Key Issues and Strategies of Aqueous Zinc-Ion Batteries
Abstract
:1. Introduction
| Element | Atomic Mass | Standard Potential (V) vs. SHE | Gravimetric Capacity (mAh/g) | Volumetric Capacity (mAh/cm3) | Ionic Radius (Å) |
|---|---|---|---|---|---|
| Li | 6.94 | −3.040 | 3680 | 2061 | 0.76 |
| Na | 23.00 | −2.713 | 1165 | 1129 | 1.02 |
| K | 39.1 | −2.924 | 685 | 610 | 1.38 |
| Mg | 24.31 | −2.356 | 2206 | 3834 | 0.72 |
| Ca | 40.08 | −2.840 | 1337 | 2072 | 1.00 |
| Zn | 65.41 | −0.763 | 820 | 5855 | 0.75 |
| Al | 26.98 | −1.676 | 2980 | 8046 | 0.53 |
2. The Challenges of the Zinc Anode
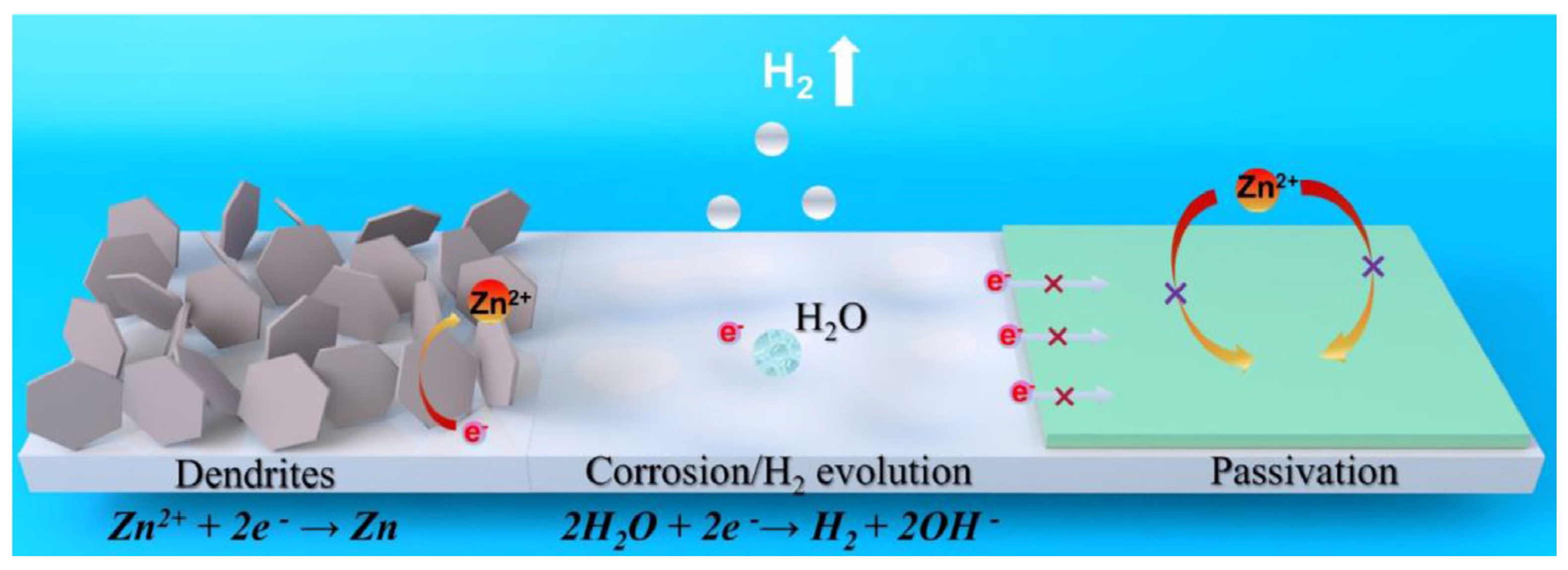
2.1. Dendrites and Protrusion
2.2. Hydrogen Evolution Reaction
2.3. Passivation Layer
3. The Strategies of Zinc Anode Protection
3.1. Surface Engineering
3.2. Electrolyte Modification
3.2.1. Highly Concentrated Electrolyte
3.2.2. Hydrogel Electrolytes
3.2.3. Functional Additives
3.3. Three-Dimensional Structural Skeleton and Alloy Strategies
4. Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.; Dahn, J.R.; Wainwright, D.S. Rechargeable lithium batteries with aqueous electrolytes. Science 1994, 264, 1115–1118. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Wang, Y.; Rong, X.; Sun, W.; Fan, X.; Xu, S.; Schroeder, M.A.; Cresce, A.V.; Wang, F. “Water-in-salt” electrolyte makes aqueous sodium-ion battery safe, green, and long-lasting. Adv. Energy Mater. 2017, 7, 1701189. [Google Scholar] [CrossRef]
- Su, D.; McDonagh, A.; Qiao, S.Z.; Wang, G. High-capacity aqueous potassium-ion batteries for large-scale energy storage. Adv. Mater. 2017, 29, 1604007. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bao, J.; Dong, X.; Truhlar, D.; Wang, Y.; Wang, C.; Xia, Y. Aqueous Mg-ion battery based on polyimide anode and prussian blue cathode. ACS Energy Lett. 2017, 2, 1115–1121. [Google Scholar] [CrossRef]
- Gheytani, S.; Liang, Y.; Wu, F.; Jing, Y.; Dong, H.; Rao, K.K.; Chi, X.; Fang, F.; Yao, Y. An aqueous Ca-ion battery. Adv. Sci. 2017, 4, 1700465. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Pan, G.; Li, G.; Gao, X. Copper hexacyanoferrate nanoparticles as cathode material for aqueous Al-ion batteries. J. Mater. Chem. A 2015, 3, 959–962. [Google Scholar] [CrossRef]
- Xu, C.; Li, B.; Du, H.; Kang, F. Energetic zinc ion chemistry: The rechargeable zinc ion battery. Angew. Chem. Int. Ed. 2012, 51, 933–935. [Google Scholar] [CrossRef]
- Liu, Y.; Holze, R. Metal-Ion Batteries. Encyclopedia 2022, 2, 1611–1623. [Google Scholar] [CrossRef]
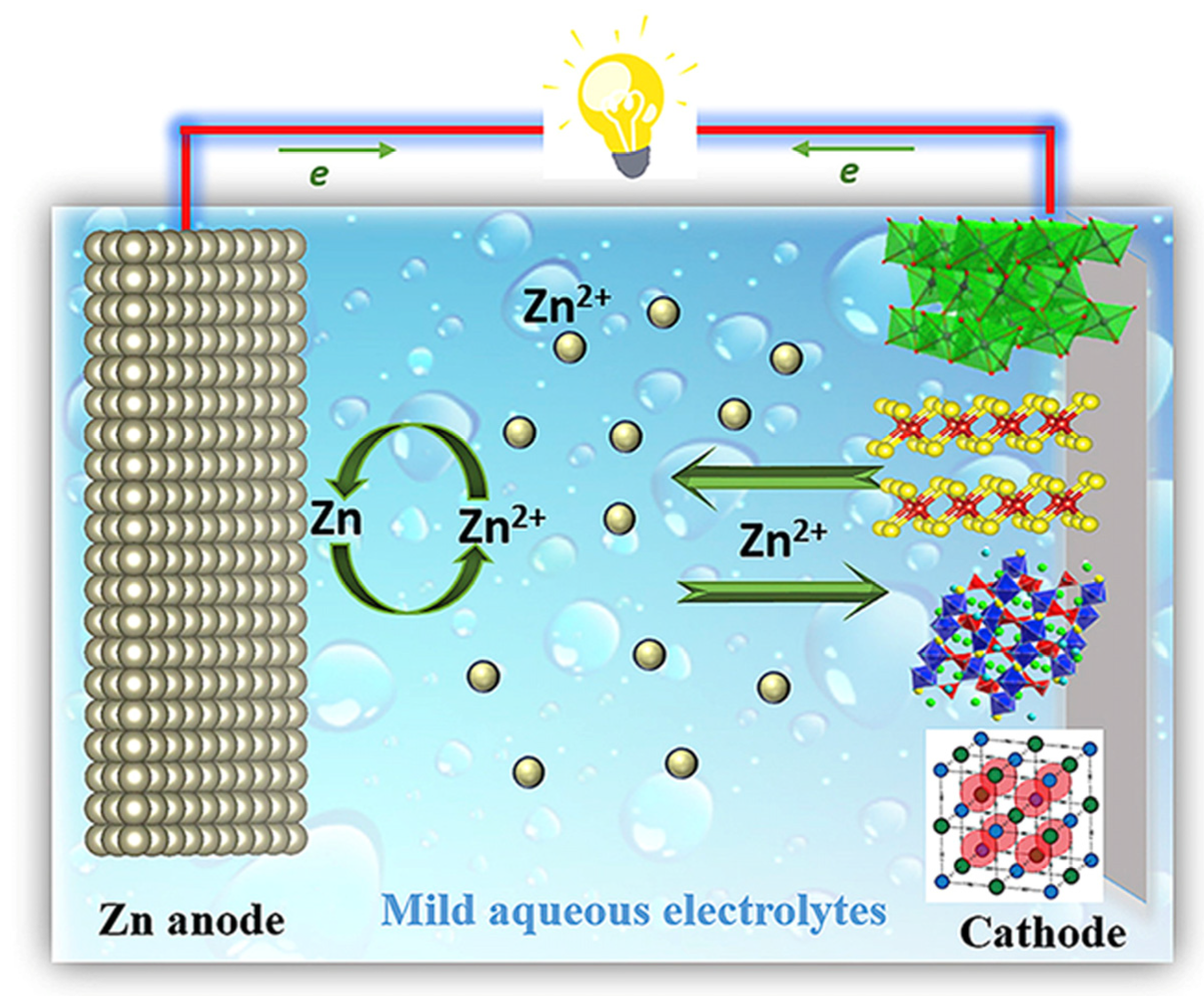
- Zeng, X.; Hao, J.; Wang, Z.; Mao, J.; Guo, Z. Recent progress and perspectives on aqueous Zn-based rechargeable batteries with mild aqueous electrolytes. Energy Storage Mater. 2019, 20, 410–437. [Google Scholar] [CrossRef]
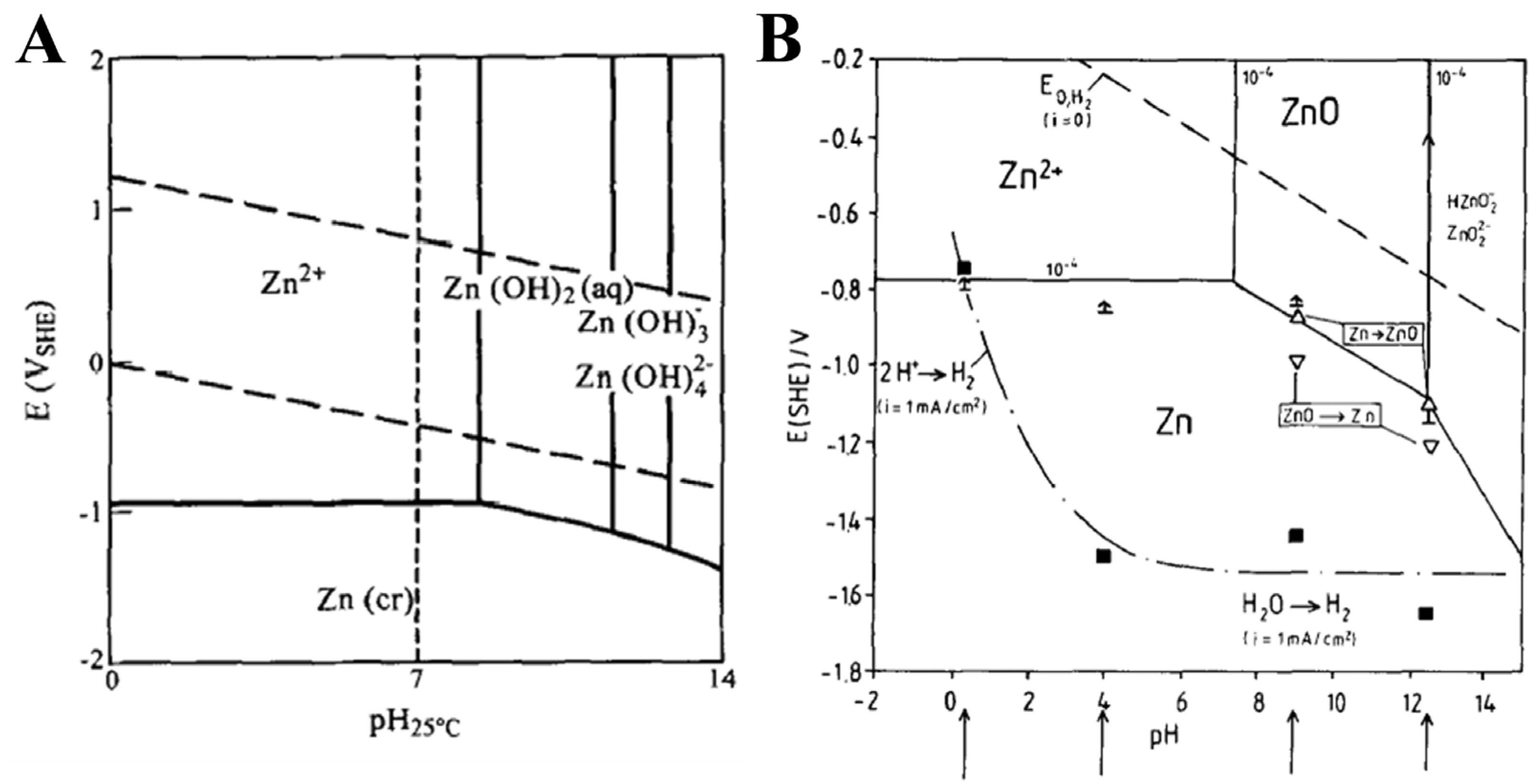
- Beverskog, B.; Puigdomenech, I. Revised pourbaix diagrams for zinc at 25–300 °C. Corros. Sci. 1997, 39, 107–114. [Google Scholar] [CrossRef]
- Wippermann, K.; Schultze, J.; Kessel, R.; Penninger, J. The inhibition of zinc corrosion by bisaminotriazole and other triazole derivatives. Corros. Sci. 1991, 32, 205–230. [Google Scholar] [CrossRef]
