Abstract
The optimization and advancement of effective catalysts in the oxygen evolution reaction (OER) are integral to the evolution of diverse green power technologies. In this study, cobalt–nitrogen–graphene (Co-N-g) catalysts are analyzed for their OER contribution via density functional theory (DFT). The influence of vacancies and nitrogen doping on catalyst performance was probed via electronic features and related Frontier Molecular Orbitals. The research reveals that the double-vacancy nitrogen-doped catalyst (DV-N4) exhibits remarkable OER effectiveness, characterized by a notably low overpotential of 0.61 V. This is primarily attributed to enhanced metal–ligand bonding interactions, a diminished energy gap indicating augmented reactivity, and advantageous charge redistribution upon water adsorption. Additionally, nitrogen doping is found to facilitate electron loss from Co, thus promoting water oxidation and improving OER performance. This research provides crucial insights into high-performance OER catalyst design, informing future developments in efficient renewable energy devices.
1. Introduction
Electrocatalysts serve a key function within the field of sustainable energy transformation and preservation systems, specifically in devices such as power cells with self-renewing abilities and metal–air batteries with rechargeable properties [1,2]. The oxygen evolution reaction (OER) represents a vital semi-cell process within these technologies. The advancement of high-performance, durable and economically favorable electrocatalysts for OER is crucial to elevate their efficacy and facilitate their broader acceptance [3]. Traditionally, electrocatalysts based on precious metals, including those derived from iridium and ruthenium, have been used in OER, due to their remarkable catalytic performance and resilience. However, their limited availability and substantial expense restrict their application on a larger scale, hence stimulating the exploration of substitutes that are more readily available and economically viable [4].
Materials centered around transition metals, specifically those that include elements such as cobalt, iron and nickel, and perovskite-based composites, have surfaced as hopeful replacements for electrocatalysts based on noble metals in relation to OER [5,6,7]. Recent advances in nanoscience have demonstrated that the electrocatalytic capabilities of these substances might be further improved by regulating their dimensions, structural characteristics, and makeup at the nanoscale [8,9]. Additionally, hybrid materials that combine transition-metal-based catalysts with carbon-based supports, such as graphene, have shown great potential in improving the catalytic performance for OER, due to their enhanced charge transfer properties and increased active sites [10,11].
Graphene, a planar carbon compound with exceptional electronic, mechanical and thermal attributes, has paved the way for advancements in electrocatalyst designs [12]. Specifically, nitrogen-infused graphene has demonstrated potential as a metal-independent electrocatalyst for OER. This is because the integration of nitrogen atoms can adjust the electronic framework and establish active zones for water attachment and oxidation [13,14]. Furthermore, nitrogen-infused graphene can also act as an efficient base for metal-derived catalysts, fostering robust interactions between the metal and the support, and enhancing charge transfer characteristics [15,16,17].
Cobalt-based materials, including oxides (e.g., perovskite-based oxides), sulfides and phosphides, are often researched as OER electrocatalysts, due to their ample presence on Earth, considerable stability and satisfactory catalytic performance [18,19,20]. Current research indicates that amalgamating cobalt-based substances with nitrogen-infused graphene can result in improved OER electrocatalytic behavior [21,22,23]. For instance, Liang and colleagues reported that a composite material, composed of Co3O4 nanocrystals developed on diminished graphene oxide, showcases comparable OER behavior but superior durability, when compared to Pt [21]. In distinct research, Wang and colleagues proved that nitrogen-infused graphene, garnished with cobalt-centered nanoparticles, showcases excellent OER behavior and resilience, due to the electric interaction involving Co and pyridinic-N integrated into graphene [24]. Additionally, cobalt sulfide and phosphide nanoparticles have also been effectively amalgamated with nitrogen-infused graphene, demonstrating enhanced OER electrocatalytic performance [25,26].
Recent research illustrates that the electronic engagement between cobalt nanoparticles and pyridinic-N-infused graphene is crucial in enhancing the OER activity of cobalt-centric materials integrated with nitrogen-infused graphene. Wang et al. [27] showed that the powerful association between cobalt and pyridinic-N-infused graphene prompts a significant decrease in the Co oxidation state, resulting in the emergence of highly reactive Co species for OER. In another study, Kim et al. [28] revealed that charge displacement near the Co (fcc)/N-Gr surface drastically enhances the catalytic efficiency and specific Cl− avoidance in a seawater electrolyte, as derived from DFT modelling, showcasing the potential for better electrochemical performance. Additionally, Chu and his team [29] managed to synthesize a nitrogen-infused graphene catalyst with Co embedded, and reported that the N dopant heightened the positive charge density on nearby carbon atoms, thereby advancing OER. Moreover, the interplay between layers of pyridinic N-integrated graphene and Co improves the electric state of reactive carbon units adjacent to the Fermi level, enhancing OER performance, as confirmed in previous research [30]. These insights provide valuable knowledge on the atomic-level mechanisms governing catalytic behavior in hybrid electrocatalysts and suggest we can be optimistic about this direction of development towards the systematic creation and construction of effective OER catalysts.
