Enhancing Hydrogen Peroxide Synthesis through Coordination Engineering of Single-Atom Catalysts in the Oxygen Reduction Reaction: A Review
Abstract
:1. Introduction
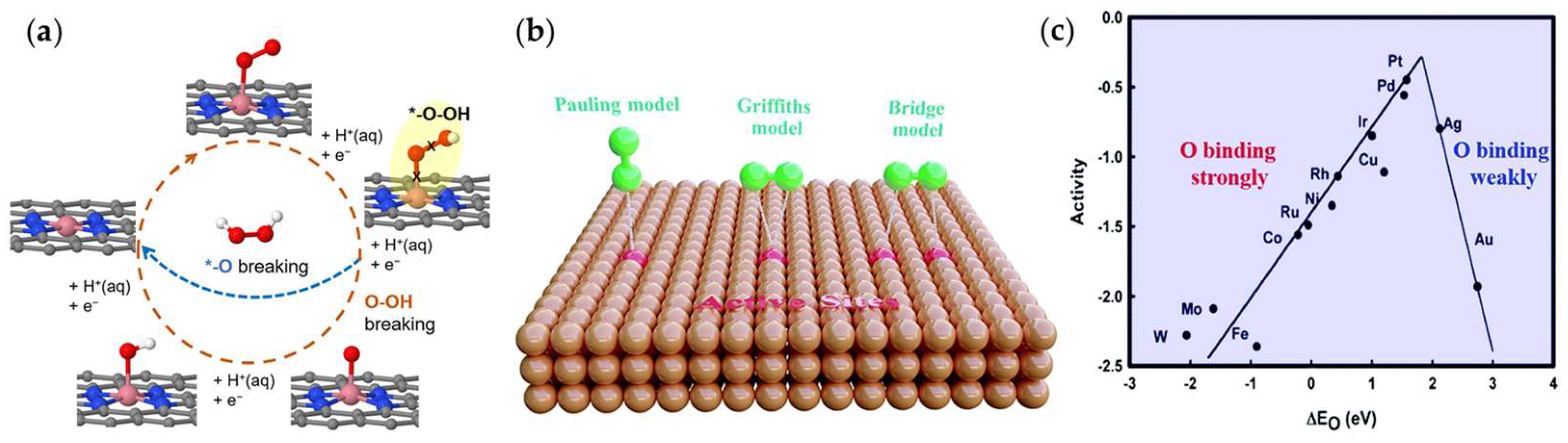
2. Mechanism of Electrocatalytic Oxygen Reduction for H2O2 Synthesis
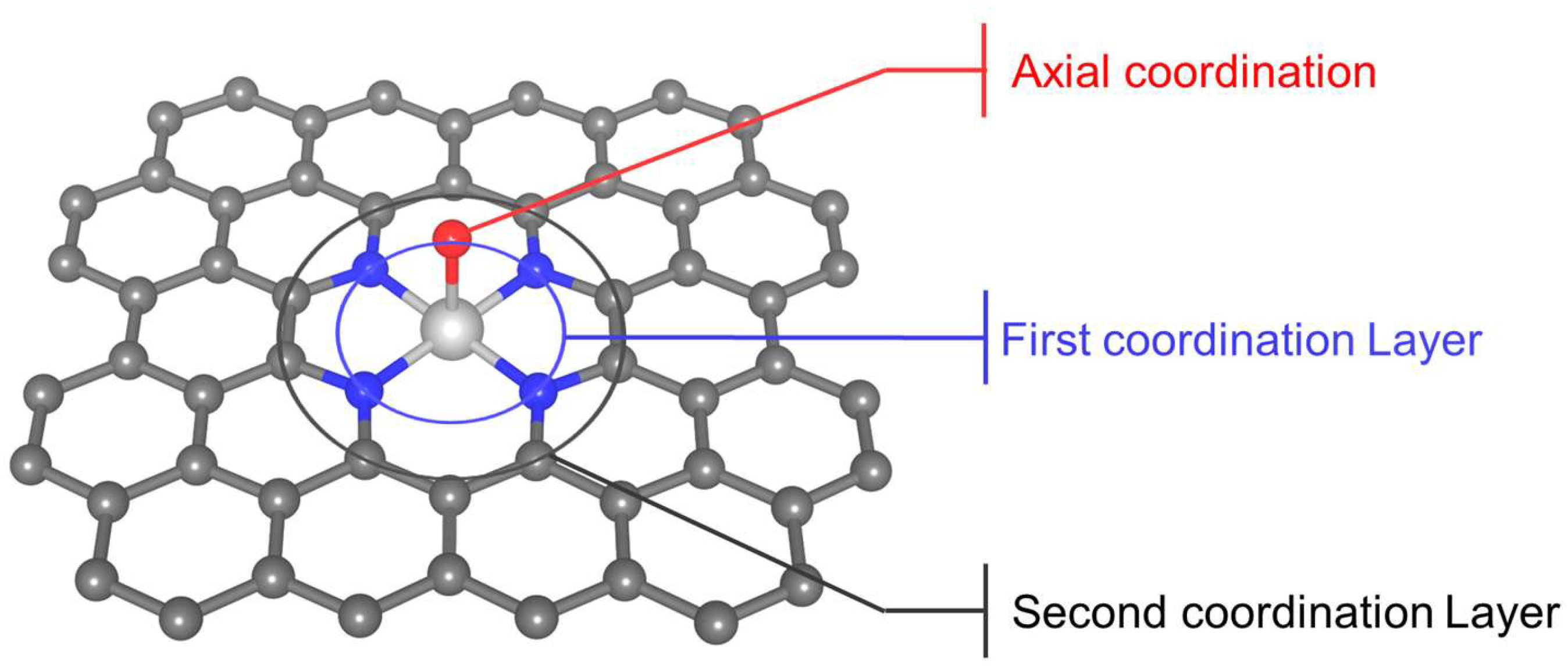
3. Regulation of Coordination Environment
3.1. Adjustment of the First Coordination Layer
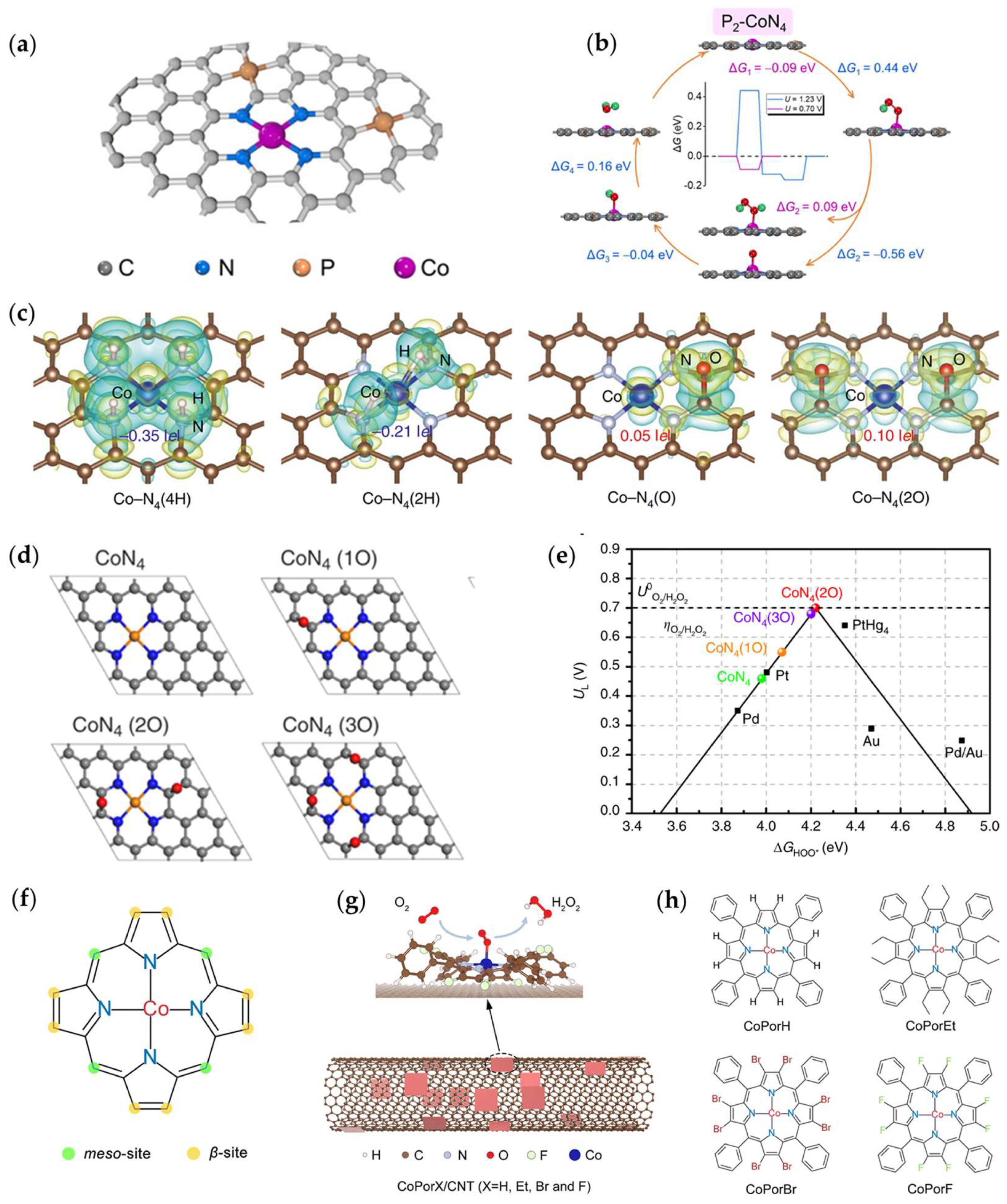
3.1.1. The Types of Coordination Atoms

3.1.2. The Number of Coordination Atoms
3.1.3. Axial Coordination

3.2. Adjustment of the Second and Higher Coordination Layers
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, K.; Liang, J.; Wang, Y.; Zhang, L.; Xu, Z.; Sun, S.; Luo, Y.; Li, T.; Liu, Q.; Li, N.; et al. Conductive Two-Dimensional Magnesium Metal-Organic Frameworks for High-Efficiency O2 Electroreduction to H2O2. ACS Catal. 2022, 12, 6092–6099. [Google Scholar] [CrossRef]
- Guo, J.; Liao, L.; Li, Y.; Liang, J.; Wang, Y.; Ying, D.; Jia, J. Enhanced wastewater treatment via direct electrocatalytic activation of hydrogen peroxide in divided cells with flow-through electrode and bipolar membrane. Sep. Purif. Technol. 2022, 292, 121030. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Yamada, Y.; Karlin, K.D. Hydrogen Peroxide as a Sustainable Energy Carrier: Electrocatalytic Production of Hydrogen Peroxide and the Fuel Cell. Electrochim. Acta 2012, 82, 493–511. [Google Scholar] [CrossRef] [PubMed]
- Kopacz, W.; Okninski, A.; Kasztankiewicz, A.; Nowakowski, P.; Rarata, G.; Maksimowski, P. Hydrogen peroxide–A promising oxidizer for rocket propulsion and its application in solid rocket propellants. FirePhysChem 2022, 2, 56–66. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, T.; Zou, J.-J.; Li, Y.; Zhang, C. Amorphous Nickel Oxides Supported on Carbon Nanosheets as High-Performance Catalysts for Electrochemical Synthesis of Hydrogen Peroxide. ACS Catal. 2022, 12, 5911–5920. [Google Scholar] [CrossRef]
- Wang, N.; Ma, S.; Zuo, P.; Duan, J.; Hou, B. Recent Progress of Electrochemical Production of Hydrogen Peroxide by Two-Electron Oxygen Reduction Reaction. Adv. Sci. 2021, 8, e2100076. [Google Scholar] [CrossRef]
- Xing, Z.H.; Wang, W.L.; Li, X.Z.; Zhang, K.; Gan, L.; Wu, Q.Y. Electrochemical Synthesis of Hydrogen Peroxide Catalyzed by Carbon Nanotubes with Surface Co-NX Sites and Encapsulated Co Nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 44282–44291. [Google Scholar] [CrossRef]
- Rawah, B.S.; Li, W. Electrocatalytic generation of hydrogen peroxide on cobalt nanoparticles embedded in nitrogen-doped carbon. Chin. J. Catal. 2021, 42, 2296–2305. [Google Scholar] [CrossRef]
- Yang, S.; Verdaguer-Casadevall, A.; Arnarson, L.; Silvioli, L.; Čolić, V.; Frydendal, R.; Rossmeisl, J.; Chorkendorff, I.; Stephens, I.E.L. Toward the Decentralized Electrochemical Production of H2O2: A Focus on the Catalysis. ACS Catal. 2018, 8, 4064–4081. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, Q.; Thomsen, L.; Gao, N.; Pan, J.; Daiyan, R.; Yun, J.; Brandt, J.; Lopez-Salas, N.; Lai, F.; et al. Constructing Interfacial Boron-Nitrogen Moieties in Turbostratic Carbon for Electrochemical Hydrogen Peroxide Production. Angew. Chem. Int. Ed. 2022, 61, e202206915. [Google Scholar] [CrossRef]
