Experimental Studies on Preheating Combustion Characteristics of Low-Rank Coal with Different Particle Sizes and Kinetic Simulation of Nitrogen Oxide
Abstract
:1. Introduction
2. Experiment
2.1. Experimental Apparatus
2.2. Fuel Characteristics
2.3. Experimental Conditions
2.4. Sample Analysis Methods
3. Experimental Results and Analyses
3.1. CFB Preheating
3.1.1. The Conversion of Lignite to Coal Gas
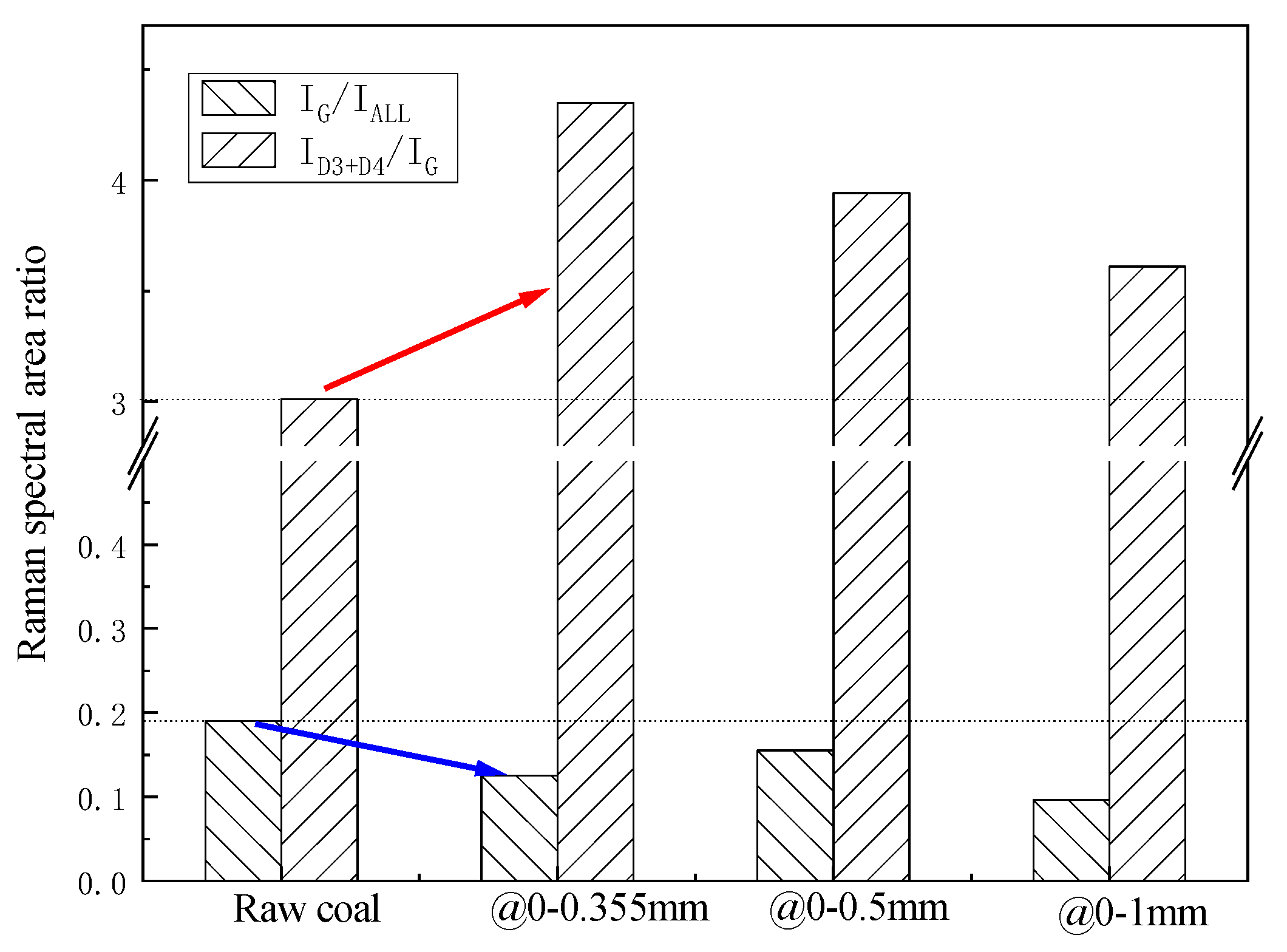
3.1.2. The Conversion of Lignite to Preheated Char
3.2. Combustion Characteristics of Preheated Fuel
3.3. The Conversion Process of Fuel-N and NO Emission
3.3.1. The Conversion of Fuel-N in the Preheating
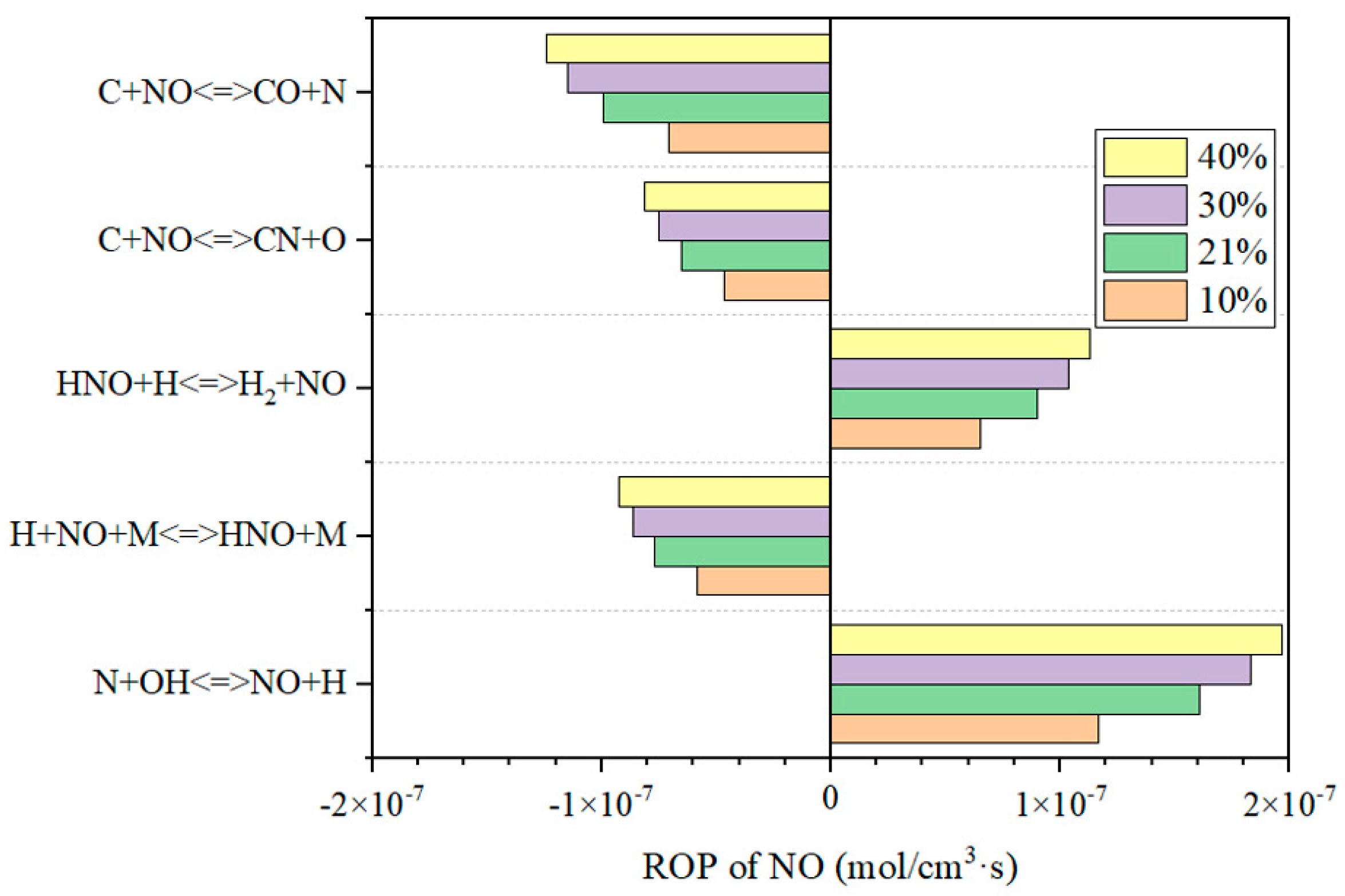