Even though these results are promising, we still lack a full interpretation of the intrinsic process that improves OER performance in cobalt-centric materials carried by nitrogen-doped graphene. Studies that delve deeper into the electronic structure, adsorption behaviors, and reaction pathways at an atomic scale are crucial to fine-tune the creation and development of these hybrid electrocatalysts. The usage of density functional theory (DFT) computations has emerged as a key methodology for generating these vital insights [6,11,25].
In this study, DFT is utilized to systematically explore the joint impact of cobalt nanoparticles and nitrogen-infused graphene on the OER. The focus extends beyond merely understanding the interplay of these components; it probes into the electronic structure, including aspects like changes in Gibbs free energy, location of the d-band center, exploration of Projected State Densities (PDOS), assessment of the Overlapping Population in Crystal Orbitals (COOP) and analysis of Frontier Molecular Orbitals. Furthermore, adsorption behaviors and atomic-level reaction pathways are closely examined, to acquire a comprehensive understanding of their enhanced OER function. By evaluating these elements that are pivotal to catalytic efficiency, our study transcends a mere theoretical exploration; we aspire to offer actionable insights and principles for the thoughtful creation and development of advanced cobalt-centric electrocatalysts carried by nitrogen-doped graphene. These catalysts are expected to offer superior OER performance, contributing to the wider progression of renewable energy applications.
2. Computational Details
2.1. DFT Calculation
In this study, DFT operations were conducted using the Vienna Ab initio Simulation Toolkit (VASP) [31]. The explanation for exchange–correlation interactions relied on the generalized gradient approach (GGA), integrating the Perdew–Burke–Ernzerhof (PBE) function [32]. To manage the significantly interrelated Co-3d electrons, the application of a DFT+U method was adopted, settling on a Hubbard U parameter of Ueff = 3.5 eV for the Co atom, aligning with the approach detailed by Cai et al. [33]. An energy cut-off at 500 eV was selected for the plane-wave basis set used in the study, establishing the energy and force thresholds at 1 × 10−6 eV/Å and 0.01 eV/Å, respectively [34]. The Brillouin zone was sampled in all calculations via a 2 × 2 × 1 Monkhorst–Pack k-point grid [35]. To safeguard against overlap amongst recurring images, a vacuum layer of 15 Å was introduced along the z-axis. The chosen substrate for the catalyst was a 4 × 4 graphene supercell. In the present exploration, four distinct catalyst configurations were thoroughly examined, namely Co-embedded single-defect graphene (SD-GN), Co-embedded single-defect graphene with nitrogen tri-substitution (SD-N3), Co-embedded dual-defect graphene (DV-GN), and Co-embedded dual-defect graphene with nitrogen quartet substitution (DV-N4), as graphically presented in Figure 1a–d, correspondingly.
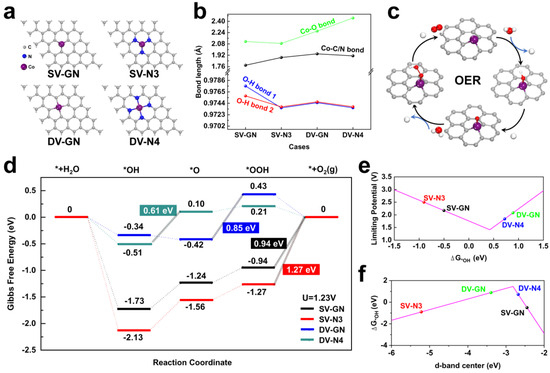
Figure 1.
Catalyst structure and OER performance. (a) Conceptual sketch of SV-GN, SV-N3, DV-GN and DV-N4 catalysts. Grey, brown and blue spheres correspond to carbon, cobalt and nitrogen constituents, respectively. (b) Evaluation of Co-O and O-H bond dimensions in H2O attached to catalysts. (c) Completed reaction cycle for the OER. (d) Depiction of Gibbs energy landscape for the OER at a potential equivalent to 1.23 V against NHE. Black, red, blue and green are indicative of SV-GN, SV-N3, DV-GN and DV-N4, respectively (Symbol * denotes the specified substrate). Correlations represented via volcano plots between: (e) ORR limiting potential and ΔG*OH at a potential equivalent to 1.23 V versus NHE; and (f) ΔG*OH and the index of the d-band center.
The computation of Gibbs free energy (ΔG) was carried out by employing the following formula [36]:
where ΔEDFT signifies the total energy variation between a particular substrate with adsorbed species (*O, *OH and *OOH) and its substrate. ΔZPE stands for the energy related to the vibrational frequencies of adsorbed species at the absolute thermal condition (T = 298.15 K). ΔS is indicative of entropy alteration, which is ignored and its contribution combined with the value of ∆ZPE.
Estimation of the d-band center occurred through mapping the projected density of states (DOS) onto the Co atom’s d-orbitals, as shown in the following equation:
where nd(ε) represents the PDOS corresponding to the d-orbitals of the Co atoms, and ε is the energy.
2.2. Molecular Orbital Calculation
Molecular orbitals of water (H2O) along with the computation of each catalyst structure’s highest occupied molecular orbital (HOMO) plus lowest unoccupied molecular orbital (LUMO) transpired via the Gaussian 09W program [37]. Investigation progress was enabled via B3LYP exchange–correlation functional interaction alongside a 6-311G basic function set. A comprehensive optimization was executed for all structures, and it was affirmed through vibration analysis that these optimized structures represent the authentic energy minima on the potential energy landscape.
