Abstract
This paper presents findings from an experimental study investigating the secondary atomization of liquid fuel droplets widely used in the heat and power industry exemplified by fuel oil and environmentally promising fuel oil/water emulsion. The scientific novelty comes from the comparative analysis of the critical conditions and integral characteristics of the secondary atomization of the liquid and composite fuels with the greatest potential for power plants. Here, we used two fuel atomization schemes: droplet–droplet collisions in a gas and droplets impinging on a heated solid wall. The temperature of the liquids under study was 80 °C. The velocities before collision ranged from 0.1 m/s to 7 m/s, while the initial droplet sizes varied from 0.3 mm to 2.7 mm. A copper substrate served as a solid wall; its temperature was varied from 20 °C to 300 °C. The main characteristics of droplet interaction were recorded by a high-speed camera. Regime maps were constructed using the experimental findings. It was established that the critical Weber number was several times lower when water and fuel oil droplets collided than during the collision of fuel oil droplets with 10 vol% of water. The secondary atomization of fuel oil/water emulsion droplets by their impingement on a heated solid wall was found to reduce the typical sizes of liquid fragments by a factor of 40–50. As shown in the paper, even highly viscous fuels can be effectively sprayed using primary and secondary droplet atomization schemes. It was established that the optimal temperature of the fuel oil to be supplied to the droplet collision zone is 80 °C, while the optimal substrate temperature for the atomization of fuel oil/water emulsion droplets approximates 300 °C.
1. Introduction
The coagulation, crushing, and fragmentation of liquid droplets during their interaction with other droplets, solid surfaces, and particles occur in many technological processes. For example, demulsification and dehydration of the oil/water mixture is accompanied by coalescence and breakup [1,2]. Particles and drops can collide under the action of thermophoretic and electrophoretic forces in fluid filtration systems for sulfur recovery [3]. An important role is played by the interaction between drops and particles in spray systems for cooling surfaces [4,5]. Minimizing the contact time of droplets and a solid surface plays a decisive role in preventing icing [6]. Droplets of spray-drying agents are deposited after collision with solid particles (the most widely used method of encapsulation) [7]. The deposition of thin multilayer films on solid surfaces of various shapes is used in the production of sensors, integrated optics [8], polymer electronic devices [9], and drug delivery systems [10]. Modern technologies of primary liquid fuel atomization are based on spray nozzles and sprinklers [11,12]. It is necessary to manage the disruption of liquid fuel jets and droplet flows, as well as obtain the necessary dependences of the jet angle and droplet size distribution on the geometry of spray nozzles [13]. That requires experimental and theoretical research findings, which have been extremely scarce so far. Therefore, secondary atomization inside the furnace becomes a promising technology [14,15].
The secondary atomization of liquid droplets is used in various applications, such as fuel spraying in boiler furnaces [16,17,18], cooling systems [18,19], and heat and mass transfer equipment [20,21]. Thus, a reliable study of secondary droplet atomization is crucial to the development of effective spraying technologies. It is necessary to analyze the global scientific advances in studying these processes and to find promising niches for further research. In [22], Bachalo generalized the research findings on the formation of sprays and further behavior of the newly generated two-phase turbulent flow. Arguments are presented regarding the need to integrate experimentation and modeling [22]. Lefebvre and McDonell discuss many atomizer types, their applications, and spray properties [23]. Secondary droplet atomization involves various principles and approaches. The most typical ones are as follows: droplet breakup due to the impingement on a solid surface (also known as wall impact) [24], disruption of droplets due to their collision with each other [25,26], puffing and micro-explosive breakup from droplet superheating to the water boiling point [27,28], and droplet disruption by an air flow (gas jet) [29,30]. The wide use of secondary atomization schemes for liquid fuel droplets in industrial heat and power systems may reduce the environmental impact and economic costs of combustion as well as improve its energy efficiency.
Fuel droplets often collide with walls in internal combustion engines and combustion chambers [31]. In [32], Buck et al. experimentally studied liquid droplet collisions with dry and wet walls using the coefficient of restitution (CoR). The CoR characterizes the energy dissipation during a collision. It is defined as the ratio of the droplet rebound velocity to its impact velocity. The CoR is found to decrease with an increase in the thickness of a liquid layer on the wall and the viscosity of that liquid. The analysis of the research findings in [32] indicates that the authors did not outline the conditions for the occurrence of different droplet collision regimes. This is especially important for heat and power equipment, because the disruption regime can provide the largest evaporation surface area of the resulting fragments. The hydrodynamics of a water droplet impinging on superheated solid horizontal surfaces were studied by Negeed et al. [33]. The authors obtained the empirical correlations between the hydrodynamic characteristics of a droplet impinging on a heated surface, and the Reynolds number and Weber number, as well as other parameters.
Orme [34] summarized the known data on the collision behavior of fuel droplets in gases with the following outcomes: bounce (BO), coalescence (CO), separation (SE), and disruption (DI). An attempt was made to relate the existing findings to a unified description of droplet behavior at the moment of collision and during their further motion. Orme hypothesized that the discrepancies in the critical We numbers between different quantitative studies [34] were caused by the effects occurring on the surface of droplets and by interfacial conditions. The presence of surface contaminants shown in [35,36,37], such as surfactants, can significantly affect the droplet collision process. This statement was corroborated in [38], where Ashgriz and Givi explored the collisions of burning fuel droplets in the Weber number range from 1 to 40. The experimentally obtained critical Weber number was 5–7 for different conditions. The collision behavior of such droplets differs significantly from the existing data on the interaction between common liquid droplets. The several liquid aerosol flows are often intermixed at different angles by intersecting the nozzle spray cones in order to intensify droplet collisions, i.e., secondary atomization. In this case, the rate of droplet disruption intensity depends heavily on the Weber number and the impact angle, as well as the viscosity, density, and surface tension of the liquid. Here, researchers focus on providing an increase in the number of small fragments produced from the collisions of initial drops, as well as on the impact of the above parameters [39,40]. Solomatin et al. [41] explored the collisions between droplets of promising multi-component fuels and determined the conditions for the stable occurrence of one of the four interaction regimes. The ranges of key parameters providing active droplet disruption were determined in the dimensional and dimensionless coordinate systems. No droplet atomization regime maps based on Weber, Reynolds, Ohnesorge, and capillary numbers have yet been constructed for promising emulsified fuels to predict the number of secondary droplets formed under different thermal conditions.
The aim of this research was to investigate the secondary atomization of fuel oil droplets and droplets of a promising fuel oil/water emulsion colliding with each other in a gas and impinging on a solid wall; to construct collision regime maps for fuel oil droplets colliding with each other based on Weber, Reynolds, Ohnesorge, and capillary numbers; and to determine the free surface area before and after the collision of initial droplets with each other and with a substrate. This is the first comparative analysis of the critical conditions and integral characteristics of secondary atomization for droplets of liquid and composite fuels that have the greatest potential for use in power plants. Scientifically, it is important to determine how small the collision-produced droplets are and how this correlates with a set of critical factors and parameters. This was the motivation for the present work.
2. Materials and Methods
2.1. Type of Fuel
Boilers of thermal power stations and heating plants fired by liquid fuel normally use fuel oil [42,43]. Fuel oil is known to have certain advantages over other fuel types: high calorific value (9500 kcal/kg) and low ash content (0.3–0.5%). It also has some drawbacks: high content of sulfur (about 3.5%), high pour point (25–30 °C), and high viscosity (µ ≈ 0.013 Pa·s at T ≈ 80 °C). In addition, spraying of this fuel poses a number of challenges, in particular, high pressure in the fuel supply system (7–35 bar), large droplet size (0.1–0.6 mm) significantly affecting the ignition delay, high jet velocities (80–300 m/s), and small jet angle (42–56°). To overcome these drawbacks, research teams all over the world focus on the secondary atomization of liquid and composite fuel droplets.
Fuel oil is promising for the power industry and maritime transport due to its low cost and high heat of combustion. Fuel oil is a highly viscous fuel that needs to be heated to 60–90 °C before use [44], which leads to a 2–6 times reduction in viscosity depending on the fuel rank and temperature. One of the main problems with fuel oil is its water absorption during storage and transportation [45,46]. In particular, as-received fuel oil contains up to 1.5% of water (GOST 10585-2013). During maritime and river transportation, water content in fuel oil increases to 3–5%. In this research, we studied the conditions of water absorption by fuel oil upon direct steam heating. Fuel oil is heated by injecting fresh steam into it using pipes or metal-reinforced hoses. This leads to significant steam leaks and water absorption by fuel oil. Up to 100 kg of steam is used per 1 ton of fuel oil, and water content in the latter reaches 10% [47]. Fuel oil with higher water concentration foams in open tanks and pulsates when injected from a nozzle. The combustion of water-containing fuel oil increases heat losses with flue gases, aerodynamic resistance, and energy demands for the on-site needs; it also reduces the theoretical combustion temperature, heat transfer in the furnace, and, hence, the boiler efficiency. Moisture in fuel oil complicates the use of fuel-oil-handling equipment; it may disrupt the regime of fuel oil combustion due to water clogs preventing the smooth supply of fuel to spray nozzles; it also complicates the operation of boiler elements. Elevated water content in sulfur fuel oil exacerbates the corrosion of fuel oil pipelines and equipment because some sulfur compounds, e.g., hydrogen sulfide, dissolve in water. At the same time, the combustion of fuel oil with 5–10% of water distributed in it improves the atomization efficiency, increases combustion stability, and reduces the content of harmful emissions (nitrogen and carbon oxides, etc.). To account for the moisture absorbed by fuel oil, we added water to it in a volume concentration of approximately 5%, 10%, 15%, and 30% and mixed the resulting composition using a homogenizer. In the experiments, we used grade M 100 fuel oil (Rosneft, Russia). Prior to mixing, the liquids were heated to 80 °C on an AMTAST MSH-2 (Amtast USA Inc., Lakeland, FL, USA) magnetic hotplate stirrer. After mixing, the temperature of the resulting two-component liquid was also maintained at approximately 80 °C. Table 1 shows the main properties of the compositions under study.

Table 1.
Fuel compositions used in the experiments and their properties. The table also contains data on the compositions used for the comparison of collision regimes and outcomes.
2.2. Secondary Droplet Atomization Schemes
Four schemes are normally used for secondary droplet atomization: droplet impingement on a heated solid substrate [33,48], droplet exposure to an air jet [49], droplet–droplet collisions [39,40], and micro-explosive breakup [50]. As fuel oil is a highly viscous liquid, using an oncoming air flow to break up fuel oil droplets is not very effective. Due to its high viscosity, a fuel oil droplet is entrained by an air flow practically without atomization; its surface only deforms slightly perpendicular to the deformation axis. Micro-explosive atomization of fuel oil in furnaces is unlikely, because the droplet heating time will be too long given the low water concentration (below 10%), and the droplet will travel all the way to the chamber wall without ignition. We focused on two secondary atomization schemes for fuel oil droplets, namely, droplet–droplet collisions and droplet impingement on a solid surface. The surface was heated by attaching a laboratory-grade autotransformer to it. The temperature was monitored using an infrared thermometer (Testo SE & Co., Titisee-Neustadt, Germany).
