1. Introduction
The environmental impact of industrial chemical processes where the primary source of thermal energy is fossil fuel combustion has generated significant attention from the global economic community and environmental groups aiming to implement urgent and sustainable greenhouse gas (GHG) emission reduction measures in order to mitigate the current and imminent reality of climate change. The industrial sector accounts for about 33% of global anthropogenic GHG emissions [
1]; this is why it is urgent to incorporate alternative technologies that contribute to lower or almost atmospheric zero carbon emissions into society’s productive schemes. In 2017, the direct combustion of fossil fuels accounted for 73% of energy use in the global industrial sector, while electricity as a form of substitution accounted for only 27%; within a similar analysis under a total global energy context, it has been estimated that the contribution of electric power is only 22% while coal, hydrocarbons, gas, biofuels, and others continue to be those of greatest relevance, with 78% [
2].
The real contribution of society to the mitigation and control of climate change will occur to the extent that energy sources or technologies with lower environmental impact (photovoltaic and wind energy, for example) are incorporated into production processes. Electricity will undoubtedly play a key role in the medium term in achieving what has been called a “zero carbon economy,” in which countries’ public policies will have to drive rapid growth in the supply of “clean” electricity to decarbonize the current electricity supply and support the growing and future demand for zero carbon electricity, a need understood by many experts worldwide [
3]. Concerning the use of electricity as a heating medium, it has been determined that only 5% of US manufacturing companies use electricity for this purpose, with technologies that include resistance heating [
4], induction heating, microwave processing, electric arc furnace heating (steel industry), electric boilers, heat pumps, and radio frequency (RF) drying [
5].
In research conducted mainly in Europe and the Netherlands, the concept of electrification has gone further by developing four main concepts of study [
6]: thermal energy consists of efficient and improved heat and steam generation through electricity for use in chemical processes. Hydrogen energy uses electricity to produce hydrogen through water electrolysis and subsequent uses in various applications, primarily as fuel cells. Energy specialty products are the direct electrochemical synthesis of intermediate products and high-value-added fine and specialty chemicals using conventional or biomass-derived feedstocks. Commodity energy is the direct electrochemical synthesis (both centralized and decentralized) of high-volume commodity chemicals used in the market (ammonia, methanol, ethylene, etc.) from conventional and waste feedstocks such as CO
2.
The above proposals show the enormous potential of electrification for the sustainability of society and the planet in the short and medium term. In the studies by [
1,
7,
8,
9,
10], a review is carried out on the adoption of new technologies in different industrial sectors and their potential for achieving decarbonization. Each of these studies highlights the use of electricity as a thermal source in industrial processes. However, it is important to note that in these reviews, the implementation of these technologies lacks depth regarding the viability and likelihood of the proposed technological pathways. In this context, the objective of this literature review is to delve deeper into the main technological schemes that allow the incorporation of electricity as an alternative to replace thermal sources (thermal energy) at both small and large scales in the industrial sector. In general terms, the use of electrical energy as a heating source in the chemical industry is more expensive than conventional gas or fuel oil combustion, except when the technological proposal uses combined schemes of electricity generation through renewable sources, hydroelectric or nuclear systems [
11]. However, the potential for electrification will increase to the extent that energy generation is cheaper, with technological developments that allow increasing the overall efficiency of equipment and processes, in addition to the policies that governments must provide for the decisive use of electricity as a primary and differentiating factor for the sustainability of society, within the framework of the energy transition and decarbonization of processes.
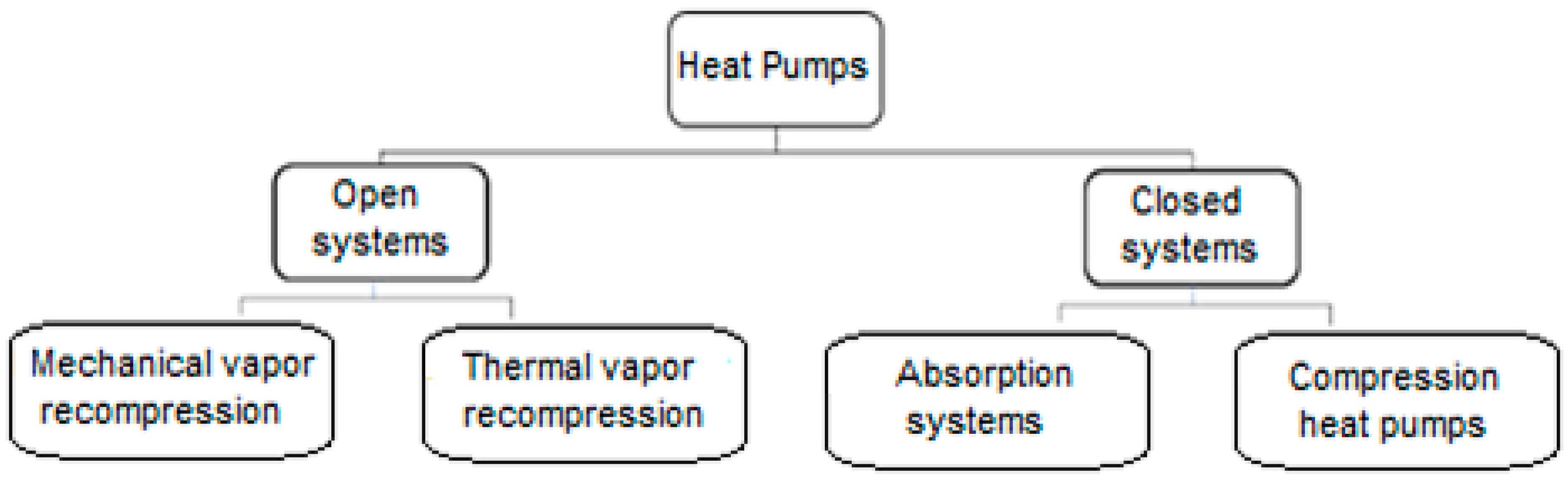
The so-called heat pump is a promising technology at the industrial level for the utilization of waste heat from an electrical source. These systems can currently extract energy at low temperatures and incorporate it into processes at conditions up to 150 °C [
12,
13]. The heat pump is a thermodynamic concept where a thermal machine extracts heat from a cold location and transfers it to a warmer point by supplying mechanical work (electricity) from outside the system, essentially representing a reverse-cycle refrigeration machine. Some studies suggest that the application of heat pumps from electric sources could reduce the use of fossil fuels by up to 43% of the total heating energy supply (currently at 70%) used in buildings in large cities. Furthermore, it is estimated that in the coming years, technological advances in heat pump technology will reduce up to 23% of fossil fuels in the housing sector [
14]. Heat pump systems are usually classified according to
Figure 1 [
15].
The so-called closed-cycle systems use a working fluid, usually a refrigerant, in a sealed circuit without contact with the process fluid. The vapor compression or adsorption principle uses a mechanical compressor specified according to the required temperature level (associated with the machine’s compression ratio) and the economic process. In open-cycle systems, the same process fluid is used to raise the temperature or energy of a system to desired conditions by vapor compression. Vapor compression is performed analogously to the closed cycle through a compressor or a high-pressure vapor thermocompression ejector.
