Abstract
Direct Air Capture (DAC) is a promising technology to fight climate change by capturing carbon dioxide (CO2) from the air. For DAC to be a negative emissions technology, the captured CO2 must be removed permanently, but can also be used as a net-zero technology to produce sustainable chemicals, fuels or other materials. This review presents a comprehensive survey of recent advancements, challenges, and potential applications of DAC technology, with an emphasis on the recent rapid increase in the number of DAC developers, the majority of them being founded in the past 4 years. Through pilot projects and recent commercial deployments, several DAC companies have made significant advances and demonstrated their scalability. Cost and energy efficiency remain significant impediments to the wide deployment of DAC. Integration with emission-free energy sources and utilization of waste heat are being researched to boost the total energy efficiency of DAC systems. Further research of electrochemical technologies for regeneration or direct capture are needed, as well as the development of new, modified, or hybrid adsorbents for improved capture efficiencies. Moreover, favorable regulations and financial incentives are crucial for enhancing the viability of DAC projects and will need to substantially increase if Paris Agreement goals are to be achieved.
1. Introduction
In the 2015 Paris Agreement, 196 nations vowed to establish a long-term goal of keeping the global average temperature rise well below 2 °C above pre-industrial levels [1]. The atmospheric carbon dioxide (CO2) concentration is currently at 423 ppm, or 3300 GtCO2, which is more than it has ever been in the last 800,000 years [2]. If the climate and economic growth targets are to be met, negative emissions technologies are certain to play a significant role in mitigating climate change by removing 10 GtCO2/year globally by mid-century and 20 GtCO2/year globally by the end of the century [3]. In this scenario, direct air capture (DAC), a possible negative emissions technology, plays an increasing role [4,5,6]. DAC is a revolutionary approach to mitigating climate change by removing CO2 directly from the atmosphere and storing or utilizing it in various applications [7]. There are currently 27 DAC plants worldwide in operation, mostly small demonstration projects, removing almost 0.01 MtCO2 annually, and 130 DAC facilities are in various stages of development for a total removal capacity of around 5 MtCO2/year [8]. To put this into perspective, the 2050 net-zero emission scenario indicates a CO2 removal from DAC of about 75 MtCO2/year in 2030 [8].
DAC technology relies on chemical or physical processes to capture CO2 from ambient air [9]. The technology typically involves three main steps: adsorption/absorption, desorption, and purification. In the adsorption/absorption step, a sorbent/solvent is used to selectively bind CO2 from the air while allowing the passage of other gases. The CO2-loaded sorbent/solvent is then processed in the desorption step, where the CO2 is separated from the sorbent before final purification. The purified CO2 can then be stored [10,11] or utilized in various applications, such as in the production of synthetic fuels or building materials [12]. Figure 1 represents the number of published articles over time with “direct air capture” in the title, using the ScienceDirect database. This figure highlights the exponential growth of research interest in DAC in the last 5–6 years.
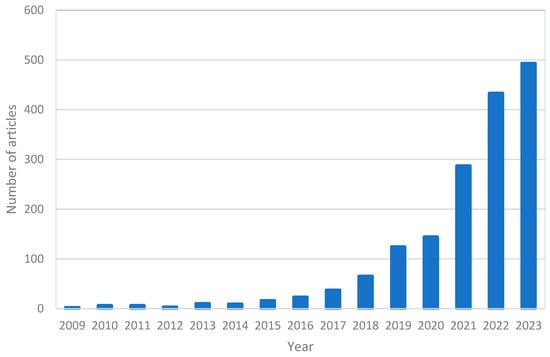
Figure 1.
Number of published articles over time containing “direct air capture” in the title from ScienceDirect database (August 2023).
Unlike carbon capture technologies developed for large stationary sources, such as power plants or industrial facilities, the location of DAC systems is more flexible. For example, it can be located close to storage sites because it is not dependent on a source of CO2 other than air, which makes it an option to offset hard-to-abate emissions [13]. One significant challenge associated with DAC is its high energy requirement, and hence its high cost [14]. Yet, DAC is a new and developing technology, and researchers and companies are actively working on developing more energy-efficient processes and reducing DAC costs [15,16].
This review paper describes the rapidly evolving DAC field. The liquid and solid sorbents explored, equipment required, and overview of DAC processes are described in the DAC Technologies section. After an overview of CO2 storage and utilization, this paper presents a comprehensive panorama of DAC-focused companies and their latest developments. Finally, an analysis of DAC energy demand and costs is presented. All tonnes reported are in metric tonnes and costs in US dollars (USD).
2. DAC Technologies
The extremely low CO2 concentration in the atmosphere and intense competition from other components in air, particularly water, impose stringent thermodynamic requirements on DAC technologies [17]. Liquid sorbent and solid sorbent technology are the two most prevalent DAC capture media [15,16]. In addition, various alternative technologies for DAC have been proposed, including electrochemical techniques [18], membrane capture [19], and ion-exchange resins [20].
Since CO2 is an acidic gas, most sorbents (solid or liquid) used to remove CO2 from gas streams must be of a basic character for sorption to take place. After each sorption step, the sorbent must release the CO2 it has trapped, which returns it to its initial condition and enables it for subsequent rounds of capture. The sorbent is a crucial component of DAC and the optimum sorbent for a DAC system must meet several requirements. Desirable characteristics of sorbents include high selectivity and affinity for CO2, stability in the relevant pH and temperature windows, high capacity for CO2, long cyclability, fast CO2 uptake and release kinetics, nontoxicity, and low cost [21]. From a process standpoint, the capture is ideally carried out at ambient temperatures and pressure because it is unprofitable to pressurize, cool, or heat large volumes of air (which eliminates membrane or cryogenic-based separation technologies), and the energy for capture should come from non-emitting sources to prevent the release of additional CO2 during the process (i.e., to attain net-zero or negative emissions) [22]. In this section, we present more details on different DAC technologies.
2.1. Liquid Sorbent Technology
2.1.1. Aqueous Alkaline Solvents
Aqueous sorbents with a high CO2 selectivity, such as sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)2), are strong bases used as a sorption technique for DAC. In 1999, Lackner et al. [23] proposed absorption using Ca(OH)2 in a three-reaction process (see Table 1). The number of OH groups that may react with CO2 and, consequently, the rate of CO2 absorption are both severely constrained by the solubility of Ca(OH)2 in water, which is very low (0.025 M) at room temperature. In 2006, Baciocchi et al. [24] proposed the use of NaOH in a four-reaction process because it enables significant CO2 binding while also increasing carbonate solubility in water. While Ca(OH)2 and NaOH both offer strong binding to CO2, NaOH has the benefit of producing a carbonate with higher water solubility, which also avoids scaling on the inner surfaces of the absorption column. However, the high solubility of sodium carbonate hinders direct precipitation. Similar procedures and regeneration techniques to those described here could also be used with KOH to capture CO2. However, KOH is more expensive than NaOH and is commercially produced at a scale that is a hundred times lower. On the other hand, KOH requires lower regeneration energy than NaOH [25].

Table 1.
Aqueous Alkaline Solvents Reactions. A in AOH can be Na or K.
The main process flow of the liquid sorbent system is depicted in Figure 2, which has two chemical cycles that work concurrently. In the first cycle, air is passed through the air contactor where it meets the aqueous solution (KOH or NaOH) and CO2 is absorbed at ambient temperature and pressure. Due to the diluted concentration of CO2 in air, contactor designs for CO2 capture from air are distinct from those of conventional packed towers used in point-source carbon capture; it has been observed that the most advantageous configuration is for very short columns with very large cross-sections [26]. In the second cycle, the pellet reactor or causticizer regenerates the sorbent solution by reacting with calcium hydroxide (Ca(OH)2) to form CaCO3. The produced CaCO3 is heated to about 900 °C in the calciner to release CO2, and the reaction outputs of CaO are sent into the slaker unit to react with water and form Ca(OH)2 for use in subsequent cycles [27]. It should be emphasized that because moisture must be eliminated before calcination in a kiln, water loss throughout the process results in a significant energy cost. Zeman et al. [28] demonstrated the importance of water loss in their system, with an average mass loss of 90 gH2O/gCO2.
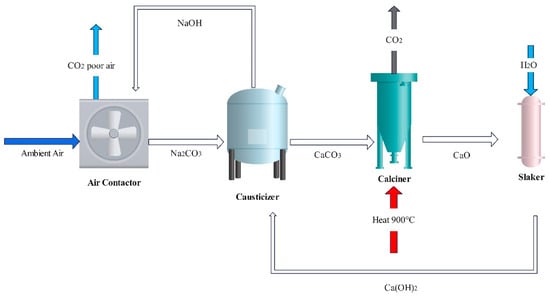
Figure 2.
Schematic diagram of a typical NaOH liquid sorbent process.
2.1.2. Aqueous Amines
Since 1930, aqueous solutions of primary and secondary amines have been used to capture CO2 from natural gas and hydrogen at high partial pressures [29]. Aqueous amines such as monoethanolamide (MEA), diethanolamine (DEA), and methyl diethanolamine (MDEA) are the most often used amines for CO2 absorption [30]. High reaction rates, appropriate CO2 absorption capacities, maturity of the technology and low cost compared to alternative processes are all benefits of amine solutions. However, the main disadvantages of amine solutions are oxidative degradation, high volatility of most amine solvents and high regeneration heat [17,31]. Recent studies have examined the use of aqueous amine solvents for DAC [32]. Miao et al. [32] have shown experimentally that primary and secondary amines function best, absorbing around 85% of the CO2 from air, similar to NaOH under the same conditions. One of the most effective sorbents for DAC are aqueous unrestricted primary amines since they are just as effective as aqueous alkali hydroxides while also having the ability to save energy because sorbent regeneration occurs at lower temperatures [33,34].
2.1.3. Ionic Liquids (IL)
Salts that are liquid at ambient conditions are known as ionic liquids (ILs). ILs offer virtually limitless structural and chemical tunability since they are mostly composed of large organic ions [35,36]. In addition to strong CO2 solubility and selectivity, ILs display excellent performance in CO2 capture due to their stability, low volatility, and flexibility of design options [37,38,39]. However, a disadvantage of ILs for CO2 capture is their higher viscosity compared to that of conventional solvents, resulting in difficult manufacturing and purification procedures [40]. On the other hand, there are several options to reduce the viscosity such as proper mix of cations and anions, length of the alkyl chain of cations, or addition of a co-solvent [39,41]. Polar solvents, such as water, have a greater effect on viscosity reduction than non-polar solvents, such as benzene and toluene [39,42]. Attaching CO2-philic functional groups to ILs can dramatically enhance their CO2 collection ability [41]. Moitra et al. [43] used CaO- and superbase-ionic liquids (SIL)-bifunctionalized sorbents to capture CO2; these sorbents demonstrated rapid interaction kinetics, high CO2 uptake capacity, facile CO2 release, and stable sorption/desorption cycles. New research proposes that immobilizing ILs in porous materials with large surface areas and specific active sites, such as MOFs, might remedy the difficulties caused by ILs [44,45,46], which is discussed further in the MOF part under Section 2.2.
