Evaluation of Pt-Co Nano-Catalyzed Membranes for Polymer Electrolyte Membrane Fuel Cell Applications
Abstract
:1. Introduction
2. Experimental details
2.1. Materials and Methods
2.1.1. Materials
2.1.2. Physical Characterizations
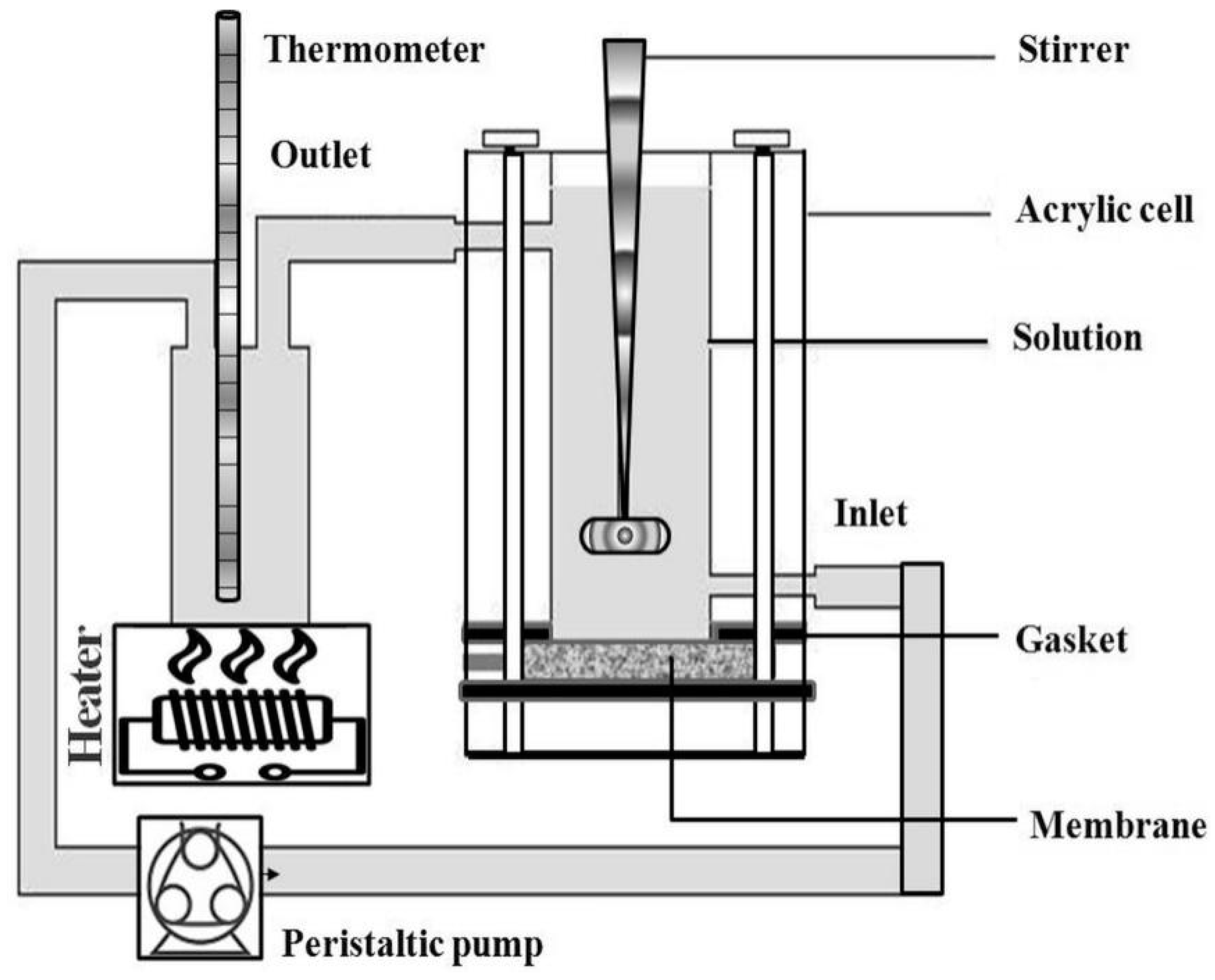
2.1.3. Electrochemical Characterizations
2.2. Membrane Pretreatment
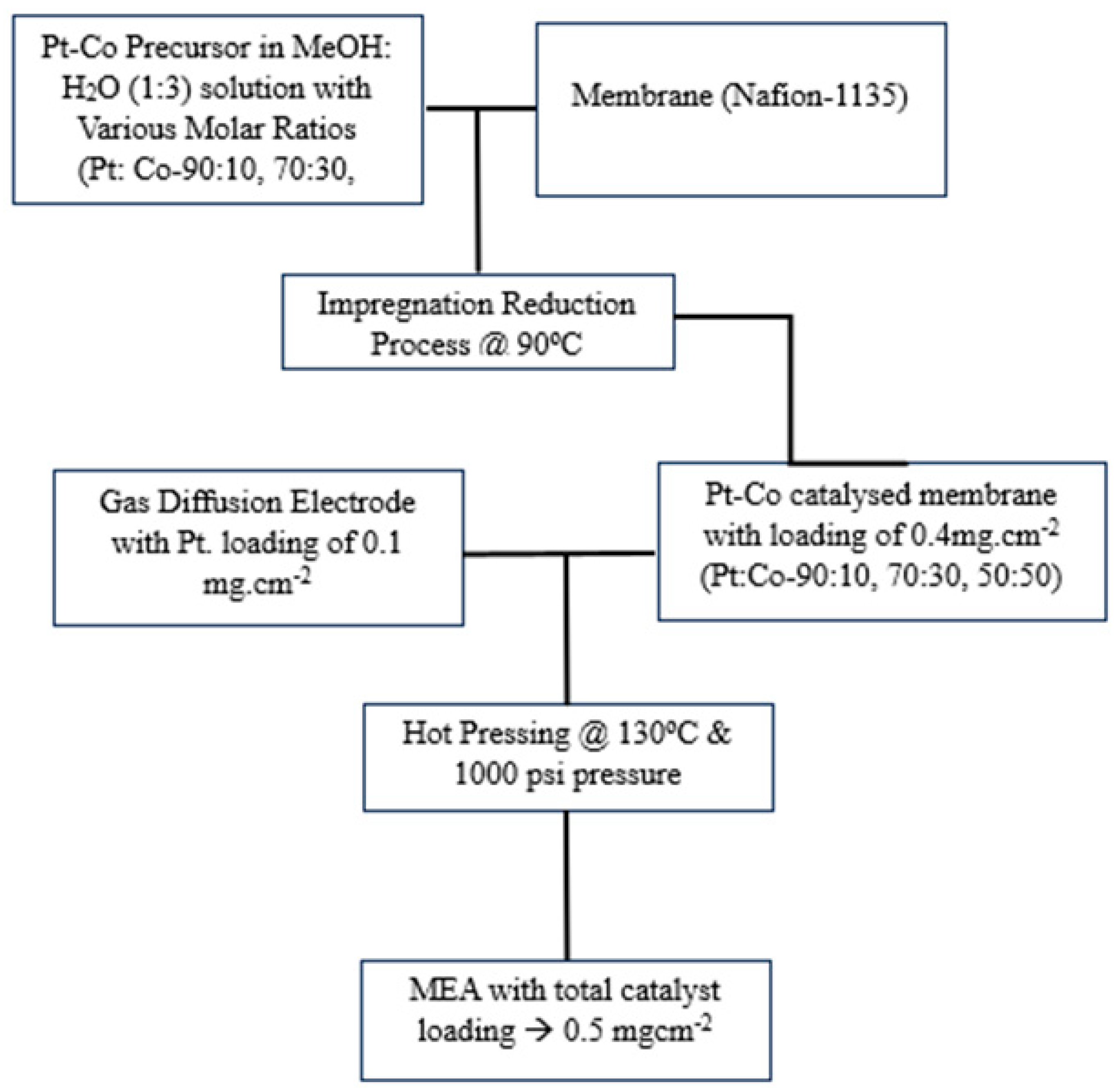
2.3. Preparation of Platinum-Cobalt (Pt-Co) Nano-Catalyzed Membranes
2.4. MEA Fabrication from Pt-Co Nano-Catalyzed Membranes
2.5. Physical Characterization of Pt-Co Nano-Catalyzed Membrane
2.5.1. XRD Analysis
2.5.2. High-Resolution Scanning Electron Microscopic Studies (HR-SEM) with EDX
2.6. Electrochemical Characterization of Pt-Co Nano-Catalyzed Membranes
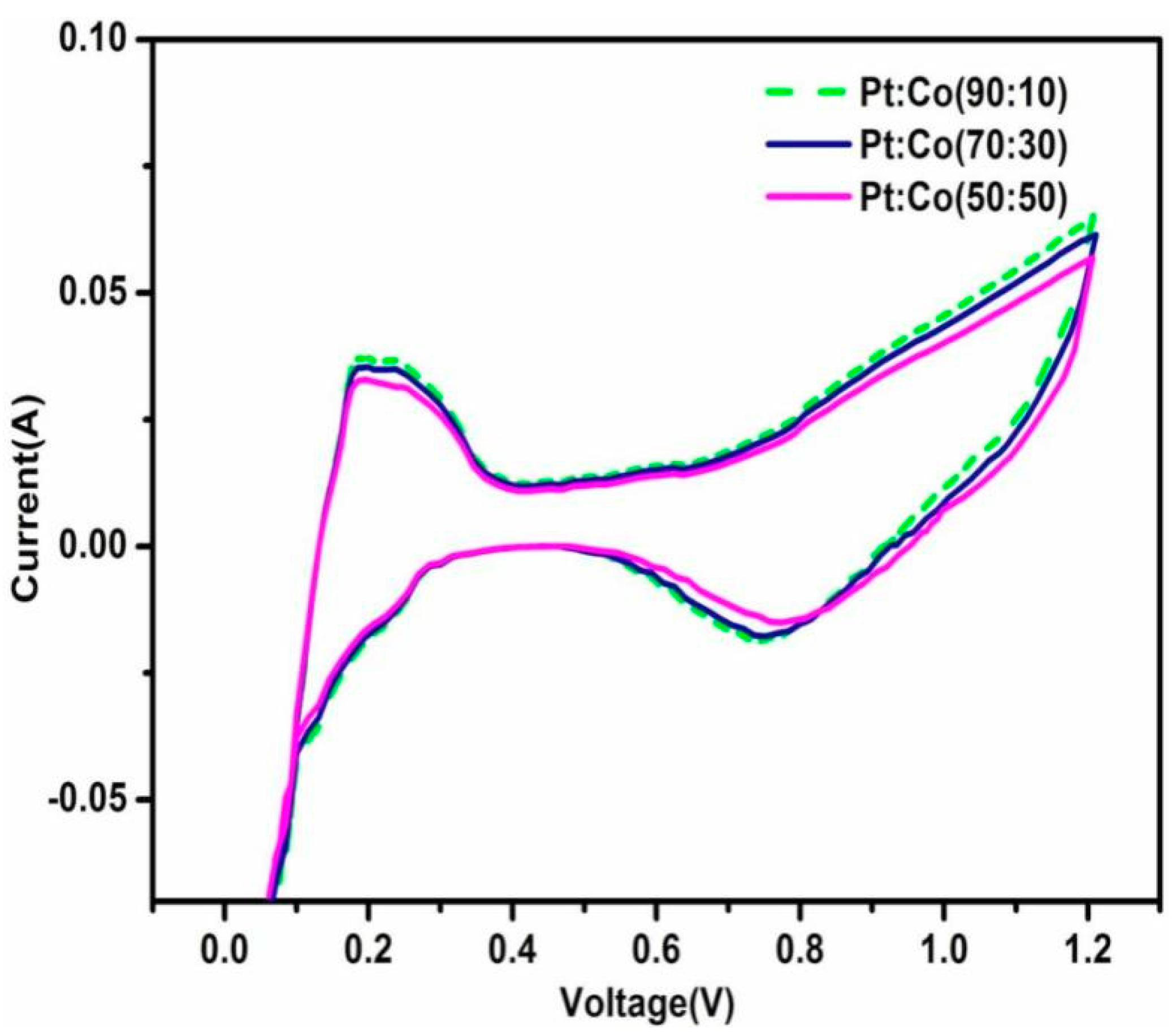
Cyclic Voltammetry Studies
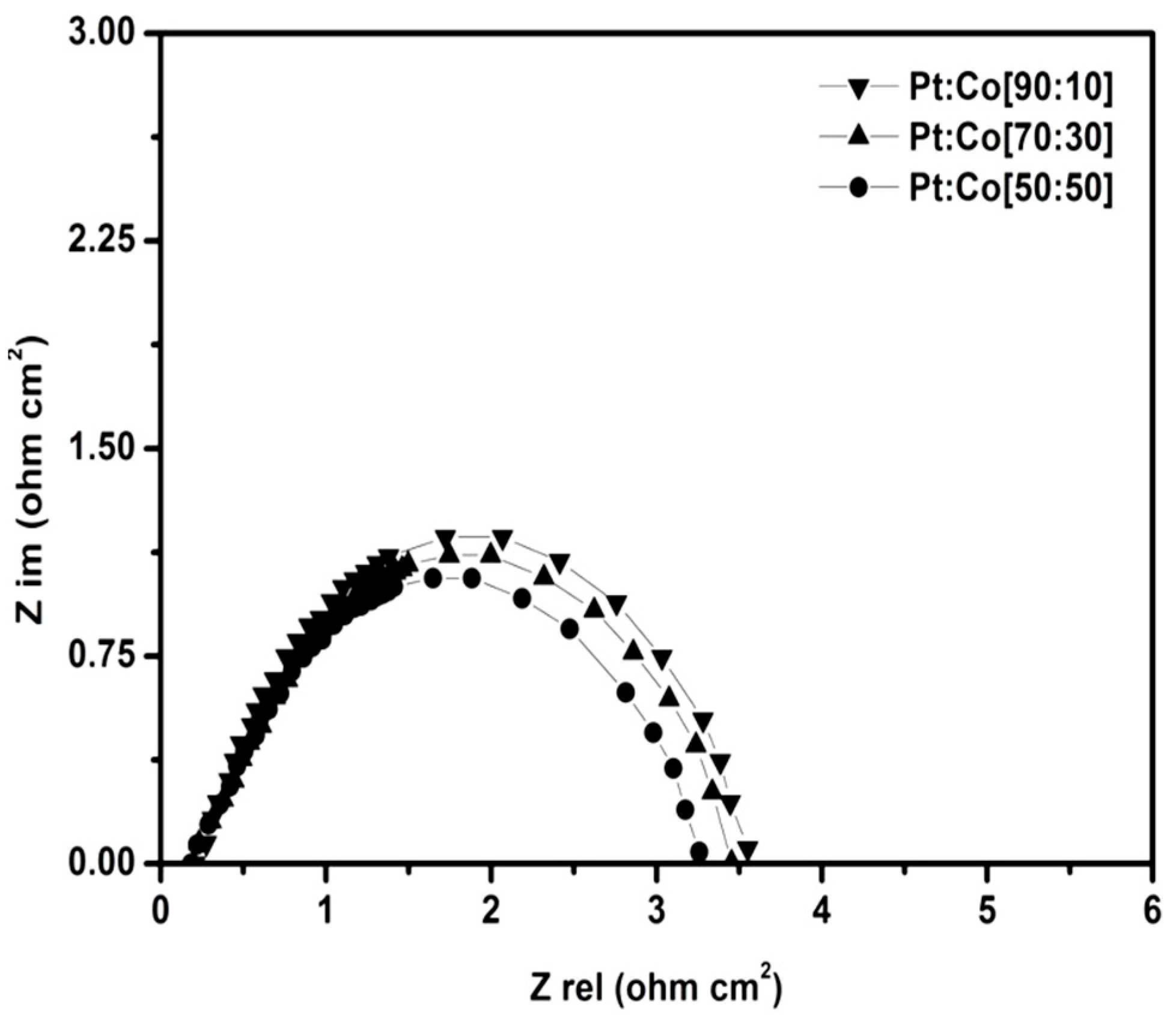
2.7. Electrochemical Impedance Spectroscopy (EIS)
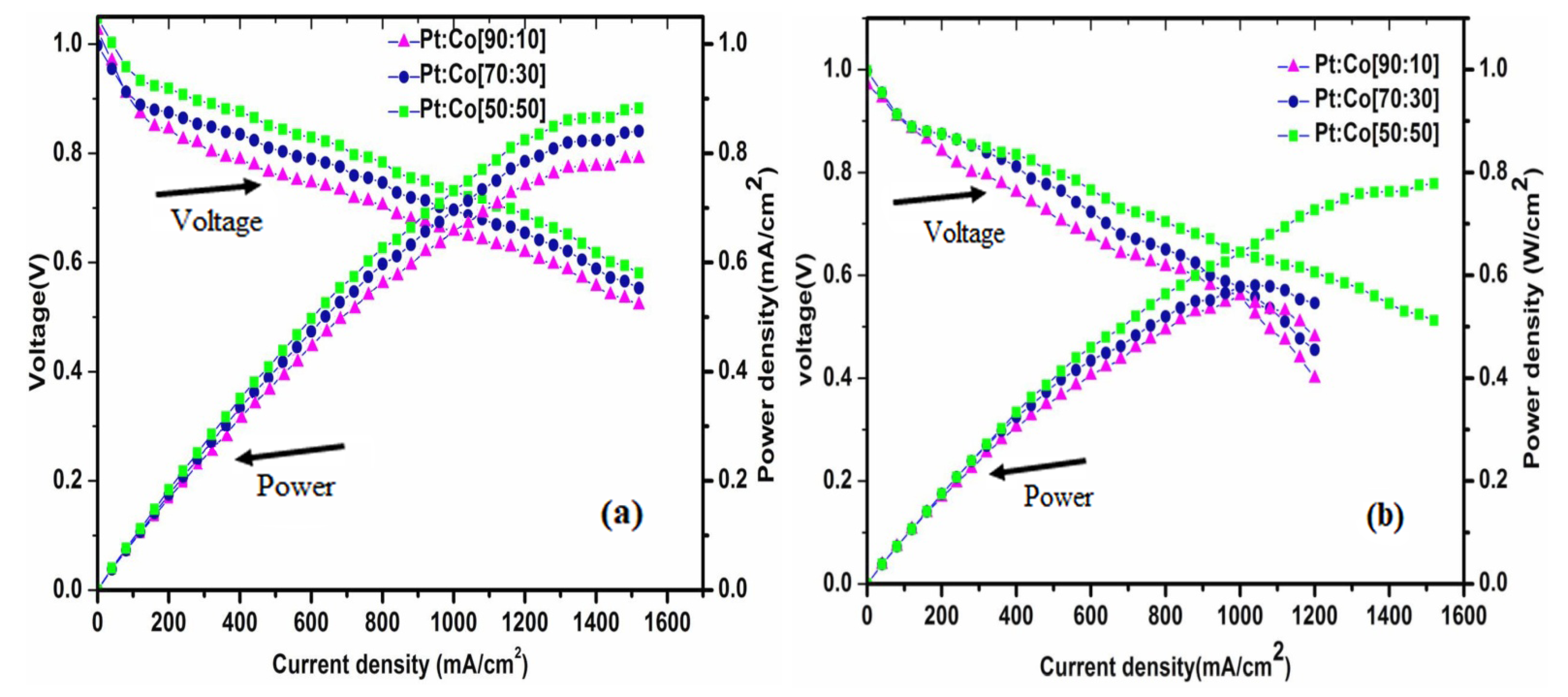
Polarization Studies
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Athanasaki, G.; Jayakumar, A.; Kannan, A. Gas diffusion layers for PEM fuel cells: Materials, properties and manufacturing—A review. Int. J. Hydrogen Energy 2023, 48, 2294–2313. [Google Scholar] [CrossRef]
- Jayakumar, A. An assessment on polymer electrolyte membrane fuel cell stack components. In Applied Physical Chemistry with Multidisciplinary Approaches; Apple Academic Press: Waretown, NJ, USA, 2018; pp. 23–49. [Google Scholar]
- Liu, C.; Li, S. Performance Enhancement of Proton Exchange Membrane Fuel Cell through Carbon Nanofibers Grown In Situ on Carbon Paper. Molecules 2023, 28, 2810. [Google Scholar] [CrossRef]
- Chen, D.; Pei, P.; Li, Y.; Ren, P.; Meng, Y.; Song, X.; Wu, Z. Proton exchange membrane fuel cell stack consistency: Evaluation methods, influencing factors, membrane electrode assembly parameters and improvement measures. Energy Convers. Manag. 2022, 261, 115651. [Google Scholar] [CrossRef]
