A Comprehensive Review on Carbon Dioxide Sequestration Methods
Abstract
1. Introduction
2. CO2 Sequestration Methods
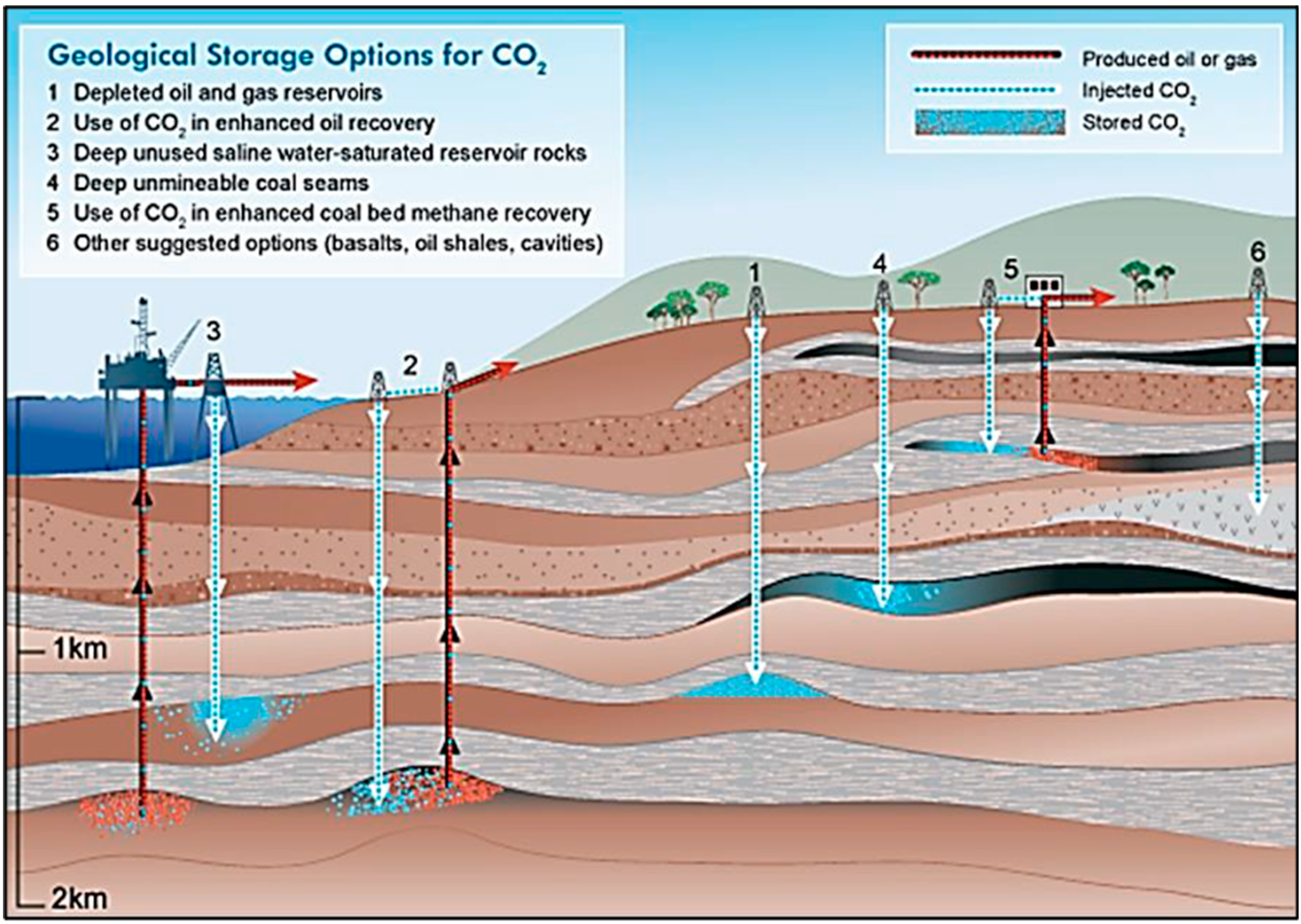
3. Storage in Subsurface Reservoir Formations
3.1. Brine Aquifers
3.2. Drained Hydrocarbon Reservoir Formations
- Additional site characterisation involves investigating potential leakage risks, such as the condition of the cap rock and any abandoned wells with integrity problems;
- Additional evaluations of surface processing plants’ fugitive and discharging emissions;
- Leakage rates may be estimated from specific locations, and the normality of the reservoir’s behaviour can be determined by increased monitoring and field surveillance.
3.3. In-Accessible Coal Seams
3.4. Subsurface Basalt Formations
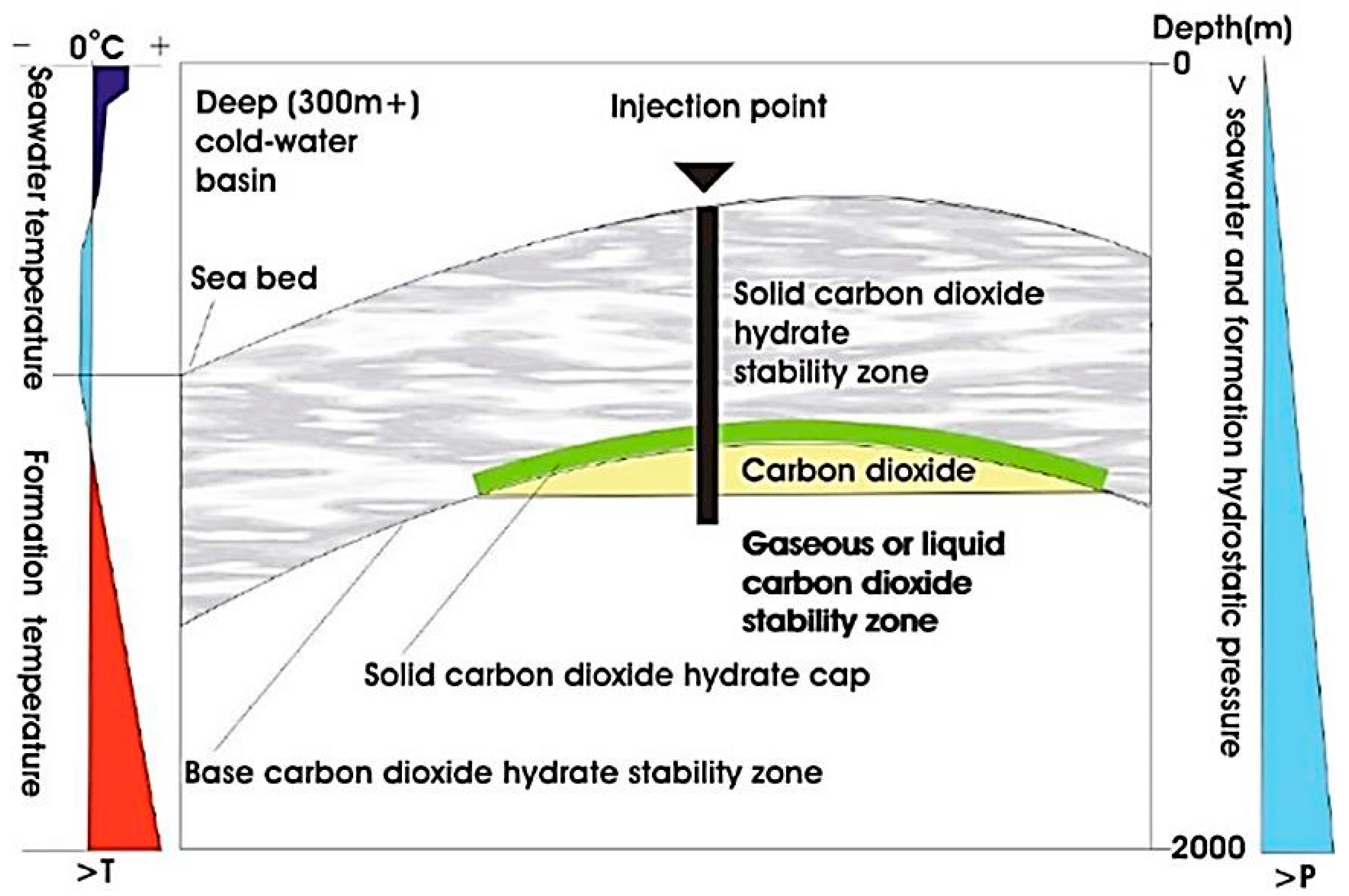
3.5. CO2 Sequestration in Hydrate Deep Formations
3.6. Enhanced Geothermal Systems Based on CO2
4. Carbonation of Mineral
Limitation and Future Work
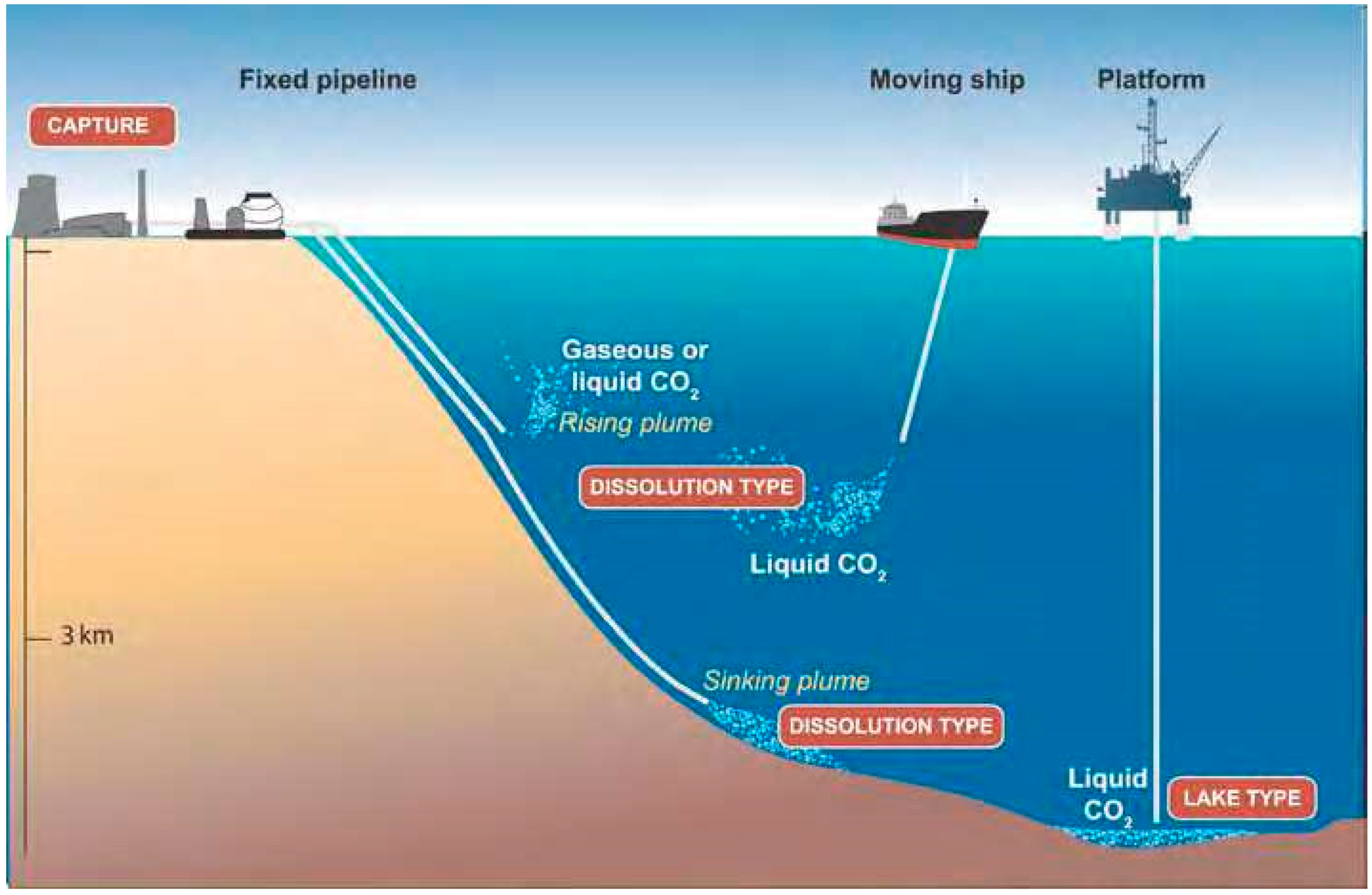
5. CO2 Sequestration on Ocean Floor
Limitation and Future Work
6. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTL | Alberta Carbon Trunk Line |
| CBM | Coal Bed Methane |
| CCS | Carbon Capture and Storage |
| CO2CRC | The Cooperative Research Centre for Greenhouse Gas Technologies |
| DOE | Department of Energy |
| ECBM | Enhanced Coal Bed Methane recovery |
| EGS | Enhanced Geothermal System |
| EOR | Enhanced Oil Recovery |
| GHG | Greenhouse Gas |
| HCPV | Hydrocarbon Pore Volume |
| IPCC | Intergovernmental Panel on Climate Change |
| LNG | Liquefied Natural Gas |
| MIT | Massachusetts Institute of Technology |
| MVA | Monitoring, Verification and Accounting |
| OGIP | Original Gas in Place |
| OOIP | Original Oil in Place |
| TRL | Technology Readiness Level |
| UKCCSRC | UK Carbon Capture and Storage Research Centre |
| US-DOE | United States Department of Energy |
| USGS | United States Geological Survey |
| VSP | Vertical Seismic Profile |
| XRD | X-ray Diffraction |
Appendix A
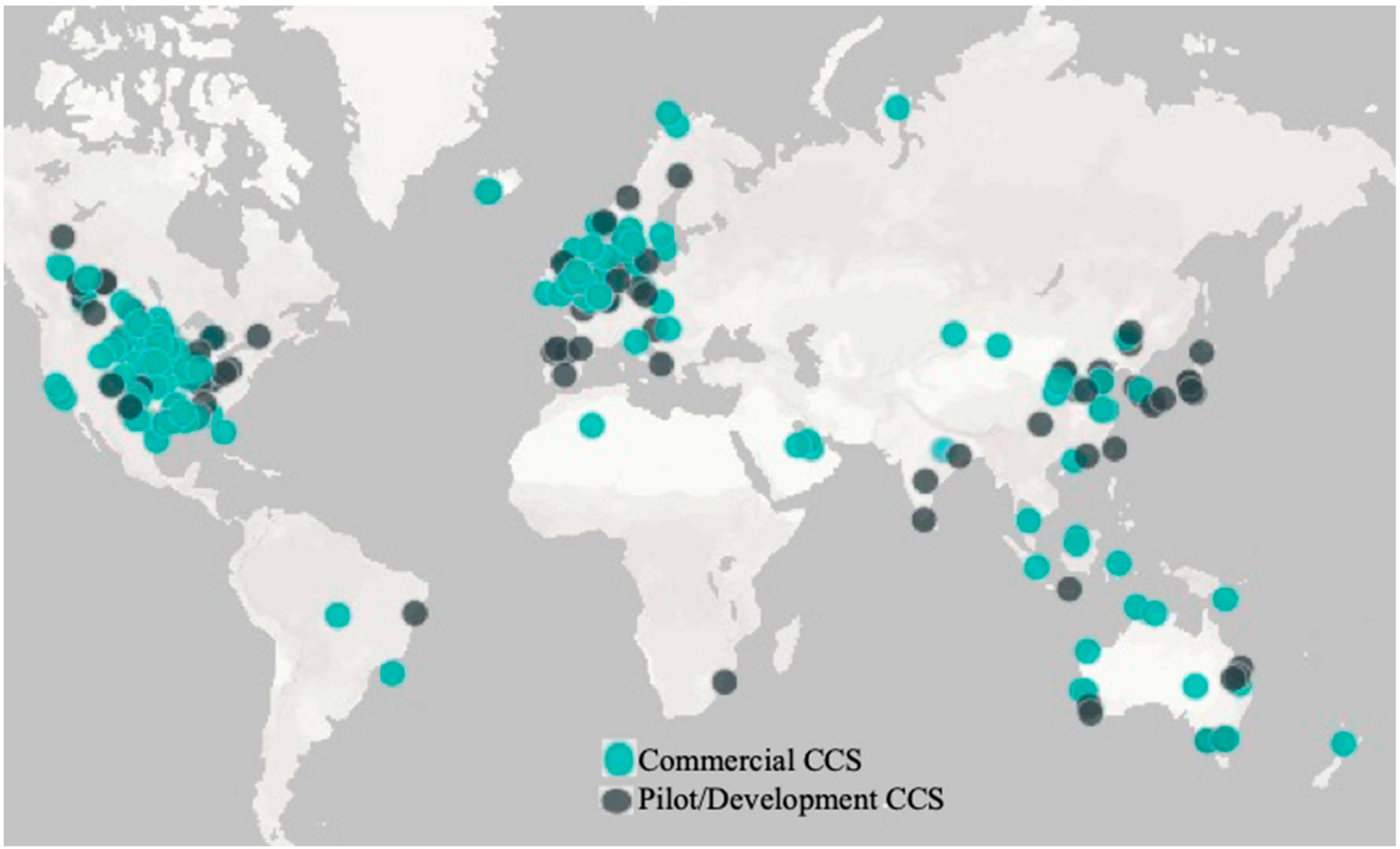
| Facility Name | Facility Category | Facility Status | Country | Operational | Facility Industry |
|---|---|---|---|---|---|
| In Salah CO2 Storage | Commercial CCS Facility | Completed | Algeria | 2004 | Natural Gas Processing |
| Bridgeport Energy Moonie CCUS project | Commercial CCS Facility | Advanced Development | Australia | 2023 | CO2 Transport and Storage |
| Burrup CCS Hub | Commercial CCS Facility | Early Development | Australia | CO2 Transport and Storage | |
| Callide Oxyfuel Project | Pilot and Demonstration CCS Facility | Completed | Australia | 2012 | Power Generation |
| CarbonNet | Commercial CCS Facility | Advanced Development | Australia | CO2 Transport and Storage | |
| Cliff Head CCS Project (Mid West Clean Energy Project) | Commercial CCS Facility | Advanced Development | Australia | 2025 | CO2 Transport and Storage |
| CO2CRC Otway | Pilot and Demonstration CCS Facility | Operational | Australia | 2008 | Natural Gas Processing |
| CTSCo Surat Basin CCS Project | Pilot and Demonstration CCS Facility | Advanced Development | Australia | 2023 | Power Generation |
| Gorgon Carbon Dioxide Injection | Commercial CCS Facility | Operational | Australia | 2019 | Natural Gas Processing |
| Hazelwood Carbon Capture and Mineral Sequestration Pilot Plant | Pilot and Demonstration CCS Facility | Completed | Australia | 2009 | Power Generation |
| Hydrogen Energy Supply Chain (HESC) project | Commercial CCS Facility | Advanced Development | Australia | Hydrogen Production | |
| Hydrogen Energy Supply Chain (HESC) project | Pilot and Demonstration CCS Facility | Completed | Australia | 2028 | Hydrogen Production |
| INPEX CCS Project Darwin | Commercial CCS Facility | Early Development | Australia | 2026 | Natural Gas Processing |
| Mid-West Modern Energy Hub | Commercial CCS Facility | Early Development | Australia | Hydrogen Production | |
| Moomba CCS hub (Santos Cooper Basin CCS Project) | Commercial CCS Facility | In Construction | Australia | 2024 | Hydrogen Production |
| National Geosequestration Laboratory (NGL) Australia | Pilot and Demonstration CCS Facility | Operational | Australia | 2015 | Research and Development |
| Otway Natural Gas Plant CCS | Commercial CCS Facility | Early Development | Australia | 2026 | Natural Gas Processing |
| Post-Combustion Capture (PCC)@CSIRO | Pilot and Demonstration CCS Facility | Operational | Australia | 2005 | Power Generation |
| South East Australia Carbon Capture Hub | Commercial CCS Facility | Early Development | Australia | 2025 | Natural Gas Processing |
| South West Hub | Pilot and Demonstration CCS Facility | Completed | Australia | Fertiliser Production | |
| Wallumbilla Renewable Methane Demonstration Project | Pilot and Demonstration CCS Facility | Advanced Development | Australia | 2021 | Direct Air Capture |
| Antwerp@C-BASF Antwerp CCS | Commercial CCS Facility | Advanced Development | Belgium | 2030 | Chemical Production |
| Antwerp@C-Exxonmobil Antwerp Refinery CCS | Commercial CCS Facility | Early Development | Belgium | 2030 | Chemical Production |
| Antwerp@C–Borealis Antwerp CCS | Commercial CCS Facility | Early Development | Belgium | 2030 | Chemical Production |
| Antwerp@C–Ineos Antwerp CCS | Commercial CCS Facility | Early Development | Belgium | 2030 | Chemical Production |
| LEILAC | Pilot and Demonstration CCS Facility | In Construction | Belgium | 2025 | Cement Production |
| Steelanol | Utilisation Facilities | Operational | Belgium | 2023 | Iron and Steel Production |
| FS Lucas do Rio Verde BECCS Project | Commercial CCS Facility | Early Development | Brazil | Ethanol Production | |
| Miranga CO2 Injection Project | Pilot and Demonstration CCS Facility | Completed | Brazil | 2009 | Fertiliser Production |
| Petrobras Santos Basin Pre-Salt Oil Field CCS | Commercial CCS Facility | Operational | Brazil | 2008 | Natural Gas Processing |
| Air Products Net-Zero Hydrogen Energy Complex | Commercial CCS Facility | Advanced Development | Canada | 2024 | Hydrogen Production |
| Alberta Carbon Conversion Technology Centre (ACCTC) | Pilot and Demonstration CCS Facility | Operational | Canada | 2018 | Power Generation |
| Alberta Carbon Trunk Line (ACTL) | Commercial CCS Facility | Operational | Canada | 2020 | CO2 Transport and Storage |
| Blue But Better | Commercial CCS Facility | In Construction | Canada | 2024 | Hydrogen Production |
| Boundary Dam Unit 3 Carbon Capture and Storage Facility (BD3 CCS facility) | Commercial CCS Facility | Operational | Canada | 2014 | Power Generation |
| Capital Power Genesee CCS Project | Commercial CCS Facility | Advanced Development | Canada | 2026 | Power Generation |
| Caroline Carbon Capture Power Complex | Commercial CCS Facility | Early Development | Canada | 2025 | Power Generation |
| CMC Research Institutes (CMCRI) | Pilot and Demonstration CCS Facility | Operational | Canada | 2018 | Research and Development |
| CO2 Solutions Valleyfield Carbon Capture Demonstration Project | Pilot and Demonstration CCS Facility | Completed | Canada | 2015 | Research and Development |
| Enhance Energy Clive CO2-EOR (ACTL) | Commercial CCS Facility | Operational | Canada | 2020 | CO2 Transport and Storage |
| Federated Co-operatives Limited (Ethanol) | Commercial CCS Facility | Advanced Development | Canada | 2024 | Ethanol Production |
| Federated Co-operatives Limited (Refinery) | Commercial CCS Facility | Advanced Development | Canada | 2026 | Oil Refining |
| Glacier Gas Plant MCCS | Commercial CCS Facility | Operational | Canada | 2022 | Natural Gas Processing |
| Husky Energy Lashburn and Tangleflags CO2 Injection in Heavy Oil Reservoirs Project | Pilot and Demonstration CCS Facility | Operational | Canada | 2012 | Ethanol Production |
| Nauticol Energy Net Zero Methanol (ACTL) | Commercial CCS Facility | Early Development | Canada | 2025 | Methanol Production |
| Northwest Redwater CO2 Recovery Unit Sturgeon Refinery (ACTL) | Commercial CCS Facility | Operational | Canada | 2020 | Oil Refining |
| Origins Project Carbon Storage Hub | Commercial CCS Facility | Early Development | Canada | 2026 | CO2 Transport and Storage |
| Pembina Cardium CO2 Monitoring Pilot | Pilot and Demonstration CCS Facility | Completed | Canada | 2005 | Natural Gas Processing |
| Polaris CCS Project | Commercial CCS Facility | Early Development | Canada | 2025 | Hydrogen Production |
| Quest | Commercial CCS Facility | Operational | Canada | 2015 | Hydrogen Production |
| Saskatchewan NET Power Plant | Commercial CCS Facility | Early Development | Canada | 2025 | Power Generation |
| Shand Carbon Capture Test Facility (CCTF) | Pilot and Demonstration CCS Facility | Operational | Canada | 2015 | Research and Development |
| Southeast Saskatchewan CCUS Hub-Storage | Commercial CCS Facility | Advanced Development | Canada | CO2 Transport and Storage | |