- Li, S.; Ma, J.; Xu, F.; Wei, L.; He, D. Fundamental principles and environmental applications of electrochemical hydrogen peroxide production: A review. Chem. Eng. J. 2023, 452, 139371. [Google Scholar] [CrossRef]
- Perry, S.C.; Pangotra, D.; Vieira, L.; Csepei, L.-I.; Sieber, V.; Wang, L.; Ponce de León, C.; Walsh, F.C. Electrochemical synthesis of hydrogen peroxide from water and oxygen. Nat. Rev. Chem. 2019, 3, 442–458. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, Q.; Mao, X.; Cheng, C.; Zhang, L.; Wang, L.; Liu, T.F.; Li, Y.; Li, Y. Theory-guided design of hydrogen-bonded cobaltoporphyrin frameworks for highly selective electrochemical H2O2 production in acid. Nat. Commun. 2022, 13, 2721. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, R.; Zhang, S.; Hong, H.; Zhao, Y.; Huang, Z.; Han, C.; Li, H.; Zhi, C. Ultrahigh oxygen-doped carbon quantum dots for highly efficient H2O2 production via two-electron electrochemical oxygen reduction. Energy Environ. Sci. 2022, 15, 4167–4174. [Google Scholar] [CrossRef]
- Sabri Rawah, B.; Albloushi, M.; Li, W. Electro-synthesis of pure aqueous H2O2 on nitrogen-doped carbon in a solid electrolyte flow cell without using anion exchange membrane. Chem. Eng. J. 2023, 466, 143282. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Cheng, M.J.; Lu, Q. Tailoring the Electrochemical Production of H2O2: Strategies for the Rational Design of High-Performance Electrocatalysts. Small 2020, 16, e1902845. [Google Scholar] [CrossRef]
- Wang, Y.; Waterhouse, G.I.N.; Shang, L.; Zhang, T. Electrocatalytic Oxygen Reduction to Hydrogen Peroxide: From Homogeneous to Heterogeneous Electrocatalysis. Adv. Energy Mater. 2020, 11, 2003323. [Google Scholar] [CrossRef]
- Asghar, A.; Abdul Raman, A.A.; Wan Daud, W.M.A. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, X.; Xia, C.; Wu, Z.Y.; Zhu, P.; Kim, J.Y.T.; Bai, X.; Gao, G.; Hu, Y.; Zhong, J.; et al. Highly active and selective oxygen reduction to H2O2 on boron-doped carbon for high production rates. Nat. Commun. 2021, 12, 4225. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, S.; Geng, J.; Wang, G.; Zhang, H. Cobalt single atom catalysts for the efficient electrosynthesis of hydrogen peroxide. Inorg. Chem. Front. 2021, 8, 2829–2834. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, W.; Guo, K.; Xiong, M.; Zhang, J.; Lu, X. A Pentagonal Defect-Rich Metal-Free Carbon Electrocatalyst for Boosting Acidic O2 Reduction to H2O2 Production. J. Am. Chem. Soc. 2023, 145, 11589–11598. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ni, P.; Chen, C.; Lu, Y.; Yang, P.; Kong, B.; Fisher, A.; Wang, X. Selective Electrochemical H2O2 Production through Two-Electron Oxygen Electrochemistry. Adv. Energy Mater. 2018, 8, 1801909. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Xia, C.; Wang, H.-F.; Tang, C. Recent advances in electrocatalytic oxygen reduction for on-site hydrogen peroxide synthesis in acidic media. J. Energy Chem. 2022, 67, 432–450. [Google Scholar] [CrossRef]
- Sheng, H.; Janes, A.N.; Ross, R.D.; Hofstetter, H.; Lee, K.; Schmidt, J.R.; Jin, S. Linear paired electrochemical valorization of glycerol enabled by the electro-Fenton process using a stable NiSe2 cathode. Nat. Catal. 2022, 5, 716–725. [Google Scholar] [CrossRef]
