Methane Emission Reduction Technologies for Natural Gas Engines: A Review
Abstract
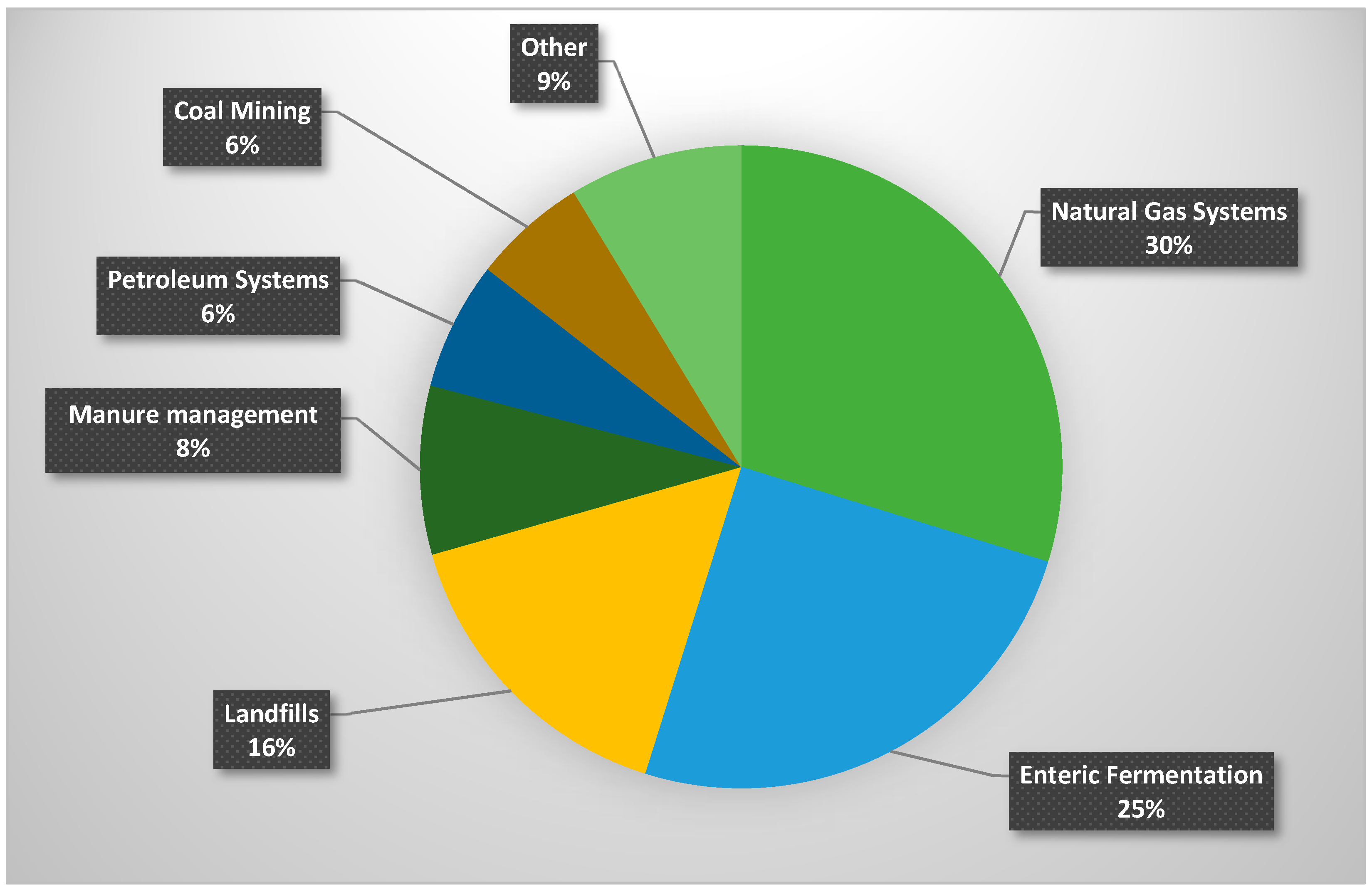
1. Introduction
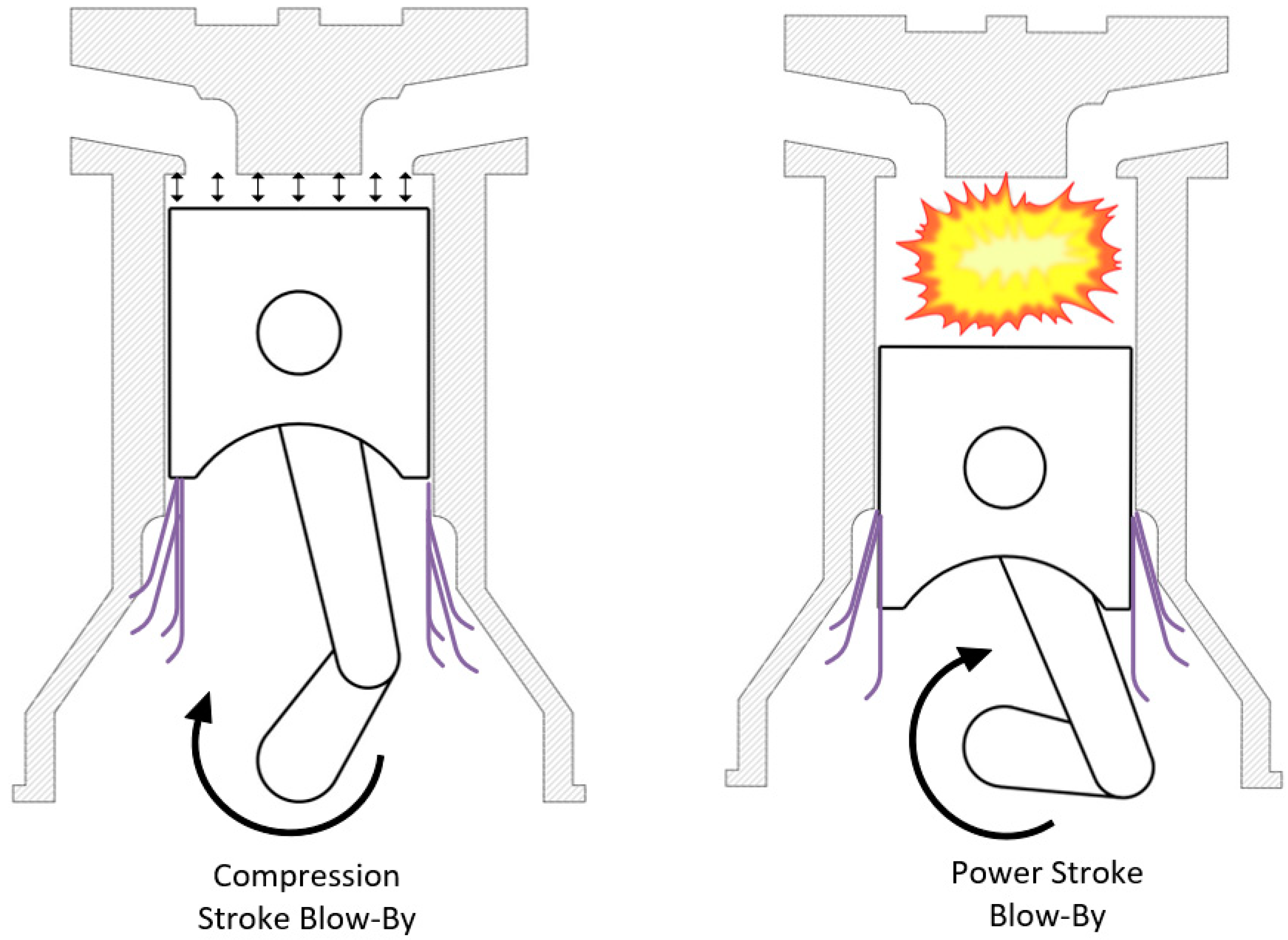
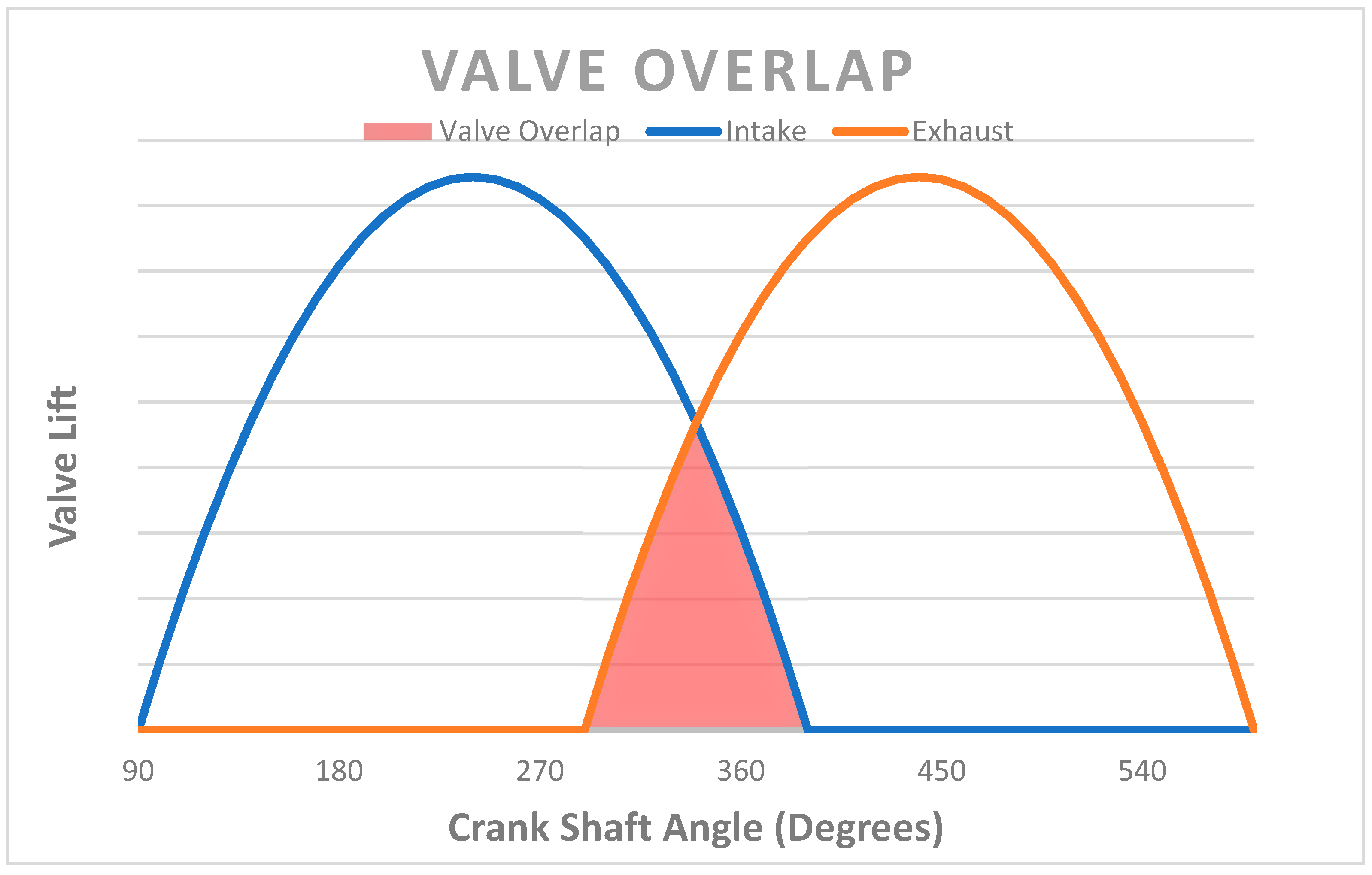
2. Sources of Methane Slip in Natural Gas Engines
3. In Cylinder Methane Emission Reduction Technologies
4. Exhaust Methane Emission Reduction Technologies
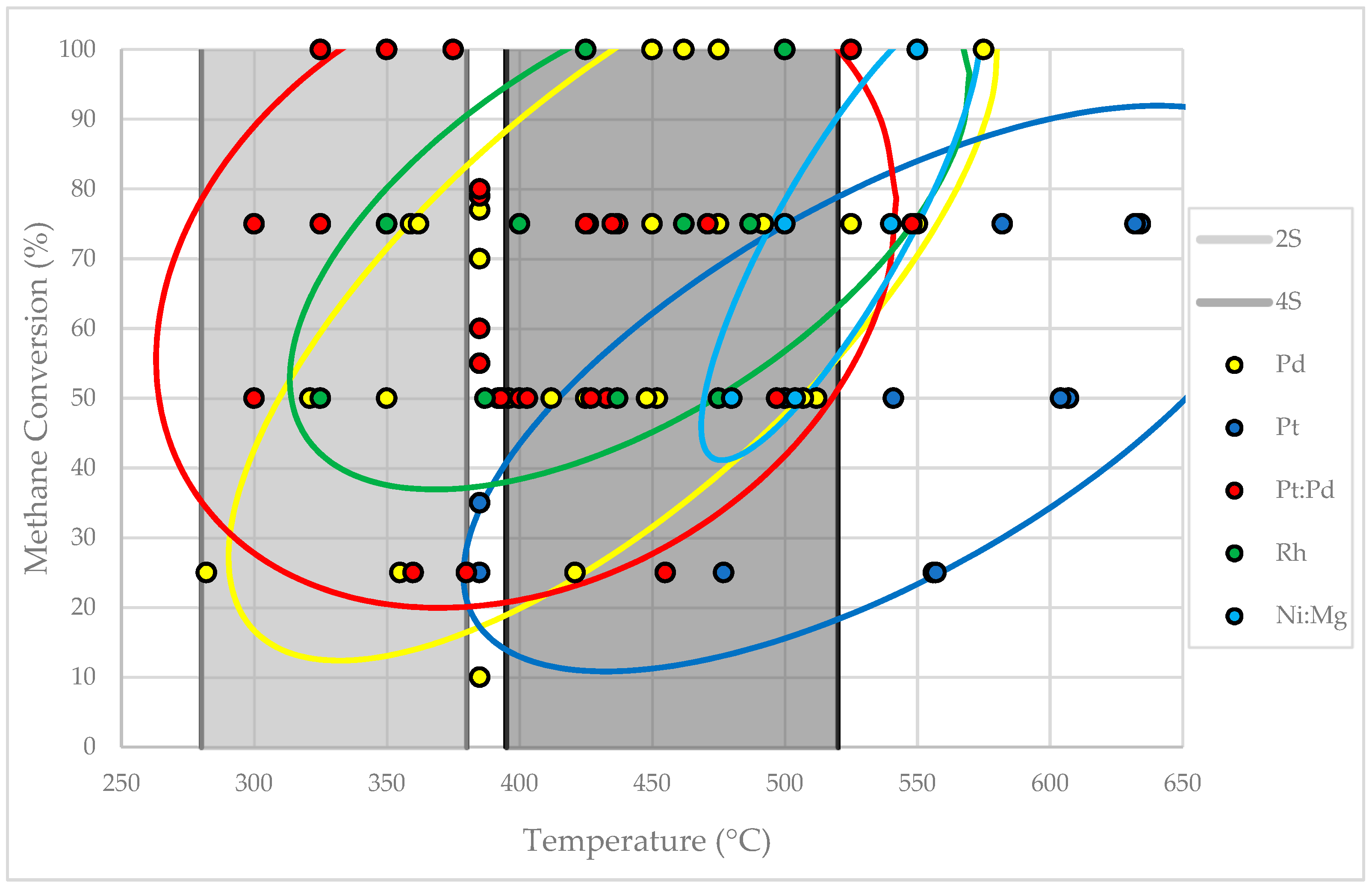
4.1. Catalytic Oxidation
4.1.1. Palladium
4.1.2. Platinum
4.1.3. Palladium-Platinum Alloy
4.1.4. Rhodium
4.1.5. Nickel-Magnesium Alloy
4.1.6. Three-Way Catalyst
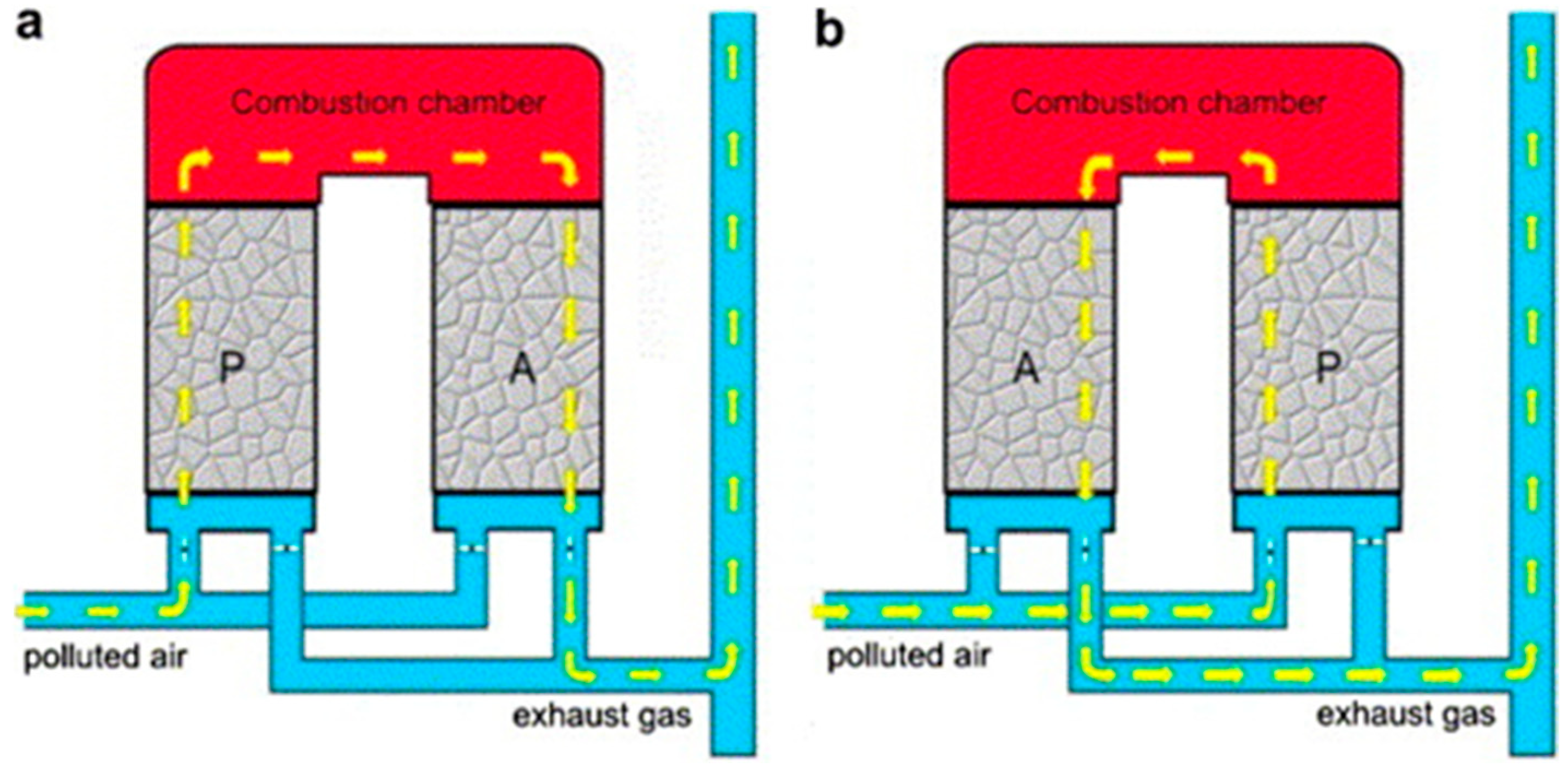
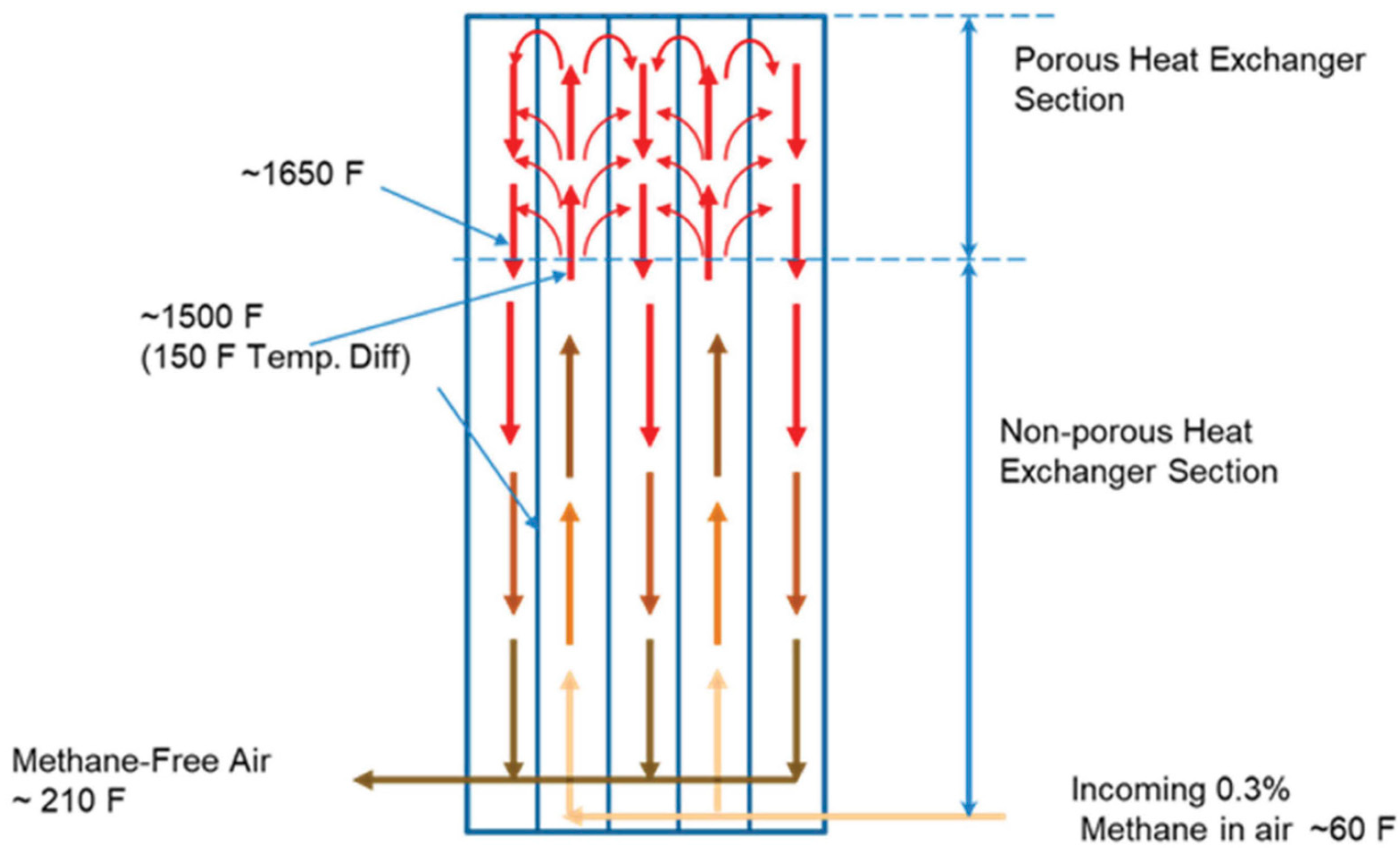
4.2. Thermal Oxidizers
| Operational Temperature | Heated Wire Power | Methane Concentration | Methane Removal Efficiency | Source |
|---|---|---|---|---|
| 300–513 °C | 40 kW | 1190 ppm | 91.5% | [97] |
| 300–382 °C | 40 kW | 21 ppm | 95.2% | [97] |
| 300–440 °C | 40 kW | 3020 ppm | 99.5% | [97] |
| 300–418 °C | 22 kW | 634 ppm | 98.5% | [97] |
| 300–436 °C | 22 kW | 595 ppm | 98.3% | [97] |
| 600–900 °C | - | 0.5–1.1% | 90–100% | [98] |
| 1000–1200 °C | - | 0.3–0.8% | 95% | [99] |
| 400–700 °C | - | 3500 ppm | 90–100% | [102] |
4.3. Exhaust Gas Recirculation
5. Hydrogen Blending
6. Conclusions
7. Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Catalyst | Ratio | Density (g/m3) | CH4 (ppm) | O2 (%) | H2O (%) | SO2 (ppm) | Aged | T (°C) | Conversion (%) | Source |
|---|---|---|---|---|---|---|---|---|---|---|
| Pd | - | 10,400 | 2000 | 10.5 | 10 | - | No | 385 | 70 | [37] |
