Synergistic Effects of Torrefaction and Alkaline Pretreatment on Sugar and Bioethanol Production from Wood Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Torrefaction
2.3. Alkali-Mediated Pretreatment
2.4. Enzymatic Hydrolysis
2.5. Bioethanol Fermentation
2.6. Characterization of Pretreated Biomass
2.6.1. Fiber Analysis
2.6.2. Ash Content
2.6.3. FTIR Analysis
2.6.4. Sugar Composition
2.6.5. HPLC Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Process Parameters in Alkaline Pretreatment
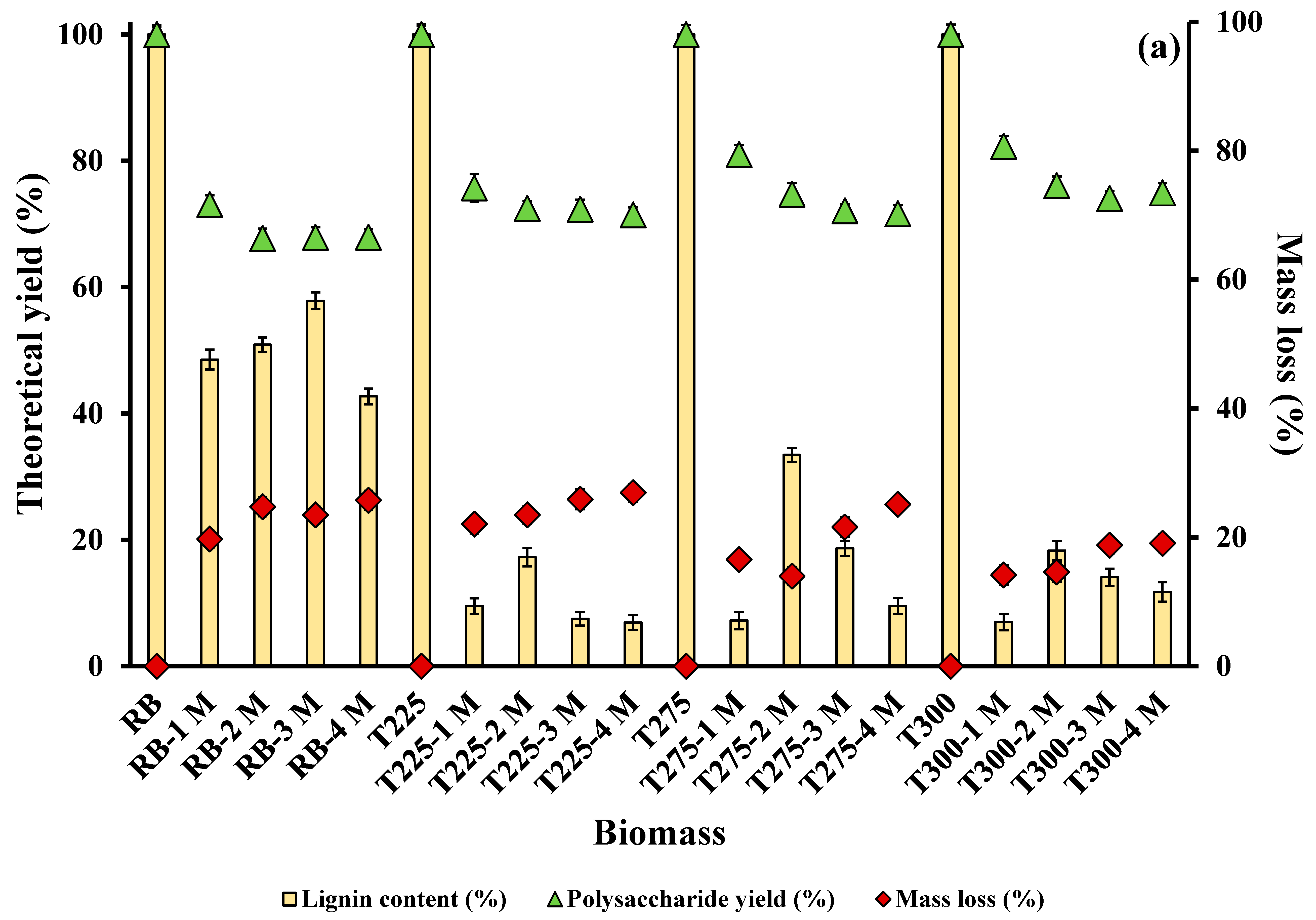
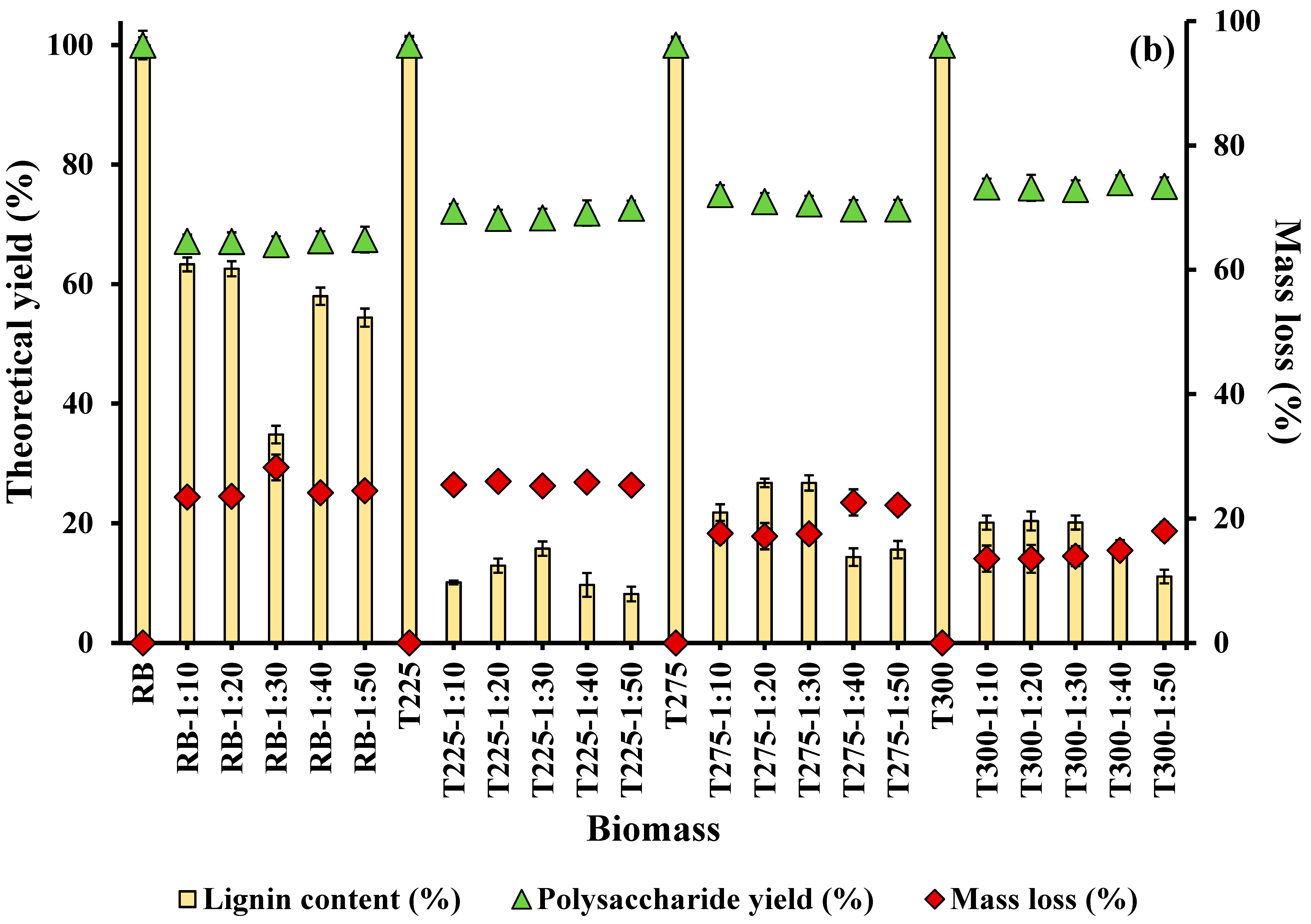
3.1.1. Effect of Alkali Concentration and Solid–Liquid Ratio on Lignin Extraction
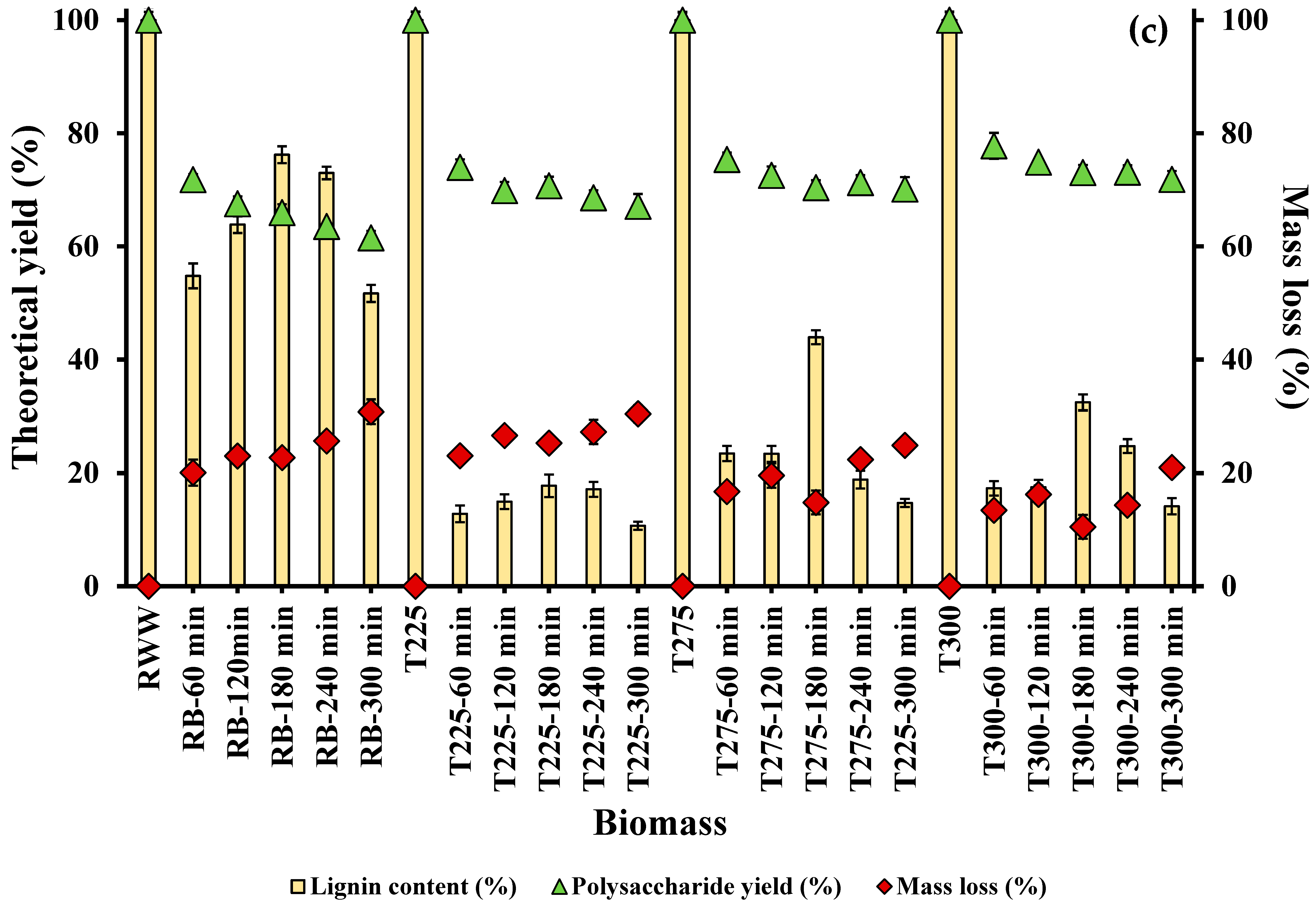
3.1.2. Effect of Residence Time on Lignin Extraction
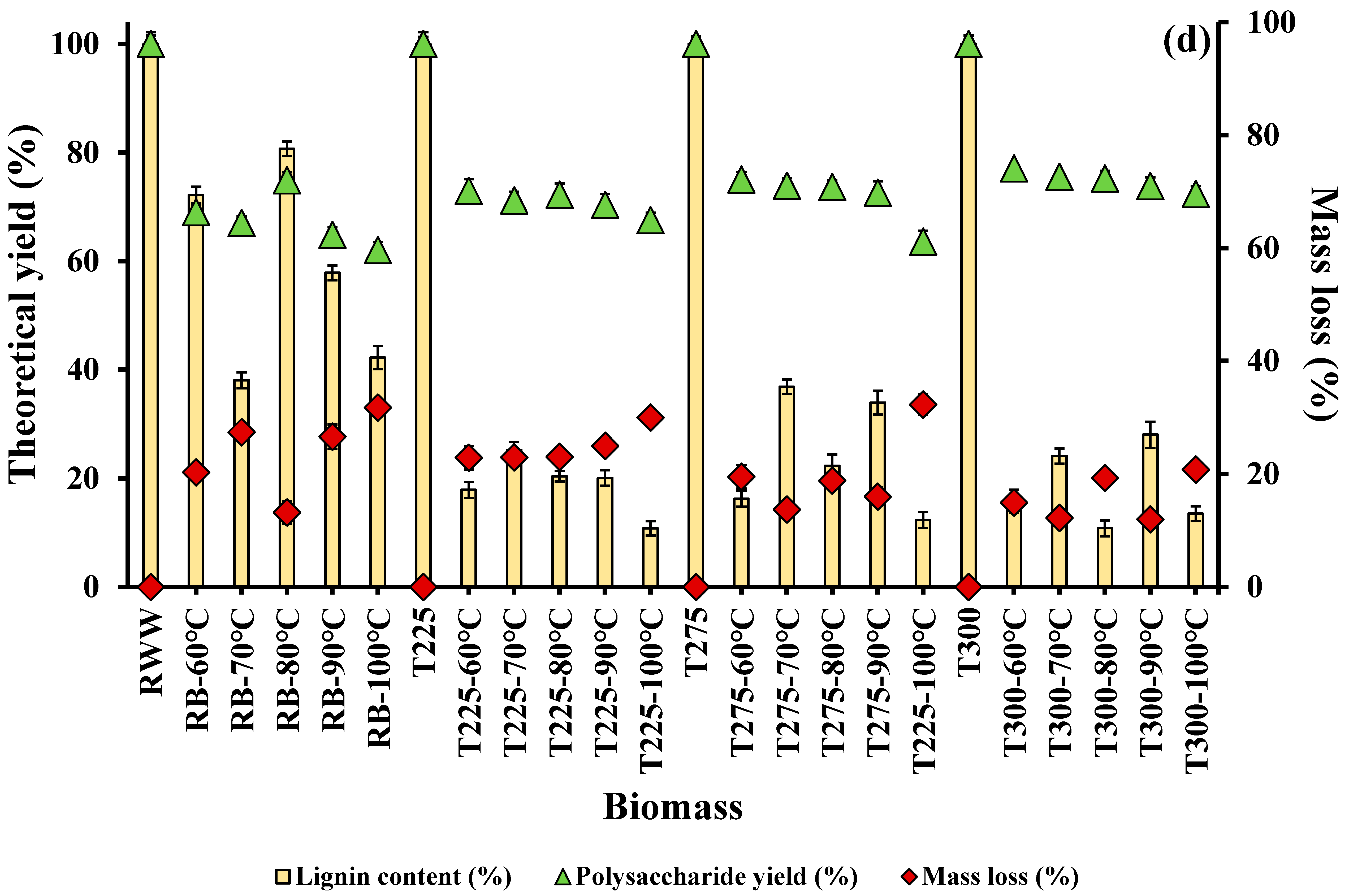
3.1.3. Effect of Temperature on Lignin Extraction
3.2. Effect of Integrated Pretreatment on Biomass Composition and Properties
3.2.1. Chemical Composition of Biomass
3.2.2. Sugar Composition of Biomass
3.2.3. Impact of Pretreatment on Cellulose and Lignin-Related Characteristics
3.3. Enzymatic Hydrolysis
3.4. Bioethanol Production
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hasanov, I.; Shanmugam, S.; Kikas, T. Extraction and isolation of lignin from ash tree (Fraxinus exselsior) with protic ionic liquids (PILs). Chemosphere 2022, 290, 133297. [Google Scholar] [CrossRef]
- Ge, S.; Yek, P.N.Y.; Cheng, Y.W.; Xia, C.; Mahari, W.A.W.; Liew, R.K.; Peng, W.; Yuan, T.-Q.; Tabatabaei, M.; Aghbashlo, M.; et al. Progress in microwave pyrolysis conversion of agricultural waste to value-added biofuels: A batch to continuous approach. Renew. Sustain. Energy Rev. 2021, 135, 110148. [Google Scholar] [CrossRef]