- Kundu, D.; Adams, B.D.; Duffort, V.; Vajargah, S.H.; Nazar, L.F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 2016, 1, 16119. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M. Zn/MnO2 battery chemistry with H+ and Zn2+ coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, L.; Dai, X.; Wang, X.; Niu, Z.; Chen, J. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat. Commun. 2018, 9, 1656. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Ran, Q.; Dai, T.-Y.; Shi, H.; Zeng, S.-P.; Zhu, Y.-F.; Wen, Z.; Zhang, W.; Lang, X.-Y.; Zheng, W.-T.; et al. Surface-Alloyed Nanoporous Zinc as Reversible and Stable Anodes for High-Performance Aqueous Zinc-Ion Battery. Nano-Micro Lett. 2022, 14, 128. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, X.; Liu, H.; Li, J.; Chen, Y.; Duan, H. 3D-Printed Multi-Channel Metal Lattices Enabling Localized Electric-Field Redistribution for Dendrite-Free Aqueous Zn Ion Batteries. Adv. Energy Mater. 2021, 11, 2003927. [Google Scholar] [CrossRef]
- Ji, S.; Qin, J.; Yang, S.; Shen, P.; Hu, Y.; Yang, K.; Luo, H.; Xu, J. A mechanically durable hybrid hydrogel electrolyte developed by controllable accelerated polymerization mechanism towards reliable aqueous zinc-ion battery. Energy Storage Mater. 2023, 55, 236–243. [Google Scholar] [CrossRef]
- Huang, S.; Hou, L.; Li, T.; Jiao, Y.; Wu, P. Antifreezing Hydrogel Electrolyte with Ternary Hydrogen Bonding for High-Performance Zinc-Ion Batteries. Adv. Mater. 2022, 34, 2110140. [Google Scholar] [CrossRef]
- Park, J.-H.; Hyun Park, S.; Joung, D.; Kim, C. Sustainable biopolymeric hydrogel interphase for dendrite-free aqueous zinc-ion batteries. Chem. Eng. J. 2022, 433, 133532. [Google Scholar] [CrossRef]
- Du, W.; Ang, E.H.; Yang, Y.; Zhang, Y.; Ye, M.; Li, C.C. Challenges in the material and structural design of zinc anode towards high-performance aqueous zinc-ion batteries. Energy Environ. Sci. 2020, 13, 3330–3360. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, C.; Zhang, H.; Li, X. Anode for Zinc-Based Batteries: Challenges, Strategies, and Prospects. ACS Energy Lett. 2021, 6, 2765–2785. [Google Scholar] [CrossRef]
- Zheng, J.; Archer, L.A. Controlling electrochemical growth of metallic zinc electrodes: Toward affordable rechargeable energy storage systems. Sci. Adv. 2021, 7, eabe0219. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Huang, Z.; Ming, F.; Zeng, Y.; Wei, B.; Jiang, Q.; Qi, Z.; Wang, Z.; Liang, H. Surface and Interface Engineering of Zn Anodes in Aqueous Rechargeable Zn-Ion Batteries. Small 2022, 18, 2200006. [Google Scholar] [CrossRef]
- He, H.; Qin, H.; Wu, J.; Chen, X.; Huang, R.; Shen, F.; Wu, Z.; Chen, G.; Yin, S.; Liu, J. Engineering interfacial layers to enable Zn metal anodes for aqueous zinc-ion batteries. Energy Storage Mater. 2021, 43, 317–336. [Google Scholar] [CrossRef]
- Li, Y.; Wu, P.; Zhong, W.; Xie, C.; Xie, Y.; Zhang, Q.; Sun, D.; Tang, Y.; Wang, H.-Y. Progressive nucleation mechanism enables stable zinc stripping-plating behavior. Energy Environ. Sci. 2021, 14, 5563–5571. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, H.; Zhu, R.; Zhou, H. High reversibility at high current density: The zinc electrodeposition principle behind the “trick”. Energy Environ. Sci. 2023, 16, 2723–2731. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, X.; Wang, N.; Lai, W.-H.; Liu, Y.; Chou, S.-L.; Liu, H.-K.; Dou, S.-X.; Wang, Y.-X. Anode optimization strategies for aqueous zinc-ion batteries. Chem. Sci. 2022, 13, 14246–14263. [Google Scholar] [CrossRef]
