Exploring the Properties of the Torrefaction Process and Its Prospective in Treating Lignocellulosic Material
Abstract
:1. Introduction
2. Description of the Torrefaction Process
3. Types of Torrefaction
| Type of Torrefaction | Advantages/Disadvantages | Biomass Source | Reference |
|---|---|---|---|
| Dry (Non-oxidative) | Advantages: Low costs, fast process Disadvantages: Low mass and energy yields, difficult process control | Wheat straw | [54] |
| Corn stalk | [55] | ||
| Agricultural biomass | [28] | ||
| Pine, eucalyptus, chestnut, holm oak, olive tree pruning and vine shoot | [56] | ||
| Dry (Oxidative) | Advantages: High mass and energy yields, simple process control Disadvantages: High initial energy or heat required, slow process | Microalgae | [57] |
| Wood sphere | [27] | ||
| Patula pine | [48] | ||
| Olive stones | [23] | ||
| Rice husk | [58] | ||
| Wet (Hydrothermal carbonization) | Advantages: No pre-drying necessary, suitable for wet biomass, by-products in liquid form, high quality of char with lower ash content than in dry torrefaction, possible addition of catalysts to enhance process Disadvantages: Lower char yield, high energy consumption due to high-pressure operation, possible corrosion of reactors, post-drying of char is required, complicated process to implement in continuous mode | Woody biomass | [13] |
| Rice husk | [43] | ||
| Olive oil cake | [44] | ||
| Miscanthus | [45] | ||
| Sewage sludge and cheese whey | [46] | ||
| Steam torrefaction | Advantages: No pre-drying necessary, suitable for wet biomass, higher pelletability of solid product Disadvantages: High costs and energy consumption due to high-pressure operation, complicated process to implement in continuous mode | Walnut oil processing wastes | [50] |
| Agro-industrial residues | [52] | ||
| Mixture of chicken manure and sawdust | [53] | ||
| Camellia shell | [51] |
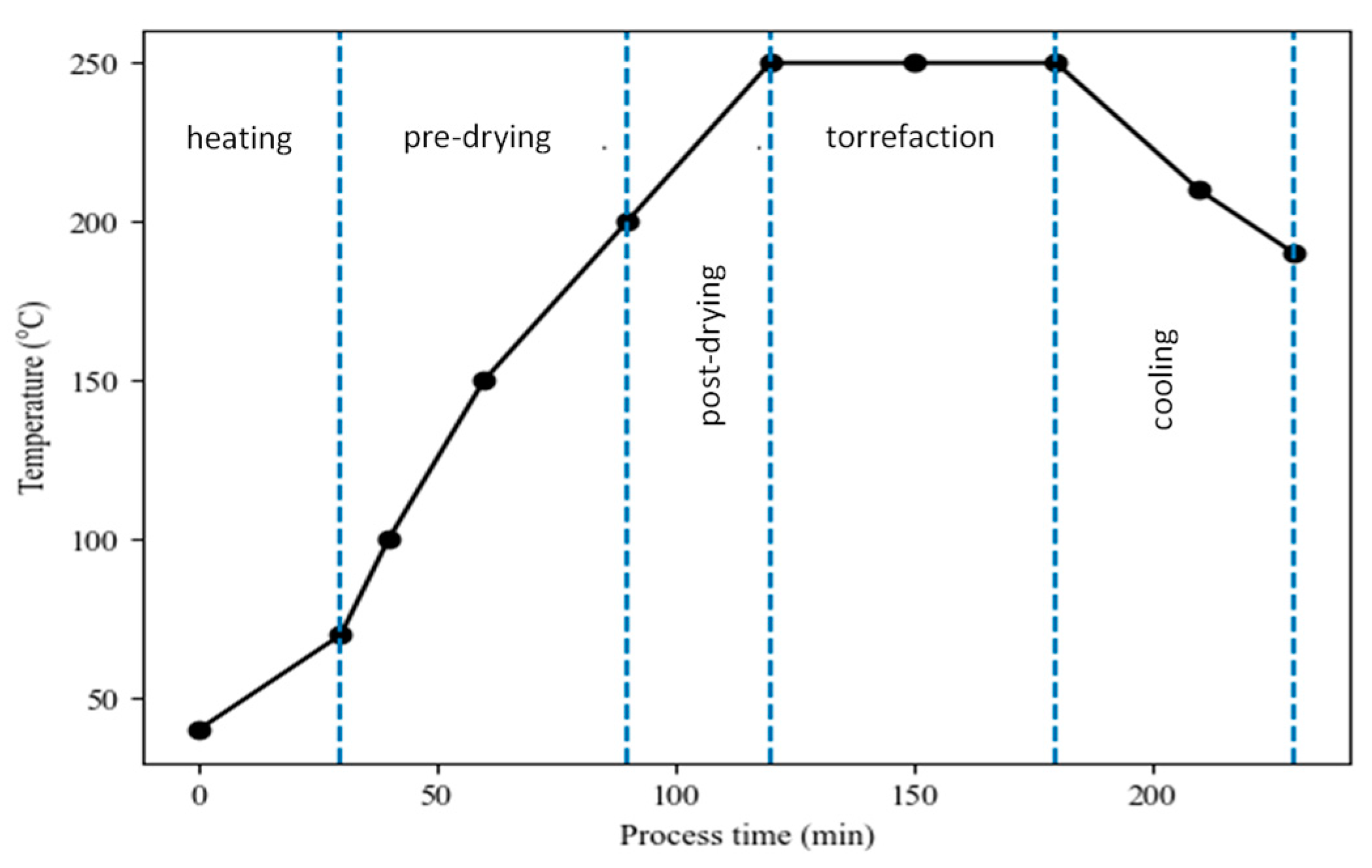
3.1. Torrefaction Rate
- Initial heating: the biomass is heated to the drying stage. This usually takes place in a temperature range of between 25 and 105 °C. The temperature rises, and at the end of this stage moisture begins to evaporate.
- Pre-drying: usually takes place in a temperature range between 105 and 200 °C. The biomass begins to slowly decompose (basic components), and the free moisture begins to evaporate from the biomass at a constant rate.
- Drying and intermediate heating: the temperature of the biomass rises to approximately 200 °C, releasing physically bound water. At this stage, the biomass contains no more moisture, so the biomass begins to gradually decompose (loss of mass) and light organic matter begins to volatilize.
- Torrefaction: in this stage, the torrefaction process practically begins. The process starts when the temperature reaches exactly 200 °C and continues until the temperature decreases below 200 °C. The temperature of torrefaction is characterized by a period of constant temperature, which can be reached even only for a short time (temperature maximum). In this stage, the mass loss is the highest.
- Cooling: the obtained solid product is cooled down from 200 °C to the final desired temperature.
