Biogas Production—The Effect of the Zinc Concentration on the Profile of Volatile Fatty Acids in Fermentation Mixtures
Abstract
:1. Introduction
1.1. Problem Statement
1.2. Current Ongoing Solution
1.3. Proposed Solution in This Work
1.4. Summary of the Current Research Novelty and Objective of This Work
2. Preparation of the Material for the Fermentation Process—Study Methodology
2.1. Thermal Analysis
- Atmosphere above the sample: air—variant I; nitrogen—variant II;
- Temperature range: 20–1000 °C;
- Heating speed: 10 °C∙min−1.
2.2. X-ray Fluorescence and Reference Method
3. Study Results and Their Interpretation
3.1. Analysis of Fermentation Processes
- -
- The volume of biogas released;
- -
- The concentrations of CH4, CO2, O2, H2S, H2, and NH3;
- -
- The contents of volatile fatty acids, such as
- methane (formic),
- ethane (acetic),
- propane (propionic),
- butane (butter),
- 2-methylpropane (iso-butyrate),
- pentane (valerian),
- 2-methylbutane (iso-valerate),
- hexane (capron),
- 2-methylpentane (iso-capron),
- heptane (enant);
- -
- The pH;
- -
- The temperature.
3.1.1. Volume of Biogas Released
3.1.2. Biogas Composition
3.1.3. Volatile Fatty Acid Analysis
- -
- The determination of the analytes at very low concentration levels;
- -
- The preparation of samples for analysis without introducing additional compounds into the environment.
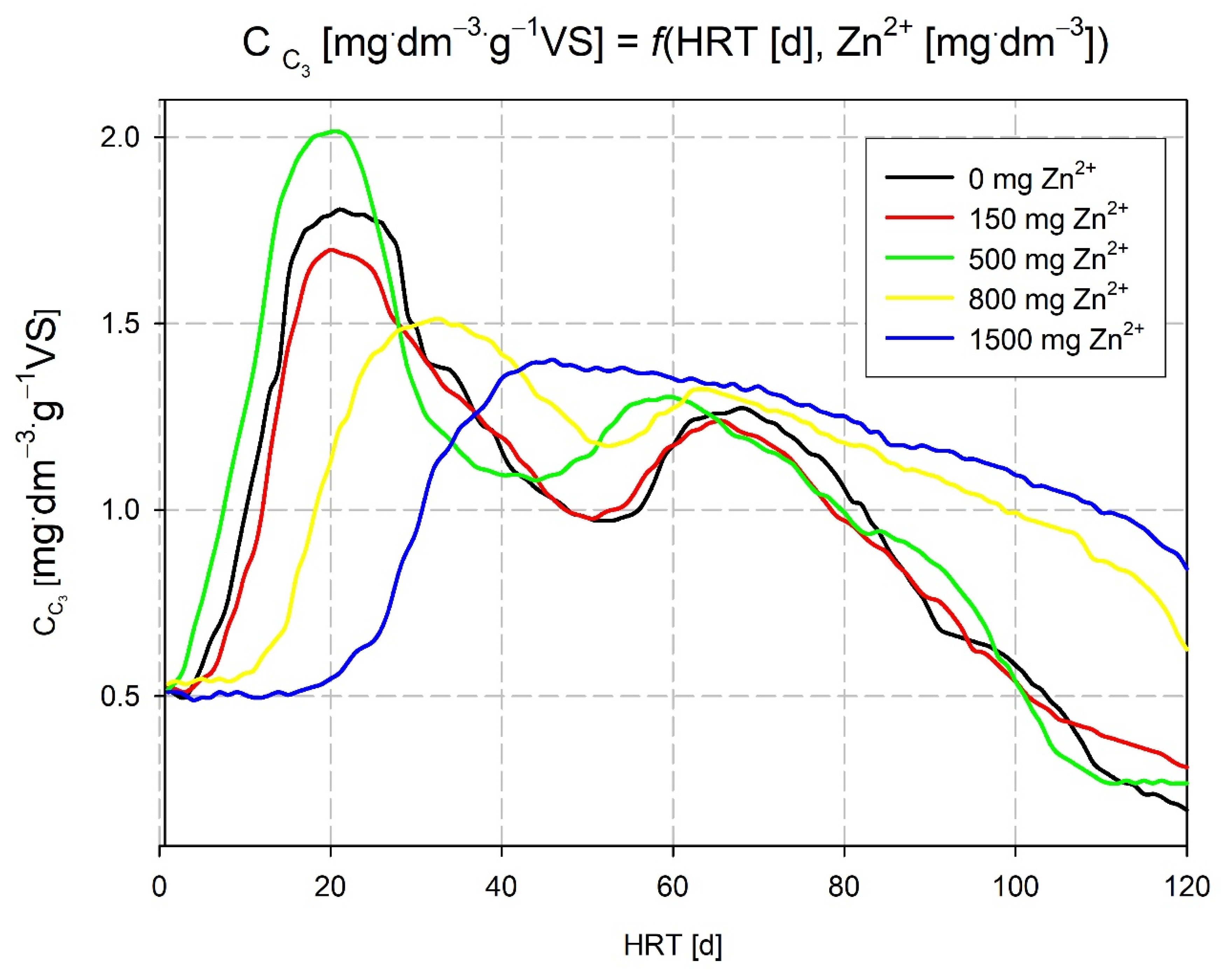
3.2. Analysis of the Effect of the Zn2+ Ion Content in the Fermentation Supernatant on the Efficiency of the Hard Corn Methane Fermentation Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ginter, M.O.; Grobicki, A.M. Analysis of anaerobic sludge containing heavy metals: A novel technique. Water Res. 1995, 29, 2780–2784. [Google Scholar] [CrossRef]
- Callander, I.J.; Barford, J.P. Precipitation, chelation, and the availability of metals as nutrients in anaerobic digestion. II. Applications. Biotechnol. Bioeng. 1983, 25, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- MacNicol, R.D.; Beckett, P.H.T. The distribution of heavy metals between the principal components of digested sewage sludge. Water Res. 1989, 23, 199–206. [Google Scholar] [CrossRef]