3. Results and Discussion
3.1. Structure of H2O Adsorption
In this investigation, Co-N-g catalysts were successfully constructed with controlled Co- and N-doping sites. As shown in Figure 1a, the optimized Co-N-g catalysts present a centrosymmetric structure, with Co located at the center of the vacancies. The lengths of bonds between Co and O, Co and C/N, as well as O and H in the H2O adsorbed on the catalysts were evaluated. These are depicted in Figure 1b and Figure S1. The shortest Co-O bond length was observed for SV-N3, while the longest was for DV-N4. The Co-C/N bond length was shortest for SV-GN and gradually increased, with DV-GN and DV-N4 having comparable Co-C/N bond lengths. The longest O-H bond length was observed for SV-GN, while the shortest was for SV-N3 and DV-N4, with DV-GN in between. It was also observed that the bond angles between the O-H and Co-O bonds varied. The height of the Co atom based on the central line of graphene in SV-GN, SV-N3, DV-GN and DV-N4 sequentially decreased from 0.08 Å to 0.00 Å, indicating that DV-N4 was better embedded within the graphene.
3.2. OER Performance
Figure 1c delineates the electron transport phases throughout the four-electron pathway of OER. The catalysts partake in a succession of redox stages, each involving electron relocation. In the initial phase, a water molecule (*H2O) is adsorbed onto the catalyst, subsequently undergoing oxidation to produce a hydroxyl entity (*OH). Further oxidation of this hydroxyl unit yields an oxygen constituent (*O). This then interacts with another water molecule, culminating in the formation of a hydroperoxyl aggregate (*OOH). In the final step, the *OOH compound is subject to further oxidation, generating an oxygen molecule (*O2). By implementing the aforementioned OER processes, alterations in Gibbs energy during the attachment of *H2O, *OH, *O, *OOH and *O2 on Co-N-g catalysts at an electrochemical potential of 1.23 V are shown in Figure 1d. The exothermic conversion of *H2O to *OH led to a decline in Gibbs energy for all catalysts, with SV-GN and SV-N3 displaying a more significant decrease compared to DV-GN and DV-N4. The transformation of *OH to *O indicated a drop in Gibbs energy for the DV-N4 catalyst, while for the remaining catalysts, an upward shift was observed. The progression from *O to *OOH is endothermic in nature, therefore resulting in a rise in Gibbs energy. During the conversion of *OOH to *O2, a surge in Gibbs energy was seen for SV-GN and SV-N3, while a reduction was noted for DV-GN and DV-N4. The steps that determined the rate, that is, the maximum endothermic steps, for SV-GN, SV-N3, DV-GN and DV-N4, were *OOH->*O2, *OOH->*O2, *O->*OOH and *OH->*O, with changes in Gibbs energy of 0.94 eV, 1.27 eV, 0.85 eV and 0.61eV, respectively. These observations suggest that DV-N4 possesses superior OER catalytic performance.
The d-band center positioning of the Co atom is intimately connected to the Fermi level and the distribution of electrons, which can exert an impact on the binding robustness [38]. Figure 1e,f show volcano diagrams illustrating the association between ORR limiting potential and ΔG*OH at an electrochemical potential of 1.23 V vs. NHE, and between ΔG*OH and the metric of the d-band center, respectively. The respective d-band center metrics are recorded in Table S1. It is widely acknowledged that a shift of the d-band center towards the Fermi level can bolster the bond’s robustness with adsorbates. In the catalysts DV-N4 and SV-GN, with their lower d-band centers, proximity to the Fermi level correlates with enhanced binding energy between Co and OH. Hence, DV-N4 is nearest to the apex of both volcano diagrams, denoting optimal binding robustness between Co and the graphene substrate, and the optimized limiting potential. These diagrams, coupled with the findings from Figure 1d, imply that the ORR limiting potential and ΔG*OH, as well as ΔG*OH and the d-band center, play pivotal roles in determining the OER activity of these Co-N-g catalysts.
3.3. Electronic Characteristics
The electronic characteristics of the catalysts, encompassing the electronic localization function (ELF) and the variation in charge density, were studied to comprehend the interplay between the atoms of the catalyst and the water molecules adsorbed onto them. Figure 2a displays the ELF of the catalysts. ELF values lie between 0 and 1, where a higher number signifies a greater likelihood of locating an electron within a specific region. For the catalysts with single vacancies, SV-GN and SV-N3, the ELF values for the Co-N bond were markedly superior to those for the Co-C bond, indicating a more potent interaction between Co and N atoms in these catalysts. Conversely, for the catalysts with double vacancies, DV-GN and DV-N4, the ELF values for Co-N and Co-C bonds were similar. This disparity in ELF values may be attributed to the differing structural arrangements of the single-vacancy and double-vacancy catalysts. This observation is contrary to the findings of [39], which documented a higher oxidation state for Fe single atoms in coordination with N than those in coordination with C.
