Downsizing Sustainable Aviation Fuel Production with Additive Manufacturing—An Experimental Study on a 3D printed Reactor for Fischer-Tropsch Synthesis
Abstract
:1. Introduction
1.1. Fischer-Tropsch Synthesis
1.2. Compact Reactors
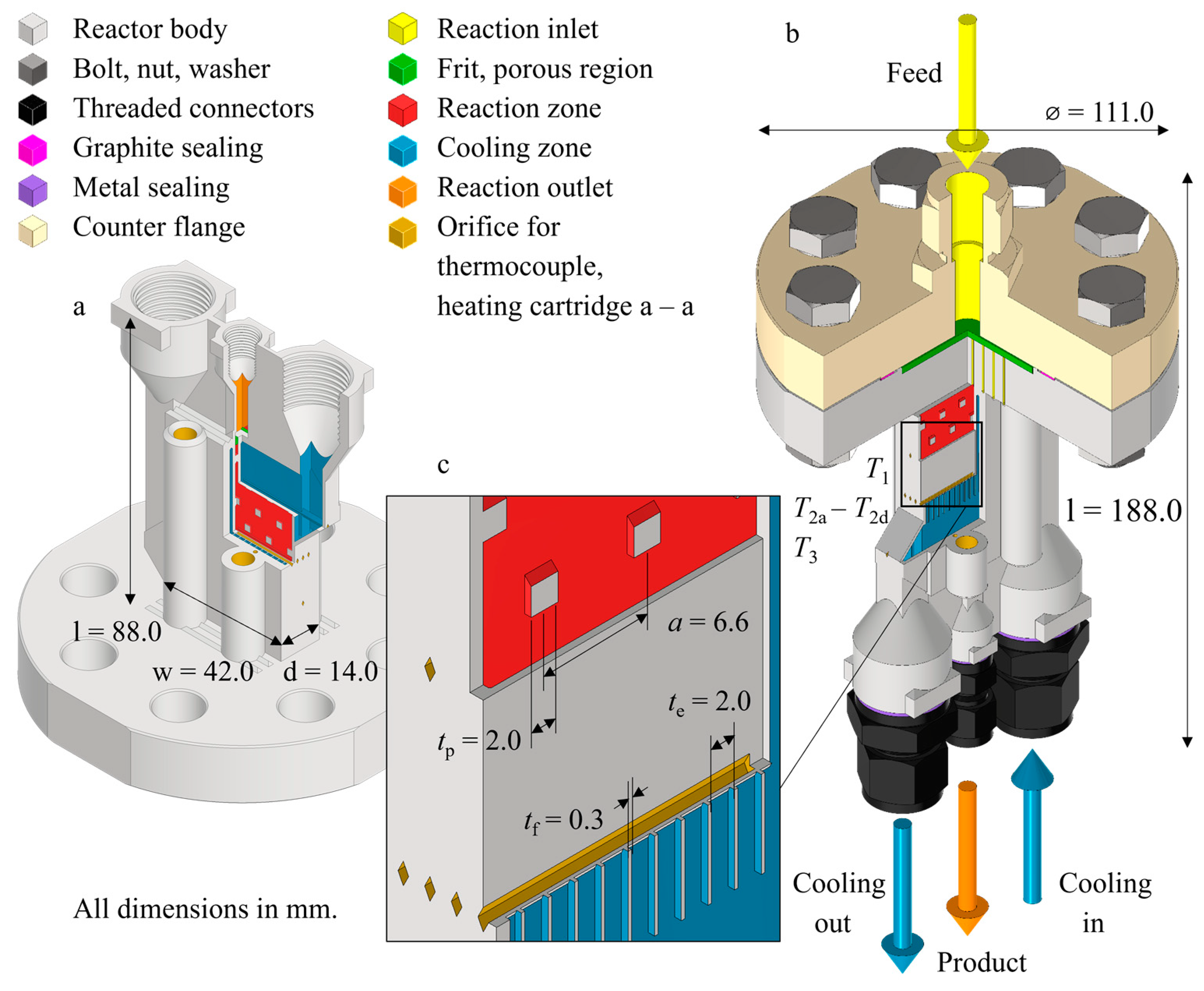
2. Compact AM Reactor
- Introduction of catalyst with a particle size of dp = 50–150 µm,
- Performance of the FT reaction with undiluted catalyst and synthesis gas at H2/CO = 2.0 and Ftotal = 6 to 30 LN h−1,
- SV = F/Vcat = 1500 to 7500 h−1; τ = 1.33 to 6.67 × 10−4 h = 0.48 to 2.4 s, and
- SVmod = F/mcat = SV/ρcat = 2 to 10 LN gcat−1 h−1; τmod = 0.1 to 0.5 h gcat LN−1; the index ‘mod’ denotes that the space velocity is related to the catalyst mass.