- Haraguchi, H. Metallomics as integrated biometal science. J. Anal. At. Spectrom. 2004, 19, 5–14. [Google Scholar] [CrossRef]
- Christ, H.; Oberholser, K.; Shank, N.E.; Nguyen, M.T. Nature of bonding between metallic ions and algal cell walls. Environ. Sci. Technol. 1981, 15, 1212–1217. [Google Scholar] [CrossRef]
- Harvey, R.W.; Leckie, J.O. Sorption of lead onto two gram-negative marine bacteria in seawater. Mar. Chem. 1985, 15, 333–344. [Google Scholar] [CrossRef]
- de Lurdes, M.; Gonçalves, S.; Sigg, L.; Reutlinger, M.; Stumm, W. Metal ion binding by biological surfaces: Voltammetric assessment in the presence of bacteria. Sci. Total Environ. 1987, 60, 105–119. [Google Scholar] [CrossRef]
- Beveridge, T.J.; Murray, R.G. Sites of metal deposition in the cell wall of Bacillus subtilis. J. Bacteriol. 1980, 141, 876–887. [Google Scholar] [CrossRef]
- Fein, J.B.; Daughney, C.J.; Yee, N.; Davis, T.A. A chemical equilibrium model for metal adsorption onto bacterial surfaces. Geochim. Et. Cosmochim. Acta 1997, 61, 3319–3328. [Google Scholar] [CrossRef]
- TienCt; Huang, C.P. Formation of surface complexes between heavy metals and sludge particles. In Heavy Metals in the Environment; Elsevier Science Publishers BV: Amsterdam, The Netherlands, 1991; pp. 195–311. [Google Scholar]
- Rosen, B.P. Bacterial resistance to heavy metals and metalloids. JBIC J. Biol. Inorg. Chem. 1996, 1, 273–277. [Google Scholar] [CrossRef]
- Konings, W.N.; Kaback, H.R.; Lolkema, J.S. Transport Processes in Eukaryotic and Prokaryotic Organisms; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Siemion, I.Z. Biostereochemia; Państwowe Wydaw: Warsaw, Poland, 1985. [Google Scholar]