3.2. Torrefaction Products
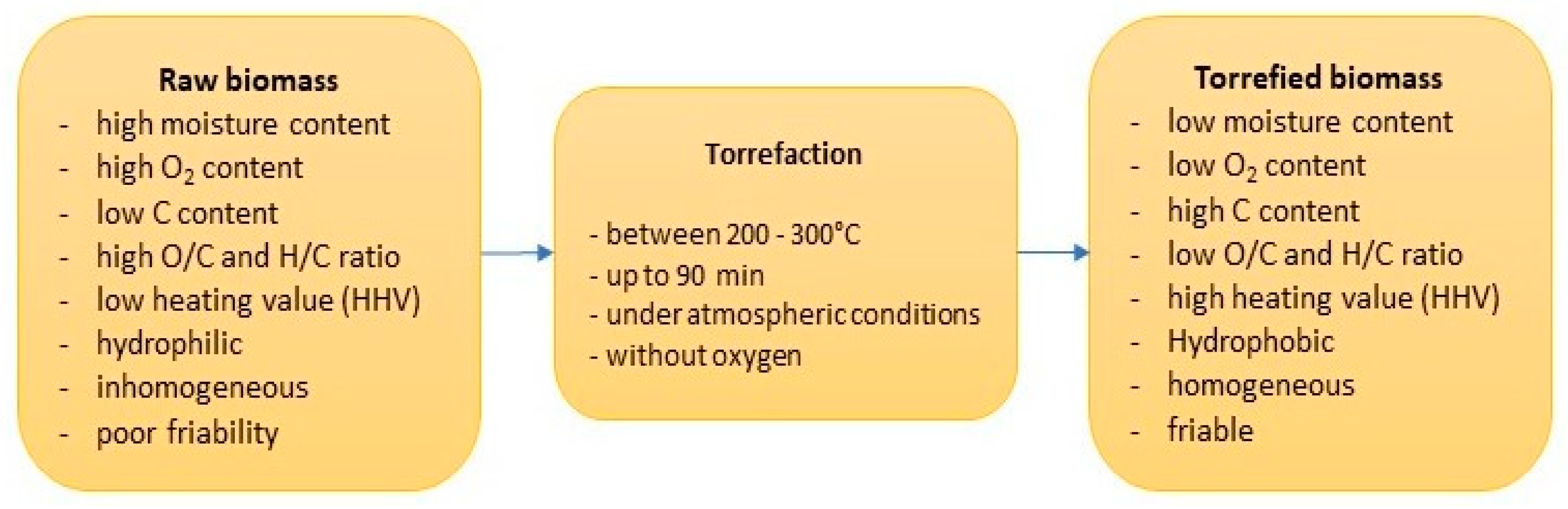
3.3. Properties of Torrefied Biomass
- Devolatilization reaction—the removal of oxygen and other volatile substances from the biomass. This usually occurs in the initial phase of the torrefaction process at a temperature of 200 °C. The result of devolatilization causes a loss of material mass in the initial phase of the process.
- Deoxygenation reaction—the removal of molecular oxygen, which in turn leads to an increase in the carbon content in the final product, as well as lower H/C and O/C ratios in the final product and to the formation of gases such as CO, CO2 and H2O.
- Depolymerization reaction—the breakdown of larger molecular compounds into smaller ones occurs, resulting in a more homogeneous and crumblier final product.
- Carbonization reaction—a thermal reaction in which an organic biomass is converted into carbon with the main objective to increase the proportion of fixed carbon and decrease the hydrocarbon content.
3.3.1. Hydrophobicity and Chemical Properties of Torrefied Biomass
3.3.2. Influential Factors
3.3.3. Temperature
3.3.4. Residence Time
3.3.5. Particle Size and Specific Area
4. Prospectives of Torrefaction Due to Reduced Impact on Environment
5. Application of the Torrefaction Process
6. Torrefaction Reactors
- Rotary kilns are cylindrical, rotating furnaces used for thermal processing, which provide a controlled environment for biomass heating. The biomass feedstock is introduced at one end of the kiln, and as it rotates, it moves through different temperature zones, undergoing torrefaction.
- Fluidized-bed reactors suspend biomass particles in an upward-flowing gas stream, offering good heat-transfer and mixing characteristics suitable for torrefaction. The fluidized bed can be adjusted to maintain a uniform temperature and residence time, resulting in consistent torrefied biomass properties.
- Moving-bed reactors involve passing biomass through a series of temperature-controlled chambers on a conveyor belt or other moving system. Each chamber exposes the biomass to progressively higher temperatures, achieving torrefaction as the biomass moves along the bed.
- Microwave torrefaction uses electromagnetic waves to generate heat within biomass. This technology offers rapid and efficient heating, resulting in shorter processing times. However, it requires careful control to ensure uniform heating and prevent overheating.
- Fixed-bed reactor: after the raw biomass is fed into the reactor, it is dried and torrefied in the furnace. Torrefied biomass is collected at the end after the torrefaction process and the reactor has cooled down.
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Lim, C.J.; Grace, J.R.; Li, H.; Parise, M.R. Effects of temperature and particle size on biomass torrefaction in a slot-rectangular spouted bed reactor. Bioresour. Technol. 2017, 244, 281–288. [Google Scholar] [CrossRef]
- Bach, Q.-V.; Skreiberg, Ø.; Lee, C.-J. Process modeling and optimization for torrefaction of forest residues. Energy 2017, 138, 348–354. [Google Scholar] [CrossRef]
- Gan, Y.Y.; Ong, H.C.; Ling, T.C.; Chen, W.-H.; Chong, C.T. Torrefaction of de-oiled Jatropha seed kernel biomass for solid fuel production. Energy 2019, 170, 367–374. [Google Scholar] [CrossRef]
- Rodriguez Alonso, E.; Dupont, C.; Heux, L.; Da Silva Perez, D.; Commandre, J.-M.; Gourdon, C. Study of solid chemical evolution in torrefaction of different biomasses through solid-state 13C cross-polarization/magic angle spinning NMR (nuclear magnetic resonance) and TGA (thermogravimetric analysis). Energy 2016, 97, 381–390. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. More efficient biomass gasification via torrefaction. Energy 2006, 31, 3458–3470. [Google Scholar] [CrossRef]
- Bridgeman, T.G.; Jones, J.M.; Shield, I.; Williams, P.T. Torrefaction of reed canary grass, wheat straw and willow to enhance solid fuel qualities and combustion properties. Fuel 2008, 87, 844–856. [Google Scholar] [CrossRef]
- Chen, W.-H.; Kuo, P.-C. A study on torrefaction of various biomass materials and its impact on lignocellulosic structure simulated by a thermogravimetry. Energy 2010, 35, 2580–2586. [Google Scholar] [CrossRef]
- Shankar Tumuluru, J.; Sokhansanj, S.; Richard Hess, J.; Wright, C.T.; Boardman, R.D. REVIEW: A review on biomass torrefaction process and product properties for energy applications. Ind. Biotechnol. 2011, 7, 384–401. [Google Scholar] [CrossRef]
- Wannapeera, J.; Worasuwannarak, N. Upgrading of woody biomass by torrefaction under pressure. J. Anal. Appl. Pyrolysis 2012, 96, 173–180. [Google Scholar] [CrossRef]
- Batidzirai, B.; Mignot, A.P.R.; Schakel, W.B.; Junginger, H.M.; Faaij, A.P.C. Biomass torrefaction technology: Techno-economic status and future prospects. Energy 2013, 62, 196–214. [Google Scholar] [CrossRef]
- Prabir, B. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory. In Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2013; pp. 1–530. [Google Scholar]