- Jayakumar, A.; Singamneni, S.; Ramos, M.; Al-Jumaily, A.M.; Pethaiah, S.S. Manufacturing the Gas Diffusion Layer for PEM Fuel Cell Using a Novel 3D Printing Technique and Critical Assessment of the Challenges Encountered. Materials 2017, 10, 796. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, S.; Xing, Y.; Kang, H.; Tan, J.; Guo, W.; Pan, M. Carbon paper coated with Metal-free C-N electrocatalyst for Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cell. Int. J. Electrochem. Sci. 2018, 13, 7020–7033. [Google Scholar] [CrossRef]
- Ren, X.; Wang, Y.; Liu, A.; Zhang, Z.; Lv, Q.; Liu, B. Current progress and performance improvement of Pt/C catalysts for fuel cells. J. Mater. Chem. A 2020, 8, 24284–24306. [Google Scholar] [CrossRef]
- Mardle, P.; Thirunavukkarasu, G.; Guan, S.; Chiu, Y.-L.; Du, S. Comparative study of ptni nanowire array electrodes toward oxygen reduction reaction by half-cell measurement and pemfc test. ACS Appl. Mater. Interfaces 2020, 12, 42832–42841. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Kuroki, H.; Ogura, S.; Fuchigami, T.; Kitamoto, Y.; Yamaguchi, T. Connected nanoparticle catalysts possessing a porous, hollow capsule structure as carbon-free electrocatalysts for oxygen reduction in polymer electrolyte fuel cells. Energy Environ. Sci. 2015, 8, 3545–3549. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.; Chen, S.; Yan, H.; Wang, C.; Wu, C.; Haleem, Y.A.; Duan, S.; Lu, J.; Ge, B.; et al. Atomically dispersed platinum sup-ported on curved carbon supports for efficient electrocatalytic hydrogen evolution. Nat. Energy 2019, 4, 512–518. [Google Scholar] [CrossRef]
- Hou, J.; Yang, M.; Ke, C.; Wei, G.; Priest, C.; Qiao, Z.; Wu, G.; Zhang, J. Platinum-group-metal catalysts for proton exchange membrane fuel cells: From catalyst design to electrode structure optimization. EnergyChem 2020, 2, 100023. [Google Scholar] [CrossRef]
- Wang, X.X.; Swihart, M.T.; Wu, G. Achievements, challenges and perspectives on cathode catalysts in proton ex-change membrane fuel cells for transportation. Nat. Catal. 2019, 2, 578–589. [Google Scholar] [CrossRef]
- Wu, D.; Shen, X.; Pan, Y.; Yao, L.; Peng, Z. Platinum Alloy Catalysts for Oxygen Reduction Reaction: Advances, Challenges and Perspectives. Chemnanomat 2020, 6, 32–41. [Google Scholar] [CrossRef]