- Zhou, Q.; Lin, Y.; Li, X.; Yang, C.; Han, Z.; Zeng, G.; Lu, L.; He, S. Effect of zinc ions on nutrient removal and growth of Lemna aequinoctialis from anaerobically digested swine wastewater. Bioresour. Technol. 2018, 249, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, M.R.A.; Borowski, T.; Himo, F.; Liao, R.-Z.; Siegbahn, P.E.M. Quantum Chemical Studies of Mechanisms for Metalloenzymes. Chem. Rev. 2014, 114, 3601–3658. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.W.W.; Jin, S.L.C.; Chen, J.-S.; Mortenson, L.E. The redox properties and activation of the F420)-non-reactive hydrogenase of Methanobacterium formicicum. Biochim. Et. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1986, 869, 37–47. [Google Scholar] [CrossRef]
- Yang, S.; Wen, Q.; Chen, Z. Impacts of Cu and Zn on the performance, microbial community dynamics and resistance genes variations during mesophilic and thermophilic anaerobic digestion of swine manure. Bioresour. Technol. 2020, 312, 123554. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Cestonaro do Amaral, A.; Kunz, A.; Radis Steinmetz, R.L.; Justi, K.C. Zinc and copper distribution in swine wastewater treated by anaerobic digestion. J. Environ. Manag. 2014, 141, 132–137. [Google Scholar] [CrossRef]
- Gordeeva, V.P.; Statkus, M.A.; Tsysin, G.I.; Zolotov, Y.A. X-ray fluorescence determination of As, Bi, Co, Cu, Fe, Ni, Pb, Se, V and Zn in natural water and soil extracts after preconcentration of their pyrrolidinedithiocarbamates on cellulose filters. Talanta 2003, 61, 315–329. [Google Scholar] [CrossRef]
- Hu Yongguang, D.Z.; Wang Sheng, M.A. Determination of shearing force by measuring NDF and ADF in tea stems with hyperspectral imaging technique. IFAC-PapersOnLine 2018, 51, 849–854. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Lo, S.-L. Predicting heating value of lignocellulosic biomass based on elemental analysis. Energy 2020, 191, 116501. [Google Scholar] [CrossRef]
- Yin, D.-m.; Mahboubi, A.; Wainaina, S.; Qiao, W.; Taherzadeh, M.J. The effect of mono- and multiple fermentation parameters on volatile fatty acids (VFAs) production from chicken manure via anaerobic digestion. Bioresour. Technol. 2021, 330, 124992. [Google Scholar] [CrossRef]