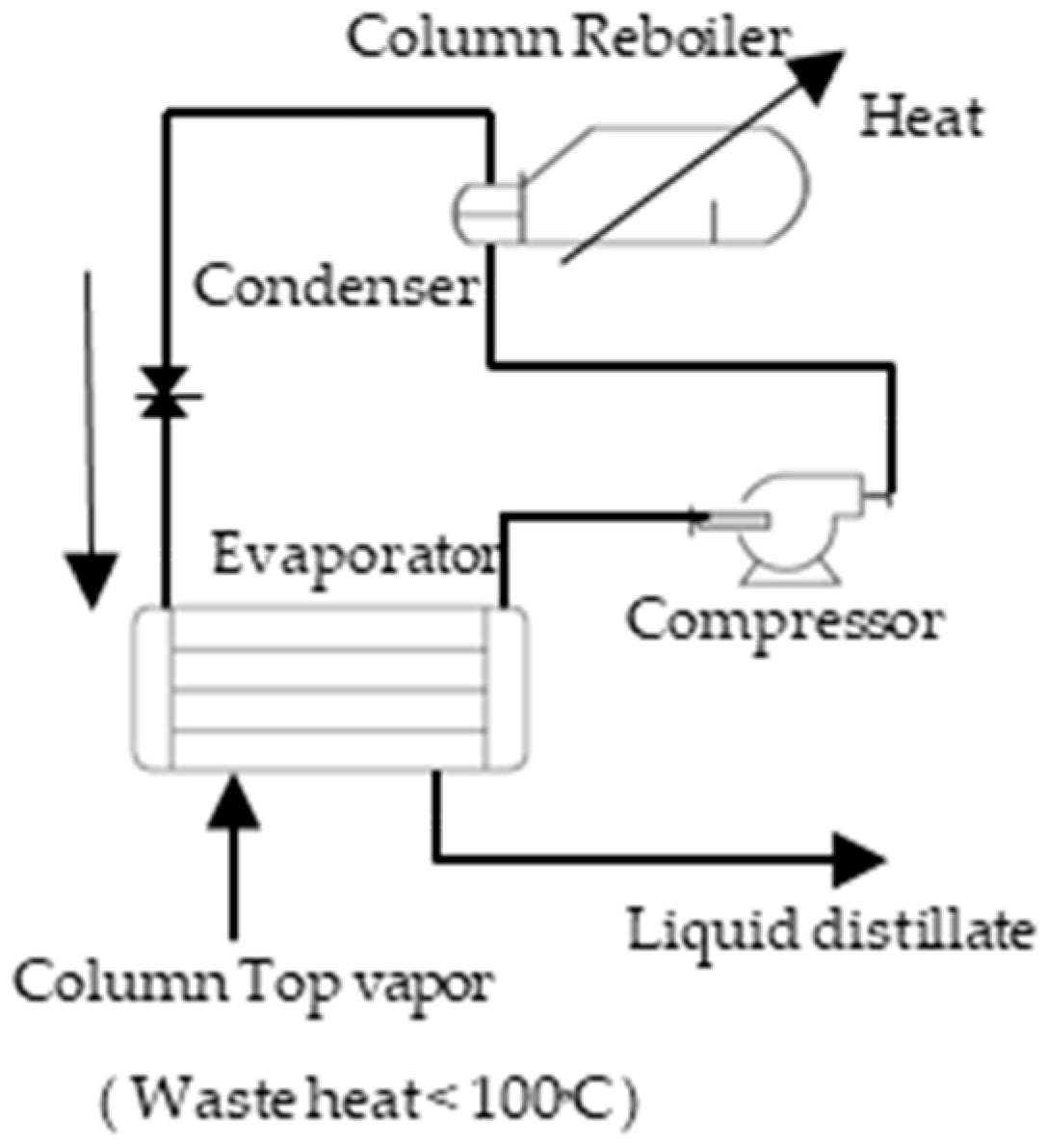
Figure 2 shows a simply closed heat pump scheme in which energy extracted from a top vapor system for a distillation column is recovered using a working fluid and energy is applied to a compressor to a heating system (Re-boiler) at the bottom of a distillation column.
At the industrial level in European countries (EU), the potential of using heat pumps for waste heat recovery has been evaluated at levels close to 35% (28.75 TWh/year) of the total heat lost, except for industries such as iron and steel, where current technologies do not allow effective recovery of waste heat from smelting processes [
16].
Continuing with alternatives or schemes of electrical use as a means of thermal supply is the conventional heating by electrical resistances used in some processes such as chemical reactors and piping systems; in this case, the heat is transferred from the surface or wall of the equipment to the center of the material (catalysts) by conduction, convection, and radiation mechanisms. The effectiveness of the process will depend on the adequate thermal and electrical conductivity of the internal material and the heat transfer efficiency of the system [
17]. Another scheme proposed for oil extraction and recovery processes has to do with the application of electric heating (EH) complemented with radiofrequency heating techniques (RH) for heavy oil recovery processes, where to date, the injection of high temperature and pressure steam has predominated as conventional techniques, it has been determined that RH has significant comparative advantages on production yields as well as better efficiency in energy conversion, making it a promising technique for crude oil recovery with minimum environmental impact due to the use of water and gas [
18]. The induction heating technique, mainly for industries such as the food industry, promises to be a good and efficient alternative; the inductive effect provides faster heating compared to gas heating and resistive heating; induction heating is a heating method that transfers energy without contact with the workpiece at high speeds [
19].
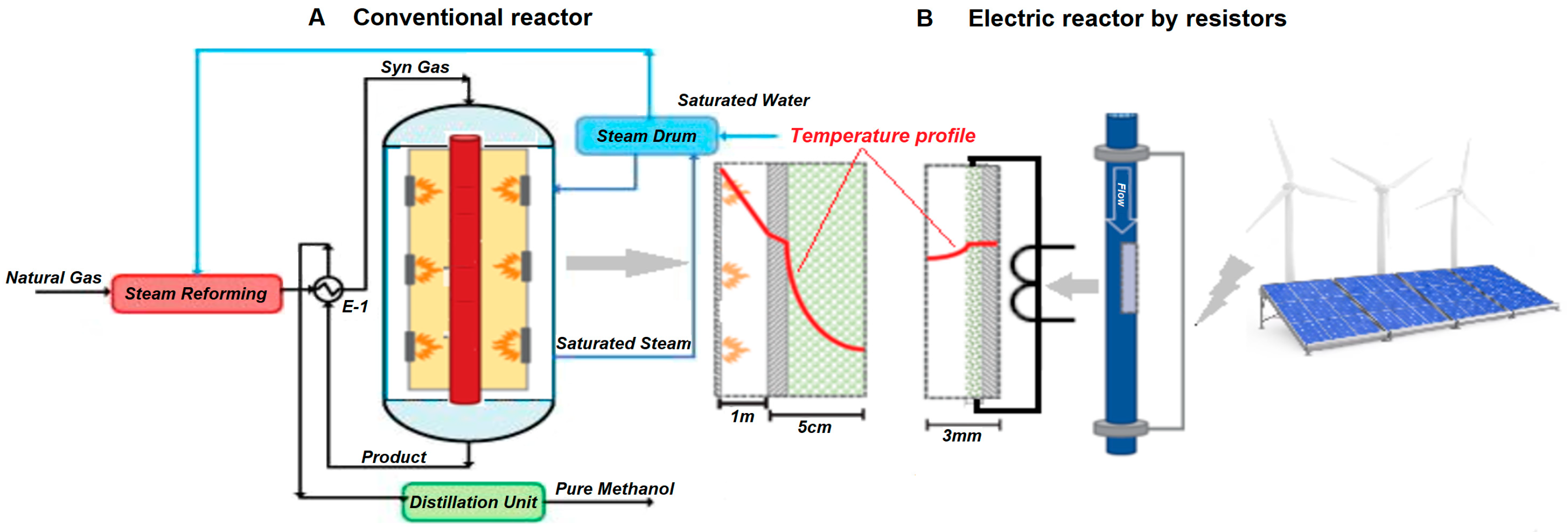
Another application of the use of electrical energy as a replacement for thermal sources is related to the use of electric furnaces in chemical reactors in replacement of conventional gas-fired combustion technologies, which have been recently potentiated to reduce the emissions of CO
2 emissions by producing more flexible and compact systems [
20]. This reactor furnace is used in hydrogen generation processes by steam methane reforming (SMR) and ammonia production, where the basic scheme can be seen in
Figure 3.