2.2. Solid Sorbent Technology
Figure 3 shows a typical adsorption process. This type of technology includes adsorption chambers containing solid sorbents where cyclic adsorption and desorption procedures are carried out. During the adsorption process, ambient air, usually propelled by fans, passes through the contactor and CO2 is adsorbed onto the solid sorbent at ambient temperature, while CO2-poor air exits the system. Air contactors for solid sorbents may take different forms including monolithic, fluidized bed, fixed bed, and moving bed designs [47]. Contactor design plays an important role in reducing the overall pressure drop of the air passing through (thereby reducing costs). After the sorbent is saturated with CO2, the system switches from adsorption to desorption. During this process, the adsorption chamber is isolated from the surrounding environment and the residual air is evacuated. The sorbent is then exposed to different conditions such as increased temperature, releasing a CO2 stream of high purity. In addition to temperature, cycles involving changing pressure, humidity, or their combinations can also be used in the capture–regeneration cycle [48]. Once CO2 desorption is complete, the sorbent is then returned to initial conditions to begin a new cycle.
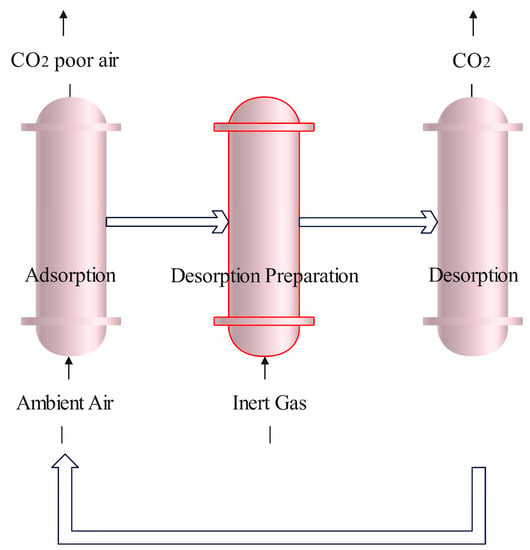
Figure 3.
Schematic diagram of a typical solid sorbent-based process. CO2 is captured during the adsorption step until the capacity of the sorbent is reached. In the desorption preparation step, the chamber is closed and, depending on the process, the pressure/temperature/humidity are changed to favor CO2 desorption. During the desorption step, the CO2 released from the sorbent is removed from the chamber.
2.2.1. Adsorbents
This section focuses on the most studied physical adsorbents for DAC application, specifically zeolites, activated carbon (AC), and metal organic frameworks (MOFs).
- Zeolites
Zeolites are three-dimensional porous crystalline aluminosilicates composed of a periodic array of TO4 tetrahedral (T = Si or Al) that are extensively utilized for gas separation. Aluminum substitution for silicon in the zeolite lattice produces isomorphism, resulting in a structure with a net negative charge and a strong affinity for quadrupolar molecules, such as CO2 [49]. Zeolites have high availability, low cost, large surface area and tailorable pore cavities which lends them to be a good molecular sieve for CO2 separation [50]. The main CO2 capture mechanism is physical adsorption where the CO2 molecules are bound to carbonate molecules by π-coordination or ion–dipole interactions; in addition, chemical modification strategies have also been explored to improve CO2 capacity such as amine modification, silica modification, and ion exchange [50]. One drawback is that the sorption capacity and CO2/N2 selectivity of zeolites for DAC applications remain problematic. Zeolite sorption capacity depends on zeolite type, therefore adding amine to create functionalized zeolites aids to increase it [51]. Zeolites are known to also adsorb moisture [50] and therefore a desiccant bed is usually required to collect the moisture from the air to offset the reduction in adsorption capacity in humid environments. This approach often requires greater energy input and expensive equipment [50,52].
- Activated Carbon (AC) and Porous Carbon
Carbon-based adsorbents may be created in several forms, including carbon allotropes (carbon nanotubes (CNTs) and graphene), activated carbons (AC), porous carbons, amorphous carbon, biomass-derived carbons, pyrogenic carbons, template-based carbons, carbon molecular sieves, carbon aerogels (CAs), and carbon nanofibers (CNFs) [53]. Carbonaceous adsorbents typically feature a range of pore sizes, consisting of micropores (<2 nm), mesopores (2–50 nm) and macropores (>50 nm). The meso- and macropores facilitate the movement of molecules to the micropores, where 90% of the adsorption takes place through van der Waals interactions [49]. Economically speaking, activated porous carbons could be more favorable for CO2 capture over other carbon-based adsorbents which require expensive precursors and chemicals for functionalization in addition to high energy consumption [53]. In general, the preparation techniques of raw materials have an impact on the properties of AC. Important variables in the preparation stages include pyrolysis, activation temperature, and holding duration [54]. Due to its low cost, large surface area, resistance to moisture, readily customizable features such as porosity, excellent stability, and low regeneration energy, AC has also attracted interest for CO2 capture [55]. Low chemical activity makes it easy to regenerate and guarantees that it does not react with the sorbate; however, this also results in low CO2 uptake capacity at low partial pressures [56] which has led to different strategies to improve capacity such as physical or chemical activations, doping (with nitrogen, oxygen or sulfur), amine addition, and carboxylation [49,53]. Kamran et al. [57] investigated the influence of different chemical activation agents on polyacrylonitrile (PAN)-based porous carbon sorbents for CO2 collection and they demonstrated stability of their sorbents over a small number (four) of adsorption–desorption cycles.
- Metal Organic Frameworks (MOFs)
MOFs are highly porous crystalline materials composed of metal-containing nodes connected by organic ligand bridges and created by powerful coordination bonds. One advantage of MOFs is the ability to customize their structure based on desired surface area, pore volume and functionalization [58]. MOFs are considered a promising separation technology for environmental applications such as water treatment and CO2 removal [59]. MOF synthesis and post-synthetic modification methods can result in different capture mechanisms such as interaction with open metal sites, Lewis basic sites (like amines), polar functional groups [60], molecular sieving through pore size control, flexible or stimuli-responsive frameworks [58,60], or improving CO2 selectivity by creating hydrophobic frameworks [60]. For example, Liu et al. [61] functionalized MIL-100(Fe), UiO-66(Zr) and MIL-100(Cr) MOFs by grafting N-(2-Aminoethyl)ethanolamine (AEEA) after the initial MOF synthesis. While the secondary amines of AEEA reacted with the metallic vacancy sites on the MOFs to produce grafts, the exposed primary amine acted as the main CO2 adsorption site, resulting in excellent thermal stability, structural stability, and improved CO2 capacity. In another study by Qiu et al. [46], a Ni-MOF was functionalized with [MeTBDH]2[HFPDO], a superbase-derived ionic liquid (SIL). The hybridized SIL-MOF demonstrated an improved CO2 uptake at 0.4 mbar (close to 400 ppm) due to a stronger interaction strength between the SIL and CO2. Uptake also improved as humidity increased; however, capture kinetics suffered due to hydrogen bonding formation and partial blocking of pores. Despite the benefits, the cost of MOFs, particularly the organic ligand, may be prohibitive to its widescale adoption [58].
2.2.2. Ion-Exchange Resin
Ion-exchange resins are cross-linked polymers to which ions can electrostatically bind and are named for their ability to exchange ions of the same charge type when in contact with an ion-containing solution. Both anion- and cation-exchange resins exist; the name indicates which type of ion can be exchanged. In the 100+ years of ion-exchange resin history, they have been used for various applications including water treatment, hydrometallurgy, catalysis and more [62]. Lackner [63] proposed the use of a strong-base ion-exchange resin with OH− charge to capture CO2. Rather than exchanging ions, the OH− reacts with CO2 to form carbonate (CO32−) and bicarbonate (HCO3−), and CO32− further reacts with CO2 to form HCO3− until full capacity of the resin is reached when it is saturated in the bicarbonate state. Moisture swing using water vapor releases the CO2 back into a gaseous state, although other studies have demonstrated regeneration of other resin types using temperature swing and even seawater [64]. Amine-functionalized ion-exchange resins (usually quaternary ammonium based) have also been studied. Wang et al. [65] found that poor adsorption kinetics occurred when temperature decreased from 20 °C to 0 °C, and humidity increased, but the effect of temperature could be mitigated by decreasing resin particle size from 30 to 5 μm. Higher porosity, such as through large inner pore sizes, can also improve uptake kinetics [66]. Although a functionalization process is required to improve the commercially available ion-exchange resins for use in DAC, the maturity of the industry is still beneficial from an economic standpoint. However, further research on these resins specifically for DAC is needed, as current studies show they may be limited to deployment in warmer and drier climates unless engineering design is used to overcome these challenges.
2.2.3. Desorption/Regeneration
Several desorption/regeneration processes have been considered for DAC, such as temperature swing adsorption (TSA), pressure swing adsorption (PSA), vacuum swing adsorption (VSA) and moisture swing adsorption (MSA). The regeneration process can be repeated multiple times, with the adsorption and desorption cycles occurring sequentially in different parts of the sorbent bed. The adsorption and desorption cycles can also be controlled by adjusting the pressure and temperature of the system, which allows for further optimization of the process.
- Temperature swing adsorption (TSA)
TSA uses increased temperature to desorb CO2 from solid sorbents [67] and is a commonly used to regeneration technique. In the case of amine-based CO2 adsorbents, lab experiments have shown that TSA regeneration is preferable [15]. Utilizing an inert gas instead of air may reduce oxidative deterioration of sorbents. In addition, TSA has the option of using pure CO2 as a stripping gas to produce high-purity CO2; however, in the case of amine-functionalized sorbents, there is a risk of urea production owing to deactivation of the adsorbent [68].
- Pressure swing adsorption (PSA) or vacuum swing adsorption (VSA)
PSA and VSA work by utilizing the different adsorption characteristics of CO2 and other gases at different pressures to selectively capture CO2 from the air. In PSA, the filtered air is compressed and then fed into a vessel filled with a solid sorbent material, such as zeolites or activated carbon, which selectively adsorbs CO2 while allowing the passage of other gases [69]. To regenerate the sorbent material and release the captured CO2, the pressure in the vessel is reduced, which causes the sorbent to desorb the CO2. The desorbed CO2 can then be collected and stored, or used for various applications, such as in the production of synthetic fuels [70]. In VSA, CO2 adsorption occurs at ambient pressure and depressurization occurs during regeneration. One of the advantages of PSA technology is its ability to capture large quantities of CO2 over time. However, PSA/VSA technology requires a significant amount of energy, particularly for the compression and desorption steps, which increases the cost of DAC systems. Researchers and companies are actively working to develop more energy-efficient PSA and VSA processes and improve the overall efficiency of DAC systems [71,72]. The combination of temperature and vacuum swing has been used several times for DAC [9,16,73].