- Nunes, L.; Causer, T.; Ciolkosz, D. Biomass for energy: A review on supply chain management models. Renew. Sustain. Energy Rev. 2020, 120, 109658. [Google Scholar] [CrossRef]
- Abdulyekeen, K.A.; Umar, A.A.; Patah, M.F.A.; Daud, W.M.A.W. Torrefaction of biomass: Production of enhanced solid biofuel from municipal solid waste and other types of biomass. Renew. Sustain. Energy Rev. 2021, 150, 111436. [Google Scholar] [CrossRef]
- Adeleke, A.A.; Odusote, J.K.; Ikubanni, P.P.; Lasode, O.A.; Malathi, M.; Paswan, D. Essential basics on biomass torrefaction, densification and utilization. Int. J. Energy Res. 2021, 45, 1375–1395. [Google Scholar] [CrossRef]
- Xin, S.; Huang, F.; Liu, X.; Mi, T.; Xu, Q. Torrefaction of herbal medicine wastes: Characterization of the physicochemical properties and combustion behaviors. Bioresour. Technol. 2019, 287, 121408. [Google Scholar] [CrossRef]
- Sarker, T.R.; Azargohar, R.; Dalai, A.K.; Meda, V. Enhancement of fuel and physicochemical properties of canola residues via microwave torrefaction. Energy Rep. 2021, 7, 6338–6353. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Rizzo, A.M.; Prussi, M.; Tedeschi, S.; Zimbardi, F.; Braccio, G.; Viola, E.; Pardelli, P.T. 2nd generation lignocellulosic bioethanol: Is torrefaction a possible approach to biomass pretreatment? Biomass Convers. Biorefinery 2011, 1, 9–15. [Google Scholar] [CrossRef]
- Chaluvadi, S.; Ujjwal, A.; Singh, R. Effect of torrefaction prior to biomass size reduction on ethanol production. Waste Biomass Valorization 2019, 10, 3567–3577. [Google Scholar] [CrossRef]
- Sheikh, M.I.; Kim, C.-H.; Park, H.-J.; Kim, S.-H.; Kim, G.-C.; Lee, J.-Y.; Sim, S.-W.; Kim, J.W. Effect of torrefaction for the pretreatment of rice straw for ethanol production. J. Sci. Food Agric. 2013, 93, 3198–3204. [Google Scholar] [CrossRef]