| Svante and Husky Energy VeloxoTherm Capture Process Test | Pilot and Demonstration CCS Facility | Advanced Development | Canada | 2018 | Oil Refining |
| WCS Redwater CO2 Recovery Unit (ACTL) | Commercial CCS Facility | Operational | Canada | 2020 | Fertiliser Production |
| Zama Field Validation Test | Pilot and Demonstration CCS Facility | Completed | Canada | 2005 | Natural Gas Processing |
| Australia-China Post Combustion Capture (PCC) Feasibility Study Project | Pilot and Demonstration CCS Facility | Completed | China | 2010 | Power Generation |
| Australia-China Post Combustion Capture (PCC) Feasibility Study Project | Pilot and Demonstration CCS Facility | Completed | China | 2010 | Power Generation |
| China Coalbed Methane Technology Sequestration Project | Pilot and Demonstration CCS Facility | Completed | China | 2004 | Research and Development |
| China National Energy Guohua Jinjie | Commercial CCS Facility | Operational | China | 2020 | Power Generation |
| China National Energy Taizhou | Commercial CCS Facility | In Construction | China | 2023 | Power Generation |
| Chinese-European Emission-Reducing Solutions (CHEERS) | Pilot and Demonstration CCS Facility | Advanced Development | China | 2022 | Oil Refining |
| CNOOC Enping CCS Offshore Project | Commercial CCS Facility | Operational | China | 2023 | Natural Gas Processing |
| CNPC Jilin Oil Field CO2 EOR | Commercial CCS Facility | Operational | China | 2018 | Natural Gas Processing |
| CNPC Jilin Oil Field EOR Demonstration Project | Pilot and Demonstration CCS Facility | Completed | China | 2008 | Natural Gas Processing |
| Daqing Oil Field EOR Demonstration Project | Pilot and Demonstration CCS Facility | Operational | China | 2003 | Natural Gas Processing |
| Guanghui Energy CCUS | Commercial CCS Facility | In Construction | China | Methanol Production | |
| Haifeng Carbon Capture Test Platform | Pilot and Demonstration CCS Facility | Operational | China | 2018 | Power Generation |
| Huaneng GreenGen IGCC Demonstration-scale System (Phase 2) | Pilot and Demonstration CCS Facility | In Construction | China | 2025 | Power Generation |
| Huaneng Longdong Energy Base Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | China | 2023 | Power Generation |
| ITRI Calcium Looping Pilot | Pilot and Demonstration CCS Facility | Operational | China | 2013 | Cement Production |
| Jinling Petrochemical CCUS (Nanjing Refinery) | Commercial CCS Facility | Operational | China | 2023 | Oil Refining |
| Karamay Dunhua Oil Technology CCUS EOR Project | Commercial CCS Facility | Operational | China | 2015 | Methanol Production |
| PetroChina Changqing Oil Field EOR CCUS | Pilot and Demonstration CCS Facility | Operational | China | 2017 | Fuel transformation |
| Shenhua Group Ordos Carbon Capture and Storage (CCS) Demonstration Project | Pilot and Demonstration CCS Facility | Completed | China | 2011 | Fuel transformation |
| Shuncheng CO2-TO-METHANOL Anyang Petrochemical | Utilisation Facilities | Operational | China | 2022 | Chemical Production |
| Sinopec Nanjing Chemical Industries CCUS Cooperation Project | Commercial CCS Facility | Operational | China | 2021 | Chemical Production |
| Sinopec Qilu-Shengli CCUS Project | Commercial CCS Facility | Operational | China | 2022 | Chemical Production |
| Sinopec Shengli Oilfield Carbon Capture Utilization and Storage Pilot Project | Pilot and Demonstration CCS Facility | Operational | China | 2010 | Power Generation |
| Sinopec Shengli Power Plant CCS | Commercial CCS Facility | Advanced Development | China | 2025 | Power Generation |
| Sinopec Zhongyuan Carbon Capture Utilization and Storage | Pilot and Demonstration CCS Facility | Completed | China | 2006 | Chemical Production |
| Yanchang Integrated CCS Demonstration | Commercial CCS Facility | Operational | China | 2012 | Chemical Production |
| Geothermal Plant with CO2 Re-injection | Pilot and Demonstration CCS Facility | Operational | Croatia | 2018 | Power Generation |
| CASTOR | Pilot and Demonstration CCS Facility | Completed | Denmark | 2006 | Power Generation |
| CESAR | Pilot and Demonstration CCS Facility | Completed | Denmark | 2008 | Power Generation |
| Copenhill (Amager Bakke) Waste to Energy CCS | Commercial CCS Facility | Advanced Development | Denmark | 2025 | Waste Incineration |
| Greenport Scandinavia | Commercial CCS Facility | Early Development | Denmark | 2025 | Bioenergy |
| Project Greensand | Commercial CCS Facility | Advanced Development | Denmark | 2025 | CO2 Transport and Storage |
| Air Liquide CalCC | Commercial CCS Facility | Early Development | France | 2028 | Lime Production |
| Air Liquide Normandy CCS | Commercial CCS Facility | Early Development | France | 2025 | Hydrogen Production |
| C2A2 Field Pilot-Le Havre | Pilot and Demonstration CCS Facility | Completed | France | 2013 | Power Generation |
| DMX™ Demonstration in Dunkirk | Pilot and Demonstration CCS Facility | Operational | France | 2022 | Iron and Steel Production |
| K6 | Commercial CCS Facility | Early Development | France | 2028 | Cement Production |
| Lacq CCS Pilot Project | Pilot and Demonstration CCS Facility | Completed | France | 2010 | Power Generation |
| CEMEX, Rüdersdorf, Germany | Commercial CCS Facility | Early Development | Germany | 2026 | Cement Production |
| Ketzin Pilot Project | Pilot and Demonstration CCS Facility | Completed | Germany | 2004 | Power Generation |
| Schwarze Pumpe Oxy-fuel Pilot Plant | Pilot and Demonstration CCS Facility | Completed | Germany | 2008 | Power Generation |
| Wilhelmshaven CO2 Capture Pilot Plant | Pilot and Demonstration CCS Facility | Completed | Germany | 2012 | Power Generation |
| MOL Szank field CO2 EOR | Commercial CCS Facility | Operational | Hungary | 1992 | Natural Gas Processing |
| CarbFix Project | Pilot and Demonstration CCS Facility | Operational | Iceland | 2012 | Power Generation |
| CODA Shipping | Commercial CCS Facility | Advanced Development | Iceland | 2026 | CO2 Transport and Storage |
| CODA Terminal Onshore Infrastructure | Commercial CCS Facility | Advanced Development | Iceland | 2026 | CO2 Transport and Storage |
| CODA Terminal Pipeline | Commercial CCS Facility | Advanced Development | Iceland | 2026 | CO2 Transport and Storage |
| CODA Terminal Storage | Commercial CCS Facility | Advanced Development | Iceland | 2026 | CO2 Transport and Storage |
| Mammoth | Commercial CCS Facility | In Construction | Iceland | 2024 | Direct Air Capture |
| Orca | Commercial CCS Facility | Operational | Iceland | 2021 | Direct Air Capture |
| Carbon Clean Solutions Solvay Vishnu Capture Project | Pilot and Demonstration CCS Facility | Completed | India | 2012 | Power Generation |
| NTPC Vindhyachal Super Thermal Power Station CCS | Utilisation Facilities | Operational | India | 2022 | Power Generation |
| Tata Steel Jamshedpur Steel Plant | Pilot and Demonstration CCS Facility | Operational | India | 2021 | Iron and Steel Production |
| Tuticorin (TTPS)-Carbon Clean Solution | Utilisation Facilities | Operational | India | 2016 | Power Generation |
| Tuticorin Alkali Chemicals and Fertilizers Ltd. | Pilot and Demonstration CCS Facility | Operational | India | 2016 | Chemical Production |
| Arun CCS Hub | Commercial CCS Facility | Early Development | Indonesia | 2029 | CO2 Transport and Storage |
| Gundih CCS Pilot | Pilot and Demonstration CCS Facility | Advanced Development | Indonesia | 2025 | Natural Gas Processing |
| PAU Central Sulawesi Clean Fuel Ammonia Production with CCUS | Commercial CCS Facility | Early Development | Indonesia | 2025 | Fertiliser Production |
| Repsol Sakakemang Carbon Capture and Injection | Commercial CCS Facility | Early Development | Indonesia | 2026 | Natural Gas Processing |
| Sukowati CCUS | Commercial CCS Facility | Early Development | Indonesia | 2028 | Oil Refining |
| Ervia Cork CCS | Commercial CCS Facility | Early Development | Ireland | 2028 | Power Generation |
| Heletz, Israel pilot CO2 injection site | Pilot and Demonstration CCS Facility | Completed | Israel | 2026 | Research and Development |
| Brindisi CO2 Capture Pilot Plant | Pilot and Demonstration CCS Facility | Completed | Italy | 2010 | Power Generation |
| Ravenna CCS Hub | Commercial CCS Facility | Early Development | Italy | 2027 | CO2 Transport and Storage |
| COURSE 50-CO2 Ultimate Reduction in Steelmaking Process by Innovative Technology for Cool Earth 50 | Pilot and Demonstration CCS Facility | Operational | Japan | 2008 | Iron and Steel Production |
| EAGLE | Pilot and Demonstration CCS Facility | Completed | Japan | 2002 | Power Generation |
| Kashiwazaki Clean Hydrogen/Ammonia Project | Pilot and Demonstration CCS Facility | In Construction | Japan | 2024 | Hydrogen Production |
| Mikawa Post Combustion Capture Demonstration Plant | Pilot and Demonstration CCS Facility | Operational | Japan | 2020 | Power Generation |