| Pd | - | 10,400 | 2000 | 10.5 | 10 | - | 1500 h of use | 385 | 10 | [37] |
| Pd | - | 51,800 | 2000 | 10.5 | 10 | - | No | 385 | 77 | [37] |
| Pd | - | 51,800 | 2000 | 10.5 | 10 | - | 1500 h of use | 385 | 25 | [37] |
| Pt | - | 9600 | 2000 | 10.5 | 10 | - | No | 385 | 35 | [37] |
| Pt | - | 9600 | 2000 | 10.5 | 10 | - | 1500 h of use | 385 | 25 | [37] |
| Pt:Pd | 1:1 | 20,800 | 2000 | 10.5 | 10 | - | No | 385 | 79 | [37] |
| Pt:Pd | 1:1 | 20,800 | 2000 | 10.5 | 10 | - | 1500 h of use | 385 | 60 | [37] |
| Pt:Pd | 2:3 | 27,900 | 2000 | 10.5 | 10 | - | No | 385 | 80 | [37] |
| Pt:Pd | 2:3 | 27,900 | 2000 | 10.5 | 10 | - | 1500 h of use | 385 | 55 | [37] |
| Pt:Pd | 1:5 | 12,000 | 7500 | NR | 4.89 | - | No | 325 | 100 | [38] |
| 300 | 75 | |||||||||
| 300 | 50 | |||||||||
| Pt:Pd | 3:41 | 4400 | 7500 | NR | 4.89 | - | No | 525 | 100 | [38] |
| 425 | 75 | |||||||||
| 400 | 50 | |||||||||
| Pt:Pd | 3:40 | 4300 | 7500 | NR | 4.89 | - | No | 350 | 100 | [38] |
| 325 | 75 | |||||||||
| 300 | 50 | |||||||||
| Pt:Pd | 10:47 | 5700 | 7500 | NR | 4.89 | - | No | 375 | 100 | [38] |
| 350 | 75 | |||||||||
| 325 | 50 | |||||||||
| Pd | - | 210 | 7500 | NR | 4.89 | - | No | N/A | 100 | [38] |
| 550 | 75 | |||||||||
| 500 | 50 | |||||||||
| Pd | - | 1400 | 7500 | NR | 4.89 | - | No | 450 | 100 | [38] |
| 400 | 75 | |||||||||
| 350 | 50 | |||||||||
| Pd | - | 5297 | 100,000 | 20 | - | - | No | 359 | 75 | [39] |
| 321 | 50 | |||||||||
| 282 | 25 | |||||||||
| Pd | - | 5297 | 100,000 | 20 | - | - | Hydrothermally | 426 | 75 | [39] |
| 394 | 50 | |||||||||
| 355 | 25 | |||||||||