- Zhang, W.; Dai, Y.; Chen, R.; Xu, Z.; Li, J.; Zong, W.; Li, H.; Li, Z.; Zhang, Z.; Zhu, J.; et al. Highly Reversible Zinc Metal Anode in a Dilute Aqueous Electrolyte Enabled by a pH Buffer Additive. Angew. Chem. Int. Ed. 2023, 62, e202212695. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Tang, Y.; Xu, Z.; Wang, K.; Li, Q.; He, B.; Liu, Y.; Ge, M.; Chen, S.; et al. Stabilizing Zn Anode Interface by Simultaneously Manipulating the Thermodynamics of Zn Nucleation and Overpotential of Hydrogen Evolution. Adv. Funct. Mater. 2022, 32, 2207898. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Zhu, M.; Fan, Y.; Lin, W.; Chan, D.; Lin, W.; Li, P.; Tang, Y.; Zhang, Y. Revitalizing zinc-ion batteries with advanced zinc anode design. Nanoscale Horiz. 2023, 8, 29–54. [Google Scholar] [CrossRef] [PubMed]
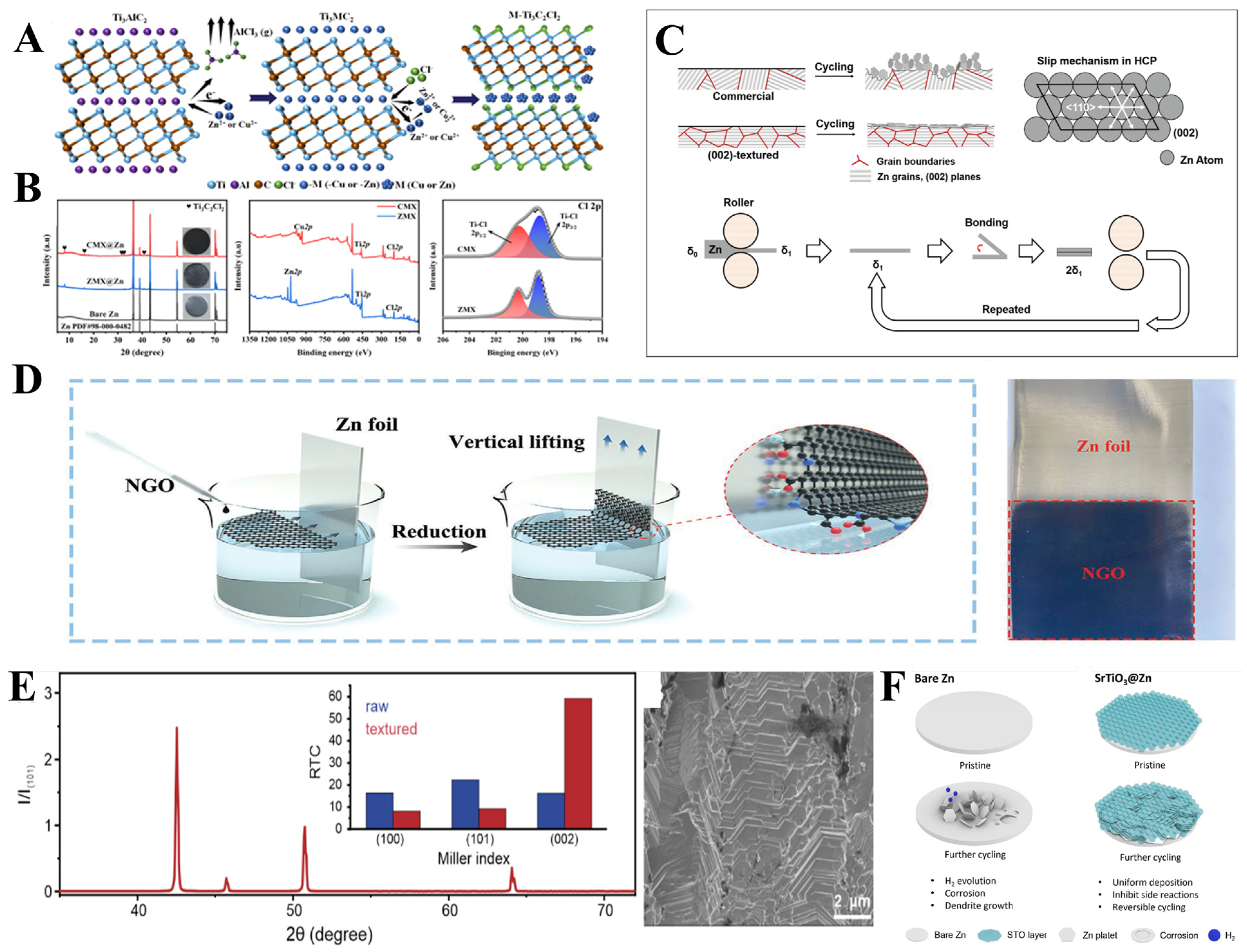
- Shen, Y.; Liu, Y.; Sun, K.; Gu, T.; Wang, G.; Yang, Y.; Pang, J.; Zheng, Y.; Yang, X.; Chen, L. Zincophilic Ti3C2Cl2 MXene and anti-corrosive Cu NPs for synergistically regulated deposition of dendrite-free Zn metal anode. J. Mater. Sci. Technol. 2024, 169, 137–147. [Google Scholar] [CrossRef]
- Zheng, J.; Deng, Y.; Yin, J.; Tang, T.; Garcia-Mendez, R.; Zhao, Q.; Archer, L.A. Textured Electrodes: Manipulating Built-In Crystallographic Heterogeneity of Metal Electrodes via Severe Plastic Deformation. Adv. Mater. 2022, 34, 2106867. [Google Scholar] [CrossRef]
- Zhou, J.; Xie, M.; Wu, F.; Mei, Y.; Hao, Y.; Huang, R.; Wei, G.; Liu, A.; Li, L.; Chen, R. Ultrathin Surface Coating of Nitrogen-Doped Graphene Enables Stable Zinc Anodes for Aqueous Zinc-Ion Batteries. Adv. Mater. 2021, 33, e2101649. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Lu, Q.; Hantusch, M.; Zhang, H.; Qu, Z.; Tang, H.; Dong, H.; Schmidt, O.G.; Holze, R.; et al. Highly enhanced reversibility of a Zn anode by in-situ texturing. Energy Storage Mater. 2022, 47, 98–104. [Google Scholar] [CrossRef]
- Ko, J.; So, S.; Kim, M.; Tae Kim, I.; Nam Ahn, Y.; Hur, J. Promoting Zn2+ migration through polar perovskite dielectric layer on Zn metal anode for the enhanced aqueous Zn-ion batteries. Chem. Eng. J. 2023, 462, 142308. [Google Scholar] [CrossRef]
- Kang, L.; Cui, M.; Jiang, F.; Gao, Y.; Luo, H.; Liu, J.; Liang, W.; Zhi, C. Nanoporous CaCO3 coatings enabled uniform Zn stripping/plating for long-life zinc rechargeable aqueous batteries. Adv. Energy Mater. 2018, 8, 1801090. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, C.; Yu, Y.; Yan, M.; Wei, Q.; He, P.; Dong, Y.; Zhang, Z.; Wang, X.; Mai, L. Ultrathin surface coating enables stabilized zinc metal anode. Adv. Mater. Interfaces 2018, 5, 1800848. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, J.; Huang, X.; Wang, Q.; Sun, D.; Tang, Y.; Ji, X.; Wang, H. Revealing the role of crystal orientation of protective layers for stable zinc anode. Nat. Commun. 2020, 11, 3961. [Google Scholar] [CrossRef]
- Yan, H.; Li, S.; Nan, Y.; Yang, S.; Li, B. Ultrafast zinc–ion–conductor interface toward high-rate and stable zinc metal batteries. Adv. Energy Mater. 2021, 11, 2100186. [Google Scholar] [CrossRef]
- Hong, L.; Wu, X.; Ma, C.; Huang, W.; Zhou, Y.; Wang, K.-X.; Chen, J.-S. Boosting the Zn-ion transfer kinetics to stabilize the Zn metal interface for high-performance rechargeable Zn-ion batteries. J. Mater. Chem. A 2021, 9, 16814–16823. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Liu, D.; Liu, M.; Zhou, T.; Qi, K.; Shi, L.; Zhu, Y.; Qian, Y. Ultra-long-life and highly reversible Zn metal anodes enabled by a desolvation and deanionization interface layer. Energy Environ. Sci. 2021, 14, 3120–3129. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Z.; Hao, R.; Huang, Y.; Liu, W.; Tan, Y.; Li, P.; Yan, J.; Liu, K. Ultra-highly stable zinc metal anode via 3D-printed g-C3N4 modulating interface for long life energy storage systems. Chem. Eng. J. 2021, 403, 126425. [Google Scholar] [CrossRef]