- Sang, Z.-y.; Hou, F.; Wang, S.-h.; Liang, J. Research progress on carbon-based non-metallic nanomaterials as catalysts for the two-electron oxygen reduction for hydrogen peroxide production. New Carbon Mater. 2022, 37, 136–151. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Wei, Z. Carbon-based catalysts of the oxygen reduction reaction: Mechanistic understanding and porous structures. Chin. J. Catal. 2023, 48, 15–31. [Google Scholar] [CrossRef]
- Jin, M.; Liu, W.; Sun, J.; Wang, X.; Zhang, S.; Luo, J.; Liu, X. Highly dispersed Ag clusters for active and stable hydrogen peroxide production. Nano Res. 2022, 15, 5842–5847. [Google Scholar] [CrossRef]
- Pizzutilo, E.; Kasian, O.; Choi, C.H.; Cherevko, S.; Hutchings, G.J.; Mayrhofer, K.J.J.; Freakley, S.J. Electrocatalytic synthesis of hydrogen peroxide on Au-Pd nanoparticles: From fundamentals to continuous production. Chem. Phys. Lett. 2017, 683, 436–442. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.; Liu, B. Catalyst: Single-Atom Catalysis: Directing the Way toward the Nature of Catalysis. Chem 2019, 5, 2733–2735. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Chen, C.; Peng, Q.; Wang, D.; Li, Y. Single-Atom Catalysts: Synthetic Strategies and Electrochemical Applications. Joule 2018, 2, 1242–1264. [Google Scholar] [CrossRef]
- Wei, Z.; Deng, B.; Chen, P.; Zhao, T.; Zhao, S. Palladium-based single atom catalysts for high-performance electrochemical production of hydrogen peroxide. Chem. Eng. J. 2022, 428, 131112. [Google Scholar] [CrossRef]
- Tang, C.; Jiao, Y.; Shi, B.; Liu, J.N.; Xie, Z.; Chen, X.; Zhang, Q.; Qiao, S.Z. Coordination Tunes Selectivity: Two-Electron Oxygen Reduction on High-Loading Molybdenum Single-Atom Catalysts. Angew. Chem. Int. Ed. 2020, 59, 9171–9176. [Google Scholar] [CrossRef]
- Gao, J.; Liu, B. Progress of Electrochemical Hydrogen Peroxide Synthesis over Single Atom Catalysts. ACS Mater. Lett. 2020, 2, 1008–1024. [Google Scholar] [CrossRef]
- Li, X.; Tang, S.; Dou, S.; Fan, H.J.; Choksi, T.S.; Wang, X. Molecule Confined Isolated Metal Sites Enable the Electrocatalytic Synthesis of Hydrogen Peroxide. Adv. Mater. 2022, 34, 2104891. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Song, P.; Feng, W.; Zhao, D.; Liu, T.; Zhang, J.; Chen, C. Tailoring the selectivity and activity of oxygen reduction by regulating the coordination environments of carbon-supported atomically dispersed metal sites. J. Mater. Chem. A 2022, 10, 17948–17967. [Google Scholar] [CrossRef]
- Jia, Y.; Xue, Z.; Yang, J.; Liu, Q.; Xian, J.; Zhong, Y.; Sun, Y.; Zhang, X.; Liu, Q.; Yao, D.; et al. Tailoring the Electronic Structure of an Atomically Dispersed Zinc Electrocatalyst: Coordination Environment Regulation for High Selectivity Oxygen Reduction. Angew. Chem. Int. Ed. 2022, 61, 202110838. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Zhang, X.; Liu, W.; Zhang, Q.; Wang, Z.; Cheng, J.; Yao, T.; Gu, L.; Du, C.; Gao, Y.; et al. Substrate strain tunes operando geometric distortion and oxygen reduction activity of CuN2C2 single-atom sites. Nat. Commun. 2021, 12, 6335. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Zhang, Z.; Yan, H.M.; Xia, G.J.; Cao, H.; Wang, Y.G. Pseudo-adsorption and long-range redox coupling during oxygen reduction reaction on single atom electrocatalyst. Nat. Commun. 2022, 13, 1734. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y. Origin of Selective Production of Hydrogen Peroxide by Electrochemical Oxygen Reduction. J. Am. Chem. Soc. 2021, 143, 9423–9428. [Google Scholar] [CrossRef]
