The Influence of Protective Coatings on High-Temperature Corrosion under Biomass Ash Deposits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Steel Grades and Coatings
2.2. Fuel Characteristics
2.3. Ash Preparation and Characteristics
2.4. Corrosion Experiments
2.5. Slagging Factors
3. Results and Discussion
3.1. Slagging Factors
3.2. Corrosion Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sandberg, J.; Karlsson, C.; Fdhila, R.B. A 7year Long Measurement Period Investigating the Correlation of Corrosion, Deposit and Fuel in a Biomass Fired Circulated Fluidized Bed Boiler. Appl. Energy 2011, 88, 99–110. [Google Scholar] [CrossRef]
- Niu, Y.; Tan, H.; Hui, S. Ash-Related Issues during Biomass Combustion: Alkali-Induced Slagging, Silicate Melt-Induced Slagging (Ash Fusion), Agglomeration, Corrosion, Ash Utilization, and Related Countermeasures. Prog. Energy Combust. Sci. 2016, 52, 1–61. [Google Scholar] [CrossRef]
- Król, D.; Motyl, P.; Poskrobko, S. Chlorine Corrosion in a Low-Power Boiler Fired with Agricultural Biomass. Energies 2022, 15, 382. [Google Scholar] [CrossRef]
- Ovčačíková, H.; Velička, M.; Vlček, J.; Topinková, M.; Klárová, M.; Burda, J. Corrosive Effect of Wood Ash Produced by Biomass Combustion on Refractory Materials in a Binary Al–Si System. Materials 2022, 15, 5796. [Google Scholar] [CrossRef]
- Lee, S.-H.; Themelis, N.J.; Castaldi, M.J. High-Temperature Corrosion in Waste-to-Energy Boilers. J. Therm. Spray Technol. 2007, 16, 104–110. [Google Scholar] [CrossRef]
- Persson, K.; Broström, M.; Carlsson, J.; Nordin, A.; Backman, R. High Temperature Corrosion in a 65 MW Waste to Energy Plant. Fuel Process. Technol. 2007, 88, 1178–1182. [Google Scholar] [CrossRef]
- Ma, W.; Wenga, T.; Frandsen, F.J.; Yan, B.; Chen, G. The Fate of Chlorine during MSW Incineration: Vaporization, Transformation, Deposition, Corrosion and Remedies. Prog. Energy Combust. Sci. 2020, 76, 100789. [Google Scholar] [CrossRef]
- Phongphiphat, A.; Ryu, C.; Yang, Y.B.; Finney, K.N.; Leyland, A.; Sharifi, V.N.; Swithenbank, J. Investigation into High-Temperature Corrosion in a Large-Scale Municipal Waste-to-Energy Plant. Corros. Sci. 2010, 52, 3861–3874. [Google Scholar] [CrossRef]
- Verbinnen, B.; De Greef, J.; Van Caneghem, J. Theory and Practice of Corrosion Related to Ashes and Deposits in a WtE Boiler. Waste Manag. 2018, 73, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Meißner, T.M.; Montero, X.; Fähsing, D.; Galetz, M.C. Cr Diffusion Coatings on a Ferritic-Martensitic Steel for Corrosion Protection in KCl-Rich Biomass Co-Firing Environments. Corros. Sci. 2020, 164, 108343. [Google Scholar] [CrossRef]
- Abu-warda, N.; López, A.J.; Pedraza, F.; Utrilla, M.V. Fireside Corrosion on T24 Steel Pipes and HVOF NiCr Coatings Exposed to Different Salt Mixtures. Corros. Sci. 2020, 173, 108747. [Google Scholar] [CrossRef]
- Zhang, J.; ur Rahman, Z.; Wang, X.; Wang, Z.; Li, P.; Wang, Y.; Bate, D.; Zhao, K.; Tan, H. Hot Corrosion Behaviors of TP347H and HR3C Stainless Steel with KCl Deposit in Oxy-Biomass Combustion. J. Environ. Manag. 2020, 263, 110411. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Song, P.; He, X.; Khan, A.; Huang, T.; Li, C.; Li, Q.; Lü, K.; Chen, K.; Lu, J. Influence of the Combined-Effect of NaCl and Na2SO4 on the Hot Corrosion Behaviour of Aluminide Coating on Ni-Based Alloys. J. Alloys Compd. 2019, 790, 228–239. [Google Scholar] [CrossRef]
- Vuelvas-Rayo, S.; Gonzalez-Rodriguez, J.G.; Porcayo-Calderon, J.; Salinas-Bravo, V.M.; Maldonado-Ruiz, S.I. Hot Corrosion Behavior of High-Chromium, High-Carbon Cast Irons in NaCl-KCl Molten Salts. Int. J. Corros. 2012, 2012, 479761. [Google Scholar] [CrossRef]
- Kiamehr, S.; Dahl, K.V.; Montgomery, M.; Somers, M.A.J. KCl-Induced High Temperature Corrosion of Selected Commercial Alloys. Mater. Corros. 2016, 67, 26–38. [Google Scholar] [CrossRef]
- Okoro, S.C.; Montgomery, M.; Frandsen, F.J.; Pantleon, K. Time and Temperature Effects on Alkali Chloride Induced High Temperature Corrosion of Superheaters during Biomass Firing. Energy Fuels 2018, 32, 7991–7999. [Google Scholar] [CrossRef]
- Okoro, S.C.; Montgomery, M.; Frandsen, F.J.; Pantleon, K. High Temperature Corrosion under Laboratory Conditions Simulating Biomass-Firing: A Comprehensive Characterization of Corrosion Products. Energy Fuels 2014, 28, 6447–6458. [Google Scholar] [CrossRef]
- Mahajan, S.; Chhibber, R. Hot Corrosion Studies of Boiler Steels Exposed to Different Molten Salt Mixtures at 950 °C. Eng. Fail. Anal. 2019, 99, 210–224. [Google Scholar] [CrossRef]
- Metsäjoki, J.; Huttunen-Saarivirta, E.; Lepistö, T. Elevated-Temperature Corrosion of Uncoated and Aluminized 9–12% Cr Boiler Steels beneath KCl Deposit. Fuel 2014, 133, 173–181. [Google Scholar] [CrossRef]