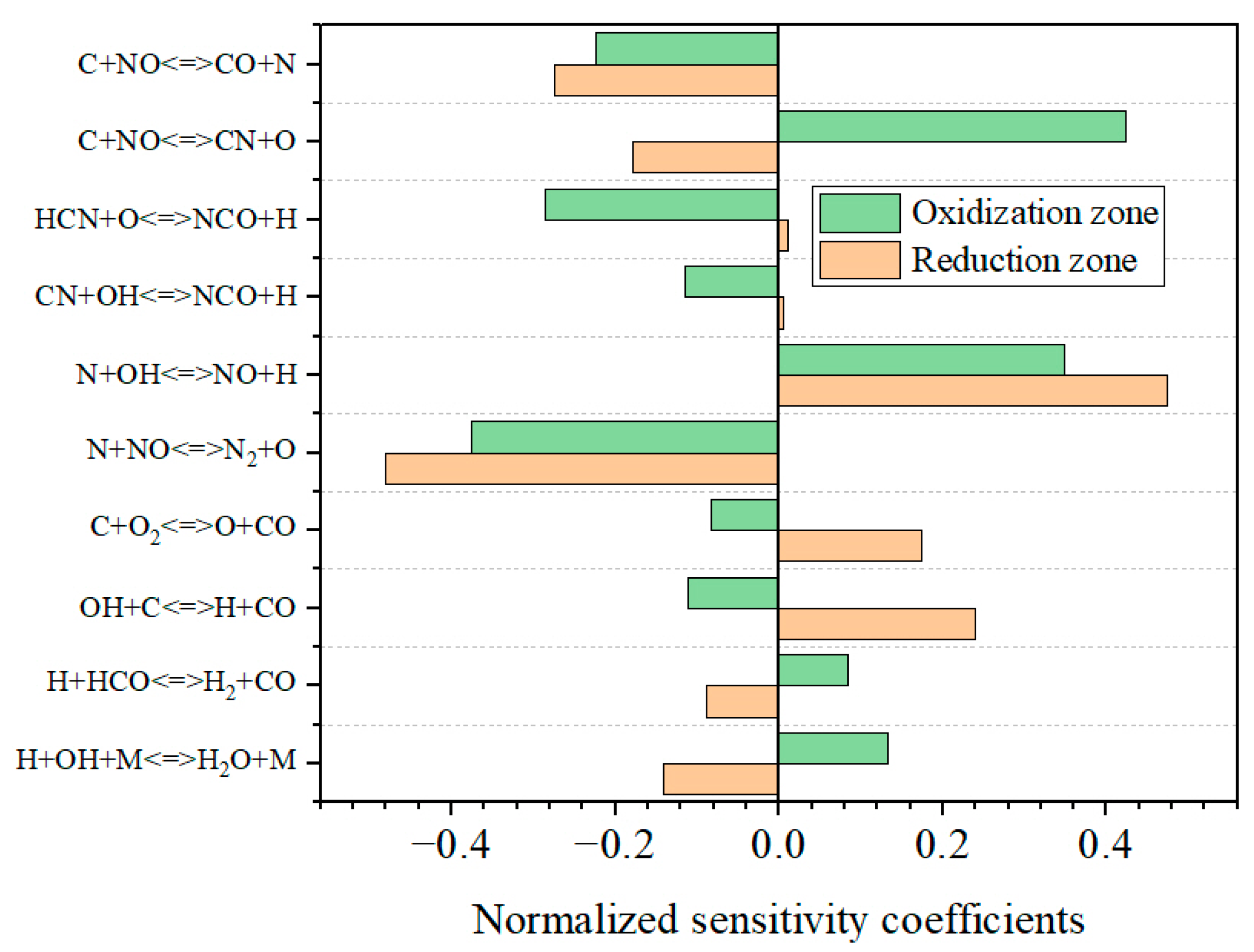
3.3.2. NO Formation in the DFC
3.4. The Kinetic Simulation of NO
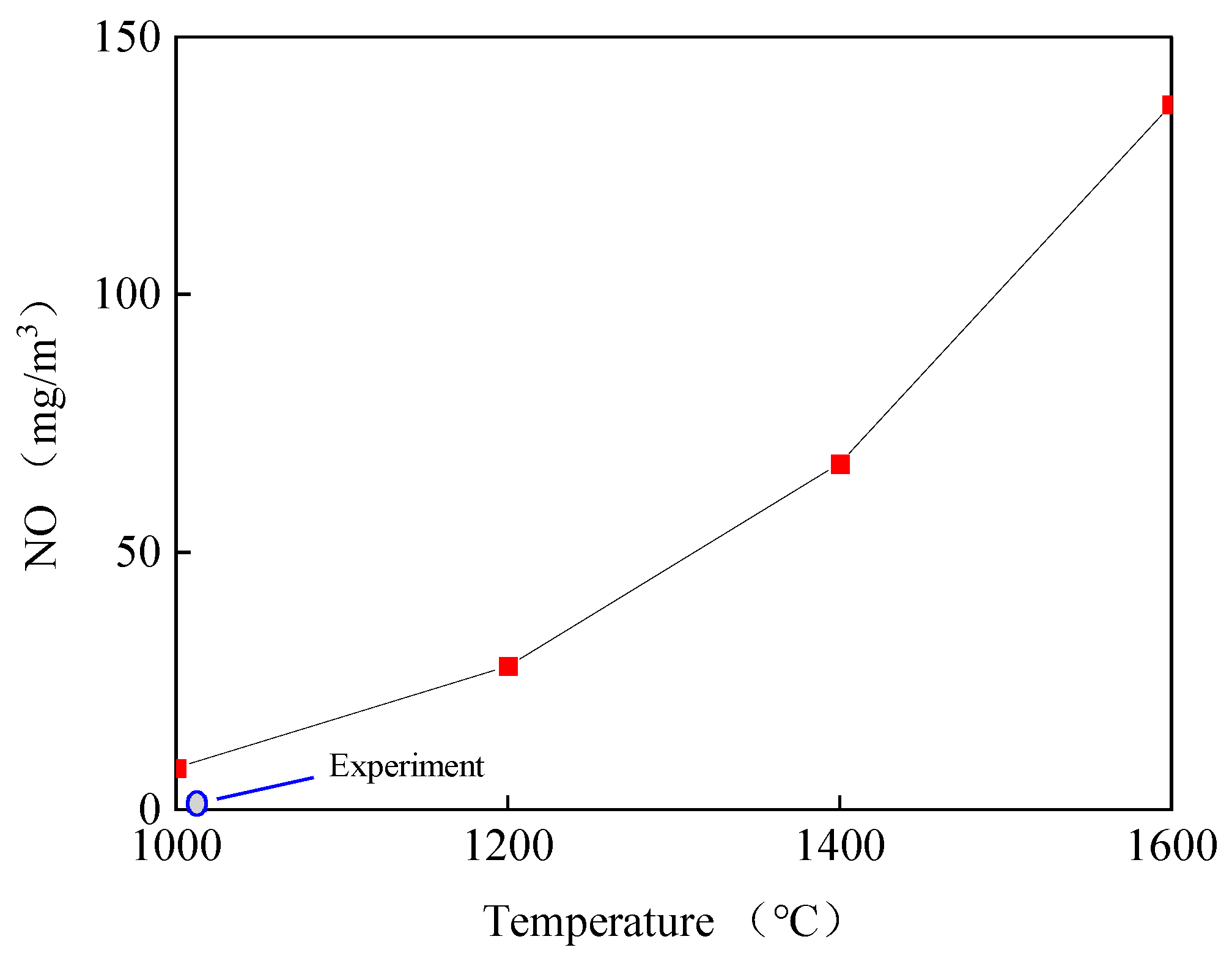
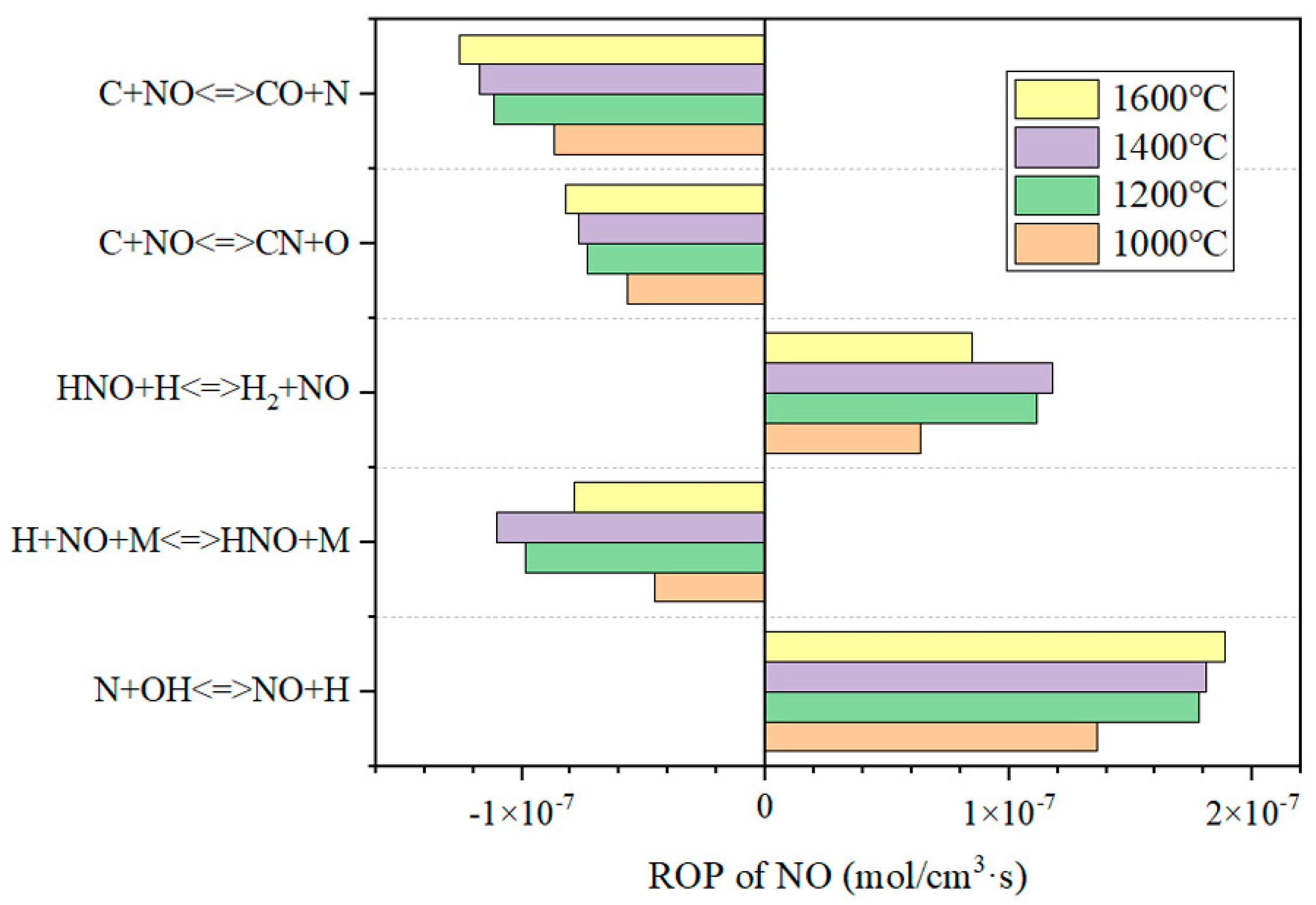
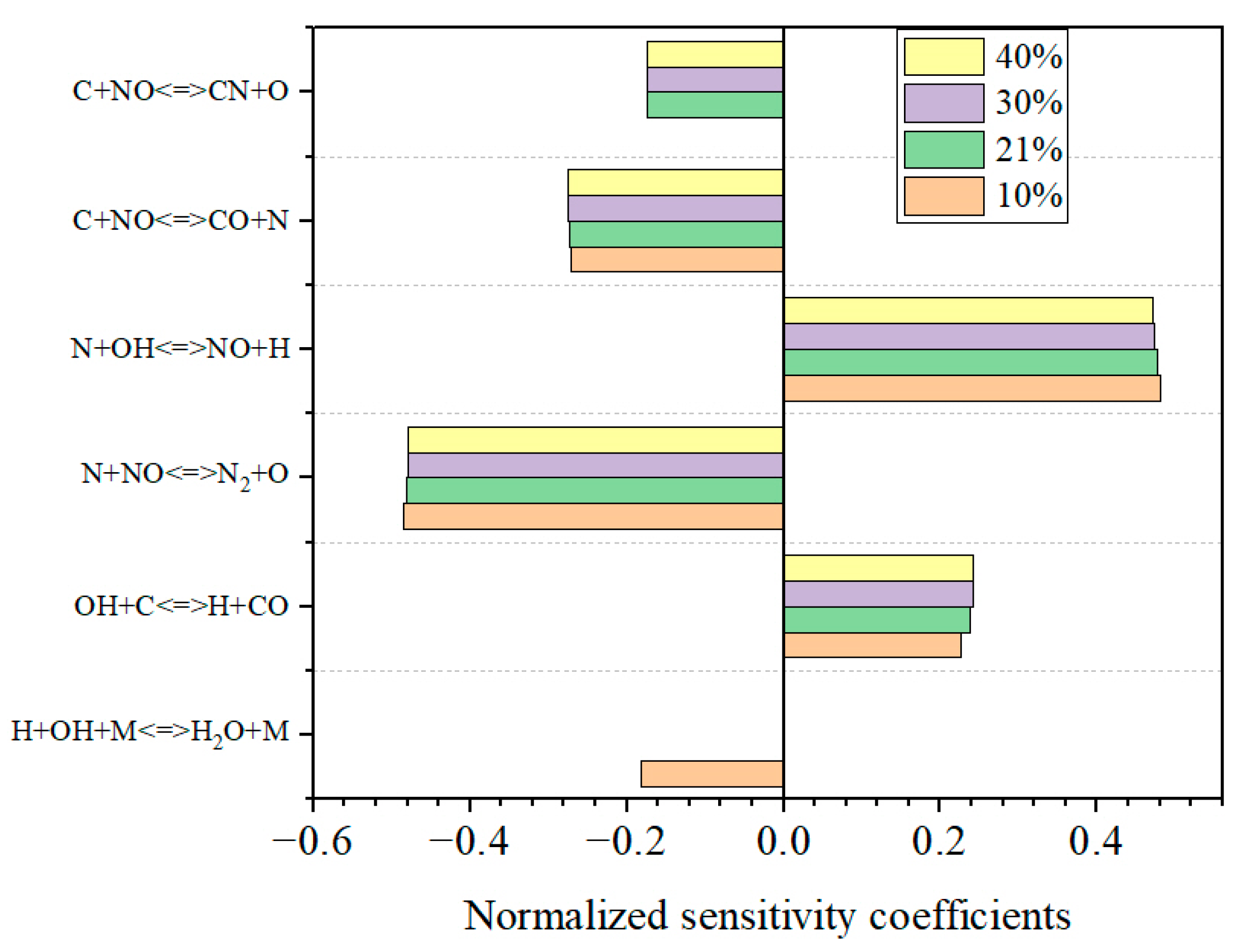
3.4.1. The Influence of Temperature on NO in the Reduction Zone
3.4.2. The Influence of Oxygen Concentration on NO in the Reduction Zone
3.4.3. The Influence of Air-Staging on NO Emission
4. Conclusions
- (1)
- With a primary air-equivalence ratio of 0.51 in the CFB, the ratio of CO/CO2 in the coal gas was lower than 1, meaning a partial combustion and gasification reaction happened in the CFB preheating. A larger particle size resulted in relatively higher CO and H2 content levels in the coal gas due to the long residence time. The release of different species for all three particle sizes followed the order H > N>C > S in the CFB preheating. Compared to raw coal, the reaction activity of preheated char intensified, forming a condition beneficial for the subsequent highly efficient combustion.