- Kodama, K.; Nagai, T.; Kuwaki, A.; Jinnouchi, R.; Morimoto, Y. Challenges in applying highly active Pt-based nanostructured catalysts for oxygen reduction reactions to fuel cell vehicles. Nat. Nanotechnol. 2021, 16, 140–147. [Google Scholar] [CrossRef]
- Yin, S.-H.; Yang, S.-L.; Li, G.; Li, G.; Zhang, B.-W.; Wang, C.-T.; Chen, M.-S.; Liao, H.-G.; Yang, J.; Jiang, Y.-X.; et al. Seizing gaseous Fe2+ to densify O2-accessible Fe–N4 sites for high-performance proton exchange membrane fuel cells. Energy Environ. Sci. 2022, 15, 3033–3040. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, X.; Shi, J.; Gan, L.; Huang, B.; Tao, L.; Wang, S. Neuron-inspired design of hierarchically porous carbon networks embedded with single-iron sites for efficient oxygen reduction. Sci. China Chem. 2022, 65, 1445–1452. [Google Scholar] [CrossRef]
- Zhu, Y.; Gan, L.; Shi, J.; Huang, G.; Gao, H.; Tao, L.; Wang, S. Co-CoF2 heterojunctions encapsulated in N, F co-doped porous carbon as bifunctional oxygen electrocatalysts for Zn-air batteries. Chem. Eng. J. 2022, 433, 133541. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Wang, J.; Liu, R.; Liu, Y.; Wang, C.; Yang, W.; Feng, X.; Wang, B. Atomically dispersed Co in a cross-channel hierarchical carbon-based electrocatalyst for high-performance oxygen reduction in Zn–air batteries. J. Mater. Chem. A 2022, 10, 18723–18729. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, Y.; Sunarso, J.; Guan, D.; Liu, B.; Liu, H.; Zhou, W.; Shao, Z. New Phosphorus-Doped Perovskite Oxide as an Oxygen Reduction Reaction Electrocatalyst in an Alkaline Solution. Chem. Eur. J. 2018, 24, 6950–6957. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, L.; Liu, C.; Li, Z.; Zou, Y.; Zhang, X.; Zhang, Y.; Zhang, Z.; Wei, G.; Yu, C. Crystal Engineering Enables Cobalt-Based Metal-Organic Frameworks as High-Performance Electrocatalysts for H2O2 Production. J. Am. Chem. Soc. 2023, 145, 7791–7799. [Google Scholar] [CrossRef]
- Lu, X.; Wang, D.; Wu, K.H.; Guo, X.; Qi, W. Oxygen reduction to hydrogen peroxide on oxidized nanocarbon: Identification and quantification of active sites. J. Colloid Interface Sci. 2020, 573, 376–383. [Google Scholar] [CrossRef]
- Li, B.Q.; Zhao, C.X.; Liu, J.N.; Zhang, Q. Electrosynthesis of Hydrogen Peroxide Synergistically Catalyzed by Atomic Co-Nx-C Sites and Oxygen Functional Groups in Noble-Metal-Free Electrocatalysts. Adv. Mater. 2019, 31, 1808173. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, S.; Ma, W.; Zhou, Z. Oxygen reduction reaction on Pt-based electrocatalysts: Four-electron vs. two-electron pathway. Chin. J. Catal. 2022, 43, 1433–1443. [Google Scholar] [CrossRef]
- Wang, K.; Huang, J.; Chen, H.; Wang, Y.; Song, S. Recent advances in electrochemical 2e oxygen reduction reaction for on-site hydrogen peroxide production and beyond. Chem. Commun. 2020, 56, 12109–12121. [Google Scholar] [CrossRef]
- Yan, X.; Shi, W.-W.; Wang, X.-z. Carbon based electrocatalysts for selective hydrogen peroxide conversion. New Carbon Mater. 2022, 37, 223–236. [Google Scholar] [CrossRef]
