6107–6114. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, G.-L.; Che, H.; Wang, H.; Yang, K.; Yang, X.; Guo, F.; Ren, Y.; Chen, Z.; Amine, K. Probing thermal and chemical stability of NaxNi1/3Fe1/3Mn1/3O2 Cathode material toward safe sodium-ion batteries. Chem. Mater. 2018, 30, 4909–4918. [Google Scholar] [CrossRef]
- Yao, H.R.; Wang, P.F.; Gong, Y.; Zhang, J.N.; Yu, X.Q.; Gu, L.; OuYang, C.Y.; Yin, Y.X.; Hu, E.Y.; Yang, X.Q.; et al. Designing Air-Stable O3-Type Cathode Materials by Combined Structure Modulation for Na-Ion Batteries. J. Am. Chem. Soc. 2017, 139, 8440–8443. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Ren, W.; Jiang, R.; Li, Q.; Yao, X.; Wang, S.; You, Y.; Mai, L. Highly crystallized Prussian blue with enhanced kinetics for highly efficient sodium storage. ACS Appl. Mater. Interfaces 2021, 13, 3999–4007. [Google Scholar] [CrossRef]
- He, S.; Zhao, J.; Rong, X.; Xu, C.; Zhang, Q.; Shen, X.; Qi, X.; Li, Y.; Li, X.; Niu, Y. Solvent-free mechanochemical synthesis of Na-rich Prussian white cathodes for high-performance Na-ion batteries. Chem. Eng. J. 2022, 428, 131083. [Google Scholar] [CrossRef]
- You, Y.; Wu, X.-L.; Yin, Y.-X.; Guo, Y.-G. High-quality Prussian blue crystals as superior cathode materials for room-temperature sodium-ion batteries. Energy Environ. Sci. 2014, 7, 1643–1647. [Google Scholar] [CrossRef]
- Wang, W.; Gang, Y.; Peng, J.; Hu, Z.; Yan, Z.; Lai, W.; Zhu, Y.; Appadoo, D.; Ye, M.; Cao, Y. Effect of eliminating water in prussian blue cathode for sodium-ion batteries. Adv. Funct. Mater. 2022, 32, 2111727. [Google Scholar] [CrossRef]
- Fadzil, S.S.M.; Woo, H.; Azzahari, A.; Winie, T.; Kufian, M. Sodium-rich prussian blue analogue coated by poly(3,4-ethylenedioxythiophene) polystyrene sulfonate as superior cathode for sodium-ion batteries. Mater. Today Chem. 2023, 30, 101540. [Google Scholar] [CrossRef]
- Adak, S.; Hartl, M.; Daemen, L.; Fohtung, E.; Nakotte, H. Study of oxidation states of the transition metals in a series of Prussian blue analogs using X-ray absorption near edge structure (XANES) spectroscopy. J. Electron Spectrosc. Relat. Phenom. 2017, 214, 8–19. [Google Scholar] [CrossRef]
- Xie, B.; Zuo, P.; Wang, L.; Wang, J.; Huo, H.; He, M.; Shu, J.; Li, H.; Lou, S.; Yin, G. Achieving long-life Prussian blue analogue cathode for Na-ion batteries via triple-cation lattice substitution and coordinated water capture. Nano Energy 2019, 61, 201–210. [Google Scholar] [CrossRef]
- Pasta, M.; Wang, R.Y.; Ruffo, R.; Qiao, R.; Lee, H.-W.; Shyam, B.; Guo, M.; Wang, Y.; Wray, L.A.; Yang, W. Manganese–cobalt hexacyanoferrate cathodes for sodium-ion batteries. J. Mater. Chem. A 2016, 4, 4211–4223. [Google Scholar] [CrossRef]
- You, Y.; Wu, X.L.; Yin, Y.X.; Guo, Y.G. A zero-strain insertion cathode material of nickel ferricyanide for sodium-ion batteries. J. Mater. Chem. A 2013, 1, 14061–14065. [Google Scholar] [CrossRef]
- Gebert, F.; Cortie, D.L.; Bouwer, J.C.; Wang, W.; Yan, Z.; Dou, S.X.; Chou, S.L. Epitaxial Nickel Ferrocyanide Stabilizes Jahn–Teller Distortions of Manganese Ferrocyanide for Sodium-Ion Batteries. Angew. Chem. 2021, 133, 18667–18674. [Google Scholar] [CrossRef]
- Wang, L.; Song, J.; Qiao, R.M.; Wray, L.A.; Hossain, M.A.; Chuang, Y.D.; Yang, W.L.; Lu, Y.H.; Evans, D.; Lee, J.J.; et al. Rhombohedral Prussian White as Cathode for Rechargeable Sodium-Ion Batteries. J. Am. Chem. Soc. 2015, 137, 2548–2554. [Google Scholar] [CrossRef] [PubMed]
- Panin, R.V.; Drozhzhin, O.A.; Fedotov, S.S.; Khasanova, N.R.; Antipov, E.V. NASICON-type NaMo2(PO4)3: Electrochemical activity of the Mo+4 polyanion compound in Na-cell. Electrochim. Acta 2018, 289, 168–174. [Google Scholar] [CrossRef]
- Hadouchi, M.; Yaqoob, N.; Kaghazchi, P.; Tang, M.; Liu, J.; Sang, P.; Fu, Y.; Huang, Y.; Ma, J. Fast sodium intercalation in Na3.41£ 0.59FeV(PO4)3: A novel sodium-deficient NASICON cathode for sodium-ion batteries. Energy Storage Mater. 2021, 35, 192–202. [Google Scholar] [CrossRef]