- Ong, H.C.; Yu, K.L.; Chen, W.-H.; Pillejera, M.K.; Bi, X.; Tran, K.-Q.; Pétrissans, A.; Pétrissans, M. Variation of lignocellulosic biomass structure from torrefaction: A critical review. Renew. Sustain. Energy Rev. 2021, 152, 111698. [Google Scholar] [CrossRef]
- Shinde, S.D.; Meng, X.; Kumar, R.; Ragauskas, A.J. Recent advances in understanding the pseudo-lignin formation in a lignocellulosic biorefinery. Green Chem. 2018, 20, 2192–2205. [Google Scholar] [CrossRef]
- Normark, M.; Pommer, L.; Gräsvik, J.; Hedenström, M.; Gorzsás, A.; Winestrand, S.; Jönsson, L.J. Biochemical Conversion of Torrefied Norway Spruce After Pretreatment with Acid or Ionic Liquid. BioEnergy Res. 2016, 9, 355–368. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-L.; Yuan, T.-Q.; Xu, F.; Sun, R.-C. Understanding the chemical and structural transformations of lignin macromolecule during torrefaction. Appl. Energy 2014, 121, 1–9. [Google Scholar] [CrossRef]
- Yang, Q.; Pan, X. Correlation between lignin physicochemical properties and inhibition to enzymatic hydrolysis of cellulose. Biotechnol. Bioeng. 2016, 113, 1213–1224. [Google Scholar] [CrossRef]
- Cahyanti, M.N.; Doddapaneni, T.R.K.C.; Madissoo, M.; Pärn, L.; Virro, I.; Kikas, T. Torrefaction of Agricultural and Wood Waste: Comparative Analysis of Selected Fuel Characteristics. Energies 2021, 14, 2774. [Google Scholar] [CrossRef]
- Rooni, V.; Sjulander, N.; Cristobal-Sarramian, A.; Raud, M.; Rocha-Meneses, L.; Kikas, T. The efficiency of nitrogen explosion pretreatment on common aspen–Populus tremula: N2–VS steam explosion. Energy 2021, 220, 119741. [Google Scholar] [CrossRef]