- Efficient cooling,
- Scalability through slit design,
- Spatially resolved T measurement,
- Possibility of connection to standard laboratory equipment,
- Streamlined workflow,
- Operating pressure of 20 bara @ 250 °C, and
- Helium standard leakage rate at q < 1 × 10−8 mbar L s−1 [31].
2.1. Design
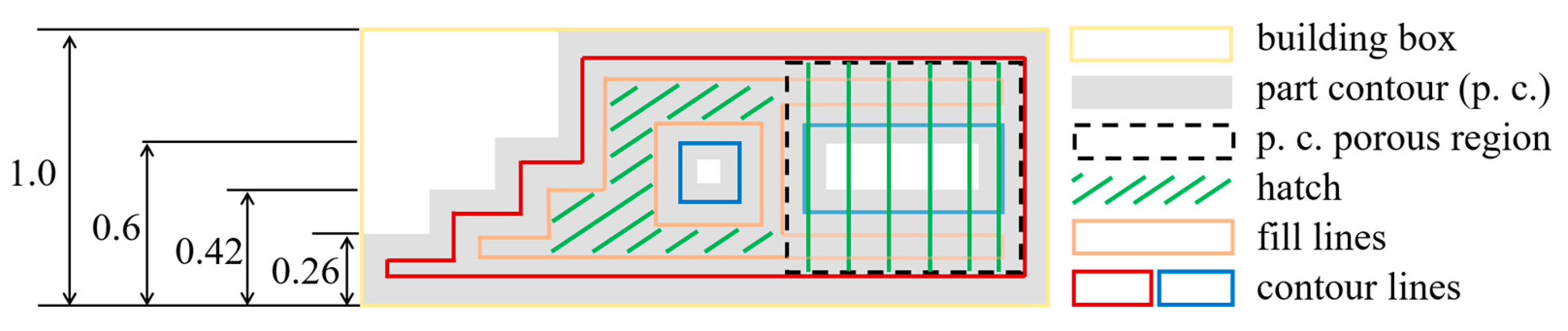
2.2. Fabrication
3. Experimental Methods
3.1. Catalyst
3.2. Reaction Test
3.3. Evaluation
4. Results and Discussion
4.1. Safety Tests and Reduction
4.2. Results of the Fischer-Tropsch Synthesis
4.3. Reactor Operation and Scale-Up
5. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviations | |
| AM | Additive manufacturing |
| BET | Brunauer Emmett Teller |
| Cn | Hydrocarbon with n carbon atoms |
| C5+ | Hydrocarbons with number of carbon atoms ≥ 5 |
| FID | Flame ionization detector |
| FTS | Fischer-Tropsch Synthesis |
| GC | Gas chromatograph |
| MFC | Mass flow controller |
| O | Olefin |
| P | Paraffin |
| PBF-LB/M | Powder bed fusion with laser beam of metals |
| PtL | Power to Liquid (liquid chemical energy carrier) |
| SAF | Sustainable Aviation Fuel |
| SLM | Selective laser melting, s. PBF-LB/M |
| STL | Standard Tessellation Language |
| TCD | Thermal conductivity detector |
| Latin Symbols | |
| A | Area (mm2) |
| a | Spacing (mm) |
| d | Diameter, depth (mm) |
| F | Volumetric flow rate (LN h−1) |
| H | Enthalpy (J) |
| l | Length (mm) |
| m | Mass (kg) |
| Molar flow (mol s−1) | |
| n | Number (-) |
| P | Productivity (g h−1) |
| p | Pressure (bar) |
| q | Standard helium leakage rate (mbar L s−1) |
| Heat flow (W) | |
| S | Selectivity (mol/mol) |
| STY | Space time yield (kg m−3 h−1) |
| SV | Space velocity at standard conditions (LN m−3 h−1) |
| t | Thickness, dimension (mm) |
| t | Time (s) |
| TOS | Time on stream (h) |
| V | Volume (mm3) |
| w | Width (mm) |
| X | Conversion (mol/mol) |
| y | Molar fraction (mol/mol) |
| Greek symbols | |
| Chain growth probability (-) | |
| Δ | Difference (diverse) |
| Angle (°) | |
| (h) | |
| Weight fraction (g/g) | |
| Subscripts | |
| c | Cooling |
| cat | Referring to catalyst or catalytic zone |
| e | Empty |
| f | Fin |
| hex | Heat transfer |
| mod | Modified, in this work: related to catalyst mass |