- Li, S.; Guo, J.; Ye, Z.; Zhao, X.; Wu, S.; Mi, J.-X.; Wang, C.-Z.; Gong, Z.; McDonald, M.J.; Zhu, Z. Zero-strain Na2FeSiO4 as novel cathode material for sodium-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 17233–17238. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Seo, D.H.; Shi, T.; Chen, S.; Tian, Y.; Kim, H.; Ceder, G. A high-energy NASICON-type cathode material for Na-ion batteries. Adv. Energy Mater. 2020, 10, 1903968. [Google Scholar] [CrossRef]
- Qin, B.; Zarrabeitia, M.; Hoefling, A.; Jusys, Z.; Liu, X.; Tübke, J.; Behm, R.J.; Cui, G.; Varzi, A.; Passerini, S. A unique polymer-inorganic cathode-electrolyte-interphase (CEI) boosts high-performance Na3V2(PO4)2F3 batteries in ether electrolytes. J. Power Source 2023, 560, 232630. [Google Scholar] [CrossRef]
- Shin, K.H.; Park, S.K.; Nakhanivej, P.; Wang, Y.; Liu, P.; Bak, S.-M.; Choi, M.S.; Mitlin, D.; Park, H.S. Biomimetic composite architecture achieves ultrahigh rate capability and cycling life of sodium ion battery cathodes. Appl. Phys. Rev. 2020, 7, 041410. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Sun, X.; Zhang, B.; He, S.; Li, L.; Wang, C. Preventing structural degradation from Na3V2(PO4)3 to V2(PO4)3: F-doped Na3V2(PO4)3/C cathode composite with stable lifetime for sodium ion batteries. J. Power Source 2018, 378, 423–432. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Sun, X.; Zhang, B.; He, S.; Wang, C. F-doping and V-defect synergetic effects on Na3V2(PO4)3/C composite: A promising cathode with high ionic conductivity for sodium ion batteries. J. Power Source 2018, 397, 307–317. [Google Scholar] [CrossRef]
- Broux, T.; Bamine, T.; Simonelli, L.; Stievano, L.; Fauth, F.; Ménétrier, M.; Carlier, D.; Masquelier, C.; Croguennec, L. VIV disproportionation upon sodium extraction from Na3V2(PO4)2F3 observed by operando X-ray absorption spectroscopy and solid-state NMR. J. Phys. Chem. C 2017, 121, 4103–4111. [Google Scholar] [CrossRef]
- Iarchuk, A.R.; Sheptyakov, D.V.; Abakumov, A.M. Hydrothermal Microwave-Assisted Synthesis of Na3+xV2-yMny(PO4)2F3 Solid Solutions as Potential Positive Electrodes for Na-Ion Batteries. Acs Appl. Energy Mater. 2021, 4, 5007–5014. [Google Scholar] [CrossRef]
- Gover, R.; Bryan, A.; Burns, P.; Barker, J. The electrochemical insertion properties of sodium vanadium fluorophosphate, Na3V2(PO4)2F3. Solid State Ion. 2006, 177, 1495–1500. [Google Scholar] [CrossRef]
- Liu, Y.M.; Wu, Z.G.; Indris, O.; Hua, W.B.; Casati, N.P.M.; Tayal, A.; Darma, M.S.D.; Wang, G.K.; Liu, Y.X.; Wu, C.J.; et al. The structural origin of enhanced stability of Na3.32Fe2.11Ca0.23(P2O7)2 cathode for Na-ion batteries. Nano Energy 2021, 79, 105417. [Google Scholar] [CrossRef]
- Oyama, G.; Pecher, O.; Griffith, K.J.; Nishimura, S.-I.; Pigliapochi, R.; Grey, C.P.; Yamada, A. Sodium intercalation mechanism of 3.8 V class alluaudite sodium iron sulfate. Chem. Mater. 2016, 28, 5321–5328. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M. Elevating the Practical Application of Sodium-Ion Batteries through Advanced Characterization Studies on Cathodes. Energies 2023, 16, 8004. https://doi.org/10.3390/en16248004
Li M. Elevating the Practical Application of Sodium-Ion Batteries through Advanced Characterization Studies on Cathodes. Energies. 2023; 16(24):8004. https://doi.org/10.3390/en16248004
Chicago/Turabian StyleLi, Mengya. 2023. "Elevating the Practical Application of Sodium-Ion Batteries through Advanced Characterization Studies on Cathodes" Energies 16, no. 24: 8004. https://doi.org/10.3390/en16248004
APA StyleLi, M. (2023). Elevating the Practical Application of Sodium-Ion Batteries through Advanced Characterization Studies on Cathodes. Energies, 16(24), 8004. https://doi.org/10.3390/en16248004