- Lori, O.; Elbaz, L. Recent Advances in Synthesis and Utilization of Ultra-low Loading of Precious Metal-based Catalysts for Fuel Cells. ChemCatChem 2020, 12, 3434–3446. [Google Scholar] [CrossRef]
- Lim, B.H.; Majlan, E.H.; Tajuddin, A.; Husaini, T.; Daud, W.R.W.; Radzuan, N.A.M.; Haque, A. Comparison of catalyst-coated membranes and catalyst-coated substrate for PEMFC membrane electrode assembly: A review. Chin. J. Chem. Eng. 2021, 33, 1–16. [Google Scholar] [CrossRef]
- Yarlagadda, V.; McKinney, S.E.; Keary, C.L.; Thompson, L.; Zulevi, B.; Kongkanand, A. Preparation of PEMFC Electrodes from Milligram-Amounts of Catalyst Powder. J. Electrochem. Soc. 2017, 164, F845–F849. [Google Scholar] [CrossRef]
- Ungan, H.; Yurtcan, A.B. PEMFC catalyst layer modification with the addition of different amounts of PDMS polymer in order to improve water management. Int. J. Energy Res. 2019, 43, 5946–5958. [Google Scholar] [CrossRef]
- Arenas, L.F.; Hadjigeorgiou, G.; Jones, S.; Van Dijk, N.; Hodgson, D.; Cruden, A.; de León, C.P. Effect of airbrush type on sprayed platinum and platinum-cobalt catalyst inks: Benchmarking as PEMFC and performance in an electrochemical hydrogen pump. Int. J. Hydrogen Energy 2020, 45, 27392–27403. [Google Scholar] [CrossRef]
- Du, S.; Guan, S.; Mehrazi, S.; Zhou, F.; Pan, M.; Zhang, R.; Chuang, P.-Y.A.; Sui, P.-C. Effect of Dispersion Method and Catalyst on the Crack Morphology and Performance of Catalyst Layer of PEMFC. J. Electrochem. Soc. 2021, 168, 114506. [Google Scholar] [CrossRef]
- Yang, D.; Guo, Y.; Tang, H.; Wang, Y.; Yang, D.; Ming, P.; Zhang, C.; Li, B.; Zhu, S. Influence of the dispersion state of ionomer on the dispersion of catalyst ink and the construction of catalyst layer. Int. J. Hydrogen Energy 2021, 46, 33300–33313. [Google Scholar] [CrossRef]
- Melo, L.; Benavides, R.; Martínez, G.; Morales-Acosta, D.; Paula, M.; Da Silva, L. Sulfonated polystyrene-co-acrylic acid membranes modified by transmembrane reduction of platinum. Int. J. Hydrogen Energy 2017, 42, 30407–30416. [Google Scholar] [CrossRef]
