Detection of Manufacturing Defects in Lithium-Ion Batteries-Analysis of the Potential of Computed Tomography Imaging
Abstract
:1. Introduction
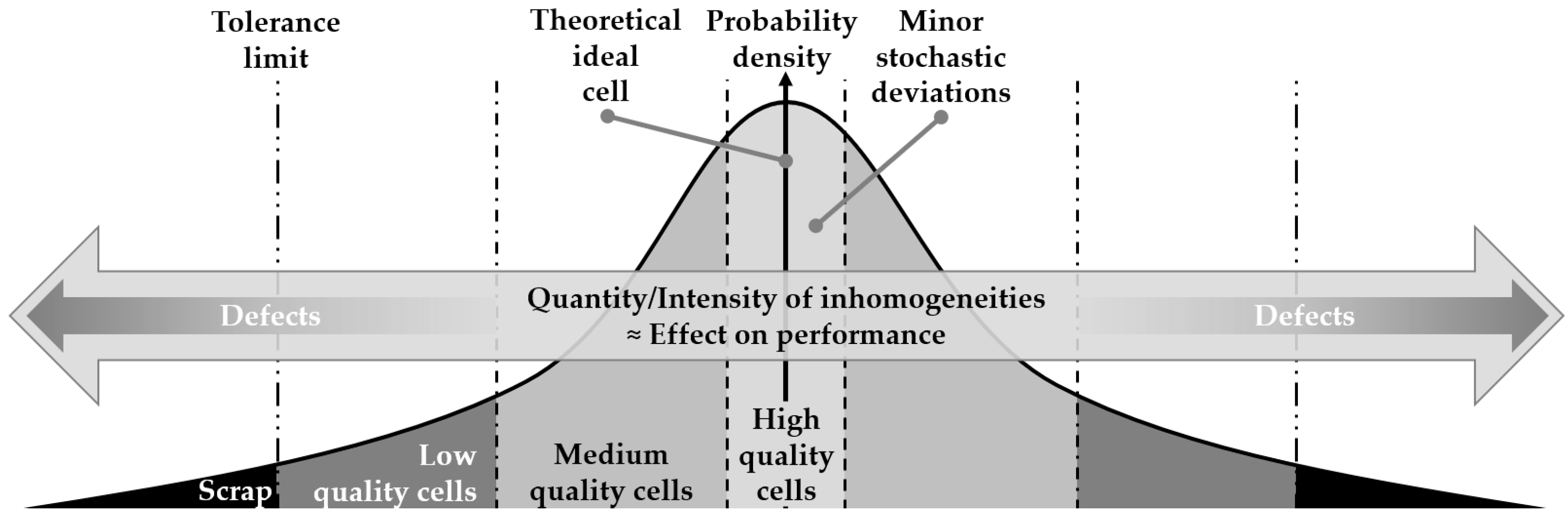
1.1. Terminology of Inhomogeneities
1.2. Intrinsic Inhomogeneities
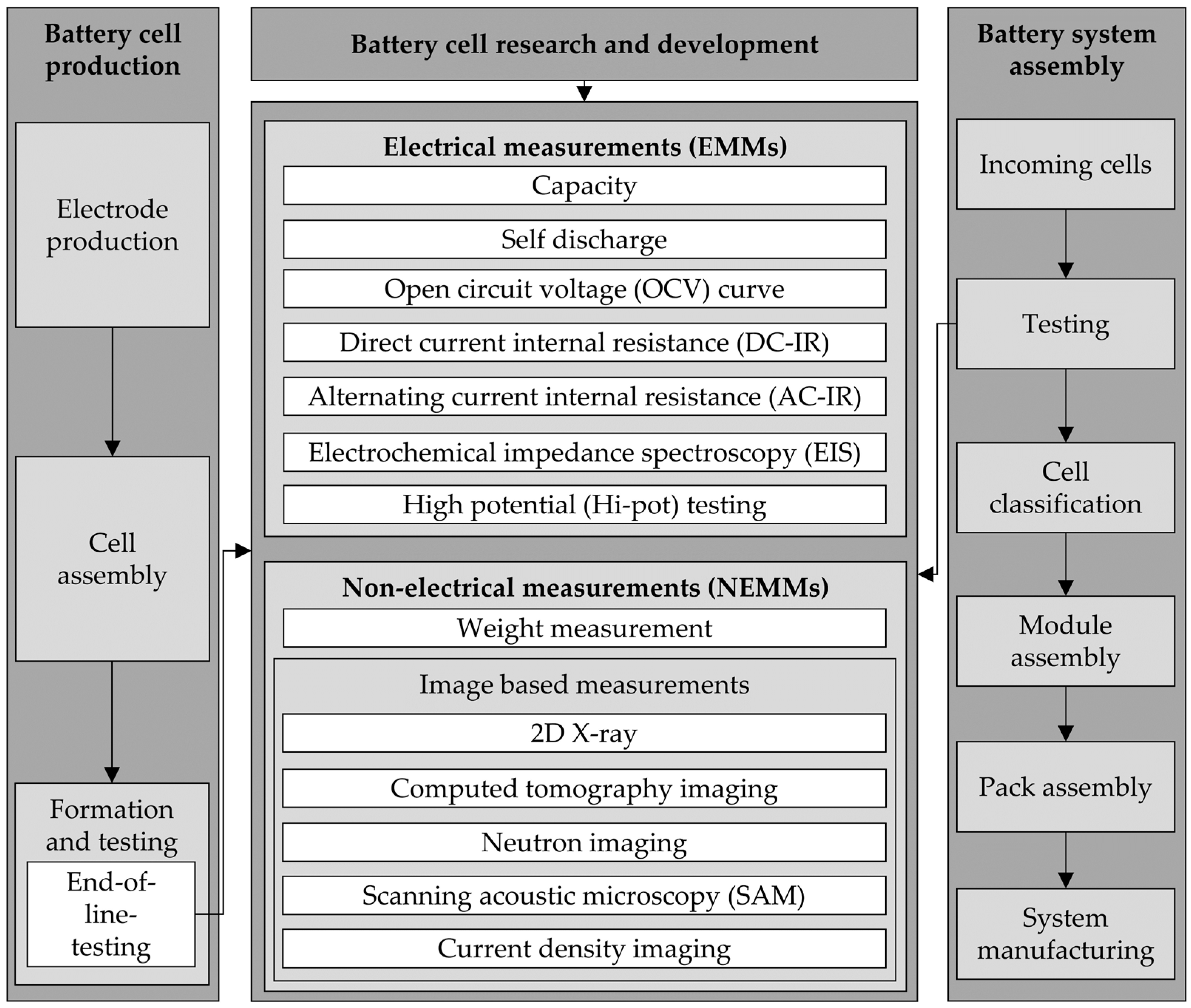
1.3. Measurement Methods
2. Experimental
2.1. Cell Specifications
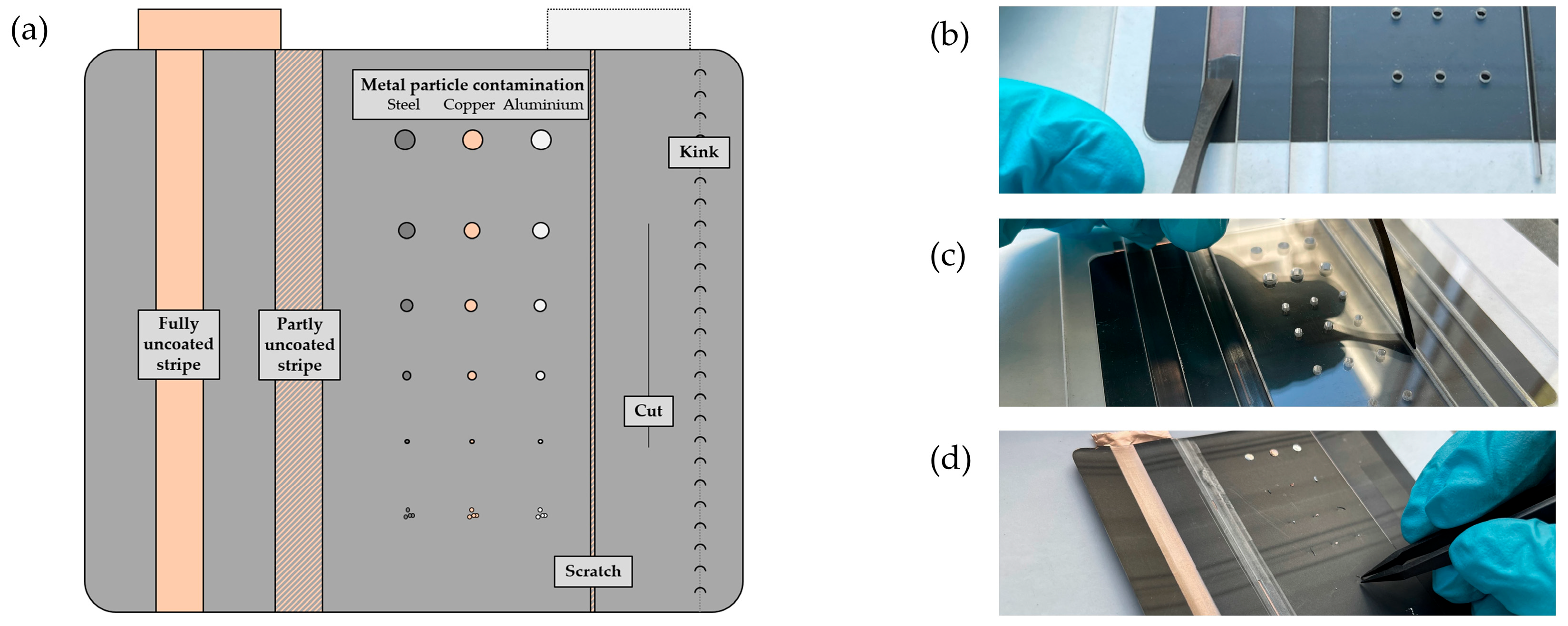
2.2. Preparation and Manipulation of the Cell
2.3. CT, 2D X-Ray and LSM Measurements
3. Results and Discussion
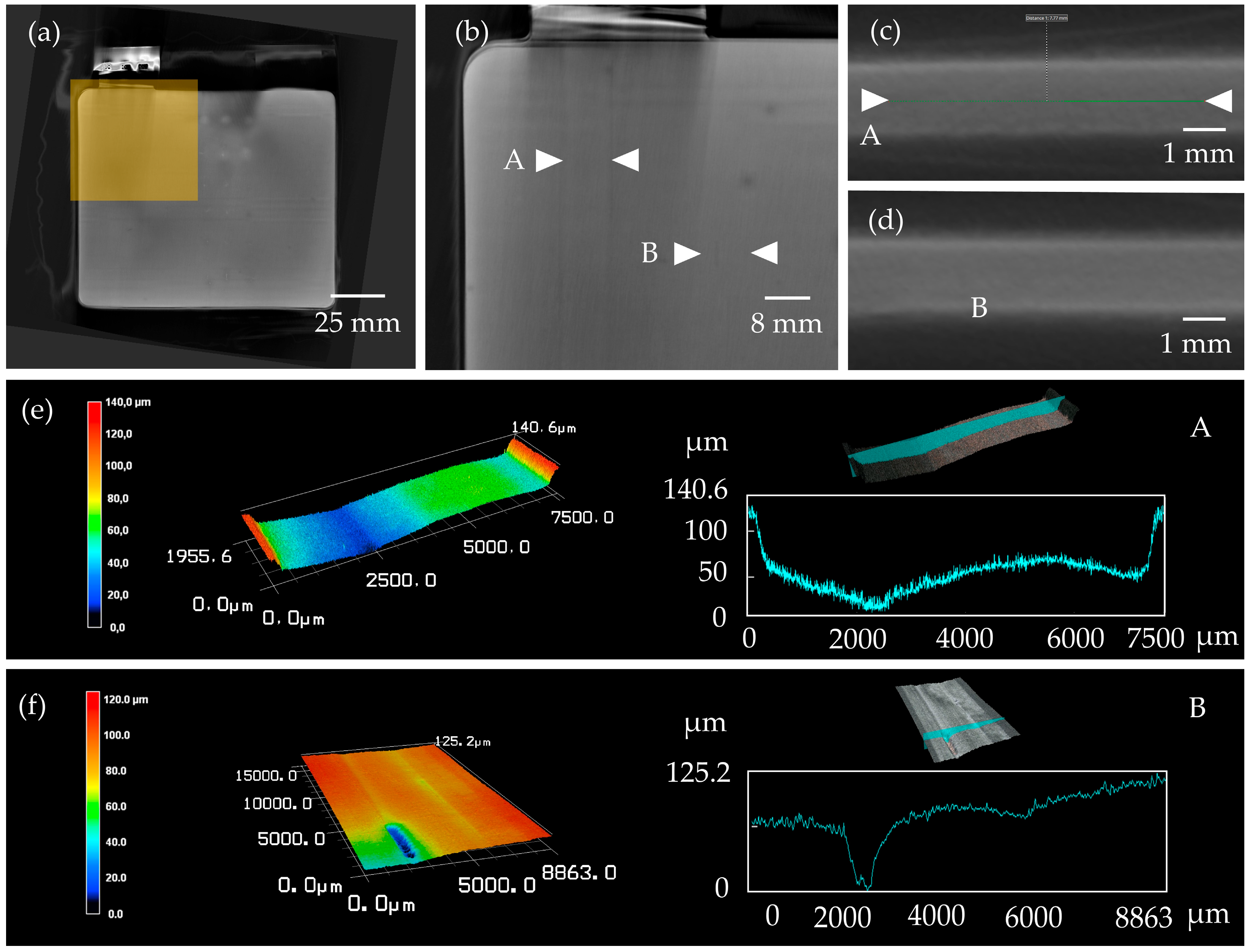
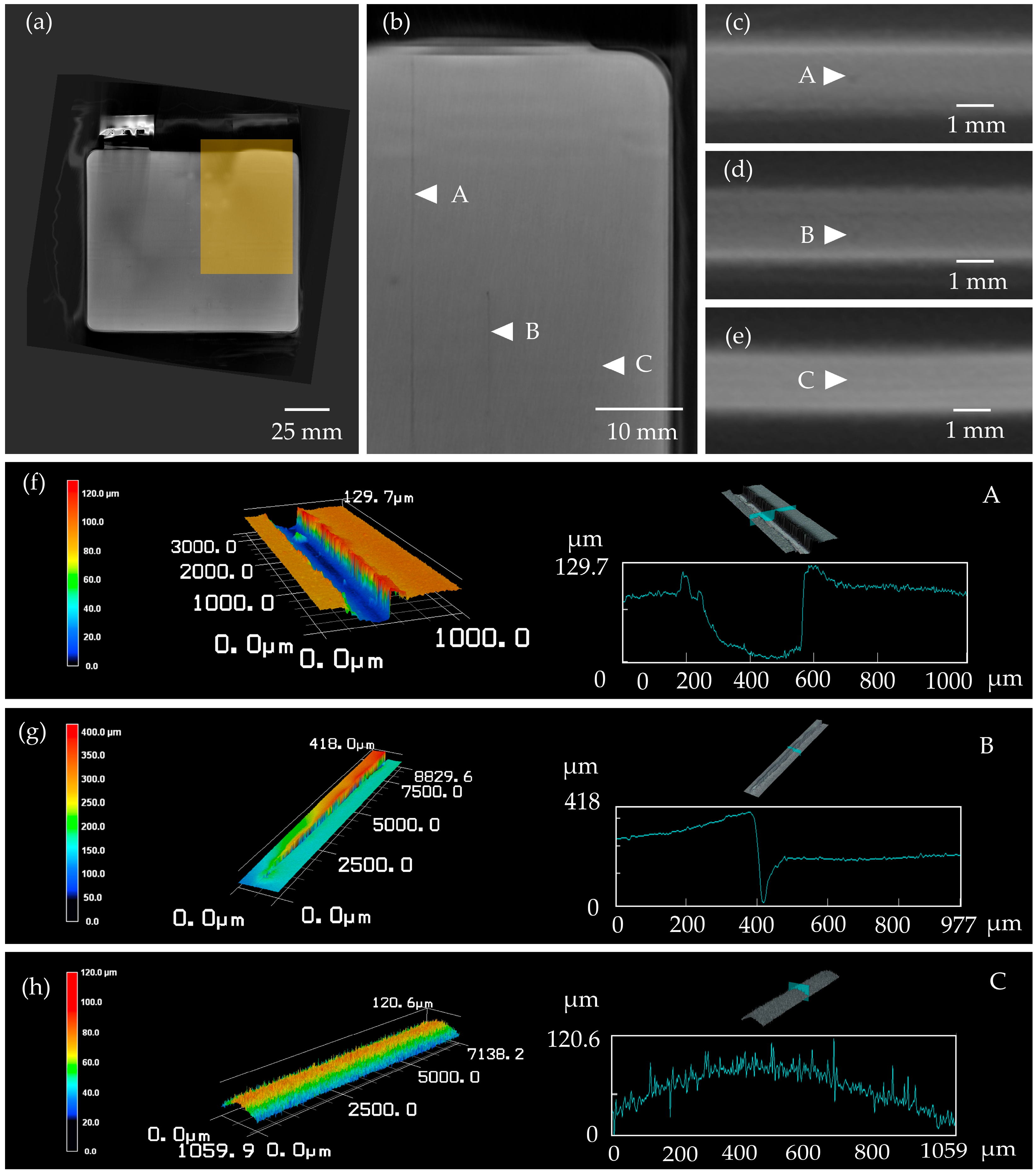
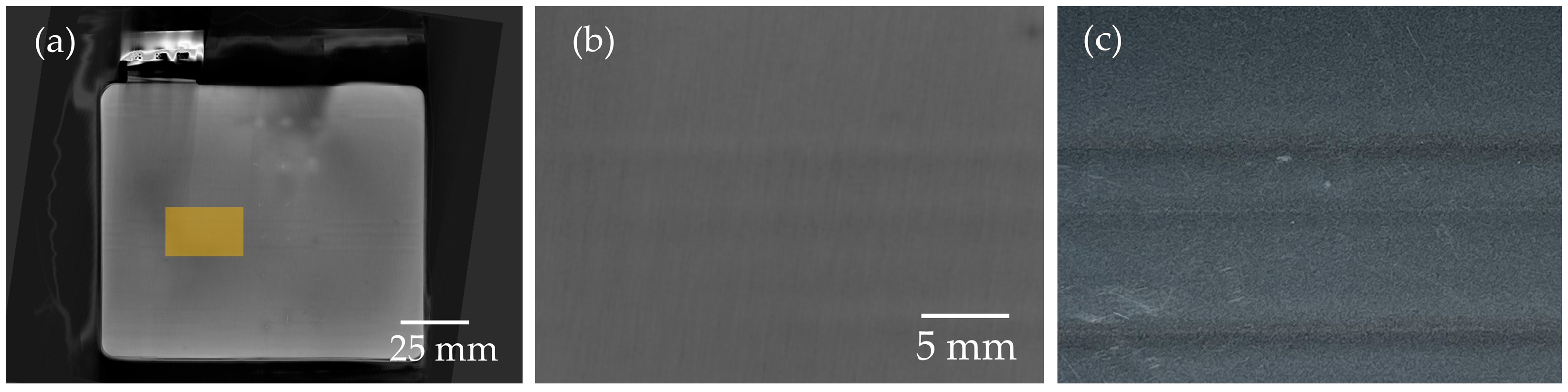
3.1. Defects on Electrode Level
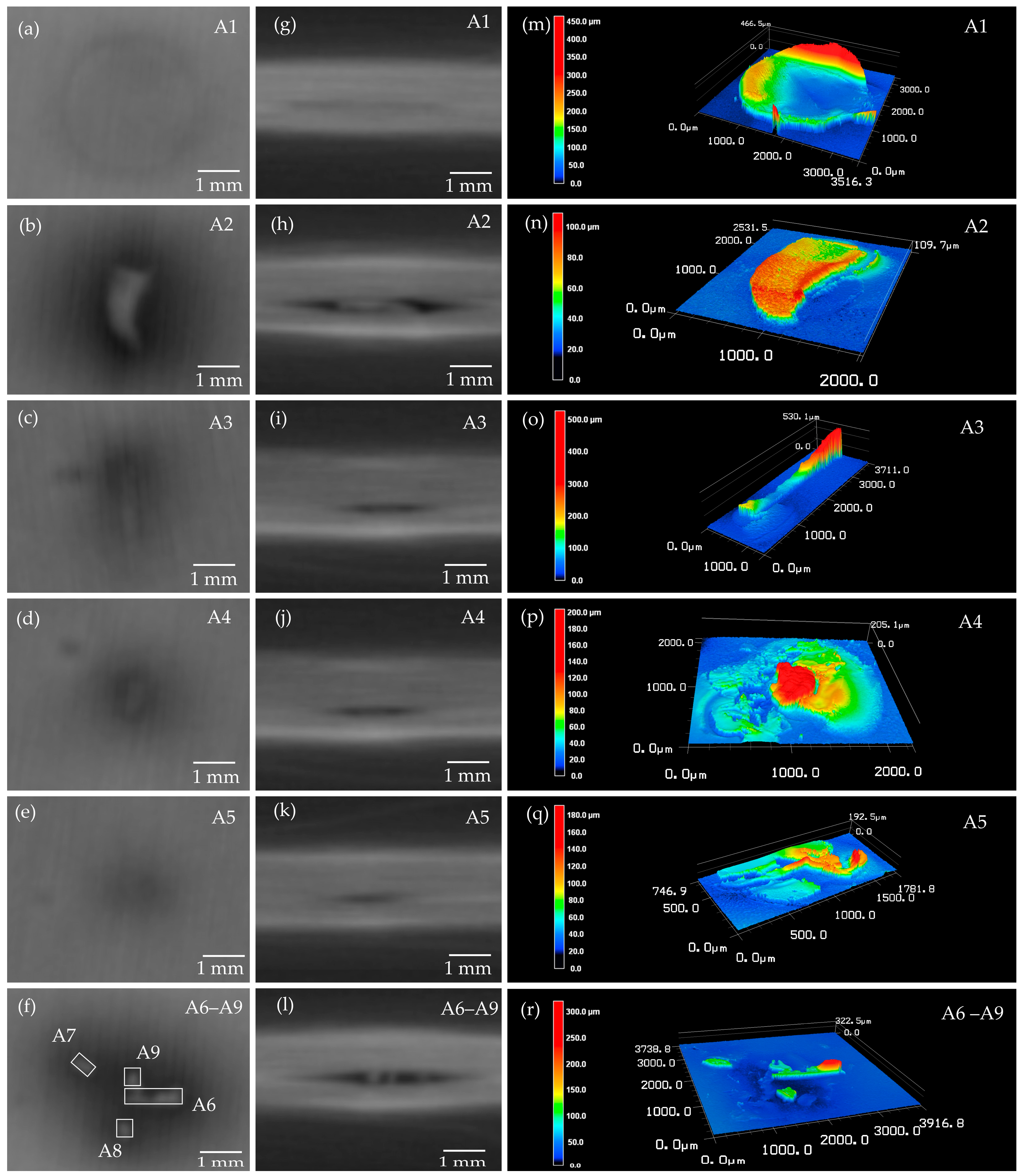
3.2. Metal-Particle Contamination on Cell Level
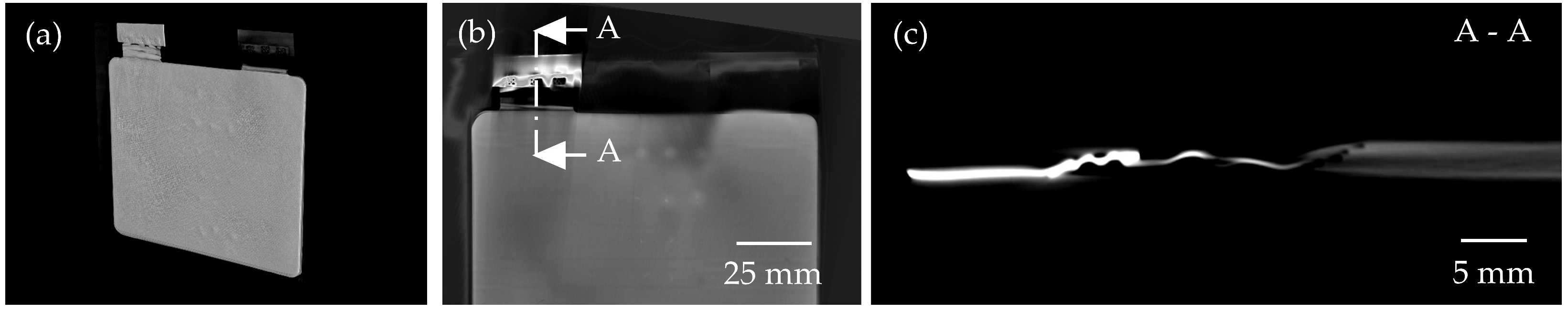
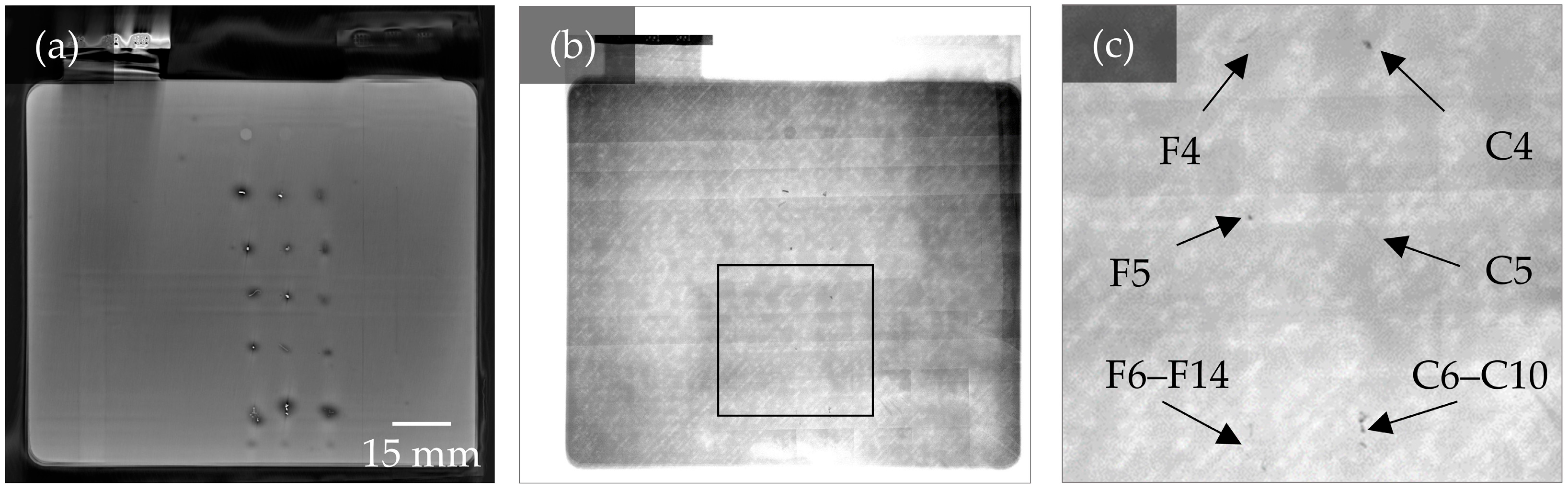
3.3. Analysis of the Potential of CT Imaging
3.4. Comparison of 3D CT Imaging and 2D X-Ray Image
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, S.; Xu, J.; Lv, M.; Zhu, Z.; Jia, J.; Li, W.; Weng, W. The Application of Industrial CT Detection Technology in Defects inspection of lithium Ion Battery. J. Phys. Conf. Ser. 2021, 2083, 32075. [Google Scholar] [CrossRef]
- Lamb, J.; Orendorff, C.J. Evaluation of mechanical abuse techniques in lithium ion batteries. J. Power Sources 2014, 247, 189–196. [Google Scholar] [CrossRef]
- Waldmann, T.; Gorse, S.; Samtleben, T.; Schneider, G.; Knoblauch, V.; Wohlfahrt-Mehrens, M. A Mechanical Aging Mechanism in Lithium-Ion Batteries. J. Electrochem. Soc. 2014, 161, A1742–A1747. [Google Scholar] [CrossRef]
- Yufit, V.; Shearing, P.; Hamilton, R.W.; Lee, P.D.; Wu, M.; Brandon, N.P. Investigation of lithium-ion polymer battery cell failure using X-ray computed tomography. Electrochem. Commun. 2011, 13, 608–610. [Google Scholar] [CrossRef]
- Villarraga-Gómez, H.; Begun, D.L.; Bhattad, P.; Mo, K.; Norouzi Rad, M.; White, R.T.; Kelly, S.T. Assessing rechargeable batteries with 3D X-ray microscopy, computed tomography, and nanotomography. Nondestruct. Test. Eval. 2022, 37, 519–535. [Google Scholar] [CrossRef]
- De Chiffre, L.; Carmignato, S.; Kruth, J.-P.; Schmitt, R.; Weckenmann, A. Industrial applications of computed tomography. CIRP Ann. 2014, 63, 655–677. [Google Scholar] [CrossRef]