| N | Standard conditions (p = 1.01325 bara, T = 273.15 K) |
| p | Particle, pin |
| R, rct | Reaction |
| w | Wall |
References
- Fuel Cells and Hydrogen 2 Joint Undertaking. Hydrogen-Powered Aviation: A Fact-Based Study of Hydrogen Technology, Economics, and Climate Impact by 2050; Publications Office of the European Union: Luxembourg, 2020; Available online: https://data.europa.eu/doi/10.2843/471510 (accessed on 25 August 2023).
- Schripp, T.; Anderson, B.E.; Bauder, U.; Rauch, B.; Corbin, J.C.; Smallwood, G.J.; Lobo, P.; Crosbie, E.C.; Shook, M.A.; Miake-Lye, R.C.; et al. Aircraft engine particulate matter emissions from sustainable aviation fuels: Results from ground-based measurements during the NASA/DLR campaign ECLIF2/ND-MAX. Fuel 2022, 325, 124764. [Google Scholar] [CrossRef]
- Lee, D.S.; Fahey, D.W.; Skowron, A.; Allen, M.R.; Burkhardt, U.; Chen, Q.; Doherty, S.J.; Freeman, S.; Forster, P.M.; Fuglestvedt, J.; et al. The contribution of global aviation to anthropogenic climate forcing for 2000 to 2018. Atmos. Environ. 2021, 244, 117834. [Google Scholar] [CrossRef]
- Bullerdiek, N.; Neuling, U.; Kaltschmitt, M. A GHG reduction obligation for sustainable aviation fuels (SAF) in the EU and in Germany. J. Air Transp. Manag. 2021, 92, 102020. [Google Scholar] [CrossRef]
- Kern, J. Decarbonisation or defossilisation? Innovative alternative fuels for the aviation in Brazil: An international reference Model. In Proceedings of the Eleventh Meeting of the SBSTA Research Dialogue (RD 11) Science for Transformation (SBSTA 50), Bonn, Germany, 20 June 2019. [Google Scholar]
- Pfeifer, P.; Biffar, L.; Timm, F.; Böltken, T. Influence of Power-to-Fuel Plant Flexibility Towards Power and Plant Utilization and Intermediate Hydrogen Buffer Size. Chem. Ing. Tech. 2020, 92, 1976–1982. [Google Scholar] [CrossRef]
- Bieringer, T.; Bramsiepe, C.; Brand, S.; Brodhagen, A.; Dreiser, C.; Fleischer-Trebes, C.; Kockmann, N.; Lier, S.; Schmalz, D.; Schwede, C.; et al. White Paper Modular Plants. 2016. Available online: https://dechema.de/en/About+DECHEMA/Press/Reports+and+position+papers/ProcessNet+Positionspapiere/2016+7+White+Paper+Modular+Plants.html (accessed on 25 August 2023).