- Agüero, A.; Baráibar, I.; Gutiérrez, M.; Hernández, M.; Muelas, R.; Rodríguez, S. Biomass Corrosion Behavior of Steels and Coatings in Contact with KCl/K2SO4 at 550 °C under an Oxy-Fuel Combustion Atmosphere: A Screening Laboratory Test. Surf. Coat. Technol. 2018, 350, 188–200. [Google Scholar] [CrossRef]
- Pooja, M.; Ravishankar, K.S.; Madav, V. High Temperature Corrosion Behaviour of Stainless Steels and Inconel 625 in Hydroxide Salt. Mater. Today Proc. 2021, 46, 2612–2615. [Google Scholar] [CrossRef]
- Ruh, A.; Spiegel, M. Thermodynamic and Kinetic Consideration on the Corrosion of Fe, Ni and Cr beneath a Molten KCl–ZnCl2 Mixture. Corros. Sci. 2006, 48, 679–695. [Google Scholar] [CrossRef]
- Li, Y.S.; Niu, Y.; Spiegel, M. High Temperature Interaction of Al/Si-Modified Fe–Cr Alloys with KCl. Corros. Sci. 2007, 49, 1799–1815. [Google Scholar] [CrossRef]
- Ma, H.T.; Zhou, C.H.; Wang, L. High Temperature Corrosion of Pure Fe, Cr and Fe–Cr Binary Alloys in O2 Containing Trace KCl Vapour at 750 °C. Corros. Sci. 2009, 51, 1861–1867. [Google Scholar] [CrossRef]
- Jonsson, T.; Folkeson, N.; Halvarsson, M.; Svensson, J.-E.; Johansson, L.-G. Microstructural Investigation of the HCl-Induced Corrosion of the Austenitic Alloy 310S (52Fe26Cr19Ni) at 500 °C. Oxid. Met. 2014, 81, 575–596. [Google Scholar] [CrossRef]
- Alcántara-Cárdenas, J.A.; Ramirez-Lopez, A.; Chávez-Alcalá, J.F.; Sanchez-Pastén, M. Evaluation of High Temperature Corrosion in Simulated Waste Incinerator Environments. Oxid. Met. 2016, 85, 611–627. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, W.; Wu, X.; Zhang, X. Chlorine-Induced High-Temperature Corrosion of Boiler Steels Combusting Sha Erhu Coal Compared to Biomass. Energy Fuels 2018, 32, 4237–4247. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Gołombek, K.; Buk, P.; Cieślik, E.; Nowak, W. The Influence of KCl on Biomass Ash Melting Behaviour and High-Temperature Corrosion of Low-Alloy Steel. Energy 2019, 188, 116062. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Magdziarz, A.; Kalemba-Rec, I.; Nowak, W. The Influence of Potassium-Rich Biomass Ashes on Steel Corrosion above 550 °C. Energy Convers. Manag. 2019, 187, 15–28. [Google Scholar] [CrossRef]
- Maj, I.; Kalisz, S.; Wejkowski, R.; Pronobis, M.; Gołombek, K. High-Temperature Corrosion in a Multifuel Circulating Fluidized Bed (CFB) Boiler Co-Firing Refuse Derived Fuel (RDF) and Hard Coal. Fuel 2022, 324, 124749. [Google Scholar] [CrossRef]
- Maj, I.; Kalisz, S.; Szymajda, A.; Łaska, G.; Gołombek, K. The Influence of Cow Dung and Mixed Straw Ashes on Steel Corrosion. Renew. Energy 2021, 177, 1198–1211. [Google Scholar] [CrossRef]
- Bankiewicz, D.; Yrjas, P.; Lindberg, D.; Hupa, M. Determination of the Corrosivity of Pb-Containing Salt Mixtures. Corros. Sci. 2013, 66, 225–232. [Google Scholar] [CrossRef]
- Duda, P.; Felkowski, Ł. Negative Impact of Thermal Loads on Pressure and Non-Pressure Boiler Parts. Energies 2023, 16, 5768. [Google Scholar] [CrossRef]
- Czupryński, A.; Adamiec, J.; Adamiak, M.; Żuk, M.; Kříž, A.; Mele, C.; Kciuk, M. High-Temperature Corrosion of Flame-Sprayed Power Boiler Components with Nickel Alloy Powders. Materials 2023, 16, 1658. [Google Scholar] [CrossRef]
- Hruska, J.; Mlnarik, J.; Cizner, J. High-Temperature Corrosion of Nickel-Based Coatings for Biomass Boilers in Chlorine-Containing Atmosphere. Coatings 2022, 12, 116. [Google Scholar] [CrossRef]
- Rozmus-Górnikowska, M.; Blicharski, M.; Kusiński, J. Influence of Weld Overlaying Methods on Microstructure and Chemical Composition of Inconel 625 Boiler Pipe Coatings. Kov. Mater.-Met. Mater. 2014, 52, 1–7. [Google Scholar] [CrossRef]
- PN-EN ISO 18122:2016-01; Solid Biofuels—Determination of Ash Content. Polski Komitet Normalizacyjny: Warszawa, Poland, 2016.
- PN-EN ISO 21656:2021-08; Solid Recovered Fuels—Determination of Ash Content. Polski Komitet Normalizacyjny: Warszawa, Poland, 2021.
- PN--EN ISO 18134-2:2017-03; Solid Biofuels—Determination of Moisture Content. Polski Komitet Normalizacyjny: Warszawa, Poland, 2018.
- CEN/TS 154-2:2010; Paliwa Wtórne—Paliwa Alternatywne: Zawartość Wilgoci Całkowitej. Energopomiar Sp. z o. o.: Gliwice, Poland, 2010.
- PN-EN ISO 18125:2017-07; Solid Biofuels—Determination of Calorific Value. Polski Komitet Normalizacyjny: Warszawa, Poland, 2021.
- PN-EN ISO 21654:2021-12; Solid Recovered Fuels—Determination of Calorific Value. Polski Komitet Normalizacyjny: Warszawa, Poland, 2021.
- PN-EN ISO 16948:2015-07; Solid Biofuels—Determination of Total Content of Carbon, Hydrogen and Nitrogen. Polski Komitet Normalizacyjny: Warszawa, Poland, 2015.
- PN-EN ISO 21663:2021-06; Solid Recovered Fuels—Determination of Total Content of Carbon, hydrogen and nitrogen. Polski Komitet Normalizacyjny: Warszawa, Poland, 2021.
