Hydration Absorption and Thermal Effects of Outcrop Porous Sandstone Based on Intelligent Experimental and Infrared Thermography Techniques
Abstract
:1. Introduction
2. Experiments and Procedures
2.1. Test Equipment
2.2. Sample Preparation
2.3. Experimental Design
3. Results and Analysis
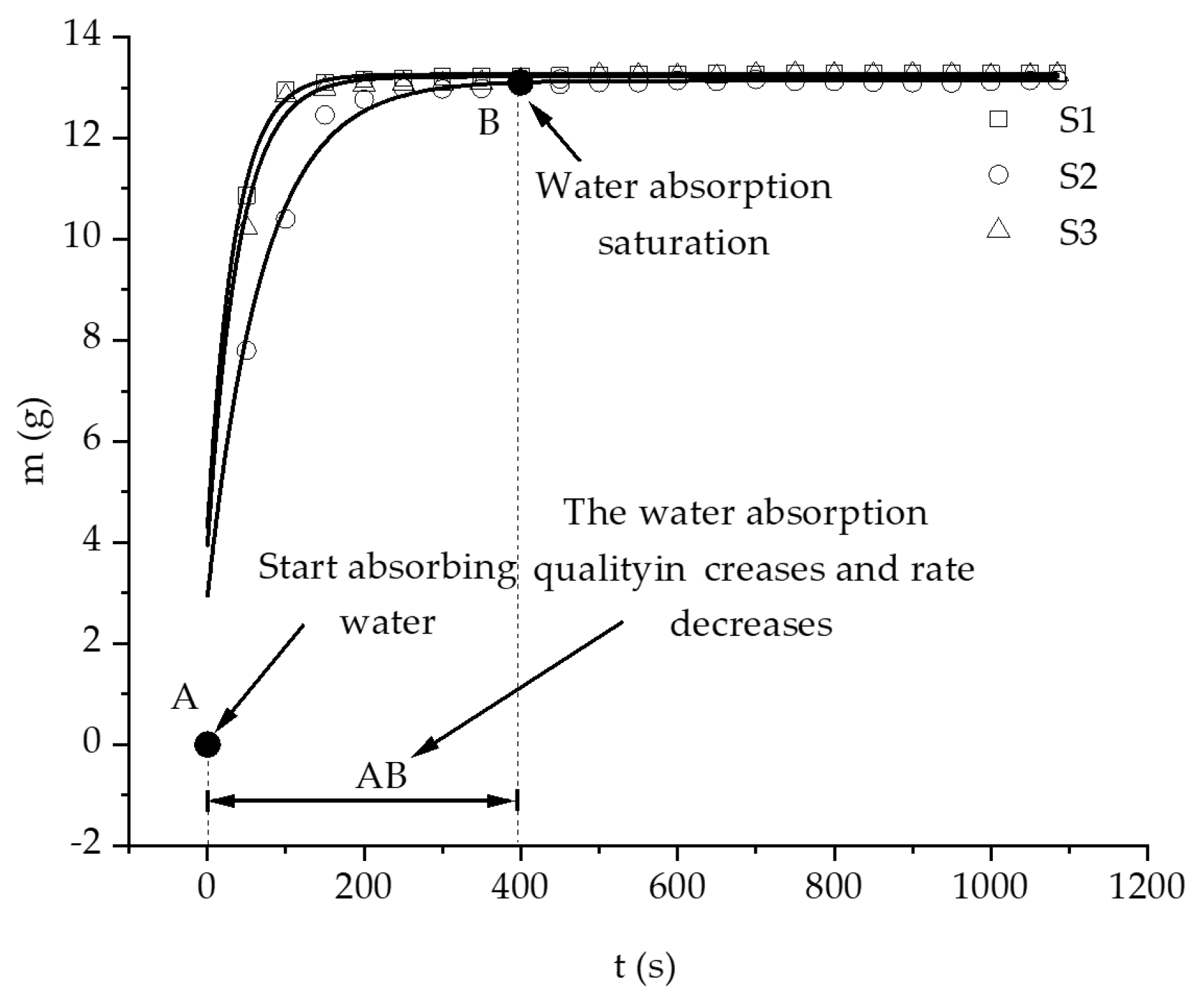
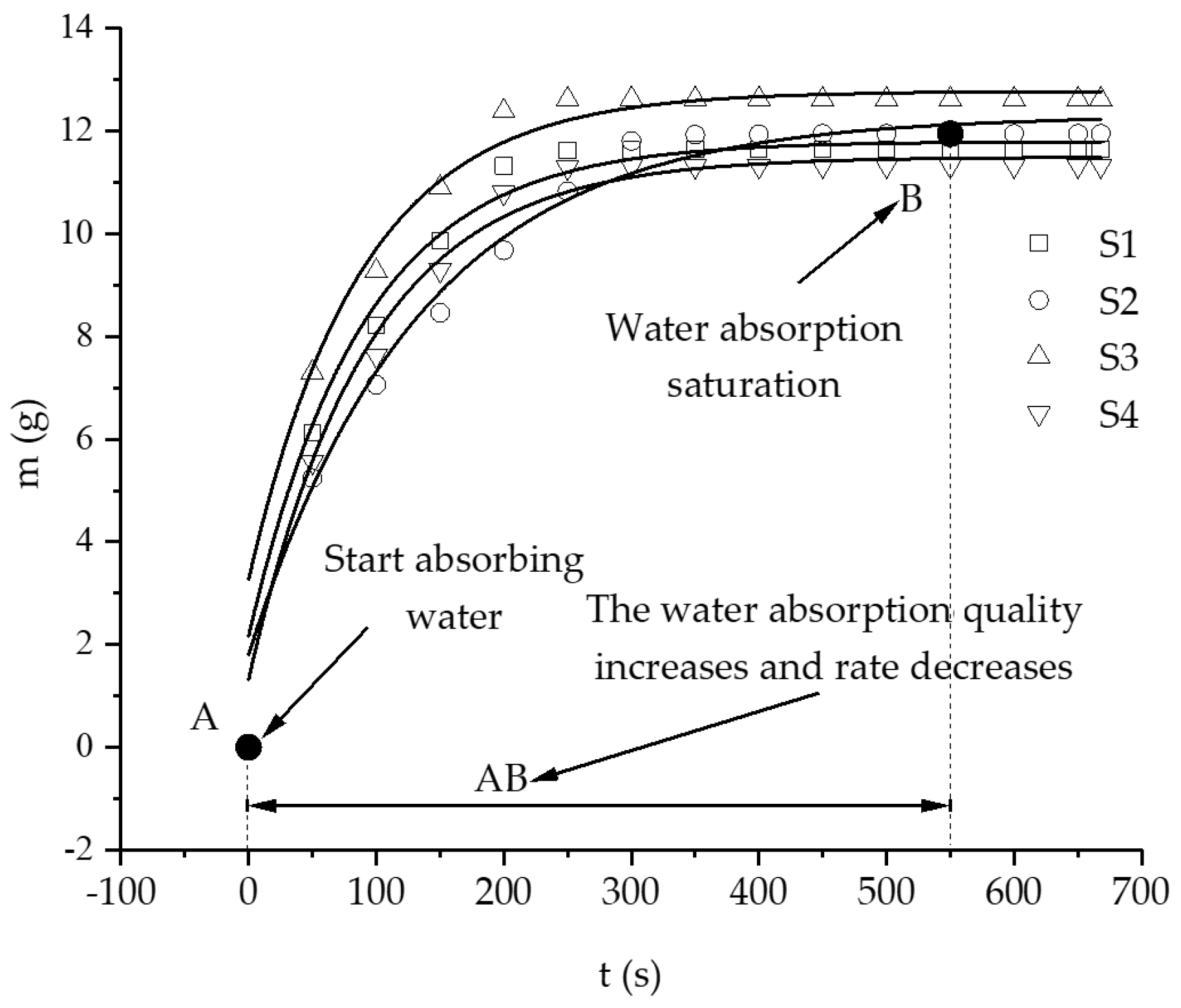
3.1. Hydration Absorption of Sandstone Samples at Different Initial Temperatures