- Hsu, C.S.; Robinson, P.R. (Eds.) Springer Handbook of Petroleum Technology, 1st ed.; Springer: Cham, Switzerland, 2017; ISBN 9783319493459. [Google Scholar]
- Van de Loosdrecht, J.; Botes, F.G.; Ciobica, I.M.; Ferreira, A.; Gibson, P.; Moodley, D.J.; Saib, A.M.; Visagie, J.L.; Weststrate, C.J.; Niemantsverdriet, J.W. Fischer-Tropsch synthesis: Catalysts and chemistry. In Comprehensive Inorganic Chemistry II; Reedijk, J., Poeppelmeier, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 525–557. ISBN 9780080965291. [Google Scholar]
- Bertoncini, F.; Marion, M.C.; Brodusch, N.; Esnault, S. Unravelling Molecular Composition of Products from Cobalt Catalysed Fischer-Tropsch Reaction by Comprehensive Gas Chromatography: Methodology and Application. Oil Gas Sci. Technol. Rev. IFP 2009, 64, 79–90. [Google Scholar] [CrossRef]
- Dry, M.E. High quality diesel via the Fischer-Tropsch process—A review. J. Chem. Technol. Biotechnol. 2002, 77, 43–50. [Google Scholar] [CrossRef]
- Almeida, L.C.; Echave, F.J.; Sanz, O.; Centeno, M.A.; Arzamendi, G.; Gandía, L.M.; Sousa-Aguiar, E.F.; Odriozola, J.A.; Montes, M. Fischer–Tropsch synthesis in microchannels. Chem. Eng. J. 2011, 167, 536–544. [Google Scholar] [CrossRef]
- Maitlis, P.M.; Klerk, A.D. Greener Fischer-Tropsch Processes for Fuels and Feedstocks, 1st ed.; Wiley-VCH: Weinheim, Germany, 2013; ISBN 9783527329458. [Google Scholar]
- Kolb, G. Review: Microstructured reactors for distributed and renewable production of fuels and electrical energy. Chem. Eng. Process. Process Intensif. 2013, 65, 1–44. [Google Scholar] [CrossRef]
- Brandner, J.J. Microfabrication in metals and polymers. In Micro Process Engineering: Fundamentals, Devices, Fabrication, and Applications; Kockmann, N., Ed.; Elektronische Ressource; Wiley-VCH: Weinheim, Germany, 2008; ISBN 3527312463. [Google Scholar]
- Cao, C.; Hu, J.; Li, S.; Wilcox, W.; Wang, Y. Intensified Fischer–Tropsch synthesis process with microchannel catalytic reactors. Catal. Today 2009, 140, 149–156. [Google Scholar] [CrossRef]
- Piermartini, P.; Böltken, T.; Selinsek, M.; Pfeifer, P. Influence of channel geometry on Fischer-Tropsch synthesis in microstructured reactors. Chem. Eng. J. 2017, 313, 328–335. [Google Scholar] [CrossRef]
- LeViness, S.; Tonkovich, A.L.Y.; Jarosch, K.; Fitzgerald, S.; Yang, B.; McDaniel, J. Improved Fischer-Tropsch Economics Enabled by Microchannel Technology. 2011. Available online: https://www.researchgate.net/publication/267236523_Improved_Fischer-Tropsch_Economics_Enabled_by_Microchannel_Technology (accessed on 25 August 2023).
- Selinsek, M. Compact Fischer-Tropsch Synthesis in Gas-to-Liquid Applications. In Proceedings of the 2nd Thematic Workshop, Prague, Czech Republic, 23–24 May 2019; Available online: https://www.comsynproject.eu/publications/ (accessed on 20 August 2023).