| Pd | - | 5297 | 100,000 | 20 | 5 | - | Hydrothermally | 492 | 75 | [39] |
| 452 | 50 | |||||||||
| 421 | 25 | |||||||||
| Pt | - | 3355 | 100,000 | 20 | - | - | No | 582 | 75 | [39] |
| 541 | 50 | |||||||||
| 477 | 25 | |||||||||
| Pt | - | 3355 | 100,000 | 20 | - | - | Hydrothermally | 634 | 75 | [39] |
| 607 | 50 | |||||||||
| 556 | 25 | |||||||||
| Pt | - | 3355 | 100,000 | 20 | 5 | - | Hydrothermally | 632 | 75 | [39] |
| 604 | 50 | |||||||||
| 557 | 25 | |||||||||
| Pt:Pd | 4:10 | 3355 | 100,000 | 20 | - | - | No | 437 | 75 | [39] |
| 403 | 50 | |||||||||
| 360 | 25 | |||||||||
| Pt:Pd | 4:10 | 3355 | 100,000 | 20 | - | - | Hydrothermally | 471 | 75 | [39] |
| 433 | 50 | |||||||||
| 380 | 25 | |||||||||
| Pt:Pd | 4:10 | 3355 | 100,000 | 20 | 5 | - | Hydrothermally | 548 | 75 | [39] |
| 497 | 50 | |||||||||
| 455 | 25 | |||||||||
| Pd | - | 2260 | 3000 | 12 | 6 | - | No | 450 | 100 | [40] |
| 400 | 75 | |||||||||
| 392 | 50 | |||||||||
| Pd | - | 2260 | 3000 | 12 | 12 | - | No | 550 | 100 | [40] |
| 450 | 75 | |||||||||
| 448 | 50 | |||||||||
| Pd | - | 2260 | 3000 | 1.5 | 12 | - | No | N/A | 50 | [40] |
| Pt | - | 2295 | 3000 | 12 | 6 | - | No | N/A | 50 | [40] |
| Pt | - | 2295 | 3000 | 12 | 12 | - | No | N/A | 50 | [40] |
| Pt | - | 2295 | 3000 | 1.5 | 12 | - | No | 550 | 100 | [40] |
| 500 | 75 | |||||||||
| 500 | 50 | |||||||||
| Pt:Pd | 1:5 | 2437 | 3000 | 12 | 6 | - | No | 500 | 100 | [40] |
| 400 | 75 | |||||||||
| 393 | 50 | |||||||||
| Pt:Pd | 1:5 | 2437 | 3000 | 12 | 12 | - | No | 500 | 100 | [40] |
| 435 | 75 | |||||||||
| 427 | 50 | |||||||||