- CEN/TS 14775; Solid Biofuels—Method for the Determination of Ash Content. European Committee for Standardization: Brussels, Belgium, 2004.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of sugars, byproducts, and degradation products in liquid fraction process samples. Gold. Natl. Renew. Energy Lab. 2006, 11, 65–71. [Google Scholar]
- Sjulander, N.; Kikas, T. Two-Step Pretreatment of Lignocellulosic Biomass for High-Sugar Recovery from the Structural Plant Polymers Cellulose and Hemicellulose. Energies 2022, 15, 8898. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, B.; Chen, H.; Wu, W.; Wu, S.; Jin, Y.; Xiao, H. Recent advances in understanding the effects of lignin structural characteristics on enzymatic hydrolysis. Biotechnol. Biofuels 2021, 14, 1–20. [Google Scholar]
- Lee, K.H.; Lee, S.K.; Lee, J.; Kim, S.; Kim, S.W.; Park, C.; Yoo, H.Y. Energy-efficient glucose recovery from chestnut shell by optimization of NaOH pretreatment at room temperature and application to bioethanol production. Environ. Res. 2022, 208, 112710. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Cheng, J.-R.; Wang, Y.-T.; Zhu, M.-J. Enhanced lignin removal and enzymolysis efficiency of grass waste by hydrogen peroxide synergized dilute alkali pretreatment. Bioresour. Technol. 2020, 301, 122756. [Google Scholar] [CrossRef]
- Woiciechowski, A.L.; Neto, C.J.D.; de Souza Vandenberghe, L.P.; de Carvalho Neto, D.P.; Sydney, A.C.N.; Letti, L.A.J.; Karp, S.G.; Torres, L.A.Z.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance–Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef]
- Morales-Martínez, J.L.; Aguilar-Uscanga, M.G.; Bolaños-Reynoso, E.; López-Zamora, L. Optimization of Chemical Pretreatments Using Response Surface Methodology for Second-Generation Ethanol Production from Coffee Husk Waste. BioEnergy Res. 2021, 14, 815–827. [Google Scholar] [CrossRef]