- Yao, X.-Y.; Pecht, M.G. Tab Design and Failures in Cylindrical Li-ion Batteries. IEEE Access 2019, 7, 24082–24095. [Google Scholar] [CrossRef]
- Finegan, D.P.; Scheel, M.; Robinson, J.B.; Tjaden, B.; Hunt, I.; Mason, T.J.; Millichamp, J.; Di Michiel, M.; Offer, G.J.; Hinds, G.; et al. In-operando high-speed tomography of lithium-ion batteries during thermal runaway. Nat. Commun. 2015, 6, 6924. [Google Scholar] [CrossRef]
- Ziesche, R.F.; Arlt, T.; Finegan, D.P.; Heenan, T.M.M.; Tengattini, A.; Baum, D.; Kardjilov, N.; Markötter, H.; Manke, I.; Kockelmann, W.; et al. 4D imaging of lithium-batteries using correlative neutron and X-ray tomography with a virtual unrolling technique. Nat. Commun. 2020, 11, 777. [Google Scholar] [CrossRef]
- Wu, Y.; Saxena, S.; Xing, Y.; Wang, Y.; Li, C.; Yung, W.; Pecht, M. Analysis of Manufacturing-Induced Defects and Structural Deformations in Lithium-Ion Batteries Using Computed Tomography. Energies 2018, 11, 925. [Google Scholar] [CrossRef]
- Kruth, J.P.; Bartscher, M.; Carmignato, S.; Schmitt, R.; de Chiffre, L.; Weckenmann, A. Computed tomography for dimensional metrology. CIRP Ann. 2011, 60, 821–842. [Google Scholar] [CrossRef]
- Ank, M.; Stock, S.; Wassiliadis, N.; Burger, T.; Daub, R.; Lienkamp, M. Influence analysis of production defects of lithium-ion cells using single-cell and multi-cell characterization. J. Energy Storage 2023, 62, 106938. [Google Scholar] [CrossRef]
- David, L.; Ruther, R.E.; Mohanty, D.; Meyer, H.M., III; Sheng, Y.; Kalnaus, S.; Daniel, C.; Wood, D.L., III. Identifying degradation mechanisms in lithium-ion batteries with coating defects at the cathode. Appl. Energy 2018, 231, 446–455. [Google Scholar] [CrossRef]
- Harris, S.J.; Lu, P. Effects of Inhomogeneities—Nanoscale to Mesoscale—On the Durability of Li-Ion Batteries. J. Phys. Chem. C 2013, 117, 6481–6492. [Google Scholar] [CrossRef]
- Qian, G.; Monaco, F.; Meng, D.; Lee, S.-J.; Zan, G.; Li, J.; Karpov, D.; Gul, S.; Vine, D.; Stripe, B.; et al. The role of structural defects in commercial lithium-ion batteries. Cell Rep. Phys. Sci. 2021, 2, 100554. [Google Scholar] [CrossRef]