| Nagaoka CO2 Storage Project | Pilot and Demonstration CCS Facility | Completed | Japan | 2003 | Natural Gas Processing |
| Osaki CoolGen Project | Pilot and Demonstration CCS Facility | In Construction | Japan | 2020 | Power Generation |
| Taiheiyo Cement Corporation | Pilot and Demonstration CCS Facility | Operational | Japan | 2021 | Cement Production |
| Tomakomai CCS Demonstration Project | Pilot and Demonstration CCS Facility | Operational | Japan | 2016 | Hydrogen Production |
| Kasawari | Commercial CCS Facility | In Construction | Malaysia | 2025 | Natural Gas Processing |
| Lang Lebah CCS | Commercial CCS Facility | Advanced Development | Malaysia | 2026 | Natural Gas Processing |
| Air Liquide Refinery Rotterdam CCS | Commercial CCS Facility | Advanced Development | The Netherlands | 2024 | Hydrogen Production |
| Air Products Refinery Rotterdam CCS | Commercial CCS Facility | Advanced Development | The Netherlands | 2024 | Hydrogen Production |
| Buggenum Carbon Capture (CO2 Catch-up) Pilot Project | Pilot and Demonstration CCS Facility | Completed | The Netherlands | 2011 | Power Generation |
| Delta Corridor Pipeline Network | Commercial CCS Facility | Early Development | The Netherlands | 2026 | CO2 Transport and Storage |
| ExxonMobil Benelux Refinery CCS | Commercial CCS Facility | Advanced Development | The Netherlands | 2024 | Hydrogen Production |
| Hydrogen 2 Magnum (H2M) | Commercial CCS Facility | Early Development | The Netherlands | 2024 | Power Generation |
| K12-B CO2 Injection Project | Pilot and Demonstration CCS Facility | Completed | The Netherlands | 2004 | Natural Gas Processing |
| L10 Carbon Capture and Storage | Commercial CCS Facility | Early Development | The Netherlands | 2026 | Hydrogen Production |
| Porthos-Compressor Station | Commercial CCS Facility | Advanced Development | The Netherlands | 2024 | CO2 Transport and Storage |
| Porthos-Offshore Pipeline | Commercial CCS Facility | Advanced Development | The Netherlands | 2024 | CO2 Transport and Storage |
| Porthos-Onshore Pipeline | Commercial CCS Facility | Advanced Development | The Netherlands | 2024 | CO2 Transport and Storage |
| Porthos Storage | Commercial CCS Facility | Advanced Development | The Netherlands | 2024 | CO2 Transport and Storage |
| Shell Energy and Chemicals Park Rotterdam | Commercial CCS Facility | In Construction | The Netherlands | 2024 | Bioenergy |
| Yara Sluiskil | Commercial CCS Facility | Early Development | The Netherlands | 2025 | Fertiliser Production |
| Zeeland Refinery Azur | Commercial CCS Facility | Early Development | The Netherlands | 2026 | Hydrogen Production |
| Project Pouakai Hydrogen Production with CCS | Commercial CCS Facility | Early Development | New Zealand | 2024 | Hydrogen Production |
| Barents Blue | Commercial CCS Facility | Early Development | Norway | 2025 | Fertiliser Production |
| Borg CO2 | Commercial CCS Facility | Early Development | Norway | CO2 Transport and Storage | |
| CEMCAP | Pilot and Demonstration CCS Facility | Completed | Norway | 2015 | Cement Production |
| CO2 Capture Test Facility at Norcem Brevik | Pilot and Demonstration CCS Facility | Completed | Norway | 2013 | Cement Production |
| Equinor Smeaheia (Norway) | Commercial CCS Facility | Early Development | Norway | 2028 | CO2 Storage |
| Fortum Oslo Varme-Shipping Route | Commercial CCS Facility | Early Development | Norway | 2025 | Waste Incineration |
| Hafslund Oslo Celsio | Commercial CCS Facility | In Construction | Norway | 2024 | Waste Incineration |
| Hafslund Oslo Celsio-Truck Route | Commercial CCS Facility | Advanced Development | Norway | 2025 | Waste Incineration |
| Norcem Brevik-Cement Plant | Commercial CCS Facility | In Construction | Norway | 2024 | Cement Production |
| Norcem Brevik-Shipping Route | Commercial CCS Facility | In Construction | Norway | 2024 | Cement Production |
| Northern Lights-Pipeline | Commercial CCS Facility | Early Development | Norway | 2024 | CO2 Transport and Storage |
| Northern Lights-Storage | Commercial CCS Facility | In Construction | Norway | 2024 | CO2 Transport and Storage |
| Polaris Carbon Storage | Commercial CCS Facility | Advanced Development | Norway | 2024 | Hydrogen Production |
| Sleipner CCS Project | Commercial CCS Facility | Operational | Norway | 1996 | Natural Gas Processing |
| Snohvit CO2 Storage | Commercial CCS Facility | Operational | Norway | 2008 | Natural Gas Processing |
| Technology Centre Mongstad (TCM) | Pilot and Demonstration CCS Facility | Operational | Norway | 2012 | Oil Refining |
| Project Hajar | Commercial CCS Facility | In Construction | Oman | 2024 | Direct Air Capture |
| Papua LNG CCS | Commercial CCS Facility | Early Development | Papua New Guinea | 2027 | Natural Gas Processing |
| GO4ECOPLANET | Commercial CCS Facility | Early Development | Poland | 2027 | Cement Production |
| North Field East Project (NFE) CCS | Commercial CCS Facility | In Construction | Qatar | 2025 | Natural Gas Processing |
| Qatar LNG CCS | Commercial CCS Facility | Operational | Qatar | 2019 | Natural Gas Processing |
| Novatek Yamal LNG CCS | Commercial CCS Facility | Early Development | Russia | 2027 | Natural Gas Processing |
| Uthmaniyah CO2-EOR Demonstration | Commercial CCS Facility | Operational | Saudi Arabia | 2015 | Natural Gas Processing |
| Pilot Carbon Storage Project (PCSP)-Zululand Basin, South Africa | Pilot and Demonstration CCS Facility | Advanced Development | South Africa | 2020 | Under Evaluation |
| Boryeong-KoSol Process for CO2 Capture (KPCC) Test | Pilot and Demonstration CCS Facility | Completed | Republic of Korea | 2010 | Power Generation |
| Hadong-Dry-sorbent CO2 Capture System Test | Pilot and Demonstration CCS Facility | Completed | Republic of Korea | 2014 | Power Generation |
| Korea-CCS 1 & 2 | Commercial CCS Facility | Early Development | Republic of Korea | 2025 | Power Generation |
| CIUDEN: CO2 Capture & Transport Technology Development Plant | Pilot and Demonstration CCS Facility | Completed | Spain | 2012 | Power Generation |
| CIUDEN: CO2 Storage Technology Development Plant | Pilot and Demonstration CCS Facility | Operational | Spain | 2015 | Research and Development |
| ELCOGAS Pre-combustion Carbon Capture Pilot Project: Puertollano | Pilot and Demonstration CCS Facility | Completed | Spain | 2010 | Power Generation |
| La Pereda Calcium Looping Pilot Plant | Pilot and Demonstration CCS Facility | Completed | Spain | 2012 | Power Generation |
| Cementa CCS (Slite Cement plant) | Commercial CCS Facility | Early Development | Sweden | 2030 | Cement Production |
| Cinfracap-Pipeline | Commercial CCS Facility | Early Development | Sweden | 2026 | CO2 Transport and Storage |
| Cinfracap-Shipping Route | Commercial CCS Facility | Early Development | Sweden | 2026 | CO2 Transport and Storage |
| Karlshamn Field Pilot | Pilot and Demonstration CCS Facility | Completed | Sweden | 2009 | Power Generation |
| Preem Refinery CCS | Commercial CCS Facility | Early Development | Sweden | 2025 | Hydrogen Production |
| STEPWISE Pilot of SEWGS Technology at Swerea/Mefos | Pilot and Demonstration CCS Facility | Operational | Sweden | 2017 | Iron and Steel Production |
| Stockholm Exergi BECCS | Commercial CCS Facility | Advanced Development | Sweden | 2027 | Bioenergy |
| Stockholm Exergi BECCS-Shipping Route | Commercial CCS Facility | Advanced Development | Sweden | 2027 | Bioenergy |
| PTTEP Arthit CCS | Commercial CCS Facility | Advanced Development | Thailand | TBC | Natural Gas Processing |
| Bayu-Undan CCS | Commercial CCS Facility | Advanced Development | Timor-Leste | 2027 | Natural Gas Processing |
| Abu Dhabi CCS (Phase 1 being Emirates Steel Industries) | Commercial CCS Facility | Operational | United Arab Emirates | 2016 | Iron and Steel Production |
| Abu Dhabi CCS Phase 2: Natural gas processing plant | Commercial CCS Facility | Advanced Development | United Arab Emirates | 2025 | Natural Gas Processing |
| Ghasha Concession Fields | Commercial CCS Facility | Advanced Development | United Arab Emirates | 2025 | Natural Gas Processing |
| Aberthaw Pilot Carbon Capture Facility | Pilot and Demonstration CCS Facility | Completed | United Kingdom | 2013 | Power Generation |
| Acorn | Commercial CCS Facility | Early Development | United Kingdom | 2024 | Hydrogen Production |
| Acorn (Minimum Viable CCS Development) | Pilot and Demonstration CCS Facility | Advanced Development | United Kingdom | 2025 | CO2 Transport and Storage |
| Acorn CO2 Pipeline | Commercial CCS Facility | Early Development | United Kingdom | 2026 | CO2 Transport and Storage |
| Acorn Direct Air Capture Facility | Commercial CCS Facility | Early Development | United Kingdom | 2026 | Hydrogen Production |
| Acorn Hydrogen | Commercial CCS Facility | Early Development | United Kingdom | 2025 | Hydrogen Production |