- Sethu, S.P.; Gangadharan, S.; Chan, S.H.; Stimming, U. Development of a novel cost effective methanol electrolyzer stack with Pt-catalyzed membrane. J. Power Sources 2011, 254, 161–167. [Google Scholar] [CrossRef]
- Alia, S.M.; Jensen, K.O.; Pivovar, B.S.; Yan, Y. Platinum-Coated Palladium Nanotubes as Oxygen Reduction Reaction Electrocatalysts. ACS Catal. 2012, 2, 858–863. [Google Scholar] [CrossRef]
- Tokarz, W.; Lota, G.; Frackowiak, E.; Czerwiński, A.; Piela, P. Fuel cell testing of Pt–Ru catalysts supported on differently prepared and pretreated carbon nanotubes. Electrochim. Acta 2013, 98, 94–103. [Google Scholar] [CrossRef]
- Kwon, S.; Ham, D.J.; Lee, S.G. Enhanced H2 dissociative phenomena of Pt–Ir electrocatalysts for PEMFCs: An integrated experimental and theoretical study. RSC Adv. 2015, 5, 54941–54946. [Google Scholar] [CrossRef]
- Garcia-Cardona, J.; Sirés, I.; Alcaide, F.; Brillas, E.; Centellas, F.; Cabot, P.L. Electrochemical performance of carbon-supported Pt(Cu) electrocatalysts for low-temperature fuel cells. Int. J. Hydrogen Energy 2020, 45, 20582–20593. [Google Scholar] [CrossRef]
- Wang, Q.; Mi, B.; Zhou, J.; Qin, Z.; Chen, Z.; Wang, H. Hollow-Structure Pt-Ni Nanoparticle Electrocatalysts for Oxygen Reduction Reaction. Molecules 2022, 27, 2524. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, M.; Radev, I.; Peinecke, V.; Drillet, J.-F. Highly active and stable pt3cr/c alloy catalyst in h2-pemfc. J. Electrochem. Soc. 2015, 162, F901. [Google Scholar] [CrossRef]
- Emery, M.; Frey, M.; Guerra, M.; Haugen, G.; Hintzer, K.; Lochhaas, K.H.; Pham, P.; Pierpont, D.; Schaberg, M.; Thaler, A.; et al. The Development of New Membranes for Proton Exchange Membrane Fuel Cells. ECS Trans. 2007, 11, 3–14. [Google Scholar] [CrossRef]
- Bragaru, A.; Vasile, E.; Danila, M.; Kusko, M.; Simion, M.; Iordanescu, A.; Pascu, R.; Craciunoiu, F.; Leca, M. Microstructural studies of platinum nanoparticles dispersed in nafion membrane. Optoelectron. Adv. Mater.-Rapid Commun. 2014, 5, 161–167. [Google Scholar]
- Baronia, R.; Goel, J.; Tiwari, S.; Singh, P.; Singh, D.; Singh, S.P.; Singhal, S.K. Efficient electro-oxidation of methanol using PtCo nanocatalysts supported reduced graphene oxide matrix as anode for DMFC. Int. J. Hydrogen Energy 2017, 42, 10238–10247. [Google Scholar] [CrossRef]