- Piersa, P.; Hilal, U.; Szufa, S.; Lewandowska, W.; Modrzewski, R.; Ślężak, R.; Ledakowicz, S. An Extensive Review and Comparison of Modern Biomass Torrefaction Reactors vs. Biomass Pyrolysis—Part 1. Energies 2022, 15, 2227. [Google Scholar] [CrossRef]
- Bach, Q.-V.; Tran, K.-Q. Dry and Wet Torrefaction of Woody Biomass—A Comparative Studyon Combustion Kinetics. Energy Procedia 2015, 75, 150–155. [Google Scholar] [CrossRef]
- Chen, W.-H.; Pillejera, M.K.; de Luna, M.D. Influence of reactor rotating speed on bamboo torrefaction. Energy Procedia 2017, 142, 131–135. [Google Scholar] [CrossRef]
- Chen, D.; Gao, A.; Cen, K.; Zhang, J.; Cao, X.; Ma, Z. Investigation of biomass torrefaction based on three major components: Hemicellulose, cellulose, and lignin. Energy Convers. Manag. 2018, 169, 228–237. [Google Scholar] [CrossRef]
- Chen, W.-H.; Cheng, C.-L.; Show, P.-L.; Ong, H.C. Torrefaction performance prediction approached by torrefaction severity factor. Fuel 2019, 251, 126–135. [Google Scholar] [CrossRef]
- Niu, Y.; Lv, Y.; Lei, Y.; Liu, S.; Liang, Y.; Wang, D.; Hui, S. Biomass torrefaction: Properties, applications, challenges, and economy. Renew. Sustain. Energy Rev. 2019, 115, 109395. [Google Scholar] [CrossRef]
- Cahyanti, M.N.; Doddapaneni, T.R.K.C.; Kikas, T. Biomass torrefaction: An overview on process parameters, economic and environmental aspects and recent advancements. Bioresour. Technol. 2020, 301, 122737. [Google Scholar] [CrossRef]
- Simonic, M.; Goricanec, D.; Urbancl, D. Impact of torrefaction on biomass properties depending on temperature and operation time. Sci. Total Environ. 2020, 740, 140086. [Google Scholar] [CrossRef]
- Sarker, T.R.; Azargohar, R.; Dalai, A.K.; Meda, V. Enhancement of fuel and physicochemical properties of canola residues via microwave torrefaction. Energy Rep. 2021, 7, 6338–6353. [Google Scholar] [CrossRef]
- Xu, J.; Huang, M.; Hu, Z.; Zhang, W.; Li, Y.; Yang, Y.; Zhou, Y.; Zhou, S.; Ma, Z. Prediction and modeling of the basic properties of biomass after torrefaction pretreatment. J. Anal. Appl. Pyrolysis 2021, 159, 105287. [Google Scholar] [CrossRef]
- Thengane, S.K.; Kung, K.S.; Gomez-Barea, A.; Ghoniem, A.F. Advances in biomass torrefaction: Parameters, models, reactors, applications, deployment, and market. Prog. Energy Combust. Sci. 2022, 93, 101040. [Google Scholar] [CrossRef]
- Soria-Verdugo, A.; Cano-Pleite, E.; Panahi, A.; Ghoniem, A.F. Kinetics mechanism of inert and oxidative torrefaction of biomass. Energy Convers. Manag. 2022, 267, 115892. [Google Scholar] [CrossRef]
- Wu, N.; Niu, Q.; Pieters, J.; Ronsse, F. Influence of torrefaction as pretreatment on the fast pyrolysis of sugarcane trash. Energy Convers. Manag. 2023, 291, 117291. [Google Scholar] [CrossRef]
- van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Mamvura, T.A.; Danha, G. Biomass torrefaction as an emerging technology to aid in energy production. Heliyon 2020, 6, e03531. [Google Scholar] [CrossRef]
- Li, X.; Lu, Z.; Chen, J.; Chen, X.; Jiang, Y.; Jian, J.; Yao, S. Effect of oxidative torrefaction on high temperature combustion process of wood sphere. Fuel 2021, 286, 119379. [Google Scholar] [CrossRef]
- Cheng, W.; Shao, J.A.; Zhu, Y.; Zhang, W.; Jiang, H.; Hu, J.; Zhang, X.; Yang, H.; Chen, H. Effect of oxidative torrefaction on particulate matter emission from agricultural biomass pellet combustion in comparison with non-oxidative torrefaction. Renew. Energy 2022, 189, 39–51. [Google Scholar] [CrossRef]
- Poudel, J.; Ohm, T.-I.; Lee, S.-H.; Oh, S.C. A study on torrefaction of sewage sludge to enhance solid fuel qualities. Waste Manag. 2015, 40, 112–118. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chen, W.-H.; Lin, B.-J.; Chang, J.-S.; Ong, H.C. Impact of torrefaction on the composition, structure and reactivity of a microalga residue. Appl. Energy 2016, 181, 110–119. [Google Scholar] [CrossRef]
- Iroba, K.L.; Baik, O.-D.; Tabil, L.G. Torrefaction of biomass from municipal solid waste fractions I: Temperature profiles, moisture content, energy consumption, mass yield, and thermochemical properties. Biomass Bioenergy 2017, 105, 320–330. [Google Scholar] [CrossRef]
- Tong, S.; Xiao, L.; Li, X.; Zhu, X.; Liu, H.; Luo, G.; Worasuwannarak, N.; Kerdsuwan, S.; Fungtammasan, B.; Yao, H. A gas-pressurized torrefaction method for biomass wastes. Energy Convers. Manag. 2018, 173, 29–36. [Google Scholar] [CrossRef]
- Nguyen, Q.; Nguyen, D.D.; Vothi, H.; He, C.; Goodarzi, M.; Bach, Q.-V. Isothermal torrefaction kinetics for sewage sludge pretreatment. Fuel 2020, 277, 118103. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, H.; Liu, J.; Evrendilek, F.; Xie, W.; He, Y.; Buyukada, M. Comparative (co-)pyrolytic performances and by-products of textile dyeing sludge and cattle manure: Deeper insights from Py-GC/MS, TG-FTIR, 2D-COS and PCA analyses. J. Hazard. Mater. 2021, 401, 123276. [Google Scholar] [CrossRef] [PubMed]