- Mondal, B.C.; Das, D.; Das, A.K. Preconcentration and separation of copper, zinc and cadmium by the use of 6-mercapto purinylazo resin and their application in microwave digested certified biological samples followed by AAS determination of the metal ions. J. Trace Elem. Med. Biol. 2002, 16, 145–148. [Google Scholar] [CrossRef]
- Le, D.M.; Sørensen, H.R.; Meyer, A.S. Elemental analysis of various biomass solid fractions in biorefineries by X-ray fluorescence spectrometry. Biomass Bioenergy 2017, 97, 70–76. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Lin, S.; Qin, Y.; Huang, X. A review on biomass thermal-oxidative decomposition data and machine learning prediction of thermal analysis. Clean. Mater. 2023, 9, 100206. [Google Scholar] [CrossRef]
- Arendt, E.K.; Zannini, E. 2—Maizes. In Cereal Grains for the Food and Beverage Industries; Arendt, E.K., Zannini, E., Eds.; Woodhead Publishing: Sawston, UK, 2013. [Google Scholar]
- Beklemishev, K.; Stoyan, A. Sorption–Catalytic Determination of Manganese Directly on a Paper-based Chelating Sorbent. Analyst 1997, 122, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Beklemishev, M.K.; Dolmanova, I.F.; Petrova, Y.Y. Sorption–catalytic testing of copper on a paper-based sorbent with attached alkylamino groups. Analyst 1999, 124, 1523–1527. [Google Scholar] [CrossRef]

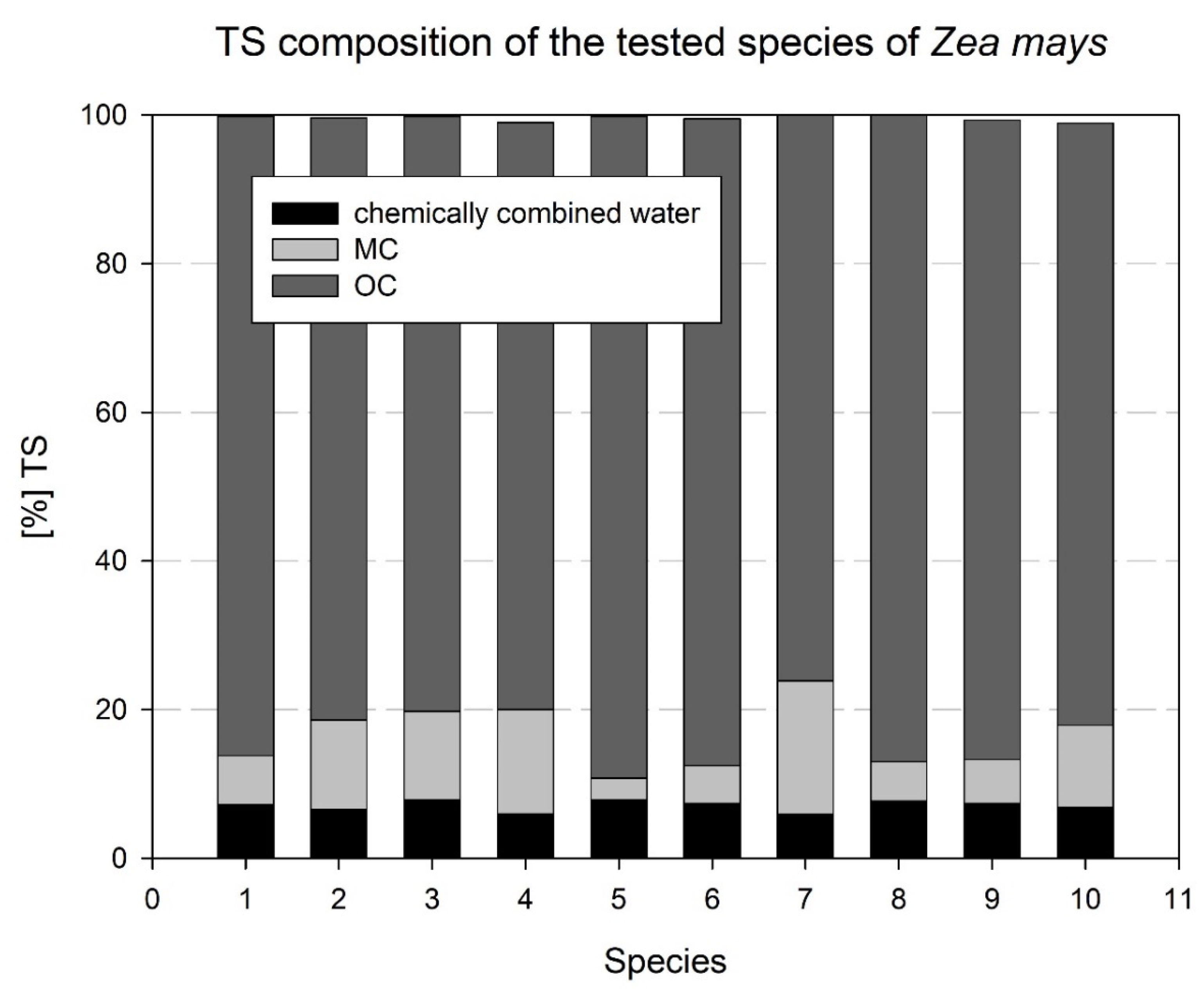
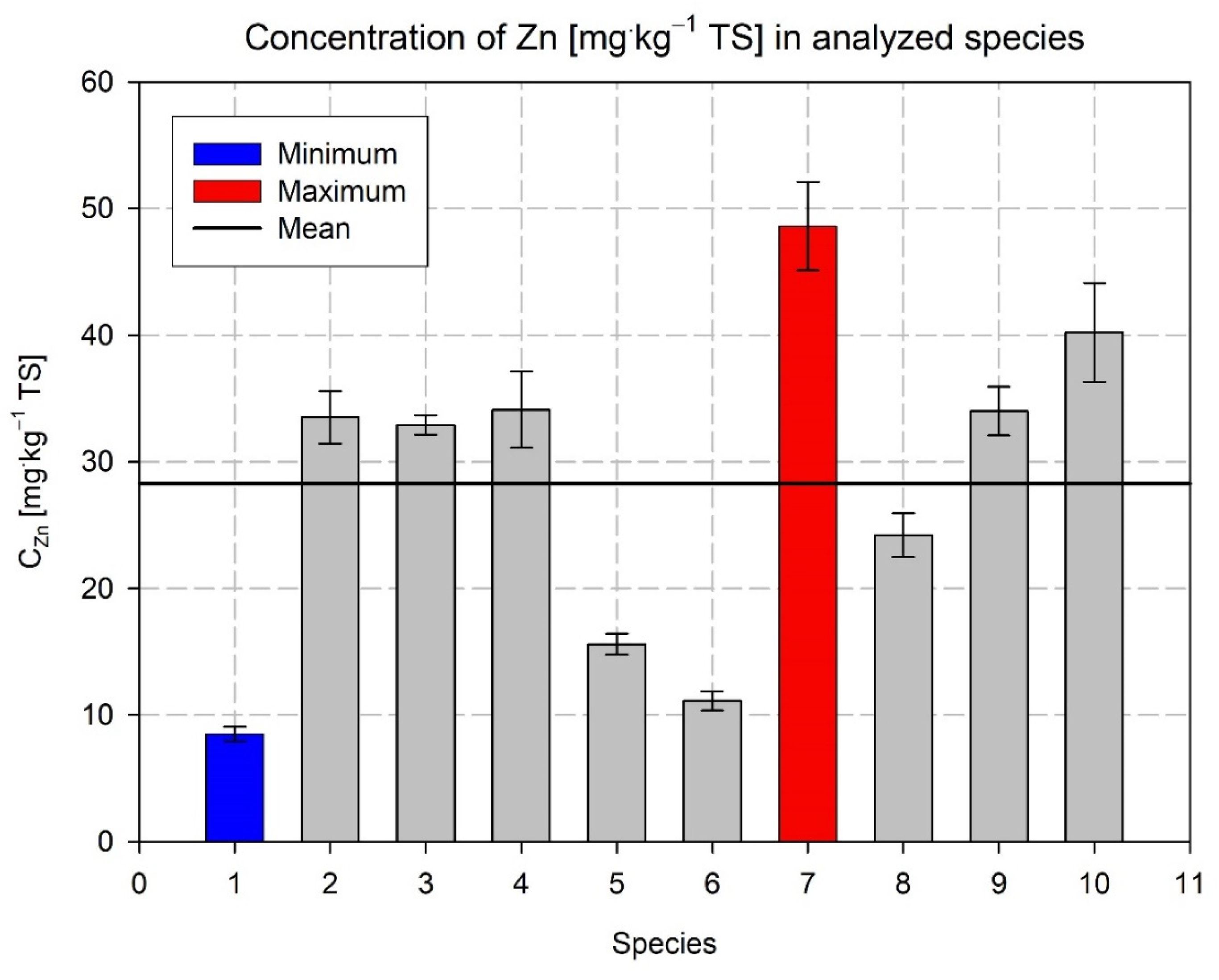






| No. (Designation) | Variety | Factor FAO | Use | Total Effective Temperature Based on 6 °C |
|---|---|---|---|---|
| 1 | MAXXIS | 330 | k/z | k—1570 °C z—1760 °C |
| 2 | MAXXYM | 310 | k | k—1520 °C |
| 3 | SILEXX | 320 | k | k—1530 °C |
| 4 | TEXXUD | 320 | k/z | k—1570 °C z—1760 °C |
| 5 | PIXXIA | 430 | k/z | k—1620 °C z—1790 °C |
| 6 | RIXXER | 380 | k/z | k—1600 °C z—1780 °C |
| 7 | SAXXOO | 380 | k | k—1590 °C |
| 8 | DK604 | 540 | k/z | k—1790 °C z—1980 °C |
| 9 | TIXXUS | 520 | k/z | k—1780 °C z—1970 °C |
| 10 | TYREXX | 550 | k/z | k—1800 °C z—1990 °C |
| Variety | TS1 (%) | TS2 (%) | TS3 (%) | TSmedium (%) | RSD (%) |
|---|---|---|---|---|---|
| 1 | 32.01 | 31.94 | 31.96 | 31.97 | 0.10 |
| 2 | 36.01 | 35.96 | 35.67 | 35.88 | 0.50 |