- Han, J.; Euchner, H.; Kuenzel, M.; Hosseini, S.M.; Groß, A.; Varzi, A.; Passerini, S. A thin and uniform fluoride-based artificial interphase for the zinc metal anode enabling reversible Zn/MnO2 batteries. ACS Energy Lett. 2021, 6, 3063–3071. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, C.; Li, T.; Yuan, Z.; Zhang, H.; Li, X. Dendrite-free zinc-based battery with high areal capacity via the region-induced deposition effect of turing membrane. J. Am. Chem. Soc. 2021, 143, 13135–13144. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, C.; Du, Y.; Wang, X.; Ding, L.; Omar, A.; Mikhailova, D. Uniform Zn Deposition Achieved by Ag Coating for Improved Aqueous Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 16869–16875. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Li, D.; Deng, T.; Li, Q.; Wang, C. Hydrophobic organic-electrolyte-protected zinc anodes for aqueous zinc batteries. Angew. Chem. Int. Ed. 2020, 59, 19292–19296. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yuan, X.; Xia, Y.; Zhang, Y.; Fu, L.; Liu, L.; Yu, N.; Huang, Q.; Wang, B.; Hu, X.; et al. An artificial polyacrylonitrile coating layer confining zinc dendrite growth for highly reversible aqueous zinc-based batteries. Adv. Sci. 2021, 8, e2100309. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhao, J.; Hu, Z.; Li, J.; Li, J.; Zhang, Y.; Wang, C.; Cui, G. Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase. Energy Environ. Sci. 2019, 12, 1938–1949. [Google Scholar] [CrossRef]
- Park, S.H.; Byeon, S.Y.; Park, J.-H.; Kim, C. Insight into the critical role of surface hydrophilicity for dendrite-free zinc metal anodes. ACS Energy Lett. 2021, 6, 3078–3085. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, Y.; Liu, G.; Zhu, R.; Huang, J.; Chen, Z.; Gao, Z.; Chen, X.; Qie, L. Redirected Zn electrodeposition by an anti-corrosion elastic constraint for highly reversible Zn anodes. Adv. Funct. Mater. 2020, 31, 2001867. [Google Scholar] [CrossRef]
- Hao, J.; Li, X.; Zhang, S.; Yang, F.; Zeng, X.; Zhang, S.; Bo, G.; Wang, C.; Guo, Z. Designing dendrite-free zinc anodes for advanced aqueous zinc batteries. Adv. Funct. Mater. 2020, 30, 2001263. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, Y.P.; Lu, H.T.; Zhang, Y.F.; Fu, C.Y.; Usman, I.; Liu, Z.X.; Feng, M.Y.; Fang, G.Z.; Cao, X.X.; et al. Anti-corrosive and Zn-ion-regulating composite interlayer enabling long-life Zn metal anodes. Adv. Funct. Mater. 2021, 31, 2104361. [Google Scholar] [CrossRef]
- Chu, Y.; Zhang, S.; Wu, S.; Hu, Z.; Cui, G.; Luo, J. In situ built interphase with high interface energy and fast kinetics for high performance Zn metal anodes. Energy Environ. Sci. 2021, 14, 3609–3620. [Google Scholar] [CrossRef]
- Li, D.; Cao, L.; Deng, T.; Liu, S.; Wang, C. Design of a solid electrolyte interphase for aqueous Zn batteries. Angew. Chem. Int. Ed. 2021, 60, 13035–13041. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zhou, W.; Huang, A.; Chen, M.; Chen, J.; Tian, Q.; Xu, J. Modifying the Zn anode with carbon black coating and nanofibrillated cellulose binder: A strategy to realize dendrite-free Zn-MnO2 batteries. J. Colloid Interface Sci. 2020, 577, 256–264. [Google Scholar] [CrossRef]
- Sawada, Y. Transition of growth form from dendrite to aggregate. Phys. A Stat. Mech. Its Appl. 1986, 140, 134–141. [Google Scholar] [CrossRef]