Demidovich et al. [51] reported on the atomization behavior of water droplets impinging on a solid surface. A polished copper (M1) substrate was found to provide the maximum efficiency in droplet breakup; it is also heated reasonably uniformly due to its high thermal conductivity. As a result, the heat fluxes are much higher for copper substrates due to higher thermal conductivity and thermal diffusivity compared to steel substrates. The higher the heat flux, the more intense the atomization. Substrate roughness plays a special part in the formation of secondary fragments. The authors of [52,53] showed the physical reasons for an increase in the number of fine droplets with an increase in the substrate roughness. When in contact with a smoother surface, droplets most often spread over it, and a subsequent droplet coalesced with the liquid film on the surface [51]. In this research, we used a copper surface with a roughness grade of 3 (arithmetical mean deviation of the profile Ra = 10–20 µm; average peak to valley height Rz = 40–80 µm).
In industrial plants, fuels are mixed using cavitators immediately before combustion, so emulsion stability is not a key indicator. We used a drop test to estimate the lamination or stability of the resulting invert emulsion [54]. The drop test was considered successful if an emulsion droplet, which was dropped on the water surface, did not mix with water. The viscosity of the emulsion was measured using a Brookfield DV3T LV viscometer (AMETEK Brookfield, Middleborough, MA, USA), which comes with four spindles to measure the viscosity of liquids with µ = 0.001–6000 Pa·s. The spindle speed ranged from 10 rpm to 250 rpm [55]. The shear rate was measured by the viscometer with an accuracy of ±1% in compliance with ASTM D445 [56]. To measure the viscosity of a liquid, the composition was placed into a cylinder, which was put into the viscometer. The parameters were recorded by the RheocalcT software, which automatically controlled and collected data.
The experiments on fuel oil droplets colliding with each other were conducted on a setup featuring a set of nozzles to provide the fixed droplet size (Rd). For droplet atomization using droplet–droplet collisions, we used a setup from [41] (Figure 1). Droplet impingement on a solid substrate was provided by an experimental setup from [51] (Figure 1).
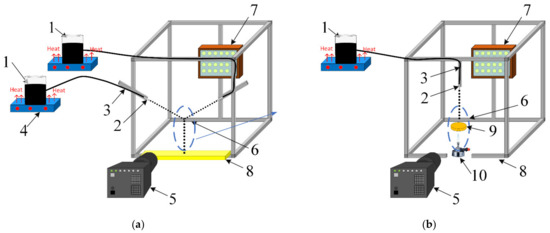
Figure 1.
Scheme of experimental setup for droplet–droplet collisions (a) and droplet impingement on solid substrates (b): 1—fuel container; 2—fuel feeding nozzle; 3—fuel oil/water emulsion feeding pipeline; 4—magnetic stirrer; 5—high-speed video camera; 6—collision area; 7—spotlight; 8—collector of liquid fragments; 9—substrate; 10—gas burner.
Liquid fuel was supplied through a pipeline (3) to detachable nozzles (2), which generated liquid droplets. Water and fuel oil were mixed using a magnetic stirrer (4). In our experiments, we consecutively generated droplets falling on a solid surface. Due to the constant droplet size and velocity, as well as the location of the liquid feeding capillaries, the time between droplets reaching the surface was also constant at approximately 0.1 s.
2.3. Variable Parameters and Recorded Characteristics; Measurement Errors
Droplet atomization was recorded with the help of a Fastcam Photron MINI UX 100 video camera (Photron, Tokyo, Japan) (with a resolution of 1280 × 1024 and a frame rate of 4000 fps). The number of droplets that can be identified in an image depends on the focal depth of the lens, and the focal depth directly depends on f-numbers. In the experiments, we used a lens with a focal length of 105 mm, f/16 aperture, and a focus distance of 0.7 m. The sizes of secondary fragments were measured when they were in focus. The accuracy of the secondary fragment size measurement on the image is determined by the value of the scale factor (0.01 mm/pix). The minimum recorded size of a droplet was 0.05 mm. The droplet velocity in the experiments was controlled by varying the liquid flow rate through the syringe in a Sino SN-50F6 syringe pump (Sino Medical-Device Technology, Shenzhen, China) (the accuracy of the flow rate setting was up to 0.1 mL/h). The velocity was measured using the Photron Fastcam software (by tracking a dynamic object on the footage). The impact angle was set by varying the angle of the nozzles installed on turntables in the same plane relative to each other. The angle was also measured using the Photron Fastcam software. The distance between the nozzles in the experiments was 36–40 cm. This choice was motivated by the long droplet formation times and distances caused by the high viscosity of fuel oil and fuel oil/water emulsion. Slight changes in the distance between nozzles were caused by the variation of their tilt to set the necessary impact angle. The equipment was calibrated before each series of experiments. For a syringe pump, we achieved this by measuring the flow rate and the volume of the liquid pumped. The scale factor for the camera was determined using a reference target. The viscometer, balance, and stirrer were calibrated in accordance with the recommendations of their respective manufacturers.
Using the Photron Fastcam Viewer software package, we determined the droplet velocity (Ud), size (Rd), and impact angle (αd). The systematic error in these experiments was as follows: 2.1% for droplet velocity measurement, 3% for air flow velocity measurement, 1.6% for droplet radius measurement, and 2.3% for impact angle measurement. Random errors are shown as confidence intervals on characteristic curves. The measured parameters were used to calculate dimensionless numbers, such as the Weber number We = (2·Rd·ρ·Ud2)/σ, Reynolds number Re = ρ·2Rd1·Urel/µ, and Ohnesorge number Oh = µ/(ρ·σ·2·Rd)0.5. These dimensionless criteria are used for generalizing the research findings because they reflect the joint contribution of different liquid properties. In particular, the Reynolds number takes into account the inertial force to viscosity force ratio and the Ohnesorge number reflects viscosity, inertia, and surface tension, whereas the Weber number determines the ratio of the liquid’s inertia to its surface tension. When calculating the values of the Weber, Ohnesorge, and Reynolds numbers, we used the size of the smaller droplet from the two colliding ones according to the methods described in [57,58,59].
The dimensionless linear interaction parameter B = b/(Rd1 + Rd2) is used to reflect the impact centricity. Figure 2 presents the schemes used for recording the measurement parameters of droplet–droplet collisions and droplet impingement on a solid wall.
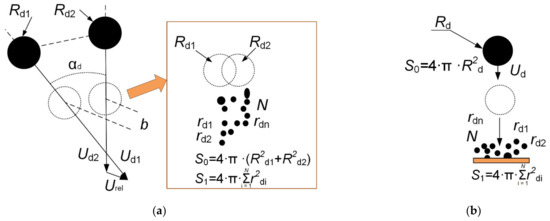
Figure 2.
Recording schemes of the measurement parameters of droplet–droplet collisions (a) and droplet impingement on a solid wall (b).
To calculate the distance between the droplets’ centers of mass (b) at the moment of approach, the vector of their resultant velocities was marked off from their centers of mass (Urel = (Ud12 + Ud22 − 2·Ud1·Ud2·cos(αd))1/2) (Figure 2). The parameter b is a segment perpendicular to the vectors marked off in parallel to the resultant velocity vector. Therefore, the linear impact parameter (B) reflects the droplet sizes and velocities, the impact angle, and the distance between the droplets’ centers of mass. The linear impact parameter is a dimensionless linear parameter of a collision describing the centricity of impacts of the droplets. The value B = 0 corresponds to a frontal collision and B = 1 corresponds to a tangent collision. The parameter B is determined before the collision and is the distance b between the centers of two droplets in a plane perpendicular to the relative velocity vector, and is normalized to the average droplet diameter [57]. The impact angle in binary collisions of droplets has a significant effect on the resulting velocity of interaction between droplets. This effect is considered when calculating the resulting droplet velocity using the least-squares method. The highest kinetic energy during the collision of droplets corresponds to impact angles close to 0° and 90° [60]. In this case, the contact area of the droplets is a maximum, their kinetic energy is also a maximum, and the interaction proceeds intensively due to internal shear stresses.
The secondary atomization characteristics of colliding droplets were determined by calculating the ratio of the total free surface area before and after the collisions. The intensity of the secondary atomization of fuel oil droplets and fuel oil/water emulsion droplets was determined in the same way. The surface area of initial droplets was given by S0 = 4·π·(Rd12 + Rd22) and the surface area of newly formed fragments was written as S1 = 4·π·∑N·rd2.
In the comparative analysis of S0 and S1, we controlled the equality of the liquid volumes before and after the collisions. For this equality to hold, the calculated number of child droplets included the liquid fragments both in and out of focus of the video camera. All the child droplets were assumed to have the same average size. Preliminary experiments using three video cameras, reproducing the conditions of three-dimensional video recording, justified this approach: the number and the average size of child droplets calculated in this way did not differ by more than 6–8% from the results obtained using three cameras. A similar calculation was performed for the collision of fuel oil/water emulsion droplets with a substrate.
The research findings are generalized in the form of the so-called interaction regime maps [34,38]. Approximately 300 impacts were analyzed to construct such maps. The number of collisions was not the same in each experiment due to the limited size of the recording area and different droplet velocities (Ud = 0.1–7 m/s). The regime maps were constructed in the coordinate system of the Weber number and linear interaction parameter B, which also indirectly reflected the impact angle αd. Using the experimental points obtained on the maps, we determined the boundary points reflecting the transition between fuel droplet interaction regimes. We then plotted a boundary connecting these points. An approximation boundary line was drawn if 95% of the points were within the domain of a certain regime. The B(Re), We(Oh), We(Re), and Re(Oh) regime maps were also constructed in a conventional form [38,61,62].
3. Results and Discussion
3.1. Main Patterns of Collisions under Study
Figure 3 presents typical experimental images illustrating the similarities and differences between the behaviors of fuel oil, water, and two-component droplets (90 vol% fuel oil and 10 vol% water).
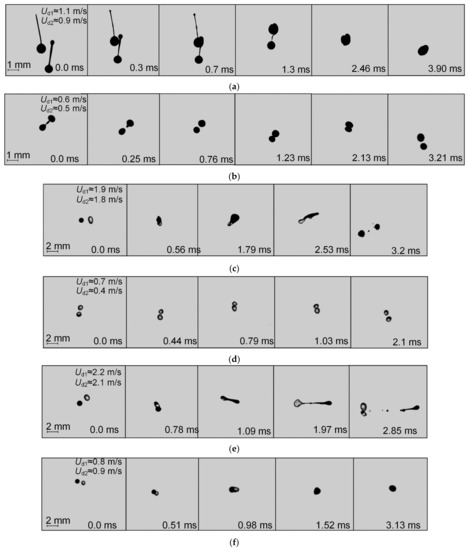
Figure 3.
Images of droplet–droplet collisions (T ≈ 80 °C): (a)—coalescence of fuel oil droplets; (b)—bounce of fuel oil droplets; (c)—disruption of two-component fuel droplets (90 vol% fuel oil, 10 vol% water); (d)—bounce of two-component fuel droplets (90 vol% fuel oil, 10 vol% water); (e)—disruption of fuel oil droplets colliding with water droplets; (f)—coalescence of fuel oil and water droplets.
Fuel oil droplets most often collided in the coalescence and bounce regimes (Figure 3a,b). When the velocity of fuel oil droplets was less than 5 m/s, the jet did not break up into a droplet flow due to the high viscosity of this fuel; hence, no disruption occurred. At higher droplet velocities, a jet broke up into an array of fuel oil droplets. Each of the two colliding droplets had a thin plume forming behind them and containing droplets less than 0.2 mm. To provide droplet disruption, water was added to fuel oil and the resulting emulsion was heated (90 vol% fuel oil and 10 vol% water, T ≈ 80 °C). Figure 3c shows the images of two-component fuel droplets colliding with each other (90 vol% fuel oil and 10 vol% water, T ≈ 80 °C). Two large fragments were formed with a radius of approximately 0.4 mm and several smaller ones with a radius of no more than 0.05 mm. A similar pattern can be seen in Video S1.