- Moisture swing adsorption (MSA)
Lackner [63] developed MSA to absorb CO2 from the air, alleviating the high regeneration costs of amine-functionalized sorbents by substituting the temperature and pressure swing processes. MSA uses the conventional method of ion hydration to trap CO2 from the air and release it in a controlled way [31]. The method involves coating a solid support with heterogeneous ion-exchange resin (usually polymeric materials). By using an amine-coated anion exchange resin, for example, it is possible to capture CO2 from dry air in the atmosphere and then release it when it is exposed to water [74]. MSA is interesting because it uses the latent heat of evaporation instead of the energy needed for PSA or TSA. All that is needed is a source of water, and the latent heat of evaporation provides all the energy needed to drive the process. The main problem with the process though, is that the capture must be performed in a dry atmosphere, which indicates this method may be less appropriate for humid areas. The energy consumption of moisture-swing sorbent has been estimated to be about 50 kJ/mol CO2 [75].
Comparing MSA technology to more traditional temperature or pressure swing adsorption reveals various advantages: (1) Instead of using expensive energy, low-cost water may be used to swing sorbents between sorption and desorption; (2) water sorption and CO2 sorption on the resin proceed in opposing directions, eliminating the energy penalty for sorption and desorption of water; (3) the moisture swing may simplify the system design by eliminating the need for heating and cooling devices. MSA also has its own disadvantages: (1) To keep anion impurities from contaminating the resin, the water must be relatively pure; (2) the system’s performance is affected by the weather, and hot, dry climates work best for MSA; (3) the partial pressure of the emitted CO2 is relatively low because of the moisture fluctuation, therefore requiring extra concentration processes prior to storage [76]. In the case of cold and humid environments, combining a moisture swing and a slight thermal swing might enhance the desorption performance.
The intended use of CO2 determines its purity requirement and may influence the DAC system regeneration method. If the use is for an agricultural greenhouse and a low concentration of CO2 is desired, the moisture swing adsorption technique can represent a good option. For example, CO2 can improve agricultural greenhouse plant performance by capturing the gas directly from the air outside and releasing it inside the greenhouse using adsorbents made of K2CO3 or Na2CO3.
2.3. Electrochemical Technology
Various electrochemical methods have been investigated for the capture and separation of CO2. Such techniques include direct CO2 capture using an electrochemical cell, CO2 capture via redox-active materials, and regeneration of CO2 binding sorbents for reuse. Advantages of electrochemical techniques include operating temperatures and pressures lower than temperature or temperature-vacuum swing adsorption [77] and easy integration with renewable energy sources [78].
2.3.1. Electrochemical Capture
One method for direct capture involves feeding air to one side of a polymer electrolyte membrane electrochemical cell and isolating the CO2 on the permeate side (see Figure 4). The advantage of this method is that it does not rely on a large pressure gradient like conventional membrane separation. This type of system was first reported by the National Aeronautics and Space Administration for CO2 removal from spacecrafts [79]. More recently, Muroyama et al. [80] tested commercial alkaline anion exchange membranes (AEM) for the capture of 0.1–100% CO2 in a N2 gas feed. At the cathode, CO2 reacts with OH− to form CO32− or HCO3−, which travel to the anode side. H2 was used to depolarize the anode, resulting in the formation of CO2, H2O at the outlet. Condensing the water out would leave a mixture of CO2 and residual H2, which could be useful for synthetic fuel and chemical production, as discussed in a later section. It was noted that at sub-percent levels of CO2, low faradaic efficiency and high energy consumption made decoupling the capture and CO2 isolation/sorbent regeneration step more sensible [80].
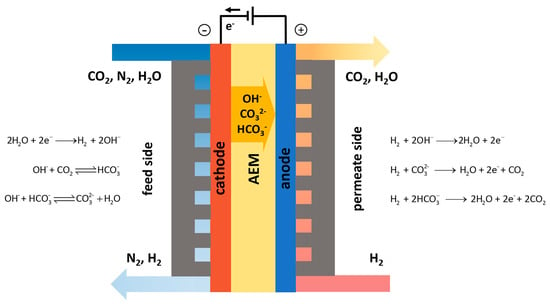
Figure 4.
Schematic of AEM CO2 separation device [80].
Another method for direct capture of CO2 uses redox-active materials. Redox-based capture has two subcategories: direct and indirect. The direct method uses a redox-active sorbent where its affinity for CO2 is electrochemically activated or deactivated. This has been called electrochemically mediated complexation separations [81]. Common sorbents studied include quinones, bipyridines, disulfides, and transition metals [81,82]. The indirect method electrochemically activates/deactivates the affinity of a redox-active competitor for the CO2-binding sorbent. When activated, the sorbent has higher attraction to the competitor than to CO2, causing the sorbent to release CO2 and bind to the competitor instead. This has been referred to as electrochemically mediated competitive complexation separation [81]. For both methods, the main steps of the cycle are activation, capture of CO2, deactivation, and desorption of CO2. Seo et al. [83] used the 1-AP nitrate to capture CO2 and then regenerate it through an electrochemical H-cell containing an anion exchange membrane separating the two (see Figure 5). The measured work required to capture from ambient air was 162 kJe/mol CO2. In a recent report by the same authors [84], the commercial organic dye, neutral red (NR), was used as a redox-active compound due to its oxygen insensitivity and was combined with a hydrotropic agent, nicotinamide (NA), to improve CO2 solubility in the aqueous solution. The estimated work required for capture from ambient air was 65 kJe/mol of CO2, which is an improvement over 162 kJe/mol CO2.
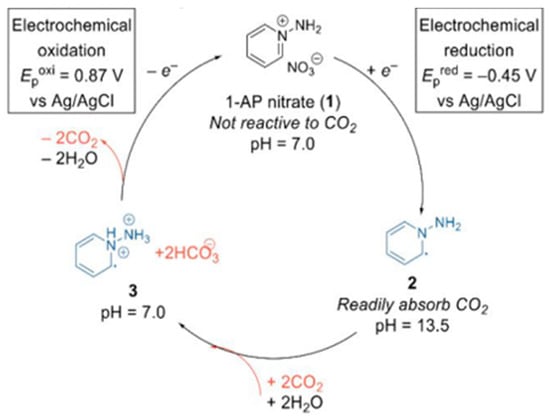
Figure 5.
Aqueous Amine and Electrochemical to capture and release CO2 [83].
2.3.2. Electrochemical Regeneration
The most common electrochemical system is to first capture CO2 using a sorbent followed by separation of CO2 and subsequent regeneration of the sorbents [77]. Electrochemical separation can involve bipolar membranes (BPM), monopolar membranes, or a combination thereof. As the most energy-demanding part of the liquid sorbent-based processes is the sorbent regeneration, which can involve heating to upwards of 900 °C, it is therefore natural for researchers to investigate alternative methods for regeneration [77]. In addition, several DAC companies are either planning to use electrochemical regeneration or exploring its use [85]. Below, we discuss monopolar and bipolar membrane electrodialysis technologies.
- Monopolar Membrane Electrodialysis
Monopolar ion exchange membranes (cationic or anionic) allow the permeation of one charge type from an electrolyte. Anion exchange membranes are positively charged and, therefore, attract and allow anions to pass through. Different arrangements using monopolar ion exchange membranes have been proposed for the electrochemical regeneration of CO2 absorption solvents for DAC, including aqueous amines or aqueous alkaline solutions [86]. Shu et al. [78] coupled CO2 capture using NaOH and absorbent regeneration using a three-chamber electrochemical stack configuration (see Figure 6). The H2-recycling electrochemical system (HRES) is connected to a membrane contactor that was first created for nitrogen recovery from wastewater. In the HRES, H+ produced from oxidized H2 reacts with the carbonate to form carbonic acid (H2CO3), which leads to CO2 desorption once the pH is low enough. NaOH regeneration occurs in the cathode compartment [78].
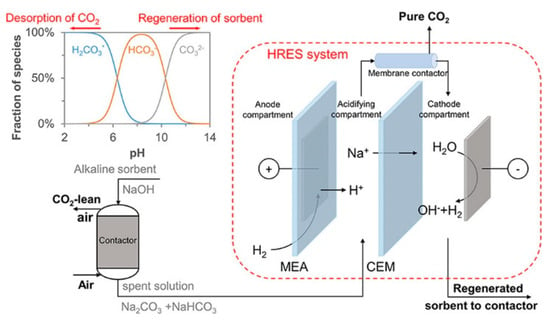
Figure 6.
Aqueous alkaline solvent regeneration and CO2 desorption in a continuous system using a H2− recycling electrochemical cell [78].
- Bipolar Membrane Electrodialysis
In the bipolar membrane electrodialysis (BPMED) process, aqueous carbonate (e.g., Na2CO3) is generated, as opposed to aqueous bicarbonate (e.g., NaHCO3) in a conventional process. The process for BPMED is shown in Figure 7. A pH of less than 10 results in NaHCO3, while a pH greater than 10 produces Na2CO3 (see Reactions (2) and (8)). NaHCO3 is then reacted with sulfuric acid to produce CO2, water, and sodium sulfate (Na2SO4) (Reaction (9)). Finally, Na2SO4 undergoes BPMED regeneration (Reaction (10)) to restart the absorption cycle.
Bipolar membranes (BPM) consist of two contacting monopolar membrane layers, one positively charged anion exchange membrane (AEM) and one negatively charged cation exchange membrane (CEM). The interfacial contact at the CEM and AEM is called the bipolar junction. BPMs can be stacked with an AEM or CEM to form a two-cell stack, or with both an AEM and CEM to form a three-cell stack. For acid-base regeneration, the aqueous products from the separation step are routed to the BPMED stack where the cells are separated into an acidic and a basic compartment [87,88]. Water molecules electro-dissociate into protons and hydroxide ions at the bipolar junction while the Na2SO4 also dissociates, and its applicable ions pass through the monopolar membrane to react with the H+ and OH-, resulting in the formation of H2SO4 and NaOH.
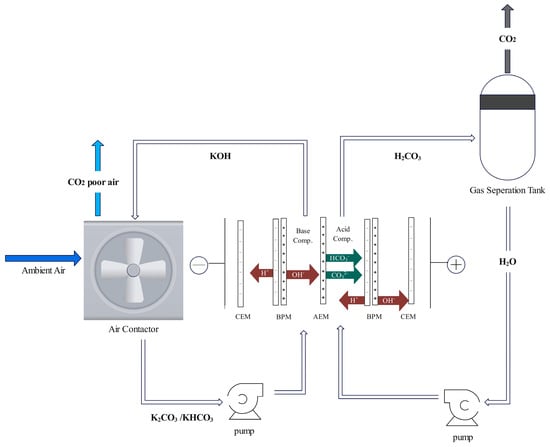
Figure 7.
Schematic of an aqueous alkali hydroxide solution regeneration with BPM cells (adapted from [88]).