- Corderi, S.; Renders, T.; Servaes, K.; Vanbroekhoven, K.; De Roo, T.; Elst, K. Strategies for the Removal of Polysaccharides from Biorefinery Lignins: Process Optimization and Techno Economic Evaluation. Molecules 2021, 26, 3324. [Google Scholar] [CrossRef]
- Tripathi, J.; Richard, T.L.; Memis, B.; Demirci, A.; Ciolkosz, D. Interactions of Torrefaction and Alkaline Pretreatment with Respect to Glucose Yield of Hydrolyzed Wheat Straw. Biomass 2022, 2, 264–278. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Zhang, Z. Waste pretreatment technologies for hydrogen production. In Waste to Renewable Biohydrogen; Elsevier: Amsterdam, The Netherlands, 2021; pp. 109–122. [Google Scholar]
- Gagić, T.; Perva-Uzunalić, A.; Knez, Ž.; Škerget, M. Hydrothermal Degradation of Cellulose at Temperature from 200 to 300 °C. Ind. Eng. Chem. Res. 2018, 57, 6576–6584. [Google Scholar] [CrossRef]
- Manokhoon, P.; Rangseesuriyachai, T. Effect of two-stage sodium hydroxide pretreatment on the composition and structure of Napier grass (Pakchong 1) (Pennisetum purpureum). Int. J. Green Energy 2020, 17, 864–871. [Google Scholar] [CrossRef]
- Kathirselvam, M.; Kumaravel, A.; Arthanarieswaran, V.; Saravanakumar, S. Characterization of cellulose fibers in Thespesia populnea barks: Influence of alkali treatment. Carbohydr. Polym. 2019, 217, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Midha, V.K.; Choudhary, A.K. Optimization of Parameters for Alkali Pretreatment on Coir Fiber for Biomass Production Using TOPSIS. J. Nat. Fibers 2022, 19, 3038–3050. [Google Scholar] [CrossRef]
- Oh, S.; Park, D.H.; Lee, S.M.; Ahn, B.J.; Ahn, S.H.; Yang, I. Effect of Torrefaction Temperature on Lignin Distribution of Larix kaempferi C. and Liriodendron tulipifera L. Cubes and the Impact of Binder on Durability of Pellets Fabricated with the Torrefied Cubes. Environ. Prog. Sustain. Energy 2019, 38, 13190. [Google Scholar] [CrossRef]