Furthermore, collisions between droplets of the two-component fuel (90 vol% fuel oil and 10 vol% water, T ≈ 80 °C) moving at a velocity of less than 2 m/s ended in bounce (Figure 3d). However, a lot more fragments with radii of approximately 0.1 mm were formed when water droplets collided with fuel oil droplets (Figure 3e). A fuel oil droplet crashed into a water droplet and a thin plume of fragments was formed behind it. Some layers of water and fuel oil were mixed, which resulted in the formation of secondary droplets of emulsified fuel oil. With an increase in the velocity to 3 m/s, coalescence occurred (Figure 3f), which led to fuel oil mixing with water.
Figure 4 presents typical experimental images illustrating the similarities and differences between the collision behaviors of two-component fuel droplets (90 vol% of fuel oil and 10 vol% of water) with a heated solid surface. To intensify the disruption of fuel oil/water emulsion, the substrate temperature must be at least 300 °C. At this temperature of the wall, droplets are heated fast enough when impinging on it. These effects are shown in Video S2. Water boils in a two-component droplet, and vapor bubbles emerge and reach the droplet surface, so it breaks up into an array of secondary fragments (Figure 4a,b). The first droplet is observed to stick to a heated substrate; it spreads over the substrate and heats up. The subsequent droplet acts on the first one, thus destroying it. As a result, a certain proportion of two-component fuel on a substrate is replaced and starts heating up. These effects increase the droplet–substrate contact time to intense fragmentation (see Video S3). However, this process did not take place at a substrate temperature of approximately 200 °C. A droplet evaporated to form a relatively concentrated gas–vapor mixture (Figure 4c,d).
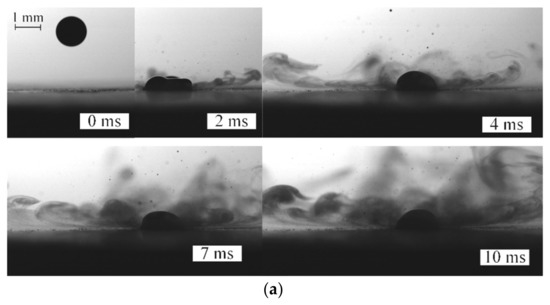
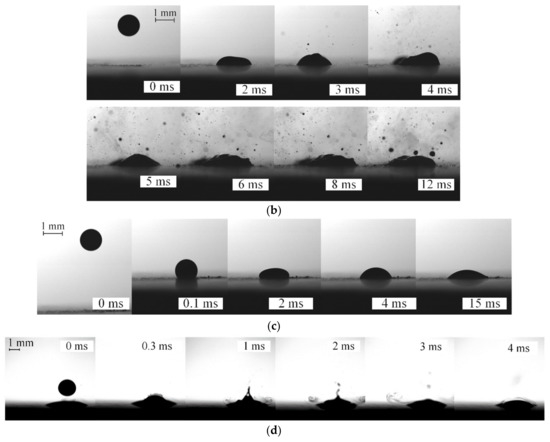
Figure 4.
Video frames of droplets impinging on a heated solid surface: (a,b)—disruption of two-component fuel droplets (90 vol% fuel oil, 10 vol% water, T ≈ 80 °C; Ud ≈ 2.1 m/s) (at Ts ≈ 300 °C); (c,d)—two-component fuel droplets (90 vol% fuel oil, 10 vol% water, T ≈ 80 °C; Ud ≈ 2.3 m/s) impinging on a solid wall (at Ts ≈ 200 °C).
No significant stratification was observed in parent droplets during the high-speed recording of fuel oil/water composition droplets colliding with each other in a gas and impinging on a solid wall. When fuel oil is used as one of the droplet components, the liquid–liquid interfaces are difficult to identify, even under extra lighting. Fuel oil/water emulsion stratifies in real production processes leading to a certain variation in collision regimes and outcomes. The collision regime maps obtained in this research predict the influence of this factor on the characteristics of the processes under study. In particular, we further comment on the collision regime maps in comparison with the known data for slurries and emulsions with significantly different water concentrations. These differences are important for understanding the potential changes in the operation of secondary atomization systems for liquid droplets. At the same time, if fuel oil/water emulsions are prepared and mixed immediately before atomization, the atomization patterns will correspond to those described above on the basis of the experimental footage analysis.
3.2. Droplet–Droplet Collision Regime Maps
Figure 5 and Figure 6 show typical collision regime maps in the coordinate systems accounting for the dimensionless linear interaction parameter, as well as the Weber and Reynolds numbers. The dimensionless linear interaction parameter accounts for the distance between the droplets’ centers of mass and collision centricity. This regime map was used to present the four collision regimes in one coordinate system for each fuel composition. It showed the conditions in which one can ensure stable droplet disruption, i.e., their extensive secondary atomization. The maps indicate the boundaries for the four droplet collision regimes: bounce, separation, coalescence, and disruption. The regime maps have similar shapes to those obtained for the collisions of milk [54], hydroxypropyl methylcellulose aqueous solutions (2%, 4%, and 8%) [63], water, and diesel oil droplets [64]. The transition boundaries between regimes are in acceptable agreement. The absolute values of critical Weber numbers, impact parameters, and other criteria differ due to a significant difference of liquid properties.
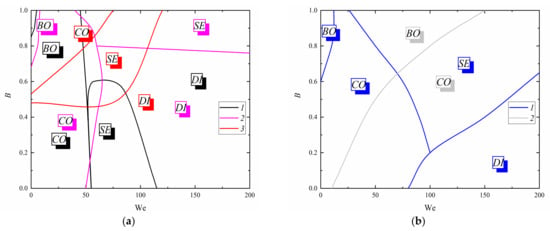
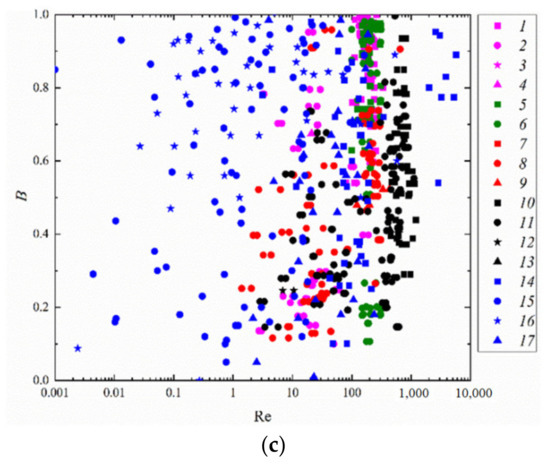
Figure 5.
Collision regime maps of typical compositions accounting for the dimensionless linear interaction parameter: (a) 1—CWS (30 wt% coal, 70 wt% water) [41]; 2—two-component fuel (30% transformer oil, 70% water) [41]; 3—CWSP (30 wt% coal, 25 wt% transformer oil, 45 wt% water) [41]; (b) 4—water [41]; 5—fuel oil; (c) 1–4—CWS (30 wt% coal, 70 wt% water) [41]; 5,6—two-component fuel (30% transformer oil, 70% water) [41]; 7–9—CWSP (30 wt% coal, 25 wt% transformer oil, 45 wt% water) [41]; 10–13—fuel oil; 14–17—water [41]; 1,5,7,10,14—disruption; 2,6,8,11,15—coalescence; 3,12,16—bounce; 4,9,13,17—separation.
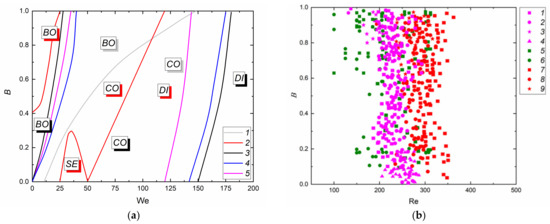
Figure 6.
Collision regime maps of typical compositions accounting for the dimensionless linear interaction parameter: (a) 1—fuel oil; 2—collision of fuel oil and water droplets; 3—two-component fuel (90 vol% fuel oil, 10 vol% water); 4—two-component fuel (85 vol% fuel oil, 15 vol% water); 5—two-component fuel (70 vol% fuel oil, 30 vol% water); (b) 1–4—collision of fuel oil and water droplets; 4–6—fuel oil; 7–9—two-component fuel (90 vol% fuel oil, 10 vol% water); 1,5,7—disruption; 2,6,8—coalescence; 3,9—bounce; 4—separation.
The experimental studies of collisions between fuel oil droplets have shown that these droplets are unlikely to break up by droplet–droplet collisions. Figure 5a shows that pure fuel oil droplets collided in the bounce and coalescence regimes. That is why we added 10% of water to fuel oil. This led to droplet breakup due to their heterogeneous composition. Droplets of fuel slurry have an unstable geometry (ellipsoid, liquid disk, etc.), which leads to their disruption at an almost 30% lower inertia compared to water and oil-in-water emulsions. Adding water to fuel oil reduces the surface tension and viscosity of droplets, which leads to droplet surface transformation. Droplet homogeneity is also disrupted because fuel oil droplets contain water droplets, so there are interfaces between liquids with different viscosity and surface tension. As a result, when such droplets collide with each other, their internal interfaces separate, and a droplet of fuel oil/water emulsion breaks up. Intense agitation of the emulsion reduces the size of water droplets within fuel, which also results in smaller child droplets. High Weber numbers (above 50) are important for the majority of practical applications based on the atomization of fuel compositions. According to the experimental data on CWS (coal–water slurries) and CWSP (coal–water slurries containing petrochemicals), adding a liquid combustible component to CWS makes them more viscous. This in turn significantly increases the number of child droplets formed from the breakup of parent drops. Therefore, CWSP droplets break up more intensely even at a lower We. Figure 5b presents the fuel droplet collision regime maps in the coordinate system of the dimensionless linear interaction parameter versus the Reynolds number. The Reynolds number reflects the viscous forces of a liquid. Heating has a greater effect on the interaction regimes for slurries than for fuel oil, water, and fuel emulsions, because heating intensifies the convective heat and mass transfer within droplets. This increases the strain in droplets, so they break up into a larger number of secondary fragments. In addition, an increase in the temperature of the liquid results in droplet heating and a reduction in liquid viscosity.
The analysis of regime maps (Figure 6) has shown that the disruption of fuel oil with added water occurred at a critical Weber number above 150. However, the disruption of fuel oil droplets colliding with water droplets occurred at a critical Weber number of approximately 50. The reason for this is that a water droplet breaks up upon collision and the resulting secondary fragments of water absorb fuel oil droplets. This results in a higher ratio of the free surface area of liquid droplets after and before collision (Figure 7). Interestingly, the ratio of the free surface areas after and before collision changes significantly at a Weber number of 100+. The greatest number of secondary fragments is observed after the collision of fuel oil droplets with water. By comparing the values of S1/S0, we found that at a Weber number of approximately 200, droplets of a two-component fuel are atomized 50% more effectively than those of fuel oil, and fuel oil colliding with water provides a 100% greater efficiency.
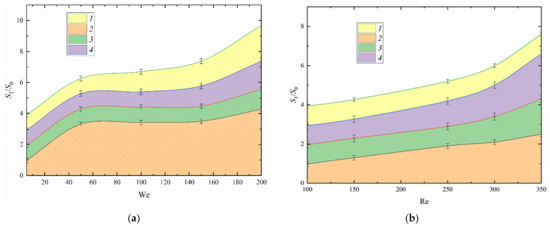
Figure 7.