| Acorn Storage Site | Commercial CCS Facility | Advanced Development | United Kingdom | 2025 | CO2 Transport and Storage |
| Buxton Lime Net Zero | Commercial CCS Facility | Early Development | United Kingdom | 2024 | Lime Production |
| Caledonia Clean Energy | Commercial CCS Facility | Early Development | United Kingdom | 2025 | Power Generation |
| CF Fertilisers Billingham Ammonia CCS | Commercial CCS Facility | Early Development | United Kingdom | 2023 | Fertiliser Production |
| Damhead Pipeline (Medway Hub) | Commercial CCS Facility | Early Development | United Kingdom | Power Generation | |
| Damhead Power Station (Medway) | Commercial CCS Facility | Early Development | United Kingdom | Power Generation | |
| Drax BECCS Project | Commercial CCS Facility | Early Development | United Kingdom | 2027 | Power Generation |
| Drax bioenergy carbon capture pilot plant | Pilot and Demonstration CCS Facility | Operational | United Kingdom | 2019 | Power Generation |
| East Coast Cluster Humber Pipeline | Commercial CCS Facility | Advanced Development | United Kingdom | 2025 | CO2 Transport and Storage |
| East Coast Cluster Teesside Pipeline | Commercial CCS Facility | Advanced Development | United Kingdom | 2025 | CO2 Transport and Storage |
| Endurance Storage Site | Commercial CCS Facility | Advanced Development | United Kingdom | 2025 | CO2 Transport and Storage |
| Esmond and Forbes Carbon Storage (Medway Hub) | Commercial CCS Facility | Early Development | United Kingdom | Power Generation | |
| Ferrybridge Carbon Capture Pilot (CCPilot100+) | Pilot and Demonstration CCS Facility | Completed | United Kingdom | 2011 | Power Generation |
| Grain Power Station (Medway) | Commercial CCS Facility | Early Development | United Kingdom | Power Generation | |
| H2NorthEast | Commercial CCS Facility | Early Development | United Kingdom | 2027 | Hydrogen Production |
| Hydrogen to Humber Saltend | Commercial CCS Facility | Early Development | United Kingdom | 2025 | Hydrogen Production |
| HyNet Hydrogen Production Project (HPP) | Commercial CCS Facility | Early Development | United Kingdom | 2025 | Hydrogen Production |
| HyNet North West | Commercial CCS Facility | Early Development | United Kingdom | 2026 | Hydrogen Production |
| HyNet North West-Hanson Cement CCS | Commercial CCS Facility | Early Development | United Kingdom | 2026 | Cement Production |
| HyNet Pipeline | Commercial CCS Facility | Early Development | United Kingdom | 2025 | CO2 Transport and Storage |
| Hynet Storage Site | Commercial CCS Facility | Early Development | United Kingdom | 2025 | CO2 Transport and Storage |
| Isle of Grain LNG Terminal (Medway Hub) | Commercial CCS Facility | Early Development | United Kingdom | 2026 | Power Generation |
| Keady 3 CCS Power Station | Commercial CCS Facility | Early Development | United Kingdom | 2027 | Power Generation |
| Killingholme Power Station | Commercial CCS Facility | Early Development | United Kingdom | 2027 | Hydrogen Production |
| Medway Hub Shipping | Commercial CCS Facility | Early Development | United Kingdom | Power Generation | |
| Medway Power Station | Commercial CCS Facility | Early Development | United Kingdom | Power Generation | |
| NET Power Plant (East Coast Cluster) | Commercial CCS Facility | Early Development | United Kingdom | 2025 | Power Generation |
| Net Zero Teesside-CCGT Facility | Commercial CCS Facility | Early Development | United Kingdom | 2025 | Power Generation |
| Net Zero Teesside—BP H2Teesside | Commercial CCS Facility | Early Development | United Kingdom | 2027 | Hydrogen Production |
| Northern Gas Network H21 North of England | Commercial CCS Facility | Early Development | United Kingdom | 2026 | Hydrogen Production |
| Pembroke Power Station | Commercial CCS Facility | Early Development | United Kingdom | 2030 | Power Generation |
| Peterhead CCS Power Station | Commercial CCS Facility | Advanced Development | United Kingdom | 2026 | Power Generation |
| Phillips 66 Humber Refinery CCS | Commercial CCS Facility | Advanced Development | United Kingdom | 2028 | Hydrogen Production |
| Prax Lindsey Carbon Capture Project (PLCCP) | Commercial CCS Facility | Advanced Development | United Kingdom | 2028 | Oil Refining |
| Redcar Energy Centre | Commercial CCS Facility | Early Development | United Kingdom | 2025 | Power Generation |
| Renfrew Oxy-fuel (Oxycoal 2) Project | Pilot and Demonstration CCS Facility | Completed | United Kingdom | 2007 | Power Generation |
| Suez Waste to Energy CCS (East Coast Cluster) | Commercial CCS Facility | Early Development | United Kingdom | 2027 | Waste Incineration |
| Tees Valley Energy Recovery Facility Project (TVERF) | Commercial CCS Facility | Early Development | United Kingdom | 2026 | Bioenergy |
| UKCCSRC Pilot-scale Advanced Capture Technology (PACT) | Pilot and Demonstration CCS Facility | Completed | United Kingdom | 2012 | Power Generation |
| Vertex Hydrogen | Commercial CCS Facility | Early Development | United Kingdom | 2025 | Oil Refining |
| Viking CCS Pipeline | Commercial CCS Facility | Advanced Development | United Kingdom | 2027 | CO2 Transport and Storage |
| Viking CCS Storage Site | Commercial CCS Facility | Advanced Development | United Kingdom | 2027 | CO2 Transport and Storage |
| Viridor Runcorn Carbon Capture | Commercial CCS Facility | Early Development | United Kingdom | Waste Incineration | |
| VPI Immingham Power Plant CCS | Commercial CCS Facility | Advanced Development | United Kingdom | 2027 | Power Generation |
| Whitetail Clean Energy | Commercial CCS Facility | Early Development | United Kingdom | Power Generation | |
| ADM Illinois Industrial | Commercial CCS Facility | Operational | USA | 2017 | Ethanol Production |
| ArcelorMittal Texas (formerly voestalpine Texas) | Commercial CCS Facility | Early Development | USA | Iron and Steel Production | |
| Arkalon CO2 Compression Facility | Commercial CCS Facility | Operational | USA | 2009 | Ethanol Production |
| Ascension Clean Energy (Louisiana) | Commercial CCS Facility | Early Development | USA | 2027 | Hydrogen Production |
| Atkinson Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Bayou Bend CCS | Commercial CCS Facility | Advanced Development | USA | 2025 | CO2 Transport and Storage |
| Baytown Low Carbon Hydrogen | Commercial CCS Facility | Advanced Development | USA | 2027 | Hydrogen Production |
| Bell Creek-Incidental CO2 Storage Associated with a Commercial EOR Project | Pilot and Demonstration CCS Facility | Operational | USA | 2010 | Natural Gas Processing |
| Bonanza BioEnergy CCUS EOR | Commercial CCS Facility | Operational | USA | 2012 | Ethanol Production |
| Borger CO2 Compression Facility | Commercial CCS Facility | Completed | USA | 2001 | Fertiliser Production |
| Bushmills Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Cal Capture | Commercial CCS Facility | Advanced Development | USA | 2027–2028 | Power Generation |
| Cane Run CCS | Commercial CCS Facility | Early Development | USA | Power Generation | |
| Carbon TerraVault I Project | Commercial CCS Facility | Early Development | USA | 2025 | CO2 Transport and Storage |
| CarbonFree Skymine | Utilisation Facilities | Operational | USA | 2015 | Cement Production |
| Casselton Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Central City Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Central Louisiana Regional Carbon Storage (CENLA) Hub | Commercial CCS Facility | In Construction | USA | 2027 | CO2 Transport and Storage |
| Century Plant | Commercial CCS Facility | Operational | USA | 2010 | Natural Gas Processing |
| Clean Energy Systems BiCRS Plant-Madera County | Commercial CCS Facility | Early Development | USA | 2027 | Power Generation |
| Clean Energy Systems Carbon Negative Energy Plant-Central Valley | Commercial CCS Facility | Early Development | USA | 2025 | Power Generation |
| CO2 Sequestration Field Test: Deep Unminable Lignite Seam | Pilot and Demonstration CCS Facility | Completed | USA | 2009 | Research and Development |
| Coastal Bend CCS | Commercial CCS Facility | Early Development | USA | 2026 | CO2 Transport and Storage |
| Coffeyville Gasification Plant | Commercial CCS Facility | Operational | USA | 2013 | Fertiliser Production |
| Core Energy CO2-EOR | Commercial CCS Facility | Operational | USA | 2003 | Natural Gas Processing |
| Coyote Clean Power Project | Commercial CCS Facility | Advanced Development | USA | 2025 | Power Generation |
| CPV Shay Energy Center (CPV West Virginia Natural Gas Power Station CCS) | Commercial CCS Facility | Early Development | USA | Power Generation | |
| Cranfield Project | Pilot and Demonstration CCS Facility | Operational | USA | 2009 | Research and Development |
| Cyclus Power Generation | Commercial CCS Facility | Early Development | USA | Bioenergy | |