- Kehrer, M.; Locke, M.; Offermanns, C.; Heimes, H.; Kampker, A. Analysis of Possible Reductions of Rejects in Battery Cell Production during Switch-On and Operating Processes. Energy Technol. 2021, 9, 2001113. [Google Scholar] [CrossRef]
- Gitis, A. Flaw Detection in the Coating Process of Lithium-Ion Battery Electrodes with Acoustic Guided Waves; RWTH Aachen University: Aachen, Germany, 2017. [Google Scholar]
- Mohanty, D.; Li, J.; Born, R.; Maxey, L.C.; Dinwiddie, R.B.; Daniel, C.; Wood, I.D.L. Non-destructive evaluation of slot-die-coated lithium secondary battery electrodes by in-line laser caliper and IR thermography methods. Anal. Methods 2014, 6, 674–683. [Google Scholar] [CrossRef]
- Han, X.; Xia, S.; Cao, J.; Wang, C.; Chen, M. Effect of Humidity on Properties of Lithium-ion Batteries. Int. J. Electrochem. Sci. 2021, 16, 210554. [Google Scholar] [CrossRef]
- Kok, M.D.R.; Robinson, J.B.; Weaving, J.S.; Jnawali, A.; Pham, M.; Iacoviello, F.; Brett, D.J.L.; Shearing, P.R. Virtual unrolling of spirally-wound lithium-ion cells for correlative degradation studies and predictive fault detection. Sustain. Energy Fuels 2019, 3, 2972–2976. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, E.; Yan, X.; Ma, C.; Wen, W.; Liao, X.-Z.; Ma, Z.-F. Influence of Structural Imperfection on Electrochemical Behavior of Prussian Blue Cathode Materials for Sodium Ion Batteries. J. Electrochem. Soc. 2016, 163, A2117–A2123. [Google Scholar] [CrossRef]
- Du Baret de Limé, A.; Lein, T.; Maletti, S.; Schmal, K.; Reuber, S.; Heubner, C.; Michaelis, A. Impact of Electrode Defects on Battery Cell Performance: A Review. Batter. Supercaps 2022, 5, e202200239. [Google Scholar] [CrossRef]
- Mühlbauer, M.J.; Petz, D.; Baran, V.; Dolotko, O.; Hofmann, M.; Kostecki, R.; Senyshyn, A. Inhomogeneous distribution of lithium and electrolyte in aged Li-ion cylindrical cells. J. Power Sources 2020, 475, 228690. [Google Scholar] [CrossRef]
- Spiegel, S.; Heckmann, T.; Altvater, A.; Diehm, R.; Scharfer, P.; Schabel, W. Investigation of edge formation during the coating process of Li-ion battery electrodes. J. Coat. Technol. Res. 2022, 19, 121–130. [Google Scholar] [CrossRef]