Post-collision to pre-collision free surface area ratio against Weber number (a) and Reynolds number (b): 1—water [41]; 2—two-component fuel (90 vol% fuel oil, 10 vol% water); 3—collision of fuel oil and water droplets; 4—fuel oil.
For a basic composition with 90% of fuel oil and 10% of water, the most effective secondary atomization by droplet impingement on a heated wall is provided at a temperature of approximately 300 °C. This can be explained by the Leidenfrost effect at a substrate temperature of approximately 300 °C [44]. When a droplet of fuel oil hits a heated substrate, it breaks up into a multitude of small fragments and bounces off due to the vapor buffer zone. With an increase in the substrate temperature, fewer secondary fragments of a large size are produced, i.e., the Leidenfrost effect is intensified and a droplet bounces off the substrate. The optimal substrate temperatures are somewhat different for other compositions used in the experiments. The optimal substrate temperature ranges are specified in our earlier published studies [51]. It is established that the effective atomization of water slurry droplets requires the substrate temperature to be higher than the water boiling point but by no more than 100 °C. For emulsions, this temperature is as high as 250–350 °C because of the liquid component, which envelops water droplets, and the corresponding increase in the intense boiling temperature.
In contrast with droplet–droplet collisions, the following two regimes were observed for fuel oil droplet impingement on a heated solid wall (Figure 6): spreading of the first droplet without disruption and fragmentation of falling droplets. Droplets of fuel oil with added water collided with each other in several regimes: coalescence, bounce, separation, and disruption (extensive atomization).
Figure 8 shows typical collision regimes in the coordinate systems accounting for inertia, viscosity, and surface tension (using the Weber, Reynolds, and Ohnesorge numbers). An effort was made to present the four collision regimes in one coordinate system for each composition in order to show the conditions that can ensure stable droplet bounce, separation, coalescence, or disruption. The figure also shows the We, Re, and Oh variation ranges, in which several regimes may occur under the same conditions. Such conditions can be considered transient between the corresponding collision regimes. The correlation of forces is balanced in these ranges, so other factors that are unaccounted for in these coordinate systems can contribute to the occurrence of separation, bounce, coalescence, or disruption.
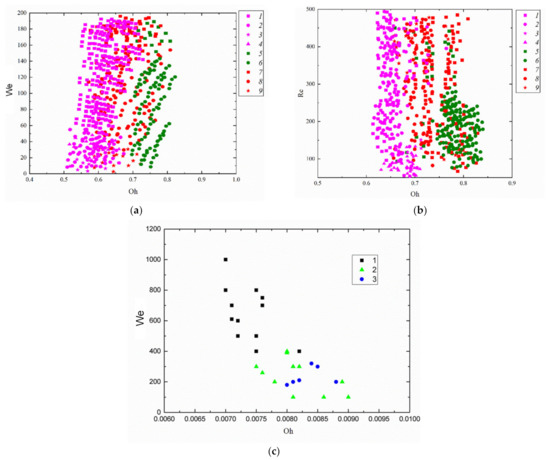
Figure 8.
Collision regime maps accounting for inertia, friction, surface tension, and viscosity according to experimental data (a,b): 1–4—collision of fuel oil and water droplets; 5,6—fuel oil; 7–9—two-component fuel (90 vol% fuel oil, 10 vol% water); 1,5,7—disruption; 2,6,8—coalescence; 3,9—bounce; 4—separation and data from [65] (c) 1—coalescence; 2—separation; 3—disruption.
Using We(Oh) and Re(Oh) interaction regime maps, one can single out the variation ranges of the Reynolds, Ohnesorge, and Weber numbers (Figure 5a,c), in which there was a relatively stable occurrence of not only disruption and coalescence but also separation and bounce. Transition zones between regimes are of special interest. In these ranges, the factors that are normally neglected play an important part, for instance, the droplet shape, head-on or off-center collision trajectories, etc. Such conditions can only be scrutinized as part of a comprehensive evaluation using three- or even multi-dimensional coordinate systems.
The analysis of the literature on the collision behavior of heterogeneous droplets (emulsions, solutions, slurries, and immiscible liquids), for instance [65], indicates the dominating influence of not just inertia and surface tension but also internal friction (viscosity) on the interaction behavior of liquid fragments. The interaction regime maps of water and liquid fuel droplets in the Re(Oh) and We(Oh) coordinate systems show that the lower the liquid viscosity, the more extensive the droplet coalescence. This holds true even at high We numbers corresponding to separation and disruption in the B(We) coordinate system, i.e., when inertial forces exceed surface tension forces.
In the case of highly viscous fuel compositions, droplet–droplet collisions in a gas followed by their breakup require relatively high resultant velocities and large initial sizes. Such conditions often imply the significant wear of atomizers due to high injection pressures and friction forces. That is why it is necessary to estimate the critical conditions for the transition to the intense fragmentation of highly viscous droplets impinging on heated solid walls. Schemes that combine droplet–droplet collisions and droplet impingement on a solid surface produce an aerosol with less energy consumption and equipment wear.
The curves of secondary fragment characteristics against the key collision parameters presented in Figure 7 are overall monotonic, so the results can be extended to higher droplet velocities and smaller sizes provided in high-potential fuel systems. Slight variations of the increase in the number of secondary fragments against droplet velocity and initial size reflected in the Weber number are caused by the experimentally established changes in the energy balance in the collision zone. The results of the corresponding energy analysis are presented further.
3.3. Fuel Oil Droplets Impinging on a Solid Wall
The experiments have shown that droplet velocity has a decisive influence on the boundary of transition from spreading to disruption. On average, droplets sized 0.5–2 mm moving at a velocity above 3–4 m/s collided with a copper substrate heated to approximately 300 °C in the stable disruption regime. The regime map of droplet interaction with a solid wall in Figure 9 accounts for the Ohnesorge number, which in turn accounts for the viscosity and surface tension forces. Thus, this map does not consider the impact angle or the substrate characteristics. This is the main reason for a relatively significant scatter of the values of the Ohnesorge number. Nonetheless, the transition boundaries between the first and second regime of droplet interaction with a wall can be tracked relatively clearly when generalizing the experimental data. This regime map can be used to select the component composition of fuel oil with a view to intensifying droplet disruption.
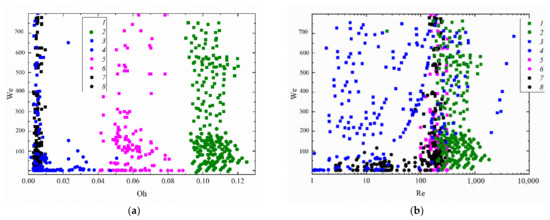
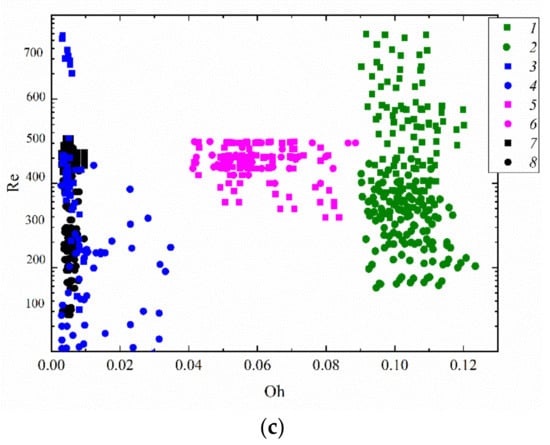
Figure 9.
Collision regime map for a droplet impinging on a surface accounting for Weber and Ohnesorge (a), Weber and Reynolds (b), and Reynolds and Ohnesorge (c) numbers. Rounds denote first droplet spreading and coalescing with subsequent ones; squares refer to disruption: 1,2—two-component fuel (90 vol% fuel oil, 10 vol% water); 3,4—water [51]; 5,6—two-component fuel (30 vol% transformer oil, 70 vol% water) [51]; 7,8—slurry (30 wt% coal, 70 wt% water) [51].
Figure 9a presents the collision regime maps of fuel oil/water droplets with a solid wall. An important consequence of the research is the established effective substrate temperature. The substrate temperature has a significant impact on the disruption behavior of falling droplets. This impact is not always obvious, however. In particular, for the most effective atomization of fuel oil droplets, the wall temperature should approximate 300 °C. At lower or higher temperatures, fewer secondary fragments were produced. There are several reasons for this. First, the fuel oil and water evaporation rates are so high on a substrate at temperatures exceeding 300 °C that a droplet does not have enough time to break up. We observed a rapid outflow of vapors, with their mixing with the oxidizer, ignition, and formation of a flue gas flow. This is also attributed to the Leidenfrost effect, which emerges when a droplet collides with a surface whose temperature is much higher than the boiling point of the liquid. This effect leads to the production of a heat-insulating vapor layer between the surface and the newly formed liquid fragment. As a result, the droplet bounces off the surface.
At lower or higher temperatures, fewer secondary fragments were produced. However, there are several reasons for this. At substrate temperatures below 300 °C, the heat flux supplied from the substrate to the droplet was enough for heating the liquid layers, reducing the viscosity and surface tension, and spreading over the solid wall, but not enough for water boiling. As a result, droplets practically stuck to the substrate, and all the subsequent impinging droplets contributed to the thickness of the fuel film on the substrate and its cooling. An analysis of experimental video frames has shown that extensive atomization of water/fuel oil droplets on a heated solid surface is possible, when a droplet does not have enough time to spread and form a large contact area. In other words, a droplet evaporates so rapidly that the resulting vapors prevent spreading over the substrate. A droplet floats over the substrate on a buffer vapor layer. In this state, a droplet is less stable. It breaks up more extensively upon collision with subsequent droplets and the wall; water also boils more actively. The footage shows that unstable droplets are constantly moving in all directions including up, towards the subsequent falling droplets. Here, the droplet shape on a substrate is far removed from spherical. As a result, the collision energy of the consecutive droplets is relatively high. This intensifies fragmentation. Obviously, the optimal substrate temperature for droplet fragmentation depends on the original droplet size. The smaller the droplets, the lower substrate temperature intensifies their fragmentation. The original droplet size is limited by the spray system capabilities and fuel rheology. Therefore, it is necessary to consider the regime maps and transient substrate temperatures in order to develop fuel droplet atomization technologies.
Fuel oil atomization provides a small number of secondary fragments (3 to 6) unlike typical slurries and emulsions. This effect results from the high viscosity of fuel oil (µ ≈ 0.013 Pa·s at T ≈ 80 °C), which is much higher than that of water (µ ≈ 0.00036 Pa·s at T ≈ 80 °C), even upon heating (Figure 10). The substrate heating leads to the formation of a heated layer of two-component fuel on the surface after the first droplet hits it. Subsequent droplets hit the liquid layer and transform it. When the first droplet of the fuel oil/water emulsion reaches the substrate, it is heated, which intensifies the evaporation of water microdroplets and their local boiling. Vapor bubbles destroy the fuel oil film and craters are formed in it. A cloud of secondary fragments is produced due to the micro-explosive boiling of water within the fuel. The atomization of two-component fuel oil droplets using this method is similar to that of water emulsions with waste oils [41]. Two-component fuels produce 10% more secondary fragments than used oil emulsions but 40–70% fewer than slurry fuels. The transition from the spreading regime to droplet disruption for droplets impinging on a solid surface on a We(Oh) map is illustrated reasonably clearly in Figure 9a. In contrast with Re(Oh), a We(Re) map can more clearly show the role of the viscosity and surface tension correlation. The experimental findings show that fuel oil is not the only composition with a high potential for extensive atomization (disruption). It is safe to conclude from the data on the collision regime maps that droplets of any liquid composite fuel break up under much lower aerodynamic forces than droplets of homogeneous liquids. The experimental data also allow us to determine the conditions in which significant droplet atomization is possible.