| 3 | 38.21 | 38.27 | 38.27 | 38.25 | 0.14 |
| 4 | 34.01 | 34.02 | 33.95 | 33.99 | 0.12 |
| 5 | 35.06 | 35.08 | 35.00 | 35.05 | 0.12 |
| 6 | 32.03 | 32.05 | 31.96 | 32.01 | 0.14 |
| 7 | 32.63 | 32.55 | 32.55 | 32.58 | 0.15 |
| 8 | 35.97 | 36.26 | 35.70 | 35.98 | 0.77 |
| 9 | 34.50 | 34.85 | 34.49 | 34.61 | 0.59 |
| 10 | 38.42 | 38.46 | 38.32 | 38.40 | 0.20 |
| Species | Sample Weight | ||
|---|---|---|---|
| m1 (g) | m2 (g) | m3 (g) | |
| 1 | 0.7030 | 0.7415 | 0.7154 |
| 2 | 0.7454 | 0.7124 | 0.7412 |
| 3 | 0.7115 | 0.7015 | 0.7144 |
| 4 | 0.7728 | 0.7325 | 0.7210 |
| 5 | 0.7030 | 0.7247 | 0.7325 |
| 6 | 0.7021 | 0.7147 | 0.7415 |
| 7 | 0.7413 | 0.7122 | 0.7524 |
| 8 | 0.7568 | 0.7423 | 0.7155 |
| 9 | 0.7418 | 0.7644 | 0.7321 |
| 10 | 0.7555 | 0.7045 | 0.7521 |
| Element | Analytical Line (mÅ) | Left Background Point (mÅ) | Right Background Point (mÅ) | Exposure Time (s) |
|---|---|---|---|---|
| Zn | Kβ 1298.6 | 1240 | 1350 | 60 |
| Analyte | Equation | R2 | sr | LOD (μg∙Filter−1) |
|---|---|---|---|---|
| Zn | y = 1855x + 720 | 0.9954 | 0.32 | 1.29 |
| Standard | Zn |
|---|---|
| NCS ZC73010 | 2.9 |
| NCS ZC73012 | 26 |
| NCS ZC73014 | 51 |
| CTA-OTL-1 | 49.9 |
| CTA-VTL-2 | 43.3 |
| Analyte | Expected Value (mg∙kg−1 TS) | Value Received (mg kg−1 TS) | Retrieved From (%) | |||
|---|---|---|---|---|---|---|
| Zn | 2.90 | 49.9 | 3.03 | 49.6 | 104.5 | 99.3 |
| Analyte | Equation | R2 | sr | LOD (mg-dm)−3 |
|---|---|---|---|---|
| Zn | y = 5.437-10−2 x + 8.129-10−3 | 0.9996 | 0.07 | 0.32 |
| Analyte | F | Fkr | f1 | f2 | s12 | S22 |
|---|---|---|---|---|---|---|
| Zn | 9.610 | 16.30 | 5 | 5 | 9.61–10−2 | 1.00–10−2 |
| Analyte | Factor Determination | Deviation Standard Regression | Detection Limit (mg-dm)−3 | Coefficient of Variation (%) | ||||
|---|---|---|---|---|---|---|---|---|
| FAAS | WD-XRF | FAAS | WD-XRF | FAAS | WD-XRF | FAAS | WD-XRF | |
| Zn | 0.9995 | 0.9957 | 0.10 | 0.31 | 0.43 | 0.12 | 2.79 | 8.51 |
| Variety | Input | TS In the Fermenter (%) | VS (g) | |
|---|---|---|---|---|
| Water (g). | Silage (g). | |||