Similar pilot-scale applications for processes with the use of methane and CO
2 as carbon capture and hydrogen generation alternatives have to do with implementing thermal reactors using plasma generated with high-frequency electricity [
21]. Plasma is generated by passing an electric current through a gas. Gasses are electrical and thermal insulators at average ambient temperatures; generating many charge carriers is necessary to convert them into electrical conductors. Plasmas are generated through many routes, such as high-intensity electric arcs, microwaves, shock waves, radio frequency (RF) induction, lasers, or high-energy particle beams [
22]. Heating gases can also generate plasmas to elevated temperatures in furnaces. However, due to the inherent temperature limitations, this route is only used with metallic vapors of low ionization potential [
23].
Microwave heating similarly generates thermal energy through the close interaction of electromagnetic fields with molecules in the material of interest. Due to penetrability properties and selectivity, microwave technology can act directly on each unit within the material [
24]. The pyrolysis process has recently been considered one of the most promising approaches for the treatment of solid wastes and sludges to produce fuel oils and synthesis gas through so-called medium heating steps. In oil extraction and drilling applications, where oil-bearing residues (OBDC) are treated using pyrolysis, one of the recent experimental alternatives has been the application of microwaves for oily muds finding some comparative advantages over the traditional method by electrical heating, thus, improving the effects of fuel recovery at lower temperatures < 600 °C and in an efficient way [
25].
In industrial process units, the application of electric and magnetic fields for thermal supply or extraction systems in heat exchangers is being studied. The predominant methods to improve heat transfer in this equipment include increasing the transfer surface area, vibrating the surfaces or transfer fluids, improving the physical properties of certain types of coolant, and finally, adding solid nanoparticles such as carbon-acetone in the presence of magnetic fields [
26]. Research in the field of nanotechnology is also contributing to the development of applications in the field of heat transfer for industrial equipment, as well as some proposed nanoparticles such as metal oxides SiO
2 and Al
2O
3 [
27] confer to the fluids some improvement in the coefficient of thermal conductivity compared to the cases of base fluids without particles. In other tests, the hydrothermal characteristics of some so-called ferrofluids (water with 4 vol%) have been evaluated in heat exchangers of different types. For example, Fe
3O
4 in countercurrent double tube type heat exchangers exposed to non-uniform transverse magnetic fields under different field strengths, finding, in the end, significant improvements in the overall heat transfer of the equipment [
28,
29].
The above paragraphs are examples of the use of electrical energy as a potential source of heat generation with industrial applications. However, as previously mentioned, electrical energy can, in some instances, be more efficient and economical for the process to the extent that the supply is low-cost and comes from renewable sources such as the sun, wind, or others.
This review paper is organized as follows.
Section 2 describes the methodological approach to the review of key search topics with the use of the VOSviewer software (Version 1.6.19);
Section 3 summarizes the concepts extracted from the body of research associated with electrification;
Section 4 analyzes and discusses key aspects to consider in future research processes on electrification: challenges for successful implementation and finally
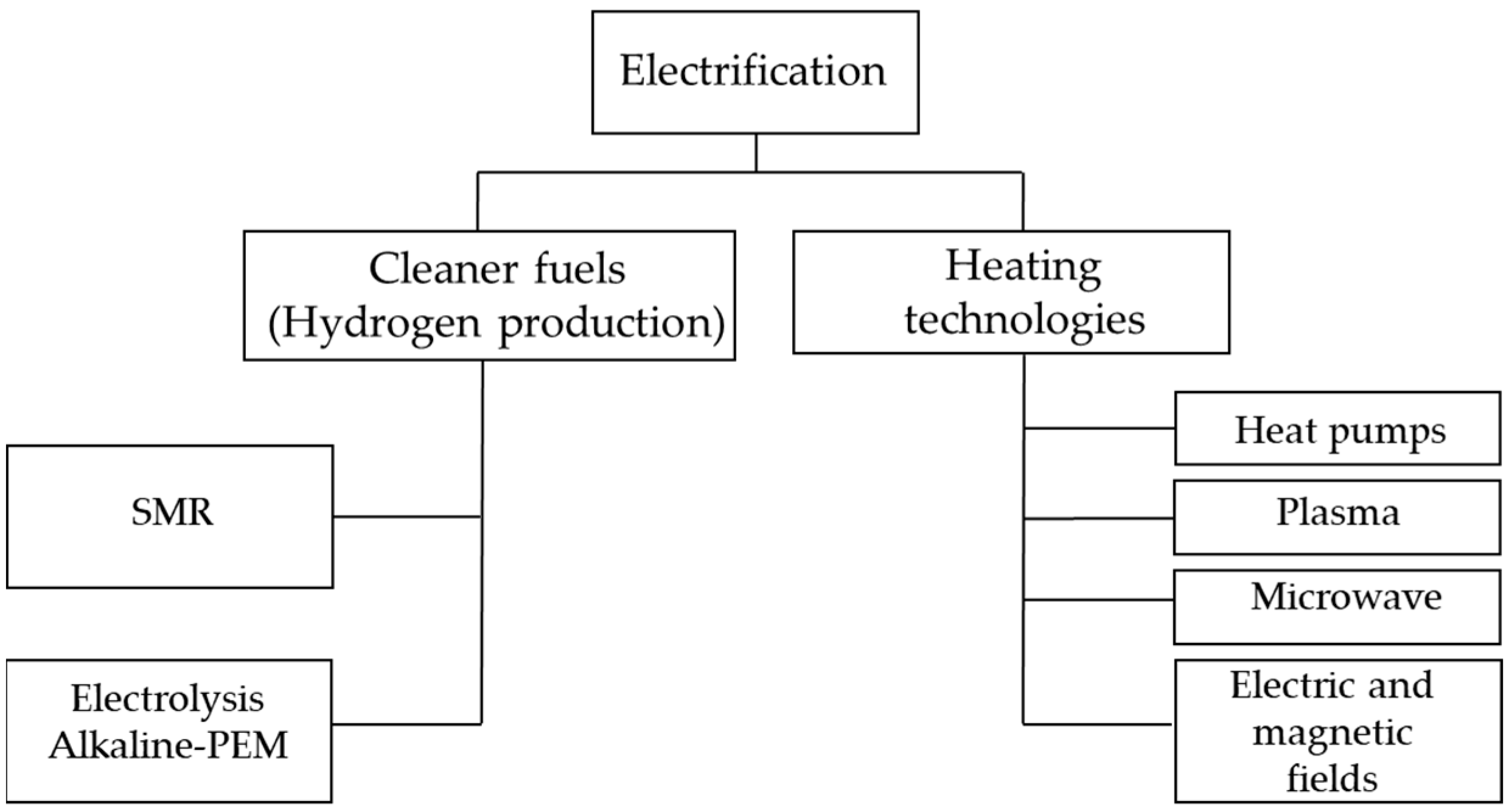
Section 5, with the conclusions of the document review. The review framework focuses on electrification as a means to obtain cleaner fuels (hydrogen production) and its use in heating technologies with applications in industrial processes (
Figure 4).