- Mackinnon, D.J.; Brannen, J.M.; Fenn, P.L. Characterization of impurity effects in zinc electrowinning from industrial acid sulphate electrolyte. J. Appl. Electrochem. 1987, 17, 1129–1143. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, S.; Li, J.; Luo, X.; Liu, Z.; Zhang, T.; Cao, X.; Long, M.; Lu, B.; Pan, A.; et al. Surface-preferred crystal plane for a stable and reversible zinc anode. Adv. Mater. 2021, 33, 2100187. [Google Scholar] [CrossRef]
- Zheng, J.; Bock, D.C.; Tang, T.; Zhao, Q.; Yin, J.; Tallman, K.R.; Wheeler, G.; Liu, X.; Deng, Y.; Jin, S.; et al. Regulating electrodeposition morphology in high-capacity aluminium and zinc battery anodes using interfacial metal–substrate bonding. Nat. Energy 2021, 6, 398–406. [Google Scholar] [CrossRef]
- Li, S.; Fu, J.; Miao, G.; Wang, S.; Zhao, W.; Wu, Z.; Zhang, Y.; Yang, X. Toward planar and dendrite-free Zn electrodepositions by regulating Sn-crystal textured surface. Adv. Mater. 2021, 33, 2008424. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Gu, C.; Wang, X.; Wang, S.; Zhang, X.; Qin, J.; Wu, Z.S. Manipulating crystallographic orientation of zinc deposition for dendrite-free zinc ion batteries. Adv. Energy Mater. 2021, 11, 2101299. [Google Scholar] [CrossRef]
- Zheng, J.; Yin, J.; Zhang, D.; Li, G.; Bock, D.C.; Tang, T.; Zhao, Q.; Liu, X.; Warren, A.; Deng, Y.; et al. Spontaneous and field-induced crystallographic reorientation of metal electrodeposits at battery anodes. Sci. Adv. 2020, 6, eabb1122. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zhao, J.; Ren, H.; Chen, Y.; Chua, R.; Jie, E.T.J.; Cai, Y.; Edison, E.; Manalastas, W., Jr.; Wong, M.W.; et al. Anion texturing towards dendrite-free Zn anode for aqueous rechargeable batteries. Angew. Chem. Int. Ed. 2021, 60, 7213–7219. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, Q.; Tang, T.; Yin, J.; Quilty, C.D.; Renderos, G.D.; Liu, X.; Deng, Y.; Wang, L.; Bock, D.C.; et al. Reversible epitaxial electrodeposition of metals in battery anodes. Science 2019, 366, 645–648. [Google Scholar] [CrossRef]
- Wang, X.; Meng, J.; Lin, X.; Yang, Y.; Zhou, S.; Wang, Y.; Pan, A. Stable zinc metal anodes with textured crystal faces and functional zinc compound coatings. Adv. Funct. Mater. 2021, 31, 2106114. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, X.; Sun, J.; Liu, Y.; Hou, L. Recent progress in “water-in-salt” electrolytes toward non-lithium based rechargeable batteries. Front. Chem. 2020, 8, 595. [Google Scholar] [CrossRef]
- Droguet, L.; Grimaud, A.; Fontaine, O.; Tarascon, J.-M. Water-in-Salt electrolyte (WiSE) for aqueous batteries: A long way to practicality. Adv. Energy Mater. 2020, 10, 2002440. [Google Scholar] [CrossRef]
- Azov, V.A.; Egorova, K.S.; Seitkalieva, M.M.; Kashin, A.S.; Ananikov, V.P. “Solvent-in-salt” systems for design of new materials in chemistry, biology and energy research. Chem. Soc. Rev. 2018, 47, 1250–1284. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.; Luo, C.; Wang, C.; Xu, K. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 2015, 350, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Borodin, O.; Gao, T.; Fan, X.; Sun, W.; Han, F.; Faraone, A.; Dura, J.A.; Xu, K.; Wang, C. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 2018, 17, 543–549. [Google Scholar] [CrossRef]
- Hu, P.; Yan, M.; Zhu, T.; Wang, X.; Wei, X.; Li, J.; Zhou, L.; Li, Z.; Chen, L.; Mai, L. Zn/V2O5 aqueous hybrid-ion battery with high voltage platform and long cycle life. ACS Appl. Mater. Interfaces 2017, 9, 42717–42722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Holoubek, J.; Wu, X.; Daniyar, A.; Zhu, L.; Chen, C.; Leonard, D.P.; Rodríguez-Pérez, I.A.; Jiang, J.-X.; Fang, C.; et al. A ZnCl2 water-in-salt electrolyte for a reversible Zn metal anode. Chem. Commun. 2018, 54, 14097–14099. [Google Scholar] [CrossRef] [PubMed]