- Medford, A.J.; Vojvodic, A.; Hummelshøj, J.S.; Voss, J.; Abild-Pedersen, F.; Studt, F.; Bligaard, T.; Nilsson, A.; Nørskov, J.K. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. J. Catal. 2015, 328, 36–42. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, K.; Ai, X.; Liang, X.; Zhang, Q.; Chen, H.; Zou, X. Theory-guided electrocatalyst engineering: From mechanism analysis to structural design. Chin. J. Catal. 2022, 43, 2987–3018. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Ren, X.; Gao, J.; Huang, Y.; Liu, B. Microenvironment modulation of single-atom catalysts and their roles in electrochemical energy conversion. Sci. Adv. 2020, 6, eabb6833. [Google Scholar] [CrossRef]
- Liu, K.; Fu, J.; Lin, Y.; Luo, T.; Ni, G.; Li, H.; Lin, Z.; Liu, M. Insights into the activity of single-atom Fe-N-C catalysts for oxygen reduction reaction. Nat. Commun. 2022, 13, 2075. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, B.; Zhang, Z.; Guo, P.; Liu, C.; Zhang, Y.; Zhao, L.; Wang, Z. Tailoring the d-Orbital Splitting Manner of Single Atomic Sites for Enhanced Oxygen Reduction. Adv. Mater. 2023, 35, e2210757. [Google Scholar] [CrossRef]
- Cui, X.; Li, W.; Ryabchuk, P.; Junge, K.; Beller, M. Bridging homogeneous and heterogeneous catalysis by heterogeneous single-metal-site catalysts. Nat. Catal. 2018, 1, 385–397. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, H.; Zuo, S.; Dong, J.; Li, Y.; Zhang, J.; Han, Y. Engineering the Coordination Sphere of Isolated Active Sites to Explore the Intrinsic Activity in Single-Atom Catalysts. Nanomicro Lett. 2021, 13, 136. [Google Scholar] [CrossRef]
- Zheng, R.; Meng, Q.; Zhang, L.; Ge, J.; Liu, C.; Xing, W.; Xiao, M. Co-based Catalysts for Selective H2O2 Electroproduction via 2-electron Oxygen Reduction Reaction. Chem. Eur. J. 2023, 29, e202203180. [Google Scholar] [CrossRef]
- Luo, E.; Chu, Y.; Liu, J.; Shi, Z.; Zhu, S.; Gong, L.; Ge, J.; Choi, C.H.; Liu, C.; Xing, W. Pyrolyzed M–Nx catalysts for oxygen reduction reaction: Progress and prospects. Energy Environ. Sci. 2021, 14, 2158–2185. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Wu, D.; Zhang, L.; Li, J.; Xu, J.; Lin, S.; Datye, A.K.; Xiong, H. Coordination structure at work: Atomically dispersed heterogeneous catalysts. Coord Chem. Rev. 2022, 460, 214469. [Google Scholar] [CrossRef]
- Fu, C.; Luo, L.; Yang, L.; Shen, S.; Yan, X.; Yin, J.; Wei, G.; Zhang, J. An In-Depth Theoretical Exploration of Influences of Non-Metal-Elements Doping on the ORR Performance of Co−gN4. ChemCatChem 2021, 13, 2303–2310. [Google Scholar] [CrossRef]
- Giulimondi, V.; Mitchell, S.; Perez-Ramirez, J. Challenges and Opportunities in Engineering the Electronic Structure of Single-Atom Catalysts. ACS Catal. 2023, 13, 2981–2997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, J.; Ge, R.; Zhang, J.; Cairney, J.M.; Li, Y.; Zhu, M.; Li, S.; Li, W. The effect of coordination environment on the activity and selectivity of single-atom catalysts. Coord Chem. Rev. 2022, 461, 214493. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y.; Xu, R.; Chen, W.; Zheng, L.; Han, A.; Zhu, Y.; Zhang, J.; Zhang, H.; Luo, J.; et al. Electronic structure engineering to boost oxygen reduction activity by controlling the coordination of the central metal. Energy Environ. Sci. 2018, 11, 2348–2352. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, X.; Zhang, R.; Yu, S.; Levell, Z.H.; Wang, C.; Ma, S.; Zou, P.; Han, L.; Qin, J.; et al. Highly Selective Oxygen Reduction to Hydrogen Peroxide on a Carbon-Supported Single-Atom Pd Electrocatalyst. ACS Catal. 2022, 12, 4156–4164. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Chen, S.; Zha, Q.; Zhu, M. In-situ monitoring of hydrogen peroxide production at nickel single-atom electrocatalyst. Chem. Eng. J. 2023, 460, 141657. [Google Scholar] [CrossRef]