- Noori, M.S.; Karimi, K. Chemical and structural analysis of alkali pretreated pinewood for efficient ethanol production. RSC Adv. 2016, 6, 65683–65690. [Google Scholar] [CrossRef]
- Bay, M.S.; Karimi, K.; Esfahany, M.N.; Kumar, R. Structural modification of pine and poplar wood by alkali pretreatment to improve ethanol production. Ind. Crop. Prod. 2020, 152, 112506. [Google Scholar] [CrossRef]
- Shi, L.; Hu, Z.; Li, X.; Li, S.; Yi, L.; Wang, X.; Hu, H.; Luo, G.; Yao, H. Gas-pressurized torrefaction of lignocellulosic solid wastes: Low-temperature deoxygenation and chemical structure evolution mechanisms. Bioresour. Technol. 2023, 385, 129414. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Chen, M.; Qiu, Z.; Fang, C.; Lidén, G.; Liu, X.; Zhang, B.; Bao, J. Simultaneous and rate-coordinated conversion of lignocellulose derived glucose, xylose, arabinose, mannose, and galactose into D-lactic acid production facilitates D-lactide synthesis. Bioresour. Technol. 2023, 377, 128950. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Liu, J.; Li, Y.; Wang, Y.; Huang, J.; Ai, Y.; Chen, P.; He, Y.; Aftab, M.N.; et al. Integrated NIRS and QTL assays reveal minor mannose and galactose as contrast lignocellulose factors for biomass enzymatic saccharification in rice. Biotechnol. Biofuels 2021, 14, 144. [Google Scholar] [CrossRef]
- Auxenfans, T.; Crônier, D.; Chabbert, B.; Paës, G. Understanding the structural and chemical changes of plant biomass following steam explosion pretreatment. Biotechnol. Biofuels 2017, 10, 36. [Google Scholar] [CrossRef]