BPMED technology has already been commercialized by several manufacturers mostly in the production, regeneration, or recovery of acid and base salt solutions [87]. Advantages include the ability to utilize renewable electricity sources, and the closed loop design allowing sorbent reuse, although water must be inputted into the system. Challenges with BPMED include the cost being 3−10 times that of monopolar membranes which is associated with the smaller production scale, more complicated preparation of bipolar membranes, and potential chemical instability of the AEM which can be exposed to highly alkaline conditions (pH > 11) [87,89].
While much progress has been made in electrochemical research, challenges for scale-up persist including the need to reduce membrane cost, improve faradaic efficiency, improve CO2 capacity [77,81], improve electron and charge transfer kinetics, as well as the presence of high operable current densities and membrane permselectivity [81].
3. Storage and Utilization of CO2
In order for DAC to become a true negative emissions technology, the CO2 captured must be permanently stored. Although utilizing CO2 to produce chemicals or fuels could at best be net zero, such options not only displace fossil fuels, but also may provide sufficient economic benefits to accelerate the development and deployment of DAC technologies.
3.1. Geological Storage
In order to limit the release of CO2 into the atmosphere and its greenhouse gas contribution to global warming, carbon sequestration can be used as a strategy for storing CO2 in deep geologic formations. In 2021, the CO2 Storage Resource Catalog estimated that the total potential storage capacity of CO2 resources worldwide was roughly 13,000 GtCO2 [90]. Geological carbon sequestration is the process of storing CO2 in naturally occurring pore spaces in rock formations that operate as long-term CO2storage reservoirs [91]. Ideal conditions for geological storage are sedimentary basins with a caprock above with minimum thickness of 20 m, a CO2 injection of at least 800 m deep (for CO2 to be in supercritical state) and less than 2500 m [92]. The National Academy of Sciences conducted a thorough investigation of the price of carbon storage and determined that the levelized price range would be between USD 8.6 and USD 20.4/tCO2, realizing that the cost of carbon storage is largely affected by pipeline needs and economies of scale [3].
3.2. Enhanced Oil Recovery (EOR)
Oil fields often undergo many phases of production. Natural pressure drives oil up the wells during the primary production phase. The pressure in the reservoir is raised in the secondary phase by using pumping fluids, such as water or natural gas, forcing more oil to the production wells. CO2-EOR can be used in the tertiary phase, aiming to change the characteristics of the oil or the flow pattern to boost the recovery of the remaining oil. CO2 interacts with oil, making it easier (e.g., changing the viscosity) for it to flow to the production wells. The resulting oil contains some CO2, which is recovered and re-injected back in the reservoir [93].
EOR can recover up to 15–20% of the original field, and it accounts for about 3.5% of US domestic oil production annually [94]. EOR now utilizes around 80 MtCO2/year, which is second only to the production of urea, which requires about 130 MtCO2/year. The International Energy Agency (IEA) estimates that a net 0.19 tCO2 is stored for each oil barrel produced using CO2 that has been captured [93]. According to a recent study conducted by the United States National Energy Technology Laboratory (NETL), EOR may extract over 67 billion barrels of oil from commercially recoverable deposits. This estimate was predicated on the possibility of capturing 20 Gt of CO2 from factories and other industrial sources. EOR may recover up to 40% of residual fossil fuel, while CO2 is permanently captured and stored in the reservoir [95]. However, it appears paradoxical to use captured CO2 to extract more oil that produces CO2 through combustion, especially if the CO2 is taken from the air using DAC. In this vein, the IEA has emphasized that, in order to meet the targets of the Paris Agreement, no new investments in fossil fuels should be undertaken, necessitating the discovery of alternative, economic, and environmentally sound uses for CO2 [96].
3.3. Fuels and Chemicals
The production of fuels (e.g., gasoline, diesel or jet fuel) and chemicals (e.g., methanol, methane, formic acid or formaldehyde) from captured CO2 is gaining momentum [97]. These synthetic fuels and chemicals are capable of directly or indirectly displacing traditional fossil fuels. In the case of fuels, the present transport infrastructure may still be utilized for the foreseeable future, and they might assist in decarbonizing difficult-to-abate sectors such as marine and air transport. Due to its high stability, thermodynamically unfavorable processes are needed to convert CO2 into these chemicals, implying additional energy and hence costs [1].
Synthetic fuels and chemicals, e.g., through the Fischer–Tropsch synthesis, can be produced using syngas, a combination of H2 and CO [98]. CO2 reforming (also known as dry reforming) is an alternate way to produce syngas by combining two of the most significant greenhouse gases, CO2, and methane (CH4) (see Reaction (11)) [99]. Dry reforming requires high temperatures (900–1200 K) and may suffer from soot deposition which deactivates the catalyst; thus, it is not yet widely utilized [100]. Reverse water gas shift (RWGS) is another known CO2 conversion method to syngas (see Reaction (12)), and various heterogeneous transition metal-based catalysts supported on metal oxides have been investigated between 200 and 600 °C [101,102,103].
Methanol is an important building block in the synthesis of olefins, dimethyl ether, and fuels, and is thus capable of displacing fossil fuels. In addition, methanol can be used as an energy carrier. As a liquid, it can be handled and transported more easily than gases or solids. Methanol is currently produced from syngas that, in turn, is created from fossil fuels. However, it can also be produced from CO2 in one of the simplest processes available to convert CO2 into liquid products [104,105]. Concentrated CO2 can be hydrogenated over a metal or metal oxide catalyst (such copper (Cu), zinc oxide (ZnO), or aluminum oxide (Al2O3)) at relatively low temperature and pressure, about 225 °C and 50 bar, respectively, to produce methanol (CH3OH) (see Reaction (13)). Renewable power to produce H2 is required to make the methanol manufacturing process economically and ecologically sustainable [106,107].
CO2 methanation is another viable method of using carbon dioxide [108]. Synthetic methane is an easily exportable fuel that may be used in the existing infrastructure for storage, transportation, and usage. CO2 hydrogenation to methane over nickel catalysts was proposed more than 100 years ago via the Sabatier reaction, Reaction (14). The reactant H2 can be generated from water electrolysis using grid electricity or renewable electricity [109].
Urea, formic acid, cyclic carbonates, and salicylic acid are the other most typical compounds produced from CO2 [110]. However, few studies have explored the conversion of CO2 captured from ambient air to these compounds [111].
3.4. Mineralization
Mineral carbonation involves the interaction of CO2 with metal-containing materials, such as naturally occurring minerals or alkaline industrial waste [112]. In this utilization, CO2 reacts with minerals rich in Ca and Mg to form carbonates, such as calcite (CaCO3), magnesite (MgCO3), dolomite (CaMg(CO3)2), and a host of metastable, hydrated carbonate minerals, such as hydro magnesite (Mg5(CO3)4(OH)2·4H2O), nesquehonite (Mg(HCO3)(OH)·2(H2O)), dypingite (Mg5(CO3)4(OH)2·5H2O) or hydrotalcite (Mg6Al2CO3(OH)16·4H2O). At upper crustal pressures and temperatures below 300 °C, silicate minerals react with aqueous fluids to form talc (Mg3Si4O12(OH)2), brucite (Mg(OH)2), serpentine (Mg3Si2O5(OH)4), and other hydrous phases. Excess magnesium creates brucite (Mg(OH)2) in rocks with a magnesium to silicon ratio greater than 1.5. Hydrous silicates and brucite may also combine with CO2 to generate carbonates [113]. Carbonate has a substantially lower standard Gibbs free energy than CO2, implying that CO2 storage by mineral carbonation is stable and permanent [114].
3.5. Biological Utilization
Biological utilization depends on living organisms using solar energy to convert CO2 into organic carbon during photosynthesis, which is an indirect way of carbon sequestration. This environmentally friendly process turns CO2 into value-added goods, such as bioethanol, through further processing [115].
Microalgae are seen as a potential alternative to terrestrial plants made of starchy and lignocellulosic materials for the production of biofuels, which could replace fossil fuels and reduce GHG emissions [116]. The benefits of CO2 conversion using microalgae are as follows: high photosynthetic efficiency, low concentration CO2 sequestration applicability, quicker sequestration rate, reduced land usage, co-production of food, fuel, fine chemicals, etc. The majority of microalgae culture currently relies on flue gas as a source of carbon, but NOx and SOx in the gas hinder microalgae growth [117,118]. When CO2 from DAC is used to produce microalgae, the impacts of gas toxicity are eliminated and site flexibility is increased. Additionally, the purity of CO2 used as a feedstock for microalgae growth should be in the range of 5–35 vol.%, which is lower than most alternatives for CO2 use, such as EOR and fuel synthesis, resulting in less energy-intensive processes [119].
Human food, protein-rich feed for animals, and slow-release fertilizer may all be made from autotrophic microbial biomass produced from CO2 that has been captured. These are mostly accomplished by the absorption of H2-oxidation bacteria which utilize CO2 and hydrogen to generate carbonaceous chemicals and energy carriers [120,121].
4. DAC Companies
DAC-focused companies emerged as early as 2009, but many of the current players were only founded in the last 4 years. While companies that emerged from research institutions are working to develop their innovations, others are focused on adapting existing technologies from more mature industries for DAC. In this section, we provide an overview of the technology used and level of commercialization reached of several DAC companies. Table 2 summarizes important information about the DAC companies considered here.
4.1. Liquid Sorbent Technology
4.1.1. Carbon Engineering
Carbon Engineering (CE) was founded in Calgary, AB, Canada in 2009 and has since moved their headquarters to Vancouver, BC, Canada. The company developed a technique for carbon capture that combines KOH and Ca(OH)2 solutions with a dual chemical loop similar to the one described above [26]. Since 2011, the company has been running a prototype, and since October 2015, it has been operating a demonstration plant in Squamish, BC, Canada with a capacity of 1 tCO2/day [12]. In the Permian Basin of Texas, a commercial facility with a 1 MtCO2/year collection capability is under construction and expected to be completed in late 2024. The CO2 captured will be used for Enhanced Oil Recovery [122].
The KOH aqueous solution is used in the company’s absorption process. The conventional closed counter-flow tower is replaced by an open-air contactor with a cross-flow and slab design, where air is forced horizontally and perpendicularly to the solution at a speed of 1.3 m/s. The aqueous solution flows downhill along a PVC structured packing. This new air contactor design allows for reduced fan power (hence a lower operating cost) and smaller absorber size (hence a lower capital cost). Instead of using a standard precipitator, a pellet reactor is used. The K2CO3 solution from the air contactor flows downwards and reacts with a slurry containing a 30% Ca(OH)2 injected from the bottom. Natural gas is used to heat the calciner to the regeneration temperature of 900 °C, resulting in 0.48 tonnes of additional CO2 generated by the process for every tonne of CO2 captured from the air. All the CO2 leaves the calciner at a purity of 97.1%, dry basis [12]. A fully electrical method is also being proposed by the business, in which high-temperature electrical heating would supply the thermal energy for calcination [27]. Rather than storage, CE has suggested a different method to handle the isolated CO2, which is known as “thermo-catalysis”. In this method, collected CO2 interacts with renewable hydrogen produced by water electrolysis to create synthetic fuels like gasoline, diesel, and jet fuel. CE’s pilot plant in British Columba is also testing this process.