- Dubarry, M.; Vuillaume, N.; Liaw, B.Y. Origins and accommodation of cell variations in Li-ion battery pack modeling. Int. J. Energy Res. 2010, 34, 216–231. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Pei, L.; Zhu, C.; Xu, B. A comparative study of sorting methods for Lithium-ion batteries. In Proceedings of the 2014 IEEE Conference and Expo Transportation Electrification Asia-Pacific (ITEC Asia-Pacific), Beijing, China, 31 August–3 September 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 1–6, ISBN 978-1-4799-4239-8. [Google Scholar]
- Liu, C.; Tan, J.; Shi, H.; Wang, X. Lithium-Ion Cell Screening With Convolutional Neural Networks Based on Two-Step Time-Series Clustering and Hybrid Resampling for Imbalanced Data. IEEE Access 2018, 6, 59001–59014. [Google Scholar] [CrossRef]
- Reynolds, C.D.; Slater, P.R.; Hare, S.D.; Simmons, M.J.; Kendrick, E. A review of metrology in lithium-ion electrode coating processes. Mater. Des. 2021, 209, 109971. [Google Scholar] [CrossRef]
- Ryll, K.; Hoffmann, L.; Landrath, O.; Lienesch, F.; Kurrat, M. Key Figure Based Incoming Inspection of Lithium-Ion Battery Cells. Batteries 2021, 7, 9. [Google Scholar] [CrossRef]
- Thomitzek, M.; Schmidt, O.; Abraham, T.; Cerdas, F.; Röder, F.; Krewer, U.; Herrmann, C. Model-based identification of production tolerances in battery production. Procedia CIRP 2021, 104, 1059–1064. [Google Scholar] [CrossRef]
- Moradpour, A.; Kasper, M.; Kienberger, F. Quantitative Cell Classification Based on Calibrated Impedance Spectroscopy and Metrological Uncertainty. Batter. Supercaps 2023, 6, e202200524. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, Y.; Lu, L.; Han, X.; Kong, X.; Wang, H.; Ouyang, M.; Gao, P.; Zheng, H.; Wang, K. A comprehensive research on internal short circuits caused by copper particle contaminants on cathode in lithium-ion batteries. eTransportation 2022, 13, 100183. [Google Scholar] [CrossRef]
- Grabow, J.; Klink, J.; Benger, R.; Hauer, I.; Beck, H.-P. Particle Contamination in Commercial Lithium-Ion Cells—Risk Assessment with Focus on Internal Short Circuits and Replication by Currently Discussed Trigger Methods. Batteries 2023, 9, 9. [Google Scholar] [CrossRef]
- Mohanty, D.; Hockaday, E.; Li, J.; Hensley, D.K.; Daniel, C.; Wood, D.L. Effect of electrode manufacturing defects on electrochemical performance of lithium-ion batteries: Cognizance of the battery failure sources. J. Power Sources 2016, 312, 70–79. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, J.; Gu, M.; Xiao, J.; Zhang, J.-G.; Wang, C.-M. Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries. Nat. Commun. 2017, 8, 14101. [Google Scholar] [CrossRef]