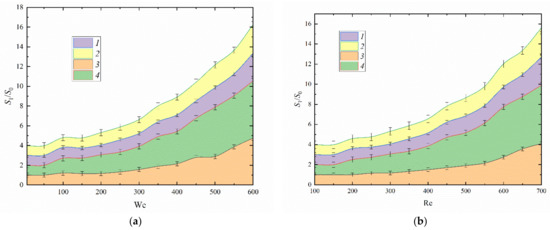
Figure 10.
Liquid surface area ratio against Weber number (a) and Reynolds number (b): 1—water [51]; 2—slurry (30 wt% coal, 70 wt% water) [51]; 3—two-component fuel (30 vol% transformer oil, 70 vol% water) [51]; 4—two-component fuel (90 vol% fuel oil, 10 vol% water).
Fuel oil is currently used in the energy sector because of its low cost and high calorific value [45,66]. Fuel oil is a mixture of residual matter and remnants from petroleum cracking containing up to 40% of diesel, which makes it applicable as a marine fuel [67]. A number of problems arise from the use of such fuel. First, if a spray contains large droplets, the fuel combustion is incomplete, which contributes to soot formation both in the combustion chamber and in the exhaust system. Second, the heterogeneous structure of a fuel jet in a combustion chamber also leads to the incomplete combustion of fuel oil, leading to droplet collisions with the equipment walls [45,66]. In this research, we studied the atomization patterns of fuel oil droplets colliding in a gas with each other and with a solid wall. Such atomization schemes are used in actual fuel spray cones, but no experimental findings have been published so far that would make it possible to determine the key spraying characteristics and critical atomization conditions. Here, we have established that the minimum Weber number must be above 150, and hence the velocity must be greater than 3 m/s with a radius of the parent droplet of at least 0.5 mm. These parameters provide consistent atomization of fuel oil droplets colliding with a heated solid wall and with neighboring droplets. The effective secondary atomization of fuel oil droplets impinging on a solid wall leads to a reduction in the local superheating of power plant walls and engines. Our experiments have shown that adding 5–30% of water to fuel oil and its heating to 80 °C reduces the size of secondary fragments by 40–70% and increases their number by 50–65%. The combustion of such blends in an experimental unit simulating the typical features of an industrial boiler unit was studied by Pei et al. [46]. They established that adding water to fuel oil reduces the SOx, NOx, and particulate matter emissions. These effects are achieved by the secondary atomization of fuel oil droplets. The experimental findings from this research can help control the secondary atomization of fuel oil droplets in combustion chambers.
The interaction of fuel oil/water emulsion droplets with heated solid surfaces provides the opportunity to intensify different mechanisms of secondary atomization. Each of the mechanisms can increase the number of secondary liquid fragments in a different way. At certain heating temperatures, it is possible to trigger several secondary atomization mechanisms. In particular, of the greatest interest is the secondary atomization of fuel oil/water emulsion droplets impinging on a solid wall and water superheating to the boiling point followed by the micro-explosive breakup. A model simulating the heating and explosive boiling of immiscible fuel oil and water droplets was presented in [68]. The simulations provided a quantitative estimate of the relative time scales of aerodynamic-induced and emulsion-induced breakup mechanisms. However, the micro-explosive breakup of mixed fuel oil/water droplets remains unstudied.
The experiments have shown that the threshold thermal conditions for this combined breakup depend on the substrate temperature and parent droplet size, as well as the water and fuel oil concentration ratio. The lower the water concentration in a droplet, the faster it heats up to boiling point. The smaller the parent droplets, the lower heat flux is required for droplet breakup through the micro-explosive mechanism. Intense secondary droplet atomization systems can be based on the schemes involving collisions of heterogeneous droplets with each other, with a gas flow, and with a solid wall. The experiments have provided the droplet breakup characteristics in two of the most promising schemes, where liquid jets are directed so that droplets collide with each other and with heated solid walls. Droplet breakup can be intensified by selecting the specific conditions suitable for the actual plants and reactors with their thermal limitations. These conditions can also be provided in plants and reactors that can be fired by emulsified and slurry fuels for thermal and flame water treatment, as well as mixing multiphase flows. They are also attainable in heat exchange systems and fire suppression. The droplet atomization regime maps provide basic information for the subsequent development of systems for the secondary atomization of liquid droplets with different component compositions and in different thermal conditions. It is important to perform the energy analysis in the droplet collision zone with solid walls to obtain the ratio of the acting forces. The next section presents the results of this analysis.
3.4. Calculation of Droplet Energies in the Interaction Zone
3.4.1. Energy Balance during Binary Fuel Droplet Collisions
Droplet–droplet collisions are characterized by a certain energy balance in the interaction zone. At the initial point of time (before the collision of two droplets), the kinetic energy KEi comprises the initial kinetic energy KEii of the part of droplets directly involved in the collision and the initial kinetic energy KEni of the part of droplets that does not take part in the interaction. The energy KEii can be represented in the form of the energy KEsii and the kinetic energy KEdii. KEsii stretches the droplet along the radial direction and gradually transforms into the bridge surface tension energy and the viscous dissipation energy. KEdii deforms the droplet along the radial direction and gradually transforms into the viscous dissipation energy. The kinetic energy of the parts that are not involved in the interaction includes the droplet-stretching energy KEsni and KErni, which deforms the droplet along the radial direction at the initial interaction stage and makes the droplet swirl when the deformation reaches its maximum.
In the course of swirling and deformation, the energy KErni gradually transforms into the viscous dissipation energy. In this case, kinetic energy can be written as [69]:
KEi = KEii + KEni = KEsii + KEdii + KEsni + KErni;
KEsii = 1/2·ρ·[VSi·(US·sin(αd))2 + VLi·(UL·sin(αd))2];
KEdii = 1/2·ρ·[VSi·(US·cos(αd))2 + VLi·(UL·cos(αd))2];
KEsni = 1/2·ρ·[(VS − VSi)·(US·sin(αd))2 + (VL − VLi)·(UL·sin(αd))2];
KErni = 1/2·ρ·[(VS − VSi)(US·cos(αd))2 + (VL − VLi)(UL·cos(αd))2].
VLi and VSi are the volumes of the interaction zones in the larger and smaller droplets and VL and VS are the volumes of the larger and smaller droplets.
VLi = χL·VL;
VSi = χS·VS;
χL = 1 − (2 − τ)2·(1 + τ)/4; for h > DL/2;
χL = τ2·(3 − τ)/4; for h ≤ DL/2;
χS = 1 − (2·Δ − τ)2·(Δ + τ)/(4·Δ3); for h > DS/2;
χS = τ2·(3·Δ − τ)/(4·Δ3); for h ≤ DS/2;
h = 0.5·(DL + DS)·(1 − B);
τ = (1 − B)·(1 + Δ).
χL and χS are the coefficients reflecting the interaction zones in the larger and smaller droplets; h is the length of the interaction zone; τ is the impact angle coefficient; DS is the initial diameter of the smaller droplet; and DL is the initial diameter of the larger droplet.
The critical Weber number required for the occurrence of droplet disruption is given by:
We ≥ 4·(1 + Δ3)(3·(1 + Δ)·(1 − B)·(Δ3·χS + χL))1/2/((1 − α3)·Δ2·B2).
Let us use Equations (6)–(14) to determine the critical Weber number for stable droplet breakup. The following initial conditions are assumed. CWS droplets (30 wt% coal and 70 wt% water, DL ≈ 0.001 m, DS ≈ 0.001 m) collide head on, i.e., B ≈ 0.8, Δ = 1. The coefficients reflecting the interaction zone of the larger and smaller droplets will take the following values: χL = 0.104; length of the interaction zone h ≤ DL/2; χS = 0.104 for h ≤ DS/2. The critical Weber number for Equation (14) will be We = 52.04. Let us perform similar calculations for the two-component fuel containing 30% of transformer oil and 70% of water: DL ≈ 0.0009 m, DS ≈ 0.00095 m, B ≈ 0.6, Δ = 0.947, χL = 0.337; χS = 0.368. The critical Weber number We = 123.17. For the two-component fuel with 90 vol% of fuel oil and 10 vol% of water, the parameters will look as follows: DL ≈ 0.0009 m, DS ≈ 0.00095 m, B ≈ 0.6, Δ = 0.947, χL = 0.337; χS = 0.368. The critical Weber number We = 166.64.
These values are in acceptable agreement with the We values obtained experimentally (Figure 5 and Figure 6). The deviations are 3.92%, 2.57%, and 2.02% for the CWS (30 wt% coal, 70 wt% water), two-component fuel containing 30% of transformer oil and 70% of water, and another two-component fuel with 90 vol% of fuel oil and 10 vol% of water, respectively. A similar calculation was performed for each regime map (Figure 5 and Figure 6) and the deviation did not exceed 5%.
The viscous dissipation energy during collision is given by:
VDE = VDEsii + VDEdii + VDEsni + VDErni.
During interaction, the components of kinetic energy KEsii, KEsni, KEdii, and KErni are converted into parts of viscous dissipation energy VDEsii, VDEdii, VDEsni, and VDErni, respectively:
where α2, α3, α4, and α5 are viscous dissipation coefficients.
VDEsii = α3·KEsii;
VDEsii = α3·KEsii;
VDEdii = α4·KEdii;
VDErni = α5·KErni,
The energy KEdii fully transforms into the viscous dissipation energy; hence, α4 = 1.
Another part of the kinetic energy of a droplet is converted into revolution energy (RE):
RE = KErni − VDErni.
During the rotation and deformation of a droplet, its kinetic energy is converted into the energy of surface tension, which prevents the droplet breakup. Thus, for droplet fragmentation to occur, the total effective stretching energy must exceed the surface tension energy of the bridge:
where SElig is the surface tension energy of the bridge:
KEes = KEi − VDE − RE ≥ SElig,
SElig = (2·σ·[π·h·(VSi + VLi)])0.5.
Using the energy balance Equation (21), as well as Equations (1)–(5) and (15)–(22), let us determine the collision parameters for the consistent fragmentation of different fuel droplets. The following initial parameters are assumed for the collision between CWS droplets (30 wt% coal, 70 wt% water): DL ≈ 0.00095 m, DS ≈ 0.0009 m, UL ≈ 1.3 m/s, US ≈ 1.1 m/s, head-on collision, i.e., B ≈ 0 and αd ≈ 90°. The total effective stretching energy KEes equals 1.06 J and the viscous dissipation energy is 4.24 J. The kinetic energy at the initial moment KEi amounts to 5.31 J. The bridge surface tension energy SElig at the moment of maximum deformation is 0.029 J. The rotation energy RE equals 6.86·10−6 J.
Thus, the energy balance (21) for CWS (30 wt% coal, 70 wt% water) will take the following form:
1.06 = 5.31 − 4.24 − 6.86·10−6 ≥ 0.029; 1.06 > 0.029.
The following is a similar estimate for the collisions between two-component fuel droplets (30% transformer oil, 70% water) with the following initial parameters: DL ≈ 0.00095 m, DS ≈ 0.0009 m, UL ≈ 1.5 m/s, US ≈ 1.2 m/s, B ≈ 0, and αd ≈ 90°. The total effective stretching energy KEes = 0.96 J and the viscous dissipation energy equals 3.83 J. The kinetic energy at the initial moment KEi amounts to 4.78 J. The bridge surface tension energy SElig at the moment of maximum deformation is 0.022 J. The rotation energy RE equals 2.1·10−5 J.