- (2)
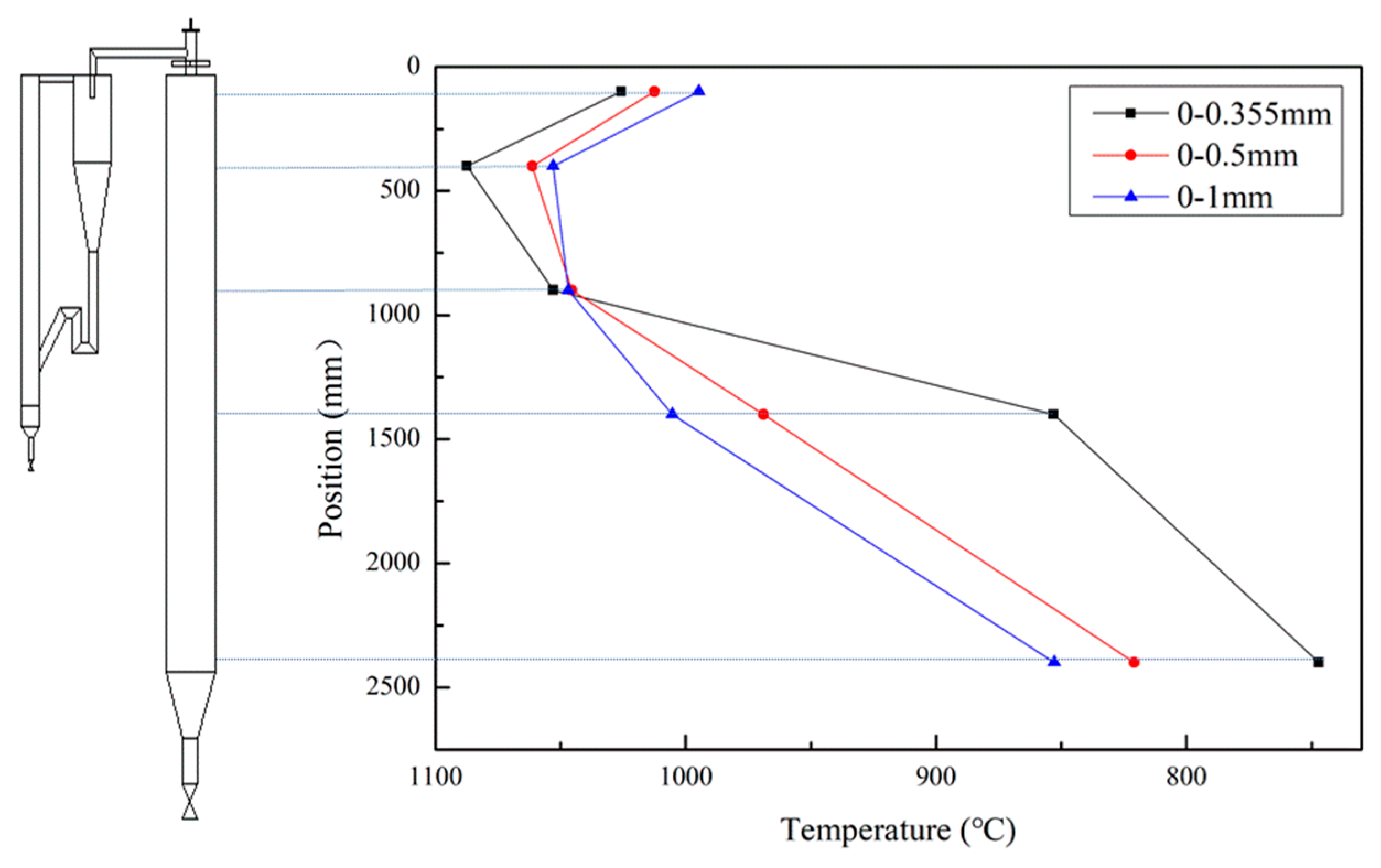
- After preheating, the samples with a fine particle size showed a fast combustion reaction near the secondary air nozzle and had the highest combustion temperature, 1080 ℃, at 400 mm below the nozzle. The largest combustion temperature for a particle size of 0–0.355 mm was about 100 ℃ higher than that for a particle size of 0–1 mm. The fast combustion reaction for a particle size of 0–0.355 mm was mainly due to the large particle-surface area, an excellent mixing of fuel with oxygen, and the preheated fuel modification itself. For the three particle sizes, the temperature distributions in the DFC were uniform, with a lower temperature difference, and the combustion efficiencies were over 98%.
- (3)
- There were three conversion paths for fuel-N into N2 in the CFB preheating: the first was a homogenous reduction with coal gas and N-containing species, the second was a heterogeneous reduction with char and N-containing species in an inner pore of the particle, and the third was a heterogeneous reduction with char and N-containing species on the outside of the particle. The largest conversion ratio of fuel-N into N2 in the CFB preheating was 52.64% for a particle size of 0–0.5 mm, corresponding to the lowest NO emission in this system. A large particle size produced more NH3, but with the lowest conversion ratio of fuel-N into N2.
- (4)
- In the reduction zone of the DFC, CO was the main species, with tiny amounts of HCN, NH3 and NO, and in the oxidation zone, CO continuously decreased and NO rapidly increased. The effect of the particle size on NO was negligible. The ultimate NO emission was in the range of 105~120 mg/m3, more 50% lower than that of conventional combustion.