3. Results
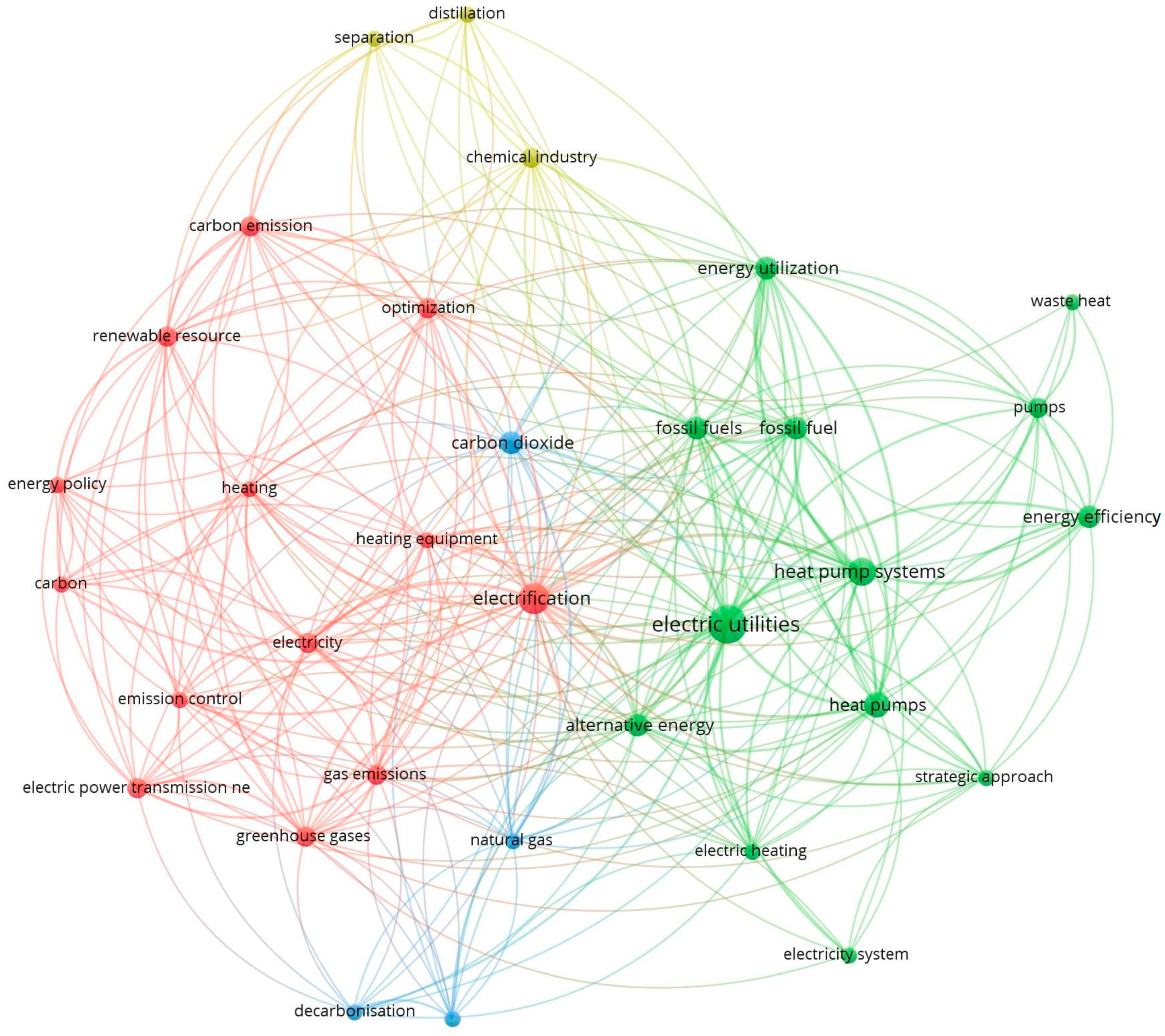
Following the methodology described in the previous section, 239 potentially relevant records were identified through the Scopus database using the following search words: electrification + process + industries.
Table 1 summarizes the occurrence results presented by VOSviewer for the selected keywords.
The tabulated results were again organized and classified by topics of interest, eliminating from
Table 1 the components: electric utilities, automotive industry, and hybrid vehicles, as they were not closely related to the research topic. The search components finally focused on electrification (
Figure 7) with association links, mainly related to heating equipment, heat pumps, carbon dioxide, gas emissions, fossil fuels, electric utilities, and alternative energies.
In principle, some relevant aspects emerge from the map in
Figure 7, such as the example of the association of electrification with the chemical industry; however, it exists as an energy supply. It is still in the early stages or phases of the development of works related to thermal and industrial decarbonization schemes, where the main published articles associated with carbon dioxide, emissions, alternative energies, and new fuels are presented after 2017. In the case of sectors such as the oil industry with high energy demand, the association of published works is almost null. However, the topic of electrification through heat pumps addresses technological components of common use in the chemical industry, such as separation systems or distillation of products.
In the deepening of electrification through heat pumps, the most widely used component is for heating applications in urban homes and buildings, with a reduced application to industrial process units or plants. Most of the published articles on electrification highlight it as one of the key factors contributing to the reduction of greenhouse gases, increase in energy use and efficiency, and incentive for implementing non-conventional renewable energies in society.
In summary, based on the results obtained from the relationship maps, it was decided to address the concept of electrification under two closely related concepts: electrification strategies and existing technologies for the use of electricity as a means of energy substitution for heating in industrial processes. The revision of the document focuses mainly on issues related to heat pumps, heating equipment, carbon dioxide capture processes, and non-conventional energies and fossil fuels.
3.1. Electrification Strategies
Electricity can play a key role in climate change mitigation, and recent literature and research on fossil fuel replacement energy transition scenarios attest to this. Electricity can be produced with a low carbon footprint by various means, including nuclear power plants, renewable sources, and combustion technologies with carbon capture and storage (CCS) [
30]. In fact, in 1992, Manne and Richels [
31] emphasized the importance of migrating to heat pump systems as a way to reduce residential consumption of fossil fuels in a clear incentive towards electrification: “natural gas burners can replace the current residential oil burners, but electric heat pump systems can also replace them...”.
From this point on, research work and developments have been focused on the exploration of technologies for the decarbonization process of industry and society in general, with prospective analyses of scenarios up to the years 2050 and 2070, where it is clear that the concept of electrification will define the frame of reference for the automotive industry, intensive power generation industry, mineral production, synthesis of basic materials, and society.
The conclusion is that electrification processes are feasible and technically possible and will have significant implications or repercussions on how industry, power systems, and hydrocarbons interact today based on current and expected future relative prices [
32]. Therefore, it is clear that to the extent that electricity comes from renewable sources, the prospects for profitability will be broader. Electricity is a very versatile form of energy that can be used in the industry not only for heating or electromechanical systems but also in electrochemical processes or for the synthesis of new products, as in the case of the production of hydrogen fuel for energy storage systems (cells). This hydrogen generated together with CO and CO
2 and surplus from some industrial processes can be used to synthesize fuels, plastics, or ammonia for the fertilizer industry. Among the main perspectives related to the literature on electrification is, as mentioned above, hydrogen generation from the electrolysis technique. Water electrolysis is an electrochemical process that splits water into its elemental components, hydrogen and oxygen. Direct electric current promotes this endothermic type reaction (Equation (1)). The process generates no direct emissions of CO
2. Therefore, this option represents an alternative hydrogen production pathway with potentially low GHG emissions [
33]. The two leading electrolysis technologies currently applied on a commercial scale are proton exchange membrane electrolysis and alkaline technology:
Conventionally, hydrogen production is performed by the steam reforming (SMR) process in the presence of alumina and nickel-based catalysts in tubular reactors, where the basic reactions are:
The primary reaction is endothermic and therefore requires high energy consumption provided by the combustion of natural gas in furnace reactors. Recent studies have proposed the use of methane decomposition to produce hydrogen using plasma-type reactors where the thermal supply electricity is obtained in two ways:
renewable energy sources (solar and wind radiation) are available worldwide without connecting to the power grid;
natural gas-fired combined cycle (CC) power plants have high utilization and low fossil fuel use.
In most cases, the electricity required and the methane demand are estimated to be close to the theoretical minimum; 221 MJ
Methane/kg H
2. The comparative advantages in emission reductions between a conventional SMR process and a plasma reactor system range from 14 kg CO
2equi/kg H
2 and 6 kg CO
2equi/kg H
2, respectively [
33].
In general, it can be said that the strategies proposed for industrial electrification in most of the research reviewed lead to the following topics:
3.2. Heat Pumps
The so-called high-grade industrial waste heat (thermal potential) emitted by flue gases or hot conditions of a system, loss of insulation of pipes or equipment, can be harnessed for energy transfer by heat pumps, the concept of heat pumps is based on transferring heat from a low-temperature medium to a higher temperature medium with the help of an external energy source or work applied to a fluid in the system.