For the two-component fuel with 30% of transformer oil and 70% of water, the energy balance (21) will take the form:
0.96 = 4.78 − 3.83 − 2.1·10−5 ≥ 0.022; 0.96 > 0.022.
For droplets of the two-component fuel containing 90 vol% of fuel oil and 10 vol% of water with initial parameters DL ≈ 0.00095 m, DS ≈ 0.0009 m, UL ≈ 1.5 m/s, US ≈ 1.2 m/s, B ≈ 0, and αd ≈ 90°, the total effective stretching energy KEes = 1.12 J and the viscous dissipation energy equals 4.48 J. The kinetic energy at the initial moment KEi amounts to 5.60 J. The bridge surface tension energy SElig at the moment of maximum deformation is 0.063 J. The rotation energy RE is 1.8·10−5 J.
For the two-component fuel with 30% of transformer oil and 70% of water, the energy balance (21) will take the form:
1.12 = 5.60 − 4.48 − 1.8·10−5 ≥ 0.063; 1.12 > 0.063.
As can be seen from the calculated energy balances, the total effective stretching energy exceeds the bridge surface tension energy for the fuel blends under study. This means that, with the assumed initial parameters, these fuel blends break up to form secondary fragments. A further increase in the droplet size and velocity will lead to even greater prevalence of the kinetic energy over the bridge surface tension energy.
3.4.2. Energy Balance during Fuel Droplet Impingement on a Solid Surface
When droplets collide with a solid surface, the energy balance (23) is defined as [70]:
where Edr is the kinetic energy of the falling droplet; ES0, EP0 and ESs, EPs are the surface and potential energy of a droplet before and after collision, respectively; Esd is the post-collision kinetic energy of droplets (energy of spreading of a droplet over a solid surface after collision); Eθ is the energy from the work performed by the radial forces applied from the wall to the contact line; and ED is the viscous dissipation energy (the dissipation losses due to viscosity when a droplet spreads after collision). Potential energy (EP0, EPs) makes the smallest contribution compared to other components of Equation (23), so it can be neglected [71].
Using the values of the energies given in [70], Equation (23) will take the following form:
Let us divide both parts of Equation (24) by πDd2σ and obtain the following equation:
where the capillary number Ca = We/Re. Assuming that , let us rearrange all the We values to the left side of the equation. Equation (23) will then take the form:
Substituting the numerical values to Equation (26), we will obtain the critical Weber number for the fuel droplet impingement on a surface (We≈162), which differs from the experimental value by 1.49%. This calculation was performed for all the experimental points and the deviation did not exceed 5%. Using the above formulas for calculating the critical Weber number and energy balance for droplet fragmentation from droplet–droplet and droplet–substrate collisions, one can also predict the outcomes of secondary droplet atomization for other fuel types. The resulting equations reflect almost all the key properties of liquids: viscosity, density, and surface tension.
4. Technology Development Using Experimental Results
The experimental findings make it possible to choose the conditions for effective secondary atomization of multi-component droplets. Since the research findings are generalized using dimensionless criteria (Weber, Reynolds, Ohnesorge, and capillary numbers), they can be extended to other conditions typical of multi-component fuel technologies. This improves the impact of this study and extends the limits to the applicability of the findings. We made the following conclusions from the experimental findings:
- (i)
- The number of secondary fragments increases exponentially with an increase in the Weber numbers. The free surface area of secondary fragments more than doubles with an increase in the parent droplet velocity. This leads to a reduction in the secondary droplet size and an increase in their number. We compared the free surface areas of droplets before and after collision to find that, at a Weber number of approximately 200, droplets of a two-component fuel are atomized 50% more effectively than those of fuel oil, and fuel oil colliding with water provides a 100% greater efficiency.
- (ii)
- The critical thermal conditions for combined breakup were found to depend on the substrate temperature and parent droplet size, as well as water to fuel oil concentration ratio. It was established that adding 5–30% of water to fuel oil and heating it to 80 °C reduced the size of secondary fragments by 40–70% and increased their number by 50–65%. The optimal wall surface temperature for the interaction with fuel oil/water droplets is 300 °C. At this temperature, we observed the maximum droplet breakup due to the absence of the Leidenfrost effect.
- (iii)
- For fuel oil droplets to be atomized effectively, their velocity must be 3 m/s and their size must range from 0.4 mm to 0.7 mm. These parameters provide consistent atomization of fuel oil droplets colliding with a heated solid wall and with each other.
The droplet collision regime patterns and maps provide the necessary database for the development of secondary atomization systems for liquid droplets with different component compositions and in different thermal conditions. We presented some examples of how the results obtained can be used in the fuel atomization technologies in industrial plants. Due to low cost and high calorific value, fuel oil is a popular fuel in many industries. According to estimates by NASA, the USA can save USD 1.6 billion annually if it manages to increase the combustion efficiency of fuel oil droplets by at least 2% [72]. The combustion efficiency of this fuel can be increased by reducing the droplet size, which will lead to more complete combustion in a shorter time. Adding water to fuel oil also reduces anthropogenic emissions [46].
Few research findings have been published so far on the collisions of fuel oil droplets with flat and curvilinear surfaces, most common to ship engines and boiler units [73,74]. Fuel oil may absorb additional water if stored or prepared incorrectly. In some cases, the cost of corrective measures exceeds the resulting economic gain several-fold. Hence, the interest in fuel oil/water emulsions is on the increase [75,76]. In this research, we studied the two fuel droplet interaction schemes that are most common to power plants: droplet–droplet collisions and droplet impingement on a solid surface. The use of fuel oil/water emulsions in industrial equipment requires specialized atomization systems based on nozzles and sprinklers [45,66,77]. The atomization of this fuel is notable for a number of weaknesses leading to the unstable jet combustion. The spray cone is uneven due to high fuel oil viscosity, and larger droplets are formed at its periphery. This effect leads to incomplete fuel combustion, droplet sublimation, and sooting [45], which in turn reduces the equipment performance. Our research findings show the necessary and sufficient conditions for the intense atomization of fuel oil/water emulsion droplets. The optimal temperature of fuel oil/water emulsion was found to be approximately 80 °C. We also determined the critical Weber numbers required for the consistent disruption of fuel oil/water emulsion droplets colliding with each other. These help predict the necessary and sufficient droplet size and velocity for intense secondary atomization. The results show that an increase in the Weber number improves the efficiency of secondary atomization and increases the ratio of the free surface area after and before droplet atomization. With excess air in the nozzle, fuel droplets do not have time to burn up completely, which leads to droplet collisions with solid surfaces. As a result, soot is formed on the surfaces of the equipment, causing future local overheating. With a lack of oxidizer, however, droplets hit the walls of the nozzle, which leads to an inhomogeneous spray cone. The optimal temperature of the wall to atomize a fuel oil/water emulsion droplet impinging on it was found to be approximately 300 °C. This temperature of the solid surface prevents the Leidenfrost effect and improves the atomization efficiency due to a 30% increase in the free surface area of a droplet after the collision. As a result, the combustion of fuel oil/water emulsion becomes more efficient.
High-speed video recording allowed us to obtain experimental data on the key interaction regimes between droplets of a high-potential fuel oil/water emulsion, water, and fuel oil, as well as their atomization through impinging on a heated solid surface. Fuel oil/water emulsions are used in heat and power plants but spraying of this fuel presents a number of difficulties. Here, we present experimental data showing an opportunity of significant secondary atomization of fuel-oil-based droplets by droplet–droplet collisions and impingement on a solid wall. The research findings substantiate the economic and environmental effects of using secondary droplet atomization in fuel generation and transformation systems. The energy efficiency of heating equipment was improved by reducing the size of droplets after the primary atomization, which enlarged the surface area of phase transitions, heat exchange, and chemical reactions.
The experimental findings on droplet–droplet and droplet-heated wall collisions are primary data to be used for further research into the spraying atomization of fuel oil/water emulsion. Such experiments will help provide the accident-free and reliable operation of the equipment destined for the preparation, supply, and stable combustion of fuel oil and liquids on this basis. As fuel oil is a very viscous liquid, it may clog nozzles. Due to the experimental findings, we obtained the appropriate parameters for the most effective droplet atomization.
5. Conclusions
- (i)
- The secondary atomization scheme for droplets of water-free fuel oil and fuel oil/water emulsion through collisions with each other and with water droplets provides a relatively significant increase in the liquid surface area (S1/S0 > 3). The required temperature of the fuel oil to be supplied to the droplet collision zone is 80 °C. The optimal substrate temperature for the atomization of two-component droplets (90 vol% fuel oil and 10 vol% water) is approximately 300 °C. The secondary atomization of fuel oil/water emulsion droplets by their impingement on a heated solid wall makes it possible to reduce the typical sizes of liquid fragments by a factor of 40–50.
- (ii)
- We have plotted droplet collision regime maps and established the critical Weber and Ohnesorge numbers, as well as the critical values of the dimensionless linear interaction parameter, which reflect the threshold conditions of extensive droplet disruption. The disruption of fuel oil droplets with 10 vol% of water colliding with each other is provided at a critical Weber number of approximately 150. For fuel oil droplets colliding with water droplets, this number decreases to approximately 50. We have also shown the role of liquid heating in the occurrence of the collision regimes under study. Droplets of pure fuel oil and fuel oil with added water undergo the most significant atomization when impinging on a heated solid wall with a certain layer of fuel adhering to it.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en16021008/s1, Video S1: Video frames showing droplets of two-component fuel colliding with each other.; Video S2: Video frames showing a droplet of two-component fuel impinging on a substrate heated to 300 °C; Video S3: Video frames showing a droplet of two-component fuel impinging on a substrate heated to 200 °C.