- Kolapkar, S.S.; Zinchik, S.; Burli, P.; Lin, Y.; Hartley, D.S.; Klinger, J.; Handler, R.; Bar-Ziv, E. Integrated torrefaction-extrusion system for solid fuel pellet production from mixed fiber-plastic wastes: Techno-economic analysis and life cycle assessment. Fuel Process. Technol. 2022, 226, 107094. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, L.; Ke, L.; Zhang, Q.; Cui, X.; Fan, L.; Dai, A.; Xu, C.; Zhang, Q.; Bob, K.; et al. Co-torrefaction of corncob and waste cooking oil coupled with fast co-pyrolysis for bio-oil production. Bioresour. Technol. 2023, 370, 128529. [Google Scholar] [CrossRef]
- Xieyuan, W.; Zhiliang, C.; Jingyong, L.; Zebin, W.; Zihong, C.; Fatih, E.; Shuiyu, S.; Zhibin, C. Co-combustion of Zn/Cd-hyperaccumulator and textile dyeing sludge: Heavy metal immobilizations, gas-to-ash behaviors, and their temperature and atmosphere dependencies. Chem. Eng. J. 2023, 451, 138683. [Google Scholar]
- Jiawei, F.; Xijian, W.; Jingyong, L.; Fatih, E.; Tao, C.; Wuming, X.; Weijie, X.; Yao, H. Co-circularity of spent coffee grounds and polyethylene via co-pyrolysis: Characteristics, kinetics, and products. Fuel 2023, 337, 127061. [Google Scholar]
- Bach, Q.-V.; Skreiberg, Ø. Upgrading biomass fuels via wet torrefaction: A review and comparison with dry torrefaction. Renew. Sustain. Energy Rev. 2016, 54, 665–677. [Google Scholar] [CrossRef]
- Roy, B.; Kleine-Möllhoff, P.; Dalibard, A. Superheated Steam Torrefaction of Biomass Residues with Valorisation of Platform Chemicals—Part 1: Ecological Assessment. Sustainability 2022, 14, 1212. [Google Scholar]
- Sharma, H.B.; Sarmah, A.K.; Dubey, B. Hydrothermal carbonization of renewable waste biomass for solid biofuel production: A discussion on process mechanism, the influence of process parameters, environmental performance and fuel properties of hydrochar. Renew. Sustain. Energy Rev. 2020, 123, 109761. [Google Scholar] [CrossRef]
- Das, P.; Chandramohan, V.P.; Mathimani, T.; Pugazhendhi, A. Recent advances in thermochemical methods for the conversion of algal biomass to energy. Sci. Total Environ. 2021, 766, 144608. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, T.; Xiong, Y.; Dong, Q. Effects of wet torrefaction on the physicochemical properties and pyrolysis product properties of rice husk. Energy Convers. Manag. 2017, 141, 403–409. [Google Scholar] [CrossRef]
- Alshareef, S.A.; Otero, M.; Alanazi, H.S.; Siddiqui, M.R.; Khan, M.A.; Alothman, Z.A. Upcycling olive oil cake through wet torrefaction to produce hydrochar for water decontamination. Chem. Eng. Res. Des. 2021, 170, 13–22. [Google Scholar] [CrossRef]
- Park, J.-H.; Choi, Y.-C.; Lee, Y.-J.; Kim, H.-T. Characteristics of Miscanthus Fuel by Wet Torrefaction on Fuel Upgrading and Gas Emission Behavior. Energies 2020, 13, 2669. [Google Scholar] [CrossRef]
- Petrovič, A.; Cenčič Predikaka, T.; Škodič, L.; Vohl, S.; Čuček, L. Hydrothermal co-carbonization of sewage sludge and whey: Enhancement of product properties and potential application in agriculture. Fuel 2023, 350, 128807. [Google Scholar] [CrossRef]
- Kota, K.B.; Shenbagaraj, S.; Sharma, P.K.; Sharma, A.K.; Ghodke, P.K.; Chen, W.-H. Biomass torrefaction: An overview of process and technology assessment based on global readiness level. Fuel 2022, 324, 124663. [Google Scholar] [CrossRef]
- Ramos-Carmona, S.; Martínez, J.D.; Pérez, J.F. Torrefaction of patula pine under air conditions: A chemical and structural characterization. Ind. Crops Prod. 2018, 118, 302–310. [Google Scholar] [CrossRef]
- Bach, Q.-V.; Trinh, T.N.; Tran, K.-Q.; Thi, N.B.D. Pyrolysis characteristics and kinetics of biomass torrefied in various atmospheres. Energy Convers. Manag. 2017, 141, 72–78. [Google Scholar] [CrossRef]
- Zhang, D.; Han, P.; Zheng, H.; Yan, Z. Torrefaction of walnut oil processing wastes by superheated steam: Effects on products characteristics. Sci. Total Environ. 2022, 830, 154649. [Google Scholar] [CrossRef]
- Tu, R.; Sun, Y.; Wu, Y.; Fan, X.; Cheng, S.; Jiang, E.; Xu, X. The fuel properties and adsorption capacities of torrefied camellia shell obtained via different steam-torrefaction reactors. Energy 2022, 238, 121969. [Google Scholar] [CrossRef]
- Brachi, P.; Miccio, F.; Ruoppolo, G.; Stanzione, F.; Miccio, M. Pressurized steam torrefaction of wet agro-industrial residues. Chem. Eng. Trans. 2018, 65, 49–54. [Google Scholar]
- Is’emin, R.L.; Kuz’min, S.N.; Konyakhin, V.V.; Milovanov, O.Y.; Mikhalev, A.V.; Muratova, N.S.; Nebyvaev, A.V.; Kokh-Tatarenko, V.S. Comparative Studies of the Biochar Production Process Using Hydrothermal Carbonization and Superheated Steam Torrefaction. Therm. Eng. 2022, 69, 981–988. [Google Scholar] [CrossRef]
- Torres Ramos, R.; Valdez Salas, B.; Montero Alpírez, G.; Coronado Ortega, M.A.; Curiel Álvarez, M.A.; Tzintzun Camacho, O.; Beleño Cabarcas, M.T. Torrefaction under Different Reaction Atmospheres to Improve the Fuel Properties of Wheat Straw. Processes 2023, 11, 1971. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, S.; Zhang, X.; Liu, H.; Sun, B.; Guo, S. Influence of air oxidative and non-oxidative torrefaction on the chemical properties of corn stalk. Bioresour. Technol. 2021, 332, 125120. [Google Scholar] [CrossRef]