- Gao, J.; Yang, H.B.; Huang, X.; Hung, S.-F.; Cai, W.; Jia, C.; Miao, S.; Chen, H.M.; Yang, X.; Huang, Y.; et al. Enabling Direct H2O2 Production in Acidic Media through Rational Design of Transition Metal Single Atom Catalyst. Chem 2020, 6, 658–674. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; He, F.; Song, M.; Huang, X.; Shen, T.; Li, J.; Zhang, C.; Zhang, J.; Wang, D. Highly dispersed Co atoms anchored in porous nitrogen-doped carbon for acidic H2O2 electrosynthesis. Chem. Eng. J. 2022, 438, 141657. [Google Scholar] [CrossRef]
- Suk, M.; Chung, M.W.; Han, M.H.; Oh, H.-S.; Choi, C.H. Selective H2O2 production on surface-oxidized metal-nitrogen-carbon electrocatalysts. Catal. Today 2021, 359, 99–105. [Google Scholar] [CrossRef]
- He, X.; Chang, L.; Han, P.; Li, K.; Wu, H.; Tang, Y.; Gao, F.; Zhang, Y.; Zhou, A. High-performance Co-N-C catalyst derived from PS@ZIF-8 @ZIF-67 for improved oxygen reduction reaction. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 130988. [Google Scholar] [CrossRef]
- Shi, S.; Wang, B.; Wang, Y.; Yang, Y.; Zhang, Z.; Xu, Y.; Suo, Y. Structure optimization of ZIF-12-derived Co-N-C for efficient oxygen reduction and oxygen evolution. Fuel 2022, 330, 125516. [Google Scholar] [CrossRef]
- Gao, J.; Hu, Y.; Wang, Y.; Lin, X.; Hu, K.; Lin, X.; Xie, G.; Liu, X.; Reddy, K.M.; Yuan, Q.; et al. MOF Structure Engineering to Synthesize Co-N-C Catalyst with Richer Accessible Active Sites for Enhanced Oxygen Reduction. Small 2021, 17, e2104684. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Luo, T.; Li, X.; Chen, K.; Fu, J.; Liu, K.; Cai, C.; Wang, Q.; Li, H.; Chen, Y.; et al. Identification of the Highly Active Co-N4 Coordination Motif for Selective Oxygen Reduction to Hydrogen Peroxide. J. Am. Chem. Soc. 2022, 144, 14505–14516. [Google Scholar] [CrossRef]
- Fan, M.; Cui, J.; Zhang, J.; Wu, J.; Chen, S.; Song, L.; Wang, Z.; Wang, A.; Vajtai, R.; Wu, Y.; et al. The modulating effect of N coordination on single-atom catalysts researched by Pt-Nx-C model through both experimental study and DFT simulation. J. Mater. Sci. Technol. 2021, 91, 160–167. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Ji, Y.; Zhao, Z.; Cui, W.; Sang, X.; Cheng, Y.; Yang, B.; Li, Z.; Zhang, Q.; et al. Single-atom Iron Catalyst with Biomimetic Active Center to Accelerate Proton Spillover for Medical-level Electrosynthesis of H2O2 Disinfectant. Angew. Chem. Int. Ed. 2023, 62, e202306491. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, C.; Ye, K.; Liang, Z.; Jiang, F.; Shen, S.; Zhao, X.; Ma, L.; Shadike, Z.; Wang, X.; et al. Manipulating the oxygen reduction reaction pathway on Pt-coordinated motifs. Nat. Commun. 2022, 13, 685. [Google Scholar] [CrossRef]
- Jing, L.; Tian, Q.; Su, P.; Li, H.; Zheng, Y.; Tang, C.; Liu, J. Mesoporous Co–O–C nanosheets for electrochemical production of hydrogen peroxide in acidic medium. J. Mater. Chem. A 2022, 10, 4068–4075. [Google Scholar] [CrossRef]
- Jin, H.; Song, T.; Paik, U.; Qiao, S.-Z. Metastable Two-Dimensional Materials for Electrocatalytic Energy Conversions. Acc. Mater. 2021, 2, 559–573. [Google Scholar] [CrossRef]
- Rong, X.; Wang, H.J.; Lu, X.L.; Si, R.; Lu, T.B. Controlled Synthesis of a Vacancy-Defect Single-Atom Catalyst for Boosting CO2 Electroreduction. Angew. Chem. Int. Ed. 2020, 59, 1961–1965. [Google Scholar] [CrossRef]