- PN_EN ISO 16994:2016-10; Solid Biofuels—Determination of Total Content of Sulfur and Chlorine. Polski Komitet Normalizacyjny: Warszawa, Poland, 2016.
- PN-EN 15408:2011; Solid Recovered Fuels—Methods for the Determination of Sulphur (S), Chlorine (Cl), Fluorine (F) and Bromine (Br) Content. Polski Komitet Normalizacyjny: Warszawa, Poland, 2011.
- CEN/TR 15404:2010; Paliwa Wtórne—Paliwa Alternatywne: Charakterystyczne Temperatury Topliwości Popiołu. Energopomiar Sp. z o. o.: Gliwice, Poland, 2010.
- Garcia-Maraver, A.; Mata-Sanchez, J.; Carpio, M.; Perez-Jimenez, J.A. Critical Review of Predictive Coefficients for Biomass Ash Deposition Tendency. J. Energy Inst. 2017, 90, 214–228. [Google Scholar] [CrossRef]
- Weber, R.; Poyraz, Y.; Beckmann, A.M.; Brinker, S. Combustion of Biomass in Jet Flames. Proc. Combust. Inst. 2015, 35, 2749–2758. [Google Scholar] [CrossRef]
- Miles, T.R.; Miles, T.R.; Baxter, L.L.; Bryers, R.W.; Jenkins, B.M.; Oden, L.L. Boiler Deposits from Firing Biomass Fuels. Biomass Bioenergy 1996, 10, 125–138. [Google Scholar] [CrossRef]
- Lachman, J.; Baláš, M.; Lisý, M.; Lisá, H.; Milčák, P.; Elbl, P. An Overview of Slagging and Fouling Indicators and Their Applicability to Biomass Fuels. Fuel Process. Technol. 2021, 217, 106804. [Google Scholar] [CrossRef]
- Jenkins, B.M.; Baxter, L.L.; Miles, T.R.; Miles, T.R. Combustion Properties of Biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Vamvuka, D.; Zografos, D. Predicting the Behaviour of Ash from Agricultural Wastes during Combustion. Fuel 2004, 83, 2051–2057. [Google Scholar] [CrossRef]