- Álvarez, A.; Nogueiro, D.; Pizarro, C.; Matos, M.; Bueno, J.L. Non-oxidative torrefaction of biomass to enhance its fuel properties. Energy 2018, 158, 1–8. [Google Scholar] [CrossRef]
- Zhang, C.; Ho, S.-H.; Chen, W.-H.; Wang, R.; Show, P.-L.; Ong, H.C. Oxidative torrefaction performance of microalga Nannochloropsis Oceanica towards an upgraded microalgal solid biofuel. J. Biotechnol. 2021, 338, 81–90. [Google Scholar] [CrossRef]
- Chen, D.; Chen, F.; Cen, K.; Cao, X.; Zhang, J.; Zhou, J. Upgrading rice husk via oxidative torrefaction: Characterization of solid, liquid, gaseous products and a comparison with non-oxidative torrefaction. Fuel 2020, 275, 117936. [Google Scholar] [CrossRef]
- Bergman, P.C.A.; Boersma, A.R.; Zwart, R.W.R.; Kiel, J.H.A. Torrefaction for Biomass Co-Firing in Existing Coal-Fired Power Stations. BIOCOAL; Energy Research Centre of the Netherlands: Petten, The Netherlands, 2005. [Google Scholar]
- Yogalakshmi, K.N.; Sivashanmugam, P.; Kavitha, S.; Kannah, Y.; Varjani, S.; AdishKumar, S.; Kumar, G. Lignocellulosic biomass-based pyrolysis: A comprehensive review. Chemosphere 2022, 286, 131824. [Google Scholar] [CrossRef]
- Tian, X.; Dai, L.; Wang, Y.; Zeng, Z.; Zhang, S.; Jiang, L.; Yang, X.; Yue, L.; Liu, Y.; Ruan, R. Influence of torrefaction pretreatment on corncobs: A study on fundamental characteristics, thermal behavior, and kinetic. Bioresour. Technol. 2020, 297, 122490. [Google Scholar] [CrossRef]
- Chen, W.-H.; Peng, J.; Bi, X.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chen, W.-H.; Lin, B.-J.; Chang, J.-S.; Ong, H.C. Fuel Property Variation of Biomass Undergoing Torrefaction. Energy Procedia 2017, 105, 108–112. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, B.-J.; Lin, Y.-Y.; Chu, Y.-S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.-S.; Ho, S.-H.; Culaba, A.B.; et al. Progress in biomass torrefaction: Principles, applications and challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar] [CrossRef]
- Chew, J.J.; Doshi, V. Recent advances in biomass pretreatment—Torrefaction fundamentals and technology. Renew. Sustain. Energy Rev. 2011, 15, 4212–4222. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Liu, Y.; Ruan, R.; He, C.; Yu, Z.; Jiang, L.; Zeng, Z.; Tian, X. Integrated process of lignocellulosic biomass torrefaction and pyrolysis for upgrading bio-oil production: A state-of-the-art review. Renew. Sustain. Energy Rev. 2019, 107, 20–36. [Google Scholar] [CrossRef]
- Ivanovski, M.; Goricanec, D.; Krope, J.; Urbancl, D. Torrefaction pretreatment of lignocellulosic biomass for sustainable solid biofuel production. Energy 2022, 240, 122483. [Google Scholar] [CrossRef]
- Richards, A.P.; Haycock, D.; Frandsen, J.; Fletcher, T.H. A review of coal heating value correlations with application to coal char, tar, and other fuels. Fuel 2021, 283, 118942. [Google Scholar] [CrossRef]
- Bridgeman, T.G.; Jones, J.M.; Williams, A.; Waldron, D.J. An investigation of the grindability of two torrefied energy crops. Fuel 2010, 89, 3911–3918. [Google Scholar] [CrossRef]
- Castells, B.; Amez, I.; Medic, L.; García-Torrent, J. Torrefaction influence on combustion kinetics of Malaysian oil palm wastes. Fuel Process. Technol. 2021, 218, 106843. [Google Scholar] [CrossRef]
- Ribeiro, J.M.C.; Godina, R.; Matias, J.C.d.O.; Nunes, L.J.R. Future Perspectives of Biomass Torrefaction: Review of the Current State-of-the-Art and Research Development. Sustainability 2018, 10, 2323. [Google Scholar] [CrossRef]
- Chen, W.-H.; Hsu, H.-C.; Lu, K.-M.; Lee, W.-J.; Lin, T.-C. Thermal pretreatment of wood (Lauan) block by torrefaction and its influence on the properties of the biomass. Energy 2011, 36, 3012–3021. [Google Scholar] [CrossRef]
- Patel, B.; Gami, B.; Bhimani, H. Improved fuel characteristics of cotton stalk, prosopis and sugarcane bagasse through torrefaction. Energy Sustain. Dev. 2011, 15, 372–375. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Lee, S.-M.; Lee, H.-W.; Lee, J.-W. Physical and chemical characteristics of products from the torrefaction of yellow poplar (Liriodendron tulipifera). Bioresour. Technol. 2012, 116, 120–125. [Google Scholar] [CrossRef]
- Bach, Q.-V.; Chen, W.-H.; Chu, Y.-S.; Skreiberg, Ø. Predictions of biochar yield and elemental composition during torrefaction of forest residues. Bioresour. Technol. 2016, 215, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cen, K.; Cao, X.; Li, Y.; Zhang, Y.; Ma, H. Restudy on torrefaction of corn stalk from the point of view of deoxygenation and decarbonization. J. Anal. Appl. Pyrolysis 2018, 135, 85–93. [Google Scholar] [CrossRef]
- Jagodzińska, K.; Czerep, M.; Kudlek, E.; Wnukowski, M.; Yang, W. Torrefaction of wheat-barley straw: Composition and toxicity of torrefaction condensates. Biomass Bioenergy 2019, 129, 105335. [Google Scholar] [CrossRef]
- Hu, J.; Song, Y.; Liu, J.; Evrendilek, F.; Buyukada, M.; Yan, Y.; Li, L. Combustions of torrefaction-pretreated bamboo forest residues: Physicochemical properties, evolved gases, and kinetic mechanisms. Bioresour. Technol. 2020, 304, 122960. [Google Scholar] [CrossRef]