| Pt:Pd | 1:5 | 2437 | 3000 | 1.5 | 12 | - | No | N/A | 50 | [40] |
| Rh | - | 2 wt% | 2500 | 10 | - | - | No | 425 | 100 | [33] |
| 350 | 75 | |||||||||
| 325 | 50 | |||||||||
| Rh | - | 2 wt% | 2500 | 10 | 5 | - | No | 500 | 100 | [33] |
| 400 | 75 | |||||||||
| 387 | 50 | |||||||||
| Rh | - | 2 wt% | 2500 | 10 | - | 20 | No | 500 | 100 | [33] |
| 462 | 75 | |||||||||
| 437 | 50 | |||||||||
| Rh | - | 2 wt% | 2500 | 10 | 5 | 20 | No | 550 | 100 | [33] |
| 487 | 75 | |||||||||
| 475 | 50 | |||||||||
| Pd | - | 2 wt% | 2500 | 10 | - | - | No | 462 | 100 | [33] |
| 362 | 75 | |||||||||
| 350 | 50 | |||||||||
| Pd | - | 2 wt% | 2500 | 10 | 5 | - | No | 475 | 100 | [33] |
| 425 | 75 | |||||||||
| 412 | 50 | |||||||||
| Pd | - | 2 wt% | 2500 | 10 | - | 20 | No | 550 | 100 | [33] |
| 475 | 75 | |||||||||
| 425 | 50 | |||||||||
| Pd | - | 2 wt% | 2500 | 10 | 5 | 20 | No | N/A | 100 | [33] |
| 525 | 75 | |||||||||
| 512 | 50 | |||||||||
| Ni:Mg | 9:1 | 6,250,000 | 10,000 | 10 | 10 | - | No | 550 | 100 | [41] |
| 500 | 75 | |||||||||
| 480 | 50 | |||||||||
| Ni:Mg | 9:1 | 6,250,000 | 10,000 | 10 | 10 | - | 40 h of use | 550 | 100 | [41] |
| 540 | 75 | |||||||||
| 504 | 50 | |||||||||
| Pd | - | 1 wt% | 10,000 | 10 | 10 | - | No | 450 | 100 | [41] |
| 425 | 75 | |||||||||
| 396 | 50 | |||||||||
| Pd | - | 1 wt% | 10,000 | 10 | 10 | - | 40 h of use | 575 | 100 | [41] |
| 540 | 75 | |||||||||
| 507 | 50 |
References
- Overview of Greenhouse Gases. Available online: https://www.epa.gov/ghgemissions/overview-greenhouse-gases (accessed on 14 August 2023).