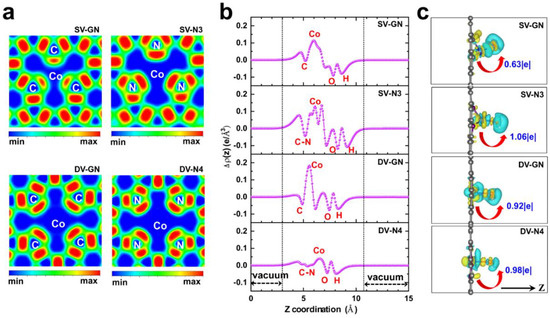
Figure 2.
Electronic characteristics. (a) ELF (electronic localization function) of catalysts. (b) Planar-average charge-density difference, and (c) charge density difference of H2O adsorbed on catalysts. Yellow signifies charge accumulation, while green indicates depletion. Red arrow indicates electron transfer from Co atom to H2O adsorbate, |e| represents Bader charge of Co atom.
As depicted in Figure 2b,c, the variation in charge density offers a clear representation of the electron movement and alterations in electron density subsequent to the interplay between the atomic fragments of the catalyst. It was noted that electrons were transferred from Co to neighboring C/N or O atoms. The results of the planar-average charge-density difference in Figure 2c reveal both electron accumulation and reduction in the Co boundary region (spanning 4–11 Å in the Z direction). A considerable degree of electron accumulation was noted close to the O or C/N atoms, followed by a marked charge depletion near the Co atom. This implies that the interaction with water molecules provokes a reorganization of the electron density in the catalysts, which might impact their catalytic efficacy.
3.4. Bader Charge Analysis and Electronic Structure
The influence of N doping on promoting electron departure from Co and the consequent oxidation of H2O is depicted in the analysis. The Bader charge analysis offers a detailed view of the charge apportioned to the O and Co atoms in Co-N-g catalysts. From SV-GN’s +0.50|e| to DV-N4’s +0.95|e|, the Bader charges (see Table S1) of Co atoms escalated before the adsorption of H2O. However, as represented in Figure 3a, the catalysts without N doping (SV-GN and DV-GN) demonstrate the most positive and most negative shift, respectively, in O’s Bader charge upon H2O adsorption. In contrast, the N-doped catalysts (SV-N3 and DV-N4) exhibit an O Bader charge that falls in between. An increased positive Bader charge on an O atom may suggest an intensified oxidation. On adsorption of H2O, the Bader charge of Co in all catalysts undergoes a positive shift (see Figure 3b and Table S1), implying that Co cedes electrons, thereby enabling the oxidation of O in H2O. This effect is accentuated in the N-doped catalysts, indicating the augmenting role of N doping in the extraction of electrons from Co and the subsequent H2O oxidation.
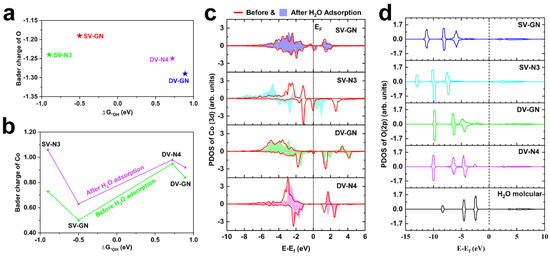
Figure 3.
Analysis of Bader charge and electronic structure. The relationships of the Bader charges of (a) O and (b) Co with ΔG*OH at U = 1.23 V vs. NHE, respectively. (c) PDOS of Co-3d states before (red) and after H2O adsorption on the catalyst surface. (d) PDOS of O-2p states for molecular H2O and the catalysts with adsorbed H2O.
In the PDOS examination (see Figure S2), a pronounced hybridization peak proximate to the Fermi energy demarcation emerges in catalysts enriched by nitrogen (SV-N3 and DV-N4), a result that can be linked with the integration of nitrogen constituents. Significantly, the Co-3d and O-2p orbitals have a crucial function during the catalytic adsorption phase. As Figure 3c highlights, with H2O adsorption, the vanishing of the Co-3d hybridization peak adjacent to the Fermi level in SV-GN, SV-N3 and DV-GN becomes apparent, indicating an electron redistribution within the Co-3d orbitals. Nevertheless, the number and position of Co-3d hybridization peaks in DV-N4 are largely maintained.
In the analysis of the PDOS of O-2p states with H2O adsorbed on the catalysts (see Figure 3d), the emergence of a mild hybridization peak between Co-3d and O-2p states near E-Ef = −0.25 eV is noted in the case of both DV-GN and DV-N4. A further analysis comparing the Co-3d and O-2p states during H2O adsorption (see Figure S3) showcases an energy coincidence at E-Ef = −0.25 eV specifically for the DV-N4 catalyst. These marked degrees of hybridization and concurrent energy states mainly contribute to the potent attraction of H2O experienced by the Co-N-g catalysts.