- (5)
- The mechanism of GRI-Mech 2.11 was applied to the kinetic simulation of NO in this preheating combustion system. Three parameters, namely, temperature, oxygen concentration, and secondary-air ratio, were varied to analyze NO-formation variations. The results showed that there was good agreement between the experiment and the simulation, illustrating the validity of the mechanism. The simulation results showed that the best secondary-air ratio was 0.9, with the lowest NO emission, 61 mg/m3.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, M.; Cheng, C.; Miao, Z.; Wan, K.; He, Q. Physicochemical properties, combustion kinetics and thermodynamics of oxidized lignite. Energy 2023, 268, 126657. [Google Scholar] [CrossRef]
- Hees, J.; Zabrodiec, D.; Massmeyer, A.; Hatzfeld, O.; Kneer, R. Experimental investigation into the influence of the oxygen concentration on a pulverized coal swirl flame in oxy-fuel atmosphere. Fuel 2019, 240, 64–74. [Google Scholar] [CrossRef]
- Jeon, M.; Lee, E.; Kim, M.; Jegal, H.; Park, S.; Chi, J.; Baek, S.; Lee, J.; Keel, S. Nitric oxide (NO) and nitrous oxide (N2O) emissions during selective non-catalytic reduction and selective catalytic reduction processes in a pulverized coal/ammonia co-fired boiler. J. Environ. Chem. Eng. 2023, 11, 109398. [Google Scholar] [CrossRef]
- Lv, Z.M.; Xiong, X.H.; Tan, H.Z.; Wang, X.B.; Liu, X.; Rahman, Z. Experimental investigation on NO emission and burnout characteristics of high-temperature char under the improved preheating combustion technology. Fuel 2022, 313, 122662. [Google Scholar] [CrossRef]
- Kuang, M.; Wang, J.L.; Wang, X.; Zhao, X.J.; Chen, Y.Y.; Du, L. In-furnace flow field, coal combustion and NOx emission characteristics regarding the staged-air location in a cascade-arch down-fired furnace. J. Energy. Inst. 2021, 98, 259–270. [Google Scholar] [CrossRef]
- Su, K.; Ouyang, Z.; Ding, H.; Wang, W.; Zhang, J.; Wang, H.; Zhu, S. Experimental investigation on effect of external circulation system on preheating characteristics of pulverized coal. Energy 2023, 278, 127781. [Google Scholar] [CrossRef]
- Li, L.; Cheng, L.; Wang, B.; Ma, Z.; Zhang, W. Experimental study on the effect of limestone on SO2 and NO emission characteristics during coal/coke combustion. J. Energy. Inst. 2023, 111, 101403. [Google Scholar] [CrossRef]
- Xu, M.; Tu, Y.; Zhou, A.; Xu, H.; Yu, W.; Li, Z.; Yang, W. Numerical study of HCN and NH3 reduction in a two-stage entrained flow gasifier by implementing MILD combustion. Fuel 2019, 251, 482–495. [Google Scholar] [CrossRef]
- Wu, C.Y.; Tree, D.; Baxter, L. Reactivity of NH3 and HCN during low-grade fuel combustion in a swirling flow burner. Proc. Combust. Inst. 2007, 31, 2787–2794. [Google Scholar] [CrossRef]
- Schafer, S.; Bonn, B. Hydrolysis of HCN as an important step in nitrogen oxide formation in fluidised combustion. Part 1. Homogeneous reactions. Fuel 2000, 79, 1239–1246. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, Z.; Bian, L.; Li, Z. Influence of over-fired air location on gas-particle flow characteristics within a coal-fired industrial boiler under radial air staging. Energy 2023, 283, 128617. [Google Scholar] [CrossRef]
- Xiang, J.; Li, M.; Sun, L.; Lu, J.; Sun, X. Comparison of nitrogen oxide emissions from boilers for a wide range of coal qualities. Int. J. Therm. Sci. 2000, 39, 833–841. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, J.; Lu, Q. Experimental study on combustion characteristics and NOx emissions of pulverized anthracite preheated by circulating fluidized bed. J. Therm. Sci. 2011, 20, 355–361. [Google Scholar] [CrossRef]
- Liang, B.; Bai, H.; Tan, B.; Fu, L.; Bai, D. Experimental investigation of circulating fluidized bed combustion of dry powders of coal slime. Fuel 2023, 348, 128566. [Google Scholar] [CrossRef]
- Ge, C.; Li, S.; Wang, L. Current investigation status of oxy-fuel circulating fluidized bed combustion. Fuel 2023, 342, 127699. [Google Scholar] [CrossRef]
- Ding, H.; Ouyang, Z.; Su, K.; Wang, W.; Zhang, J.; Wang, H.; Zhu, S. Experimental research on effects of multi-layer injection of the high-temperature preheated pulverized coal on combustion characteristics and NOx emission. Fuel 2023, 347, 128424. [Google Scholar] [CrossRef]
- Ding, H.; Ouyang, Z.; Shi, Y.; Chen, R.; Zhang, Z.; Zhu, S.; Lyu, Q. Effects of the T-abrupt exit configuration of riser on fuel properties, combustion characteristics and NOx emissions with coal self-preheating technology. Fuel 2023, 337, 126860. [Google Scholar] [CrossRef]
- Saha, M.; Dally, B.; Medwell, P.; Chinnici, A. Effect of particle size on the MILD combustion characteristics of pulverized brown coal. Fuel. Process. Technol. 2017, 155, 74–87. [Google Scholar] [CrossRef]