Author Contributions
Conceptualization, G.K.; methodology, N.S. and P.T.; investigation, A.I., N.S. and P.T.; writing—original draft preparation, A.I. and N.S.; writing—review and editing, A.I., G.K. and P.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Russian Science Foundation (project 18-71-10002-π, https://rscf.ru/en/project/21-71-03001/, accessed on 30 July 2021).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| B | linear approach parameter, mm; |
| B | dimensionless linear interaction parameter; |
| Dd | initial droplet diameter, mm; |
| Dmax | diameter at the moment of droplet spreading on the surface, mm; |
| dimensionless diameter; | |
| DS | initial diameter of the smaller droplet, m; |
| DL | initial diameter of the larger droplet, m; |
| ED | viscous dissipation energy, J; |
| Eθ | energy from the work performed by the radial forces applied from the wall to the contact line, J; |
| Edr | kinetic energy transformed into rotational energy and viscous dissipation energy, J; |
| Esd | post-collision kinetic energy of droplets, J; |
| EP0 and EPs | potential energy of a droplet before and after collision, J; |
| ES0 and ESs | surface energy of a droplet before and after collision, J; |
| h | deformed droplet height, m; |
| hmax | droplet height at the moment of droplet spreading on the surface, mm; |
| Oh | Ohnesorge number; |
| N | number of newly formed post-collision droplets, pcs; |
| Re | Reynolds number; |
| Rd1, Rd2 | radius of the first and second droplets, m; |
| rd | radius of the post-collision droplet, m; |
| Ra | arithmetical mean deviation of the profile, m; |
| Rav | average droplet radius; |
| Rz | average peak-to-valley roughness, m; |
| S0 | free surface area of droplets before collision, m2; |
| S1 | free surface area of post-collision droplets, m2; |
| S1/S0 | ratio of the free surface area of droplets after and before collision; |
| KEi, KEii, KEni, KEsii, KEdii, KEsni, KErni | kinetic energies of stretching separation, J; |
| KEes | kinetic energy of stretching separation, J; |
| SElig | surface tension energy of the bridge, J; |
| T | temperature of the composition, °C; |
| Ts | temperature of the solid wall, °C; |
| Ud1, Ud2 | velocity of the first and second droplets, m/s; |
| US | velocity of the smaller droplet accounting for relative droplet velocity, m/s; |
| UL | velocity of the larger droplet accounting for relative droplet velocity, m/s; |
| Ud | velocity of the droplet before collision, m/s; |
| Urel | resulting (relative) velocity of droplet, m/s; |
| VDE | dissipation energy, J; |
| VDEsii, VDEdii, VDEsni, VDErni | dissipation energy during stretching separation, J; |
| RE | revolution energy, J; |
| Vli | volume of the interaction zone of the large droplet, m3; |
| Vsi | volume of the interaction zone of the smaller droplet, m3; |
| VL | volume of the large droplet, m3; |
| VS | volume of the smaller droplet, m3; |
| We | Weber number; |
| , | dimensionless constants. |
| Greek symbols | |
| αd | impact angle, °; |
| α1, α2, α3, α4, α5 | dissipation factors; |
| ρ | density, kg/m3; |
| σ | surface tension, N/m; |
| µ | dynamic viscosity, Pa∙s; |
| Δ | ratio of droplet–droplet radii; |
| τ | impact angle coefficient; |
| χL | coefficient accounting for the interaction zone of the larger droplet; |
| χS | coefficient accounting for the interaction zone of the smaller droplet; |
| ⟨θ⟩ | average dynamic wettability angle, °. |
| Abbreviations | |
| BO | bounce; |
| CO | coalescence; |
| CoR | coefficient of restitution; |
| DI | disruption; |
| SE | separation. |
References
- Hamza, J.E.; Al-Kayiem, H.H.; Lemma, T.A. Experimental Investigation of the Separation Performance of Oil/Water Mixture by Compact Conical Axial Hydrocyclone. Therm. Sci. Eng. Prog. 2020, 17, 100358. [Google Scholar] [CrossRef]
- Huang, B.; Nan, X.; Fu, C.; Liu, W.; Guo, W.; Wang, S.; Zhang, L. Probing the Coalescence Mechanism of Oil Droplets in Fluids Produced by Oil Wells and the Microscopic Interaction between Molecules in Oil Films. Energies 2022, 15, 4274. [Google Scholar] [CrossRef]
- Wu, G.; Chen, S. Comparison of Droplet-Particle Interaction on a Stationary and a Moving Particle. Chem. Eng. Sci. 2022, 253, 117552. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, A. Numerical and Experimental Analysis of Rapid Solidification Considering Undercooling Effect during Water Droplet Impact on a Substrate. Therm. Sci. Eng. Prog. 2020, 20, 100722. [Google Scholar] [CrossRef]
- Wang, L.; Ding, Y.; Qiu, Y.; Yu, Y.; Xie, J.; Chen, J. Numerical Study on Spreading and Vaporization Process of Liquid Nitrogen Droplet Impinging on Heated Wall. Energies 2022, 15, 8700. [Google Scholar] [CrossRef]
- Khojasteh, D.; Kazerooni, N.M.; Marengo, M. A Review of Liquid Droplet Impacting onto Solid Spherical Particles: A Physical Pathway to Encapsulation Mechanisms. J. Ind. Eng. Chem. 2019, 71, 50–64. [Google Scholar] [CrossRef]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An Overview of Encapsulation Technologies for Food Applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Zhao, Q.; Yin, M.; Zhang, A.P.; Prescher, S.; Antonietti, M.; Yuan, J. Hierarchically Structured Nanoporous Poly(Ionic Liquid) Membranes: Facile Preparation and Application in Fiber-Optic PH Sensing. J. Am. Chem. Soc. 2013, 135, 5549–5552. [Google Scholar] [CrossRef]
- Ho, P.K.H.; Kim, J.I.S.; Burroughes, J.H.; Becker, H.; Li, S.F.Y.; Brown, T.M.; Cacialli, F.; Friend, R.H. Molecular-Scale Interface Engineering for Polymer Light-Emitting Diodes. Nature 2000, 404, 481–484. [Google Scholar] [CrossRef]
- Wood, K.C.; Boedicker, J.Q.; Lynn, D.M.; Hammond, P.T. Tunable Drug Release from Hydrolytically Degradable Layer-by-Layer Thin Films. Langmuir 2005, 21, 1603–1609. [Google Scholar] [CrossRef]
- Muddapur, A.; Sahu, S.; Jose, J.V.; Sundararajan, T. Spray–Wall Impingement in a Multi-Hole GDI Injector for Split Injection at Elevated Wall Temperature and Ambient Conditions. Therm. Sci. Eng. Prog. 2022, 33, 101367. [Google Scholar] [CrossRef]
- Mukhtar, M.N.A.; Aziz, A.R.A.; Hagos, F.Y.; Noor, M.M.; Kadirgama, K.; Mamat, R.; Abdullah, A.A. The Influence of Formulation Ratio and Emulsifying Settings on Tri-Fuel (Diesel–Ethanol–Biodiesel) Emulsion Properties. Energies 2019, 12, 1708. [Google Scholar] [CrossRef]
- Safiullah; Mahmud, R.; Nishida, K.; Ogata, Y. Experimental and Computational Study of Diesel Spray under Nonevaporating and Evaporating Conditions—Effects of Nozzle Hole Diameter and Injection Pressure. At. Sprays 2020, 30, 627–649. [Google Scholar] [CrossRef]
- Antonov, D.V.; Volkov, R.S.; Strizhak, P.A. An Explosive Disintegration of Heated Fuel Droplets with Adding Water. Chem. Eng. Res. Des. 2018, 140, 292–307. [Google Scholar] [CrossRef]
- Malgarinos, I.; Nikolopoulos, N.; Gavaises, M. Numerical Investigation of Heavy Fuel Droplet-Particle Collisions in the Injection Zone of a Fluid Catalytic Cracking Reactor, Part I: Numerical Model and 2D Simulations. Fuel Process. Technol. 2017, 156, 317–330. [Google Scholar] [CrossRef]
- Suzuki, Y.; Harada, T.; Watanabe, H.; Shoji, M.; Matsushita, Y.; Aoki, H.; Miura, T. Visualization of Aggregation Process of Dispersed Water Droplets and the Effect of Aggregation on Secondary Atomization of Emulsified Fuel Droplets. Proc. Combust. Inst. 2011, 33, 2063–2070. [Google Scholar] [CrossRef]
- Watanabe, H.; Harada, T.; Matsushita, Y.; Aoki, H.; Miura, T. The Characteristics of Puffing of the Carbonated Emulsified Fuel. Int. J. Heat Mass Transf. 2009, 52, 3676–3684. [Google Scholar] [CrossRef]
- Yin, Z.; Nau, P.; Meier, W. Responses of Combustor Surface Temperature to Flame Shape Transitions in a Turbulent Bi-Stable Swirl Flame. Exp. Therm. Fluid Sci. 2017, 82, 50–57. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Pradhan, S.; Pati, A.R.; Barik, K. Theoretical and Experimental Investigation of the Role of Viscosity and Surface Tension in Dropwise Evaporation at Very High Substrate Temperature. Therm. Sci. Eng. Prog. 2019, 9, 200–214. [Google Scholar] [CrossRef]
- Warncke, K.; Gepperth, S.; Sauer, B.; Sadiki, A.; Janicka, J.; Koch, R.; Bauer, H.-J. Experimental and Numerical Investigation of the Primary Breakup of an Airblasted Liquid Sheet. Int. J. Multiph. Flow 2017, 91, 208–224. [Google Scholar] [CrossRef]
- Tarlet, D.; Allouis, C.; Bellettre, J. The Balance between Surface and Kinetic Energies within an Optimal Micro-Explosion. Int. J. Therm. Sci. 2016, 107, 179–183. [Google Scholar] [CrossRef]
- Bachalo, W.D. Spray Diagnostics for the Twenty-First Century. At. Sprays 2000, 10, 439–474. [Google Scholar] [CrossRef]
- Lefebvre, A.H.; McDonell, V.G. Atomization and Sprays, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781498736268. [Google Scholar]
- Cen, C.; Wu, H.; Lee, C.; Liu, F.; Li, Y. Experimental Investigation on the Characteristic of Jet Break-up for Butanol Droplet Impacting onto a Heated Surface in the Film Boiling Regime. Int. J. Heat Mass Transf. 2018, 123, 129–136. [Google Scholar] [CrossRef]
- Jung, S.; Hoath, S.D.; Martin, G.D.; Hutchings, I.M. Experimental Study of Atomization Patterns Produced by the Oblique Collision of Two Viscoelastic Liquid Jets. J. Nonnewton. Fluid Mech. 2011, 166, 297–306. [Google Scholar] [CrossRef]
- Planchette, C.; Lorenceau, E.; Brenn, G. Liquid Encapsulation by Binary Collisions of Immiscible Liquid Drops. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 365, 89–94. [Google Scholar] [CrossRef]
- Zhang, X.; Li, T.; Wang, B.; Wei, Y. Superheat Limit and Micro-Explosion in Droplets of Hydrous Ethanol-Diesel Emulsions at Atmospheric Pressure and Diesel-like Conditions. Energy 2018, 154, 535–543. [Google Scholar] [CrossRef]
- Avulapati, M.M.; Ganippa, L.C.; Xia, J.; Megaritis, A. Puffing and Micro-Explosion of Diesel-Biodiesel-Ethanol Blends. Fuel 2016, 166, 59–66. [Google Scholar] [CrossRef]
- Cao, X.-K.; Sun, Z.-G.; Li, W.-F.; Liu, H.-F.; Yu, Z.-H. A New Breakup Regime of Liquid Drops Identified in a Continuous and Uniform Air Jet Flow. Phys. Fluids 2007, 19, 57103. [Google Scholar] [CrossRef]