To gain insights into the bonding and anti-bonding interactions between O-Co and O-H within Co-N-g catalysts, the projected COHP were investigated. The pCOHP analysis for both H2O and Co-N-g catalysts with adsorbed H2O is provided in Figure 4a,b. As per the evaluation, negative quantities correspond to states of anti-bonding, while bonding states are denoted by positive values. The occurrence of bonding states takes place below the Fermi point, in the region of the valence band, whereas most anti-bonding states lie over the Fermi point, within the conduction band. For the H2O molecule, the molecular orbitals relevant to O-2p and O-2s states vary from lower to higher energy, and they correspond to σ2s, π, π, π*, π* and σ* (Figure 4a). Here, π* and σ* symbolize anti-bonding orbitals, as displayed in the orbital interaction diagram for the H2O molecule (Figure 4d). The anti-bonding states (Co-O and O-H) appear to be diminished in DV-GN and DV-N4 when contrasted with the single-vacancy catalysts SV-GN and SV-N3. This decrease in anti-bonding states promotes stronger bond formation. The total COHP (ICOHP), which is derived from the COHP integration (Figure 4c), suggests that the Co-O and O-H bonds in the DV-N4 catalyst exhibit the highest ICOHP values, corresponding to weaker bond strengths. However, the opposite trend is observed in the SV-N3 catalyst.
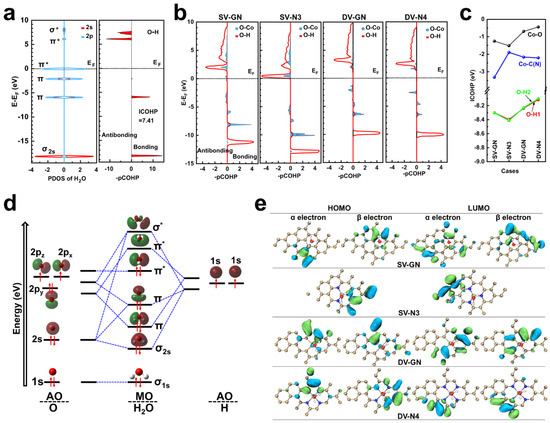
Figure 4.
pCOHP and Frontier Molecular Orbital examination. (a) PDOS of 2s- and 2p-states, and pCOHP for molecular H2O. (b) pCOHP and (c) ICOHP of Co-O (black), Co-C(N) (blue), O-H1 (red) and O-H1 (green) associated with catalysts featuring adsorbed H2O on the surface. (d) Diagram illustrating the orbital interaction of molecular H2O. Orbital energy levels are represented as solid bars. The bars on the left and right sides correspond to the AOs. The bars in the middle correspond to the complex molecular orbitals of H2O. The red arrows indicate electron spin direction: upward for up spin, downward for down spin. (e) Spatial distribution pertaining to α and β electrons’ foremost occupied and lowest unoccupied molecular orbitals on catalysts with surface-adsorbed H2O. In (d,e), the H, O, N, C and Co atoms are colored white, red, blue, silvery and grey, respectively. Green and brown, cyan and azure, indicate different signs of the orbital wave function. In (a,d), * symbolizes anti bonding molecular orbitals.
3.5. Analysis of Frontier Molecular Orbitals
The LUMO and HOMO serve as reliable indicators for assessing the chemical reactivity of a particular system [40]. Assessment of the HOMO and LUMO dispersal in Co-N-g catalysts during H2O adsorption was undertaken (Figure 4e). It can be observed that LUMO and HOMO are more diffused over the surface of double-vacancy catalysts compared to their counterparts in single-vacancy catalysts. Both LUMO and HOMO are predominantly positioned near the periphery of graphene, that has been infused with Co/N adjacent to the graphene defect. When C is replaced by N, LUMO clearly shifts towards the opposing side of the Co-N-g catalyst, denoting that atomic charge reshuffling can result in alterations in the molecular orbitals’ distribution.
A smaller HOMO-LUMO gap indicates that less energy is required for an electron to jump from the HOMO to the LUMO, typically resulting in higher reactivity. Accordingly, the HOMO, LUMO and HOMO-LUMO gap were computed for a variety of Co-N-g catalysts upon H2O adsorption (see Table S2). For SV-N3, being devoid of unpaired electrons, it is considered a closed-shell system, so α and β electrons were not evaluated separately. Excluding SV-N3, the HOMO-LUMO gap narrows from the maximum 8.66 eV for SV-GN to the minimum 7.28 eV for DV-N4. This narrower HOMO-LUMO gap contributes to the heightened catalytic activity of the DV-N4 catalyst. Interestingly, despite SV-N3 having a low HOMO-LUMO gap, our earlier ICOHP calculation (see Figure 4c) demonstrates that SV-N3 exhibits the strongest bond strengths, as reflected by the lowest ICOHP values. This might be a contributing factor to the reduced catalytic activity of SV-N3.
4. Conclusions
In this investigation, DFT was employed to comprehensively evaluate an array of Co-N-g catalysts. The findings reveal that the nitrogen-doped catalyst with a double vacancy (DV-N4) exhibited exceptional performance in the oxygen evolution reaction (OER) about limiting potential 0.61 V. Evidence for the superior catalytic prowess of DV-N4 was gathered through the analysis of Gibbs free energy alterations and the assessment of the d-band center positioning of Co atoms. COHP analyses further confirmed the catalytic superiority of DV-N4, demonstrating that it has strong metal–ligand bonding interactions, indicative of a more stable catalyst structure. Our Frontier Molecular Orbital analysis indicated a smaller energy gap for DV-N4, which suggests a higher catalytic activity. The observed redistribution of electron density in the catalysts upon water adsorption could influence catalytic activities. Additionally, Bader charge analysis showed that nitrogen doping promotes electron loss from Co and enhances the oxidation of water, thereby improving OER performance. The results revealed the crucial function of nitrogen doping in boosting electron acquisition from cobalt active catalytic sites, instigating water’s oxidation process. The overpotential of the OER for DV-N4 is the lowest, standing at 0.61 V.