- McLennan, A.R.; Bryant, G.W.; Bailey, C.W.; Stanmore, B.R.; Wall, T.F. Index for Iron-Based Slagging for Pulverized Coal Firing in Oxidizing and Reducing Conditions. Energy Fuels 2000, 14, 349–354. [Google Scholar] [CrossRef]
- Maj, I.; Kalisz, S.; Ciukaj, S. Properties of Animal-Origin Ash—A Valuable Material for Circular Economy. Energies 2022, 15, 1274. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Magdziarz, A.; Gajek, M.; Nowińska, K.; Nowak, W. Alkali Metals Association in Biomass and Their Impact on Ash Melting Behaviour. Fuel 2020, 261, 116421. [Google Scholar] [CrossRef]
- Balint, R.; Engblom, M.; Niemi, J.; Silva da Costa, D.; Lindberg, D.; Yrjas, P.; Hupa, L.; Hupa, M. Temperature Gradient Induced Changes within Superheater Ash Deposits High in Chlorine. Energy 2021, 226, 120439. [Google Scholar] [CrossRef]
- Zahs, A.; Spiegel, M.; Grabke, H.J. Chloridation and Oxidation of Iron, Chromium, Nickel and Their Alloys in Chloridizing and Oxidizing Atmospheres at 400–700 °C. Corros. Sci. 2000, 42, 1093–1122. [Google Scholar] [CrossRef]
- Kofstad, P. High Temperature Corrosion; Elsevier: London, UK, 1988. [Google Scholar]
- Song, B.; Voisey, K.T.; Hussain, T. High Temperature Chlorine-Induced Corrosion of Ni50Cr Coating: HVOLF, HVOGF, Cold Spray and Laser Cladding. Surf. Coat. Technol. 2018, 337, 357–369. [Google Scholar] [CrossRef]



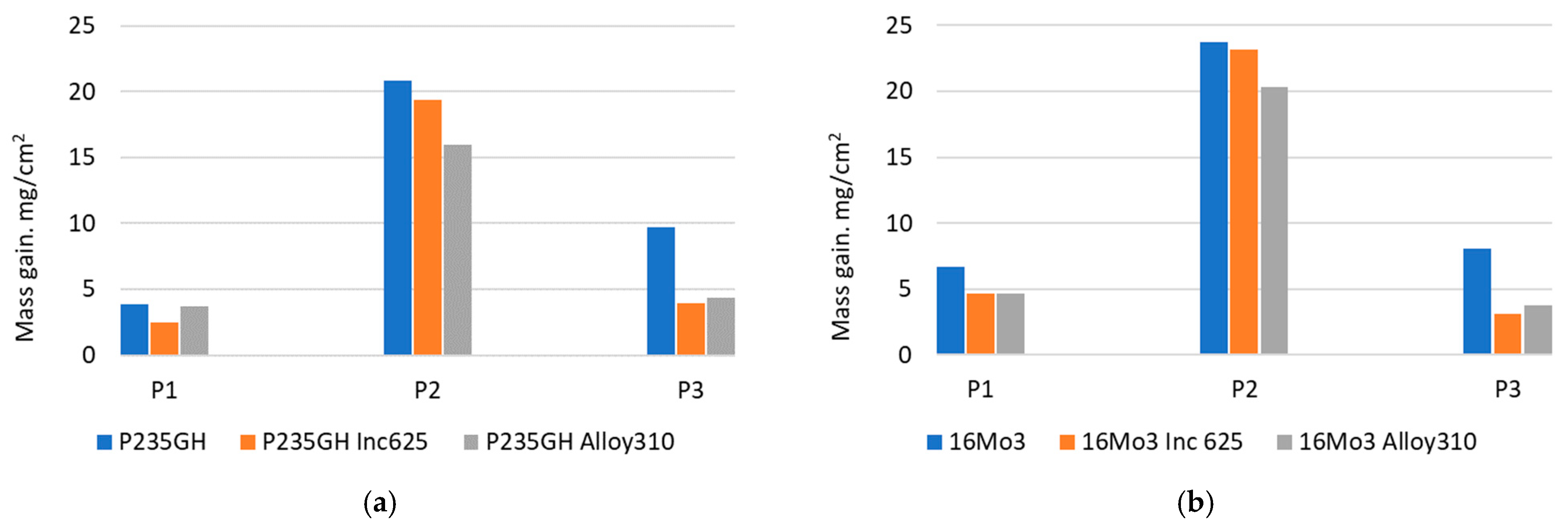