| Dave Johnston Plant Carbon Capture | Commercial CCS Facility | Early Development | USA | 2025 | Power Generation |
| Deer Park Energy Centre CCS Project | Commercial CCS Facility | Advanced Development | USA | Power Generation | |
| Diamond Vault CCS | Commercial CCS Facility | Early Development | USA | 2028 | Power Generation |
| Donaldsonville | Commercial CCS Facility | In Construction | USA | 2025 | Ammonia Production |
| Dry Fork Integrated Commercial Carbon Capture and Storage (CCS) | Commercial CCS Facility | Early Development | USA | 2025 | Power Generation |
| E.W. Brown 0.7 MWe Pilot Carbon Capture Unit | Pilot and Demonstration CCS Facility | Operational | USA | 2014 | Power Generation |
| El Dorado CCS Project | Commercial CCS Facility | Early Development | USA | 2026 | Fertiliser Production |
| Enid Fertilizer | Commercial CCS Facility | Operational | USA | 1982 | Fertiliser Production |
| Fairmont Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Farley DAC Project | Commercial CCS Facility | Advanced Development | USA | Direct Air Capture | |
| Farnsworth Unit EOR Field Project-Development Phase | Pilot and Demonstration CCS Facility | Operational | USA | 2013 | Ethanol Production |
| Freeport LNG CCS project | Commercial CCS Facility | Cancelled | USA | 2024 | Natural Gas Processing |
| Frio Brine Pilot | Pilot and Demonstration CCS Facility | Completed | USA | 2004 | Research and Development |
| Fuel Cell Carbon Capture Pilot Plant | Pilot and Demonstration CCS Facility | Operational | USA | 2016 | Power Generation |
| G2 Net-Zero LNG | Commercial CCS Facility | Early Development | USA | Natural Gas Processing | |
| Galva Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Gerald Gentleman Station Carbon Capture | Commercial CCS Facility | Advanced Development | USA | 2025 | Power Generation |
| Goldfield Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Grand Forks Blue Ammonia Capture plant | Commercial CCS Facility | Early Development | USA | Natural Gas Processing | |
| Grand Junction Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Granite Falls Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Great Plains Synfuels Plant and Weyburn-Midale | Commercial CCS Facility | Operational | USA | 2000 | Hydrogen Production |
| Hackberry Carbon Sequestration Project (Sempra) | Commercial CCS Facility | Early Development | USA | CO2 Transport and Storage | |
| Haynesville Gas Processing (CENLA Hub) | Commercial CCS Facility | In Construction | USA | 2027 | Natural Gas Processing |
| Heartland Greenway Storage | Commercial CCS Facility | Early Development | USA | 2025 | Ethanol Production |
| Heartland Hydrogen Hub | Commercial CCS Facility | Advanced Development | USA | Power Generation | |
| HeidelbergCement CCS | Commercial CCS Facility | Advanced Development | USA | 2023 | Cement Production |
| Heron Lake Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Huron Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Illinois Allam-Fetvedt cycle power plant | Commercial CCS Facility | Early Development | USA | 2025 | Power Generation |
| Illinois Basin Decatur Project (CO2 Injection Completed, Monitoring Ongoing) | Pilot and Demonstration CCS Facility | Completed | USA | 2011 | Ethanol Production |
| James M. Barry Electric Generating Plant CCS Project | Commercial CCS Facility | Advanced Development | USA | 2030 | Power Generation |
| Kevin Dome Carbon Storage Project-Development Phase | Pilot and Demonstration CCS Facility | Completed | USA | 2013 | Research and Development |
| LafargeHolcim Cement Carbon capture | Commercial CCS Facility | Early Development | USA | 2025 | Cement Production |
| LafargeHolcim Ste. Genevieve Cement Plant CCS | Commercial CCS Facility | Early Development | USA | Cement Production | |
| Lake Charles Methanol | Commercial CCS Facility | Advanced Development | USA | 2025 | Chemical Production |
| Lamberton Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Lawler Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Linde hydrogen plant for OCI fertilizer blue ammonia Beaumont | Commercial CCS Facility | In Construction | USA | 2025 | Hydrogen Production |
| Lone Cypress Hydrogen Project | Commercial CCS Facility | Early Development | USA | 2025 | Hydrogen Production |
| Lost Cabin Gas Plant | Commercial CCS Facility | Operational | USA | 2013 | Natural Gas Processing |
| Louisiana Clean Energy Complex | Commercial CCS Facility | In Construction | USA | 2025 | Hydrogen Production |
| Marcus Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Marshall County ECBM Project | Pilot and Demonstration CCS Facility | Completed | USA | 2009 | Research and Development |
| Mason City Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Mendota BECCS | Commercial CCS Facility | Early Development | USA | 2025 | Bioenergy |
| Merrill Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| MGSC Validation Phase (Phase II): CO2 Storage and Enhanced Oil Recovery: Bald Unit Oil Field Test Site | Pilot and Demonstration CCS Facility | Completed | USA | 2009 | Research and Development |
| MGSC Validation Phase (Phase II): CO2 Storage and Enhanced Oil Recovery: Sugar Creek Oil Field Test Site | Pilot and Demonstration CCS Facility | Completed | USA | 2009 | Research and Development |
| Michigan Basin (Phase II) Geologic CO2 Sequestration Field Test | Pilot and Demonstration CCS Facility | Completed | USA | 2008 | Natural Gas Processing |
| Michigan Basin Large-Scale Injection Test | Pilot and Demonstration CCS Facility | Operational | USA | 2013 | Natural Gas Processing |
| Midwest AgEnergy Blue Flint ethanol CCS | Commercial CCS Facility | Early Development | USA | 2022 | Ethanol Production |
| Mina Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Mountaineer Validation Facility | Pilot and Demonstration CCS Facility | Completed | USA | 2009 | Power Generation |
| Mt. Simon CCS Hub (Iowa Illinois Carbon Pipeline) | Commercial CCS Facility | Early Development | USA | CO2 Transport and Storage | |
| Mustang Station of Golden Spread Electric Cooperative Carbon Capture | Commercial CCS Facility | Advanced Development | USA | Power Generation | |
| National Carbon Capture Center (NCCC) | Pilot and Demonstration CCS Facility | Operational | USA | 2011 | Research and Development |
| NET Power Clean Energy Large-scale Pilot Plant | Pilot and Demonstration CCS Facility | Operational | USA | 2018 | Power Generation |
| Nevada Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| NextDecade Rio Grande LNG CCS | Commercial CCS Facility | Early Development | USA | 2025 | Natural Gas Processing |
| Norfolk Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Northern Delaware Basin CCS | Commercial CCS Facility | Advanced Development | USA | 2023 | Natural Gas Processing |
| NuDACCS-Nuclear Direct Air CCS Project | Pilot and Demonstration CCS Facility | Advanced Development | USA | Direct Air Capture | |
| OCI Fertiliser | Commercial CCS Facility | In Construction | USA | 2025 | Fertiliser Production |
| One Earth Energy facility Carbon Capture | Commercial CCS Facility | Advanced Development | USA | 2025 | Ethanol Production |
| Onida Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Otter Tail Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Oxy-combustion of Heavy Liquid Fuels-15 MW Pilot Test | Pilot and Demonstration CCS Facility | Completed | USA | 2012 | Power Generation |
| PCS Nitrogen | Commercial CCS Facility | Operational | USA | 2013 | Fertiliser Production |
| Petra Nova Carbon Capture Project | Commercial CCS Facility | Operational | USA | 2017 | Power Generation |
| Plainview Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Plant Barry & Citronelle Integrated Project | Pilot and Demonstration CCS Facility | Completed | USA | 2012 | Power Generation |
| Plant Daniel Carbon Capture | Commercial CCS Facility | Advanced Development | USA | Power Generation | |
| Pleasant Prairie Power Plant Field Pilot | Pilot and Demonstration CCS Facility | Completed | USA | 2008 | Power Generation |
| Polk Power Station CCS | Commercial CCS Facility | Advanced Development | USA | Under Evaluation | Power Generation |
| Prairie State Generating Station Carbon Capture | Commercial CCS Facility | Advanced Development | USA | 2025 | Power Generation |
| Project Interseqt-Hereford Ethanol Plant | Commercial CCS Facility | Early Development | USA | 2023 | Ethanol Production |
| Project Interseqt-Plainview Ethanol Plant | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Project Tundra | Commercial CCS Facility | Advanced Development | USA | 2026 | Power Generation |
| Red Trail Energy CCS | Commercial CCS Facility | Operational | USA | 2022 | Ethanol Production |
| Redfield Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| River Bend CCS Louisiana Pipeline | Commercial CCS Facility | Early Development | USA | 2026 | CO2 Transport and Storage |