- U.S. Environmental Protection Agency. Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2020—Main Text. EPA 430-R-22-003. Available online: https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks-1990-2020 (accessed on 14 August 2023).
- Importance of Methane. Available online: https://www.epa.gov/gmi/importance-methane (accessed on 14 August 2023).
- Methane and Climate Change—Global Methane Tracker 2022—Analysis. Available online: https://www.iea.org/reports/global-methane-tracker-2022/methane-and-climate-change (accessed on 14 August 2023).
- Di Lullo, G.; Oni, A.O.; Kumar, A. Blending Blue Hydrogen with Natural Gas for Direct Consumption: Examining the Effect of Hydrogen Concentration on Transportation and Well-to-Combustion Greenhouse Gas Emissions. Int. J. Hydrogen Energy 2021, 46, 19202–19216. [Google Scholar] [CrossRef]
- Kahraman, N.; Çeper, B.; Akansu, S.O.; Aydin, K. Investigation of Combustion Characteristics and Emissions in a Spark-Ignition engine fuelled with natural gas–hydrogen blends. Int. J. Hydrogen Energy 2009, 34, 1026–1034. [Google Scholar] [CrossRef]
- Park, C.; Kim, C.; Choi, Y.; Won, S.; Moriyoshi, Y. The Influences of Hydrogen on the Performance and Emission Characteristics of a Heavy Duty Natural Gas Engine. Int. J. Hydrogen Energy 2011, 36, 3739–3745. [Google Scholar] [CrossRef]
- Katsampes, N.; Montgomery, D.; Arney, G.; Olsen, D.B. Hydrogen-Natural Gas Fuel Blending in a Caterpillar CG137-8 “Rich Burn” Engine with 3-Way Catalyst. In Proceedings of the 13th U.S. National Combustion Meeting Organized by the Central States Section of the Combustion Institute, College Station, TX, USA, 19–22 March 2023. [Google Scholar]
- Akansu, S.O.; Kahraman, N.; Çeper, B. Experimental Study on a Spark Ignition Engine Fuelled by Methane–Hydrogen Mixtures. Int. J. Hydrogen Energy 2007, 32, 4279–4284. [Google Scholar] [CrossRef]
- Ma, F.; Wang, Y.; Liu, H.; Li, Y.; Wang, J.; Zhao, S. Experimental Study on Thermal Efficiency and Emission Characteristics of a Lean Burn Hydrogen Enriched Natural Gas Engine. Int. J. Hydrogen Energy 2007, 32, 5067–5075. [Google Scholar] [CrossRef]
- Overview of Natural Gas: Background. Available online: http://naturalgas.org/overview/background/ (accessed on 29 September 2023).
- Natural Gas Explained. Available online: https://www.eia.gov/energyexplained/natural-gas/use-of-natural-gas.php#:~:text=Electricity%20generation%20and%20heating%20are,other%20uses%20for%20natural%20gas (accessed on 29 September 2023).
- August 2023 Form EIA-860M. Available online: https://www.eia.gov/electricity/data/eia860m/ (accessed on 29 September 2023).
- About, U.S. Natural Gas Pipelines—Transporting Natural Gas. Available online: https://www.eia.gov/naturalgas/archive/analysis_publications/ngpipeline/index.html (accessed on 29 September 2023).
- CIMAC WG17|Methane and Formaldehyde Emissions of Gas Engines. Available online: https://www.cimac.com/publications/publications350/cimac-wg17-methane-and-formaldehyde-emissions-of-gas-engines.html (accessed on 15 August 2023).
- Vaughn, T.L.; Luck, B.; Williams, L.; Marchese, A.J.; Zimmerle, D. Methane Exhaust Measurements at Gathering Compressor Stations in the United States. Environ. Sci. Technol. 2021, 55, 1190–1196. [Google Scholar] [CrossRef]
- Learn about the Greenhouse Gas Reporting Program (GHGRP)|Greenhouse Gas Reporting Program (GHGRP)|US EPA. Available online: https://climatechange.chicago.gov/ghgreporting/learn-about-greenhouse-gas-reporting-program-ghgrp#:~:text=The%20GHGRP%20(codified%20at%2040,sites%20in%20the%20United%20States (accessed on 14 August 2023).
- Subpart W—Petroleum and Natural Gas Systems. Available online: https://www.epa.gov/ghgreporting/subpart-w-petroleum-and-natural-gas-systems (accessed on 14 August 2023).
- Methane Emissions Reduction Program. Available online: https://www.epa.gov/inflation-reduction-act/methane-emissions-reduction-program (accessed on 14 August 2023).
- EPA Proposes to Strengthen Air Quality Standards to Protect the Public from Harmful Effects of Soot. Available online: https://www.epa.gov/newsreleases/epa-proposes-strengthen-air-quality-standards-protect-public-harmful-effects-soot (accessed on 14 August 2023).
- Subpart JJJJ—Standards of Performance for Stationary Spark Ignition Internal Combustion Engines. Available online: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-C/part-60/subpart-JJJJ (accessed on 29 September 2023).
- What Is the Definition of VOC? Available online: https://www.epa.gov/air-emissions-inventories/what-definition-voc (accessed on 29 September 2023).