The potential for experimental synthesis of the DV-N4 catalyst was previously reported by Han et al. [41] and Wu et al. [42]. The current study’s findings underscore the value of doping and vacancy engineering in designing high-performance OER catalysts and enhances our understanding of Co-N-g catalysts’ performance in electrochemical reactions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en16247981/s1, Figure S1: Bond lengths and bond angles of the stable Co-N-g catalyst of (a) SV-GN, (b) SV-N3, (c) DV-GN, and (d) DV-N4; Figure S2: PDOS of specified elements C (blue), Co (red) and N (green), for Co-N-g catalyst of (a) SV-GN, (b) SV-N3, (c) DV-GN, and (d) DV-N4; Figure S3: PDOS of Co-3d (blue) and O-2p states (red) after H2O adsorption on the catalyst surface; Table S1: Bader charge and d-band center values of SV-GN, SV-N3, DV-GN, and DV-N4 catalysts with H2O adsorbed; Table S2: HOMO, LUMO, and HOMO-LUMO gapa of α electrons and β electrons for SV-GN, SV-N3, DV-GN, and DV-N4 catalysts with H2O adsorbed. The unit is eV.
Author Contributions
Conceptualization, J.W. and C.Y.; Methodology, Y.W.; Software, J.W., H.H. and R.L.; Formal analysis, H.H.; Investigation, J.W., H.H. and C.Y.; Data curation, H.Z.; Writing—original draft, J.W.; Writing—review & editing, J.W., W.C. and H.Z.; Visualization, J.W., H.H. and R.L.; Supervision, W.C., H.Z. and H.C.; Project administration, C.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China under grants numbers 21875224 and 52101320.
Data Availability Statement
Data is unavailable due to privacy or ethical restrictions.
Acknowledgments
This study was conducted using the high-performance computing platform of China University of Geosciences (Wuhan) and was supported by the National Natural Science Foundation of China under grants numbers 21875224 and 52101320.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yaqoob, L.; Noor, T.; Iqbal, N.; Nasir, H.; Sohail, M.; Zaman, N.; Usman, M. Nanocomposites of cobalt benzene tricarboxylic acid MOF with rGO: An efficient and robust electocatalyst for oxygen evaluation reaction (OER). Renew. Energy 2020, 156, 1040–1054. [Google Scholar] [CrossRef]
- Luque-Centeno, J.M.; Martinez-Huerta, M.V.; Sebastian, D.; Lemes, G.; Pastor, E.; Lazaro, M.J. Bifunctional N-doped graphene Ti and Co nanocomposites for the oxygen reduction and evolution reactions. Renew. Energy 2018, 125, 182–192. [Google Scholar] [CrossRef]
- Ghouri, Z.K.; Elsaid, K.; Nasef, M.M.; Badreldin, A.; Wubulikasimu, Y.; Abdel-Wahab, A. Incorporation of manganese carbonyl sulfide ((Mn2S2 (CO)7) and mixed metal oxides-decorated reduced graphene oxide (MnFeCoO4/rGO) as a selective anode toward efficient OER from seawater splitting under neutral pH conditions. Renew. Energy 2022, 190, 1029–1040. [Google Scholar] [CrossRef]
- Chen, X.; Lin, S.Y.; Zhang, H. Screening of single-atom catalysts sandwiched by boron nitride sheet and graphene for oxygen reduction and oxygen evolution. Renew. Energy 2022, 189, 502–509. [Google Scholar] [CrossRef]
- Xu, X.M.; Pan, Y.L.; Ge, L.; Chen, Y.B.; Mao, X.; Guan, D.Q.; Li, M.R.; Zhong, Y.J.; Hu, Z.W.; Peterson, V.K.; et al. High-Performance Perovskite Composite Electrocatalysts Enabled by Controllable Interface Engineering. Small 2021, 17, 2101573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Li, J.; Yang, Y.; Zhang, S.; Zhu, H.S.; Zhu, X.Q.; Xing, H.H.; Zhang, Y.L.; Huang, B.L.; Guo, S.J.; et al. Co3O4/Fe0.33Co0.66P Interface Nanowire for Enhancing Water Oxidation Catalysis at High Current Density. Adv. Mater. 2018, 30, 1803551. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tu, W.G.; Zhang, B.W.; Yin, S.M.; Huang, Y.Z.; Kraft, M.; Xu, R. Nickel Nanoparticles Encapsulated in Few-Layer Nitrogen-Doped Graphene Derived from Metal–Organic Frameworks as Efficient Bifunctional Electrocatalysts for Overall Water Splitting. Adv. Mater. 2017, 29, 1605957. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.W.; Zheng, D.; Liu, D.; Harris, J.; Si, J.Y.; Ding, T.Y.; Qu, D.Y. Highly Efficient Ni-Fe Based Oxygen Evolution Catalyst Prepared by A Novel Pulse Electrochemical Approach. Electrochim. Acta 2017, 247, 722–729. [Google Scholar] [CrossRef]