| San Juan Basin ECBM Storage Test | Pilot and Demonstration CCS Facility | Completed | USA | 2008 | Research and Development |
| Shenandoah Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Shute Creek Gas Processing Plant | Commercial CCS Facility | Operational | USA | 1986 | Natural Gas Processing |
| Sioux Center Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Steamboat Rock Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| STRATOS (1PointFive Direct Air Capture) | Commercial CCS Facility | In Construction | USA | 2024 | Direct Air Capture |
| Summit Carbon Solutions-Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | CO2 Transport and Storage |
| Summit Pipeline | Commercial CCS Facility | Advanced Development | USA | 2024 | Bioenergy |
| Superior Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Terrell Natural Gas Processing Plant (formerly Val Verde Natural Gas Plants) | Commercial CCS Facility | Operational | USA | 1972 | Natural Gas Processing |
| The Illinois Clean Fuels Project | Commercial CCS Facility | Early Development | USA | 2025 | Chemical Production |
| Valero Port Arthur Refinery | Commercial CCS Facility | Operational | USA | 2013 | Hydrogen Production |
| Velocys’ Bayou Fuels Negative Emission Project | Commercial CCS Facility | Early Development | USA | 2026 | Chemical Production |
| Wabash CO2 Sequestration | Commercial CCS Facility | Advanced Development | USA | 2022 | Fertiliser Production |
| Watertown Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Wentworth Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| West Pearl Queen CO2 Sequestration Pilot Test and Modelling Project | Pilot and Demonstration CCS Facility | Completed | USA | 2002 | Research and Development |
| Wood River Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
| Wyoming Integrated Test Center (ITC) | Pilot and Demonstration CCS Facility | Operational | USA | 2018 | Power Generation |
| York Biorefinery Carbon Capture and Storage | Commercial CCS Facility | Advanced Development | USA | 2024 | Ethanol Production |
References
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennell, P. An overview of CO2 capture technologies. Energy Environ. Sci. 2013, 3, 1645–1669. [Google Scholar] [CrossRef]
- International Energy Agency IEA. Storing CO2 through Enhanced Oil Recovery, Combining EOR with CO2 Storage (EOR) for Profit; IEA: Paris, France, 2015. [Google Scholar]
- IEA. World Energy Outlook; IEA: Paris, France, 2009. [Google Scholar]
- ECF. Roadmap 2050: A Practical Guide to a Prosperous, Low-Carbon Europe; European Climate Foundation: The Hague, The Netherlands, 2010. [Google Scholar]
- Hanak, D.P.; Anthony, E.J.; Manovic, V. A review of developments in pilot-plant testing and modelling of calcium looping process for CO2 capture from power generation systems. Energy Environ. Sci. 2015, 8, 2199–2249. [Google Scholar] [CrossRef]
- Yamasaki, A. An overview of CO2 mitigation options for global warming-emphasizing CO2 sequestration options. J. Chem. Eng. Jpn. 2003, 36, 361–375. [Google Scholar] [CrossRef]
- Mabon, L.; Shackley, S. Public engagement in discussing carbon capture and storage. In World Social Science Report 2013—Changing Global Environments; OECD: Paris, France, 2013; pp. 398–403. [Google Scholar]
- Bachu, S. Review of CO2 storage efficiency in deep saline aquifers. Int. J. Greenh. Gas Control. 2015, 40, 188–202. [Google Scholar] [CrossRef]
- Bai, X.; van der Leeuw, S.; O’Brien, K.; Berkhout, F.; Biermann, F.; Brondizio, E.S.; Cudennec, C.; Dearing, J.; Duraiappah, A.; Glaser, M.; et al. Plausible and desirable futures in the Anthropocene: A new research agenda. Glob. Environ. Chang. 2016, 39, 351–362. [Google Scholar] [CrossRef]
- Na, J.; Xu, T.; Yuan, Y.; Feng, B.; Tian, H.; Bao, X. An integrated study of fluid–rock interaction in a CO2-based enhanced geothermal system: A case study of Songliao Basin, China. Appl. Geochem. 2015, 59, 166–177. [Google Scholar] [CrossRef]
- GCCSI. Alberta Carbon Trunk Line (“ACTL”) with North West Sturgeon Refinery CO2 Stream. Glob CCS Inst. 2017. Available online: https://co2re.co/FacilityData (accessed on 1 January 2020).
- DECC. CCS Roadmap—Supporting Deployment of Carbon Capture and Storage in the UK. 2012. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/48317/4899-the-ccs-roadmap.pdf (accessed on 1 January 2020).
- Bachu, S. Screening and selection criteria, and characterisation techniques for the geological sequestration of carbon dioxide (CO2). In Developments and Innovation in Carbon Dioxide (CO2) Capture and Storage Technology; Woodhead Publishing Ltd.: Cambridge, UK, 2010. [Google Scholar] [CrossRef]
- Bachu, S.; Brulotte, M.; Grobe, M.; Stewart, S. Suitability of the Alberta Subsurface for Carbon Dioxide Sequestration in Geological Media; Alberta Energy and Utilities Board: Calgary, AB, Canada, 2000. [Google Scholar]
- Han, Y.; Ho, W.S.W. Recent advances in polymeric facilitated transport membranes for carbon dioxide separation and hydrogen purification. J. Polym. Sci. 2020, 58, 2435–2449. [Google Scholar] [CrossRef]
- MIT. Carbon Capture and Sequestration Technologies. Massachusetts Inst Technol. 2015. Available online: https://sequestration.mit.edu/tools/projects/index.html (accessed on 1 October 2022).
- Shukla, R.; Ranjith, P.; Haque, A.; Choi, X. A review of studies on CO2 sequestration and caprock integrity. Fuel 2010, 89, 2651–2664. [Google Scholar] [CrossRef]
- CO2CRC. Injection & Storage 2015. 2021. Available online: https://co2crc.com.au/research/storage-research/depleted-hydrocarbon-reservoir-co2-storage/ (accessed on 20 June 2022).
- Li, Q.; Wei, Y.-N.; Liu, G.; Lin, Q. Combination of CO2 geological storage with deep saline water recovery in western China: Insights from numerical analyses. Appl. Energy 2014, 116, 101–110. [Google Scholar] [CrossRef]
- Javaheri, M.; Jessen, K. Residual Trapping in Simultaneous Injection of CO2 and Brine in Saline Aquifers. In Proceedings of the SPE Western North American Region Meeting, Anchorage, AK, USA, 7–11 May 2011. [Google Scholar]
- Yang, F.; Pang, Z.; Lin, L.; Jia, Z.; Zhang, F.; Duan, Z.; Zong, Z. Hydrogeochem-ical and isotopic evidence for trans-formational flow in a sedimentary basin: Im-plications for CO2 storage. Appl. Geochem. 2013, 30, 4–15. [Google Scholar] [CrossRef]
- Frerichs, J.; Rakoczy, J.; Ostertag-Henning, C.; Krüger, M. Viability and Adaptation Potential of Indigenous Microorganisms from Natural Gas Field Fluids in High Pressure Incubations with Supercritical CO2. Environ. Sci. Technol. 2014, 48, 1306–1314. [Google Scholar] [CrossRef]
- Burnol, A.; Thinon, I.; Ruffine, L.; Herri, J.M. Influence of impurities (nitro-gen and methane) on the CO2 storage capacity as sediment-hosted gas hydrate—Application in the area of the Celtic Sea and the Bay of Biscay. Int. J. Greenh. Gas Control 2015, 35, 96–109. [Google Scholar] [CrossRef]
- Trémosa, J.; Castillo, C.; Vong, C.Q.; Kervévan, C.; Lassin, A.; Audigane, P. Long-term assessment of geochemical reactivity of CO2 storage in highly saline aquifers: Application to Ketzin, In Salah and Snøhvit storage sites. Int. J. Greenh. Gas Control 2014, 20, 2–26. [Google Scholar] [CrossRef]
- Procesi, M.; Cantucci, B.; Buttinelli, M.; Armezzani, G.; Quattrocchi, F.; Boschi, E. Strategic use of the underground in an energy mix plan: Synergies among CO2, CH4 geological storage and geothermal energy. Latium Region case study (Central Italy). Appl. Energy 2013, 110, 104–131. [Google Scholar] [CrossRef]
- Quattrocchi, F.; Boschi, E.; Spena, A.; Buttinelli, M.; Cantucci, B.; Procesi, M. Synergic and conflicting issues in planning underground use to produce energy in densely populated countries, as Italy. Geological storage of CO2, natural gas, geothermics and nuclear waste disposal. Appl. Energy 2013, 101, 393–412. [Google Scholar]
- Li, S.; Wang, P.; Wang, Z. Strategy to Enhance Geological CO2 Storage Capacity in Saline Aquifer. Geophys. Res. Lett. 2023, 50, e2022GL101431. [Google Scholar] [CrossRef]
- Bachu, S. Screening and ranking of sedimentary basins for sequestration of CO2 in geological media in response to climate change. Environ. Geol. 2003, 44, 277–289. [Google Scholar] [CrossRef]
- Wei, N.; Li, X.; Jiao, Z.; Stauffer, P.H.; Liu, S.; Ellett, K.; Middleton, R.S. A Hierarchical Framework for CO2 Storage Capacity in Deep Saline Aquifer Formations. Front. Earth Sci. 2022, 9, 777323. [Google Scholar] [CrossRef]
- Le Gallo, Y.; Couillens, P.; Manai, T. CO2 Sequestration in Depleted Oil or Gas Reservoirs. In Proceedings of the SPE International Conference on Health, Safety and Environment in Oil and Gas Exploration and Production, Kuala Lumpur, Malaysia, 20–22 March 2002; pp. 1390–1392. [Google Scholar] [CrossRef]
- Cantucci, B.; Montegrossi, G.; Vaselli, O.; Tassi, F.; Quattrocchi, F.; Perkins, E.H. Geochemical modeling of CO2 storage in deep reservoirs: The Weyburn Project (Canada) case study. Chem. Geol. 2009, 265, 181–197. [Google Scholar] [CrossRef]