Conventional or everyday use of heat pump techniques have been in the US, EU, and China’s residential heating [
37] supplemented with solar energy systems. However, the use of industrial-scale heat pumps for low-grade (low-temperature) waste heat recovery has not been widely deployed globally. Therefore, in 2010, the International Energy Agency (IEA) launched a new incentive project for these technologies called “industrial heat pump application”, which mainly focused on high-temperature industrial and commercial applications [
38].
In China, where industrial energy efficiency is considered lower than the world average, and waste heat in exhaust gases is only 30% utilized, the government is promoting the implementation of heat pumps. Systems that are being studied for application have to do with wastewater sludge drying processes, applications for the heating of crude oil extracted from oil fields, and applications for the printing and dyeing industry where in general, the conclusion is that more research is required on the types of refrigerants used in pumps for high-temperature services, as well as efficiency studies for the systems and pump components: this includes exploring and building innovative systems concerning increasing the COP of the system [
39]. The steady-state efficiency of a heat pump cycle is evaluated by a coefficient called the coefficient of performance (COP). COP is defined as:
where Q is the proper heat delivered (extracted from a lower temperature source) and P is the high-grade (primary) energy (work) input to the heat pump.
Based on temperature and waste heat continuity, some industrial heat pump systems present COP values for vapor compression heat pump systems in the order of 4.2 [
40] and for renewable solar source-assisted heat pump systems in the order of 5.207 [
41]. This means that through a heat pump, it is feasible to recover four to five times more energy compared to the energy applied to the pump.
The two main application types of heat pump systems considered for fractionation columns in chemical plants are:
A conventional heat pump system schematic is shown in
Figure 7:
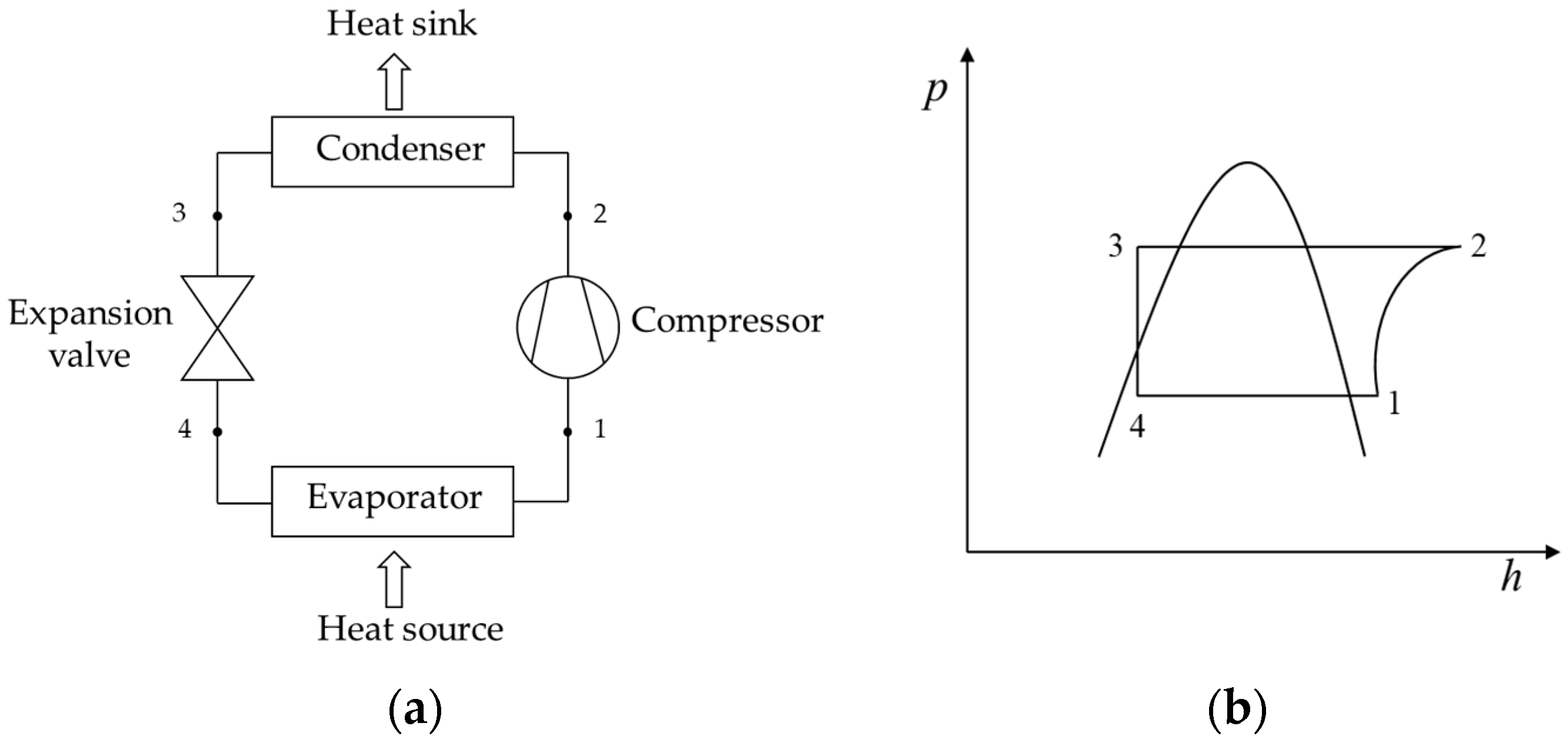
The vapor compression cycle used in a heat pump generally consists of a mechanical compressor, an expansion valve, and two heat exchangers called an evaporator and condenser. These devices usually form a closed circuit where a refrigerant fluid circulates throughout the cycle.
Figure 8 shows the operation scheme of the cycle with its corresponding diagram p—h (pressure–enthalpy). In the evaporator, the evaporated refrigerant absorbs heat from a source (state point 1). The superheated vapor flows through the compressor (state point 2), moved by electrical energy to subsequently enter the so-called high-pressure condenser, where the hot vapor is condensed by transferring the heat of condensation to a user of interest (indicate point 3). Finally, the condensed liquid refrigerant at high pressure passes through an isoenthalpy expansion valve (state point 4), vaporizing at low pressure to return to the evaporator, completing a complete cycle.