- Deshmukh, S.R.; Tonkovich, A.L.Y.; Jarosch, K.T.; Schrader, L.; Fitzgerald, S.P.; Kilanowski, D.R.; Lerou, J.J.; Mazanec, T.J. Scale-Up of Microchannel Reactors for Fischer-Tropsch Synthesis. Ind. Eng. Chem. Res. 2010, 49, 10883–10888. [Google Scholar] [CrossRef]
- Choudhury, H.A.; Cheng, X.; Afzal, S.; Prakash, A.V.; Tatarchuk, B.J.; Elbashir, N.O. Understanding the deactivation process of a microfibrous entrapped cobalt catalyst in supercritical fluid Fischer-Tropsch Synthesis. Catal. Today 2020, 343, 112–124. [Google Scholar] [CrossRef]
- Milewski, J.O. Additive Manufacturing of Metals: From Fundamental Technology to Rocket Nozzles, Medical Implants, and Custom Jewelry; Springer International Publishing: Cham, Switzerland, 2017; ISBN 9783319582047. [Google Scholar]
- Grinschek, F.; Ladewig, B.; Navarrete Munoz, A.; Klahn, C.; Dittmeyer, R. Getting Chemical and Biochemical Engineers Excited about Additive Manufacturing. Chem. Ing. Tech. 2022, 94, 931–938. [Google Scholar] [CrossRef]
- Xie, D. Additively Manufactured Permeable-Dense Composites and Its Applications in Microstructured Reactors. Doctoral Dissertation, Karlsruher Institut für Technologie, Karlsruhe, Germany, 2021. [Google Scholar]
- Stoll, P.; Spierings, A.; Wegener, K. Impact of a process interruption on tensile properties of SS 316L parts and hybrid parts produced with selective laser melting. Int. J. Adv. Manuf. Technol. 2019, 103, 367–376. [Google Scholar] [CrossRef]
- González-Castaño, M.; Baena-Moreno, F.M.; Navarro de Miguel, J.C.; Miah, K.U.M.; Arroyo-Torralvo, F.; Ossenbrink, R.; Odriozola, J.A.; Benzinger, W.; Hensel, A.; Wenka, A.; et al. 3D-printed structured catalysts for CO2 methanation reaction: Advancing of gyroid-based geometries. Energy Convers. Manag. 2022, 258, 115464. [Google Scholar] [CrossRef]
- Avril, A.; Hornung, C.H.; Urban, A.; Fraser, D.; Horne, M.; Veder, J.-P.; Tsanaktsidis, J.; Rodopoulos, T.; Henry, C.; Gunasegaram, D.R. Continuous flow hydrogenations using novel catalytic static mixers inside a tubular reactor. React. Chem. Eng. 2017, 2, 180–188. [Google Scholar] [CrossRef]
- Fratalocchi, L.; Groppi, G.; Visconti, C.G.; Lietti, L.; Tronconi, E. Adoption of 3D printed highly conductive periodic open cellular structures as an effective solution to enhance the heat transfer performances of compact Fischer-Tropsch fixed-bed reactors. Chem. Eng. J. 2020, 386, 123988. [Google Scholar] [CrossRef]
- Hauser, A.; Weitzer, M.; Gunsch, S.; Neubert, M.; Karl, J. Dynamic hydrogen-intensified methanation of synthetic by-product gases from steelworks. Fuel Process. Technol. 2021, 217, 106701. [Google Scholar] [CrossRef]
- Wei, Q.; Li, H.; Liu, G.; He, Y.; Wang, Y.; Tan, Y.E.; Wang, D.; Peng, X.; Yang, G.; Tsubaki, N. Metal 3D printing technology for functional integration of catalytic system. Nat. Commun. 2020, 11, 4098. [Google Scholar] [CrossRef]
- Grinschek, F.; Charles, A.; Elkaseer, A.; Klahn, C.; Scholz, S.G.; Dittmeyer, R. Gas-tight means zero defects—Design considerations for thin-walled fluidic devices with overhangs by laser powder bed fusion. Mater. Des. 2022, 223, 111174. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. Additive Manufacturing Technologies; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar]
- Klahn, C. Laseradditiv Gefertigte, Luftdurchlässige Mesostrukturen: Herstellung und Eigenschaften für die Anwendung; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 9783662477601. [Google Scholar]
- Myrstad, R.; Eri, S.; Pfeifer, P.; Rytter, E.; Holmen, A. Fischer-Tropsch synthesis in a microstructured reactor. Catal. Today 2009, 147, S301–S304. [Google Scholar] [CrossRef]
- Kirsch, H.; Lochmahr, N.; Staudt, C.; Pfeifer, P.; Dittmeyer, R. Production of CO2-neutral liquid fuels by integrating Fischer-Tropsch synthesis and hydrocracking in a single micro-structured reactor: Performance evaluation of different configurations by factorial design experiments. Chem. Eng. J. 2020, 393, 124553. [Google Scholar] [CrossRef]
- van der Laan, G.P.; Beenackers, A.A.C.M. Kinetics and Selectivity of the Fischer–Tropsch Synthesis: A Literature Review. Catal. Rev. 1999, 41, 255–318. [Google Scholar] [CrossRef]
- Griffiths, L. TCT Buyer’s Guide 2022; Rapid News Group: Chester, UK, 2022. [Google Scholar]
- Dittmeyer, R.; Klumpp, M.; Kant, P.; Ozin, G. Crowd oil not crude oil. Nat. Commun. 2019, 10, 1818. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Vázquez, F.V.; Koponen, J.; Ruuskanen, V.; Bajamundi, C.; Kosonen, A.; Simell, P.; Ahola, J.; Frilund, C.; Elfving, J.; Reinikainen, M.; et al. Power-to-X technology using renewable electricity and carbon dioxide from ambient air: SOLETAIR proof-of-concept and improved process concept. J. CO2 Util. 2018, 28, 235–246. [Google Scholar] [CrossRef]
- DIN EN ISO 4022:2018-12; Durchlässige Sintermetallwerkstoffe: Bestimmung der Flüssigkeitsdurchlässigkeit. Beuth Verlag GmbH: Berlin, Germany, 2018. Available online: https://www.nautos.de/OJG/search (accessed on 20 August 2023).