- Park, K.-J.; Hwang, J.-Y.; Ryu, H.-H.; Maglia, F.; Kim, S.-J.; Lamp, P.; Yoon, C.S.; Sun, Y.-K. Degradation Mechanism of Ni-Enriched NCA Cathode for Lithium Batteries: Are Microcracks Really Critical? ACS Energy Lett. 2019, 4, 1394–1400. [Google Scholar] [CrossRef]
- Liu, H.; Wolfman, M.; Karki, K.; Yu, Y.-S.; Stach, E.A.; Cabana, J.; Chapman, K.W.; Chupas, P.J. Intergranular Cracking as a Major Cause of Long-Term Capacity Fading of Layered Cathodes. Nano Lett. 2017, 17, 3452–3457. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, Z.; Luo, Z.; He, Z.; Zheng, J.; Li, Y.; Mao, J.; Dai, K. Microcrack generation and modification of Ni-rich cathodes for Li-ion batteries: A review. Sustain. Mater. Technol. 2021, 29, e00305. [Google Scholar] [CrossRef]
- Kumberg, J.; Müller, M.; Diehm, R.; Spiegel, S.; Wachsmann, C.; Bauer, W.; Scharfer, P.; Schabel, W. Drying of Lithium-Ion Battery Anodes for Use in High-Energy Cells: Influence of Electrode Thickness on Drying Time, Adhesion, and Crack Formation. Energy Technol. 2019, 7, 1900722. [Google Scholar] [CrossRef]
- Gao, T.; Kim, A.; Lu, W. Modeling electrode-level crack and quantifying its effect on battery performance and impedance. Electrochim. Acta 2020, 363, 137197. [Google Scholar] [CrossRef]
- Heenan, T.M.M.; Wade, A.; Tan, C.; Parker, J.E.; Matras, D.; Leach, A.S.; Robinson, J.B.; Llewellyn, A.; Dimitrijevic, A.; Jervis, R.; et al. Identifying the Origins of Microstructural Defects Such as Cracking within Ni-Rich NMC811 Cathode Particles for Lithium-Ion Batteries. Adv. Energy Mater. 2020, 10, 2002655. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Xie, H.; Luo, F. Surface Quality Assurance Method for Lithium-Ion Battery Electrode Using Concentration Compensation and Partiality Decision Rules. IEEE Trans. Instrum. Meas. 2020, 69, 3157–3169. [Google Scholar] [CrossRef]
- Schoo, A.; Moschner, R.; Hülsmann, J.; Kwade, A. Coating Defects of Lithium-Ion Battery Electrodes and Their Inline Detection and Tracking. Batteries 2023, 9, 111. [Google Scholar] [CrossRef]