4.1.2. Carbon Blade
Established in 2021 in San Diego, CA, USA, Carbon Blade uses technology developed by RoCo® [123] which combines a sodium hydroxide (NaOH) solution and electrodialysis bipolar membrane (EDBM) technology for regeneration [124]. All equipment, including integrated wind turbines, solar panels and rechargeable batteries to power this system, are designed to fit within or attached to the DAC container which can be placed at a carbon utilization or storage site. In 2022, the company created its first gas–liquid contactor prototype. For 2023, the company has plans for various levels of testing in Iceland and California, with a goal for their first 1000 units to be in operation in 2025. Estimated cost is near USD 100/tCO2 [124], and the energy requirement is up to 1.5 MJ/kg CO2 compared to 3.5–4 MJ/kg CO2 for liquid amine solvent systems [125].
The contactor is placed on a vertical rotor, allowing for rotation in low wind speeds much like a vertical axis wind turbine. The leaf-like contactor surface consists of sandwiched hydrophobic polymer membranes, which allows the passage of air and contact with the basic solution flowing on the inner surface of the membrane. CO2 is captured as an aqueous sodium bicarbonate and is returned to the gaseous state using a reaction with sulfuric acid (H2SO4), which simultaneously creates water and sodium sulfate with CO2. The CO2 is separated while the sodium sulfate and water move onto the regeneration step with the help of bipolar membrane electrodialysis. While the contactor design takes advantage of natural convection even in low wind speeds to reduce overall system energy requirements, this is a trade-off for the rate of capture. The company estimates that wind speeds of 7–8 km/h are required to remove 1 tCO2/day for a contactor surface area of 200 m2 [125]. In addition, this liquid sorbent technology is likely to suffer the same concern of freezing conditions. Also, because water is an input into the system, this design is less suitable for arid locations.
4.1.3. Greenlyte Carbon Technologies
Greenlyte Carbon Technologies (GCT) is a spinoff from the Universität Duisburg-Essen in Germany. Founded in 2022, the company plans to complete its first 100 tCO2/year demonstration plant named Greenberry 2 by September 2023 [126], install their first commercial demonstration plant in 2024, and partner with a hydrocarbon synthesizer to commission a 1 ktCO2/year plant in 2026 [127]. GCT plans to build modular containerized systems that can be used for direct air capture or flue gas capture with a capture rate of 10 kg/h. The absorbent formulation can be adjusted to a specific CO2 concentration.
In the GCT process, CO2 is first absorbed with a liquid sorbent mixture consisting of water, polyethylene glycol (PEG) or polyols, and potassium carbonate, sodium carbonate, amino acids or mixtures of these components, to form a hydrogen carbonate. The liquid mixture can be tailored to operate within −30 °C to 50 °C. The hydrogen carbonate then undergoes either two-chamber or three-chamber electrolysis to generate gaseous oxygen, carbon dioxide, and hydrogen, or releases the CO2 by applying waste heat [127]. The advantage of the three-chamber bipolar membrane process is that all gaseous products are generated within separate chambers, avoiding the need for a post-separation process. Alternatively, two-chamber electrolysis with a Ni(OH)2 anode results in the O2 binding to the electrode and only CO2 and H2 being initially released, which can be used in synthetic hydrocarbon production.
4.1.4. Mission Zero Technologies
Mission Zero was founded June 2020 as a spinout from Deep Science Ventures in London, Great Britain, that is currently focused on utilizing off-the-shelf technologies for fast DAC deployment. By 2024, they expect to have an integrated 1000 tCO2/year capture and storage plant utilizing carbon storage company 44.01′s peridotite mineralization technology in the Al Hajar mountains in Oman. Project DRIVE in the UK is another planned 120 tCO2/year pilot capture and storage plant, integrating capture and manufactured limestone (M-LS) production using the process of O.C.O Technology [128].
The company uses an aqueous mixture containing cationic polymeric amine, polyethyleneimine (PEI), to absorb CO2. The CO2 reacts with water to form carbonic acid and subsequently dissociates into H+ and HCO3−. Carbonic anhydrase or a zinc-cyclen catalyst in the solution accelerates the ion formation process. The contactor is a gas sparger or a falling film reactor [129]. After leaving the contactor, the absorbent solution enters the electrochemical regeneration step where it passes over anion and cation exchange electrolyte beads to dissociate the H+ and HCO3− from the capture solution. The ions then pass through their respective ion-exchange membranes into a second solution using either capacitive deionization ion-separation or electrodialysis. Both of these technologies are well established in the water purification industry. As the concentration of ions increase in the non-aqueous second solution, chemical equilibrium causes the ions to reform carbonic acid which dissociate into CO2 gas and water. The CO2 can be isolated at this point with a purity of 98% [130]. At least some of the anions in the second solution react with a mineral or salt to form a precipitated material that is released from the second solution. The core process energy consumption is less than 800 kWh/tCO2 [131].
4.2. Solid Sorbent Technology
4.2.1. Climeworks
Climeworks, a Swiss company that is a spinoff of ETH Zurich established in 2009, is a significant DAC development company [16].The company progressed from creating its initial prototype in 2013 to operating the first commercial facility in the world in 2017, with a 900 tCO2/year capture capability, in Hinwil, Switzerland. The Climeworks design is scalable and modular, and one collector can capture 135 kgCO2/day [132]. A Class II amine-functionalized sorbent, such as 3-aminopropylmethyldiethoxysilane (APDES), loaded upon nano fibrillated cellulose (NFC), is used by Climeworks (APDES-NFC-FD). By using temperature vacuum swing adsorption (TVSA) and waste heat from a nearby incinerator, regeneration is accomplished at a temperature of 100 °C. A complete cycle lasts 4–6 h. Another commercial facility which began operation in September 2021, dubbed Orca, is located in Hellisheidi, Iceland, and is able to capture and store CO2 at 700 m underground with a capacity of 4000 tCO2/year. The captured CO2 is combined with water and pumped underground, where it interacts with basalt rocks via mineral carbonation. Climeworks collaborates on mineralization with the Icelandic company, Carbfix, which specializes in underground CO2 mineralization. For the regeneration step of the adsorbent bed, waste heat from a geothermal power plant is used. Including its 14 pilot plants [73] across Europe, the company’s total operating plant capacity is 6900 tCO2/year) [14], with another 36,000 tCO2/year plant (Mammoth) expected to complete construction in 2024. The plants use renewable energy or waste heat to capture CO2 for storage, fuel, food, and beverage manufacturing. For example, the 150 tCO2/year pilot plant in Troia, Italy utilizes the CO2 to make methane, while the plant in Dresden, Germany aims to capture CO2 to create carbon-neutral fuels (mostly diesel) while also utilizing renewable energy for hydrogen generation [122]. In 2017 or prior, the company planned to have removed 225 MtCO2 (approximately 1% of total global emissions) by 2025 [48], which is highly unlikely now. As of 2019, the company was capturing at USD 600–800/tCO2 but has a roadmap to achieving USD 100–150/tCO2 by 2030 [133]. In 2022, they were selling carbon credits at USD 1000/tCO2, but this could drop to USD 300–400/tCO2 by 2030 [134]. Climeworks plans to expand operations in the US and announced in 2023 that it applied to participate in three hubs as part of the US Department of Energy’s (DOE) Regional Direct Air Capture Hubs program [135].
4.2.2. Global Thermostat
In 2010, a Columbia University professor founded Global Thermostat in New York (NY, USA), and in 2020, the company relocated to a new research and development headquarters in Colorado. The company uses a Class I amine-functionalized polymer (most recent patents use poly(allylamine) or poly(vinyl amine)) [136] on a porous ceramic honeycomb monolith structure for capture. The regeneration ideally uses waste heat from nearby industry and is based on TVSA with a desorption temperature of 60 °C to 120 °C, but preferably below 100 °C [136,137], and a pressure of 0.2 bar or below [138]. The process produces CO2 with a purity greater than 95% [139]. To date, the company has had four pilot plants from 2011 to 2021 [140], with the first located at the Stanford Research Institute (SRI) International in Menlo Park (California), having a capacity of 1000 tCO2/year [48]. In April 2023, they unveiled a 1000 tCO2/year commercial-scale demonstration plant in Colorado; however, the site does not yet have the capability to store the CO2 and is therefore venting any captured material [141]. Future plans include a 100,000 tCO2/year capacity plant (front-end engineering and design (FEED) is already underway) [140] and potentially a small DAC unit in Chile [141]. While the cost for their former pilot plant in Huntsville, Alabama was previously quoted as USD 150/tCO2 [142], costs have since increased as the company now believes they can reach USD 300/tCO2 in 2025 [143]. Cost and energy consumption for the Colorado plant have not been disclosed.
4.2.3. Hydrocell and Soletair Power
Hydrocell, located in Järvenpää, Finland, was created in 1993 and initially focused on fuel cell technology but eventually led to developments in DAC and heat exchangers. The company’s technology consists of a HCell brush-type heat exchanger and regenerative CO2 scrubber using amine-functionalized polystyrene spherical beads. The system fits into a conventional shipping container and can capture 1.387 tCO2/year. A TVSA cycle is used, where the adsorption lasts approximately 20 h, followed by 15 min of air evacuation, and heating using a glycol–water heat exchanger system for 30 min [144]. Their TVSA regeneration temperature ranges from 70 °C to 80 °C (the lowest value among the other systems considered) [145] and a vacuum of 5 mbar is used [146]. From 2017 to 2018, the company supplied their DAC system for the Soletair demonstration plant led by the Lappeenranta-Lahti University of Technology (LUT) and the VTT Technical Research Centre of Finland. The plant incorporated a solar power system, DAC system, electrolysis hydrogen production, and synthetic fuel production using a Fischer–Tropsch process. The goal after the piloting phase was to use the synthesis units in future EU projects [147]. Hydrocell is partners with Soletair Power. Established in 2016, Soletair Power uses technologies developed by LUT and the VTT Technical Research Centre of Finland. The company is focused on integrating DAC into commercial building HVAC systems, both indoors and outdoors. The CO2 is stored in containers and supplied to concrete manufacturers for permanent storage. The company is also involved in utilization technology development. In 2021, they demonstrated a compact methane production module at Expo 2020 Dubai and installed an outdoor DAC unit at the ZBT hydrogen and fuel cell center in Duisburg, Germany in 2022. In 2023, they installed their HVAC-integrated DAC system at seed funding company Wärtsilä’s Sustainable Technology Hub building in Vaasa, Finland. The HVAC-integrated system can capture up to 20 tCO2/year [148].