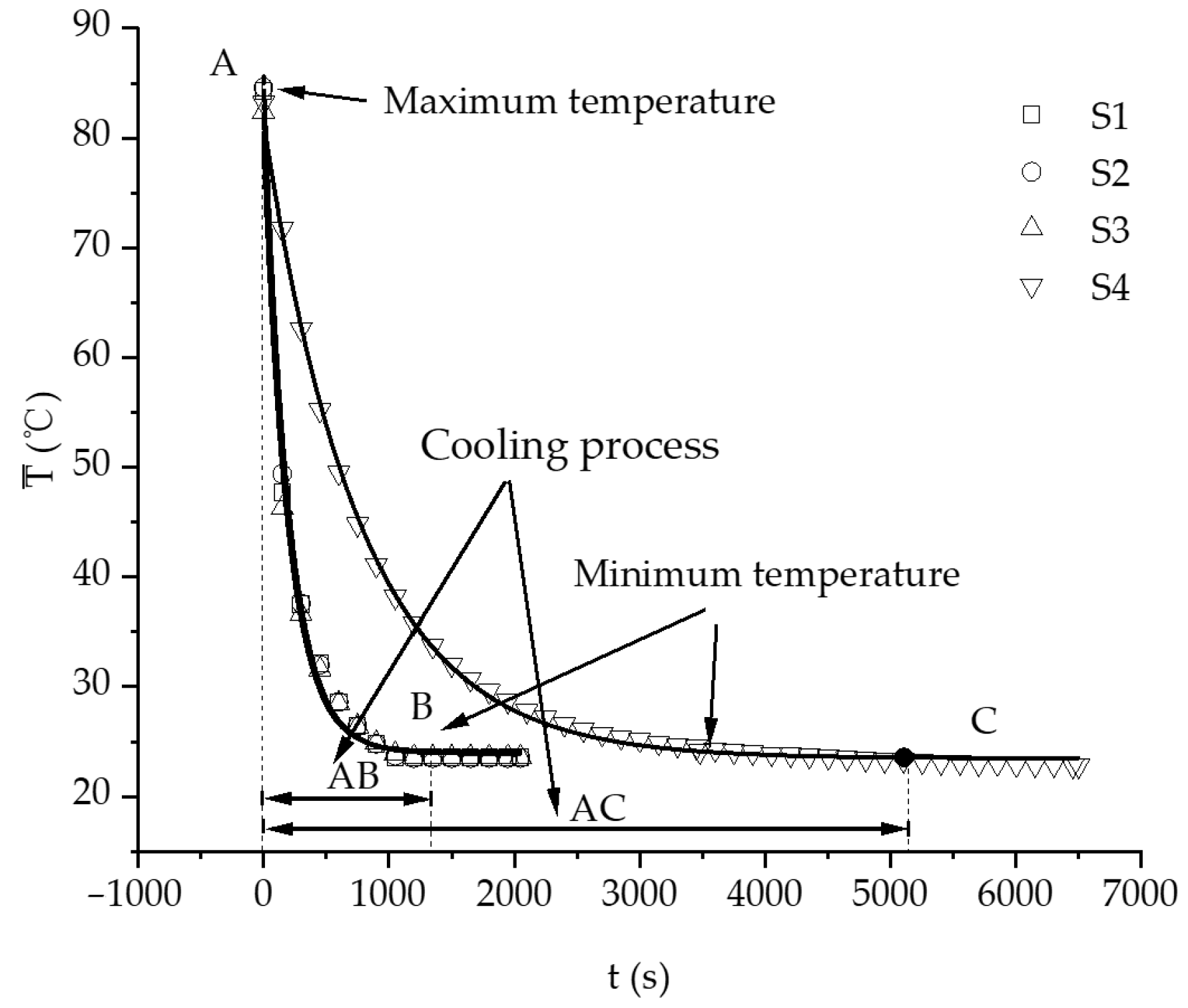
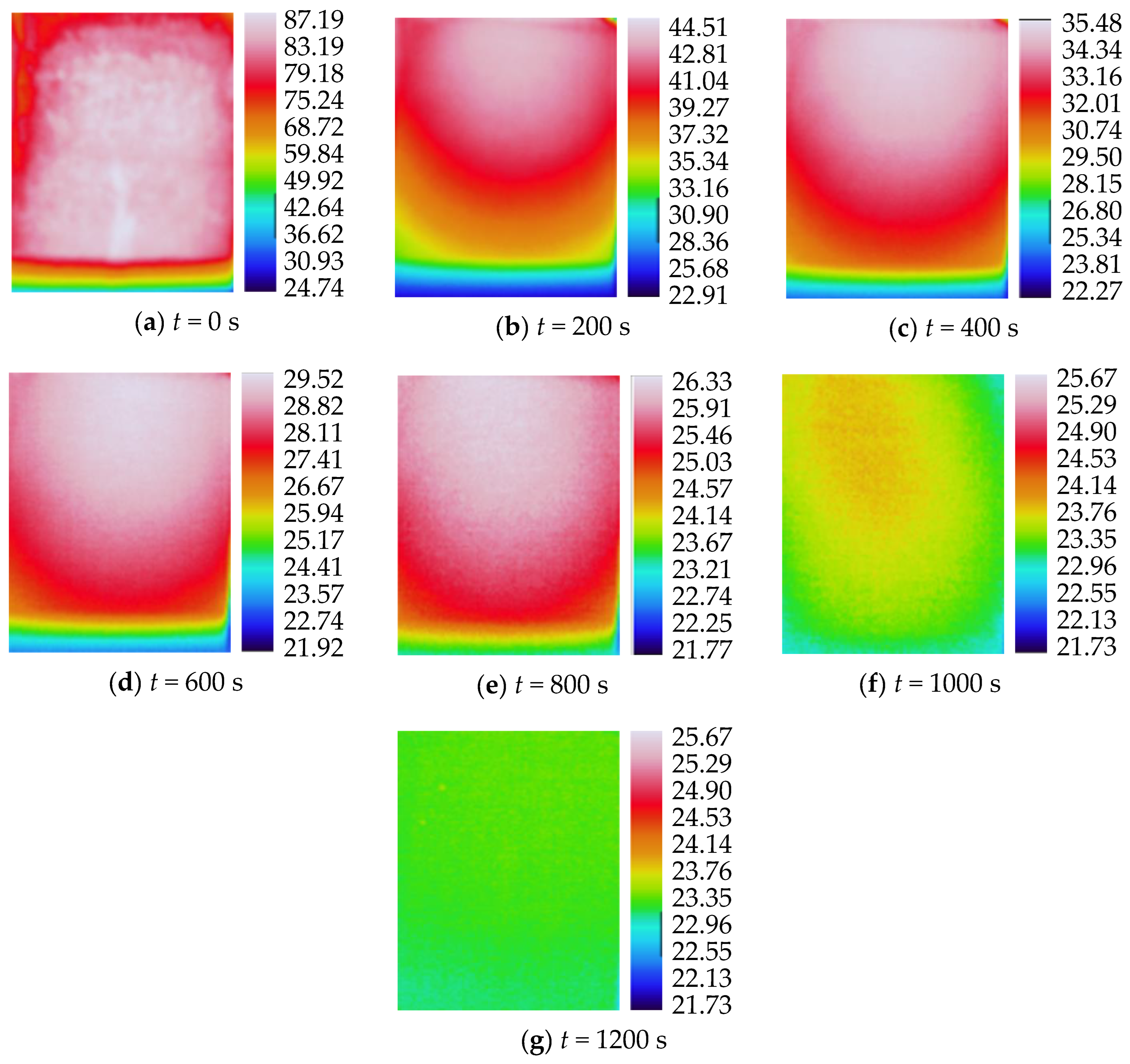
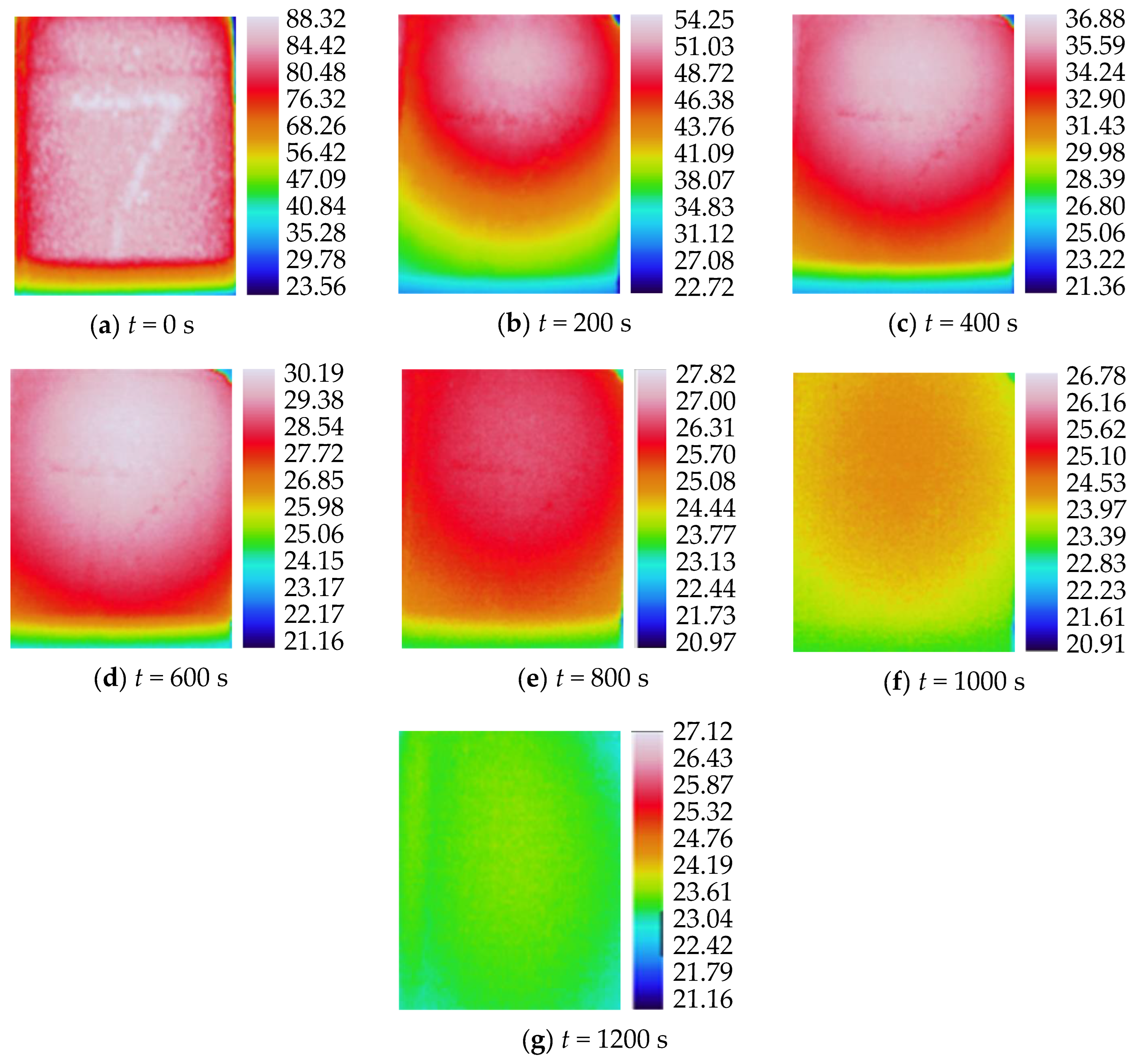
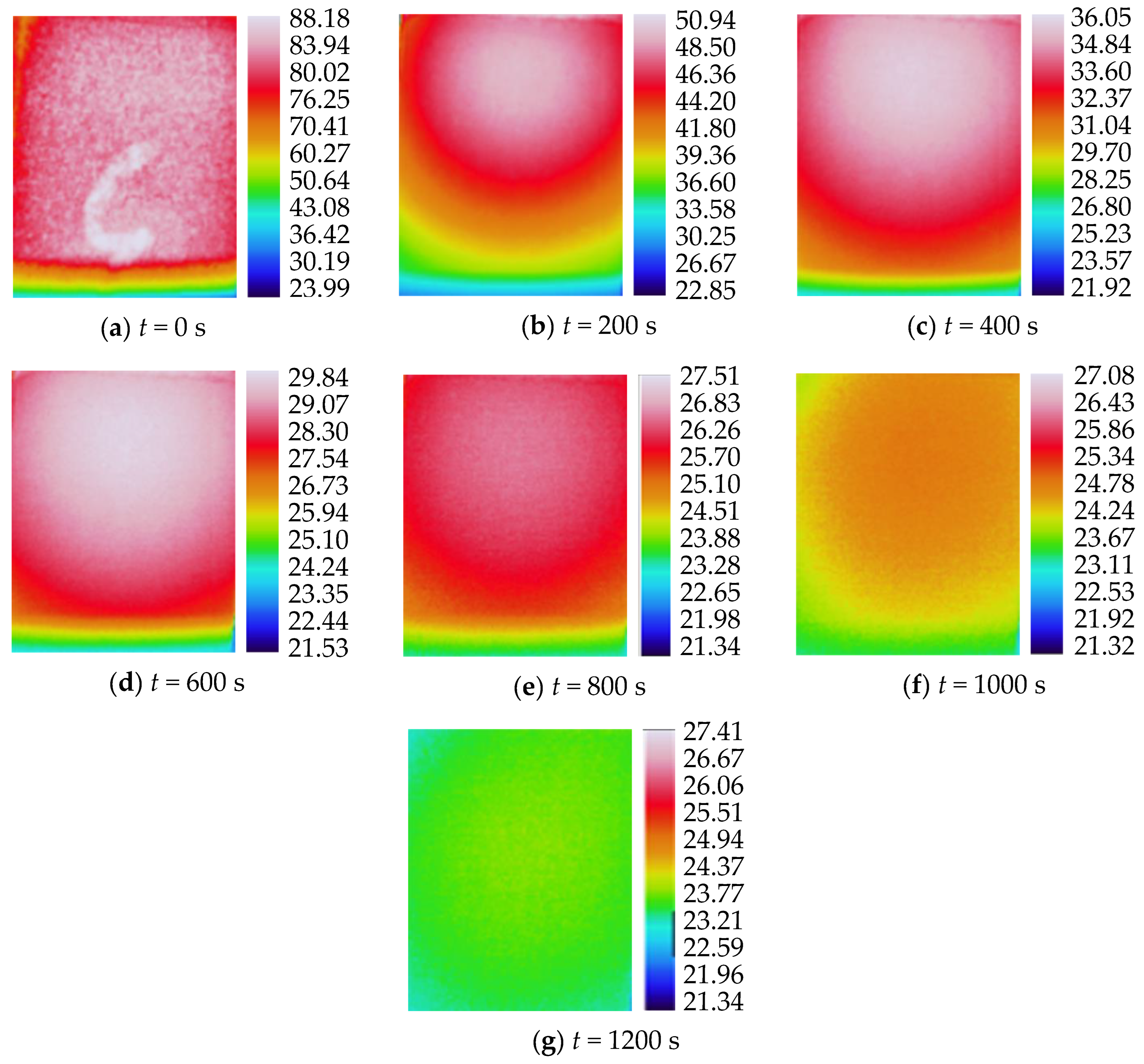
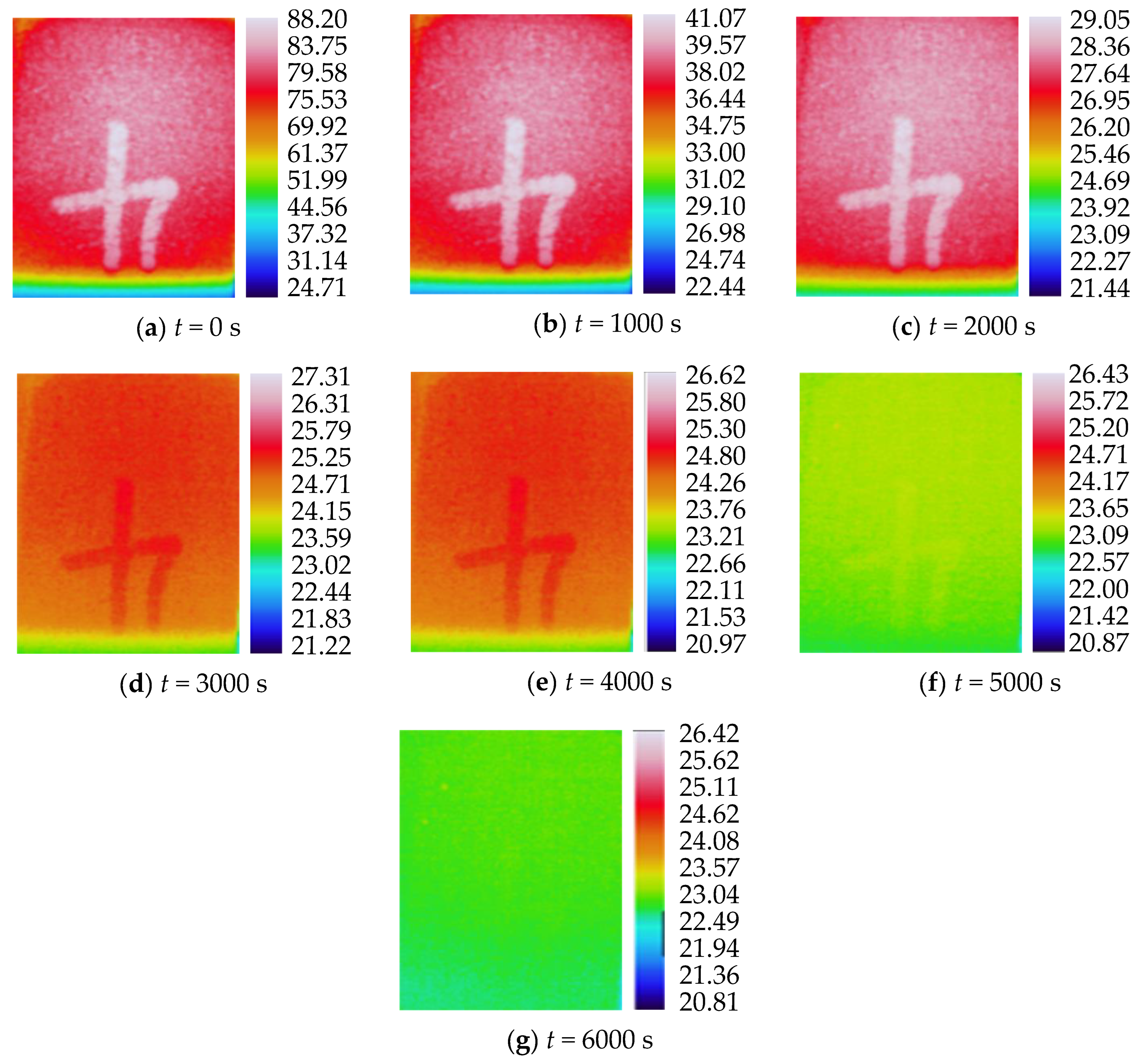
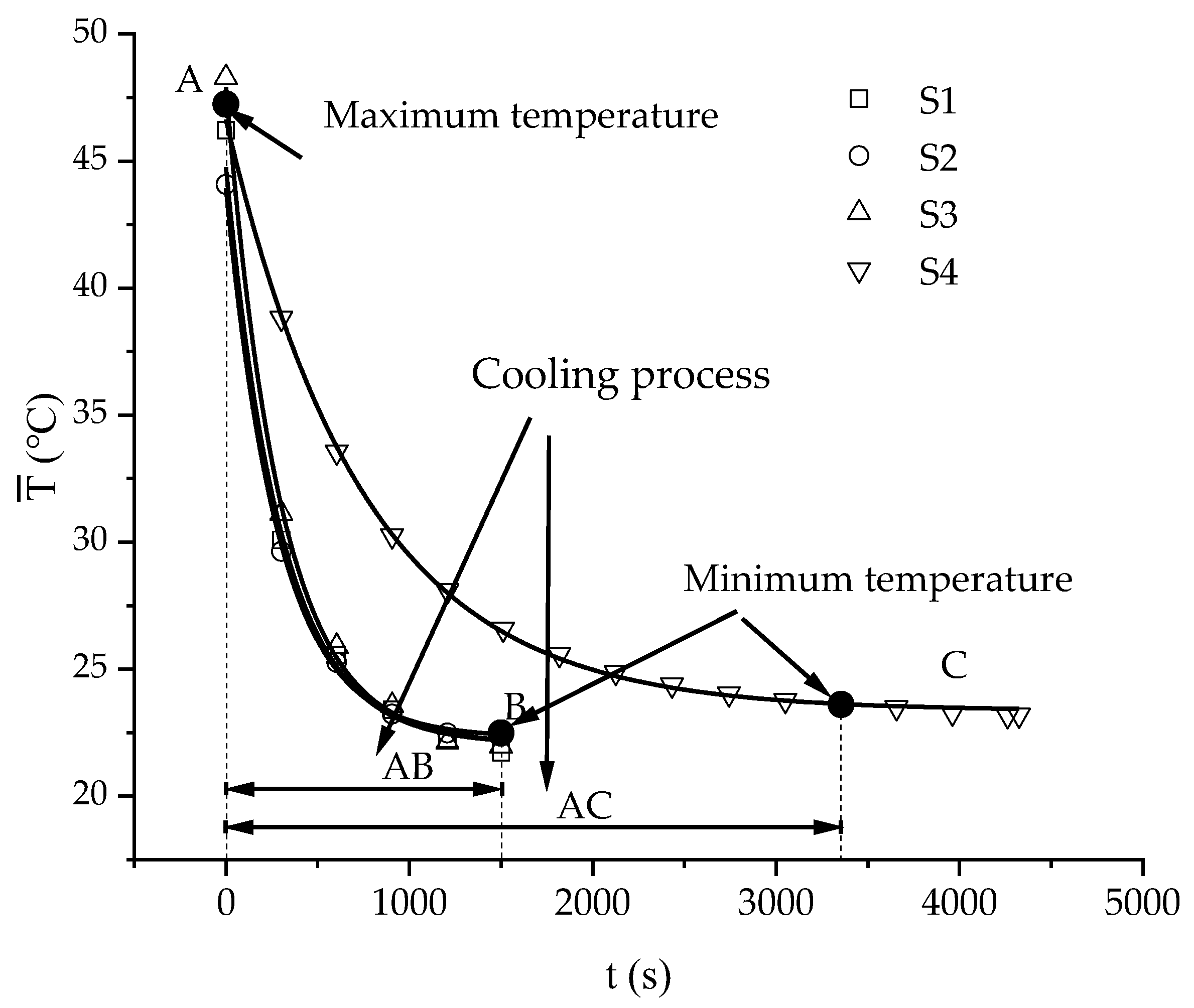