- Abdulyekeen, K.A.; Daud, W.M.A.W.; Patah, M.F.A.; Abnisa, F. Torrefaction of organic municipal solid waste to high calorific value solid fuel using batch reactor with helical screw induced rotation. Bioresour. Technol. 2022, 363, 127974. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Huang, S.; Wu, Y.; Wu, S.; Yang, J. A comprehensive study on torrefaction of penicillin mycelial residues: Analysis of product characteristics and conversion mechanisms of N. Fuel 2022, 330, 125703. [Google Scholar] [CrossRef]
- Devaraja, U.M.A.; Senadheera, S.S.; Gunarathne, D.S. Torrefaction severity and performance of Rubberwood and Gliricidia. Renew. Energy 2022, 195, 1341–1353. [Google Scholar] [CrossRef]
- Zhang, C.; Ho, S.-H.; Chen, W.-H.; Xie, Y.; Liu, Z.; Chang, J.-S. Torrefaction performance and energy usage of biomass wastes and their correlations with torrefaction severity index. Appl. Energy 2018, 220, 598–604. [Google Scholar] [CrossRef]
- Ivanovski, M.; Petrovic, A.; Ban, I.; Goricanec, D.; Urbancl, D. Determination of the Kinetics and Thermodynamic Parameters of Lignocellulosic Biomass Subjected to the Torrefaction Process. Materials 2021, 14, 7877. [Google Scholar] [CrossRef]
- Ivanovski, M.; Urbancl, D.; Petrovič, A.; Stergar, J.; Goričanec, D.; Simonič, M. Improving Lignocellulosic and Non-Lignocellulosic Biomass Characteristics through Torrefaction Process. Appl. Sci. 2022, 12, 12210. [Google Scholar] [CrossRef]
- Ivanovski, M.; Goričanec, D.; Urbancl, D. The Evaluation of Torrefaction Efficiency for Lignocellulosic Materials Combined with Mixed Solid Wastes. Energies 2023, 16, 3694. [Google Scholar] [CrossRef]
- Ivanovski, M.; Goričanec, D.; Urbancl, D. The Thermochemical Conversion of Municipal Solid Waste by Torrefaction Process. Thermo 2023, 3, 277–288. [Google Scholar] [CrossRef]
- Namkung, H.; Park, J.-H.; Lee, Y.-J.; Song, G.-S.; Choi, J.W.; Park, S.-J.; Kim, S.; Liu, J.; Choi, Y.-C. Performance evaluation of biomass pretreated by demineralization and torrefaction for ash deposition and PM emissions in the combustion experiments. Fuel 2021, 292, 120379. [Google Scholar] [CrossRef]
- Ubando, A.T.; Rivera, D.R.T.; Chen, W.-H.; Culaba, A.B. A comprehensive review of life cycle assessment (LCA) of microalgal and lignocellulosic bioenergy products from thermochemical processes. Bioresour. Technol. 2019, 291, 121837. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, W.; Chen, W.-H.; Ho, S.-H.; Pétrissans, A.; Pétrissans, M. Effect of torrefaction on the structure and reactivity of rice straw as well as life cycle assessment of torrefaction process. Energy 2022, 240, 122470. [Google Scholar] [CrossRef]
- Acharya, B.; Sule, I.; Dutta, A. A review on advances of torrefaction technologies for biomass processing. Biomass Convers. Biorefinery 2012, 2, 349–369. [Google Scholar] [CrossRef]
- Potrč, S. Production of Solid Biofuels with Torrefaction of Biomass. Master’s Thesis, Faculty of Chemistry and Chemical Engineering, University of Maribor, Maribor, Slovenia, 2018. [Google Scholar]

| Thermochemical Process | Temperature (°C) | Pressure (kPa) | Pre-Drying |
|---|---|---|---|
| Liquefaction | 250–330 | 5000–20,000 | Not required |
| Torreaction | 200–300 | 100 | Required |
| Pyrolysis | 300–600 | 100–500 | Required |
| Gasification | 500–1300 | ≥100 | Required |
| Combustion | 700–1400 | ≥100 | Not required |
| Type of Biomass | The Aim of the Research | Reaction Conditions | Reference |
|---|---|---|---|
| Waste sludge from a municipal treatment plant | A study on the physio-chemical variation in sewage sludge during torrefaction in a horizontal tubular reactor under nitrogen flow | 150–400 °C 50 min | [29] |
| Algae residue | A study on the composition, structure, and reactivity of a microalga residue after torrefaction. | 200, 250 and 300 °C 15, 30 and 60 min | [30] |
| Municipal waste | The microwave-assisted torrefaction of construction demolition and grass clippings were studied | 250, 500 and 750 W * 15, 30 and 60 min | [31] |
| Different types of waste | A study on a gas-pressurized (GP) torrefaction method to torrefy biomass wastes | 200, 250 and 300 °C 15 min | [32] |
| Algae residue (Arthrospira platensis and Chlamydomonas sp.) | To develop a torrefaction severity factor (TSF) to account for the relationship between operating conditions, biomass nature, and torrefaction severity | 200, 250, 275 and 300 °C 15, 30, 45 and 60 min | [16] |
| Waste sludge from municipal treatment plants | An isothermal kinetic study of the torrefaction of sewage sludge | 220, 240, 260, 280 and 300 °C 5 min | [33] |
| Textile dyeing sludge and cattle manure | In-depth analysis of the co-pyrolytic performance between textile dyeing sludge and cattle manure using TGA apparatus | 35 to 1000 °C in a N2 atmosphere at heating rates of 5, 10, 20, and 40 °C/min | [34] |
| Mixture of fiber (biomass) and plastic wastes | A study on the techno-economic analysis and life-cycle assessment of an integrated torrefaction–extrusion system for solid fuel pellet production | / | [35] |