4.2.4. Skytree
Skytree was created in 2014 in Amsterdam, The Netherlands, as a spinoff from the European Space Agency incubator program. The capture process, using benzylamine-based ion-exchange resin beads supported on crosslinked polystyrene, is based on electrostatic absorption and moisturizing desorption at 60 to 80 °C, utilizing low-grade heat [149]. The company currently plans to offer two modular models with capacities of 10 kg/day and 200 kg/day. Target utilization include enhancing growth in controlled horticultural environments, algae cultivation, and building material mineralization [150]. In July 2023, the company announced its first commercial DAC units for Growy vertical farms in the Netherlands after successfully completing field tests [151].
4.2.5. Carbon Collect
Carbon Collect Limited, formerly Silicon Kingdom Holdings, was founded in 2018 in Dublin, Ireland. The company’s Mechanical TreeTM technology was developed by Professor Klaus Lackner, director of the Center for Negative Carbon Emissions at Arizona State University (ASU). The Mechanical TreeTM is a tower over 10 m tall consisting of 150 stacked sorbent layers designed to allow the passage of wind through them, foregoing the need for powered air flow. Each stack is about 1.5 m (5 ft) in diameter and holds six “leaves” [152]. For regeneration, the tower retracts into the 2.7 m (9 ft) tall base. The system is designed to use TVSA, MVSA, or MTVSA depending on the climate conditions. The sorbent is an anionic exchange resin such as quaternary ammonium functionalized poly(arylene ether sulfone) with iodide counterion [153] or quaternary ammonium functionalized polystyrene [154]. For MVTSA, the chamber is first partially evacuated to remove air contamination and then the water in the inner conduit is heated to 40–100 °C to create sub-atmospheric steam to release the CO2 [155]. The CO2-rich gas (5 kPa [155], or about 1% concentration [156]) and water vapor are collected. The CO2 is then isolated by reacting CO2, with sodium carbonate to form sodium bicarbonate [156]. NaHCO3 may then undergo heating [156] or an electrochemical process to regenerate and release the CO2 [154] at a purity between 95% and 99.9% [157]. The remaining water vapor is condensed, and energy is recovered. Depending on the ambient wind, sorption may take 30 to 60 min.
The first commercial-scale machine was installed at ASU’s Tempe campus in Q1 2022 with a capacity of 90 kg/day if ran continuously, and the company plans to initially deploy farms with a 1000 tCO2 capacity [152]. A total of 250 large-scale Mechanical TreeTM farms can capture 1 GtCO2 [158]. While the company is currently aiming for below USD 100/tCO2 at full commercial scale, Lackner believes his technology could eventually bring the cost of carbon down to USD 30/tCO2 [159]. Using MVTSA, the system consumes less than 70 kJe/mol or 440 kWh/tCO2 [155].
4.2.6. Carbon Capture
Founded in 2019 in Los Angeles, CA, USA, Carbon Capture Inc. is focused on deploying deeply modular DAC systems designed for high-volume manufacturing and quicker development cycles. In October 2021, the company announced a feasibility study for a planned pilot project at the proposed Tamarack nickel mine near Duluth, Minnesota [160]. In September 2022 they announced Project Bison, a partnership with Frontier Carbon Solutions, to capture 5 MtCO2/year by 2030 and inject it into Class VI wells for permanent storage in deep saline aquifers in Wyoming [161]. While the company has patents for using zeolites as their adsorbent, they have intentionally designed their system to accept a variety of sorbents including but not limited to amines and MOFs [162]. The company uses at least 25% by volume of CO2 as the heat transfer medium for the temperature vacuum swing regeneration of the zeolites. Dry CO2 is produced by condensing the moisture from the gas [163].
The DAC system runs at minimum in pairs to overcome the hydrophilic nature of zeolites. The incoming air first passes through a desiccant containing rotating wheel to remove 80–95% of the moisture from the air before the first DAC module captures the CO2 and the remaining moisture. The desiccant is regenerated via a reverse dry air swing process where the dry air exiting a second DAC module passes through the first desiccant wheel; this method takes advantage of the water concentration difference between the desiccant and the exiting air stream rather than thermal energy. The desiccant wheel from the second stream is regenerated by the air exiting the first DAC module. The moisture from both desiccants is released back into the ambient air [164].
4.2.7. Verdox
As a spinoff from the Massachusetts Institute of Technology, Professor T. Alan Hatton, a specialist in electrochemical processes and separation, and Dr. Sahag Voskian first published their technology in 2019 in Energy & Environmental Science [82,165] and subsequently formed Verdox to commercialize their electro-swing adsorption technology. In early 2021, the company began testing its technology with aluminum manufacturer Norsk Hydro with a goal of reaching industrial scale by 2030. Norsk Hydro has a minority ownership of Verdox [166].
Verdox uses a reduced polyanthraquinone adsorbent in a sandwich electrode structure. In each sandwich center is a ferrocene electrode layered between two electrolyte membrane separators, followed by a quinone electrode on either side. Each electrode is synthesized by impregnating a non-woven carbon fiber mat with either a quinone– or ferrocene–carbon nanotube–polymer solution. The porous electrodes are assembled and lastly moistened with a liquid salt electrolyte. CO2 adsorption is triggered when voltage is applied, initiating a redox reaction where the ferrocene is oxidized and polyanthraquinone is reduced. In its reduced state, the quinone has a high affinity for CO2, and this affinity is reversed by applying voltage in the opposite direction to release the CO2 [167].
Lab tests showed a capture rate of 90% for CO2 concentrations of 0.6 to 10%, a capture capacity drop of 30% over 7000 charge–discharge cycles, and the energy use of 1 GJ/tCO2 captured or 40–90 kJ/mol CO2 [82]. Projected capital and operating costs are USD 50-100/tCO2 [167].
4.2.8. TerraFixing
TerraFixing is a Canadian company founded in 2020 in Ottawa, ON, Canada. The company utilizes proprietary zeolites for CO2 adsorption with a capture capacity of approximately 1.0–2.5 mmol/g, where capacity increases as temperature decreases [168]. Their zeolites also tout a lower heat of sorption than Climeworks, Global Thermostat and Heirloom, reducing energy requirements to 1 MWh/tCO2 [168]. If electricity cost is USD 0.03/kWh or less, the company can capture CO2 for USD 40/tCO2 or less [169]. To take advantage of thermodynamics, the company is focused on deployment in cold and dry climates including Canada, Norway, Alaska, Russia, Finland, Greenland, Tibetan plateau, and Antarctica. Access to and price of renewable energy varies widely between these locations.
Incoming air passes through a particulate filter, water capture bed, and CO2 adsorbent bed. Desiccant in the water capture bed dehumidifies the air to ensure water is not adsorbed by the zeolites. A five-step cycle is used: adsorption, blowdown, evacuation, pressurization, waterbed regeneration. The blowdown removes weakly adsorbed components of N2, O2, and Ar by applying a vacuum, the evacuation steps release the adsorbed CO2 via a temperature–vacuum swing, and then dry air is pushed through the adsorbent bed in the reverse direction to return the bed back to ambient pressure. This dry air picks up the residual heat from the CO2 desorption step and flows through the water capture bed to regenerate the desiccant [170].
4.2.9. Noya
Noya was founded in 2020 in San Francisco, CA, USA. The company’s initial aim was to take advantage of various types of existing cooling tower infrastructures ranging from commercial buildings to industrial cooling towers. In August 2021, Noya’s first pilot plant was installed in San Leandro, California at Alexandre Family Farm, where an estimated 0.55–1.1 tCO2/year is captured as heat is removed from the dairy pasteurization process [171]. CO2 captured using this method costs less than USD 100/tCO2 [172]. In 2022, the US Inflation Reduction Act increased the incentive for geologic storage to USD 180/tCO2 for companies that capture 1000+ tCO2 per deployment, which resulted in Noya pivoting towards developing modular DAC systems in Q4 2022, which can be sited at geologic storage locations [173].
Despite the pivot, aspects of their technology have not changed. The DAC system utilizes activated carbon monolith supports [174] for their sorbent, and a temperature–vacuum swing is used for regeneration. The sorbent material may include MgO, Al2O3, K2CO3, activated carbon, monoethylamine, glycine or sarcosine.
4.2.10. Heirloom Carbon Technologies
Heirloom was founded in April 2021 in San Francisco, CA, USA. The company uses alkaline solid Ca(OH)2 powder (limestone) on large trays, stacked vertically to minimize land area requirements and increase air-to-sorbent contact. By using natural convection, they avoid using fans to force air through the contactor, which reduces energy use to less than 0.05 GJ/tCO2 from 0.5–1 GJ/tCO2 [175]. The capture and regeneration processes follows a similar method as that of aqueous Ca(OH)2, where CaCO3 is formed upon reaction with CO2 and then undergoes calcination at 900 °C and hydration regeneration steps (see Reaction(4)). Where their process differs is in the use of an electric kiln to avoid additional generation of CO2 from direct fossil fuel burning in their process [176].
The carbonation process requires less than 3 days for 85% carbonation, even at low wind speeds. Heirloom has a long-term target of USD 50/tCO2 removed [177], and while they currently use 2500 kWh/tCO2, they have targeted total energy requirement of less than 1500 kWh/tCO2, or 54 GJ/tCO2 [175,178]. Key areas of interest for deployment in the US based on existing low-carbon power plants, power plant capacities, and limestone sources include San Francisco, Columbia River Valley in Washington, and East Texas and Louisiana. The humidity of these regions is also advantageous due to the ability to accelerate the rate of CO2 uptake [48]. In February 2023, captured CO2 from Heirloom’s headquarters in Brisbane, California was permanently stored in concrete in partnership with CarbonCure and Central Concrete. In March 2023, non-profit science and technology development company Battelle in collaboration with Heirloom and Climeworks submitted a proposal to the US DOE for Project Cypress DAC Hub in Louisiana [179].
4.2.11. Sustaera
Sustaera spun off from the US research and development company Susteon in June 2021 and is based in Durham, NC. The company utilizes a low-cost sodium carbonate-based adsorbent on a monolith substrate. The monolith reduces pressure drop during capture, reducing the power required to move air through their contactor. While their current bench scale unit captures 1–2 kgCO2/day, their commercial units capture about 8 tCO2/day or less than 3000 tCO2/year. Each unit contains 16 modules and each module contains approximately 400 monoliths. A regeneration temperature of ~80 °C is used and the systems use only solar and wind energy. The adsorbent has a desorption energy requirement of about –65 kJ/mol. The company has chosen to use widely available materials so that no new infrastructure needs to be developed. Its first 300 tCO2/year pilot project is underway, with expected operation in 2023. In 2024, they plan to operate 3000 tCO2/year commercial units and continuously scale up from there. While their first module cost is about USD 175/tCO2, they expect to reduce that to USD 100 or less at commercial scale [180].