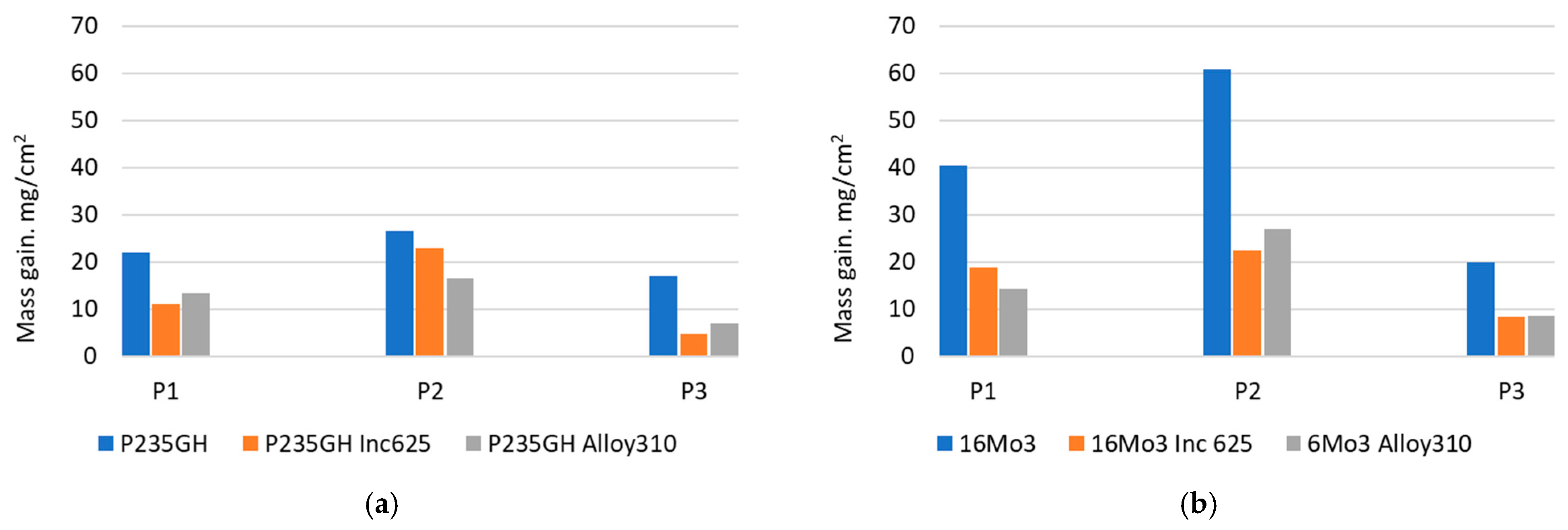
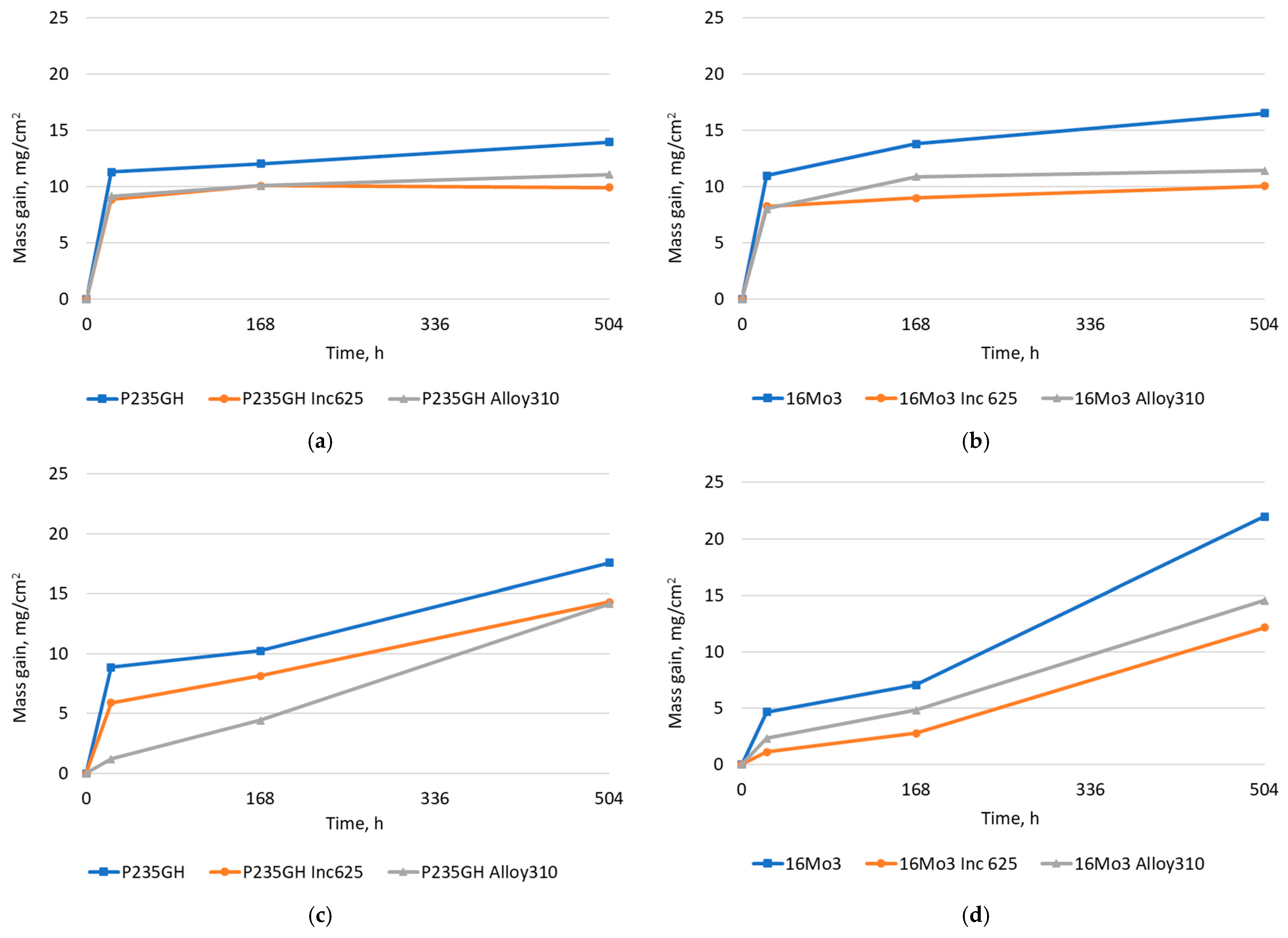
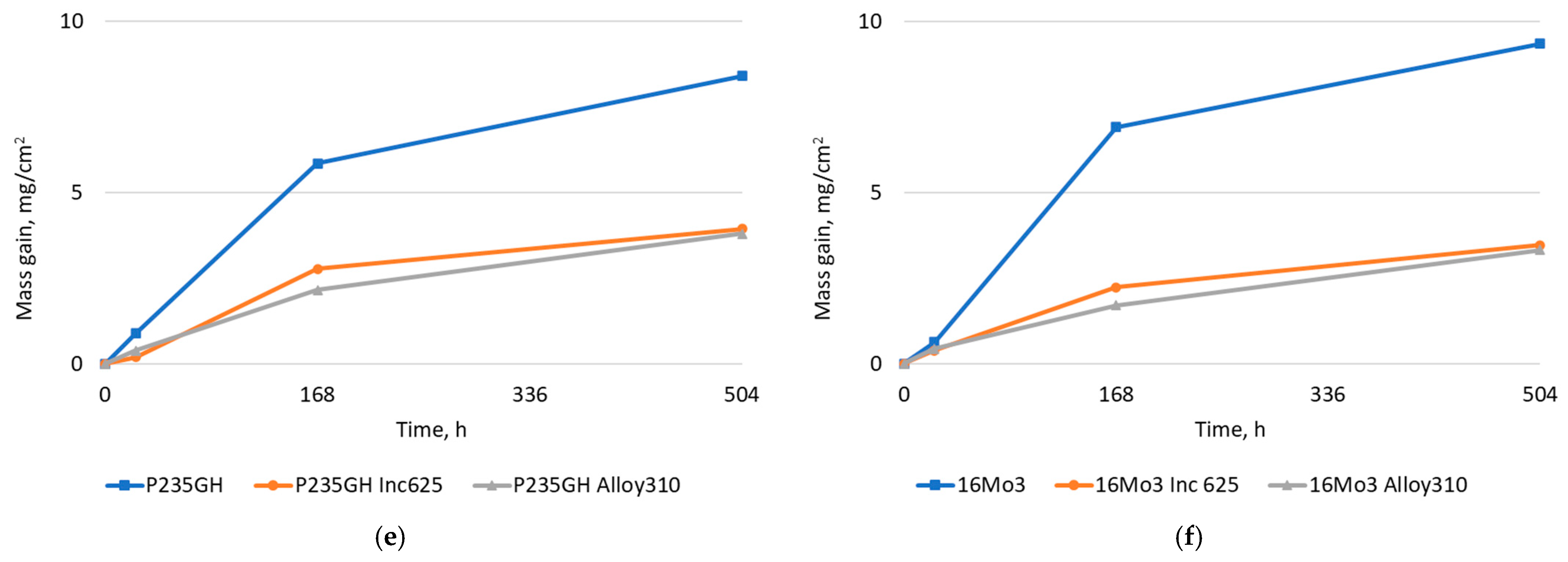
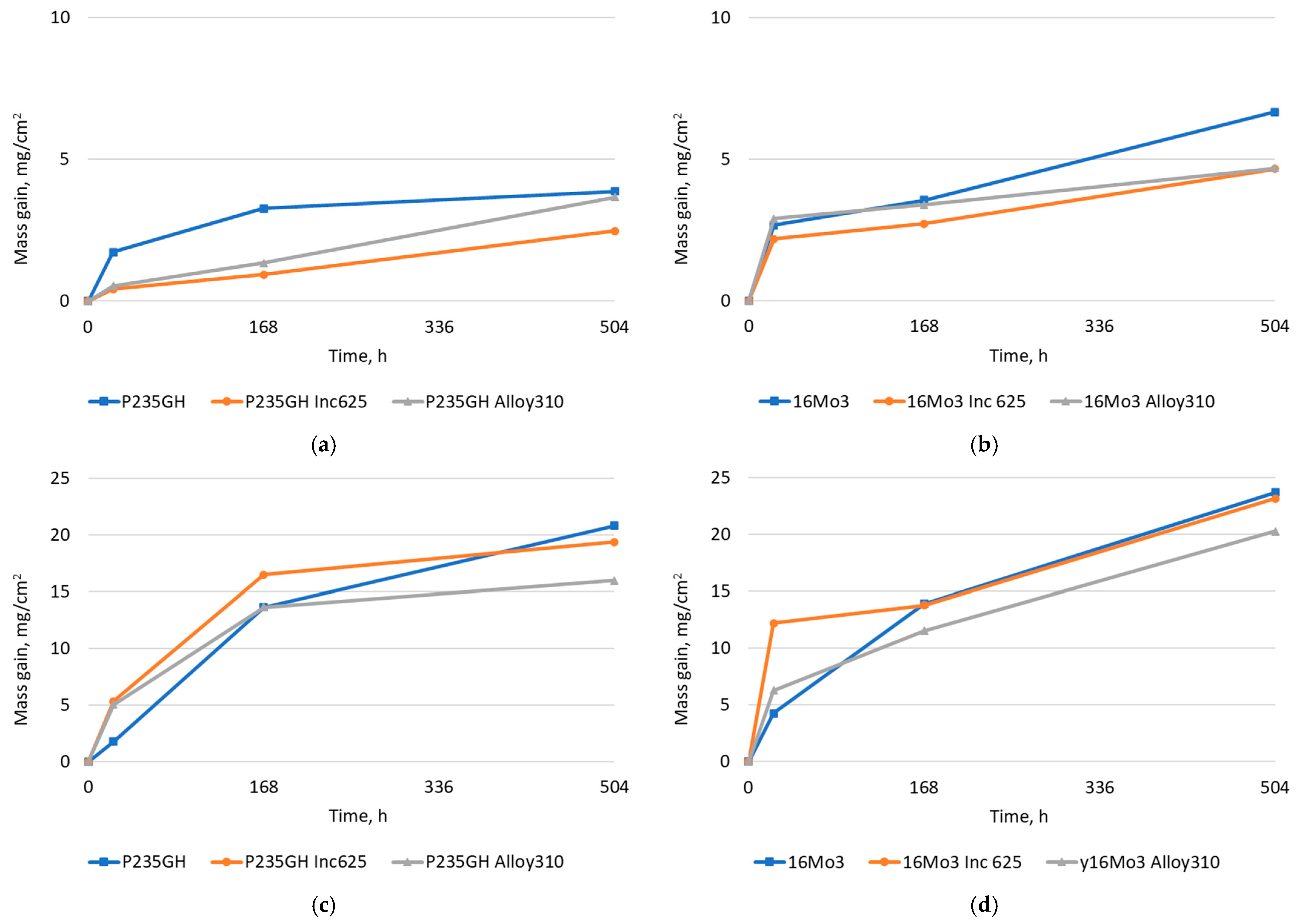
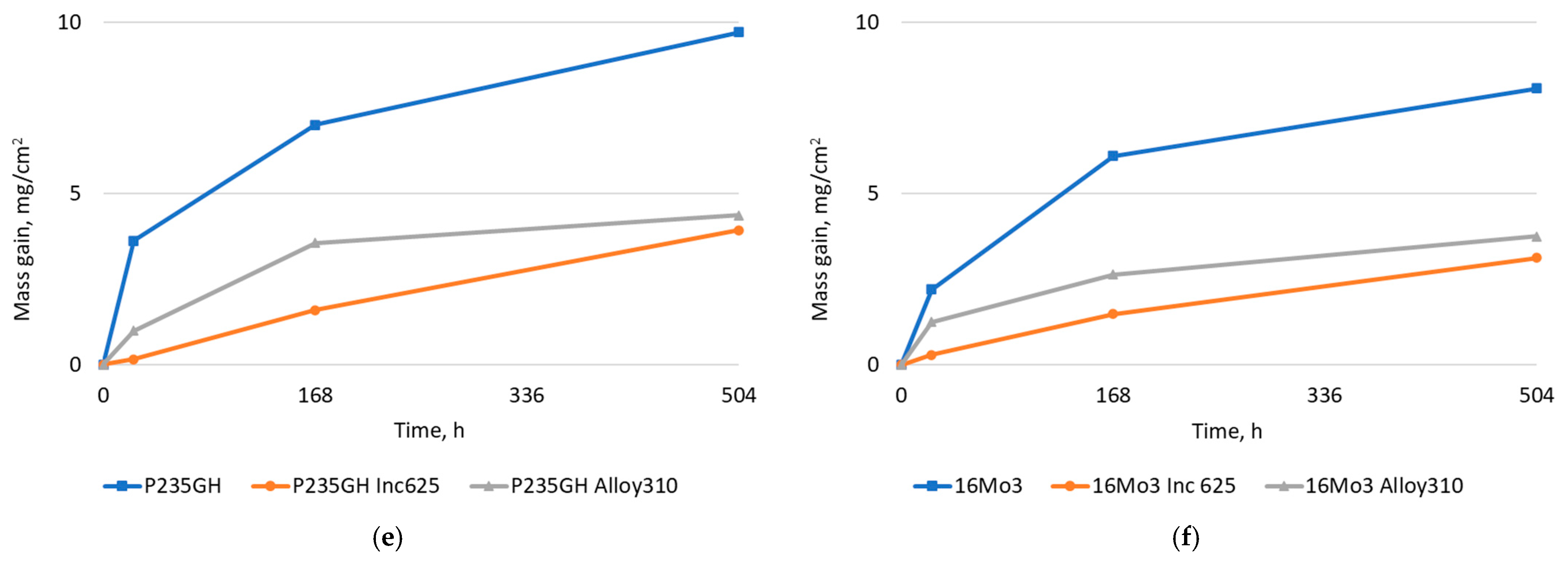
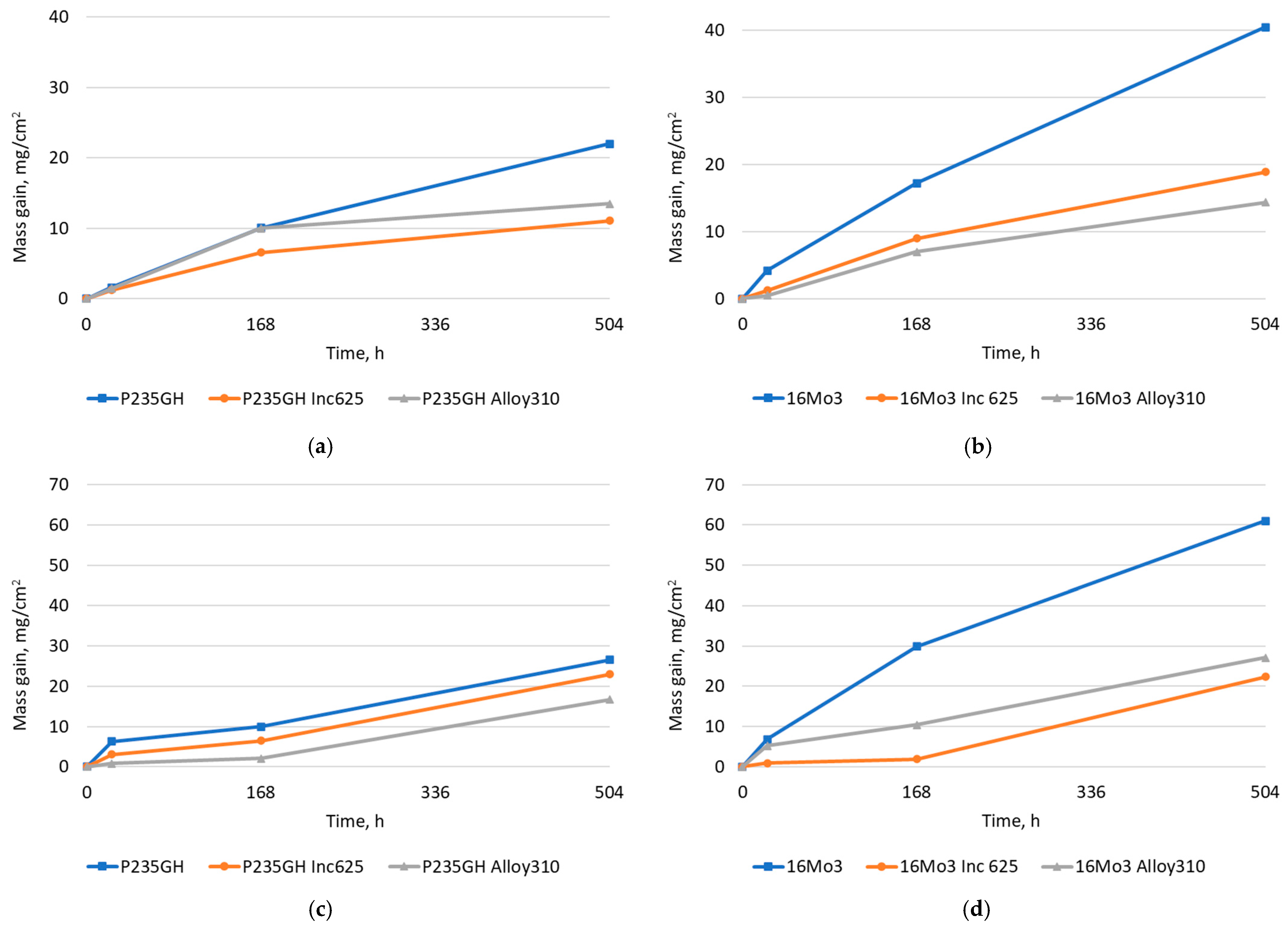
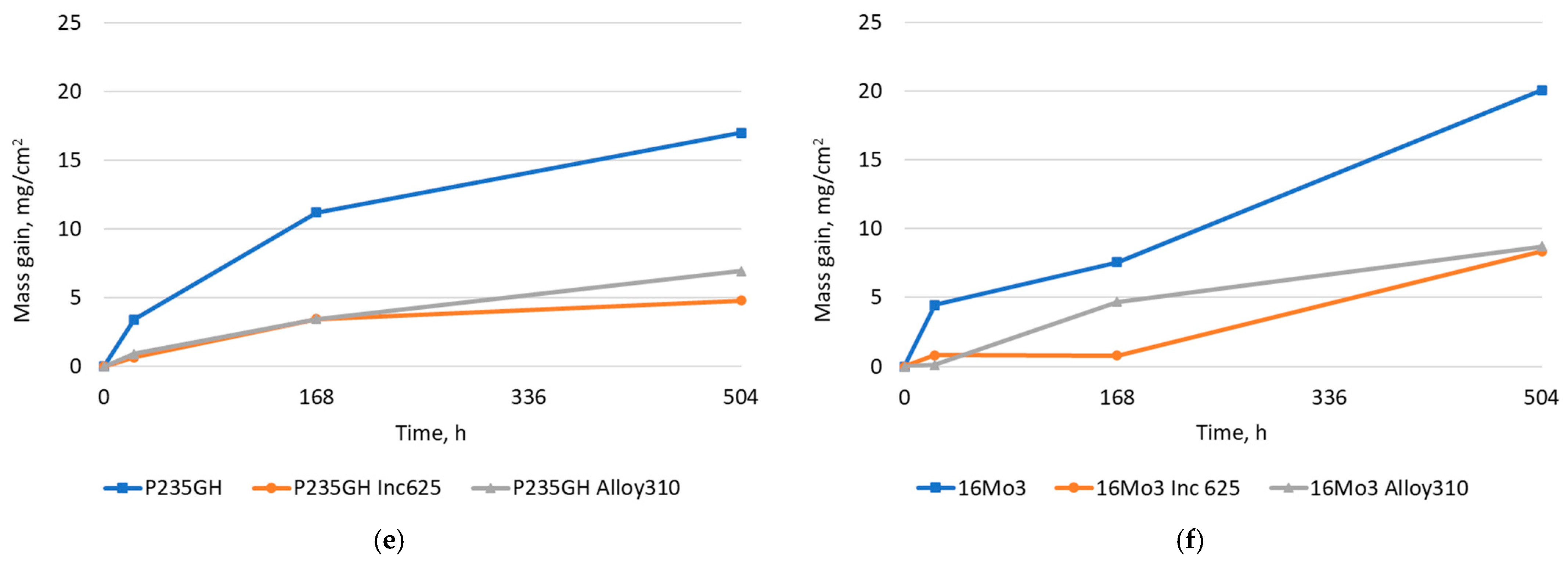
| Element | 16Mo3 | P235GH | Inconel 625 | Alloy 310 |
|---|---|---|---|---|
| C | 0.12—0.2 | max. 0.16 | 0.03–0.1 | 0.08–0.15 |
| Si | max. 0.35 | max. 0.35 | max. 0.5 | max. 0.65 |
| Mn | 0.4–0.9 | 0.6–1.2 | max. 0.5 | 01.02.2005 |
| Ni | max. 0.3 | max. 0.3 | min 58 | 20–22.5 |
| P | max. 0.025 | max. 0.025 | max. 0.02 | max. 0.03 |
| S | max. 0.01 | max. 0.015 | max. 0.015 | max. 0.03 |
| Cr | max. 0.3 | max. 0.3 | 20–23 | 25–28 |
| Mo | 0.25–0.35 | max. 0.08 | 8–10 | max. 0.75 |
| N | max. 0.012 | max. 0.012 | - | - |
| Cu | max. 0.3 | max. 0.3 | max. 0.5 | max. 0.75 |
| Nb | - | max. 0.02 | - | - |
| Ti | - | max. 0.03 | max. 0.4 | - |
| Al. | - | max. 0.02 | max. 0.4 | - |
| V | - | max. 0.02 | - | - |
| Fe | - | - | max. 5 | - |
| Co | - | - | max. 1 | - |
| Cr + Cu + Mo + Ni | - | <0.7 | - | - |
| Sample | Moisture | Ash | HHV | LHV | C | H | N | S | Cl |
|---|---|---|---|---|---|---|---|---|---|
| % | % | MJ/kg | MJ/kg | % | % | % | % | % | |
| P1 | 42.6 | 2.1 | 19.84 | 18.55 | 50.5 | 5.91 | 0.31 | 0.04 | 0.013 |
| P2 | 15.4 | 33.85 | 19.33 | 18.3 | 44.59 | 5.56 | 1.28 | 0.35 | 0.56 |
| P3 | 59.1 | 12.3 | 18.98 | 17.75 | 47.86 | 5.66 | 0.54 | 0.05 | 0.033 |