- Bhatia, R.; Lad, J.B.; Bosch, M.; Bryant, D.N.; Leak, D.; Hallett, J.P.; Franco, T.T.; Gallagher, J.A. Production of oligosaccharides and biofuels from Miscanthus using combinatorial steam explosion and ionic liquid pretreatment. Bioresour. Technol. 2021, 323, 124625. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Chen, W.-H.; Colin, B.; Pétrissans, A.; Quirino, R.L.; Pétrissans, M. Thermodegradation characterization of hardwoods and softwoods in torrefaction and transition zone between torrefaction and pyrolysis. Fuel 2022, 310, 122281. [Google Scholar] [CrossRef]
- Li, Y.; Yan, C.; Chen, Y.; Han, X.; Shao, Z.; Qi, H.; Li, X.; Nishiyama, Y.; Hu, T.; Chen, P. The major role of London dispersion interaction in the assembly of cellulose, chitin, and chitosan. Cellulose 2023, 30, 8127–8138. [Google Scholar] [CrossRef]
- Haldar, D.; Dey, P.; Patel, A.K.; Dong, C.-D.; Singhania, R.R. A Critical Review on the Effect of Lignin Redeposition on Biomass in Controlling the Process of Enzymatic Hydrolysis. BioEnergy Res. 2022, 15, 863–874. [Google Scholar] [CrossRef]
- Kim, S. Enhancing Bioethanol Productivity Using Alkali-Pretreated Empty Palm Fruit Bunch Fiber Hydrolysate. BioMed Res. Int. 2018, 2018, 5272935. [Google Scholar] [CrossRef]
- Toquero, C.; Bolado, S. Effect of four pretreatments on enzymatic hydrolysis and ethanol fermentation of wheat straw. Influence of inhibitors and washing. Bioresour. Technol. 2014, 157, 68–76. [Google Scholar] [CrossRef]
- Ellison, C.; Garcia-Perez, M.; Mullen, C.A.; Yadav, M.P. Thermochemical behavior of alkali pretreated biomass–a thermogravimetric and Py-GC/FID study. Sustain. Energy Fuels. 2023, 7, 3306–3315. [Google Scholar] [CrossRef]
- Salehian, P.; Karimi, K. Alkali Pretreatment for Improvement of Biogas and Ethanol Production from Different Waste Parts of Pine Tree. Ind. Eng. Chem. Res. 2013, 52, 972–978. [Google Scholar] [CrossRef]