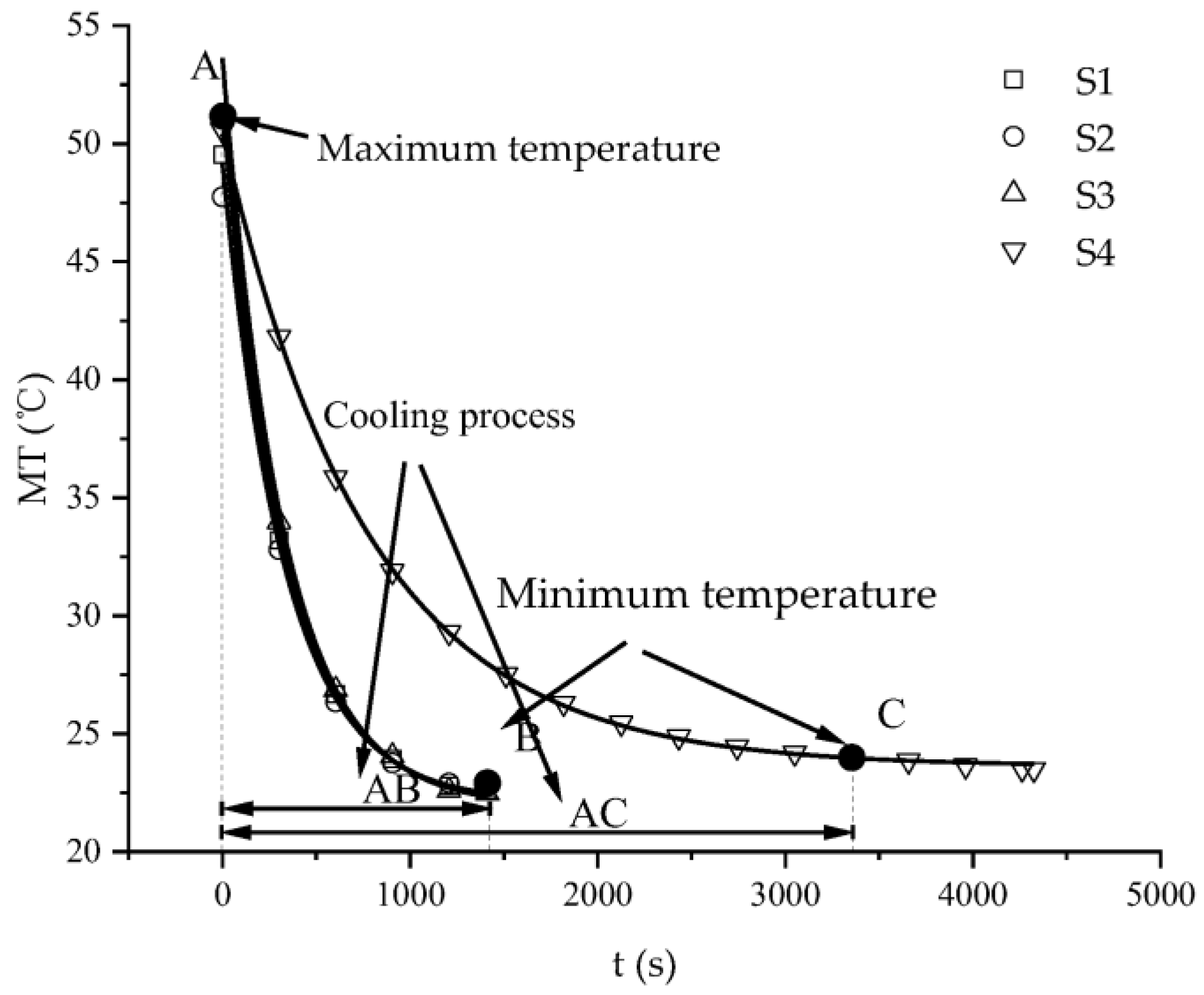
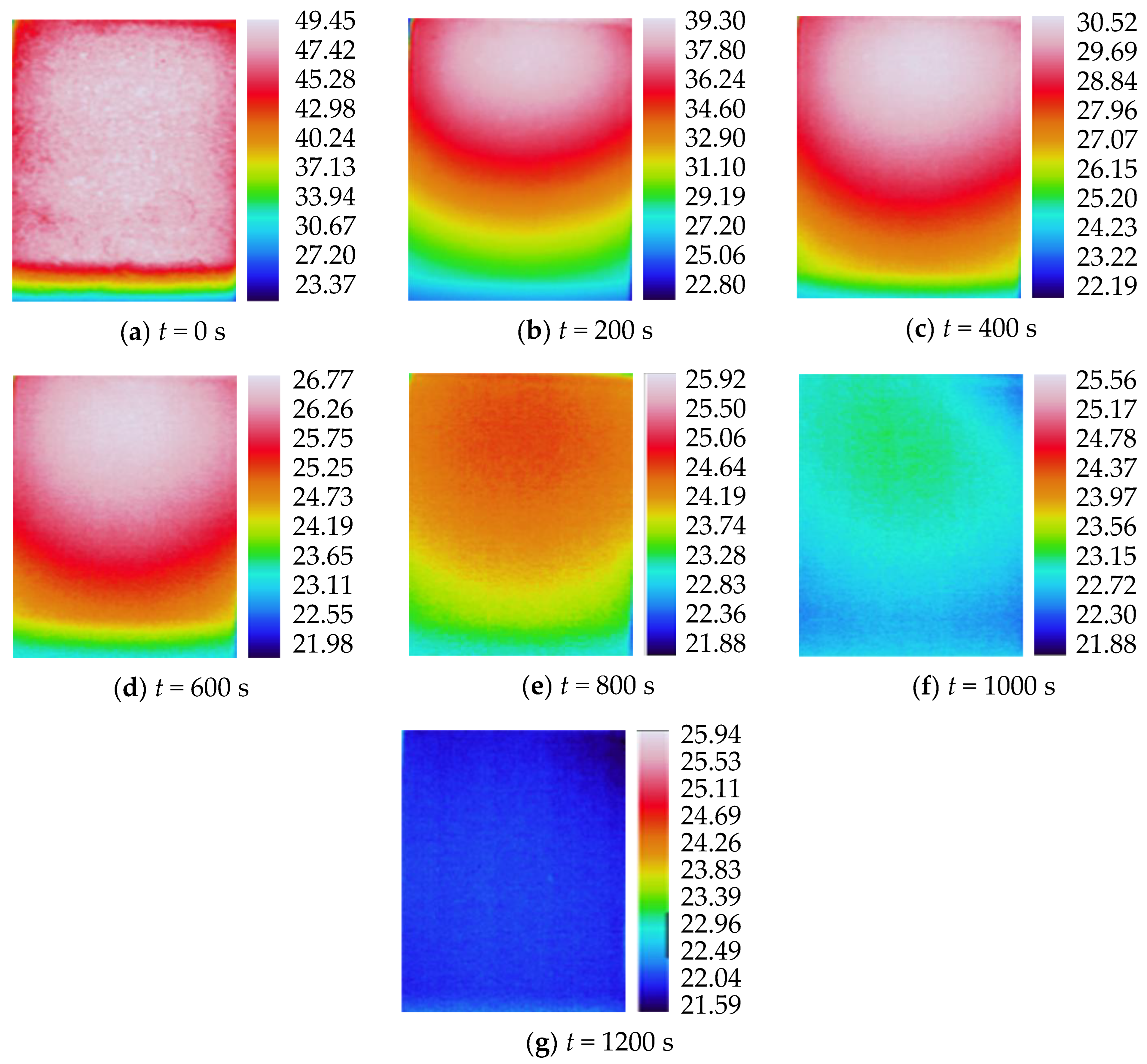
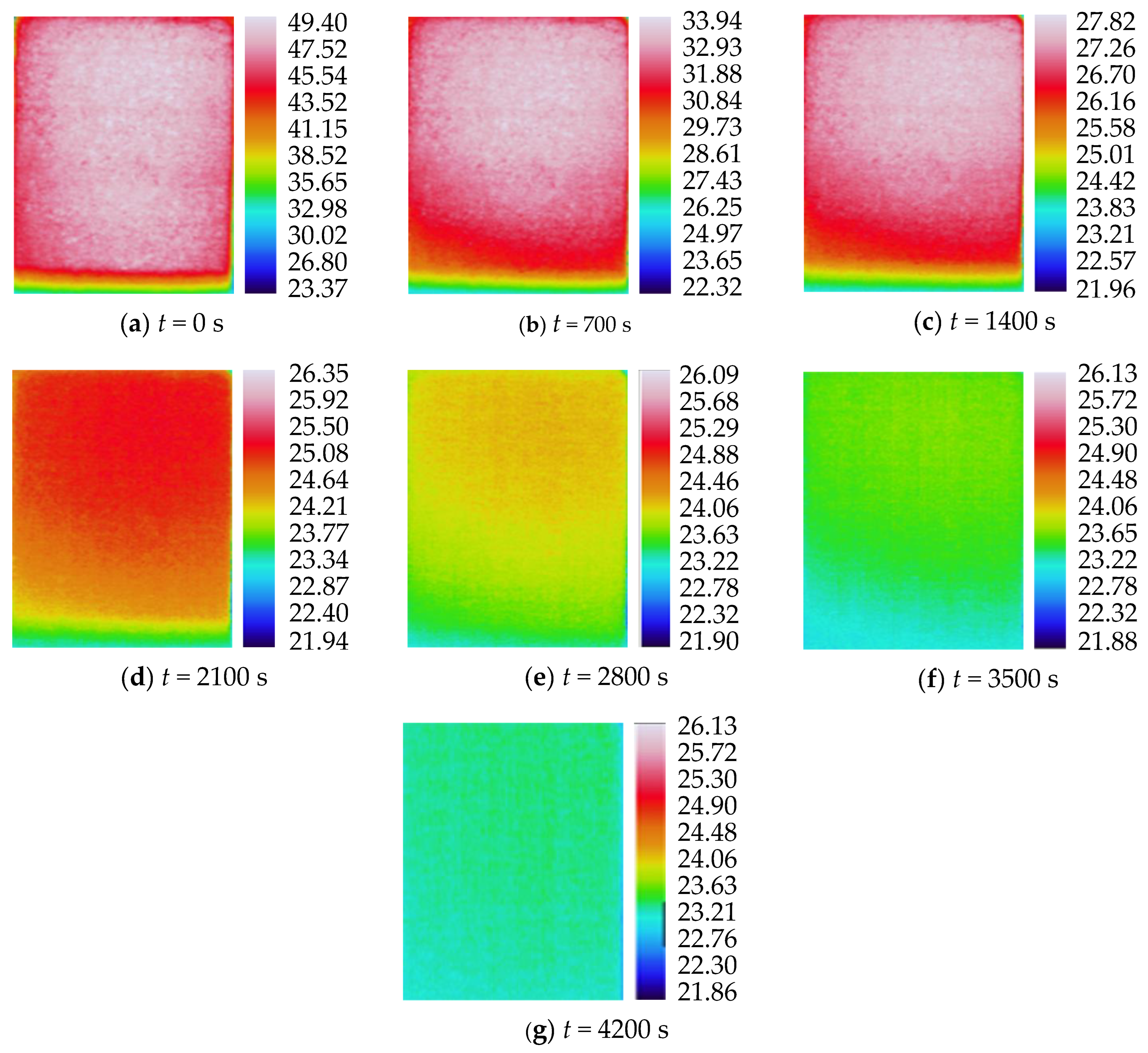
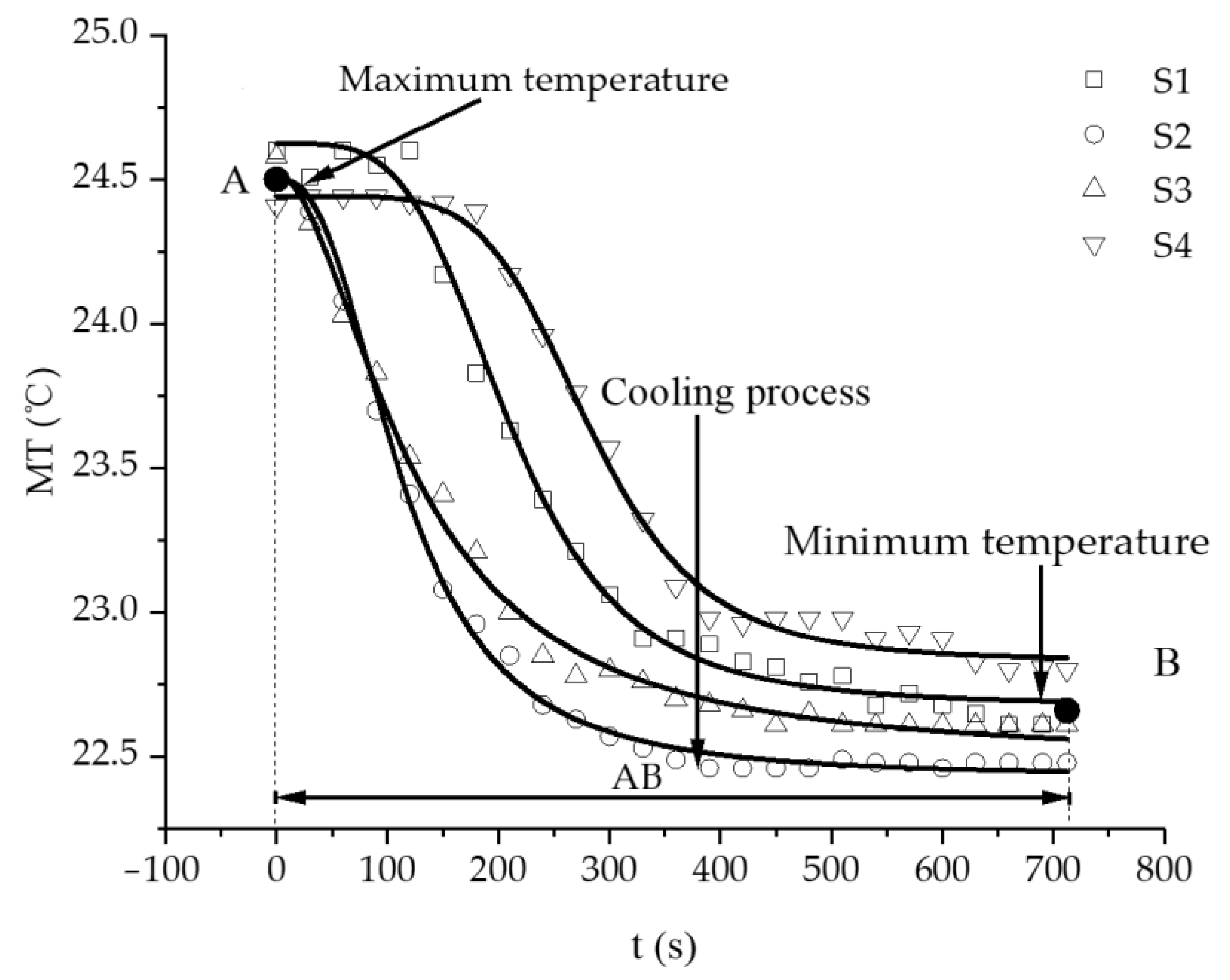
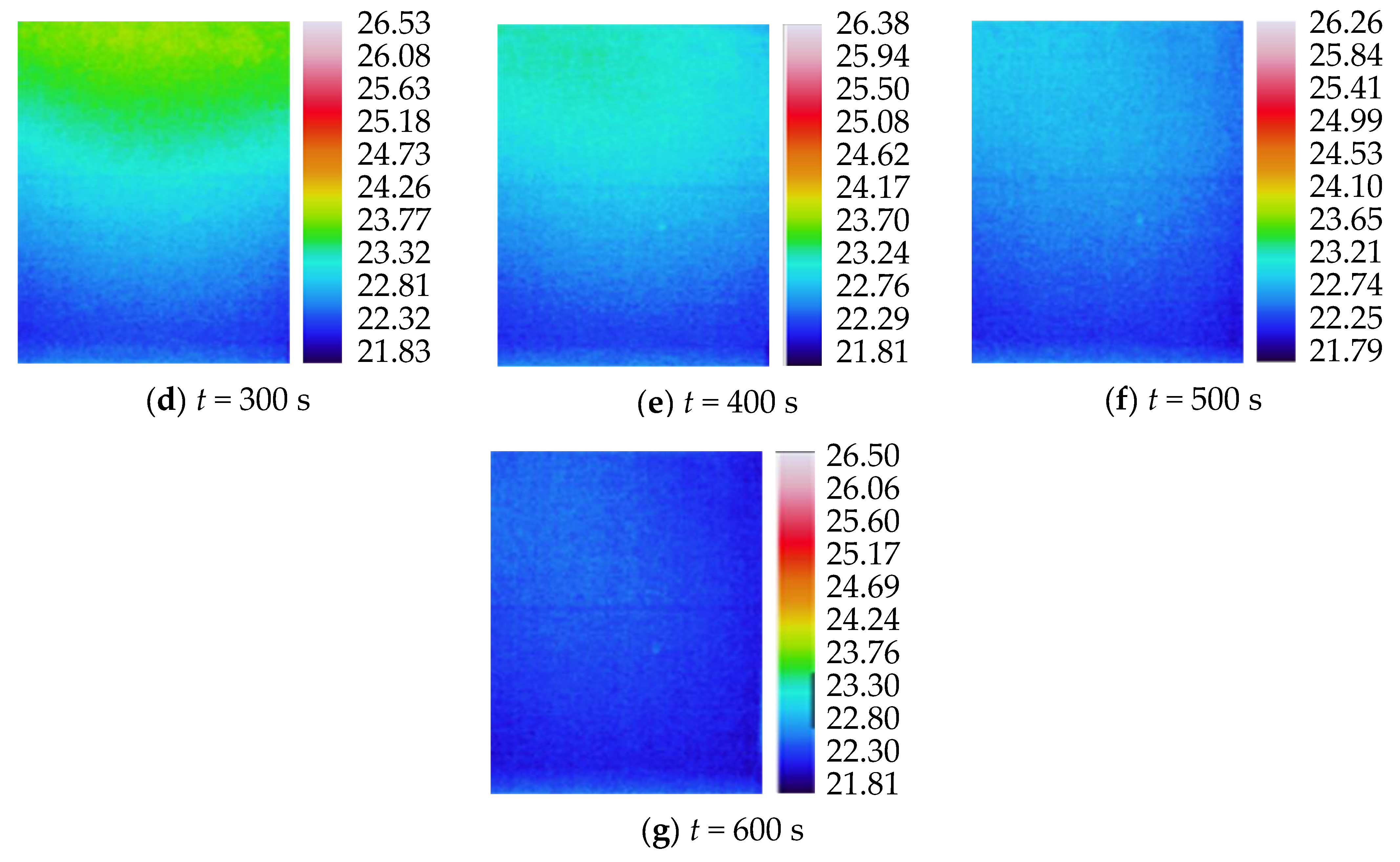
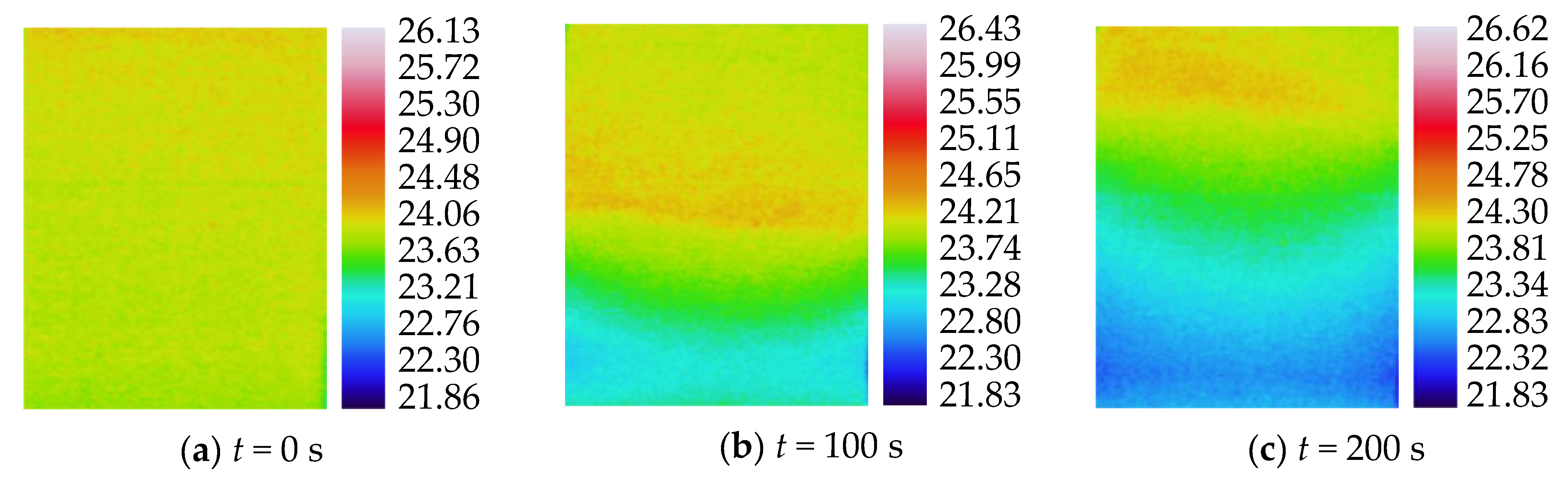