| MAXXIS | 6000 | 2010 | 8.02 | 139 |
| MAXXYM | 1720 | 7.99 | 111 | |
| SILEXX | 1790 | 8.02 | 115 | |
| TEXXUD | 1850 | 8.01 | 117 | |
| PIXXIA | 1770 | 7.98 | 126 | |
| RIXXER | 2000 | 8.00 | 139 | |
| SAXXOO | 1950 | 7.99 | 118 | |
| DK604 | 1710 | 7.98 | 119 | |
| TIXXUS | 1800 | 7.99 | 124 | |
| TYREXX | 1580 | 8.00 | 102 | |
| Variety | Parameter | r2 | Vbiogas | |||||
|---|---|---|---|---|---|---|---|---|
| a | b | c | x0 | y0 | (dm3) | (dm3.g VS−1) | ||
| MAXXIS | 6454.39 | 43.46 | 1.71 | 57.89 | 241.38 | 0.9884 | 374.5 | 2.70 |
| MAXXYM | 6615.15 | 42.05 | 1.96 | 49.47 | 243,03 | 0.9826 | 356.5 | 3.20 |
| SILEXX | 6367.14 | 50.18 | 1.64 | 55.92 | 233.14 | 0.9912 | 416.2 | 3.62 |
| TEXXUD | 5598.85 | 58.44 | 1.63 | 54.67 | 120.35 | 0.9890 | 399.2 | 3.41 |
| PIXXIA | 6012.20 | 48.62 | 1.32 | 45.51 | 691.45 | 0.9463 | 440.9 | 3.51 |
| RIXXER | 4802.01 | 65.95 | 1.30 | 52.38 | 128.76 | 0.9746 | 348.3 | 2.50 |
| SAXXOO | 5214.41 | 58.48 | 1.46 | 51.12 | 244.58 | 0.9842 | 387.8 | 3.28 |
| DK604 | 6231.82 | 41.87 | 1.92 | 48.57 | 244.81 | 0.9904 | 340.0 | 2.86 |
| TIXXUS | 5486.68 | 51.26 | 1.79 | 51.03 | 208.47 | 0.9875 | 363.7 | 2.94 |
| TYREXX | 5978.28 | 45.33 | 1.82 | 56.27 | 198.67 | 0.9910 | 350.2 | 3.42 |
| Analyte | Scope Determinability | Accuracy | Response Time (s) |
|---|---|---|---|
| CH4 | 0–100% | 0.2% at 5% | 20 |
| 1% at 30% | |||
| 3% at 100% | |||
| CO2 | 0–100% | 0.1% at 10% | 30 |
| 3% at 50% | |||
| 5% at 100% | |||
| O2 | 0–25% | 0.5% | 20 |
| H2 S | 0–1500 ppm | 5% | 30 |
| H2 | 0–1000 ppm | 5% | 30 |
| NH3 | 0–1000 ppm | 5% | 30 |
| Variety | Parameter Values for CH4 | Parameter Values for CO2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g | h | i | xc | yc | r2 | a | b | r2 | c | d | e | f | r2 | |
| MAXXIS | 58.49 | 68.52 | 1.72 | 51.62 | 1.12 | 0.9929 | 6.12 | −4.30 | 0.9548 | −0.0002 | 0.06 | −4.14 | 130.8 | 0.9852 |
| MAXXYM | 61.36 | 71.11 | 1.66 | 55.01 | 1.53 | 0.9853 | 3.69 | −5.72 | 0.9789 | −0.0002 | 0.06 | −4.40 | 139.1 | 0.9606 |