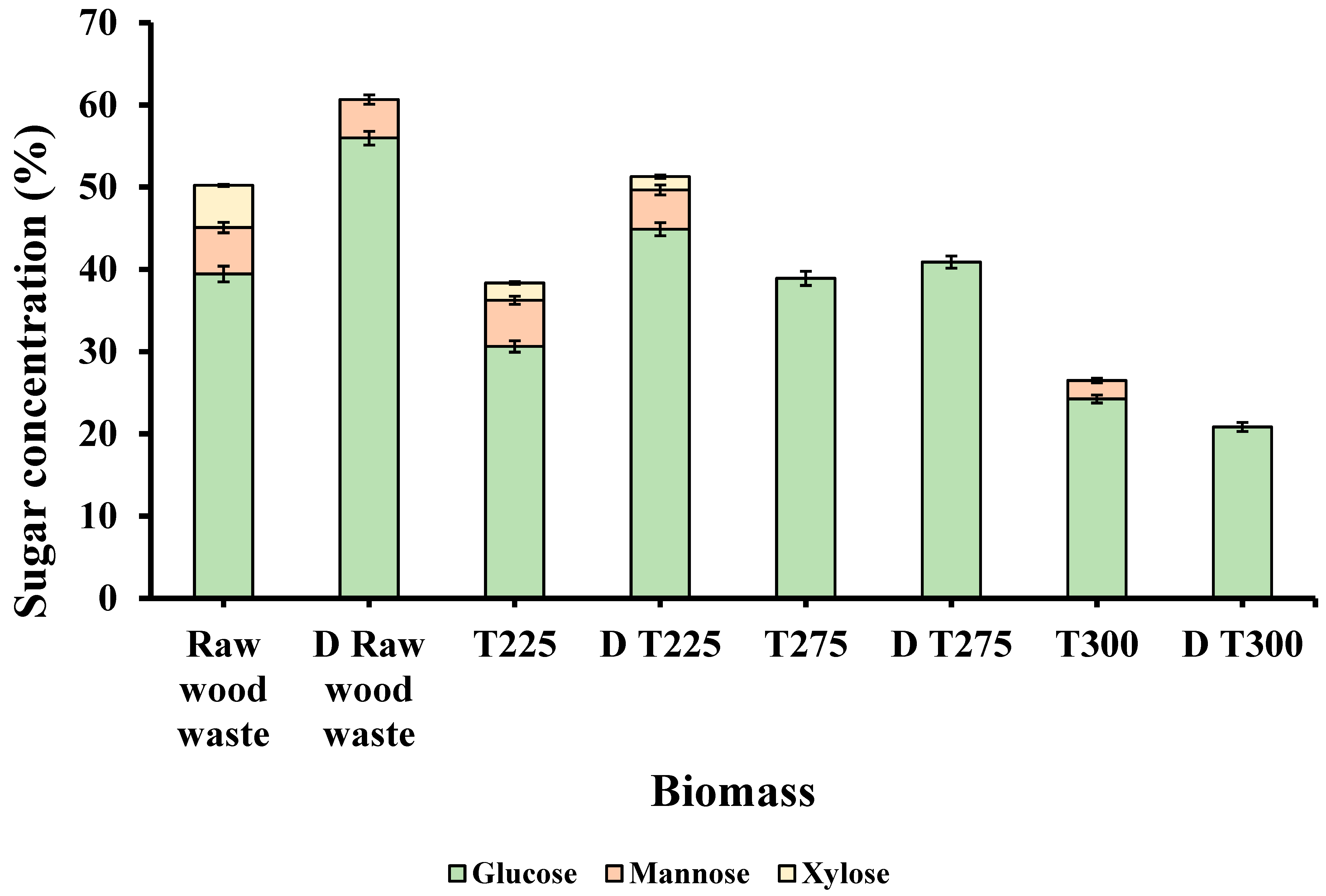

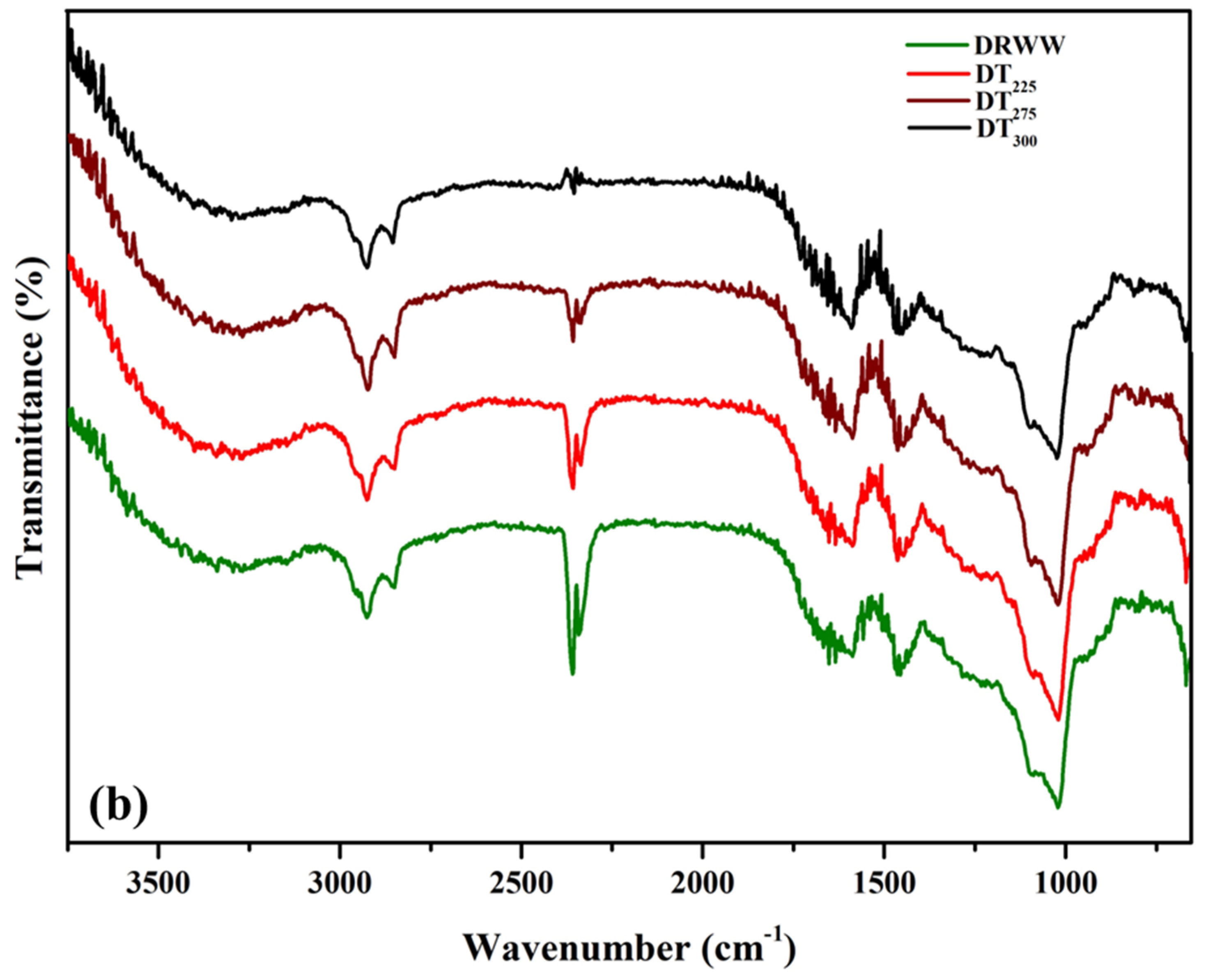
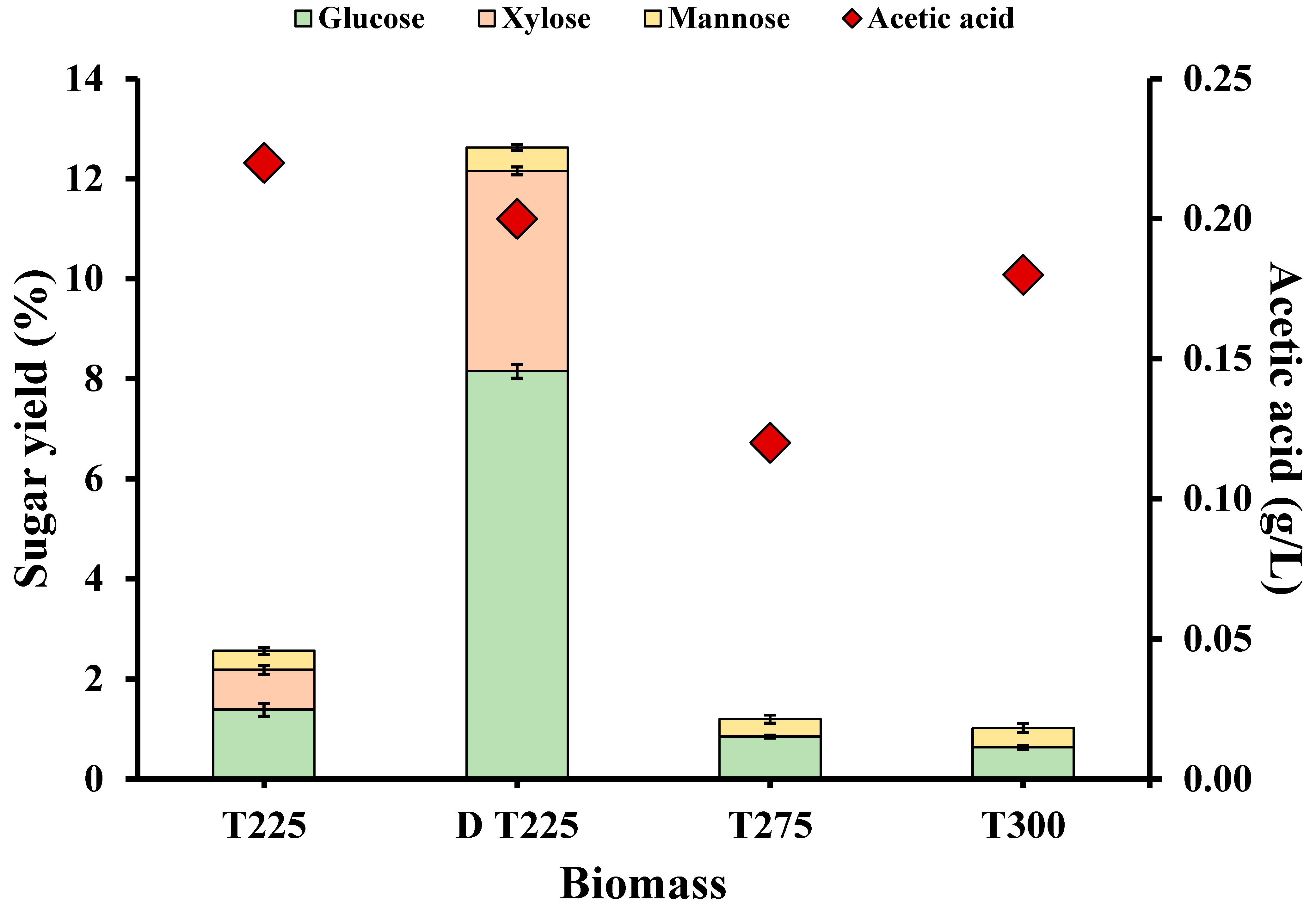
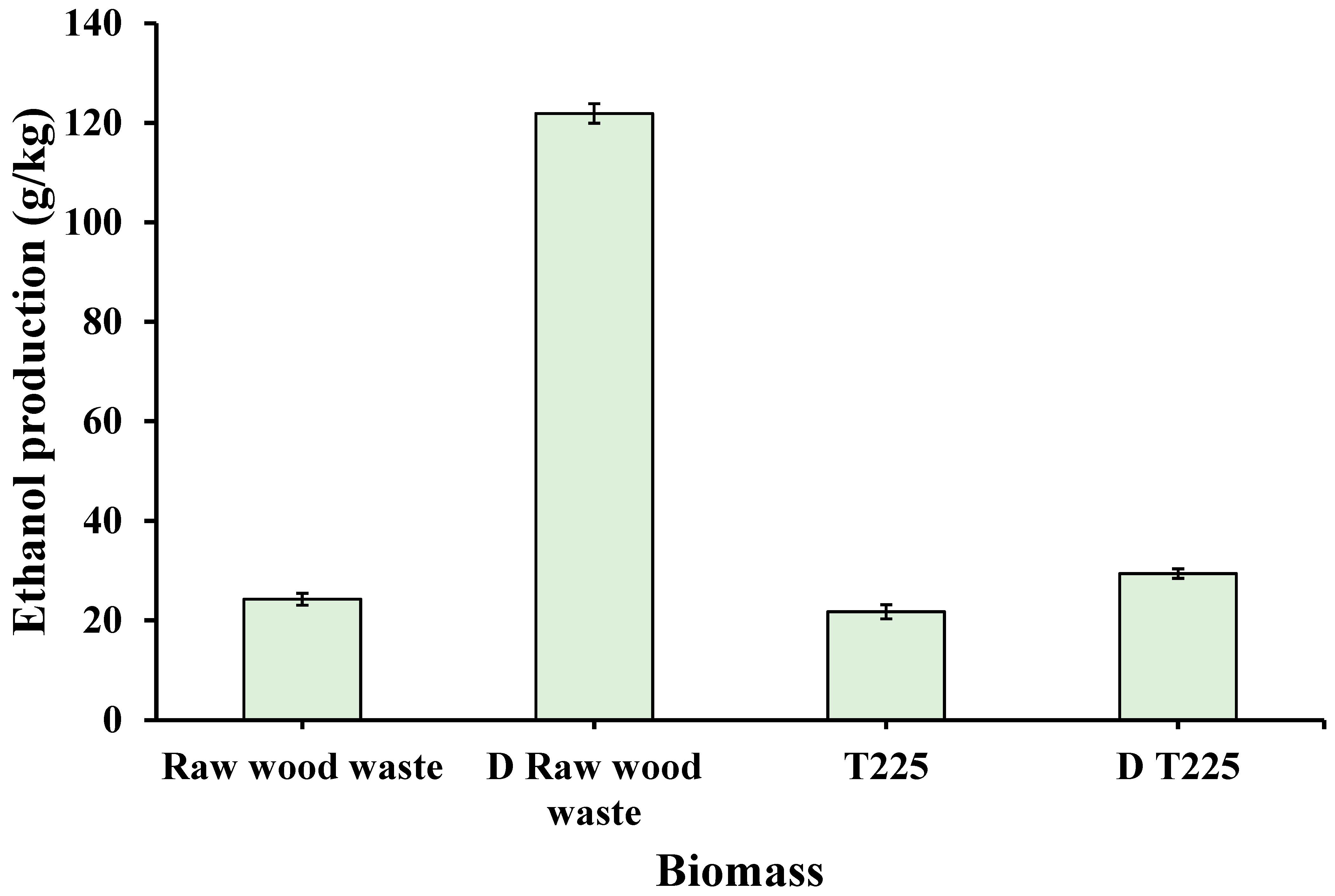
| Biomass | LOI (A1423/A897) | HBI (A3400/A1323) | CLL (A1508/A1600) |
|---|---|---|---|
| Raw wood waste | 0.98 ± 0.003 | 2.20 ± 0.001 | 1.13 ± 0.004 |
| Torrefied wood waste | |||
| T225 | 0.97 ± 0.007 | 2.21 ± 0.005 | 1.15 ± 0.007 |
| T275 | 1.20 ± 0.005 | 2.85 ± 0.004 | 1.23 ± 0.003 |
| T300 | 1.00 ± 0.008 | 2.97 ± 0.007 | 1.20 ± 0.007 |
| Delignified torrefied wood waste | |||
| DT225 | 0.99 ± 0.008 | 2.21 ± 0.007 | 1.16 ± 0.006 |
| DT275 | 1.09 ± 0.006 | 2.55 ± 0.004 | 1.18 ± 0.005 |
| DT300 | 1.14 ± 0.007 | 2.64 ± 0.008 | 1.24 ± 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cahyanti, M.N.; Shanmugam, S.; Kikas, T. Synergistic Effects of Torrefaction and Alkaline Pretreatment on Sugar and Bioethanol Production from Wood Waste. Energies 2023, 16, 7606. https://doi.org/10.3390/en16227606
Cahyanti MN, Shanmugam S, Kikas T. Synergistic Effects of Torrefaction and Alkaline Pretreatment on Sugar and Bioethanol Production from Wood Waste. Energies. 2023; 16(22):7606. https://doi.org/10.3390/en16227606
Chicago/Turabian StyleCahyanti, Margareta Novian, Sabarathinam Shanmugam, and Timo Kikas. 2023. "Synergistic Effects of Torrefaction and Alkaline Pretreatment on Sugar and Bioethanol Production from Wood Waste" Energies 16, no. 22: 7606. https://doi.org/10.3390/en16227606
APA StyleCahyanti, M. N., Shanmugam, S., & Kikas, T. (2023). Synergistic Effects of Torrefaction and Alkaline Pretreatment on Sugar and Bioethanol Production from Wood Waste. Energies, 16(22), 7606. https://doi.org/10.3390/en16227606