- Kirsch, H. Dezentrale Synthese Strombasierter Flüssiger Kraftstoffe über die Fischer-Tropsch Route. Ph.D. Thesis, Karlsruher Institut für Technologie, Karlsruhe, Germany, 2020. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular Size Distribution in Linear Condensation Polymers. J. Am. Chem. Soc. 1936, 58, 1877–1885. [Google Scholar] [CrossRef]
- Kaiser, R.E. Gas-Chromatographie. 2. Aufl. Bd. 22. B I Hochschultaschenbücher; Bibliographisches Institut: Mannheim, Germany, 1973; ISBN 3411000228. [Google Scholar]
- Brübach, L.T. Katalytische Hydrierende Spaltung von Fischer-Tropsch Produkten im Technikumsmaßstab. Master’s Thesis, Karlsruher Institut für Technologie, Karlsruhe, Germany, 2018. [Google Scholar]
- Pabst, K.; González, M.I.; Kraushaar-Czarnetzki, B.; Schaub, G. Combination of Fischer–Tropsch Synthesis and Hydroprocessing in a Single-Stage Reactor. Part I. Mathematical Modeling of the Reaction Kinetics. Ind. Eng. Chem. Res. 2013, 52, 8978–8987. [Google Scholar] [CrossRef]
- Shell. Pearl GTL Overview. Available online: https://www.shell.com/about-us/major-projects/pearl-gtl/pearl-gtl-an-overview.html (accessed on 3 July 2023).
- MAN Energy Solutions. Shell Pearl GTL—Gas-to-Liquid Reactor in Qatar for Shell. Available online: https://www.man-es.com/process-industry/products/chemical-reactors/water--air--oil-operated-reactors (accessed on 3 July 2023).
- Hydrocarbons Technology. Oryx. Available online: https://www.hydrocarbons-technology.com/projects/oryx/ (accessed on 3 July 2023).
- 2011. Available online: https://docplayer.net/45263490-Our-projects-oryx-gtl.html (accessed on 25 August 2023).
- Berger, R. EUROKIN Spreadsheet on Requirements for Measurement of Intrinsic Kinetics in the Gas-Solid Fixed-Bed Reactor. 2010. Available online: https://eurokin.org/wp-content/uploads/downloads/2019/08/EUROKIN_fixed-bed_html_guide.pdf (accessed on 15 September 2023).