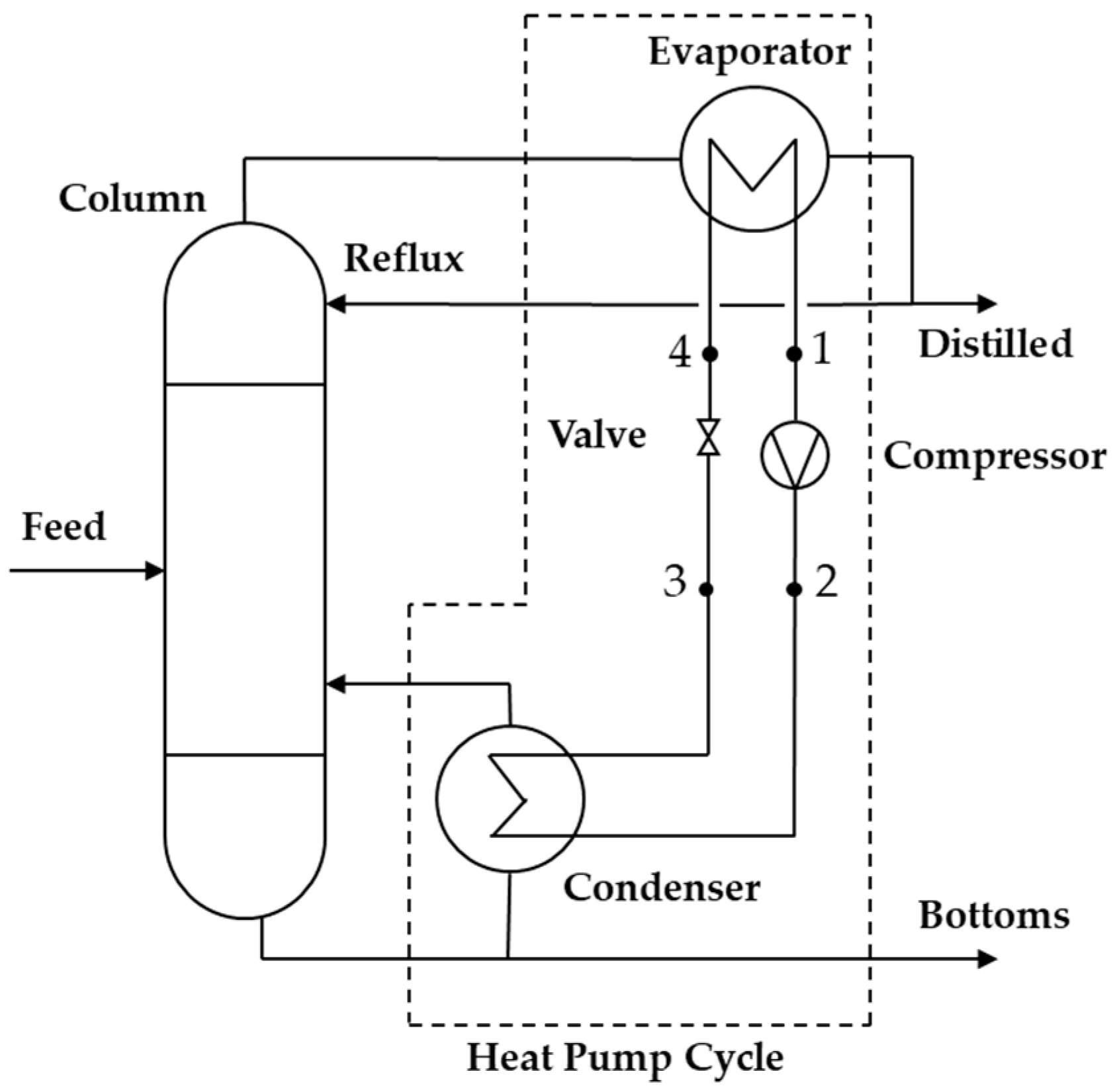
This cycle can be applied to a distillation column to separate chemical components of interest using the so-called MVR system, in which the applications can be open or closed cycles depending on the separated substances’ characteristics.
Figure 9 shows a closed-cycle heat pump system where the working fluid is a refrigerant with specific thermodynamic properties that facilitate the energy transfer processes in the system.
The Shell Company references applying the heat pump concept for a propylene recovery system [
6]. Propylene is produced by the distillation separation of a mixed propylene and propane stream in a fuel refinery. Traditionally, column heating is carried out using low-pressure steam or hot fluid. The Shell Pernis refinery (The Netherlands) has incorporated a propylene–propane distillation column that applies a mechanical vapor re-compression (MVR) system. In MVR, an electric compressor increases the pressure of the upper vapors. As the column operates independently of the refrigerant fluid, the column pressure can be reduced, resulting in better separation between propylene and propane, reaching purities up to 99.5%. As it is known, propylene is the raw material used to produce a thermoplastic material that is very versatile industrially due to its thermal, mechanical, chemical, and electrical properties, such as polypropylene [
42].
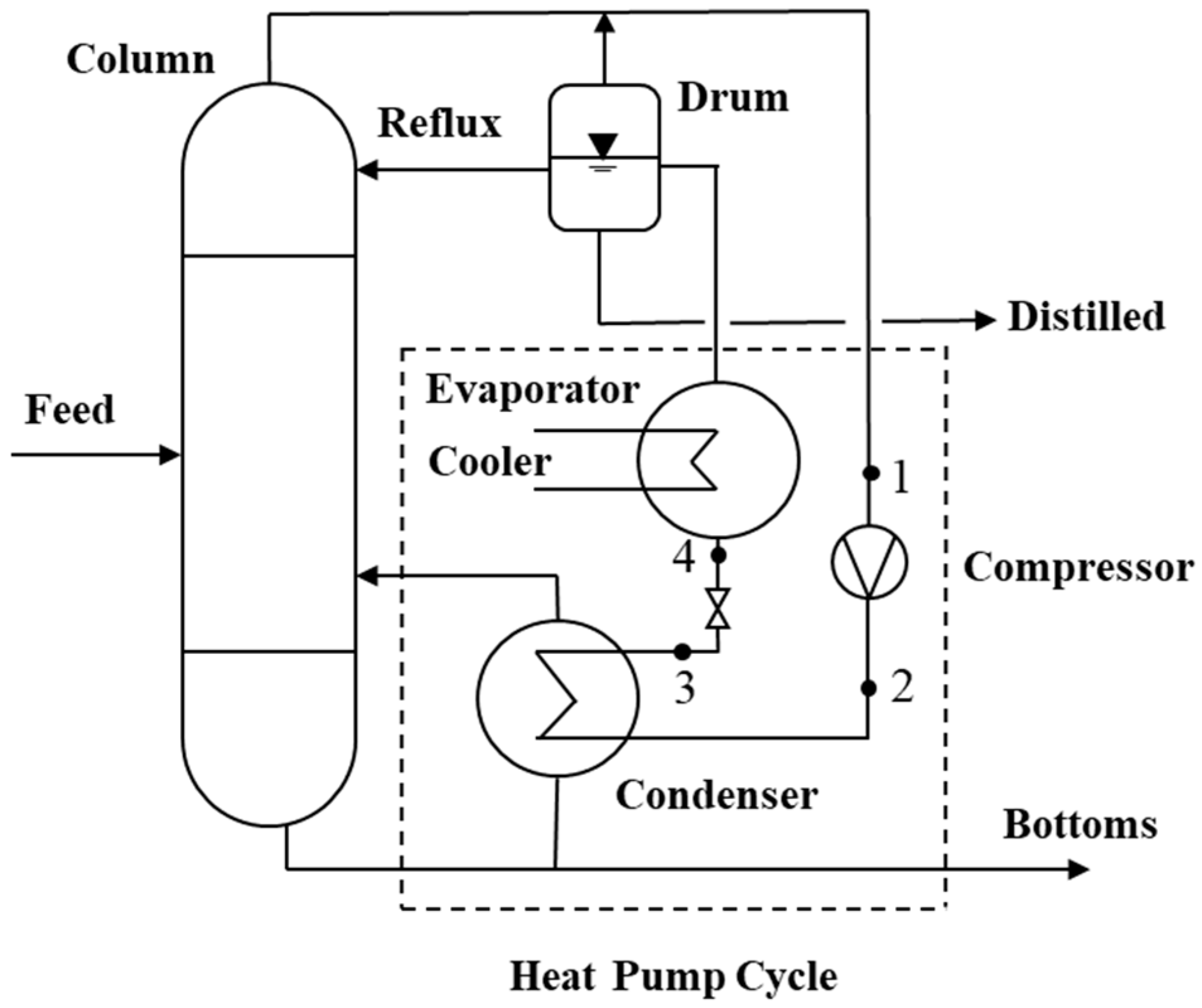
Figure 10 shows the application of an open-cycle MVR system where the cooling fluid is the same as the process fluid.
It is important to clarify the use of the terms evaporator and condenser of a heat pump system about the meaning conventionally used for a distillation process. For example, for a distillation column, the condenser represents the heat pump’s evaporator and the evaporator represents the pump’s condenser.