4.2.12. Octavia Carbon
Founded in 2022, Octavia Carbon is the Global South’s first DAC company, headquartered in Nairobi, Kenya. The company leverages Kenya’s basaltic geology and highly renewable powered grid, including geothermal and hydropower, which make it ideal for both CO2 capture and carbon mineralization. Its Wangari 1 pilot plant is planned to be operational by 2024, with a capacity of 1000 tCO2/year [181]. In July 2023, the company also announced another 1000 tCO2/year pilot called Project Hummingbird, in partnership with carbon mineralization company, Cella, to be built in Kenya’s Rift Valley [182]. Once scaled to 40,000 tCO2/year, the expected carbon credits sale price is between USD 300 and 500/tCO2 [183]. Few technological details have been disclosed; however, it is speculated that it will be very similar operationally to Climeworks’ Orca plant in Iceland, utilizing waste heat from geothermal power production for the regeneration process.

Table 2.
DAC Company Information Summary.
Table 2.
DAC Company Information Summary.
| Sorbent State | Company | Sorbent | Desorption Method | Capacity (CO2/year) 1 | Capture Cost (USD/tCO2) | Country of Origin | Representative Patent(s) 2 |
|---|---|---|---|---|---|---|---|
| Liquid | Carbon Engineering | KOH | Calcination | 365 t; 500 kt-1 Mt (2024) | USD 94–232 | Canada | [184,185] |
| Liquid | Carbon Blade | NaOH | EDBM | 1000 units (2025) | ~USD 100 | United States | [125] |
| Liquid | Greenlyte Carbon Technologies | PEG or polyols, and K2CO3, Na2CO3, amino acids or mixtures of these | Electrolysis | 100 tCO2 (Q4 2023); 1 ktCO2 (2026/2027) | Unknown | Germany | [186] |
| Liquid | Mission Zero Technologies | PEI (polyethyleneimine) | Electrochemical | 1000 tCO2 (2024) | Unknown | Great Britain | [129] |
| Solid | Climeworks | Amine-functionalized nanofibrillated cellulose | TVSA | 6.9 kt; 42.9 kt (2024) | USD 600–800; USD 100 (2030) | Switzerland | [187,188,189] |
| Solid | Global Thermostat | Aminopolymer | TVSA | 1 kt CO2 3; 100 kt CO2 (under FEED) | USD 300 (2025) | United States | [136,137,138,139] |
| Solid | Hydrocell/ Soletair Power | Amine-functionalized polystyrene spherical beads | TVSA | 20 t | Unknown | Finland | [145,190] |
| Solid | Skytree | Benzylamine-based ion-exchange resin beads | TSA | 10 Mt cumulative capture (2030) | Unknown | Netherlands | [191] |
| Solid | Carbon Collect | Ammonium functionalized polymer | MVTSA (TVSA and MVSA capable) | 33 t | USD 100 target | Ireland | [153,154] |
| Solid | Carbon Capture | Zeolites | TVSA | 5 Mt (2030) | United States | [163,164] | |
| Solid | Verdox | Polyanthraquinone | Electroswing adsorption | Unknown | USD 50–100 | United States | [191,192,193] |
| Solid | TerraFixing | Zeolites | TVSA | Unknown | USD 40 4 | Canada | [170] |
| Solid | Noya | May include MgO, Al2O3, K2CO3, activated carbon, monoethylamine, glycine or sarcosine. | TVSA | ~1 t | <USD 100 5 | United States | [194] |
| Solid | Heirloom | Limestone Ca(OH)2 | TSA | Unknown | USD 50 target | United States | [195] |
| Solid | Sustaera | Sodium carbonate supported on monolith | TSA | 300 ton (2023); 3000 ton (2024) | USD 175; ≤USD 100 target | United States | [196,197] |
| Solid | Octavia Carbon | Unknown | Not disclosed, likely TSA | 1000 tCO2 (2024) | USD 300–500 | Kenya | Not available |
1 Capacity may include current and planned capacity. 2 Active or pending patents applicable to DAC. Not an exhaustive list of patents. 3 Only includes most recent pilot plant. 4 Assuming electricity cost of USD 0.03/kWh. 5 Cost prior to company pivoting business plan. See section on company.
5. DAC Energy Demand and Cost
Reducing energy demand and cost is essential for a large-scale deployment of DAC. Both gross cost of capture and the net removal cost of capture may be reported in the literature [3,48]. Gross cost of capture is the cost per unit of CO2 at which the CO2 must be sold in order to cover the process of removal. The net removal cost of capture takes into account the net life-cycle emissions of the capture process, including upstream emissions and is, therefore, higher than gross cost [48].
The key variables that affect DAC cost are described below:
- Capital cost, which involves the cost of equipment and the cost of land. The plant’s capacity affects these factors.
- Operating costs are the costs associated with running a business, or with running a machine, part, piece of equipment, or facility. Energy, equipment maintenance, and labor costs are critical elements in DAC plants.
- Every technology has certain unique costs attached to it. The cost of the solid sorbent and the lifetime of solid sorbent are crucial in solid sorbent technology. Since reaction efficiency is never 100%, liquid technology requires make-up inputs, such as water and sorbent [12]. In DAC plants, the air contactor’s design is also crucial. Adding several fans to increase the air velocity in the air contactor improves the plant’s performance but adds to the capital and operating cost.
The mean energy requirement for operation of either solid sorbent or liquid solvent DAC methods consists of approximately 80% thermal energy and 20% electrical energy; therefore, the source of energy is a major aspect influencing both net carbon removal and the cost of DAC systems [73,198]. For solid sorbent systems, estimated heating requirements are 2.9–5.5 GJ/tCO2 and the electricity requirements are 0.6–1.1 GJ/tCO2 [14]. The largest consumer of energy in solid sorbent systems is the regeneration process, including thermal energy to heat both the sorbent and contactor, and electrical energy for the vacuum pumps to remove residual air from the contactor, if VSA/TVSA is used. It has been estimated that sorbents with lower regeneration energies may decrease thermal energy needs by 3 GJ/tCO2, with certain sorbents claiming regeneration energies as low as 1 GJ/tCO2 [12,198,199,200]. The contactor fans, which are needed to counteract the pressure loss though the contactor, are also a major consumer of electricity [3], although it should be noted that some DAC companies have purposely designed contactors relying on natural ambient convection to eliminate this power demand [124,152,177].
Thermal energy needs for the liquid solvent systems have been reported to range from 5.25 to 8.1 GJ/tCO2, with electricity requirements of 1.3 to 1.8 GJ/tCO2 [14]. The range of energy requirements stems from differences in the packing material and contactor configuration utilized in the liquid solvent DAC process [12,201]. The highest energy usage in conventional liquid DAC processes occurs during the thermal regeneration of CaO in the calciner which produces high-purity CO2. The energy to dry and warm the CaCO3 pellets prior to feeding into the calciner comes in second [3]. Electrochemical systems may be able to reduce the cost of regeneration for liquid solvent systems. For example, the calciner in Carbon Engineering’s pilot plant uses 5.25 GJ/tCO2 released from calcination [12], whereas the minimum separation energy needed for BPM is ~2.73 kJ/tCO2 for bicarbonate and ~5.45 kJ/tCO2 for carbonate [81].
Current cost estimates from DAC companies vary widely, as shown in Table 2, and not all have released the information. Companies founded earlier generally have higher reported costs. Climeworks’ first commercial plant reported an actual cost of USD 600/tCO2 [202]. Carbon Engineering’s estimated cost for a ~1 MtCO2/year plant based on their pilot is USD 94–232/tCO2 [12]. Global Thermostat has not released energy or cost information for its most recent demonstration plant in Colorado but estimates they can decrease their costs to USD 300/tCO2 by 2025 [143]. Companies established more recently have the benefit of learning from these pioneer companies and generally have lower estimated costs. TerraFixing, under cold climate conditions, has estimated USD 40/tCO2 assuming an electricity cost of USD 0.03/kWh [169]; however, securing such low electricity costs in their ideal climate zones may pose a challenge. For instance, the 2022 costs of electricity in Iceland and Finland for a >15,000 kWh consumption was USD 0.079 and USD 0.16, respectively, with all taxes and fees included [203]. Electricity costs in Canada vary widely, with Manitoba on the low end at ~USD 0.078/kWh and the Nunavut at the high end with ~USD 0.47/kWh in 2018, including fees and taxes, for residential consumption of 2000 kWh [204]. Assuming large consumer rates are half of residential rates, the rates are still above USD 0.03/kWh. Noya’s original business plan of retrofitting cooling towers with DAC modules was estimated to be less than USD 100/tCO2 [172], but changing their business plan towards the deployment of DAC at geologic storage sites will likely increase capital costs.
In addition to company-specific cost estimates, several research institutions have evaluated cost estimates for different DAC technologies, as listed in Table 3. Comparing the upper limit of estimated costs, modeling shows that liquid sorbent-based DAC has the potential to cost more than solid sorbent-based DAC. Given the high energy demand to run DAC, the cost of different energy sources has also been examined in the literature (see Table 4). For instance, the modeled net removal cost of capture for a liquid DAC system using electricity from wind and heat from natural gas is USD 156–293/tCO2, and for a a solid DAC system it is USD 113–326/tCO2. Using natural gas for both electricity and heating provides USD 199–357/tCO2 for liquid DAC systems and USD 124–407/tCO2 for solid DAC systems [3]. If an electric calciner is used in a liquid DAC system, the estimated cost using geothermal power is USD 270–480/tCO2 [205]. While emission-free power sources like geothermal, solar, wind, and nuclear energy are desirable, plant location may dictate the use of natural gas or coal for the provision of heat, particularly for high-temperature regeneration processes like calcination [27]. The rise of hydrogen as a fuel source could eventually replace fossil fuels, but it only makes sense to do so if it is generated from a carbon-free source. For solid sorbent systems, waste heat from other industrial processes could be used, but this may not align with storage locations. Also, if the industrial process emits CO2, then flue gas capture makes more sense.

Table 3.
Summary of DAC cost from research organizations.

Table 4.
Cost comparison based on source of electricity and thermal energy.
6. Conclusions and Outlook
A review of various DAC technologies was performed. Solid sorbent DAC is distinguished by its cyclic adsorption–desorption process and high sorbent flexibility, where considerations must be made for sorbent cost, CO2 selectivity and degradation. Its low temperature regeneration requirement means thermal energy may be supplied by low-grade waste heat or renewable-based heat. In comparison, conventional liquid solvent DAC can run as a continuous process but has a more complex regeneration system requiring high temperatures. Water is a possible by-product isolated from solid DAC, whereas water is lost prior to the calcination stage of liquid DAC systems and, therefore, requires makeup water. While current solid sorbents rely mostly on micro- and mesopore structures to capture CO2, the high customizability of MOFs could enable other mechanisms to improve capture capacity and CO2 selectivity. Nonetheless, a balance of cost and performance needs to be considered and sorbent technology as a whole will benefit from further research to improve both these aspects. Electrochemical technologies are a promising alternative to the high-temperature regeneration process in liquid DAC systems, and many companies are investigating this approach to reduce overall energy needs. In addition, electrochemical direct capture systems and ion-exchange resins introduce new methods to capture CO2 which use electro-swing and moisture swing desorption techniques, respectively. Air contactor design plays an important role in reducing overall pressure drop of the air passing through, thereby reducing energy and cost, while balancing surface area contact with the solvent or sorbent.