| Sample | SiO2 | Fe2O3 | Al2O3 | Mn3O4 | TiO2 | CaO | MgO | SO3 | P2O5 | Na2O | K2O | BaO | SrO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 54.50 | 1.60 | 3.97 | 1.11 | 0.25 | 16.80 | 2.64 | 1.63 | 1.73 | 0.49 | 5.75 | 0.11 | 0.05 |
| P2 | 48.60 | 3.23 | 12.8 | 0.10 | 1.19 | 15.10 | 2.28 | 1.66 | 0.72 | 5.74 | 1.16 | 0.21 | 0.07 |
| P3 | 72.80 | 1.49 | 3.09 | 0.53 | 0.25 | 8,16 | 1.09 | 0.81 | 1.50 | 0.50 | 4.00 | 0.05 | 0.02 |
| P1 | P2 | P3 | |
|---|---|---|---|
| Shrinkage Starting Temperature (SST) | 1180 ± 60 | 1040 ± 80 | 1270 ± 95 |
| Deformation temperature (DT) | 1200 ± 60 | 1070 ± 80 | >1500 ± 75 |
| Hemisphere temperature (HT) | 1220 ± 65 | 1190 ± 65 | >1500 ± 75 |
| Flow temperature (FT) | 1310 ± 70 | 1200 ± 60 | >1500 ± 75 |
| Factor | Formula | Evaluation |
|---|---|---|
| Silica content in the ash | SiO2 [%] | <20—low 20–25—moderate >25—high |
| Quotient (SA) of silica oxide and alumina oxide | <1.87—niskie 1.87–2.65—moderate >2.65 bardzo wysokie | |
| Chlorine content in the fuel | Cl [%] as received | <0.2—low 0.2–0.3—moderate 0.3–0.5—high >0.5—very high |
| Basic-to-acidic oxide ratio | <0.5—low 0.5–1.0—moderate 1.0–1.75—high >1.75—very high | |
| Basic-to-acidic oxide ratio + potassium | <0.5—low 0.5–1.0—moderate 1.0–1.75—high >1.75—very high | |
| Basic-to-acidic oxide ratio simplified | <0.5—low 0.5–1.0—moderate 1.0–1.75—high >1.75—very high | |
| Babcock Index | where: Sd—sulfur content in dry fuel | <0.6—low 0.6–2.0—moderate 2.0–2.6—high >2.6—very high |