- Wen, Y.-H.; Huang, R. Effect of Chemical Ordering on Thermal Stability of Pt–Co Nanoparticles. J. Phys. Chem. C 2019, 123, 12007–12014. [Google Scholar] [CrossRef]
- Hargreaves, J.S.J. Some considerations related to the use of the Scherrer equation in powder X-ray diffraction as applied to heterogeneous catalysts. Catal. Struct. React. 2016, 2, 33–37. [Google Scholar] [CrossRef]
- Woo, J.-Y.; Lee, K.-M.; Jee, B.-C.; Ryu, C.-H.; Yoon, C.-H.; Chung, J.-H.; Kim, Y.-R.; Moon, S.-B.; Kang, A.-S. Electrocatalytic char-acteristics of Pt–Ru–Co and Pt–Ru–Ni based on covalently cross-linked sulfonated poly (ether ketone)/heteropolyacids com-posite membranes for water electrolysis. J. Ind. Eng. Chem. 2010, 16, 688–697. [Google Scholar] [CrossRef]
- Antolini, E. Structural parameters of supported fuel cell catalysts: The effect of particle size, inter-particle distance and metal loading on catalytic activity and fuel cell performance. Appl. Catal. B Environ. 2016, 181, 298–313. [Google Scholar] [CrossRef]
- Xu, J.; Aili, D.; Li, Q.; Christensen, E.; Jensen, J.O.; Zhang, W.; Hansen, M.K.; Liu, G.; Wang, X.; Bjerrum, N.J. Oxygen evolution catalysts on supports with a 3-D ordered array structure and intrinsic proton conductivity for proton exchange membrane steam electrolysis. Energy Environ. Sci. 2014, 7, 820–830. [Google Scholar] [CrossRef]
- Muntean, R.; Pascal, D.-T.; Mărginean, G.; Vaszilcsin, N. Carbon Nanofibers Decorated with Pt-Co Alloy Nanoparticles as Catalysts for Electrochemical Cell Applications. I. Synthesis and Structural Characterization. Int. J. Electrochem. Sci. 2017, 12, 4597–4609. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, W.; Ge, H.; Chen, C.; Yan, W.; Gao, Z.; Gan, J.; Zhang, B.; Duan, X.; Qin, Y. Synergistic effects in atom-ic-layer-deposited ptcox/cnts catalysts enhancing hydrolytic dehydrogenation of ammonia borane. Appl. Catal. B Environ. 2018, 235, 256–263. [Google Scholar] [CrossRef]