- Lindblom, J.; Su, W. Methane Slip Prevention in the Combustion Chamber. Bachelor’s Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2021. [Google Scholar]
- Jensen, M.V.; Cordtz, R.F.; Schramm, J. Numerical Analysis of Methane Slip Source Distribution in a Four-Stroke Dual-Fuel Marine Engine. J. Mar. Sci. Technol. 2021, 26, 606–617. [Google Scholar] [CrossRef]
- Castillo, A.Q.; Zdanowicz, A.; Windom, B.; Olsen, D. Characterization of Crankcase Ventilation Gas on Stationary Natural Gas Engines. In Proceedings of the 13th U.S. National Combustion Meeting Organized by the Central States Section of the Combustion Institute, College Station, TX, USA, 19–22 March 2023. [Google Scholar]
- Hiltner, J. Unburned Hydrocarbon Emissions from Lean Burn Natural Gas Engines; Sources and Solutions; CIMAC Congress: Helsinki, Finland, 2016. [Google Scholar]
- Innovative Environmental Solutions, Inc. Optimized Technical Solutions. Availability and Limitations of NOx Emission Control Resources for Natural Gas-Fired Reciprocating Engine Prime Movers Used in the Interstate Natural Gas Transmission Industry; The INGAA Foundation, Inc.: Washington, DC, USA, 2014. [Google Scholar]
- Hampson, G.J. High Efficiency Natural Gas Engine Combustion Using Controlled Auto-Ignition. In Proceedings of the ASME 2019 Internal Combustion Engine Division Fall Technical Conference; American Society of Mechanical Engineers, Chicago, IL, USA, 20 October 2019; p. V001T03A019. [Google Scholar]
- Hopoko, M.S. Test Report on Exhaust Emissions from One Solar Mars T-1200 Gas Turbine, Four Cooper Bessemer GMVA-10 Engines, Eight Copper Messemer GMV-10 Engines, and Five Ingersol Rand KVS-12 Engines at the Holbrook Compressor Station; Division of Technical Services and Monitoring Bureau of Air Quality Control: Houston, TX, USA, 1991. [Google Scholar]
- Olsen, D.B.; Willson, B.D. The Effect of Retrofit Technologies on Formaldehyde Emissions from a Large Bore Natural Gas Engine. Energy Power Eng. 2011, 03, 574–579. [Google Scholar] [CrossRef][Green Version]
- Vieira, G.; Beurlot, K.; Xie, N.; Patterson, M.; Olsen, D. Precombustion Chamber Nozzle Design Effect on Unburned Me-thane Emissions of a Large Bore Two-Stroke Lean- Burn Natural Gas Engine. In Proceedings of the 13th U.S. National Combustion Meeting Organized by the Central States Section of the Combustion Institute, College Station, TX, USA, 19–22 March 2023. [Google Scholar]
- Forzatti, P.; Lietti, L. Catalyst deactivation. Catal. Today 1999, 52, 165–181. [Google Scholar] [CrossRef]
- Gélin, P.; Primet, M. Complete Oxidation of Methane at Low Temperature over Noble Metal Based Catalysts: A Review. Appl. Catal. B Environ. 2002, 39, 1–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Glarborg, P.; Johansen, K.; Andersson, M.P.; Torp, T.K.; Jensen, A.D.; Christensen, J.M. A Rhodium-Based Methane Oxidation Catalyst with High Tolerance to H2O and SO2. ACS Catal. 2020, 10, 1821–1827. [Google Scholar] [CrossRef]
- Olsen, D.; Hull, W.; Ginger, P.; Olson, C.; Willson, B. Humidity Control for Power and Emissions; Gas Research Institute: Des Plaines, IL, USA, 2003. [Google Scholar]
- Defoort, M.; Olsen, D.; Willson, B. Performance Evaluation of Oxidation Catalysts for Natural Gas Reciprocating Engines. In Proceedings of the GMRC Gas Machinery Conference, Nashville, TN, USA, 7–9 October 2002. [Google Scholar]
- Yamamoto, H.; Uchida, H. Oxidation of Methane over Pt and Pd Supported on Alumina in Lean-Burn Natural-Gas Engine Exhaust. Catal. Today 1998, 45, 147–151. [Google Scholar] [CrossRef]
- Worth, D.J.; Stettler, M.E.J.; Dickinson, P.; Hegarty, K.; Boies, A.M. Characterization and Evaluation of Methane Oxidation Catalysts for Dual-Fuel Diesel and Natural Gas Engines. Emiss. Control. Sci. Technol. 2016, 2, 204–214. [Google Scholar] [CrossRef]
- Abbasi, R.; Huang, G.; Istratescu, G.M.; Wu, L.; Hayes, R.E. Methane Oxidation over Pt, Pt:Pd, and Pd Based Catalysts: Effects of Pre-Treatment. Can. J. Chem. Eng. 2015, 93, 1474–1482. [Google Scholar] [CrossRef]
- Gremminger, A.; Pihl, J.; Casapu, M.; Grunwaldt, J.-D.; Toops, T.J.; Deutschmann, O. PGM Based Catalysts for Exhaust-Gas after-Treatment under Typical Diesel, Gasoline and Gas Engine Conditions with Focus on Methane and Formaldehyde Oxidation. Appl. Catal. B Environ. 2020, 265, 118571. [Google Scholar] [CrossRef]
- Caravaggio, G.; Nossova, L.; Turnbull, M.J. Nickel-Magnesium Mixed Oxide Catalyst for Low Temperature Methane Oxida-tion. Chem. Eng. J. 2021, 405, 126862. [Google Scholar] [CrossRef]
- Gélin, P.; Urfels, L.; Primet, M.; Tena, E. Complete Oxidation of Methane at Low Temperature over Pt and Pd Catalysts for the Abatement of Lean-Burn Natural Gas Fuelled Vehicles Emissions: Influence of Water and Sulphur Containing Compounds. Catal. Today 2003, 83, 45–57. [Google Scholar] [CrossRef]
- Nilsson, J.; Carlsson, P.-A.; Martin, N.M.; Adams, E.C.; Agostini, G.; Grönbeck, H.; Skoglundh, M. Methane oxidation over Pd/Al2O3 under rich/lean cycling followed by operando XAFS and modulation excitation spectroscopy. J. Catal. 2017, 356, 237–245. [Google Scholar] [CrossRef]
- Farrauto, R.; Hobson, M.; Kennelly, T.; Waterman, E. Catalytic chemistry of supported palladium for combustion of methane. Appl. Catal. A Gen. 1992, 81, 227–237. [Google Scholar] [CrossRef]
- Fujimoto, K.-I.; Ribeiro, F.H.; Avalos-Borja, M.; Iglesia, E. Structure and Reactivity of PdOx/ZrO2Catalysts for Methane Oxidation at Low Temperatures. J. Catal. 1998, 179, 431–442. [Google Scholar] [CrossRef]
- Kinnunen, N.M.; Hirvi, J.T.; Venäläinen, T.; Suvanto, M.; Pakkanen, T.A. Procedure to Tailor Activity of Methane Combustion Catalyst: Relation between Pd/PdOx Active Sites and Methane Oxidation Activity. Appl. Catal. A Gen. 2011, 397, 54–61. [Google Scholar] [CrossRef]
- Miller, J.B.; Malatpure, M. Pd Catalysts for Total Oxidation of Methane: Support effects. Appl. Catal. A Gen. 2015, 495, 54–62. [Google Scholar] [CrossRef]
- Narui, K.; Yata, H.; Furuta, K.; Nishida, A.; Kohtoku, Y.; Matsuzaki, T. Effects of Addition of Pt to PdO/Al2O3 Catalyst on Catalytic Activity for Methane Combustion and TEM Observations of Supported Particles. Appl. Catal. A Gen. 1999, 179, 165–173. [Google Scholar] [CrossRef]
- Strobel, R.; Grunwaldt, J.-D.; Camenzind, A.; Pratsinis, S.E.; Baiker, A. Flame-made Alumina Supported Pd–Pt Nanoparticles: Structural Properties and Catalytic Behavior in Methane Combustion. Catal. Lett. 2005, 104, 9–16. [Google Scholar] [CrossRef]
- Lapisardi, G.; Gélin, P.; Kaddouri, A.; Garbowski, E.; Da Costa, S. Pt–Pd bimetallic catalysts for methane emissions abatement. Top. Catal. 2007, 42–43, 461–464. [Google Scholar] [CrossRef]
- Kinnunen, N.M.; Hirvi, J.T.; Suvanto, M.; Pakkanen, T.A. Methane combustion activity of Pd–PdOx–Pt/Al2O3 catalyst: The role of platinum promoter. J. Mol. Catal. A Chem. 2012, 356, 20–28. [Google Scholar] [CrossRef]
- Chin, Y.-H.; Buda, C.; Neurock, M.; Iglesia, E. Consequences of Metal–Oxide Interconversion for C–H Bond Activation during CH4 Reactions on Pd Catalysts. J. Am. Chem. Soc. 2013, 135, 15425–15442. [Google Scholar] [CrossRef]
- Lapisardi, G.; Urfels, L.; Gélin, P.; Primet, M.; Kaddouri, A.; Garbowski, E.; Toppi, S.; Tena, E. Superior catalytic behaviour of Pt-doped Pd catalysts in the complete oxidation of methane at low temperature. Catal. Today 2006, 117, 564–568. [Google Scholar] [CrossRef]