3.3. Plasma
The application of using plasma dates back to the 19th century when industries working with metals developed and employed plasma to deliver elevated temperatures in furnaces [
43,
44]. Plasma is generated by passing an electric current through a gas (air, nitrogen, argon, steam, etc.). In general, gases naturally insulate at normal ambient temperatures; therefore, generating a significant amount of electrical charge carriers is necessary to convert them into electrical conductors. Plasma is generated from many routes, such as high-intensity electric arc systems, microwaves, shock waves, radio frequency (RF) induction, and laser or particle beams at high energy. In addition, plasmas can be generated by heating gases in furnaces at high temperatures. However, this route is only used for metal vapors that have low ionization potentials and, therefore, low inherent temperatures [
45].
The use of plasma-based reactors has been used for the treatment of industrial solid wastes generating temperatures ranging from 12,000 to 22,000 K. In the case of plasma generated by an electric arc, the main attraction is the requirement of minimum plasma gas flow rates. Reactors employing this type of transferred arc are available in a wide power range, from a few hundred watts to tens of megawatts.
Figure 11 shows the diagram of a plasma reactor employing steam to produce synthesis gas (high temperature) by applying propane for dissociation using argon as the carrier gas [
46,
47].
The plasma reactor (
Figure 11) consists of three main sections. The first part is the input of superheated steam, which decomposes into free radicals O, H, and OH after collision with argon molecules at high temperatures. The second part consists of the propane gas inlet to the gasification part of the reactor with a volume of 352 mm
3. In this section, propane gas reacts with free radicals from the dissociation of water vapor leading to the gasification of propane at high temperatures, reported as 2800 K, to produce ethylene and hydrogen.
Another application of plasma heating for solid waste pyrolysis processes concerns its use as a dedicated technology for the remediation of soils contaminated with heavy metals. Phytoremediation is one of them. This emerging technique is an ecological engineering technology that uses metal-accumulating plants to assimilate heavy metals in the soil. These plants are called hyperaccumulator plants which process heavy metals by volatilization, stabilization, and extraction of metals such as cadmium (Cd), copper (Cu), and lead (Pb), typically by incineration processes; this is where plasma heating methodologies have been explored to achieve efficient results [
48].
Pyrolysis/gasification processes of solid wastes to produce synthesis gas offers an effective potential energy alternative versus that supplied by fossil fuels. Furthermore, since the syngas generated contains essentially molecular hydrogen and carbon monoxide, these mixtures can be used as a high-quality fuel. The use of pyrolysis or gasification using a plasmatron device offers unique advantages for solid waste conversion, providing high temperatures at fast heating rates for specific municipal solid waste (MSW) gasification processes, in this case, processing capacities of up to 10 tons/day have been achieved, using for this purpose an integrated furnace with two thermal plasma torches transferred [
49].
The above is just one example of the feasibility of using a plasma torch heating process for sludge handling waste pyrolysis processes. Another fundamental approach concerns the transformation of coal into liquid fuel. The sustainability of this production process is mainly affected by the challenges associated with high capital requirements and the high emissions generated compared to conventional hydrocarbons [
50] compared to conventional hydrocarbons. Therefore, plasma arc methane reformer developments have been proposed in coal-to-liquid plants.
Within the concept of carbon capture, mechanisms have been explored to generate valuable synthesis gas from waste carbon dioxide through the following endothermic reaction Equation (6). also known as dry reforming methane. The reaction is strongly endothermic and therefore requires high heat supplies.
Typical catalysts for this process are metal-impregnated alumina bases. Al
2O
3, Ag/Al
2O
3, Pt/Al
2O
3, and Cu/Al
2O
3. Some studies indicate the possibility of obtaining similar conversion results for this process using plasma alone (without catalysts). Conversions for this process can be between 32.8% and 22.4%. CH
4 and 22.4% CO
2, where an important factor calculated is the energy efficiency of the reaction (η). This factor determines how much energy is required for the conversion of the CH
4 and CO
2; based on the results obtained, it has been determined that only ~4% of the input energy is utilized in the conversion of the reactants; this low utilization is a significant drawback for the technology and should be improved by further studies of the plasma/catalyst/configuration combinations [
51]. Finally, another application under study uses combined systems to gasify and decompose aromatic products or residues (tar) by plasma. A plasma and microwave reactor system was developed and tested for converting tar substitutes, i.e., benzene, toluene, and 1-methylnaphthalene, into a nitrogen stream. It was demonstrated that the efficiency of the process could be as high as 98% [
52]. The above have been examples of potential applications of the use of plasma for chemical reaction systems.
3.4. Microwave Usage
Microwaves are oscillating electromagnetic energy with frequencies in ranges from 300 MHz to 300 GHz, with the most effective range for dielectric heating being between 0.915 and 2.45 GHz. It is estimated that the ability of a material to be heated by this technique depends on the following relationship [
53]:
where tanδ is the ratio between the dissipative (including electrical conductivity losses) and capacitive behavior of the materials.
ε′ is the dielectric constant, representing the ability of a material to be polarized by an external electric field and is, therefore, a relative measure of the microwave energy density.
ε″ is the loss factor, quantifying the efficiency with which electromagnetic energy is converted into heat [
54].
The value of tanδ value is easily related to the ability of the materials to be heated; the higher the value, the better this ability; the application of microwave heating will depend on the nature of the component, as can be seen in
Table 2.
The peculiarities of microwave heating are derived from the generation of heat by the transfer of energy during the interaction between electromagnetic radiation and the chemical compounds of the material. Over the years, many industrial production plants have been applying this technique, seeking significant improvements in heating operating efficiency. The main fields of application include [
35] the food industry: e.g., for tempering large or small food blocks from −20 °C to −2 °C can be realized by compact 60 kW installed installations.
In the rubber industry: MW is used to heat rubber blocks to several hundred kilograms in weight replacing conventional vulcanization, which uses hot air or salt baths with the usual limitation associated with thermal conduction and heating effects.
In the wood industry: In wood manufacturing, the MW process creates a uniformly heated product with extremely consistent properties, such as density in all product dimensions. The strength of the finished product is higher than that of conventional wood-based building materials. The drying of wood paint is especially efficient with MW as the residual water in the paint can be reduced by up to 2%, allowing for optimal UV-vis polymerization without unwanted white spots or quality effects.
In waste processing: It is used for processing automobile tires, hospital and municipal waste treatment, vitrification of nuclear waste, treatment of highly toxic substances, recovery of plastic waste, heating of resins, polymerization, heating of oil sands, and soil remediation.
In the field of so-called fine chemical processes, it has been used in the last 10 years for the extraction of synthetic organic compounds and for chemical manufacturing in reaction vessels or chemical reactors. However, the most widespread applications of microwaves are for drying food pastes or pharmaceutical species at very low temperatures using a vacuum. For these types of products, there is a requirement to keep the entire batch at sufficiently low temperatures to avoid spoilage.
One large-scale MW application concerns the waste gasification process. Gasification is the reaction of fuel in the presence of air, oxygen, and steam to produce a gaseous product that can be used as an energy source or as a feedstock in subsequent stages of chemical synthesis, liquid fuels or gaseous fuels in one application example, Indonesian lignite with ash and a moisture content of 38.12% by weight was injected into the air/oxygen flare with a 4 kW power microwave source, finding relative concentrations of synthesized gases at a ratio of 1.36 coal to steam of 48% hydrogen. In the end, it was found that the improvement of combustion using MW was due to the heating of the gases in the flame zone producing higher flame temperatures facilitating the conversion of the gases into hydrogen, 23% carbon monoxide, 25% carbon dioxide, and 4% methane [
55].
3.5. Electric and Magnetic Fields
Studies related to electric and magnetic fields (EFs) are mainly focused on how these fields can influence some catalytic processes in terms of their activity or how these fields have effects at the level of metallic nanoparticles in heat exchange processes, which in turn can generate energy and efficiency benefits for the facility. In the food industry, for example, research has been carried out on the effect of electric fields or so-called electric pulses (PEF) on food nutrients and how these are affected to a greater extent by conventional thermal treatment [
56].