| Mixture of corncob and waste cooking oil | Co-torrefaction of corncob and waste cooking oil | 180, 210, and 240 °C 30, 60, 90, 120 and 150 min | [36] |
| Textile dyeing sludge | A study on the characterization and quantification of interactions among Zn, Cd, Cl, S, and minerals and their migration and transformation behaviors in the air (N2/O2) versus the oxy-fuel (CO2/O2) co-combustions of SAH and TDS through TGA, thermodynamic equilibrium simulations, and joint optimization | 650, 750, 850, and 950 °C | [37] |
| Spent coffee grounds and polyethylene | Co-pyrolysis performances of CG and PE, interaction effects, kinetics, and product characterization in response to the varying temperature and blend ratio, using TGA apparatus | 35 to 1000 °C in an N2 atmosphere at heating rates of 10, 20, and 40 °C/min | [38] |
| Stage | Heat Equation | Parameter | |
|---|---|---|---|
| Pre-heating | m—mass (kg), Cp—heat capacity (kJ/kgK), —temperatures of raw and feed biomass (K), huf—the heat utilization factor for pre-heating | (2) | |
| Drying | L—latent heat (kJ/kg), xm—the moisture (%), hud—heat utilization factor during drying | (3) | |
| Intermediate heating | Cpd—specific heat capacity of dry biomass (J/kgK), Ttor—torrefaction temperature (K) —heat utilization factor for intermediate heating | (4) | |
| Torrefaction | Hloss—the heat loss (kJ), Xt—absorbed heat during torrefaction (kJ/kg) | (5) | |
| Cooling | MYdb—mass yield (%), Cpt—specific heat capacity of biomass (J/kgK) Tp—temperature of torrefied biomass at the end of the process (K) | (6) |
| Characteristic | Wood | Wood Pellets | Torrefied Wood Pellets | Charcoal | Coal |
|---|---|---|---|---|---|
| Moisture content (wt %) | 30–45 | 7–10 | 1–5 | 1–5 | 10–15 |
| LHV (MJ/kg) * | 9–12 | 15–18 | 20–24 | 30–32 | 23–28 |
| Volatile matter (wt %) | 70–75 | 70–75 | 55–65 | 10–12 | 15–30 |
| Fixed carbon (wt %) | 20–25 | 20–25 | 28–35 | 85–87 | 50–55 |
| Density (kg/dm3) | 0.2–0.25 | 0.55–0.75 | 0.75–0.85 | 0.2–0.4 | 0.8–0.85 |
| Energy density (GJ/m3) | 2–3 | 7.5–10.4 | 15–18.7 | 6–6.4 | 18.4–23.8 |
| Hygroscopicity | Hydrophilic | Hydrophilic | Hydrophobic | Hydrophobic | Hydrophobic |
| Biological decomposition | Yes | Yes | No | No | No |
| Grindability | Bad | Bad | Good | Good | Good |
| Biomass | Operating Conditions (T, t, atmosphere) | General Remarks | Reference |
|---|---|---|---|
| Wood biomass (Lauan) | 220, 250, 280 °C, 30–120 min | The properties of the torrefied wood improved at a temperature > 250 °C and a torrefaction time > 60 min. These conditions were recommended to increase the heating value and grinding, and to prevent the excessive loss of wood mass. | [72] |
| Cotton, Sugar cane | 300 °C, 60 min | Torrefaction improved the gross heating value (27–41%) of research biomasses, reduced their moisture and VM contents (3–6% and 14–18%, respectively), improved their fixed carbon (9–24%) and reduced mass loss (27–46%). | [73] |
| Yellow poplar (Liriodendron tulipifera) | 240, 260, 280 °C, 30 min | The carbon share increased, while the oxygen and hydrogen share decreased with increased temperature. The energy density, mass reduction and energy efficiency increased. | [74] |
| Stem wood and forest residue biomass | 220–300 °C, 120 min, inert atmosphere (N2) | The qualities of torrefied biomass exhibited enhancement when contrasted with those of raw biomass. However, as the temperature increased, both the mass and energy efficiency tended to diminish. | [75] |
| Rice husks | 150, 180, 210, 240 °C, 60 min | Examination of the physicochemical attributes of untreated and pretreated samples revealed that wet torrefaction enhanced fuel properties. Additionally, a significant quantity of alkaline earth metal species were effectively eliminated. This dual benefit mirrors the advantages associated with both the dry torrefaction and demineralization processes. | [43] |
| Corn stalk | 200, 230, 260, 290 °C, 30 min, inert atmosphere (N2) | Elevating the torrefaction temperature in the range of 200–290 °C brought about a notable reduction in the oxygen share and a gradual augmentation in the carbon content in the corn stalk. During the torrefaction process, cornstalk oxygen transformed into CO2 and CO as a gaseous product, or into H2O and oxygen-containing compounds, such as acids and phenols. These results showed that dehydration reactions and gas generation prevail throughout the decarbonization or deoxygenation phase of torrefaction. | [76] |
| Wheat–barley straw | 240–320 °C, 75 min, inert atmosphere (N2) | As temperature increased, the more differentiated the structure of the torrefied biomass condensates became. Acids, aldehydes, and ketones dominated in the analyzed temperature range. | [77] |
| Sludge from municipal wastewater treatment plants | 220–300 °C, 120 min, inert atmosphere (N2) | Non-lignocellulosic biomass was less heat-resistant and decomposed much faster than lignocellulosic biomass. In addition, the mass yield of torrefied sewage sludge was at temperatures below 280 °C, lower than that of woody biomass. | [33] |