- Burch, R.; Urbano, F.; Loader, P. Methane combustion over palladium catalysts: The effect of carbon dioxide and water on activity. Appl. Catal. A Gen. 1995, 123, 173–184. [Google Scholar] [CrossRef]
- Gholami, R.; Alyani, M.; Smith, K.J. Deactivation of Pd Catalysts by Water during Low Temperature Methane Oxidation Relevant to Natural Gas Vehicle Converters. Catalysts 2015, 5, 561–594. [Google Scholar] [CrossRef]
- Schwartz, W.R.; Ciuparu, D.; Pfefferle, L.D. Combustion of Methane over Palladium-Based Catalysts: Catalytic Deactivation and Role of the Support. J. Phys. Chem. C 2012, 116, 8587–8593. [Google Scholar] [CrossRef]
- Persson, K.; Pfefferle, L.D.; Schwartz, W.; Ersson, A.; Järås, S.G. Stability of Palladium-Based Catalysts during Catalytic Combustion of Methane: The Influence of Water. Appl. Catal. B Environ. 2007, 74, 242–250. [Google Scholar] [CrossRef]
- Hurtado, P.; Ordóñez, S.; Sastre, H.; Dıez, F.V. Combustion of Methane over Palladium Catalyst in the Presence of Inorganic Compounds: Inhibition and Deactivation Phenomena. Appl. Catal. B Environ. 2004, 47, 85–93. [Google Scholar] [CrossRef]
- Sadokhina, N.; Smedler, G.; Nylén, U.; Olofsson, M. The Influence of Gas Composition on Pd-Based Catalyst Activity in Methane Oxidation—Inhibition and Promotion by NO. Appl. Catal. B Environ. 2017, 200, 351–360. [Google Scholar] [CrossRef]
- Lampert, J.K.; Kazi, M.S.; Farrauto, R.J. Palladium Catalyst Performance for Methane Emissions Abatement from Lean Burn Natural Gas Vehicles. Appl. Catal. B Environ. 1997, 14, 211–223. [Google Scholar] [CrossRef]
- Monai, M.; Montini, T.; Melchionna, M.; Duchoň, T.; Kúš, P.; Tsud, N.; Prince, K.C.; Matolin, V.; Gorte, R.J.; Fornasiero, P. Phosphorus Poisoning during Wet Oxidation of Methane over Pd@CeO2/Graphite Model Catalysts. Appl. Catal. B Environ. 2016, 197, 271–279. [Google Scholar] [CrossRef]
- Kang, S.B.; Hazlett, M.; Balakotaiah, V.; Kalamaras, C.; Epling, W. Effect of Pt:Pd ratio on CO and hydrocarbon oxidation. Appl. Catal. B Environ. 2018, 223, 67–75. [Google Scholar] [CrossRef]
- Maillet, T.; Solleau, C.; Jacques, B., Jr.; Duprez, D. Oxidation of Carbon Monoxide, Propene, Propane and Methane over a Pd/A1203 Catalyst. Effect of the Chemical State of Pd. Appl. Catal. B Environ 1997, 14, 85–95. [Google Scholar] [CrossRef]
- Ribeiro, F.H.; Chow, M.; Dalla Betta, R.A. Kinetics of the Complete Oxidation of Methane over Supported Palladium Catalysts. J. Catal. 1994, 146, 537–544. [Google Scholar] [CrossRef]
- Sadokhina, N.; Ghasempour, F.; Auvray, X.; Smedler, G.; Nylén, U.; Olofsson, M.; Olsson, L. An Experimental and Kinetic Modelling Study for Methane Oxidation over Pd-based Catalyst: Inhibition by Water. Catal. Lett. 2017, 147, 2360–2371. [Google Scholar] [CrossRef]
- Monai, M.; Montini, T.; Melchionna, M.; Duchoň, T.; Kúš, P.; Chen, C.; Tsud, N.; Nasi, L.; Prince, K.C.; Veltruská, K.; et al. The Effect of Sulfur Dioxide on the Activity of hieraRCHical Pd-Based Catalysts in Methane Combustion. Appl. Catal. B Environ. 2017, 202, 72–83. [Google Scholar] [CrossRef]
- Sadokhina, N.; Smedler, G.; Nylén, U.; Olofsson, M.; Olsson, L. Deceleration of SO2 poisoning on PtPd/Al2O3 catalyst during complete methane oxidation. Appl. Catal. B Environ. 2018, 236, 384–395. [Google Scholar] [CrossRef]
- Lou, Y.; Ma, J.; Hu, W.; Dai, Q.; Wang, L.; Zhan, W.; Guo, Y.; Cao, X.; Hu, P.; Lo, G. Low-Temperature Methane Combustion over Pd/H-ZSM-5: Active Pd Sites with Specific Electronic Properties Modulated by Acidic Sites of H-ZSM-5. ACS Catal. 2021, 6, 8127–8139. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K.; Laffir, F.; Curtin, T.; Thompson, J.M.; Rooney, D.W. A Bimetallic Catalyst on a Dual Component Support for Low Temperature Total Methane Oxidation. Appl. Catal. B Environ. 2016, 187, 408–418. [Google Scholar] [CrossRef]
- Petrov, A.W.; Ferri, D.; Tarik, M.; Kröcher, O.; van Bokhoven, J.A. Deactivation Aspects of Methane Oxidation Catalysts Based on Palladium and ZSM-5. Top. Catal. 2017, 60, 123–130. [Google Scholar] [CrossRef]
- Petrov, A.W.; Ferri, D.; Kröcher, O.; van Bokhoven, J.A. Design of Stable Palladium-Based Zeolite Catalysts for Complete Methane Oxidation by Postsynthesis Zeolite Modification. ACS Catal. 2019, 9, 2303–2312. [Google Scholar] [CrossRef]
- Losch, P.; Huang, W.; Vozniuk, O.; Goodman, E.D.; Schmidt, W.; Cargnello, M. Modular Pd/Zeolite Composites Demonstrating the Key Role of Support Hydrophobic/Hydrophilic Character in Methane Catalytic Combustion. ACS Catal. 2019, 9, 4742–4753. [Google Scholar] [CrossRef]
- Venezia, A.; Di Carlo, G.; Liotta, L.; Pantaleo, G.; Kantcheva, M. Effect of Ti(IV) Loading on CH4 Oxidation Activity and SO2 tolerance of Pd Catalysts Supported on Silica SBA-15 and HMS. Appl. Catal. B Environ. 2011, 106, 529–539. [Google Scholar] [CrossRef]
- Jones, J.; Dupont, V.; Brydson, R.; Fullerton, D.; Nasri, N.; Ross, A.; Westwood, A. Sulphur Poisoning and Regeneration of Precious Metal Catalysed Methane Combustion. Catal. Today 2003, 81, 589–601. [Google Scholar] [CrossRef]
- Muto, K.-I.; Katada, N.; Niwa, M. Complete Oxidation of Methane on Supported Palladium Catalyst: Support Effect. Appl. Catal. A Gen. 1996, 134, 203–215. [Google Scholar] [CrossRef]
- Araya, P.; Guerrero, S.; Robertson, J.; Gracia, F. Methane Combustion over Pd/SiO2 Catalysts with Different Degrees of hydrophobicity. Appl. Catal. A Gen. 2005, 283, 225–233. [Google Scholar] [CrossRef]
- Deng, Y.; Nevell, T.G.; Ewen, R.J.; Honeybourne, C.L. Sulfur Poisoning, Recovery and Related Phenomena over Supported Palladium, Rhodium and Iridium Catalysts for Methane Oxidation. Appl. Catal. A Gen. 1993, 101, 51–62. [Google Scholar] [CrossRef]
- Hayes, R.E.; Kolaczkowski, S.T.; Li, P.K.C.; Awdry, S. The Palladium Catalysed Oxidation of Methane: Reaction Kinetics and the e Ect of Di Usion Barriers. Chem. Eng. Sci. 2001, 56, 4815–4835. [Google Scholar] [CrossRef]
- Becker, E.; Carlsson, P.-A.; Grönbeck, H.; Skoglundh, M. Methane Oxidation over Alumina Supported Platinum Investigated by Time-Resolved in Situ XANES Spectroscopy. J. Catal. 2007, 252, 11–17. [Google Scholar] [CrossRef]
- Amin, A.; Abedi, A.; Hayes, R.; Votsmeier, M.; Epling, W. Methane oxidation hysteresis over Pt/Al2O3. Appl. Catal. A Gen. 2014, 478, 91–97. [Google Scholar] [CrossRef]
- Abbasi, R.; Wu, L.; Wanke, S.; Hayes, R. Kinetics of methane combustion over Pt and Pt–Pd catalysts. Chem. Eng. Res. Des. 2012, 90, 1930–1942. [Google Scholar] [CrossRef]
- Burch, R.; Loader, P.K. Investigation of Pt/Al2O3 and Pd/Al2O3 catalysts for the combustion of methane at low concentrations. Appl. Catal. B Environ. 1994, 5, 149–164. [Google Scholar] [CrossRef]
- Bugosh, G.S.; Easterling, V.G.; Rusakova, I.A.; Harold, M.P. Anomalous Steady-State and Spatio-Temporal Features of Methane Oxidation on Pt/Pd/Al2O3 Monolith Spanning Lean and Rich Conditions. Appl. Catal. B Environ. 2015, 165, 68–78. [Google Scholar] [CrossRef]
- Gremminger, A.T.; de Carvalho, H.W.P.; Popescu, R.; Grunwaldt, J.-D.; Deutschmann, O. Influence of Gas Composition on Activity and Durability of Bimetallic Pd-Pt/Al2O3 Catalysts for Total Oxidation of Methane. Catal. Today 2015, 258, 470–480. [Google Scholar] [CrossRef]
- Daily Metal Price: Copper Price Chart (USD/Pound) for the Last Month. Available online: https://www.dailymetalprice.com/metalpricecharts.php (accessed on 15 August 2023).