Further studies on the application of electric and magnetic fields have been carried out to evaluate boiling processes in rehervid ores, where improvements in the heat transfer of boiling liquids have been visualized by increasing the transfer coefficients for the process equipment. The most recent review on boiling enhancement by applying an electric field (EF) was provided by Shahriari et al. [
57], which related the effects of the electrical conductivity of the evaporating liquid. Di Marco [
58] also reviewed the EF effects on boiling heat transfer (HT) performance and, likewise, the behavior in the presence of magnetic fields but found no review articles in the literature on the actual effects of magnetic field (MF) on boiling HT.
In general, it can be said that the different results of studies on the application of these energy forms for boiling systems are inconclusive and sometimes present inconsistencies on the potential effects of FE and MF applied for energy exchange systems [
59]. The steam reforming process to produce hydrogen is carried out at high temperatures because it is an endothermic reaction, in a study by Takise et al. [
60] using aromatic hydrocarbons such as toluene found that the application of an electric field to the reforming process could be carried out at lower temperature conditions and with scalable efficiencies for hydrogen production where the effect of the electric field influences the activation of the reaction water minimizing the generation and deposition of coke on the catalyst. In some catalytic processes mentioned above, MW and Plasma applications have been studied, as well as the application of FE to accelerate certain chemical reactions.
A study by Kucherov et al. [
61] evaluated the effects of passing an electric current through a Mo-V- Fe-Nb-Ox catalytic bed for a dehydrogenation process of ethane, which evidenced evident energy savings without changes or electronic type effects on the molecular components of the catalyst with additional advantages on the kinetics of the reaction (endothermic process).
An interesting application of electric fields in biofuels is the improvement in the production of biodiesel from soybean and its chemical kinetics. Although the applied electric field, in principle, increases the rate of molecular collisions, the hypothesis at this point is that the reaction rate could be accelerated due to a reduction in the surface tension between methanol and soybean oil compared to the conventional method of biodiesel preparation, it is estimated that the reaction and separation time could be shortened (enhanced transesterification) with the use of electric field [
62].
The main application of electric and magnetic fields in thermal sciences is integrated with heat exchange equipment. It is known that some conventional heat transfer fluids such as water, oil, and ethylene glycol have lower thermal conductivity than solid or metallic materials; then how to greatly improve this thermal property of the system is a crucial question for researchers. Nanofluid is a concept introduced by Choi in 1995 [
63], which consists of two main components: a base carrier fluid and quantities of solid nanoparticles (usually less than 100 nm in diameter) together with a surfactant added to the suspension in order to maintain mixing stability and avoid precipitation. The enhanced thermal conductivity of nanofluids is generating much research in the field and simultaneous applications with electrical fields.
Once steam generation processes are evidenced in r kettles, it has been observed that the electric field affects the size and shape of the bubbles in the system; therefore, electroconvection is the mechanism that affects the frequency of bubble exit or escapes contributing to the improvement in HT [
64].
The food industry is receiving most of the developments related to the application of electricity as a heating source in what is called ohmic-type heating, which is described as the process of passing an electric current through materials for heating purposes. The food products treated by this technique act as electrical resistors which heat up and dissipate energy; some aspects involved in the heating rate in an ohmic process include electrical conductivity and the strength of the applied electric field [
65]. These techniques are used for fermentation processes, microbial inactivation, pasteurization, and extraction of essential oils, among others. Finally, it is important to mention that heating processes for catalytic systems are being studied in the laboratory, seeking to incorporate electricity in a more relevant way [
66]. One example is the methanation process of CO
2 (the Sabatier reaction) with surplus hydrogen as a promising alternative to provide renewable chemical energy (CH
4) and simultaneously reduce emissions of CO
2 [
67].
Heating through electrical resistors is a heating method that can be considered easy and efficient for certain services [
68]. However, it has not been widely accepted because traditional catalysts are in powder or granular form with low electrical conductivity. Recently, Wismann et al. [
69] have reported the development of an electrically heated reactor for an alkali reforming process, which consists of a tube with an alloy and an alloy tube with a CH
4 which consists of a FeCrAl alloy tube coated with a nickel layer, which allows a consistent direct heat supply over the catalysts by applying alternating current along the tube, it is evidenced that the catalyst improves its heat conductive properties and the active components heat up quickly.
4. Discussion
From the exposed topics, there is no doubt of the immense opportunity that industrial waste heat offers for its use and exploitation in society, as demonstrated by the statistical data of large world economies such as China and the United States. The strategies proposed by the world and under the concept of electrification are fundamental to advancing toward a more balanced and sustainable society that is not dependent on current fossil fuels or coal. Today it is clear that at the industrial level, many of the technologies for the use of electrical energy as a heating source are not very widespread, being more validated on a small scale at the laboratory level. The cost of using electricity in thermal processes is still an important limitation, not to mention the environmental effects it can generate. Using or incorporating electricity with a lower or almost zero carbon footprint is the primary objective where hydropower and renewable sources such as solar, wind, and biomass play a leading role for the coming decades [
70]. The oil and fuel refining industry is one of the sectors that can provide the most opportunities to incorporate or adapt more aggressive electrification processes. This industry bases its primary source of energy on the combustion of natural gas for use mostly in furnaces and boilers, which of course, generates important contributions of CO
2 emissions to the atmosphere and therefore contribute directly to global warming. It is estimated that the top 60 oil and gas companies in the world ranking contributed more than 40% of cumulative global industrial emissions in the last 30 years [
71]. On the other hand, the concept of energy transition and the migration to cleaner fuel systems forces large energy-generating companies, and industry itself in general, to seek alternatives where electricity not only substitutes their primary sources of energy supply but also allows them to incorporate electric energy as a means of synthesis for new products, fuels or value generation systems.
Hydrogen generation from electricity (electrolysis) is seen as a good opportunity where electrolyzer technologies are maturing and positioning themselves in recent years on an industrial scale due to their relatively high efficiency (0.75–0.85) compared to the conventional reforming process (0.53–0.68) [
72].
Likewise, the opportunity to use this hydrogen as a reagent to produce other fuels, such as methanol, from the capture of CO
2 is seen as a promising economy that can be developed with a fuel that is not only less carbon [
73], a fuel that not only has a lower carbon load but also converts to a fuel that can be more efficient in the case of methanol. CO
2 a capture process that can be more economical (at the energy level) compared to the current schemes of reinjection into the earth’s subsoil, is one of the most evaluated options in recent years.
The application of heat pumps appears to be one of the most reliable mechanisms to establish optimal GHG emission reduction pathways. In addition, these electrification technologies can be applied to a wide range of industrial processes without requiring major modifications to the existing infrastructure, contributing to the total or partial replacement of fossil fuels depending on the degree of temperature the process may require.
Some studies have made it possible to quantify the contribution of industrial heat pump technologies to actual climate change mitigation, estimating as an example for Germany savings of up to 15% in final energy consumption and up to 17% in total emissions of CO
2 emissions associated with conventional energy use schemes [
74].
The implementation of heat pump systems for fractionation systems or separation of components in chemical processes through the so-called MVR systems seems to be promising alternatives in the short term. Today it is clear that replacing 100% of the fuel gas supply by industrial energy processes is not competitive, mainly for countries that have ample availability of fossil resources, considering that the cost ratio to generate 1 kW of energy for an industrial process can be between 3000 and 3500 times more to do it with electric energy than with conventional gas combustion, emphasizing without a doubt that the advantage in GHG reduction with the use of electric energy has a superlative value contribution for our society in the short and medium term.
Other electrification alternatives, such as the use of plasma, microwaves, and electric and magnetic fields, have specific applications mainly for processes such as waste gasification, pyrolysis, iron and steel industry, among others, with a wide range of experimental applications that allow us to visualize good prospects soon, mainly at the level of catalytic processes and chemical synthesis reactions.