- Xu, X.M.; Wang, W.; Zhou, W.; Shao, Z.P. Recent Advances in Novel Nanostructuring Methods of Perovskite Electrocatalysts for Energy-Related Applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Qu, L.T.; Liu, Y.; Baek, J.-B.; Dai, L.M. Nitrogen-Doped Graphene as Efficient Metal-Free Electrocatalyst for Oxygen Reduction in Fuel Cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef]
- Cui, X.J.; Ren, P.J.; Deng, D.H.; Deng, J.; Bao, X.H. Single layer graphene encapsulating non-precious metals as high-performance electrocatalysts for water oxidation. Energy Environ. Sci. 2016, 9, 123–129. [Google Scholar] [CrossRef]
- Ghuge, A.D.; Shirode, A.R.; Kadam, V.J. Graphene: A comprehensive review. Curr. Drug Targets 2017, 18, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.K.; Li, J.; Yang, M.J.; Fang, Z.S.; Jian, J.H.; Yu, D.S.; Chen, X.D.; Dai, L.M. Ultrathin Black Phosphorus-on-Nitrogen Doped Graphene for Efficient Overall Water Splitting: Dual Modulation Roles of Directional Interfacial Charge Transfer. J. Am. Chem. Soc. 2019, 141, 4972–4979. [Google Scholar] [CrossRef]
- Li, X.Y.; Su, Z.H.; Zhao, Z.F.; Cai, Q.H.; Li, Y.F.; Zhao, J.X. Single Ir atom anchored in pyrrolic-N4 doped graphene as a promising bifunctional electrocatalyst for the ORR/OER: A computational study. J. Colloid Interf. Sci. 2022, 607, 1005–1013. [Google Scholar] [CrossRef]
- Lee, W.J.; Maiti, U.N.; Lee, J.M.; Lim, J.; Han, T.H.; Kim, S.O. Nitrogen-doped carbon nanotubes and graphene composite structures for energy and catalytic applications. Chem. Commun. 2014, 50, 6818–6830. [Google Scholar] [CrossRef]
- Li, S.; Yang, Y.; Liu, L.; Zhao, Q. Electron transfer-induced catalytic enhancement over bismuth nanoparticles supported by N-doped graphene. Chem. Eng. J. 2018, 334, 1691–1698. [Google Scholar] [CrossRef]
- Wu, X.J.; Feng, B.M.; Li, W.; Niu, Y.L.; Yu, Y.N.; Lu, S.Y.; Zhong, C.Y.; Liu, P.Y.; Tian, Z.Q.; Chen, L.; et al. Metal-support interaction boosted electrocatalysis of ultrasmall iridium nanoparticles supported on nitrogen doped graphene for highly efficient water electrolysis in acidic and alkaline media. Nano Energy 2019, 62, 117–126. [Google Scholar] [CrossRef]
- Tang, J.H.; Xu, X.M.; Tang, T.; Zhong, Y.J.; Shao, Z.P. Perovskite-Based Electrocatalysts for Cost-Effective Ultrahigh-Current-Density Water Splitting in Anion Exchange Membrane Electrolyzer Cell. Small Methods 2022, 6, 2201099. [Google Scholar] [CrossRef]
- Deng, X.H.; Tüysüz, H. Cobalt-Oxide-Based Materials as Water Oxidation Catalyst: Recent Progress and Challenges. ACS Catal. 2014, 4, 3701–3714. [Google Scholar] [CrossRef]
- Zhao, R.G.; Ni, B.X.; Wu, L.M.; Sun, P.C.; Chen, T.H. Carbon-based iron-cobalt phosphate FeCoP/C as an effective ORR/OER/HER trifunctional electrocatalyst. Colloids. Surf. A. 2022, 635, 128118. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Li, Y.G.; Wang, H.L.; Zhou, J.G.; Wang, J.; Regier, T.; Dai, H.J. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef]
- Hou, Y.; Wen, Z.H.; Cui, S.M.; Ci, S.Q.; Mao, S.; Chen, J.H. An Advanced Nitrogen-Doped Graphene/Cobalt-Embedded Porous Carbon Polyhedron Hybrid for Efficient Catalysis of Oxygen Reduction and Water Splitting. Adv. Funct. Mater. 2015, 25, 872–882. [Google Scholar] [CrossRef]
- Singh, A.K.; Ji, S.; Singh, B.; Das, C.; Choi, H.; Menezes, P.W.; Indra, A. Alkaline oxygen evolution: Exploring synergy between fcc and hcp cobalt nanoparticles entrapped in N-doped graphene. Mater. Today Chem. 2022, 23, 100668. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, H.H.; Estudillo-Wong, L.A.; Li, H.Y.; Alonso-Vante, N.; Li, D.Q.; Tang, P.G.; Feng, Y.J. Synthesis and electrocatalytic performance of N-doped graphene embedded with Co/CoO nanoparticles towards oxygen evolution and reduction reactions. Catal. Commun. 2022, 164, 106428. [Google Scholar] [CrossRef]