- Liu, F.; Sang, Y.; Ma, H.; Li, Z.; Gao, Z. Nickel Oxide as an Effective Catalyst for Catalytic Combustion of Methane. J. Nat. Gas Sci. Eng. 2017, 41, 1–6. [Google Scholar] [CrossRef]
- Ordóñez, S.; Paredes, J.R.; Díez, F.V. Sulphur Poisoning of Transition Metal Oxides Used as Catalysts for Methane Combustion. Appl. Catal. A Gen. 2008, 341, 174–180. [Google Scholar] [CrossRef]
- González-Cortés, S.L.; Aray, I.; Rodulfo-Baechler, S.M.A.; Lugo, C.A.; Del Castillo, H.L.; Loaiza-Gil, A.; Imbert, F.E.; Figueroa, H.; Pernía, W.; Rodríguez, A.; et al. On the Structure and Surface Properties of NiO/MgO–La2O3 Catalyst: Influence of the Support Composition and Preparation Method. J. Mater. Sci. 2007, 42, 6532–6540. [Google Scholar] [CrossRef]
- Pakulska, M.M.; Grgicak, C.M.; Giorgi, J.B. The Effect of Metal and Support Particle Size on NiO/CeO2 and NiO/ZrO2 Catalyst Activity in Complete Methane Oxidation. Appl. Catal. A Gen. 2007, 332, 124–129. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Hu, Y.H. Methane Partial Oxidation over NiO/MgO Solid Solution Catalysts. Appl. Catal. A Gen. 1999, 183, 85–92. [Google Scholar] [CrossRef]
- 99/00356 Partial Oxidation of Methane to Syngas over Ni/ MgO, NI/CaO and NI/CeO2. Fuel Energy Abstr. 1999, 40, 35. [CrossRef]
- Hu, Y.H.; Ruckenstein, E. The Characterization of a Highly Effective NiO /MgO Solid Solution Catalyst in the CO2 Reforming of CH4. Cata. Lett. 1997, 43, 71–77. [Google Scholar] [CrossRef]
- Saanum, I.; Bysveen, M.; Tunestål, P.; Johansson, B. Lean Burn versus Stoichiometric Operation with EGR and 3-Way Catalyst of an Engine Fueled with Natural Gas and Hydrogen Enriched Natural Gas; Lund University: Lund, Sweden, 2007; p. 2007-01-0015. [Google Scholar]
- Matam, S.K.; Chiarello, G.L.; Lu, Y.; Weidenkaff, A.; Ferri, D. PdO x /Pd at Work in a Model Three-Way Catalyst for Methane Abatement Monitored by Operando XANES. Top. Catal. 2013, 56, 239–242. [Google Scholar] [CrossRef]
- Prabhu, E.; Prabhu, M.; Fox, J.E. The Oxiperator for Ventilation Air Methane (VAM). In Underground Ventilation; CRC Press: London, UK, 2023; pp. 462–466. ISBN 978-1-00-342924-1. [Google Scholar]
- Amelio, M.; Morrone, P. Numerical Evaluation of the Energetic Performances of Structured and Random Packed Beds in Regenerative Thermal Oxidizers. Appl. Therm. Eng. 2007, 27, 762–770. [Google Scholar] [CrossRef]
- Cheng, W.-H.; Chou, M.-S.; Lee, W.-S.; Huang, B.-J. Applications of Low-Temperature Regenerative Thermal Oxidizers to Treat Volatile Organic Compounds. J. Environ. Eng. 2002, 128, 313–319. [Google Scholar] [CrossRef]
- Li, Q.; Lin, B.; Yuan, D.; Chen, G. Demonstration and Its Validation for Ventilation Air Methane (VAM) Thermal Oxidation and Energy Recovery Project. Appl. Therm. Eng. 2015, 90, 75–85. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Y.; Gao, Z. Methane Emission Control by Thermal Oxidation in a Reverse Flow Reactor. In Proceedings of the 2008 2nd International Conference on Bioinformatics and Biomedical Engineering, Shanghai, China, 18 May 2008; pp. 3952–3955. [Google Scholar]
- Marín, P.; Díez, F.V.; Ordóñez, S. Reverse Flow Reactors as Sustainable Devices for Performing Exothermic Reactions: Applications and Engineering Aspects. Chem. Eng. Process.-Process. Intensif. 2018, 135, 175–189. [Google Scholar] [CrossRef]
- Liu, B.; Hayes, R.; Checkel, M.; Zheng, M.; Mirosh, E. Reversing Flow Catalytic Converter For A Natural Gas/Diesel Dual Fuel Engine. Chem. Eng. Sci. 2001, 56, 2641–2658. [Google Scholar] [CrossRef]
- Marín, P.; Hevia, M.A.; Ordóñez, S.; Díez, F.V. Combustion of Methane Lean Mixtures in Reverse Flow Reactors: Comparison between Packed and Structured Catalyst Beds. Catal. Today 2005, 105, 701–708. [Google Scholar] [CrossRef]
- Bayliff, S.; Marchese, A.; Windom, B.; Olsen, D. The Effect of EGR on Knock Suppression, Efficiency, and Emissions in a Stoichiometric, Spark Ignited, Natural Gas Engine. In Proceedings of the 2019 WSSCI Fall Technical Meeting Organized by the Western States Section of the Combustion Institute, Albuquerque, NM, USA, 14–15 October 2019. [Google Scholar]
- Qu, J.; Feng, Y.; Xu, G.; Zhang, M.; Zhu, Y.; Zhou, S. Design and Thermodynamics Analysis of Marine Dual Fuel Low Speed Engine with Methane Reforming Integrated High Pressure Exhaust Gas Recirculation System. Fuel 2022, 319, 123747. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huonder, A.; Olsen, D. Methane Emission Reduction Technologies for Natural Gas Engines: A Review. Energies 2023, 16, 7054. https://doi.org/10.3390/en16207054
Huonder A, Olsen D. Methane Emission Reduction Technologies for Natural Gas Engines: A Review. Energies. 2023; 16(20):7054. https://doi.org/10.3390/en16207054
Chicago/Turabian StyleHuonder, Andrew, and Daniel Olsen. 2023. "Methane Emission Reduction Technologies for Natural Gas Engines: A Review" Energies 16, no. 20: 7054. https://doi.org/10.3390/en16207054
APA StyleHuonder, A., & Olsen, D. (2023). Methane Emission Reduction Technologies for Natural Gas Engines: A Review. Energies, 16(20), 7054. https://doi.org/10.3390/en16207054