- Sung, Y.; Moon, C.; Eom, S.; Chor, G.; Kim, D. Coal-particle size effects on NO reduction and burnout characteristics with air-staged combustion in a pulverized coal-fired furnace. Fuel 2016, 182, 558–567. [Google Scholar] [CrossRef]
- Hu, P.; Li, P.; Wang, K.; Li, W.; Guo, J.; Liu, L.; Liu, Z. Evaluation, development, and application of a new skeletal mechanism for fuel-NO formation under air and oxy-fuel combustion. Fuel. Process. Technol. 2020, 199, 106256. [Google Scholar] [CrossRef]
- Cellek, M. Flameless combustion investigation of CH4/H2 in the laboratory-scaled furnace. Int. J. Hydrogen Energy. 2020, 45, 35208–352226. [Google Scholar] [CrossRef]
- Pan, H.; Geng, S.; Yang, H.; Zhang, G.; Bian, H.; Liu, Y. Influence of H2 blending on NOx production in natural gas combustion: Mechanism comparison and reaction routes. Int. J. Hydrogen Energy. 2023, 48, 784–797. [Google Scholar] [CrossRef]
- Ding, H.; Ouyang, Z.; Zhang, X.; Zhu, S. The effects of particle size on flameless combustion characteristics and NOx emissions of semi-coke with coal preheating technology. Fuel 2021, 297, 120758. [Google Scholar] [CrossRef]
- Liang, C.; Wang, X.; Lyu, Q. Experimental investigation on fluidized modification in gasification of preheated coal using oxygen and steam. Fuel 2021, 304, 121375. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, J.; Shi, Y.; Wang, T. Gas generation characteristics of Shenmu bituminous coal in fluidized preheating. Clean Coal Technol. 2023, 29, 149–154. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, J.; Lyu, Q.; Liu, J.; Zhang, J. Experimental study on the effect of preheating temperature on the modification of pulverized coal through a circulating fuidized bed. J. Therm. Anal. Calorim. 2022, 150, 9591–9601. [Google Scholar] [CrossRef]
- Sheng, C. Char structure characterized by Raman spectroscopy and its correlations with combustion reactivity. Fuel 2007, 86, 2316–2324. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, K.; Singh, R.; Lou, H.; Hao, J.; Wang, B.; Chen, F. Numerical simulation and cold experiment research of a low-NOx combustion technology for pulverized low-volatile coal. Appl. Therm. Eng. 2017, 114, 498–510. [Google Scholar] [CrossRef]
- Liu, Y.H.; Liu, J.Z.; Lyu, Q.G.; Zhu, J.G.; Zhang, X.Y.; Zhang, J.H.; Cao, X.Y. Comparison of oxy-fuel preheated combustion characteristics of high- and low-volatility carbon-based fuels. Fuel 2022, 330, 125583. [Google Scholar] [CrossRef]











| Item | Proximate Analysis (wt.%) | Ultimate Analysis (wt.%) | Low Heating Value (MJ/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | Cad | Had | Nad | Sad | Qnet,ad | |
| Data | 6.18 | 10.98 | 37.93 | 44.91 | 56.6 | 4.1 | 0.75 | 0.28 | 23.13 |
| Item | Case One | Case Two | Case Three |
|---|---|---|---|
| Feeding rate (kg/h) | 3.57 | 4.08 | 3.64 |
| 0.51 | 0.51 | 0.51 | |
| 0.89 | 0.87 | 0.83 | |
| 1.20 | 1.20 | 1.20 | |
| Particle size (mm) | 0–0.355 | 0–0.5 | 0–1 |
| Item | Proximate Analysis (wt.%) | Ultimate Analysis (wt.%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | Cad | Had | Nad | Sad | |
| 0–0.355 mm | 4.59 | 28.61 | 8.52 | 58.28 | 61.21 | 1.67 | 0.66 | 0.61 |
| 0–0.5 mm | 4.94 | 28.8 | 9.23 | 57.04 | 61.63 | 1.37 | 0.75 | 0.59 |
| 0–1 mm | 5.74 | 26.94 | 9.72 | 57.59 | 60.07 | 1.33 | 0.70 | 0.54 |
| Conversion ratio (%) | ||||||||
| @ 0–0.355 mm | 71.50 | / | 91.43 | 50.23 | 58.51 | 84.42 | 66.21 | 16.41 |
| @ 0–0.5 mm | 69.51 | / | 90.72 | 51.61 | 58.53 | 87.31 | 61.92 | 19.72 |
| @ 0–1 mm | 62.12 | / | 89.61 | 47.72 | 56.72 | 86.82 | 62.01 | 21.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhu, J.; Liu, J. Experimental Studies on Preheating Combustion Characteristics of Low-Rank Coal with Different Particle Sizes and Kinetic Simulation of Nitrogen Oxide. Energies 2023, 16, 7078. https://doi.org/10.3390/en16207078
Zhang J, Zhu J, Liu J. Experimental Studies on Preheating Combustion Characteristics of Low-Rank Coal with Different Particle Sizes and Kinetic Simulation of Nitrogen Oxide. Energies. 2023; 16(20):7078. https://doi.org/10.3390/en16207078
Chicago/Turabian StyleZhang, Jiahang, Jianguo Zhu, and Jingzhang Liu. 2023. "Experimental Studies on Preheating Combustion Characteristics of Low-Rank Coal with Different Particle Sizes and Kinetic Simulation of Nitrogen Oxide" Energies 16, no. 20: 7078. https://doi.org/10.3390/en16207078
APA StyleZhang, J., Zhu, J., & Liu, J. (2023). Experimental Studies on Preheating Combustion Characteristics of Low-Rank Coal with Different Particle Sizes and Kinetic Simulation of Nitrogen Oxide. Energies, 16(20), 7078. https://doi.org/10.3390/en16207078