- Jess, A.; Wasserscheid, P. Chemical Technology: An Integral Textbook; Wiley-VCH: Weinheim, Germany, 2013; ISBN 978-3-527-30446-2. [Google Scholar]
- Pfeifer, P. Application of catalysts to metal microreactor systems. In Chemical Kinetics; Patel, V., Ed.; InTech Europe: Rijeka, Croatia, 2012; pp. 325–345. ISBN 978-953-51-0132-1. [Google Scholar]

| N. | T/°C | SVmod/LN gcat−1 h−1 | Ftotal/ LN h−1 | PC5+/ g h−1 | XCO/ % | XH2/ % | SC1/ % | SC2/ % | SC3/ % | SC4/ % | SC5+/% | O/P/ - | α */ - |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 200 | 3.59 | 12.0 | 0.43 | 39.3 | 36.7 | 4.3 | 0.6 | 2.1 | 2.2 | 90.8 | 1.81 | |
| 2 | 209 | 3.59 | 12.0 | 0.55 | 52.6 | 51.5 | 5.2 | 0.6 | 2.3 | 1.0 | 90.8 | 0.94 | |
| 3 | 219 | 3.59 | 12.0 | 2.82 | 70.9 | 72.4 | 6.4 | 0.8 | 2.5 | 1.1 | 89.2 | 0.63 | |
| 4 | 219 | 5.38 | 17.9 | 1.38 | 56.0 | 55.8 | 6.8 | 0.8 | 2.8 | 1.4 | 88.1 | 0.79 | |
| 5 | 229 | 5.38 | 17.9 | 2.77 | 79.0 | 80.6 | 7.5 | 0.9 | 2.6 | 1.3 | 87.7 | 0.47 | |
| 6 | 209 | 5.38 | 17.9 | 0.81 | 43.3 | 41.2 | 6.1 | 0.7 | 2.5 | 1.2 | 89.6 | 0.92 | |
| 7 | 200 | 5.38 | 17.9 | 0.46 | 27.8 | 26.5 | 5.2 | 0.6 | 2.0 | 2.1 | 90.0 | 2.06 | |
| 8 | 209 | 1.85 | 6.2 | 1.41 | 65.1 | 67.7 | 6.9 | 0.9 | 2.9 | 1.3 | 88.1 | 0.62 | |
| 9 | 200 | 1.85 | 6.2 | 0.49 | 53.0 | 53.5 | 6.4 | 0.8 | 3.2 | 1.6 | 88.1 | 0.83 | |
| 10 | 190 | 1.85 | 6.2 | 0.28 | 31.4 | 31.2 | 6.5 | 0.8 | 3.3 | 2.8 | 86.5 | 1.50 | |
| 11 | 209 | 9.00 | 30.0 | 0.58 | 16.9 | 16.6 | 9.2 | 1.2 | 3.4 | 3.7 | 82.5 | 2.15 | |
| 12 | 229 | 9.00 | 30.0 | 2.07 | 48.5 | 50.5 | 8.8 | 1.1 | 3.4 | 1.7 | 85.0 | 0.81 | |
| 13 | 219 | 9.00 | 30.0 | 1.15 | 38.5 | 38.4 | 6.2 | 0.8 | 2.4 | 1.0 | 89.6 | 1.14 | |
| 14 | 234 | 9.00 | 30.0 | 3.43 | 69.2 | 71.0 | 8.6 | 1.1 | 3.0 | 1.6 | 85.7 | 0.54 | 0.862 |
| 1R | 200 | 3.59 | 12.0 | 0.54 | 42.3 | 41.2 | 4.8 | 0.6 | 2.2 | 1.6 | 90.9 | 1.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metzger, D.F.; Klahn, C.; Dittmeyer, R. Downsizing Sustainable Aviation Fuel Production with Additive Manufacturing—An Experimental Study on a 3D printed Reactor for Fischer-Tropsch Synthesis. Energies 2023, 16, 6798. https://doi.org/10.3390/en16196798
Metzger DF, Klahn C, Dittmeyer R. Downsizing Sustainable Aviation Fuel Production with Additive Manufacturing—An Experimental Study on a 3D printed Reactor for Fischer-Tropsch Synthesis. Energies. 2023; 16(19):6798. https://doi.org/10.3390/en16196798
Chicago/Turabian StyleMetzger, David F., Christoph Klahn, and Roland Dittmeyer. 2023. "Downsizing Sustainable Aviation Fuel Production with Additive Manufacturing—An Experimental Study on a 3D printed Reactor for Fischer-Tropsch Synthesis" Energies 16, no. 19: 6798. https://doi.org/10.3390/en16196798
APA StyleMetzger, D. F., Klahn, C., & Dittmeyer, R. (2023). Downsizing Sustainable Aviation Fuel Production with Additive Manufacturing—An Experimental Study on a 3D printed Reactor for Fischer-Tropsch Synthesis. Energies, 16(19), 6798. https://doi.org/10.3390/en16196798