- Kohli, R. Chapter 16—Applications of Ionic Liquids in Removal of Surface Contaminants. In Developments in Surface Contamination and Cleaning: Applications of Cleaning Techniques; Kohli, R., Mittal, K.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 619–680. [Google Scholar]
- Wu, J.; Liang, Q.; Yu, X.; Lü, Q.-F.; Ma, L.; Qin, X.; Chen, G.; Li, B. Deep eutectic solvents for boosting electrochemical energy storage and conversion: A review and perspective. Adv. Funct. Mater. 2021, 31, 2011102. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Shi, J.; Sun, T.; Bao, J.; Zheng, S.; Du, H.; Li, L.; Yuan, X.; Ma, T.; Tao, Z. “Water-in-deep eutectic solvent” electrolytes for high-performance aqueous Zn-ion batteries. Adv. Funct. Mater. 2021, 31, 2102035. [Google Scholar] [CrossRef]
- Sun, T.; Zheng, S.; Du, H.; Tao, Z. Synergistic effect of cation and anion for low-temperature aqueous zinc-ion battery. Nano-Micro Lett. 2021, 13, 204. [Google Scholar] [CrossRef]
- Yang, W.; Du, X.; Zhao, J.; Chen, Z.; Li, J.; Xie, J.; Zhang, Y.; Cui, Z.; Kong, Q.; Zhao, Z.; et al. Hydrated eutectic electrolytes with ligand-oriented solvation shells for long-cycling Zinc-organic batteries. Joule 2020, 4, 1557–1574. [Google Scholar] [CrossRef]
- Ghareh Bagh, F.S.; Shahbaz, K.; Mjalli, F.S.; Hashim, M.A.; AlNashef, I.M. Zinc (II) chloride-based deep eutectic solvents for application as electrolytes: Preparation and characterization. J. Mol. Liq. 2015, 204, 76–83. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.; Yang, W.; Chen, B.; Zhao, Z.; Qiu, H.; Dong, S.; Zhou, X.; Cui, G.; Chen, L. “Water-in-deep eutectic solvent” electrolytes enable zinc metal anodes for rechargeable aqueous batteries. Nano Energy 2019, 57, 625–634. [Google Scholar] [CrossRef]
- Qiu, H.; Du, X.; Zhao, J.; Wang, Y.; Ju, J.; Chen, Z.; Hu, Z.; Yan, D.; Zhou, X.; Cui, G. Zinc anode-compatible in-situ solid electrolyte interphase via cation solvation modulation. Nat. Commun. 2019, 10, 5374. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, X.; Meng, Y.; Yu, M.; Yi, J.; Wu, Y.; Lu, X.; Tong, Y. Achieving ultrahigh energy density and long durability in a flexible rechargeable quasi-solid-state Zn–MnO2 battery. Adv. Mater. 2017, 29, 1700274. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, N.; Zeng, S.; Zhou, S.; Chen, M.; Di, J.; Li, Q. An adaptive and stable bio-electrolyte for rechargeable Zn-ion batteries. J. Mater. Chem. A 2018, 6, 12237–12243. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C.; Li, Q.; Pan, Z.; Sun, J.; Zhou, Z.; He, B.; Man, P.; Xie, L.; Kang, L. Flexible and high-voltage coaxial-fiber aqueous rechargeable zinc-ion battery. Nano Lett. 2019, 19, 4035–4042. [Google Scholar] [CrossRef]
- McEvoy, H.; Ross-Murphy, S.; Clark, A. Large deformation and ultimate properties of biopolymer gels: 1. Single biopolymer component systems. Polymer 1985, 26, 1483–1492. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Wang, D.; Yang, Q.; Mo, F.; Liang, G.; Li, N.; Zhang, H.; Zapien, J.A.; Zhi, C. Super-stretchable Zinc-Air batteries based on an alkaline-tolerant dual-network hydrogel electrolyte. Adv. Energy Mater. 2019, 9, 1803046. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.; Liang, G.; Huang, Y.; Huang, Y.; Zhu, M.; Pei, Z.; Xue, Q.; Tang, Z.; Wang, Y.; et al. Waterproof and tailorable elastic rechargeable yarn zinc ion batteries by a cross-linked polyacrylamide electrolyte. ACS Nano 2018, 12, 3140–3148. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xie, X.; Liang, S.; Zhou, J. Issues and future perspective on zinc metal anode for rechargeable aqueous zinc-ion batteries. Energy Environ. Mater. 2020, 3, 146–159. [Google Scholar] [CrossRef]