- Choudhary, N.; Clever, H.; Ludwigs, R.; Rath, M.; Gannouni, A.; Schmetz, A.; Hülsmann, T.; Sawodny, J.; Fischer, L.; Kampker, A.; et al. Autonomous Visual Detection of Defects from Battery Electrode Manufacturing. Adv. Intell. Syst. 2022, 4, 2200142. [Google Scholar] [CrossRef]
- Günther, T.; Schreiner, D.; Metkar, A.; Meyer, C.; Kwade, A.; Reinhart, G. Classification of Calendering-Induced Electrode Defects and Their Influence on Subsequent Processes of Lithium-Ion Battery Production. Energy Technol. 2020, 8, 1900026. [Google Scholar] [CrossRef]
- Pfrang, A.; Kersys, A.; Kriston, A.; Sauer, D.U.; Rahe, C.; Käbitz, S.; Figgemeier, E. Geometrical Inhomogeneities as Cause of Mechanical Failure in Commercial 18650 Lithium Ion Cells. J. Electrochem. Soc. 2019, 166, A3745–A3752. [Google Scholar] [CrossRef]
- Schilling, A.; Schmitt, J.; Dietrich, F.; Dröder, K. Analyzing Bending Stresses on Lithium-Ion Battery Cathodes induced by the Assembly Process. Energy Technol. 2016, 4, 1502–1508. [Google Scholar] [CrossRef]
- Beck, D.; Dechent, P.; Junker, M.; Sauer, D.U.; Dubarry, M. Inhomogeneities and Cell-to-Cell Variations in Lithium-Ion Batteries, a Review. Energies 2021, 14, 3276. [Google Scholar] [CrossRef]
- R-Smith, N.A.-Z.; Ragulskis, M.; Kasper, M.; Wagner, S.; Pumsleitner, J.; Zollo, B.; Groebmeyer, A.; Kienberger, F. Multiplexed 16 × 16 Li-Ion Cell Measurements Including Internal Resistance for Quality Inspection and Classification. IEEE Trans. Instrum. Meas. 2021, 70, 2006109. [Google Scholar] [CrossRef]
- Westerhoff, U. Klassifizierung von Lithium-Ionen-Batteriezellen Mittels Kenngrößenbasierter Methoden; Dr. Hut: München, Germany, 2019; ISBN 978-3-8439-4179-2. [Google Scholar]
- Pan, Y.; Kong, X.; Yuan, Y.; Sun, Y.; Han, X.; Yang, H.; Zhang, J.; Liu, X.; Gao, P.; Li, Y.; et al. Detecting the foreign matter defect in lithium-ion batteries based on battery pilot manufacturing line data analyses. Energy 2023, 262, 125502. [Google Scholar] [CrossRef]
- Sazhin, S.V.; Dufek, E.J.; Gering, K.L. Enhancing Li-Ion Battery Safety by Early Detection of Nascent Internal Shorts. J. Electrochem. Soc. 2016, 164, A6281–A6287. [Google Scholar] [CrossRef]