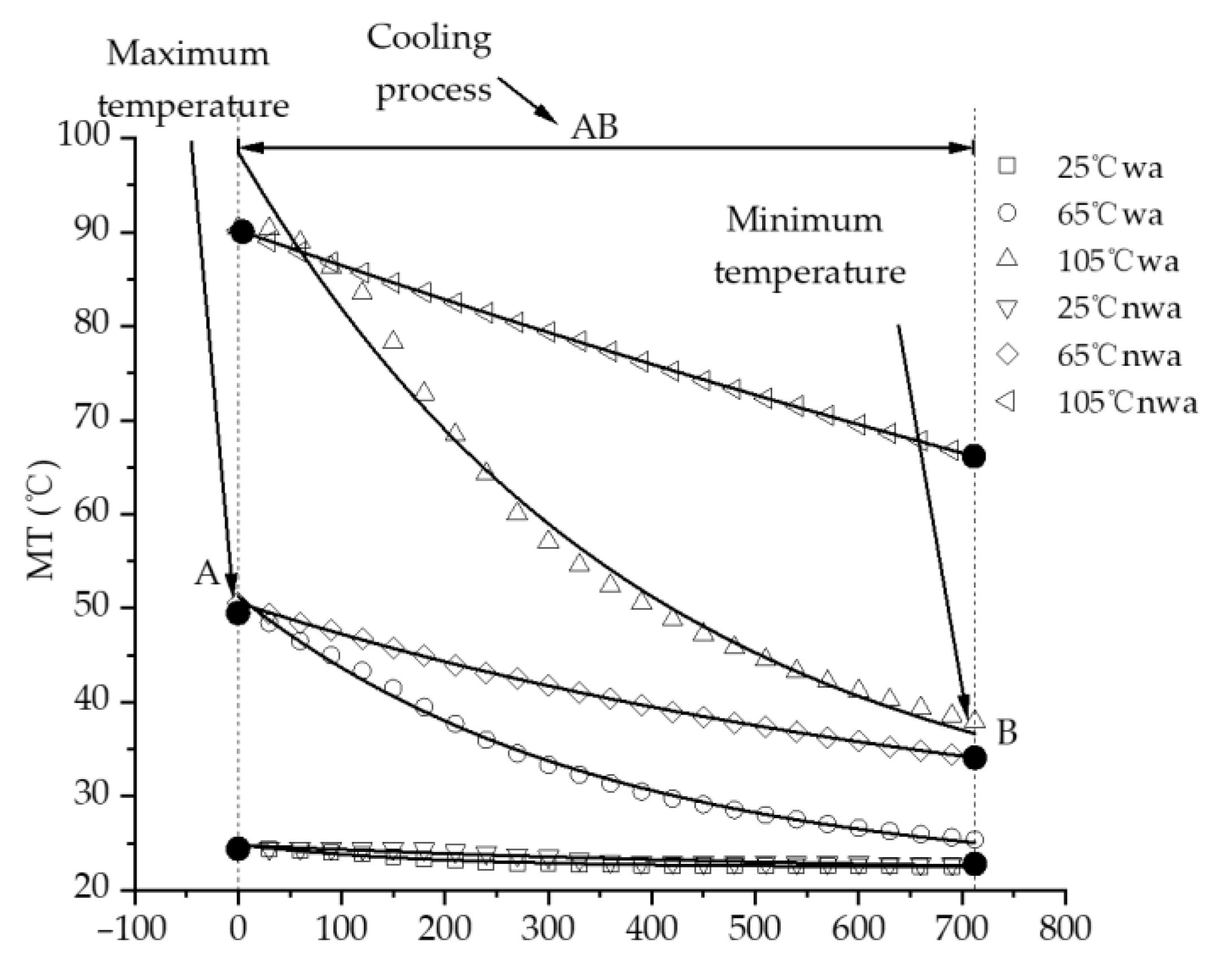
3.2. Characteristics of Temperature Variation during Hydration Absorption of Sandstone Samples
- (1)
- Surface temperature change during hydration absorption of samples at 105 °C
- (2)
- Surface temperature changes during hydration absorption of samples at 65 °C
- (3)
- Surface temperature change during hydration absorption of samples at 25 °C
- (4)
- Average and maximum surface temperature of rock samples.