- Sun, K.E.K.; Hoang, T.K.A.; Doan, T.N.L.; Yu, Y.; Chen, P. Highly sustainable zinc anodes for a rechargeable hybrid aqueous battery. Chemistry 2018, 24, 1667–1673. [Google Scholar] [CrossRef]
- Trudgeon, D.P.; Qiu, K.; Li, X.; Mallick, T.; Taiwo, O.O.; Chakrabarti, B.; Yufit, V.; Brandon, N.P.; Crevillen-Garcia, D.; Shah, A. Screening of effective electrolyte additives for zinc-based redox flow battery systems. J. Power Sources 2019, 412, 44–54. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, J.; Feng, J.; Wang, Y.; Wu, X.; Ma, P.; Zhang, X.; Wang, G.; Yan, X. A rechargeable aqueous zinc/sodium manganese oxides battery with robust performance enabled by Na2SO4 electrolyte additive. Energy Storage Mater. 2021, 38, 299–308. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Z.; Li, J.; Luo, N.; Chai, G.-L.; Miller, T.S.; Lai, F.; Shearing, P.; Brett, D.J.L.; Han, D.; et al. Alleviation of dendrite formation on zinc anodes via electrolyte additives. ACS Energy Lett. 2021, 6, 395–403. [Google Scholar] [CrossRef]
- Bayaguud, A.; Luo, X.; Fu, Y.; Zhu, C. Cationic surfactant-type electrolyte additive enables three-dimensional dendrite-free zinc anode for stable zinc-ion batteries. ACS Energy Lett. 2020, 5, 3012–3020. [Google Scholar] [CrossRef]
- Jin, Y.; Han, K.S.; Shao, Y.; Sushko, M.L.; Xiao, J.; Pan, H.; Liu, J. Stabilizing zinc anode reactions by polyethylene oxide polymer in mild aqueous electrolytes. Adv. Funct. Mater. 2020, 30, 2003932. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, J.; Fu, L.; Wu, S.; Tang, Y.; Ji, X.; Wang, H. The three-dimensional dendrite-free zinc anode on a copper mesh with a zinc-oriented polyacrylamide electrolyte additive. Angew. Chem. Int. Ed. 2019, 58, 15841–15847. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Li, D.; Hu, E.; Xu, J.; Deng, T.; Ma, L.; Wang, Y.; Yang, X.Q.; Wang, C. Solvation structure design for aqueous Zn metal batteries. J. Am. Chem. Soc. 2020, 142, 21404–21409. [Google Scholar] [CrossRef]
- Xu, Z.; Li, H.; Liu, Y.; Wang, K.; Wang, H.; Ge, M.; Xie, J.; Li, J.; Wen, Z.; Pan, H.; et al. Durable modulation of Zn(002) plane deposition via reproducible zincophilic carbon quantum dots towards low N/P ratio zinc-ion batteries. Mater. Horiz. 2023, 10, 3680–3693. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, W.; Huang, Q.; Guan, C.; Zong, W.; Dai, Y.; Du, Z.; Zhang, Z.; Li, J.; Guo, F.; et al. Trace Amounts of Triple-Functional Additives Enable Reversible Aqueous Zinc-Ion Batteries from a Comprehensive Perspective. Nano-Micro Lett. 2023, 15, 81. [Google Scholar] [CrossRef]
- Wang, S.B.; Ran, Q.; Yao, R.Q.; Shi, H.; Wen, Z.; Zhao, M.; Lang, X.Y.; Jiang, Q. Lamella-nanostructured eutectic zinc-aluminum alloys as reversible and dendrite-free anodes for aqueous rechargeable batteries. Nat. Commun. 2020, 11, 1634. [Google Scholar] [CrossRef]
- Liang, Y.; Ding, W.; Yao, B.; Zheng, F.; Smirnova, A.; Gu, Z. Mediating Lithium Plating/Stripping by Constructing 3D Au@Cu Pentagonal Pyramid Array. Batteries 2023, 9, 279. [Google Scholar] [CrossRef]
- Su, S.; Xu, Y.; Wang, Y.; Wang, X.; Shi, L.; Wu, D.; Zou, P.; Nairan, A.; Lin, Z.; Kang, F.; et al. Holey nickel nanotube reticular network scaffold for high-performance flexible rechargeable Zn/MnO2 batteries. Chem. Eng. J. 2019, 370, 330–336. [Google Scholar] [CrossRef]