| Fouling factor | <0.6—low 0.6–40.0—moderate >40.0—high | |
| Quotient of sulfur and chlorine | 2S/Cl | >4—low <1—high |
| Sintering Indicator | <0.75 and >2.0—low 0.75–2.0—high and very high | |
| Bed Agglomeration Index | >0.15—low <0.15 high | |
| Ash Fusibility Index | where: IT—Initial Deformation Temperature; HT—Hemisphere temperature | >1342—low 1232–1342—moderate 1052–1232—high <1052—very high |
| Iron to Calcium ratio | <0.3 and >3.0—low 0.3–3.0—high | |
| Slag Viscosity Index | >72—low 65–72—moderate <65—high | |
| Initial Deformation Temperature | IDT [°C] | >1100—low 900–1100—moderate <900—high |
| Factor | Symbol | P1 | P2 | P3 | |||
|---|---|---|---|---|---|---|---|
| Result | Evaluation | Result | Evaluation | Result | Evaluation | ||
| Silica content in the ash | SiO2 [%] | 54.3 | high | 48.6 | high | 72.8 | high |
| Quotient (SA) of silica oxide and alumina oxide | SA | 13.68 | very high | 3.80 | very high | 23.56 | very high |
| Chlorine content in the fuel | Cl [%] | 0.013 | low | 0.56 | very high | 0.033 | low |
| Basic-to-acidic oxide ratio | B/A | 0.466 | low | 0.44 | low | 0.20 | low |
| Basic-to-acidic oxide ratio + potassium | (B/A) + P | 0.496 | low | 0.451 | low | 0.22 | low |
| Basic-to-acidic oxide ratio simplified | B/Asimplified | 0.361 | low | 0.336 | low | 0.141 | low |
| Babcock Index | Rs | 0.019 | low | 0.154 | low | 0.01 | low |
| Fouling index Fu | Fu | 2.909 | moderate | 3.033 | moderate | 0.90 | moderate |
