Performance and Thermal Stress Evaluation of Full-Scale SOEC Stack Using Multi-Physics Modeling Method
Abstract
:1. Introduction
2. Model Development
- (1)
- All gases are ideal and incompressible, while the flow is laminar.
- (2)
- Thermal radiation inside the SOEC is negligible.
- (3)
- External environmental temperature remains stable at 800 °C.
- (4)
- The materials employed for the cathode, electrolyte layer and anode are isotropic and homogeneous, as well as linear and elastic following the isotropic Hooke’s law.
- (5)
- Certain physical parameters of the cathode, electrolyte layer, and anode materials (e.g., coefficient of thermal expansion, elastic modulus, and Poisson’s ratio) evaluated at 800 °C are unaffected by the local temperature.
- (6)
- The interface between the anodes/electrolyte layers/cathodes, as well as between the inter-connectors/sealing materials is treated as continuous, which can be freely deformed as a whole without any fractures.
2.1. Full-Scale Stack Geometric Parameters and Modeling Methods
2.2. Governing Equations
2.2.1. Electrochemical Reactions
2.2.2. Gas Flow and Momentum Equations
2.2.3. Gas Species Transport Equation
2.2.4. Heat Transfer Equation
2.2.5. Thermal Stress-Strain Relation and Failure Probability Analysis Equations
2.3. Boundary Conditions
2.4. Model Validation
3. Results and Discussion
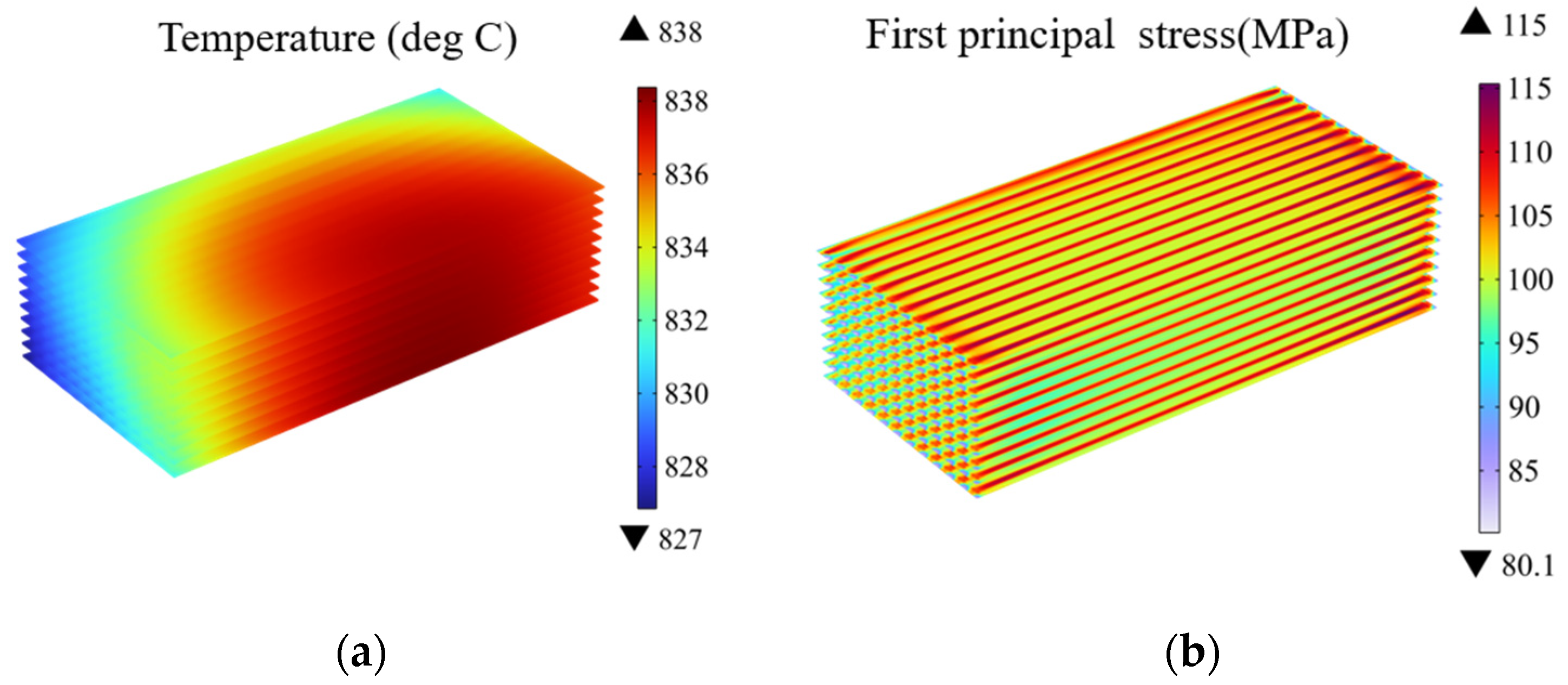