- Zhang, B.W.; Zheng, T.; Wang, Y.X.; Du, Y.; Chu, S.Q.; Xia, Z.; Amal, R.; Dou, S.X.; Dai, L. Highly efficient and selective electrocatalytic hydrogen peroxide production on Co-O-C active centers on graphene oxide. Commun. Chem. 2022, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Wei, Z.; Gong, Z.; Liu, J.; Ye, G.; Yan, M.; Dong, J.; Allen, C.; Liu, J.; Huang, K.; et al. Low-Coordinated Co-N-C on Oxygenated Graphene for Efficient Electrocatalytic H2O2 Production. Adv. Funct. Mater. 2021, 32, 2106886. [Google Scholar] [CrossRef]
- Wang, X.; An, Y.; Liu, L.; Fang, L.; Liu, Y.; Zhang, J.; Qi, H.; Heine, T.; Li, T.; Kuc, A.; et al. Atomically Dispersed Pentacoordinated-Zirconium Catalyst with Axial Oxygen Ligand for Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2022, 61, e202209746. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, S.; Cao, R.; Yuan, K.; Lu, C.; Huang, B.; Tang, X.; Hu, T.; Zhuang, X.; Chen, Y. Optimizing Microenvironment of Asymmetric N,S-Coordinated Single-Atom Fe via Axial Fifth Coordination toward Efficient Oxygen Electroreduction. Small 2022, 18, e2105387. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Y.; Lai, W.-H.; Xiao, F.; Lyu, Y.; Liao, C.; Shao, M. Approaching a high-rate and sustainable production of hydrogen peroxide: Oxygen reduction on Co–N–C single-atom electrocatalysts in simulated seawater. Energy Environ. Sci. 2021, 14, 5444–5456. [Google Scholar] [CrossRef]
- Fan, W.; Duan, Z.; Liu, W.; Mehmood, R.; Qu, J.; Cao, Y.; Guo, X.; Zhong, J.; Zhang, F. Rational design of heterogenized molecular phthalocyanine hybrid single-atom electrocatalyst towards two-electron oxygen reduction. Nat. Commun. 2023, 14, 1426. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Yang, W.; Feng, X.; Wang, B. Controlled Modification of Axial Coordination for Transition-Metal Single-Atom Electrocatalyst. Chem. Eur. J. 2022, 28, e202201471. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, D.; Kim, J.; Lim, J.S.; Paidi, V.K.; Shin, T.J.; Jeong, H.Y.; Lee, K.S.; Kim, H.; Joo, S.H. Reversible Ligand Exchange in Atomically Dispersed Catalysts for Modulating the Activity and Selectivity of the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2021, 60, 20528–20534. [Google Scholar] [CrossRef]
- Cao, P.; Quan, X.; Nie, X.; Zhao, K.; Liu, Y.; Chen, S.; Yu, H.; Chen, J.G. Metal single-site catalyst design for electrocatalytic production of hydrogen peroxide at industrial-relevant currents. Nat. Commun. 2023, 14, 172. [Google Scholar] [CrossRef]
- Xiao, C.; Cheng, L.; Zhu, Y.; Wang, G.; Chen, L.; Wang, Y.; Chen, R.; Li, Y.; Li, C. Super-Coordinated Nickel N4Ni1O2 Site Single-Atom Catalyst for Selective H2O2 Electrosynthesis at High Current Densities. Angew. Chem. Int. Ed. 2022, 61, e202206544. [Google Scholar] [CrossRef]
- Tang, C.; Chen, L.; Li, H.; Li, L.; Jiao, Y.; Zheng, Y.; Xu, H.; Davey, K.; Qiao, S.Z. Tailoring Acidic Oxygen Reduction Selectivity on Single-Atom Catalysts via Modification of First and Second Coordination Spheres. J. Am. Chem. Soc. 2021, 143, 7819–7827. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wei, Z.; Gong, Z.; Yan, M.; Hu, Y.; Zhao, S.; Ye, G.; Fei, H. Single-atom CoN4 sites with elongated bonding induced by phosphorus doping for efficient H2O2 electrosynthesis. Appl. Catal. B 2023, 324, 122267. [Google Scholar] [CrossRef]