- Wdowin, M.; Tarkowski, R.; Manecki, M. Petrographic-mineralogical and textural changes in reservoir and sealing rocks (Zaosie anticline) as a result of a long-term experiment in CO2-brine-rock interactions. Gospod. Surowcami Miner.-Miner. Resour. Manag. 2013, 29, 137–154. [Google Scholar] [CrossRef]
- Tapia, J.F.D.; Lee, J.Y.; Ooi, R.E.H.; Foo, D.C.Y.; Tan, R.R. A review of optimi-zation and decision-making models for the planning of CO2 capture, utilization and storage (CCUS) systems. Sustain. Prod. Consum. 2018, 13, 1–15. [Google Scholar] [CrossRef]
- Armitage, P.J.; Worden, R.H.; Faulkner, D.R.; Aplin, A.C.; Butcher, A.R.; Espie, A.A. Mercia Mudstone Formation caprock to carbon capture and storage sites: Petrology and petrophysical characteristics. J. Geol. Soc. 2013, 170, 119–132. [Google Scholar] [CrossRef]
- Buttinelli, M.; Procesi, M.; Cantucci, B.; Quattrocchi, F.; Boschi, E. The geo-database of caprock quality and deep saline aquifers distribution for geological storage of CO2 in Italy. Energy 2011, 36, 2968–2983. [Google Scholar] [CrossRef]
- Kim, Y.; Jang, H.; Kim, J.; Lee, J. Prediction of storage efficiency on CO2 sequestration in deep saline aquifers using artificial neural network. Appl. Energy 2017, 185, 916–928. [Google Scholar] [CrossRef]
- Zhao, X.; Liao, X.; Wang, W.; Chen, C.; Rui, Z.; Wang, H. The CO2 storage capacity evaluation: Methodology and determination of key factors. J. Energy Inst. 2014, 87, 297–305. [Google Scholar] [CrossRef]
- Kneafsey, T.J.; Pruess, K. Laboratory Flow Experiments for Visualizing Carbon Dioxide-Induced, Density-Driven Brine Convection. Transp. Porous Media 2010, 82, 123–139. [Google Scholar] [CrossRef]
- Gunter, W.D.; Bachu, S.; Benson, S. The role of hydrogeological and geochemical trapping in sedimentary basins for secure geological storage of carbon dioxide. Geol. Soc. Lond. Spéc. Publ. 2004, 233, 129–145. [Google Scholar] [CrossRef]
- Sundal, A.; Hellevang, H.; Miri, R.; Dypvik, H.; Nystuen, J.P.; Aagaard, P. Variations in mineralization potential for CO2 related to sedimentary facies and burial depth—A comparative study from the North Sea. Energy Procedia 2014, 63, 5063–5070. [Google Scholar] [CrossRef]
- Sigman, D.M.; Fripiat, F.; Studer, A.S.; Kemeny, P.C.; Martínez-García, A.; Hain, M.P.; Ai, X.; Wang, X.; Ren, H.; Haug, G.H. The Southern Ocean during the ice ages: A review of the Antarctic surface isolation hypothesis, with comparison to the North Pacific. Quat. Sci. Rev. 2021, 254, 106732. [Google Scholar] [CrossRef]
- Heinemann, N.; Stewart, R.; Wilkinson, M.; Pickup, G.; Haszeldine, R. Hydrodynamics in subsurface CO2 storage: Tilted contacts and increased storage security. Int. J. Greenh. Gas Control 2016, 54, 322–329. [Google Scholar] [CrossRef]
- Zangeneh, H.; Jamshidi, S.; Soltanieh, M. Coupled optimization of enhanced gas recovery and carbon dioxide sequestration in natural gas reservoirs: Case study in a real gas field in the south of Iran. Int. J. Greenh. Gas Control 2013, 17, 515–522. [Google Scholar] [CrossRef]
- Gao, R.S.; Sun, A.Y.; Nicot, J.P. Identification of a representative dataset for long-term monitoring at the Weyburn CO2-injection enhanced oil recovery site, Saskatchewan, Canada. Int. J. Greenh. Gas Control 2016, 54, 454–465. [Google Scholar] [CrossRef]
- BGS. Man-Made (Anthropogenic) Greenhouse Gases. 2017. Available online: https://www.bgs.ac.uk/discovering-geology/climate-change/CCS/Anthropogenic.html (accessed on 23 September 2022).
- Jaramillo, P.; Griffin, W.M.; Matthews, H.S. Comparative Analysis of the Production Costs and Life-Cycle GHG Emissions of FT Liquid Fuels from Coal and Natural Gas. Environ. Sci. Technol. 2008, 42, 7559–7565. [Google Scholar] [CrossRef]
- IPCC. Special Report on Global Warming, Summary for Policymakers; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Marston, P. Bridging the Gap: An Analysis and Comparison of Legal and Regulatory Frameworks for CO2-EOR and CO2-CCS; Global CCS Institute: Melbourne, Australia, 2013. [Google Scholar]
- Porter, R.T.; Fairweather, M.; Pourkashanian, M.; Woolley, R.M. The range and level of impurities in CO2 streams from different carbon capture sources. Int. J. Greenh. Gas Control. 2015, 36, 161–174. [Google Scholar] [CrossRef]
- Jarrell, P.M.; Fox, C.E.; Stein, M.H.; Webb, S.L. Practical Aspects of CO2 Flood-ing; SPE Monograph Series No. 22; Society of Petroleum Engineers: Richardson, TX, USA, 2002; 220p. [Google Scholar]
- IEAGHG. Long Term Integrity of CO2 Storage—Well Abandonment; IEAGHG: Cheltenham, UK, 2009. [Google Scholar]
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low-Carbon Technol. 2012, 9, 157–177. [Google Scholar] [CrossRef]
- White, D. Monitoring CO2 storage during EOR at the Weyburn-Midale Field. Geophysics 2009, 28, 838–842. [Google Scholar] [CrossRef]
- Thomas, S. Enhanced Oil Recovery—An Overview. Oil Gas Sci. Technol. Rev. L’ifp 2008, 63, 9–19. [Google Scholar] [CrossRef]
- Zaluski, W.; El-Kaseeh, G.; Lee, S.-Y.; Piercey, M.; Duguid, A. Monitoring technology ranking methodology for CO2-EOR sites using the Weyburn-Midale Field as a case study. Int. J. Greenh. Gas Control 2016, 54, 466–478. [Google Scholar] [CrossRef]
- Verdon, J.P. Using microseismic data recorded at the Weyburn CCS-EOR site to assess the likelihood of induced seismic activity. Int. J. Greenh. Gas Control 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Hutcheon, I.; Shevalier, M.; Durocher, K.; Bloch, J.; Johnson, G.; Nightingale, M.; Mayer, B. Interactions of CO2 with formation waters, oil and minerals and CO2 storage at the Weyburn IEA EOR site, Saskatchewan, Canada. Int. J. Greenh. Gas Control 2016, 53, 354–370. [Google Scholar] [CrossRef]
- Perera, M.S.A.; Gamage, R.P.; Rathnaweera, T.D.; Ranathunga, A.S.; Koay, A.; Choi, X.A. Review of CO2-Enhanced Oil Recovery with a Simulated Sensitivity Analysis. Energies 2016, 9, 481. [Google Scholar] [CrossRef]
- Tenasaka, I. Bridging the Commercial Gap for Carbon Capture and Storage; Global CCS Institute: Chevy Chase, MD, USA, 2011. [Google Scholar]
- Kuuskraa, V.; Ferguson, R. Storing CO2 with Enhanced Oil Recovery; IEA: Washington, DC, USA, 2008. [Google Scholar]
- Krooss, B.; van Bergen, F.; Gensterblum, Y.; Siemons, N.; Pagnier, H.; David, P. High-pressure methane and carbon dioxide adsorption on dry and moisture-equilibrated Pennsylvanian coals. Int. J. Coal Geol. 2002, 51, 69–92. [Google Scholar] [CrossRef]
- Gilliland, E.S.; Ripepi, N.; Conrad, M.; Miller, M.J.; Karmis, M. Selection of monitoring techniques for a carbon storage and enhanced coalbed methane recovery pilot test in the Central Appalachian Basin. Int. J. Coal Geol. 2013, 118, 105–112. [Google Scholar] [CrossRef]
- Lakeman, B. Alberta Research Council Enhanced Coalbed Methane Recovery Project in Alberta, Canada; Advanced Resources International: Arlington, VA, USA, 2016. [Google Scholar]
- McGrail, B.P.; Schaef, H.T.; Ho, A.M.; Chien, Y.-J.; Dooley, J.J.; Davidson, C.L. Potential for carbon dioxide sequestration in flood basalts. J. Geophys. Res. Solid Earth 2006, 111, B12201. [Google Scholar] [CrossRef]
- Pollyea, R.M.; Fairley, J.P.; Podgorney, R.K.; Mcling, T.L. Physical constraints on geologic CO2 sequestration in low-volume basalt formations. GSA Bull. 2014, 126, 344–351. [Google Scholar] [CrossRef]
- Matter, J.M.; Stute, M.; Snæbjörnsdottir, S.Ó.; Oelkers, E.H.; Gislason, S.R.; Aradottir, E.S.; Sigfusson, B.; Gunnarsson, I.; Sigurdardottir, H.; Gunnlaugsson, E.; et al. Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions. Science 2016, 352, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Anthonsen, K.; Aagaard, P.; Bergmo, P.E.; Gislason, S.; Lothe, A.; Mortensen, G.; Snæbjörnsdóttir, S. Characterisation and Selection of the Most Prospective CO2 Storage Sites in the Nordic Region. Energy Procedia 2014, 63, 4884–4896. [Google Scholar] [CrossRef]
- Van Pham, T.H.; Aagaard, P.; Hellevang, H. On the potential for CO2 mineral storage in continental flood basalts—PHREEQC batch- and 1D diffusion–reaction simulations. Geochem. Trans. 2012, 13, 5. [Google Scholar] [CrossRef]
- Seifritz, W. CO2 disposal by means of silicates. Nature 1990, 345, 486. [Google Scholar] [CrossRef]
- Andreani, M.; Luquot, L.; Gouze, P.; Godard, M.; Hoisé, E.; Gibert, B. Experimental Study of Carbon Sequestration Reactions Controlled by the Percolation of CO2-Rich Brine through Peridotites. Environ. Sci. Technol. 2009, 43, 1226–1231. [Google Scholar] [CrossRef]
- Nobre, C.A.; Sellers, P.J.; Shukla, J. Amazonian Deforestation and Regional Climate Change on JSTOR. 2012. Available online: https://www.jstor.org/stable/26196408?saml_data=eyJzYW1sVG9rZW4iOiJjNGVkZDY0OC1kOTNlLTQxZWMtODE0ZS04M2NhMzA3ZjJhYjIiLCJpbnN0aXR1dGlvbklkcyI6WyI1ZjhlMTUzZS02MzI1LTQ5NTgtOWRmZC0xOTBjNjg1YjE0MDQiXX0#metadata_info_tab_contents (accessed on 28 August 2022).