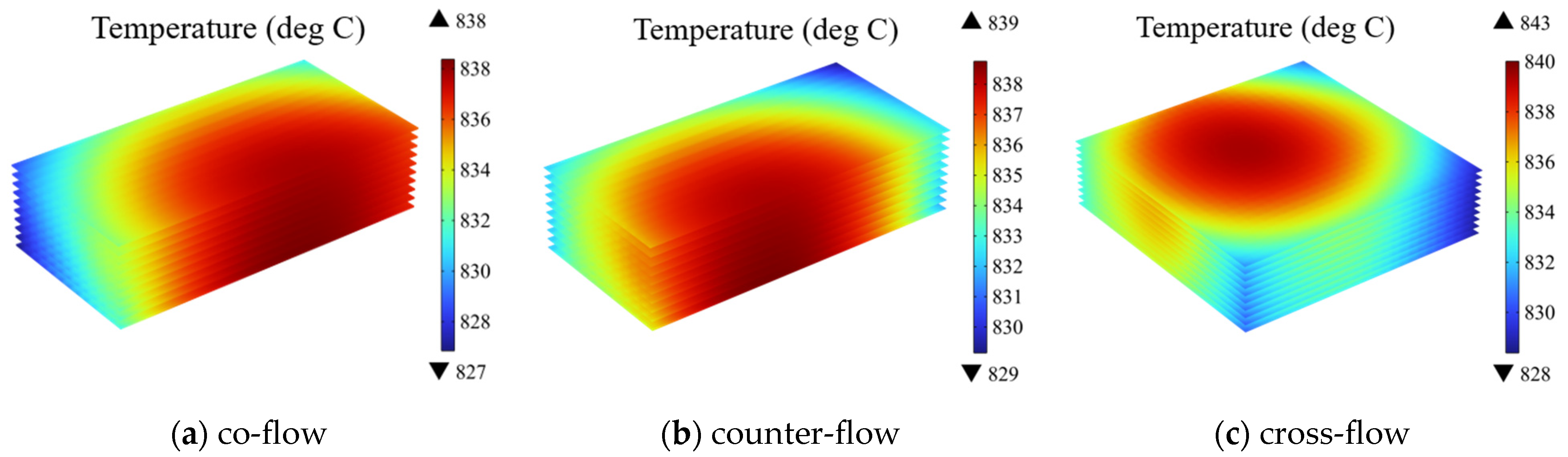
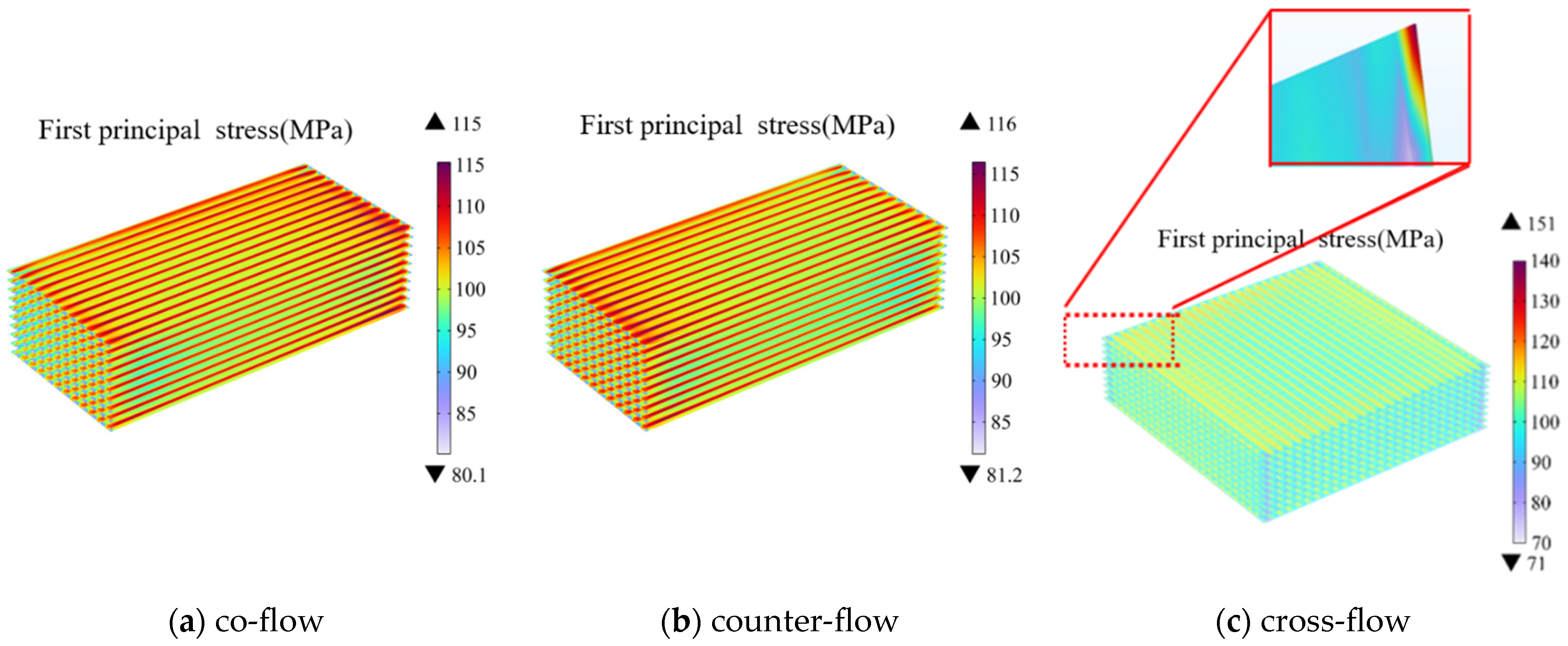
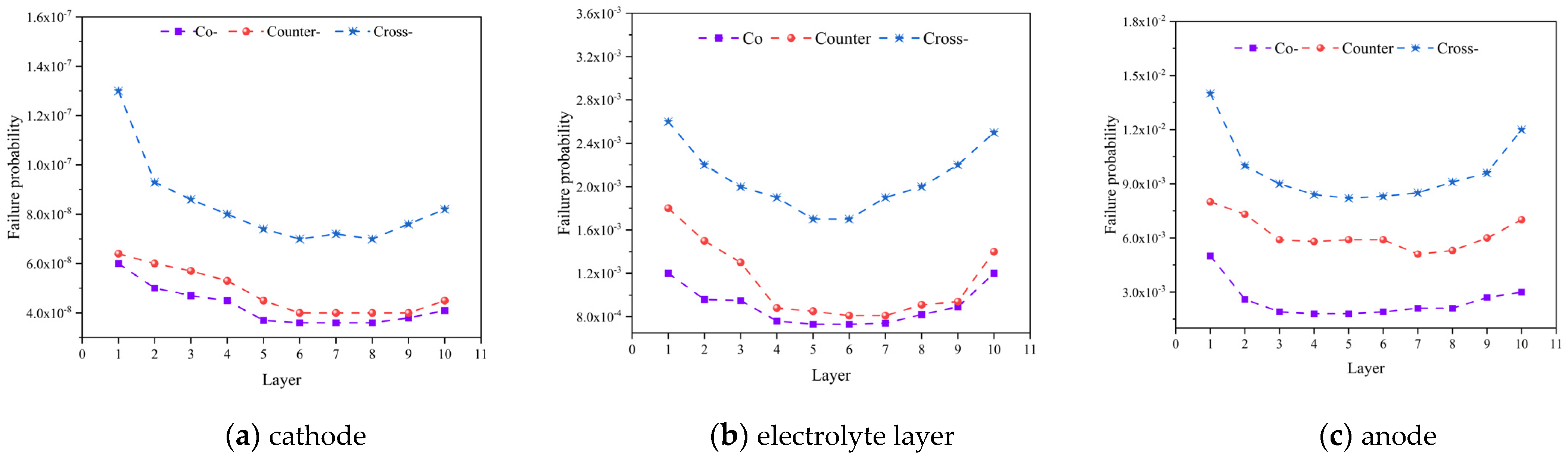
3.1. Effect of Gas Flow Arrangement and Stack Configuration
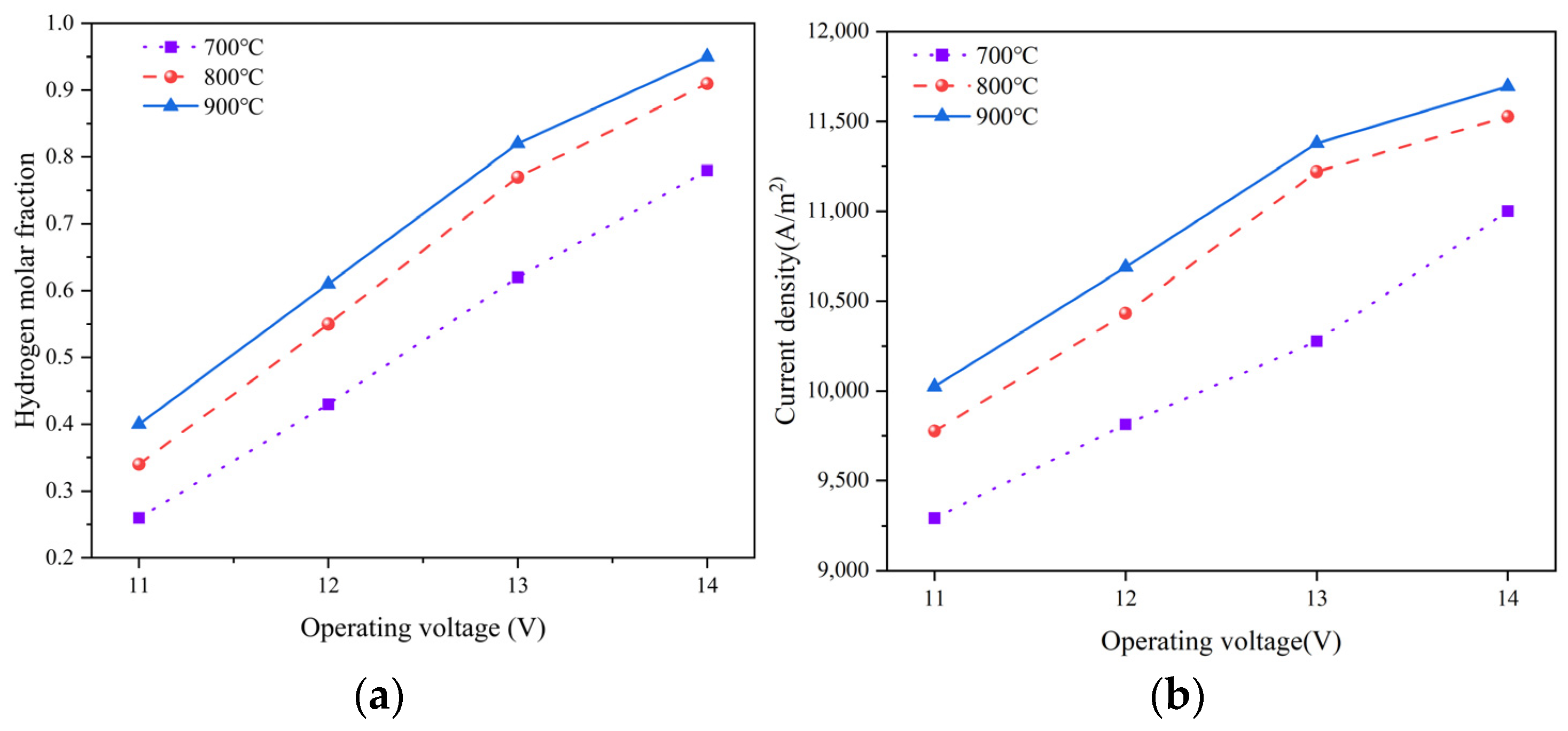
3.2. Effect of Operating Temperature
3.3. Effect of Manifold Layout
4. Conclusions
- (1)
- Among the three flow patterns, the cross-flow mode exhibits the highest electrolysis performance with 96.6% average hydrogen molar fraction predicted for the outlet region. Due to the complexity of its structure, the cross-flow mode also generates the highest thermal stress of 151 MPa, which is about 36 MPa higher than that in other flow cases. Consequently, the failure probability of this mode is notably higher.