- Kang, Z.; Wu, C.L.; Dong, L.B.; Liu, W.B.; Mou, J.; Zhang, J.W.; Chang, Z.W.; Jiang, B.Z.; Wang, G.X.; Kang, F.Y.; et al. 3D porous copper skeleton supported zinc anode toward high capacity and long cycle life zinc ion batteries. Acs Sustain. Chem. Eng. 2019, 7, 3364–3371. [Google Scholar] [CrossRef]
- Qiu, W.; Li, Y.; You, A.; Zhang, Z.; Li, G.; Lu, X.; Tong, Y. High-performance flexible quasi-solid-state Zn–MnO2 battery based on MnO2 nanorod arrays coated 3D porous nitrogen-doped carbon cloth. J. Mater. Chem. A 2017, 5, 14838–14846. [Google Scholar] [CrossRef]
- Li, M.; Meng, J.; Li, Q.; Huang, M.; Liu, X.; Owusu, K.A.; Liu, Z.; Mai, L. Finely crafted 3D electrodes for dendrite-free and high-performance flexible fiber-shaped Zn-Co batteries. Adv. Funct. Mater. 2018, 28, 1802016. [Google Scholar] [CrossRef]
- Wang, X.; Wang, F.; Wang, L.; Li, M.; Wang, Y.; Chen, B.; Zhu, Y.; Fu, L.; Zha, L.; Zhang, L.; et al. An aqueous rechargeable Zn//Co3O4 battery with high energy density and good cycling behavior. Adv. Mater. 2016, 28, 4904–4911. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, X.; Qin, R.; Liu, X.; Fang, P.; Zheng, D.; Tong, Y.; Lu, X. Dendrite-free zinc deposition induced by multifunctional CNT frameworks for stable flexible Zn-ion batteries. Adv. Mater. 2019, 31, e1903675. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Wei, C.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Flexible and free-standing Ti3C2TxMXene@Zn paper for dendrite-free aqueous zinc metal batteries and nonaqueous lithium metal batteries. ACS Nano 2019, 13, 11676–11685. [Google Scholar] [CrossRef]
- Liu, C.; Luo, Z.; Deng, W.; Wei, W.; Chen, L.; Pan, A.; Ma, J.; Wang, C.; Zhu, L.; Xie, L.; et al. Liquid alloy interlayer for aqueous zinc-ion battery. ACS Energy Lett. 2021, 6, 675–683. [Google Scholar] [CrossRef]
- Wang, L.; Huang, W.; Guo, W.; Guo, Z.H.; Chang, C.; Gao, L.; Pu, X. Sn alloying to inhibit hydrogen evolution of Zn metal anode in rechargeable aqueous batteries. Adv. Funct. Mater. 2021, 32, 2108533. [Google Scholar] [CrossRef]
- Qi, Z.; Xiong, T.; Chen, T.; Yu, C.; Zhang, M.; Yang, Y.; Deng, Z.; Xiao, H.; Lee, W.S.V.; Xue, J. Dendrite-free anodes enabled by a composite of a ZnAl alloy with a copper mesh for high-performing aqueous zinc-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 28129–28139. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Zhou, X.; Wei, A.; Meng, Q.; Ye, H.; Liu, W.; Tang, J.; Yang, J. Fast-charging and ultrahigh-capacity zinc metal anode for high-performance aqueous zinc-ion batteries. Adv. Funct. Mater. 2021, 31, 2100398. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, H.; Li, Q.; Zhou, L.; Zhao, P.; Holze, R. Key Issues and Strategies of Aqueous Zinc-Ion Batteries. Energies 2023, 16, 7443. https://doi.org/10.3390/en16217443
Liu Y, Wang H, Li Q, Zhou L, Zhao P, Holze R. Key Issues and Strategies of Aqueous Zinc-Ion Batteries. Energies. 2023; 16(21):7443. https://doi.org/10.3390/en16217443
Chicago/Turabian StyleLiu, Yi, Huibo Wang, Qingyuan Li, Lingfeng Zhou, Pengjun Zhao, and Rudolf Holze. 2023. "Key Issues and Strategies of Aqueous Zinc-Ion Batteries" Energies 16, no. 21: 7443. https://doi.org/10.3390/en16217443
APA StyleLiu, Y., Wang, H., Li, Q., Zhou, L., Zhao, P., & Holze, R. (2023). Key Issues and Strategies of Aqueous Zinc-Ion Batteries. Energies, 16(21), 7443. https://doi.org/10.3390/en16217443