| Quotient of sulfur and chlorine | 2S/Cl | 6.154 | low | 1.25 | moderate | 3.03 | moderate |
| Sintering Indicator | SI | 0.496 | low | 0.451 | low | 0.220 | low |
| Bed Agglomeration Index | BAI | 0.256 | low | 0.468 | low | 0.331 | low |
| Ash Fusibility Index | Fs | 6020 | low | 5470 | low | 7500 | low |
| Iron to Calcium ratio | IC | 0.095 | low | 0214 | low | 0.183 | low |
| Slag Viscosity Index | SR | 72.073 | low | 70.221 | moderate | 87.154 | low |
| Initial Deformation Temperature | IDT [°C] | 1200 | low | 1070 | moderate | 1500 | low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciejczyk, A.; Maj, I.; Ciukaj, S.; Hernik, B.; Osuch, A. The Influence of Protective Coatings on High-Temperature Corrosion under Biomass Ash Deposits. Energies 2023, 16, 7221. https://doi.org/10.3390/en16217221
Maciejczyk A, Maj I, Ciukaj S, Hernik B, Osuch A. The Influence of Protective Coatings on High-Temperature Corrosion under Biomass Ash Deposits. Energies. 2023; 16(21):7221. https://doi.org/10.3390/en16217221
Chicago/Turabian StyleMaciejczyk, Anna, Izabella Maj, Szymon Ciukaj, Bartłomiej Hernik, and Arkadiusz Osuch. 2023. "The Influence of Protective Coatings on High-Temperature Corrosion under Biomass Ash Deposits" Energies 16, no. 21: 7221. https://doi.org/10.3390/en16217221
APA StyleMaciejczyk, A., Maj, I., Ciukaj, S., Hernik, B., & Osuch, A. (2023). The Influence of Protective Coatings on High-Temperature Corrosion under Biomass Ash Deposits. Energies, 16(21), 7221. https://doi.org/10.3390/en16217221