- Sievers, G.W.; Jensen, A.W.; Quinson, J.; Zana, A.; Bizzotto, F.; Oezaslan, M.; Dworzak, A.; Kirkensgaard, J.J.K.; Smitshuysen, T.E.L.; Kadkhodazadeh, S.; et al. Self-supported Pt–CoO networks combining high specific activity with high surface area for oxygen reduction. Nat. Mater. 2020, 20, 208–213. [Google Scholar] [CrossRef]
- Wu, H.; Wexler, D.; Liu, H.; Savadogo, O.; Ahn, J.; Wang, G. Pt1−xCox nanoparticles as cathode catalyst for proton exchange membrane fuel cells with enhanced catalytic activity. Mater. Chem. Phys. 2010, 124, 841–844. [Google Scholar] [CrossRef]
- Rao, C.V.; Parrondo, J.; Ghatty, S.L.; Rambabu, B. High temperature polymer electrolyte membrane fuel cell performance of PtxCoy/C cathodes. J. Power Sources 2010, 195, 3425–3430. [Google Scholar] [CrossRef]
- Pethaiah, S.S.; Kalaignan, G.P.; Sasikumar, G.; Ulaganathan, M. Evaluation of platinum catalyzed MEAs for PEM fuel cell applications. Solid State Ion. 2011, 190, 88–92. [Google Scholar] [CrossRef]
- Shroti, N.; Daletou, M.K. The Pt–Co alloying effect on the performance and stability of high temperature PEMFC cathodes. Int. J. Hydrogen Energy 2022, 47, 16235–16248. [Google Scholar] [CrossRef]
- Tang, Z.; Poh, C.K.; Chin, K.C.; Chua, D.H.C.; Lin, J.; Wee, A.T.S. Cobalt coated electrodes for high efficiency PEM fuel cells by plasma sputtering deposition. J. Appl. Electrochem. 2009, 39, 1821–1826. [Google Scholar] [CrossRef]
- Giubileo, F.; Di Bartolomeo, A. The role of contact resistance in graphene field-effect devices. Prog. Surf. Sci. 2017, 92, 143–175. [Google Scholar] [CrossRef]
- Sharma, S.; Pollet, B.G. Support materials for PEMFC and DMFC electrocatalysts—A review. J. Power Sources 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Sundar Pethaiah, S.; Paruthimal Kalaignan, G.; Sasikumar, G.; Ulaganathan, M.; Swaminathan, V. Development of nano-catalyzed membrane for PEM fuel cell applications. J. Solid State Electrochem. 2013, 17, 2917–2925. [Google Scholar] [CrossRef]
- Liu, Z.; Yin, Y.; Yang, D.; Zhang, C.; Ming, P.; Li, B.; Yang, S. Efficient synthesis of Pt–Co nanowires as cathode catalysts for proton exchange membrane fuel cells. RSC Adv. 2020, 10, 6287–6296. [Google Scholar] [CrossRef]