- Roth, T.; Streck, L.; Graule, A.; Niehoff, P.; Jossen, A. Relaxation Effects in Self-Discharge Measurements of Lithium-Ion Batteries. J. Electrochem. Soc. 2023, 170, 20502. [Google Scholar] [CrossRef]
- Bauermann, L.P.; Mesquita, L.V.; Bischoff, C.; Drews, M.; Fitz, O.; Heuer, A.; Biro, D. Scanning acoustic microscopy as a non-destructive imaging tool to localize defects inside battery cells. J. Power Sources Adv. 2020, 6, 100035. [Google Scholar] [CrossRef]
- Masuch, S.; Gümbel, P.; Kaden, N.; Dröder, K. Applications and Development of X-ray Inspection Techniques in Battery Cell Production. Processes 2023, 11, 10. [Google Scholar] [CrossRef]
- Cai, Z.; Mendoza, S.; Goodman, J.; McGann, J.; Han, B.; Sanchez, H.; Spray, R. The influence of cycling, temperature, and electrode gapping on the safety of prismatic lithium-ion batteries. J. Electrochem. Soc. 2020, 167, 160515. [Google Scholar] [CrossRef]
- Bason, M.G.; Coussens, T.; Withers, M.; Abel, C.; Kendall, G.; Krüger, P. Non-invasive current density imaging of lithium-ion batteries. J. Power Sources 2022, 533, 231312. [Google Scholar] [CrossRef]
- Heenan, T.M.M.; Mombrini, I.; Llewellyn, A.; Checchia, S.; Tan, C.; Johnson, M.J.; Jnawali, A.; Garbarino, G.; Jervis, R.; Brett, D.J.L.; et al. Mapping internal temperatures during high-rate battery applications. Nature 2023, 617, 507–512. [Google Scholar] [CrossRef]
- Ran, A.; Chen, S.; Zhang, S.; Liu, S.; Zhou, Z.; Nie, P.; Qian, K.; Fang, L.; Zhao, S.-X.; Li, B.; et al. A gradient screening approach for retired lithium-ion batteries based on X-ray computed tomography images. RSC Adv. 2020, 10, 19117–19123. [Google Scholar] [CrossRef] [PubMed]


| Component | Thickness in μm | Dimensions in mm × mm × mm |
|---|---|---|
| Anode (double-sided) | 110.0 | 116 × 99 × 0.110 |
| Anode substrate | 20.0 | |
| Anode coating | 45.0 | |
| Cathode (double-sided) | 115.0 | 115 × 98 × 0.115 |
| Cathode substrate | 30.0 | |
| Cathode coating | 42.5 |
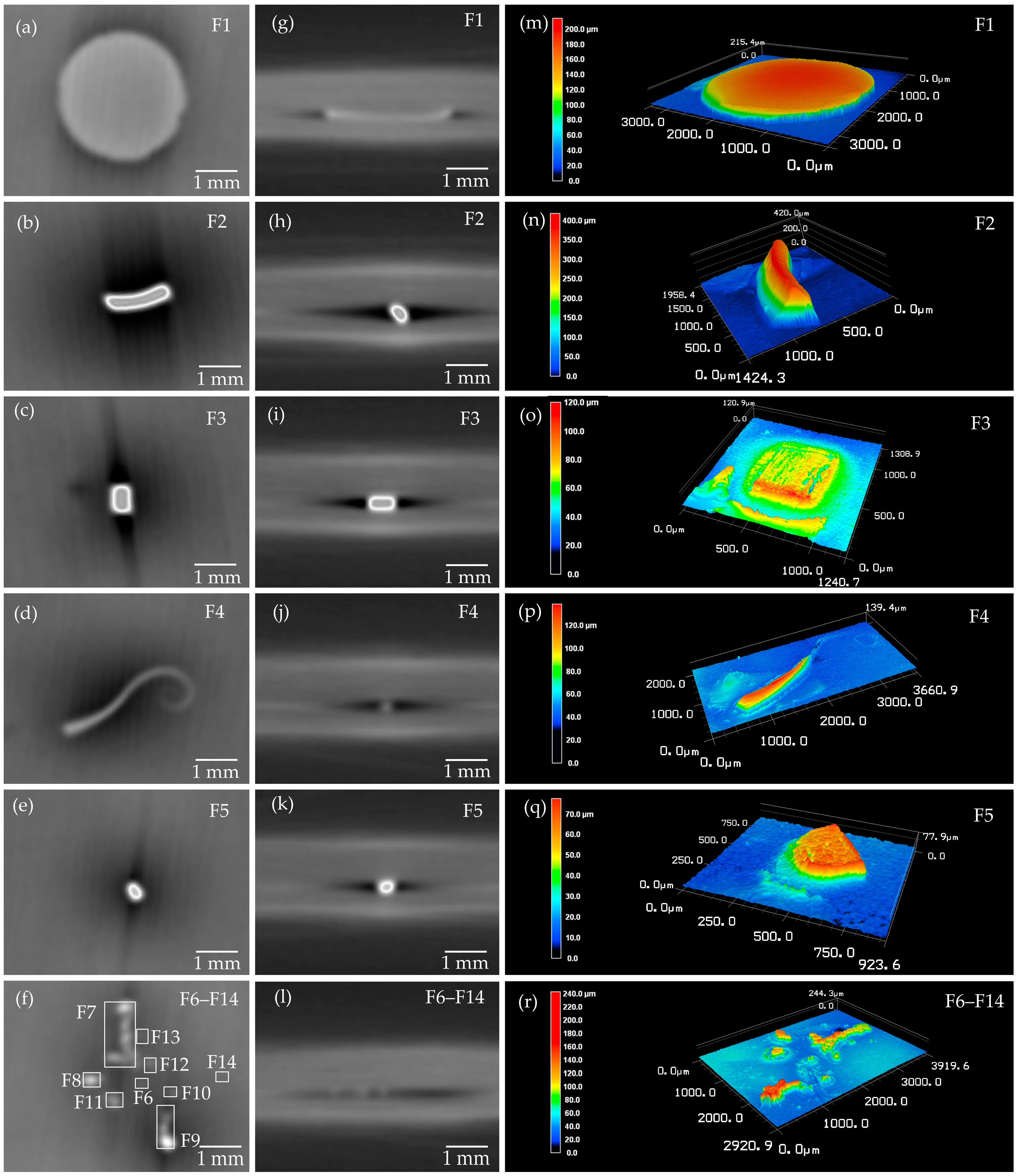
| No. | Material | Area (LSM) (in 103 μm2) | Volume (2.5D) (in 106 μm3) | Volume (3D) (in 106 μm3) | Shape |
|---|---|---|---|---|---|
| A1 | Al | 6951.02 | 738.02 | 139.02 | Punched disk |
| A2 | Al | 1578.13 | 75.62 | ~50.00 | Unspecific (-) |
| A3 | Al | 687.12 | 96.74 | ~50.00 | Needle-shaped |