- Jung, E.; Shin, H.; Lee, B.H.; Efremov, V.; Lee, S.; Lee, H.S.; Kim, J.; Hooch Antink, W.; Park, S.; Lee, K.S.; et al. Atomic-level tuning of Co-N-C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 2020, 19, 436–442. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, X.; Bedford, N.M.; Han, Z.; Thomsen, L.; Smith, S.; Amal, R.; Lu, X. Direct insights into the role of epoxy groups on cobalt sites for acidic H2O2 production. Nat. Commun. 2020, 11, 4181. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Z.; She, F.; Chen, J.; Liu, F.; Qu, J.; Cairney, J.M.; Wu, C.; Liu, K.; Yang, W.; et al. Heterogeneous molecular Co–N–C catalysts for efficient electrochemical H2O2 synthesis. Energy Environ. Sci. 2023, 16, 446–459. [Google Scholar] [CrossRef]
- Yan, M.; Wei, Z.; Gong, Z.; Johannessen, B.; Ye, G.; He, G.; Liu, J.; Zhao, S.; Cui, C.; Fei, H. Sb2S3-templated synthesis of sulfur-doped Sb-N-C with hierarchical architecture and high metal loading for H2O2 electrosynthesis. Nat. Commun. 2023, 14, 368. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Xu, H.; Zhang, Q.; Huang, Y.C.; Shi, C.; Chang, Y.C.; Xu, X.; Tang, J.; Gu, Y.; Pao, C.W.; et al. Identifying A Universal Activity Descriptor and a Unifying Mechanism Concept on Perovskite Oxides for Green Hydrogen Production. Adv. Mater. 2023, 2305074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Y.; Chen, C.; Huang, Y.C.; Dong, C.L.; Chen, C.J.; Liu, R.S.; Wang, C.; Yan, K.; Li, Y.; et al. Tuning the Coordination Environment in Single-Atom Catalysts to Achieve Highly Efficient Oxygen Reduction Reactions. J. Am. Chem. Soc. 2019, 141, 20118–20126. [Google Scholar] [CrossRef]
- Zhang, E.; Tao, L.; An, J.; Zhang, J.; Meng, L.; Zheng, X.; Wang, Y.; Li, N.; Du, S.; Zhang, J.; et al. Engineering the Local Atomic Environments of Indium Single-Atom Catalysts for Efficient Electrochemical Production of Hydrogen Peroxide. Angew. Chem. Int. Ed. 2022, 61, e202117347. [Google Scholar] [CrossRef]
- Hu, J.; Shang, W.; Xin, C.; Guo, J.; Cheng, X.; Zhang, S.; Song, S.; Liu, W.; Ju, F.; Hou, J.; et al. Uncovering Dynamic Edge-Sites in Atomic Co-N-C Electrocatalyst for Selective Hydrogen Peroxide Production. Angew. Chem. Int. Ed. 2023, 62, e202304754. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Wang, J.; Shi, J.; Li, J.; Cai, W. Enhancing Hydrogen Peroxide Synthesis through Coordination Engineering of Single-Atom Catalysts in the Oxygen Reduction Reaction: A Review. Energies 2023, 16, 6616. https://doi.org/10.3390/en16186616
He H, Wang J, Shi J, Li J, Cai W. Enhancing Hydrogen Peroxide Synthesis through Coordination Engineering of Single-Atom Catalysts in the Oxygen Reduction Reaction: A Review. Energies. 2023; 16(18):6616. https://doi.org/10.3390/en16186616
Chicago/Turabian StyleHe, Huawei, Jiatang Wang, Jiawei Shi, Jing Li, and Weiwei Cai. 2023. "Enhancing Hydrogen Peroxide Synthesis through Coordination Engineering of Single-Atom Catalysts in the Oxygen Reduction Reaction: A Review" Energies 16, no. 18: 6616. https://doi.org/10.3390/en16186616
APA StyleHe, H., Wang, J., Shi, J., Li, J., & Cai, W. (2023). Enhancing Hydrogen Peroxide Synthesis through Coordination Engineering of Single-Atom Catalysts in the Oxygen Reduction Reaction: A Review. Energies, 16(18), 6616. https://doi.org/10.3390/en16186616