- (2)
- Under all three flow patterns, the high-temperature regions within the electrolyte layer are located at the downstream of the air inlet, the biggest temperature difference of 15 ℃ predicted for the cross-flow case (about 5 ℃ higher than that in other two cases). And, the failure probability of the first cell is higher than that of the others. Within the PEN structure, the anode poses the highest risk of failure.
- (3)
- Increasing the operating temperature benefits the hydrogen production; however, due to limitations imposed by the reactant concentration at the TPB, when the operating temperature exceeds 800 °C, a minimal improvement in current density and hydrogen production is achieved with further elevation of the operating temperature. Therefore, 800 °C is the most suitable operating temperature.
- (4)
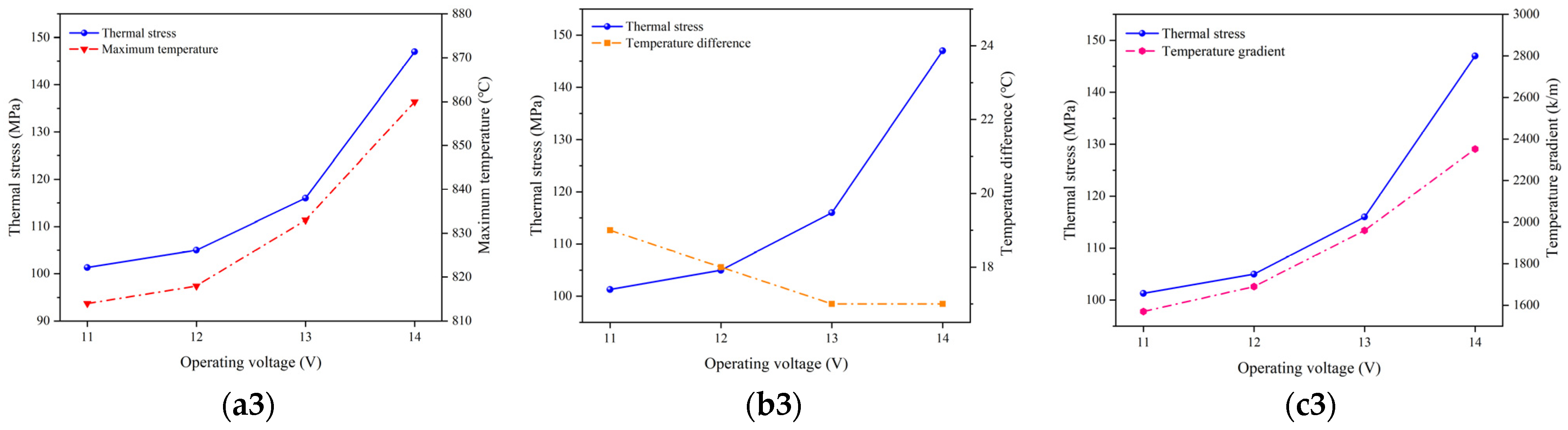
- Thermal stress is significantly higher in the bottom- and top-end cells compared to the others. Under three different operating temperatures, the maximum thermal stress in different electrolyte layers consistently occurs in the 1st cell. The higher operating voltages lead to more pronounced increases in the thermal stress. Changes in the thermal stress are primarily influenced by both the highest temperature and temperature gradient, with the latter one playing the dominant role.