- Su, L.; Jia, W.; Li, C.-M.; Lei, Y. Mechanisms for Enhanced Performance of Platinum-Based Electrocatalysts in Proton Exchange Membrane Fuel Cells. ChemSusChem 2014, 7, 361–378. [Google Scholar] [CrossRef]
- Scofield, M.E.; Liu, H.; Wong, S.S. A concise guide to sustainable PEMFCs: Recent advances in improving both oxygen reduction catalysts and proton exchange membranes. Chem. Soc. Rev. 2015, 44, 5836–5860. [Google Scholar] [CrossRef]
- Lin, R.; Cai, X.; Zeng, H.; Yu, Z. Stability of High-Performance Pt-Based Catalysts for Oxygen Reduction Reactions. Adv. Mater. 2018, 30, e1705332. [Google Scholar] [CrossRef]
- Cui, Y.; Wu, Y.; Wang, Z.; Yao, X.; Wei, Y.; Kang, Y.; Du, H.; Li, J.; Gan, L. Mitigating Metal Dissolution and Redeposition of Pt-Co Catalysts in PEM Fuel Cells: Impacts of Structural Ordering and Particle Size. J. Electrochem. Soc. 2020, 167, 064520. [Google Scholar] [CrossRef]
- Jung, W.S.; Popov, B.N. New method to synthesize highly active and durable chemically ordered fct-PtCo cathode catalyst for PEMFCs. ACS Appl. Mater. Interfaces 2017, 9, 23679–23686. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Hwang, S.; Pan, Y.-T.; Chen, K.; He, Y.; Karakalos, S.G.; Zhang, H.; Spendelow, J.S.; Su, D.; Wu, G. Ordered Pt3Co Intermetallic Nanoparticles Derived from Metal–Organic Frameworks for Oxygen Reduction. Nano Lett. 2018, 18, 4163–4171. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Spendelow, J.S. Recent developments in Pt–Co catalysts for proton-exchange membrane fuel cells. Curr. Opin. Electrochem. 2021, 28, 100715. [Google Scholar] [CrossRef]
- Yang, B.; Yu, X.; Hou, J.; Xiang, Z. Secondary reduction strategy synthesis of Pt–Co nanoparticle catalysts towards boosting the activity of proton exchange membrane fuel cells. Particuology 2023, 79, 18–26. [Google Scholar] [CrossRef]
- Pethaiah, S.S.; Kalaignan, G.P.; Ulaganathan, M.; Arunkumar, J. Preparation of durable nanocatalyzed MEA for PEM fuel cell applications. Ionics 2011, 17, 361–366. [Google Scholar] [CrossRef]