- Lee, C.H.; Reitz, R.D. An Experimental Study of the Effect of Gas Density on the Distortion and Breakup Mechanism of Drops in High Speed Gas Stream. Int. J. Multiph. Flow 2000, 26, 229–244. [Google Scholar] [CrossRef]
- Xu, C.; Cho, H. Effect of Methanol/Water Mixed Fuel Compound Injection on Engine Combustion and Emissions. Energies 2021, 14, 4491. [Google Scholar] [CrossRef]
- Buck, B.; Tang, Y.; Deen, N.G.; Kuipers, J.A.M.; Heinrich, S. Dynamics of Wet Particle–Wall Collisions: Influence of Wetting Condition. Chem. Eng. Res. Des. 2018, 135, 21–29. [Google Scholar] [CrossRef]
- Negeed, E.-S.R.; Ishihara, N.; Tagashira, K.; Hidaka, S.; Kohno, M.; Takata, Y. Experimental Study on the Effect of Surface Conditions on Evaporation of Sprayed Liquid Droplet. Int. J. Therm. Sci. 2010, 49, 2250–2271. [Google Scholar] [CrossRef]
- Orme, M. Experiments on Droplet Collisions, Bounce, Coalescence and Disruption. Prog. Energy Combust. Sci. 1997, 23, 65–79. [Google Scholar] [CrossRef]
- Li, N.; Sun, Z.; Pang, Y.; Qi, Z.; Liu, W.; Li, W.; Sun, M.; Li, B.; Wang, Z. Microscopic Mechanism for Electrocoalescence of Water Droplets in Water-in-Oil Emulsions Containing Surfactant: A Molecular Dynamics Study. Sep. Purif. Technol. 2022, 289, 120756. [Google Scholar] [CrossRef]
- Wang, Z.; Li, N.; Sun, Z.; Wang, X.; Chen, Q.; Liu, W.; Qi, Z.; Wei, L.; Li, B. Molecular Dynamics Study of Droplet Electrocoalescence in the Oil Phase and the Gas Phase. Sep. Purif. Technol. 2021, 278, 119622. [Google Scholar] [CrossRef]
- Rommel, W.; Blass, E. Hydrodynamic Modeling of Droplet Coalescence at Liquid-Liquid Interfaces. Sep. Sci. Technol. 1992, 27, 129–159. [Google Scholar] [CrossRef]
- Ashgriz, N.; Givi, P. Coalescence Efficiencies of Fuel Droplets in Binary Collisions. Int. Commun. Heat Mass Transf. 1989, 16, 11–20. [Google Scholar] [CrossRef]
- Szakáll, M.; Urbich, I. Wind Tunnel Study on the Size Distribution of Droplets after Collision Induced Breakup of Levitating Water Drops. Atmos. Res. 2018, 213, 51–56. [Google Scholar] [CrossRef]
- Kuan, C.-K.; Pan, K.-L.; Shyy, W. Study on High-Weber-Number Droplet Collision by a Parallel, Adaptive Interface-Tracking Method. J. Fluid Mech. 2014, 759, 104–133. [Google Scholar] [CrossRef]
- Solomatin, Y.; Shlegel, N.E.E.; Strizhak, P.A.A. Atomization of Promising Multicomponent Fuel Droplets by Their Collisions. Fuel 2019, 255, 115751. [Google Scholar] [CrossRef]
- Daho, T.; Vaitilingom, G.; Sanogo, O. Optimization of the Combustion of Blends of Domestic Fuel Oil and Cottonseed Oil in a Non-Modified Domestic Boiler. Fuel 2009, 88, 1261–1268. [Google Scholar] [CrossRef]
- Blinov, E.A. Fuel and Combustion Theory; SPB Publishing House of SZTU: Saint Petersburg, Russia, 2007; 120p. [Google Scholar]
- Šlančiauskas, A.; Kalpokaitė, R. Behaviour of a Heavy Fuel Oil Droplet on a Hot Surface. Int. J. Heat Mass Transf. 2006, 49, 1050–1057. [Google Scholar] [CrossRef]
- Alekseenko, S.V.; Maltsev, L.I.; Bogomolov, A.R.; Chernetskiy, M.Y.; Kravchenko, I.V.; Kravchenko, A.I.; Lapin, D.A.; Shevyrev, S.A.; Lyrshchikov, S.Y. Results of Pilot-Operating Combustion of Coal-Water Fuel in a Low-Capacity Hot Water Boiler. Bull. Tomsk Polytech. Univ. Geo Assets Eng. 2017, 328, 16–28. [Google Scholar]
- Pei, X.; Guida, P.; AlAhmadi, K.M.; Al Ghamdi, I.A.; Saxena, S.; Roberts, W.L. Cenosphere Formation of Heavy Fuel Oil/Water Emulsion Combustion in a Swirling Flame. Fuel Process. Technol. 2021, 216, 106800. [Google Scholar] [CrossRef]
- Lisienko, V.G.; Shchelokov, Y.M.; Rozin, S.E.; Ladygichev, M.G.; Druzhinina, O.G. Questions Regarding Standardization of the Indicators of Efficiency in Using Fuel and Energy Resources. Therm. Eng. 2004, 51, 847–851. [Google Scholar]
- Tang, C.; Qin, M.; Weng, X.; Zhang, X.; Zhang, P.; Li, J.; Huang, Z. Dynamics of Droplet Impact on Solid Surface with Different Roughness. Int. J. Multiph. Flow 2017, 96, 56–69. [Google Scholar] [CrossRef]
- Ithnin, A.M.; Noge, H.; Kadir, H.A.; Jazair, W. An Overview of Utilizing Water-in-Diesel Emulsion Fuel in Diesel Engine and Its Potential Research Study. J. Energy Inst. 2014, 87, 273–288. [Google Scholar] [CrossRef]
- Antonov, D.; Bellettre, J.; Tarlet, D.; Massoli, P.; Vysokomornaya, O.; Piskunov, M. Impact of Holder Materials on the Heating and Explosive Breakup of Two-Component Droplets. Energies 2018, 11, 3307. [Google Scholar] [CrossRef]
- Demidovich, A.V.; Kropotova, S.S.; Piskunov, M.V.; Shlegel, N.E.; Vysokomornaya, O.V. The Impact of Single-and Multicomponent Liquid Drops on a Heatedwall: Child Droplets. Appl. Sci. 2020, 10, 942. [Google Scholar] [CrossRef]
- Liang, G.; Mudawar, I. Review of Mass and Momentum Interactions during Drop Impact on a Liquid Film. Int. J. Heat Mass Transf. 2016, 101, 577–599. [Google Scholar] [CrossRef]
- Šikalo, Š.; Marengo, M.; Tropea, C.; Ganić, E.N. Analysis of Impact of Droplets on Horizontal Surfaces. Exp. Therm. Fluid Sci. 2002, 25, 503–510. [Google Scholar] [CrossRef]
- Finotello, G.; Kooiman, R.F.; Padding, J.T.; Buist, K.A.; Jongsma, A.; Innings, F.; Kuipers, J.A.M. The Dynamics of Milk Droplet–Droplet Collisions. Exp. Fluids 2018, 59, 17. [Google Scholar] [CrossRef]
- Williams, Y.O.; Roas-Escalona, N.; Rodríguez-Lopez, G.; Villa-Torrealba, A.; Toro-Mendoza, J. Modeling Droplet Coalescence Kinetics in Microfluidic Devices Using Population Balances. Chem. Eng. Sci. 2019, 201, 475–483. [Google Scholar] [CrossRef]
- Subcommittee, A. Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity); ASTM International: West Conshohocken, PA, USA, 2006. [Google Scholar]
- Finotello, G.; Padding, J.T.; Deen, N.G.; Jongsma, A.; Innings, F.; Kuipers, J.A.M. Effect of Viscosity on Droplet-Droplet Collisional Interaction. Phys. Fluids 2017, 29, 67102. [Google Scholar] [CrossRef]
- Qian, J.; Law, C.K. Regimes of Coalescence and Separation in Droplet Collision. J. Fluid Mech. 1997, 331, 59–80. [Google Scholar] [CrossRef]
- Ko, G.H.; Ryou, H.S. Modeling of Droplet Collision-Induced Breakup Process. Int. J. Multiph. Flow 2005, 31, 723–738. [Google Scholar] [CrossRef]
- Shlegel, N.E.; Ya, S.; Strizhak, P.A. Experimental Research into the Characteristics of Child Droplets Formed Due to Collisions of Liquid Fragments in a Gas. Powder Technol. 2019, 363, 122–134. [Google Scholar] [CrossRef]
- Finotello, G.; Padding, J.T.; Buist, K.A.; Jongsma, A.; Innings, F.; Kuipers, J.A.M. Droplet Collisions of Water and Milk in a Spray with Langevin Turbulence Dispersion. Int. J. Multiph. Flow 2019, 114, 154–167. [Google Scholar] [CrossRef]
- Sommerfeld, M.; Pasternak, L. Advances in Modelling of Binary Droplet Collision Outcomes in Sprays: A Review of Available Knowledge. Int. J. Multiph. Flow 2019, 117, 182–205. [Google Scholar] [CrossRef]
- Al-Dirawi, K.H.; Bayly, A.E. An Experimental Study of Binary Collisions of Miscible Droplets with Non-Identical Viscosities. Exp. Fluids 2020, 61, 50. [Google Scholar] [CrossRef]
- Chen, R.-H.; Chen, C.-T. Collision between Immiscible Drops with Large Surface Tension Difference: Diesel Oil and Water. Exp. Fluids 2006, 41, 453–461. [Google Scholar] [CrossRef]
- Charalampous, G.; Hardalupas, Y. Collisions of Droplets on Spherical Particles. Phys. Fluids 2017, 29, 103305. [Google Scholar] [CrossRef]
- Shebeleva, A.A.; Minakov, A.V.; Chernetskii, M.Y.; Strizhak, P.A. Deformation of a Droplet of an Organic Water-Coal Fuel in a Gas Flow. J. Appl. Mech. Technol. Phys. 2018, 59, 653–661. [Google Scholar] [CrossRef]
- Vedachalam, S.; Dalai, A.K. Hydrotreating and Oxidative Desulfurization of Heavy Fuel Oil into Low Sulfur Marine Fuel over Dual Function NiMo/γ–Al2O3 Catalyst. Catal. Today 2022, 407, 165–171. [Google Scholar] [CrossRef]
- Fostiropoulos, S.; Strotos, G.; Nikolopoulos, N.; Gavaises, M. Numerical Investigation of Heavy Fuel Oil Droplet Breakup Enhancement with Water Emulsions. Fuel 2020, 278, 118381. [Google Scholar] [CrossRef]
- Hu, C.; Xia, S.; Li, C.; Wu, G. Three-Dimensional Numerical Investigation and Modeling of Binary Alumina Droplet Collisions. Int. J. Heat Mass Transf. 2017, 113, 569–588. [Google Scholar] [CrossRef]
- Roisman, I.V.; Rioboo, R.; Tropea, C. Normal Impact of a Liquid Drop on a Dry Surface: Model for Spreading and Receding. Proc. R. Soc. A Math. Phys. Eng. Sci. 2002, 458, 1411–1430. [Google Scholar] [CrossRef]
- Mitra, S.; Nguyen, T.B.T.; Doroodchi, E.; Pareek, V.; Joshi, J.B.; Evans, G.M. On Wetting Characteristics of Droplet on a Spherical Particle in Film Boiling Regime. Chem. Eng. Sci. 2016, 149, 181–203. [Google Scholar] [CrossRef]
- Ocampo-Barrera, R.; Villasenor, R.; Diego-Marin, A. An Experimental Study of the Effect of Water Content on Combustion of Heavy Fuel Oil/Water Emulsion Droplets. Combust. Flame 2001, 126, 1845–1855. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Meng, X.; Dong, D.; Jiang, L.; Long, W.; Dan, J. An Investigation of Emulsified Heavy Fuel Oil with High Water Content Applied to a High-Speed Diesel Engine. J. Energy Eng. 2022, 148, 04022023. [Google Scholar] [CrossRef]
- Darbandi, M.; Fatin, A.; Bordbar, H. Numerical Study on NOx Reduction in a Large-Scale Heavy Fuel Oil-Fired Boiler Using Suitable Burner Adjustments. Energy 2020, 199, 117371. [Google Scholar] [CrossRef]
- Chen, R.H.; Lai, C.-M. Collision Outcome of a Water Drop on the Surface of a Deep Diesel Fuel Pool. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2011, 225, 1638–1648. [Google Scholar] [CrossRef]
- Shirvani, S.; Shirvani, S.; Shamekhi, A.H. Effects of Injection Parameters and Injection Strategy on Emissions and Performance of a Two-Stroke Opposed-Piston Diesel Engine; SAE Techical Paper 2020-01-5064; SAE International: Warrendale, PA, USA, 2020. [Google Scholar] [CrossRef]
- Gad, M.S.; Mahfouz, A.; Emara, A. Spray and Combustion Characteristics for Light Diesel/Waste Cooking Oils Blended with Fuel Additives inside an Industrial Boiler. Fuel 2021, 286, 119247. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).