- (5)
- Uniformity of the gas distribution within the cells is influenced by the number of both the manifold inlet and outlet. The 2-inlet and 1-outlet manifold design effectively enhances the gas uniformity within the channels.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Li, W.; Ma, L.; Li, W. Degradation of solid oxide electrolysis cells: Phenomena, mechanisms, and emergingmitigation strategies—A review. J. Mater. Sci. Technol. 2020, 55, 35–55. [Google Scholar] [CrossRef]
- Graves, C.; Ebbesen, S.D.; Mogensen, M. Sustainable hydrocarbon fuels by recycling CO2 and H2O with renewable or nuclear energy. Renew. Sustain. Energy Rev. 2011, 15, 1–23. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, S.; Wang, M. Thermal stress analysis at the interface of cathode and electrolyte in solid oxide fuel cells. Int. Commun. Heat Mass Transf. 2020, 118, 104831. [Google Scholar] [CrossRef]
- Xu, M.; Li, T.; Yang, M. Modeling of an anode supported solid oxide fuel cell focusing on thermal stresses. Int. J. Hydrogen Energy 2016, 41, 14927–14940. [Google Scholar] [CrossRef]
- Zhang, S.; Peters, R.; Varghese, B. Modeling of reversible solid oxide cell stacks with an open-source library. J. Electrochem. Soc. 2022, 169, 114501. [Google Scholar] [CrossRef]
- Li, J.; Lin, Z. Effects of electrode composition on the electrochemical performance and mechanical property of micro-tubular solid oxide fuel cell. Int. J. Hydrogen Energy 2012, 37, 12925–12940. [Google Scholar] [CrossRef]
- Nerat, M.; Juricic, D. Modelling of anode delamination in solid oxide electrolysis cell and analysis of its effects on electrochemical performance. Int. J. Hydrogen Energy 2018, 43, 8179–8189. [Google Scholar] [CrossRef]
- Navasa, M.; Graves, C.; Chatzichristodoulou, C. A three dimensional multi-physics model of a solid oxide electrochemical cell: A tool for understanding degradation. Int. J. Hydrogen Energy 2018, 43, 11913–11931. [Google Scholar] [CrossRef]
- Du, Y.; Qin, Y.; Zhang, G. Modelling of effect of pressure on co-electrolysis of water and carbon dioxide in solid oxide electrolysis cell. Int. J. Hydrogen Energy 2019, 44, 3456–3469. [Google Scholar] [CrossRef]
- Navasa, M.; Miao, X.; Frandsen, H.L. A fully-homogenized multi-physics model for a reversible solid oxide cell stack. Int. J. Hydrogen Energy 2019, 44, 23330–23347. [Google Scholar] [CrossRef]
- Stempien, J.P.; Ding, O.L.; Sun, Q. Energy and exergy analysis of solid oxide electrolyser cell (SOEC) working as a CO2 mitigation device. Int. J. Hydrogen Energy 2012, 37, 14518–14527. [Google Scholar] [CrossRef]
- Wang, C.; Chen, M.; Liu, M. Dynamic modeling and parameter analysis study on reversible solid oxide cells during mode switching transient processes. Appl. Energy 2020, 263, 114601. [Google Scholar] [CrossRef]
- Bernadet, L.; Gousseau, G.; Chatroux, A. Influence of pressure on solid oxide electrolysis cells investigated by experimental and modeling approach. Int. J. Hydrogen Energy 2015, 40, 12918–12928. [Google Scholar] [CrossRef]