- Zhang, J.K.; Cui, B.L.; Jiang, S.; Liu, H.T.; Dou, M.L. Construction of three-dimensional cobalt sulfide/multi-heteroatom co-doped porous carbon as an efficient trifunctional electrocatalyst. Nanoscale 2022, 14, 9849–9859. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.F.; Lin, S.M.; Yu, Y.; Meng, F.Y.; Du, G.H.; Xu, B.S. In situ phosphating Co@Nitrogen-doping graphene boosts overall water splitting under alkaline condition. J. Electroanal. Chem. 2022, 904, 115882. [Google Scholar] [CrossRef]
- Wang, H.B.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Kim, S.; Ji, S.; Yang, H.; Son, H.; Choi, H.; Kang, J.; Li, O.L. Near surface electric field enhancement: Pyridinic-N rich few-layer graphene encapsulating cobalt catalysts as highly active and stable bifunctional ORR/OER catalyst for seawater batteries. Appl. Catal. B. 2022, 310, 121361. [Google Scholar] [CrossRef]
- Chu, W.H.; Yu, Y.; Sun, D.F.; Qu, Y.N.; Meng, F.Y.; Qiu, Y.Y.; Lin, S.M.; Huang, L.Y.; Ren, J.; Su, Q.M.; et al. Uniform cobalt nanoparticles embedded in nitrogen-doped graphene with abundant defects as high-performance bifunctional electrocatalyst in overall water splitting. Int. J. Hydrog. Energy 2022, 47, 21191–21203. [Google Scholar] [CrossRef]
- Gao, Y.; Kong, D.B.; Cao, F.L.; Teng, S.; Liang, T.; Luo, B.; Wang, B.; Yang, Q.-H.; Zhi, L.J. Synergistically tuning the graphitic degree, porosity, and the configuration of active sites for highly active bifunctional catalysts and Zn-air batteries. Nano Res. 2022, 15, 7959–7967. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B. 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Bi, Y.M.; Hu, E.Y.; Liu, W.; Dwarica, N.; Tian, Y.; Li, X.L.; Kuang, Y.; Li, Y.P.; Yang, X.-Q.; et al. Single-Crystalline Ultrathin Co3O4 Nanosheets with Massive Vacancy Defects for Enhanced Electrocatalysis. Adv. Energy Mater. 2018, 8, 1701694. [Google Scholar] [CrossRef]
- Thang, H.V.; Pacchioni, G. Oxygen Vacancy in Wurtzite ZnO and Metal-Supported ZnO/M(111) Bilayer Films (M = Cu, Ag and Au). J. Phys. Chem. C. 2018, 122, 20880–20887. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B. 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Li, M.T.; Zhang, L.P.; Xu, Q.; Niu, J.B.; Xia, Z.H. N-doped graphene as catalysts for oxygen reduction and oxygen evolution reactions: Theoretical considerations. J. Catal. 2014, 314, 66–72. [Google Scholar] [CrossRef]
- Mamand, D. Theoretical calculations and spectroscopic analysis of gaussian computational examination-NMR, FTIR, UV-Visible, MEP on 2, 4, 6-Nitrophenol. J. Phys. Chem. Funct. Mater. 2019, 2, 77–86. [Google Scholar]
- Liu, M.J.; Lee, J.; Yang, T.C.; Zheng, F.Y.; Zhao, J.; Yang, C.M.; Lee, L.Y.S. Synergies of Fe Single Atoms and Clusters on N-Doped Carbon Electrocatalyst for pH-Universal Oxygen Reduction. Small Methods 2021, 5, 2001165–2001174. [Google Scholar] [CrossRef]
- Yang, J.; Fan, Y.; Liu, P.F. Theoretical insights into heterogeneous single-atom Fe1 catalysts supported by graphene-based substrates for water splitting. Appl. Surf. Sci. 2020, 540, 148245. [Google Scholar] [CrossRef]
- Zhang, L.P.; Xia, Z.H. Mechanisms of Oxygen Reduction Reaction on Nitrogen-Doped Graphene for Fuel Cells. J. Phys. Chem. C. 2011, 115, 11170–11176. [Google Scholar] [CrossRef]
- Han, Y.H.; Wang, Y.G.; Chen, W.X.; Xu, R.R.; Zheng, L.R.; Zhang, J.; Luo, J.; Shen, R.A.; Zhu, Y.Q.; Cheong, W.C.; et al. Hollow N-Doped Carbon Spheres with Isolated Cobalt Single Atomic Sites: Superior Electrocatalysts for Oxygen Reduction. J. Am. Chem. Soc. 2017, 139, 17269–17272. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Pan, C.; He, C.T.; Han, Y.H.; Ma, W.J.; Wei, H.; Ji, W.L.; Chen, W.X.; Mao, J.J.; Yu, P.; et al. Single-Atom Co-N4 Electrocatalyst Enabling Four-Electron Oxygen Reduction with Enhanced Hydrogen Peroxide Tolerance for Selective Sensing. J. Am. Chem. Soc. 2020, 142, 16861–16867. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).