- Rajalakshmi, N.; Ryu, H.; Dhathathreyan, K. Platinum catalysed membranes for proton exchange membrane fuel cells—Higher performance. Chem. Eng. J. 2004, 102, 241–247. [Google Scholar] [CrossRef]

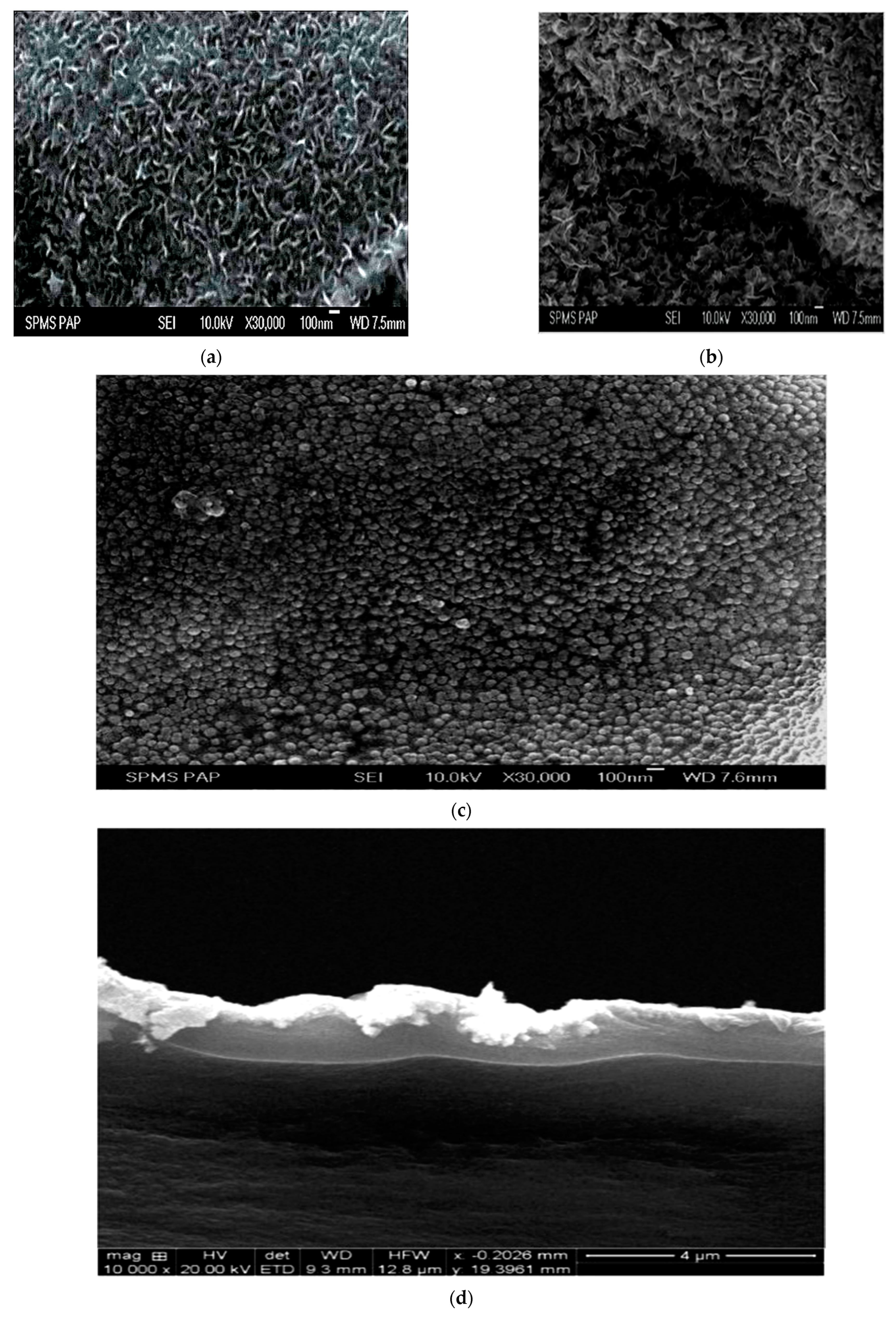
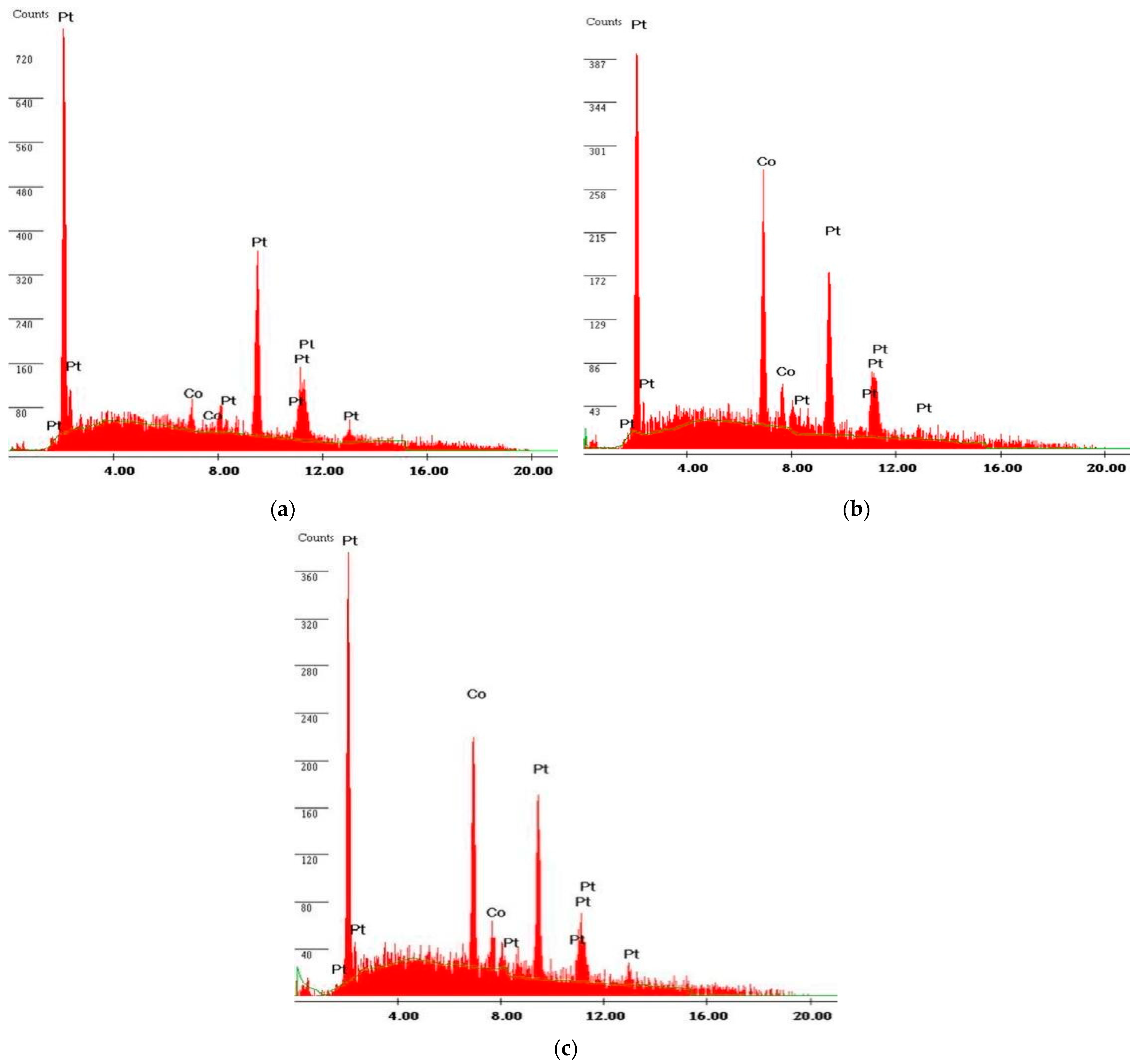


| Samples | Lattice Parameter (μm) | Pt-Pt Inter-Atomic Distance (μm) | Particle Size (μm) |
|---|---|---|---|
| Pt-Co (90:10) | 0.3948 | 0.227977 | 5.1 |
| Pt-Co (70:30) | 0.3914 | 0.225990 | 5.1 |
| Pt-Co (50:50) | 0.3892 | 0.224752 | 5.3 |
| Pt:Co in Solution at % | Pt:Co in Membrane at % |
|---|---|
| 90:10 | 93:7 |
| 70:30 | 72:28 |
| 50:50 | 51:49 |
| Pt:Co Atomic Ratios | ECSA (cm2) | RF | EAS (m2g−1) | ECSA (m2g−1) | Catalyst Utilization (%) |
|---|---|---|---|---|---|
| 90:10 | 1150 | 230 | 46 | 62.67 | 73.40 |
| 70:30 | 1100 | 220 | 44 | 62.67 | 70.21 |
| 50:50 | 1050 | 210 | 42 | 61.81 | 67.95 |
| Pt:Co Atomic Ratio % | R1 | R2 |
|---|---|---|
| 90:10 | 0.22 | 3.56 |
| 70:30 | 0.19 | 3.46 |
| 50:50 | 0.18 | 3.26 |
| Pt:Co Atomic Ratios | Current Density (mAcm−2) | Power Density (Wcm−2) | ||
|---|---|---|---|---|
| H2/O2 | H2/Air | H2/O2 | H2/Air | |
| 90:10 | 1280 | 880 | 0.76 | 0.52 |
| 70:30 | 1360 | 920 | 0.82 | 0.55 |
| 50:50 | 1480 | 1200 | 0.88 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pethaiah, S.S.; Jayakumar, A.; Palanichamy, K. Evaluation of Pt-Co Nano-Catalyzed Membranes for Polymer Electrolyte Membrane Fuel Cell Applications. Energies 2023, 16, 7713. https://doi.org/10.3390/en16237713
Pethaiah SS, Jayakumar A, Palanichamy K. Evaluation of Pt-Co Nano-Catalyzed Membranes for Polymer Electrolyte Membrane Fuel Cell Applications. Energies. 2023; 16(23):7713. https://doi.org/10.3390/en16237713
Chicago/Turabian StylePethaiah, Sethu Sundar, Arunkumar Jayakumar, and Kalyani Palanichamy. 2023. "Evaluation of Pt-Co Nano-Catalyzed Membranes for Polymer Electrolyte Membrane Fuel Cell Applications" Energies 16, no. 23: 7713. https://doi.org/10.3390/en16237713
APA StylePethaiah, S. S., Jayakumar, A., & Palanichamy, K. (2023). Evaluation of Pt-Co Nano-Catalyzed Membranes for Polymer Electrolyte Membrane Fuel Cell Applications. Energies, 16(23), 7713. https://doi.org/10.3390/en16237713