- Udagawa, J.; Aguiar, P.; Brandon, N.P. Hydrogen production through steam electrolysis: Model-based dynamic behaviour of a cathode-supported intermediate temperature solid oxide electrolysis cell. J. Power Sources 2008, 180, 46–55. [Google Scholar] [CrossRef]
- Jin, X.; Xue, X. Mathematical modeling analysis of regenerative solid oxide fuel cells in switching mode conditions. J. Power Sources 2010, 195, 6652–6658. [Google Scholar] [CrossRef]
- Kazempoor, P.; Braun, R.J. Model validation and performance analysis of regenerative solid oxide cells: Electrolytic operation. Int. J. Hydrogen Energy 2014, 39, 2669–2684. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, X.; Li, G. Comparative performance investigation of different gas flow configurations for a planar solid oxide electrolyzer cell. Int. J. Hydrogen Energy 2017, 42, 10785–10801. [Google Scholar] [CrossRef]
- Cui, T.; Xiao, G.; Yan, H. Numerical simulation and analysis of the thermal stresses of a planar solid oxide electrolysis cell. Int. J. Green Energy 2022, 20, 432–444. [Google Scholar] [CrossRef]
- Buttler, A.; Koltun, R.; Wolf, R. A detailed techno-economic analysis of heat integration in high temperature electrolysis for efficient hydrogen production. Int. J. Hydrogen Energy 2015, 40, 38–50. [Google Scholar] [CrossRef]
- Ni, M. Modeling of a solid oxide electrolysis cell for carbon dioxide electrolysis. Chem. Eng. J. 2010, 164, 246–254. [Google Scholar] [CrossRef]
- Hernández-Pacheco, E.; Singh, D.; Hutton, P.N. A macro-level model for determining the performance characteristics of solid oxide fuel cells. J. Power Sources 2004, 138, 174–186. [Google Scholar] [CrossRef]
- Costamagna, P.; Honegger, K. Modeling of solid oxide heat exchanger integrated stacks and simulation at high fuel utilization. J. Electrochem. Soc. 1998, 145, 3995–4007. [Google Scholar] [CrossRef]
- Jiang, C.; Gu, Y.; Guan, W. 3D thermo-electro-chemo-mechanical coupled modeling of solid oxide fuel cell with double-sided cathodes. Int. J. Hydrogen Energy 2020, 45, 904–915. [Google Scholar] [CrossRef]
- Zheng, J.; Xiao, L.; Wu, M. Numerical analysis of thermal stress for a stack of planar solid oxide fuel cells. Energies 2022, 15, 343. [Google Scholar] [CrossRef]
- Zhang, Z.; Yue, D.; Yang, G. Three-dimensional CFD modeling of transport phenomena in multi-channel anode-supported planar SOFCs. Int. J. Heat Mass Transf. 2015, 84, 942–954. [Google Scholar] [CrossRef]
- Frandsen, H.L. Weibull statistics effective area and volume in the ball-on-ring testing method. Mech. Mater. 2014, 73, 28–37. [Google Scholar] [CrossRef]
- Li, Z.; Yang, G.; Cui, D. Modeling and evaluating of thermo-electro-chemo-mechanical behavior for pre-reformed methane-fueled solid oxide fuel cell. J. Power Sources 2022, 522, 230981. [Google Scholar] [CrossRef]