4. Conclusions
- (1)
- The intelligent test system of rock hydration independently developed in this study can provide different hydration absorption test environments and accurate real-time measurements. This test system can be used to study the behaviors of surrounding rock with obvious hydration (such as in the Dunhuang Mogao Grottoes) and provide a powerful research and development means and equipment guarantee for the evaluation of protection technology schemes.
- (2)
- In this study, the self-developed intelligent test system for rock hydration combined with an infrared thermal imager was used to conduct liquid water adsorption experiments on rock samples at different initial temperatures. The curve of the hydration absorption process could be fitted with an exponential function, and the change trend of the curve of the hydration absorption characteristics of the rocks first rapidly increased, then slowly increased, and finally remained unchanged.
- (3)
- According to the self-developed intelligent test system for rock hydration, the stage of rapid increase in the hydration absorption of rock samples is the stage when the wetting peak rises from the bottom surface to the top surface. The average time required for this stage for rock samples at 25 °C was 211 s, for rock samples at 65 °C it was 158 s, and for rock samples at 105 °C it was 96 s. As the initial temperature increases, the effect of hydration absorption on the sandstone’s temperature increases. The faster the hydration absorption rate of the samples before reaching saturation, the shorter the time it takes to reach saturation.
- (4)
- By using the self-developed intelligent rock hydrology test system and combining it with an infrared thermal imager, the mean temperature and maximum temperature of each rock sample in the Mogao Grottoes showed a nonlinear change trend when the temperature of the sandstone decreased; the higher the initial temperature, the greater the influence of hydration absorption on the temperature of the sandstone, and the longer the time required for the rock samples to cool to room temperature.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Wang, J.; Li, L. Dynamic propagation behaviors of hydraulic fracture networks considering hydro-mechanical coupling effects in tight oil and gas reservoirs: A multi-thread parallel computation method. Comput. Geotech. 2022, 152, 105016. [Google Scholar] [CrossRef]
- Wang, Y. Adaptive Analysis of Damage and Fracture in Rock with Multiphysical Fields Coupling; Springer Press: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Hao, N.; Wang, Y.; Wu, X.; Duan, Y.; Li, P.; He, M. Real-time experimental monitoring for hydration absorption evolution behaviors of sandstone in Mogao Grottoes, China. Energies 2022, 15, 8504. [Google Scholar] [CrossRef]
- Meng, L.; Han, L.; Zhu, H.; Dong, W.; Li, W. Study on the influence of formation temperature on the water absorption and weathering of swelling rock in marine coal mines. KSCE J. Civ. Eng. 2021, 25, 4207–4220. [Google Scholar] [CrossRef]
- Tao, Z.; Xu, H.; Peng, H.; Liu, W. Experimental system of hydrodynamic action of dunhuang mogao grottoes under temperature-humidity-mechanical (THM) coupling. Geofluids 2020, 2020, 8831284. [Google Scholar]
- Alonso-Buenaposada, I.D.; Calvo, E.G.; Montes-Morán, M.; Narciso, J.; Menéndez, J.A.; Arenillas, A. Desiccant capability of organic xerogels: Surface chemistry vs porous texture. Microporous Mesoporous Mater. 2016, 232, 70–76. [Google Scholar] [CrossRef]
- Miao, C.; Zhu, M. Experimental study on the evolution of micropore structure softened by non-pressure water absorption strength of sandstone. J. Porous Media 2022, 26, 73–86. [Google Scholar]
- Zhang, F.; Liu, W.; Wang, Z.; Fu, Y.; Wang, S.; Liu, R.; He, M. The Influence of Water Absorption Increment on Infrared Radiation Characteristics of Rammed Earth; Springer: Singapore, 2018. [Google Scholar]
- Wang, J.; Sun, Q.; Xue, S.; Yang, X.; Guo, H.; Geng, J. Study on the effect of high-temperature dry-wet cycles on argillaceous sandstone. Bull. Eng. Geol. Environ. 2023, 82, 318. [Google Scholar] [CrossRef]
- Sun, X.; Shi, F.; Ding, J.; Tao, Z.; Zhang, Y.; Miao, C.; Cui, L. Study on Microscopic mechanism and failure mode of water-rock coupling in fine sandstone under uniaxial compression. Rock Mech. Rock Eng. 2023, 56, 6823–6842. [Google Scholar] [CrossRef]
- Kim, E.; Garcia, A.; Changani, H. Fragmentation and energy absorption characteristics of Red, Berea and Buff sandstones based on different loading rates and water contents. Geomech. Eng. 2018, 14, 151–159. [Google Scholar]
- Zhang, N.; Liu, L.; Hou, D.; He, M.; Liu, Y. Geomechanical and water vapor absorption characteristics of clay-bearing soft rocks at great depth. J. Mater. Sci. Technol. 2018, 24, 811–818. [Google Scholar] [CrossRef]
- Cui, G.; Zhou, C.; Liu, Z. Prediction method of water absorption of soft rock considering the influence of composition, porosity, and solute quantitatively. Appl. Sci. 2022, 12, 5938. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Z.; Du, Y.; Zhang, S.; Shi, Z.; Li, F. Effect of high temperature on pore characteristics, yield stress, and deformation property of sandstone. Bull. Eng. Geol. Environ. 2022, 81, 43. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Tan, H.; Wang, J.; Shi, Z.; Li, K. Study on fatigue characteristics and thermal damage mechanism of red sandstone under high temperature-cyclic load coupling. Int. J. Fatigue 2023, 168, 107405. [Google Scholar] [CrossRef]
- Pan, X.; Zhou, X. Damage analysis of sandstone during the creep stage after high-temperature heat treatment based on NMR technology. Rock Mech. Rock Eng. 2022, 55, 7569–7586. [Google Scholar] [CrossRef]
- Liu, X.; Li, D.; Li, C. Laboratory testing and modeling of creep deformation for sandstone including initial temperature damage. Rock Mech. Rock Eng. 2023, 56, 2479–2495. [Google Scholar] [CrossRef]
- Luo, J.; He, J. Constitutive model and fracture failure of sandstone damage under high temperature–cyclic stress. Materials 2022, 15, 4903. [Google Scholar] [CrossRef] [PubMed]
- Caccia, M.; Camarano, A.; Sergi, D.; Ortona, A.; Narciso, J. Chapter Wetting and Navier-Stokes Equation—The Manufacture of Composite Materials. In Wetting and Wettability; InTechOpen: London, UK, 2015. [Google Scholar]