- Circone, S.; Stern, L.A.; Kirby, S.H.; Durham, W.B.; Chakoumakos, B.C.; Rawn, C.J.; Rondinone, A.J.; Ishii, Y. CO2 hydrate: Synthesis, composition, structure, dissociation behavior, and a comparison to structure I CH4 hydrate. J. Phys. Chem. B 2003, 107, 5529–5539. [Google Scholar] [CrossRef]
- Rochelle, C.A.; Camps, A.P.; Long, D.; Milodowski, A.; Bateman, K.; Gunn, D.; Jackson, P.; Lovell, M.A.; Rees, J. Can CO2 hydrate assist in the underground storage of carbon dioxide? Geol. Soc. Spec. Publ. 2009, 319, 171–183. [Google Scholar] [CrossRef]
- Oldenburg, C.M. Carbon Sequestration in Natural Gas Reservoirs: Enhanced Gas Recovery and Natural Gas Storage; Lawrence Berkeley National Lab.: Berkeley, CA, USA, 2003. [Google Scholar]
- Jemai, K.; Kvamme, B.; Vafaei, M.T. Theoretical studies of CO2 hydrates formation and dissociation in cold aquifers using retrasocodebright simulator. WSEAS Trans. Heat. Mass. Transf. 2014, 9, 150–168. [Google Scholar]
- Talaghat, M.; Esmaeilzadeh, F.; Fathikaljahi, J. Experimental and theoretical investigation of simple gas hydrate formation with or without presence of kinetic inhibitors in a flow mini-loop apparatus. Fluid Phase Equilibria 2009, 279, 28–40. [Google Scholar] [CrossRef]
- Ghavipour, M.; Ghavipour, M.; Chitsazan, M.; Najibi, S.H.; Ghidary, S.S. Experimental study of natural gas hydrates and a novel use of neural network to predict hydrate formation conditions. Chem. Eng. Res. Des. 2013, 91, 264–273. [Google Scholar] [CrossRef]
- Ruffine, L.; Donval, J.; Charlou, J.; Cremière, A.; Zehnder, B. Experimental study of gas hydrate formation and destabilisation using a novel high-pressure apparatus. Mar. Pet. Geol. 2010, 27, 1157–1165. [Google Scholar] [CrossRef]
- Rehder, G.; Leifer, I.; Brewer, P.G.; Friederich, G.; Peltzer, E.T. Controls on methane bubble dissolution inside and outside the hydrate stability field from open ocean field experiments and numerical modeling. Mar. Chem. 2009, 114, 19–30. [Google Scholar] [CrossRef]
- Khabibullin, T.; Falcone, G.; Teodoriu, C. Drilling through gas-hydrate sediments: Managing wellbore-stability risks. SPE Drill. Complet. 2011, 26, 287–294. [Google Scholar] [CrossRef]
- Garapati, N.; Randolph, J.B.; Saar, M.O. Brine displacement by CO2, energy extraction rates, and lifespan of a CO2-limited CO2-Plume Geothermal (CPG) system with a horizontal production well. Geothermics 2015, 55, 182–194. [Google Scholar] [CrossRef]
- Pruess, K. Enhanced geothermal systems (EGS) using CO2 as working fluid—A novel approach for generating renewable energy with simultaneous sequestration of carbon. Geothermics 2006, 35, 351–367. [Google Scholar] [CrossRef]
- Plaksina, T.; White, C. Modeling coupled convection and carbon dioxide injection for improved heat harvesting in geopressured geothermal reservoirs. Geotherm. Energy 2016, 4, 2. [Google Scholar] [CrossRef][Green Version]
- Assima, G.P.; Larachi, F.; Molson, J.; Beaudoin, G. Impact of temperature and oxygen availability on the dynamics of ambient CO2 mineral sequestration by nickel mining residues. Chem. Eng. J. 2014, 240, 394–403. [Google Scholar] [CrossRef]
- Johnson, E.E.; Scherwath, M.; Moran, K.; Dosso, S.E.; Rohr, K.M. Fault Slip Tendency Analysis for a Deep-Sea Basalt CO2 Injection in the Cascadia Basin. Geohazards 2023, 4, 121–135. [Google Scholar] [CrossRef]
- Bobicki, E.R.; Liu, Q.; Xu, Z.; Zeng, H. Carbon capture and storage using alkaline industrial wastes. Prog. Energy Combust. Sci. 2012, 38, 302–320. [Google Scholar] [CrossRef]
- Lim, M.; Han, G.-C.; Ahn, J.-W.; You, K.-S. Environmental Remediation and Conversion of Carbon Dioxide (CO2) into Useful Green Products by Accelerated Carbonation Technology. Int. J. Environ. Res. Public Health 2010, 7, 203–228. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, A.; Deiana, P.; Fiorini, P.; Girardi, G.; Stendardo, S. Possible optimal configurations for the ZECOMIX high efficiency zero emission hydrogen and power plant. Energy 2008, 33, 952–962. [Google Scholar] [CrossRef]
- Olajire, A.A. A review of mineral carbonation technology in sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Cipolli, F.; Gambardella, B.; Marini, L.; Ottonello, G.; Zuccolini, M.V. Geo-chemistry of high-pH waters from serpentinites of the Gruppo di Voltri (Genova, Italy) and reaction path modeling of CO2 sequestration in serpentinite aquifers. Appl. Geochem. 2004, 19, 787–802. [Google Scholar] [CrossRef]
- Bruni, J.; Canepa, M.; Chiodini, G.; Cioni, R.; Cipolli, F.; Longinelli, A.; Marini, L.; Ottonello, G.; Zuccolini, M.V. Irreversible water–rock mass transfer accompanying the generation of the neutral, Mg–HCO3 and high-pH, Ca–OH spring waters of the Genova province, Italy. Appl. Geochem. 2002, 17, 455–474. [Google Scholar] [CrossRef]
- Peletiri, S.P.; Rahmanian, N.; Mujtaba, I.M. CO2 Pipeline Design: A Review. Energies 2018, 11, 2184. [Google Scholar] [CrossRef]
- Kheirinik, M.; Ahmed, S.; Rahmanian, N. Comparative techno-economic analysis of carbon capture processes: Pre-combustion, post-combustion, and oxy-fuel combustion operations. Sustainability 2021, 13, 13567. [Google Scholar] [CrossRef]
- Huijgen, W.; Witkamp, G.; Comans, R. Carbondioxide Sequestration by Mineral Carbonation; Energy Research Centre of the Netherlands (ECN): Petten, The Netherlands, 2005. [Google Scholar]
- Adams, R.A.; Hayes, M.A. Water availability and successful lactation by bats as related to climate change in arid regions of western North America. J. Anim. Ecol. 2008, 77, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Tanhua, T.; Orr, J.C.; Lorenzoni, L.; Hansson, L. Monitoring ocean carbon and ocean acidification. WMO Bull. 2015, 64, 1. [Google Scholar]
- Hofmann, M.; Schellnhuber, H.J. Ocean acidification: A millennial challenge. Energy Environ. Sci. 2010, 3, 1883–1896. [Google Scholar] [CrossRef]
- Geochemistry of Geologic CO2 Sequestration; Mineralogical Society of America: Chantilly, VA, USA, 2017. Available online: https://www.osti.gov/servlets/purl/1165067 (accessed on 1 January 2020).
- Zero CO2. CCS-International Legislation. Zero Emiss Resour Organ. 2015. Available online: http://www.zeroco2.no/introduction/ccs-international-legislation (accessed on 3 September 2021).
- Xu, Y.; Ishizaka, J.; Aoki, S. Simulations of the distribution of sequestered CO2 in the North Pacific using a regional general circulation model. Energy Convers. Manag. 1999, 40, 683–691. [Google Scholar] [CrossRef]
- Masuda, Y.; Yamanaka, Y.; Sasai, Y.; Magi, M.; Ohsumi, T. Site selection in CO2 ocean sequestration: Dependence of CO2 injection rate on eddy activity distribution. Int. J. Greenh. Gas Control 2009, 3, 67–76. [Google Scholar] [CrossRef]
- Szizybalski, A.; Kollersberger, T.; Möller, F.; Martens, S.; Liebscher, A.; Kühn, M. Communication Supporting the Research on CO2 Storage at the Ketzin Pilot Site, Germany—A Status Report after Ten Years of Public Outreach. Energy Procedia 2014, 51, 274–280. [Google Scholar] [CrossRef]
- Bachu, S.; Bonijoly, D.; Bradshaw, J.; Burruss, R.; Holloway, S.; Christensen, N.P.; Mathiassen, O.M. CO2 storage capacity estimation: Methodology and gaps. Int. J. Greenh. Gas Control 2007, 1, 430–443. [Google Scholar] [CrossRef]
- Chen, M.; Masum, S.A.; Thomas, H.R. 3D hybrid coupled dual continuum and discrete fracture model for simulation of CO2 injection into stimulated coal reservoirs with parallel implementation. Int. J. Coal Geol. 2022, 262, 104103. [Google Scholar] [CrossRef]
- Fleury, M.; Pironon, J.; Le Nindre, Y.M.; Bildstein, O.; Berne, P.; Lagneau, V.; Broseta, D.; Pichery, T.; Fillacier, S.; Lescanne, M.; et al. Evaluating Sealing Efficiency of Caprocks for CO2 Storage: An Overview of the Geocarbone-Integrity Program and Results. Oil Gas Sci. Technol. Rev. IFP 2010, 65, 435–444. [Google Scholar] [CrossRef]
- GCCSI. Accelerating the Uptake of CCS: Industrial Use of Captured Carbon Dioxide; Global CCS Institute: Melbourne, Australia, 2011. [Google Scholar]
- Gilliland, E.; Ripepi, N.; Karmis, M.; Conrad, M. An examination of MVA techniques applicable for CCUS in thin, stacked coals of the central appalachian basin. In Proceedings of the 29th the International Pittsburgh Coal Conference, Pittsburgh, PA, USA, 15–18 October 2012; Volume 3, pp. 1931–1938. [Google Scholar]
- IEAGHG. Effects of Impurities on Geological Storage of CO2; IEAGHG: Cheltenham, UK, 2011. [Google Scholar]
- IEAGHG. CO2 Storage in Depleted Gas Fields; IEAGHG: Oxford, UK, 2009. [Google Scholar]
- Iglauer, S.; Pentland, C.H.; Busch, A. CO2 wettability of seal and reservoir rocks and the implications for carbon geo-sequestration. Water Resour. Res. 2015, 51, 729–774. [Google Scholar] [CrossRef]
- IPCC. Special Report on Carbon Dioxide Capture and Storage; IPCC: Cambridge, UK, 2005. [Google Scholar]
- Luo, T.; Zhou, L.; Jiao, Z.; Bai, Y.; Wang, S. The Ordos Basin: A Premier Basin for Integrating geological CO2 Storage with Enhanced oil Recovery Projects in China. Energy Procedia 2014, 63, 7772–7779. [Google Scholar] [CrossRef]
- Matter, J.M.; Broecker, W.S.; Gislason, S.R.; Gunnlaugsson, E.; Oelkers, E.H.; Stute, M.; Sigurdardóttir, H.; Stefansson, A.; Alfreðsson, H.A.; Aradóttir, E.S.; et al. The CarbFix Pilot Project—Storing carbon dioxide in basalt. Energy Procedia 2011, 4, 5579–5585. [Google Scholar] [CrossRef]
- Song, J.; Zhang, D. Comprehensive review of caprock-sealing mechanisms for geologic carbon sequestration. Environ. Sci. Technol. 2013, 47, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Wang, Y.; Chen, Z.; Liu, H. An integrated model with stable numerical methods for fractured underground gas storage. J. Clean. Prod. 2023, 393, 136268. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwenketishi, G.T.; Benkreira, H.; Rahmanian, N. A Comprehensive Review on Carbon Dioxide Sequestration Methods. Energies 2023, 16, 7971. https://doi.org/10.3390/en16247971
Mwenketishi GT, Benkreira H, Rahmanian N. A Comprehensive Review on Carbon Dioxide Sequestration Methods. Energies. 2023; 16(24):7971. https://doi.org/10.3390/en16247971
Chicago/Turabian StyleMwenketishi, Gregory Tarteh, Hadj Benkreira, and Nejat Rahmanian. 2023. "A Comprehensive Review on Carbon Dioxide Sequestration Methods" Energies 16, no. 24: 7971. https://doi.org/10.3390/en16247971
APA StyleMwenketishi, G. T., Benkreira, H., & Rahmanian, N. (2023). A Comprehensive Review on Carbon Dioxide Sequestration Methods. Energies, 16(24), 7971. https://doi.org/10.3390/en16247971