| Bamboo | 200, 250, 300 °C, 60 min, inert atmosphere (N2) | The results showed that bamboo is a suitable biomass for the torrefaction process, as the properties of the biomass improved during the process. | [78] |
| Microalgae Nannochloropsis Oceanica | 200–300 °C, 15–60 min | The outcomes of this study offer valuable insights into evaluating the fuel characteristics of solid microalgae biofuel. These insights hold the potential to expedite the advancement of industrial-scale oxidative torrefaction processes. | [57] |
| Municipal waste | / | Review of the torrefaction of municipal waste in Malezia. | [79] |
| Remains penicillin mycelium | 230, 260, 290, 320 °C | Gases during torrefaction were analyzed with the aim of removing antibiotic residues and achieving antimicrobial resistance. The results showed that the gaseous products during torrefaction are mainly CO2, CO, CH4 and H2. The results of gas chromatography showed that the sample mainly contains ketones, furan, ester, and phenolic and N-containing compounds, among which the relative content of N-containing compounds was the highest. | [80] |
| Rubberwood and Gliricidia | 250, 275, 300 °C, 30, 45, 60 min | The energy–mass co-benefit index of rubber and gliricidia was calculated. The optimum residence times of 60 min at 275 °C and less than 60 min at 300 °C were determined for rubberwood. The optimum residence time of 60 min at 300 °C was favorable for Gliricidia. | [81] |
| Oak waste wood, mixed waste wood, municipal sludge | 220–400 °C, 30–120 min | From an energy point of view, the optimal torrefaction temperature is 260 °C, and the optimal torrefaction time is 80 min. | [19] |
| Spent coffee grounds, Chinese medicine residue, microalgal residues | 200, 250, 275, 300 °C, 15–60 min | The amounts of single biomass can be predicted with the help of the torrefaction severity index (TSI). | [82] |
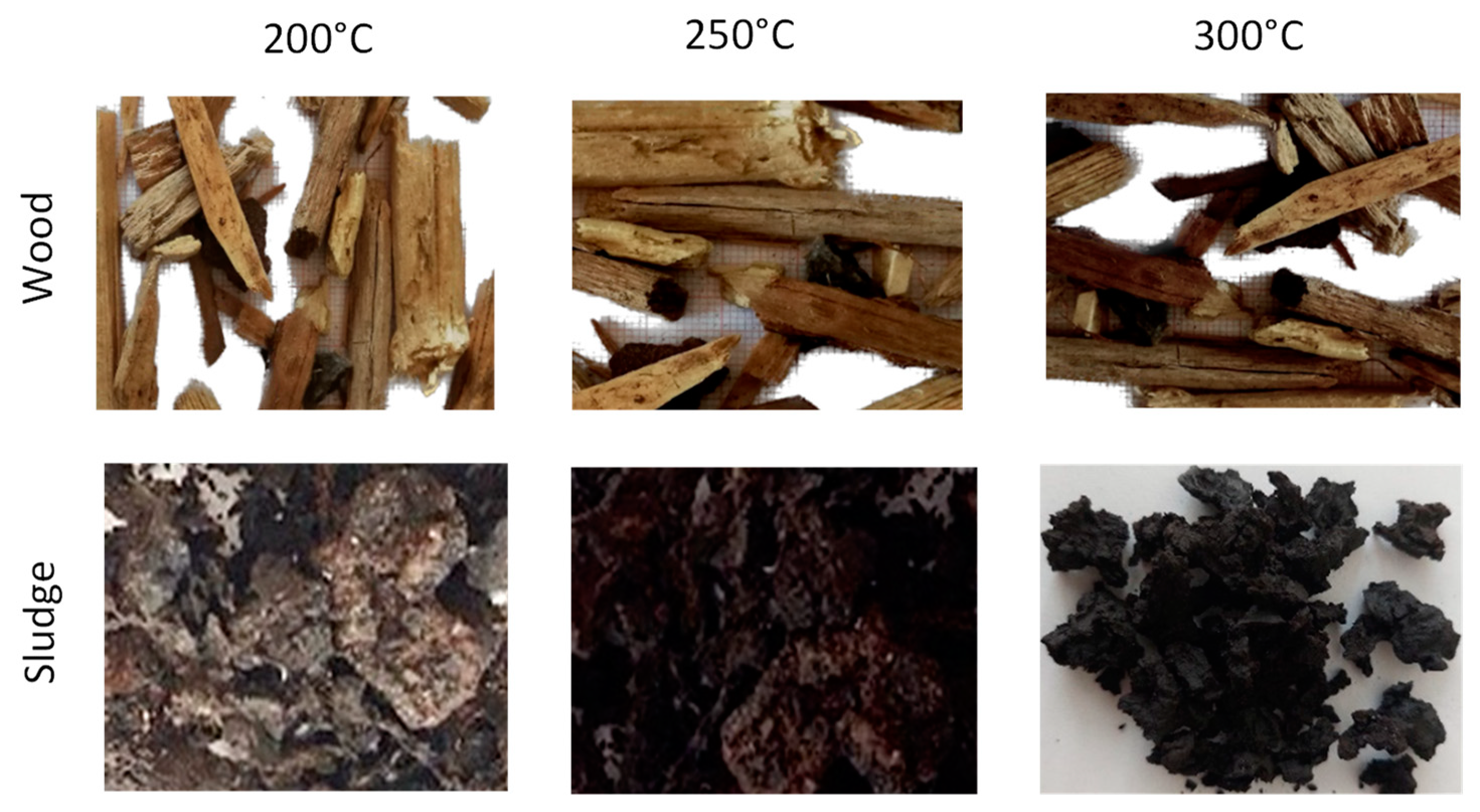
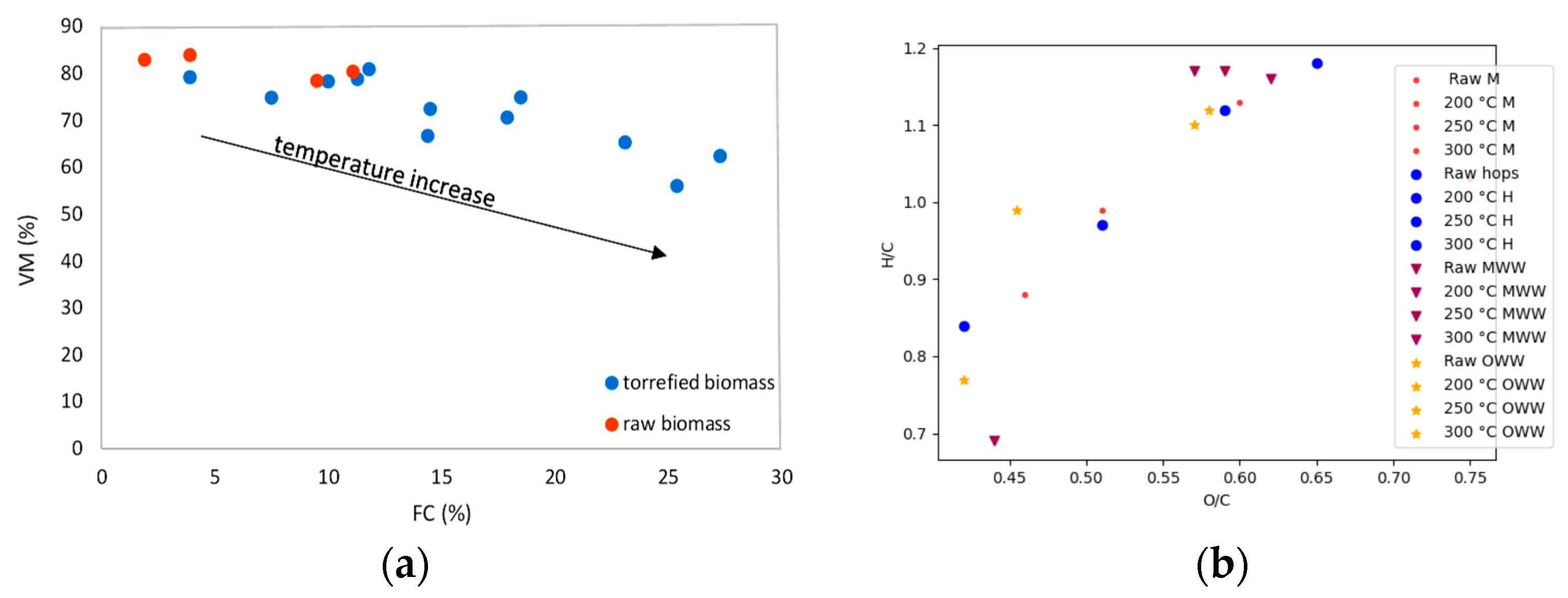
| Miscanthus, waste hops, waste mixed wood and oak wood | 200, 250, 300 °C, 90 min, semi-inert atmosphere | The results showed that the higher the torrefaction temperature, the lower the mass and energy efficiency of the torrefied samples. Significant changes in the thermo-gravimetric curves (TGA) were observed after torrefaction of the samples. The FTIR and XRD spectra showed the breaking of bonds in the cellulose molecules. The same was shown using SEM analysis. | [83] |
| Miscanthus, waste hops, municipal sludge and blends | 250, 300, 350 °C, 10–60 min, inert atmosphere (N2) | The results showed that the higher the torrefaction temperature, the lower the mass and energy efficiency of all the research samples. The optimal torrefaction conditions were found at 260 °C and 10 min. The degree of torrefaction index and the EMCI were calculated, and the proximate and elemental composition was determined. The FTIR spectra were recorded. | [84] |
| Miscanthus, waste hops, mixed municipal waste and blends | 250, 300, 350 °C, 30–60 min, inert atmosphere (N2) | Proximate and elemental composition and HHV values were determined. The FR (fuel ratio) and EROI (Energy return on investment) were subsequently calculated. The results showed that the higher the torrefaction temperature, the lower the mass and energy efficiency of all the research samples. HHV and FR, on the other hand, rose with a higher temperature. The highest EROI, 28, was calculated for the thermally treated sample of mixed municipal waste. | [85] |
| Mixed municipal sludge | 200, 250, 300 °C, 90 min, semi-inert atmosphere | Proximate and elemental composition and HHV values were determined. TGA analyses were performed and compared with each other. The results showed that the higher the torrefaction temperature, the lower the mass and energy yield of all the research samples, and the higher the HHV. | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanovski, M.; Petrovič, A.; Goričanec, D.; Urbancl, D.; Simonič, M. Exploring the Properties of the Torrefaction Process and Its Prospective in Treating Lignocellulosic Material. Energies 2023, 16, 6521. https://doi.org/10.3390/en16186521
Ivanovski M, Petrovič A, Goričanec D, Urbancl D, Simonič M. Exploring the Properties of the Torrefaction Process and Its Prospective in Treating Lignocellulosic Material. Energies. 2023; 16(18):6521. https://doi.org/10.3390/en16186521
Chicago/Turabian StyleIvanovski, Maja, Aleksandra Petrovič, Darko Goričanec, Danijela Urbancl, and Marjana Simonič. 2023. "Exploring the Properties of the Torrefaction Process and Its Prospective in Treating Lignocellulosic Material" Energies 16, no. 18: 6521. https://doi.org/10.3390/en16186521
APA StyleIvanovski, M., Petrovič, A., Goričanec, D., Urbancl, D., & Simonič, M. (2023). Exploring the Properties of the Torrefaction Process and Its Prospective in Treating Lignocellulosic Material. Energies, 16(18), 6521. https://doi.org/10.3390/en16186521