- Narciso, J.; Ramos-Fernandez, E.V.; Delgado-Marín, J.J.; Affolter, C.W.; Olsbye, U.; Redekop, E.A. New route for the synthesis of Co-MOF from metal substrates. Microporous Mesoporous Mater. 2021, 324, 111310. [Google Scholar] [CrossRef]
- Molina, J.; Rodriguezguerrero, A.; Bahraini, M.; Weber, L.; Narciso, J.; Rodriguezreinoso, F.; Louis, E.; Mortensen, A. Infiltration of graphite preforms with Al–Si eutectic alloy and mercury. Scr. Mater. 2007, 56, 991–994. [Google Scholar] [CrossRef]
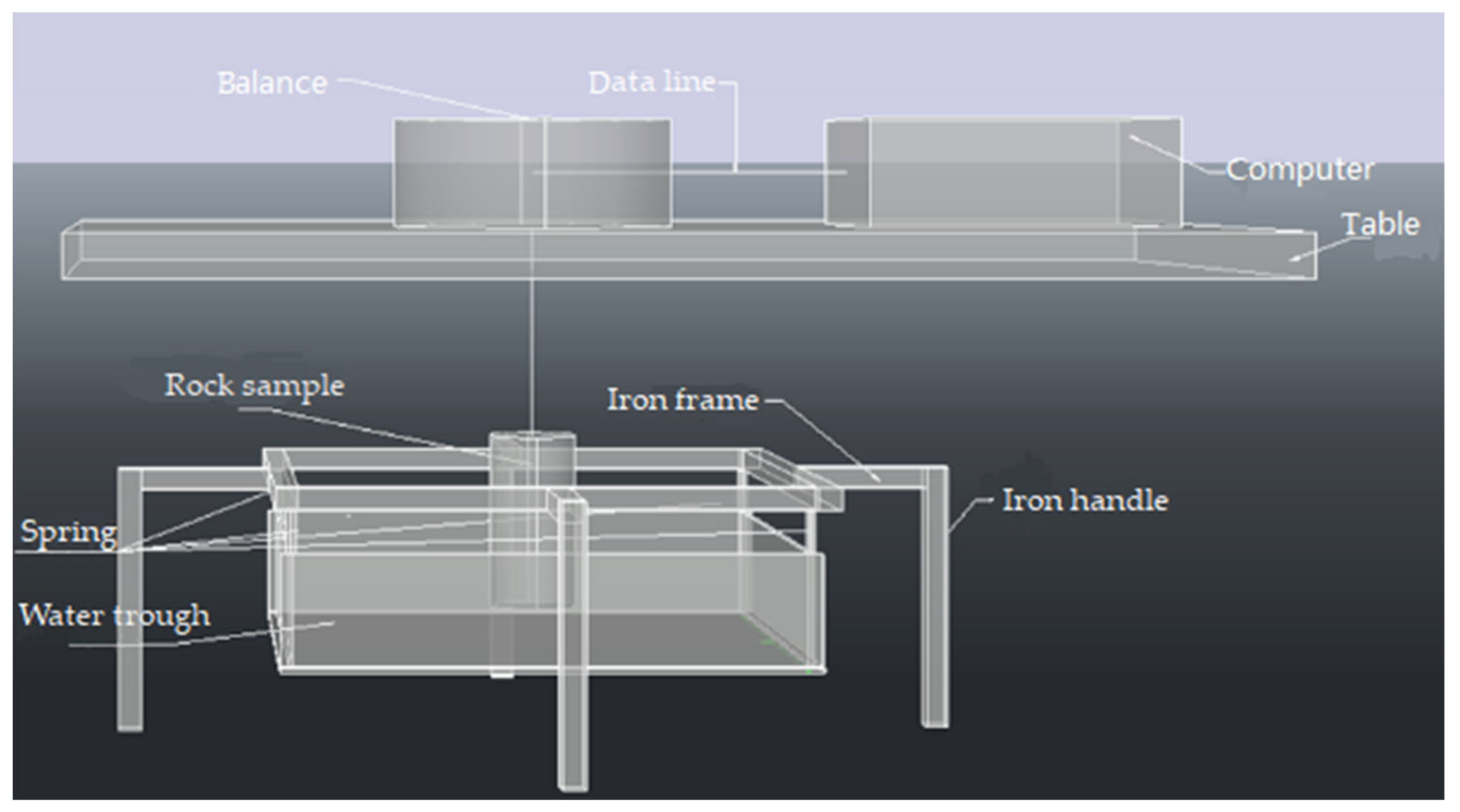















| Temperature Sensitivity (mk) | Measure Precision | Image Resolution (pixel) | Frame Frequency (Hz) | Emissivity |
|---|---|---|---|---|
| 18 | ±1 °C, ±1% | 640 × 512 | 1004 | 0.92 |
| Sample Number | Long (mm) | Wide (mm) | High (mm) | Mass (g) |
|---|---|---|---|---|
| S1 | 29.7 | 29.3 | 50.0 | 79.26 |
| S2 | 30.0 | 29.8 | 49.4 | 80.04 |
| S3 | 30.0 | 29.7 | 49.5 | 79.31 |
| S4 | 30.0 | 29.6 | 49.8 | 79.24 |
| Quartz | Potassium Feldspar | Plagioclase | Calcite | Dolomite | Hornblende | Clay Mineral |
|---|---|---|---|---|---|---|
| 29.0 | 4.1 | 31.9 | 12.3 | 7.6 | 4.9 | 10.2 |
| Temperature (°C) | S1 | S2 | S3 | S4 |
|---|---|---|---|---|
| 105 | Absorb water | Absorb water | Absorb water | No water |
| 65 | Absorb water | Absorb water | Absorb water | No water |
| 25 | Absorb water | Absorb water | Absorb water | Absorb water |
| Sample Number | Parameters of Hydration Absorption Curve Fitting at 105 °C | |||
|---|---|---|---|---|
| a | b | m0 | R2 | |
| S1 | −8.968 | 34.430 | 13.262 | 0.977 |
| S2 | −10.199 | 70.630 | 13.139 | 0.989 |
| S3 | −9.274 | 39.862 | 13.221 | 0.978 |
| Sample Number | Parameters of Hydration Absorption Curve Fitting at 65 °C | |||
|---|---|---|---|---|
| a | b | m0 | R2 | |
| S1 | −9.734 | 66.551 | 10.617 | 0.991 |
| S2 | −9.324 | 101.510 | 10.804 | 0.987 |
| S3 | −9.928 | 68.475 | 11.218 | 0.988 |
| Sample Number | Parameters of Hydration Absorption Curve Fitting at 25 °C | |||
|---|---|---|---|---|
| a | b | m0 | R2 | |
| S1 | −9.628 | 89.738 | 11.797 | 0.985 |
| S2 | −10.487 | 134.475 | 12.302 | 0.986 |
| S3 | −9.500 | 88.394 | 12.774 | 0.981 |
| S4 | −10.168 | 92.118 | 11.494 | 0.986 |
| Sample Number | The Average Temperature of 105 °C Fitting Parameters | |||
|---|---|---|---|---|
| a | b | t0 | R2 | |
| S1 | 56.471 | 203.315 | 23.992 | 0.984 |
| S2 | 61.721 | 198.930 | 23.901 | 0.991 |
| S3 | 55.279 | 195.284 | 24.099 | 0.983 |
| S4 | 58.020 | 773.033 | 23.473 | 0.999 |
| Sample Number | The Maximum Temperature Fitting Parameter of 105 °C | |||
|---|---|---|---|---|
| A | B | t0 | R2 | |
| S1 | 68.304 | 206.544 | 24.646 | 0.992 |
| S2 | 76.245 | 219.704 | 24.046 | 0.989 |
| S3 | 72.583 | 199.920 | 24.805 | 0.990 |
| S4 | 66.485 | 794.469 | 23.732 | 0.999 |
| Sample Number | The Average Temperature of 65 °C Fitting Parameters | |||
|---|---|---|---|---|
| a | b | t0 | R2 | |
| S1 | 22.701 | 300.828 | 22.044 | 0.994 |
| S2 | 21.604 | 287.664 | 22.319 | 0.998 |
| S3 | 25.861 | 297.541 | 22.050 | 0.996 |
| S4 | 23.264 | 748.680 | 23.368 | 0.999 |
| Sample Number | The Maximum Temperature Fitting Parameter of 65 °C | |||
|---|---|---|---|---|
| a | b | t0 | R2 | |
| S1 | 29.424 | 321.016 | 22.133 | 0.998 |
| S2 | 27.084 | 332.618 | 22.089 | 0.998 |
| S3 | 31.715 | 325.884 | 21.918 | 0.996 |
| S4 | 26.791 | 776.588 | 23.621 | 1.000 |
| Sample Number | The Average Temperature of 25 °C Fitting Parameters | ||||
|---|---|---|---|---|---|
| a | b | t0 | c | R2 | |
| S1 | 24.023 | 20.499 | 403.485 | 1.644 | 0.994 |
| S2 | 23.923 | 21.583 | 281.857 | 2.423 | 0.996 |
| S3 | 23.981 | 21.105 | 356.922 | 2.047 | 0.995 |
| S4 | 23.867 | 20.688 | 427.359 | 1.680 | 0.995 |
| Sample Number | The Maximum Temperature Fitting Parameter of 25 °C | ||||
|---|---|---|---|---|---|
| a | b | t0 | c | R2 | |
| S1 | 24.625 | 22.678 | 210.342 | 4.063 | 0.991 |
| S2 | 24.501 | 22.430 | 112.772 | 2.562 | 0.996 |
| S3 | 24.521 | 22.487 | 121.778 | 1.856 | 0.992 |
| S4 | 24.443 | 22.833 | 282.545 | 5.532 | 0.995 |
| Sample Environments | Mean Temperature Mean Fitting Parameter | ||||
|---|---|---|---|---|---|
| a | b | t0 | c | R2 | |
| Hydration absorption at 25 °C | 3.425 | 481.487 | 20.871 | 3.425 | 0.982 |
| Hydration absorption at 65 °C | 22.971 | 252.336 | 23.294 | 22.971 | 0.996 |
| Hydration absorption at 105 °C | 56.564 | 247.507 | 31.708 | 56.564 | 0.987 |
| 25 °C; does not absorb water | 3.644 | 636.914 | 20.446 | 3.644 | 0.916 |
| 65 °C; does not absorb water | 3.425 | 481.487 | 20.871 | 3.425 | 1.000 |
| 105 °C; does not absorb water | 22.971 | 252.336 | 23.294 | 22.971 | 1.000 |
| Sample Environments | Maximum Temperature Average Fitting Parameter | ||||
|---|---|---|---|---|---|
| a | b | t0 | c | R2 | |
| Hydration absorption at 25 °C | 2.412 | 172.823 | 22.473 | 2.412 | 0.982 |
| Hydration absorption at 65 °C | 29.949 | 342.081 | 21.324 | 29.949 | 0.996 |
| Hydration absorption at 105 °C | 73.790 | 390.832 | 24.743 | 73.790 | 0.987 |
| 25 °C; does not absorb water | 3.223 | 608.853 | 21.605 | 3.223 | 0.916 |
| 65 °C; does not absorb water | 27.090 | 759.415 | 23.509 | 27.090 | 1.000 |
| 105 °C; does not absorb water | 108.999 | 2854.339 | -18.789 | 108.999 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, N.; Wang, Y.; Cheng, H.; Wu, X.; Zhao, Y. Hydration Absorption and Thermal Effects of Outcrop Porous Sandstone Based on Intelligent Experimental and Infrared Thermography Techniques. Energies 2023, 16, 7540. https://doi.org/10.3390/en16227540
Hao N, Wang Y, Cheng H, Wu X, Zhao Y. Hydration Absorption and Thermal Effects of Outcrop Porous Sandstone Based on Intelligent Experimental and Infrared Thermography Techniques. Energies. 2023; 16(22):7540. https://doi.org/10.3390/en16227540
Chicago/Turabian StyleHao, Nai, Yongliang Wang, Hao Cheng, Xiaochong Wu, and Yi Zhao. 2023. "Hydration Absorption and Thermal Effects of Outcrop Porous Sandstone Based on Intelligent Experimental and Infrared Thermography Techniques" Energies 16, no. 22: 7540. https://doi.org/10.3390/en16227540
APA StyleHao, N., Wang, Y., Cheng, H., Wu, X., & Zhao, Y. (2023). Hydration Absorption and Thermal Effects of Outcrop Porous Sandstone Based on Intelligent Experimental and Infrared Thermography Techniques. Energies, 16(22), 7540. https://doi.org/10.3390/en16227540