| SILEXX | 60.97 | 70.83 | 1.61 | 53.56 | 1.91 | 0.9931 | 3.18 | −7.22 | 0.9756 | −0.0002 | 0.04 | −3.90 | 131.1 | 0.9766 |
| TEXXUD | 62.90 | 74.41 | 1.70 | 57.40 | 2.11 | 0.9864 | 4.66 | −9.35 | 0.9494 | −0.0002 | 0.05 | −3.80 | 129.1 | 0.9630 |
| PIXXIA | 59.36 | 63.27 | 1.51 | 46.51 | 6.17 | 0.9779 | 2.91 | −2.68 | 0.9812 | −0.0003 | 0.07 | −4.50 | 125.9 | 0.9913 |
| RIXXER | 48.89 | 81.35 | 1.27 | 48.34 | 6.62 | 0.9579 | 5.22 | −4.58 | 0.9914 | −0.0003 | 0.07 | −4.81 | 149.9 | 0.9432 |
| SAXXOO | 52.14 | 78.44 | 1.68 | 49.56 | 4.81 | 0.9814 | 4.58 | −4.11 | 0.9984 | −0.0002 | 0.06 | −4.92 | 137.4 | 0.9844 |
| DK604 | 56.29 | 74.16 | 1.59 | 52.83 | 3.88 | 0.9943 | 3.61 | −2.57 | 0.9916 | −0.0003 | 0.06 | −3.84 | 142.8 | 0.9949 |
| TIXXUS | 60.27 | 71.47 | 1.62 | 57.34 | 4.12 | 0.9896 | 5.91 | −6.64 | 0.9862 | −0.0003 | 0.06 | −4.24 | 168.6 | 0.9919 |
| TYREXX | 53.35 | 64.91 | 1.61 | 49.16 | 5.73 | 0.9962 | 4.29 | −5.37 | 0.9975 | −0.0002 | 0.06 | −4.73 | 148.7 | 0.9823 |
| Variety | PL | PQ | Border | PW | PC | %CH4 |
|---|---|---|---|---|---|---|
| MAXXIS | 686 | 6804 | 15.6951 | 4694 | 12,184 | 38.5 |
| MAXXYM | 624 | 6224 | 19.7252 | 5012 | 11,860 | 42.3 |
| SILEXX | 657 | 3272 | 22.7093 | 5036 | 8965 | 56.2 |
| TEXXUD | 578 | 2831 | 17.8933 | 5297 | 8706 | 60.8 |
| PIXXIA | 689 | 3657 | 22.7093 | 5170 | 9516 | 54.3 |
| RIXXER | 736 | 4402 | 17.6972 | 4607 | 9746 | 47.3 |
| SAXXOO | 789 | 3332 | 19.4854 | 5080 | 9201 | 55.2 |
| DK604 | 602 | 6269 | 18.9875 | 5044 | 11,915 | 42.3 |
| TIXXUS | 787 | 6251 | 17.4861 | 5179 | 12,217 | 42.4 |
| TYREXX | 685 | 5794 | 19.1657 | 4730 | 11,209 | 42.2 |
| Variety | |||
|---|---|---|---|
| MAXXIS | 23.96 | 6.44 | 0.2687 |
| MAXXYM | 22.00 | 6.73 | 0.3060 |
| SILEXX | 21.99 | 10.44 | 0.4749 |
| TEXXUD | 23.26 | 10.84 | 0.4660 |
| PIXXIA | 22.33 | 10.69 | 0.4787 |
| RIXXER | 23.04 | 7.35 | 0.3192 |
| SAXXOO | 22.47 | 9.56 | 0.4253 |
| DK604 | 22.64 | 6.42 | 0.2836 |
| TIXXUS | 22.19 | 6.88 | 0.3102 |
| TYREXX | 22.04 | 6.60 | 0.2993 |
| Acid | Mass (g∙mol−1) | C of the Standard (mmol∙dm−3) | C (mg∙dm−3) |
|---|---|---|---|
| C1 | 46.03 | 10.85 | 499.4 |