| A6 | Al | 408.86 | 45.80 | ~30.00 | Needle-shaped |
| A4 | Al | 304.06 | 34.91 | ~28.00 | - |
| A7 | Al | 107.67 | 8.25 | ~8.00 | - |
| A8 | Al | 100.40 | 9.18 | ~5.00 | - |
| A9 | Al | 99.32 | 5.75 | ~5.00 | - |
| A5 | Al | 82.88 | 5.13 | ~2.00 | - |
| F1 | Fe | 7322.46 | 857.80 | 732.25 | Punched disk |
| F2 | Fe | 547.94 | 134.16 | ~100 | - |
| F7 | Fe | 547.87 | 31.63 | ~28.00 | Swarf cluster |
| F4 | Fe | 392.02 | 19.67 | ~18.00 | - |
| F9 | Fe | 177.56 | 18.47 | ~16.00 | Needle-shaped |
| F3 | Fe | 311.66 | 9.30 | ~9.00 | - |
| F8 | Fe | 90.57 | 6.63 | ~6.00 | - |
| F5 | Fe | 139.66 | 5.82 | ~5.00 | - |
| F11 | Fe | 93.37 | 5.03 | ~4.50 | - |
| F10 | Fe | 25.87 | 1.28 | ~1.00 | - |
| F6 | Fe | 12.95 | 0.55 | ~0.50 | - |
| F13 | Fe | 12.54 | 0.35 | ~0.30 | - |
| F14 | Fe | 5.03 | 0.09 | ~0.08 | - |
| C1 | Co | 7260.82 | 1119.88 | 217.82 | Punched disk |
| C2 | Co | 927.96 | 100.69 | ~100.00 | - |
| C4 | Co | 434.54 | 39.59 | ~28.00 | - |
| C3 | Co | 679.53 | 28.49 | ~25.00 | - |
| C5 | Co | 371.86 | 15.04 | ~13.00 | Needle-shaped |
| C7 | Co | 310.15 | 16.26 | ~13.00 | Swarf cluster |
| C8 | Co | 162.46 | 16.36 | ~13.00 | - |
| C9 | Co | 102.34 | 8.06 | ~7.00 | - |
| C10 | Co | 9.72 | 0.30 | ~0.25 | - |
| C6 | Co | 9.60 | 0.09 | ~0.08 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, D.; Luc, P.-M.; Tebruegge, C.; Kowal, J. Detection of Manufacturing Defects in Lithium-Ion Batteries-Analysis of the Potential of Computed Tomography Imaging. Energies 2023, 16, 6958. https://doi.org/10.3390/en16196958
Evans D, Luc P-M, Tebruegge C, Kowal J. Detection of Manufacturing Defects in Lithium-Ion Batteries-Analysis of the Potential of Computed Tomography Imaging. Energies. 2023; 16(19):6958. https://doi.org/10.3390/en16196958
Chicago/Turabian StyleEvans, Daniel, Paul-Martin Luc, Claas Tebruegge, and Julia Kowal. 2023. "Detection of Manufacturing Defects in Lithium-Ion Batteries-Analysis of the Potential of Computed Tomography Imaging" Energies 16, no. 19: 6958. https://doi.org/10.3390/en16196958
APA StyleEvans, D., Luc, P.-M., Tebruegge, C., & Kowal, J. (2023). Detection of Manufacturing Defects in Lithium-Ion Batteries-Analysis of the Potential of Computed Tomography Imaging. Energies, 16(19), 6958. https://doi.org/10.3390/en16196958