- Radovic, M.; Lara-Curzio, E. Mechanical properties of tape cast nickel-based anode materials for solid oxide fuel cells before and after reduction in hydrogen. Acta Mater. 2004, 52, 5747–5756. [Google Scholar] [CrossRef]
- Banerjee, A.; Wang, Y.; Diercks, J. Hierarchical modeling of solid oxide cells and stacks producing syngas via H2O/CO2 Co-electrolysis for industrial applications. Appl. Energy 2018, 230, 996–1013. [Google Scholar] [CrossRef]
- Frandsen, H.L.; Makowska, M.; Greco, F. Accelerated creep in solid oxide fuel cell anode supports during reduction. J. Power Sources 2016, 323, 78–89. [Google Scholar] [CrossRef]








| Parameter | Value | Unit |
|---|---|---|
| Cell length | 89 | mm |
| Cell width | 89 | mm |
| Channel height | 0.5 | mm |
| Channel width | 2 | mm |
| Rib width | 1.5 | mm |
| Number of channels | 25 | - |
| Cathode thickness | 410 | μm |
| Electrolyte layer thickness | 10 | μm |
| Anode thickness | 60 | μm |
| Inter-connector thickness | 1 | mm |
| γ/A·m−2 | Eact/J·mol−1 | |
|---|---|---|
| Cathode | 1.34 × 1010 | 1 × 105 |
| Anode | 2.05 × 108 | 1.2 × 105 |
| Parameter | Heat Capacity (W·kg−1·K−1) | Thermal Conductivity (W·m−1·K−1) | Porosity | Permeability (m2) |
|---|---|---|---|---|
| Seal | 0.064 | |||
| Anode | 430 | 6 | 0.3 | 2 × 10−11 |
| Electrolyte | 550 | 2.7 | - | - |
| Cathode | 450 | 11 | 0.3 | 2 × 10−11 |
| Inter-connector | 475 | 44.5 | - | - |
| Components | σw (MPa) | m |
|---|---|---|
| Cathode | 128 | 13 |
| Electrolyte layer | 282 | 8 |
| Anode | 75 | 4 |
| Boundary Conditions | Value | Unit |
|---|---|---|
| Cathode inlet gas | 90% H2O, 10%H2 | - |
| Flow rate of the cathode | 1.37 | m/s |
| Flow rate of the anode | 2.72 | m/s |
| Environment temperature | 800 | °C |
| Convective heat transfer coefficient | 2 | W∙m−2∙K−1 |
| Surface emissivity | 0.3 | - |
| Free-stress temperature | 800 | °C |
| Anode inlet gas | 21%O2, 79%N2 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Xiao, L.; Liu, Y.; Zhang, X.; Zhou, R.; Liu, F.; Yuan, J. Performance and Thermal Stress Evaluation of Full-Scale SOEC Stack Using Multi-Physics Modeling Method. Energies 2023, 16, 7720. https://doi.org/10.3390/en16237720
Wang H, Xiao L, Liu Y, Zhang X, Zhou R, Liu F, Yuan J. Performance and Thermal Stress Evaluation of Full-Scale SOEC Stack Using Multi-Physics Modeling Method. Energies. 2023; 16(23):7720. https://doi.org/10.3390/en16237720
Chicago/Turabian StyleWang, Hao, Liusheng Xiao, Yingqi Liu, Xueping Zhang, Ruidong Zhou, Fangzheng Liu, and Jinliang Yuan. 2023. "Performance and Thermal Stress Evaluation of Full-Scale SOEC Stack Using Multi-Physics Modeling Method" Energies 16, no. 23: 7720. https://doi.org/10.3390/en16237720
APA StyleWang, H., Xiao, L., Liu, Y., Zhang, X., Zhou, R., Liu, F., & Yuan, J. (2023). Performance and Thermal Stress Evaluation of Full-Scale SOEC Stack Using Multi-Physics Modeling Method. Energies, 16(23), 7720. https://doi.org/10.3390/en16237720