| C2 | 60.05 | 10.16 | 610.1 |
| C3 | 74.08 | 10.49 | 777.1 |
| i-C4 | 88.11 | 10.08 | 888.1 |
| n-C4 | 88.11 | 10.08 | 888.1 |
| i-C5 | 102.13 | 10.32 | 1054 |
| n-C5 | 102.13 | 10.29 | 1051 |
| i-C6 | 116.16 | 9,92 | 1152 |
| n-C6 | 116.16 | 10.03 | 1165 |
| n-C7 | 130.19 | 10.23 | 1332 |
| Acid | Quantity Declared (mg-dm−3) | Quantity Received (Recovery) (%) |
|---|---|---|
| C2 | 315.4 | 101.5 |
| C3 | 194.3 | 99.2 |
| i-C4 | 222.0 | 101.9 |
| n-C4 | 222.0 | 101.2 |
| i-C5 | 263.5 | 102.2 |
| n-C5 | 262.7 | 99.0 |
| i-C6 | 288.1 | 100.5 |
| n-C6 | 291.3 | 100.3 |
| n-C7 | 333.0 | 99.5 |
| Bioreactor | PC | PW | r2 for PW | % CH4 | 1 | ||
|---|---|---|---|---|---|---|---|
| 1 | 9531 | 5173 | 0.9770 | 54.3 | 11.7 | 22.6 | 0.5177 |
| 2 | 9928 | 5417 | 0.9744 | 54.6 | 12.3 | 22.1 | 0.5566 |
| 3 | 9542 | 5217 | 0.9159 | 54.7 | 13.8 | 22.3 | 0.6188 |
| 4 | 7893 | 4331 | 0.9187 | 54.9 | 10.7 | 22.3 | 0.4798 |
| 5 | 7217 | 3911 | 0.9775 | 54.2 | 8.8 | 22.3 | 0.3946 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cichosz, M.; Łazarski, S.; Butarewicz, A.; Kiełkowska, U. Biogas Production—The Effect of the Zinc Concentration on the Profile of Volatile Fatty Acids in Fermentation Mixtures. Energies 2023, 16, 7425. https://doi.org/10.3390/en16217425
Cichosz M, Łazarski S, Butarewicz A, Kiełkowska U. Biogas Production—The Effect of the Zinc Concentration on the Profile of Volatile Fatty Acids in Fermentation Mixtures. Energies. 2023; 16(21):7425. https://doi.org/10.3390/en16217425
Chicago/Turabian StyleCichosz, Marcin, Sławomir Łazarski, Andrzej Butarewicz, and Urszula Kiełkowska. 2023. "Biogas Production—The Effect of the Zinc Concentration on the Profile of Volatile Fatty Acids in Fermentation Mixtures" Energies 16, no. 21: 7425. https://doi.org/10.3390/en16217425
APA StyleCichosz, M., Łazarski, S., Butarewicz, A., & Kiełkowska, U. (2023). Biogas Production—The Effect of the Zinc Concentration on the Profile of Volatile Fatty Acids in Fermentation Mixtures. Energies, 16(21), 7425. https://doi.org/10.3390/en16217425