The high thermal and electrical energy requirements for DAC from non-GHG emitting sources is a major bottleneck to the success of DAC deployment. The use of an electric calciner or electrochemical regeneration could allow meeting all energy needs by electricity. More renewable plants will need to come online not only to replace fossil fuel powered generation, but to power DAC systems. Reliance on intermittent renewables alone could prove challenging without on-site storage systems. While many locations have promising capacity for renewables, location access and grid access can be a challenge that governments, grid managers, and companies will need to consider and plan ahead for.
In the last 4 years, there has been a significant increase in the number of companies developing DAC. Several DAC companies have already deployed pilot-scale DAC systems and at least one commercial scale plant. These systems have demonstrated the feasibility of DAC technology and have provided valuable data for future research and development. This research will be critical in reducing the current high cost of DAC systems. The entry of more DAC companies could benefit the industry through the introduction of novel technology, repurposing of established technologies, and opportunity for research partnerships.
Scaling up DAC systems to the level required to make a significant impact on global CO2 emissions remains a significant challenge. To remove 10 GtCO2/year, the current total capacity of DAC projects in development will need to be 2000 times the current figure. Major funding for the industry and political support, such as the US Inflation Reduction Act of 2022, are vital to reaching the scale required as DAC companies are incentivized to build projects with an annual capacity of 1000 tonnes or greater in order to receive the tax credit of USD 180/tCO2 for geologic storage or USD 130/tCO2 for CO2 utilization. However, funding for CO2 removal technologies globally will need to increase 30 times by 2030 and by 1300 times by 2050 to achieve Paris targets [214]. Social acceptance of DAC is also a potential barrier, as it can be viewed as supporting continued fossil fuel use [1].
In conclusion, DAC technology is a promising solution to reduce atmospheric CO2 concentrations and mitigate the impacts of climate change. While the technology is still in its early stages of development, researchers and companies are making significant progress in improving the efficiency and effectiveness of DAC systems. As more DAC companies enter the industry, increased collaborations or research partnerships are also expected to improve technology and accelerate the rate at which the industry expands. The authors predict accelerated rise in research and development of new, modified, or hybrid adsorbents, and of electrochemical technologies both in the areas of direct capture and regeneration of liquid sorbents due to the ease of integration with emission-free energy sources. With increasing concern over the environmental impact of greenhouse gas emissions, DAC technology has the potential to play a critical role in achieving net-zero emissions and addressing climate change.
Author Contributions
Investigation, M.H. and V.B. (equal contribution); writing—original draft preparation M.H. and V.B. (equal contribution); writing—review and editing, E.C.; supervision, E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Environment and Climate Change Canada through the Climate Action and Awareness Fund, grant number EDF-CA-2021i010.
Data Availability Statement
This is a review paper and as such there are no new data being created.
Acknowledgments
For helpful discussions, the authors would like to thank Vanessa Schweizer, Juan Moreno-Cruz, Neil Craik, as well as Kasra Motlaghzade and Stephanie Cortinovis.
Conflicts of Interest
The authors declare no conflict of interest. This is a review paper; as such, the funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Erans, M.; Sanz-Pérez, E.S.; Hanak, D.P.; Clulow, Z.; Reiner, D.M.; Mutch, G.A. Direct Air Capture: Process Technology, Techno-Economic and Socio-Political Challenges. Energy Environ. Sci. 2022, 15, 1360–1405. [Google Scholar] [CrossRef]
- Historical CO2 Datasets. Available online: https://www.co2.earth/historical-co2-datasets (accessed on 28 March 2023).
- Board, Ocean Studies, and National Academies of Sciences, Engineering, and Medicine. Negative Emissions Technologies and Reliable Sequestration: A Research Agenda; The National Academies Press: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- Kumar, S.; Mondal, M.K. Equilibrium Solubility of CO2 in Aqueous Blend of 2-(Diethylamine)Ethanol and 2-(2-Aminoethylamine)Ethanol. J. Chem. Eng. Data 2018, 63, 1163–1169. [Google Scholar] [CrossRef]
- Li, K.; Van Der Poel, P.; Conway, W.; Jiang, K.; Puxty, G.; Yu, H.; Feron, P. Mechanism Investigation of Advanced Metal-Ion-Mediated Amine Regeneration: A Novel Pathway to Reducing CO2 Reaction Enthalpy in Amine-Based CO2 Capture. Environ. Sci. Technol. 2018, 52, 14538–14546. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Kumar, Y.; Shrivastava, S.; Patel, S.K.; Sangwai, J.S. A Review on the Recent Scientific and Commercial Progress on the Direct Air Capture Technology to Manage Atmospheric CO2 Concentrations and Future Perspectives. Energy Fuels 2023, 37, 10733–10757. [Google Scholar] [CrossRef]
- Motlaghzadeh, K.; Schweizer, V.; Craik, N.; Moreno-Cruz, J. Key Uncertainties behind Global Projections of Direct Air Capture Deployment. Appl. Energy 2023, 348, 121485. [Google Scholar] [CrossRef]
- Budinis, S. Direct Air Capture—Energy System—IEA. Available online: https://www.iea.org/energy-system/carbon-capture-utilisation-and-storage/direct-air-capture (accessed on 29 July 2023).
- Jiang, L.; Liu, W.; Wang, R.Q.; Gonzalez-Diaz, A.; Rojas-Michaga, M.F.; Michailos, S.; Pourkashanian, M.; Zhang, X.J.; Font-Palma, C. Sorption Direct Air Capture with CO2 Utilization. Prog. Energy Combust. Sci. 2023, 95, 101069. [Google Scholar] [CrossRef]
- Freyman, M.C.; Huang, Z.; Ravikumar, D.; Duoss, E.B.; Li, Y.; Baker, S.E.; Pang, S.H.; Schaidle, J.A. Reactive CO2 Capture: A Path Forward for Process Integration in Carbon Management. Joule 2023, 7, 631–651. [Google Scholar] [CrossRef]
- Garcia, J.A.; Villen-Guzman, M.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Technical Analysis of CO2 capture Pathways and Technologies. J. Environ. Chem. Eng. 2022, 10, 108470. [Google Scholar] [CrossRef]
- Keith, D.W.; Holmes, G.; St. Angelo, D.; Heidel, K. A Process for Capturing CO2 from the Atmosphere. Joule 2018, 2, 1573–1594. [Google Scholar] [CrossRef]
- Marinič, D.; Likozar, B. Direct Air Capture Multiscale Modelling: From Capture Material Optimization to Process Simulations. J. Clean. Prod. 2023, 408, 137185. [Google Scholar] [CrossRef]
- Ozkan, M.; Nayak, S.P.; Ruiz, A.D.; Jiang, W. Current Status and Pillars of Direct Air Capture Technologies. iScience 2022, 25, 103990. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef] [PubMed]
- Fasihi, M.; Efimova, O.; Breyer, C. Techno-Economic Assessment of CO2 Direct Air Capture Plants. J. Clean. Prod. 2019, 224, 957–980. [Google Scholar] [CrossRef]
- Custelcean, R. Direct Air Capture of CO2 Using Solvents. Annu. Rev. Chem. Biomol. Eng. 2022, 13, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Eisaman, M.D.; Schwartz, D.E.; Amic, S.; Larner, D.; Zesch, J.; Torres, F.; Littau, K. Energy-Efficient Electrochemical CO2 Capture from the Atmosphere. Technical Proceedings of the 2009 Clean Technology Conference and Trade Show. TechConnect Briefs 2009, 175–178. [Google Scholar]
- Castel, C.; Bounaceur, R.; Favre, E. Membrane Processes for Direct Carbon Dioxide Capture From Air: Possibilities and Limitations. Front. Chem. Eng. 2021, 3, 668867. [Google Scholar] [CrossRef]
- Goldberg, D.S.; Lackner, K.S.; Han, P.; Slagle, A.L.; Wang, T. Co-Location of Air Capture, Subseafloor CO2 Sequestration, and Energy Production on the Kerguelen Plateau. Environ. Sci. Technol. 2013, 47, 7521–7529. [Google Scholar] [CrossRef] [PubMed]
- Sifat, N.S.; Haseli, Y. A Critical Review of CO2 Capture Technologies and Prospects for Clean Power Generation. Energies 2019, 12, 4143. [Google Scholar] [CrossRef]
- Quang, D.V.; Milani, D.; Abu Zahra, M. A Review of Potential Routes to Zero and Negative Emission Technologies via the Integration of Renewable Energies with CO2 Capture Processes. Int. J. Greenh. Gas Control 2023, 124, 103862. [Google Scholar] [CrossRef]
- Lackner, K.; Ziock, H.; Grimes, P. Carbon Dioxide Extraction from Air: Is It an Option? Los Alamos National Lab. (LANL): Los Alamos, NM, USA, 1999.
- Baciocchi, R.; Storti, G.; Mazzotti, M. Process Design and Energy Requirements for the Capture of Carbon Dioxide from Air. Chem. Eng. Process. Process Intensif. 2006, 12, 1047–1058. [Google Scholar] [CrossRef]
- Sodiq, A.; Abdullatif, Y.; Aissa, B.; Ostovar, A.; Nassar, N.; El-Naas, M.; Amhamed, A. A Review on Progress Made in Direct Air Capture of CO2. Environ. Technol. Innov. 2023, 29, 102991. [Google Scholar] [CrossRef]
- Keith, D.W.; Ha-Duong, M.; Stolaroff, J.K. Climate Strategy with CO2 Capture from the Air. Clim. Change 2006, 74, 17–45. [Google Scholar] [CrossRef]
- Carbon Engineering|Direct Air Capture of CO2|Home. Available online: https://carbonengineering.com/ (accessed on 2 February 2023).
- Zeman, F. Experimental Results for Capturing CO2 from the Atmosphere. AIChE J. 2008, 54, 1396–1399. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, H.; Azarabadi, H.; Song, J.; Wu, X.; Chen, X.; Lackner, K.S. Sorbents for the Direct Capture of CO2 from Ambient Air. Angew. Chem.-Int. Ed. 2020, 59, 6984–7006. [Google Scholar] [CrossRef] [PubMed]
- El Hadri, N.; Quang, D.V.; Goetheer, E.L.V.; Abu Zahra, M.R.M. Aqueous Amine Solution Characterization for Post-Combustion CO2 Capture Process. Appl. Energy 2017, 185, 1433–1449. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, H.; Liao, X.; Armstrong, M.; Chen, X.; Lackner, K.S. Humidity Effect on Ion Behaviors of Moisture-Driven CO2 Sorbents. J. Chem. Phys. 2018, 149, 164708. [Google Scholar] [CrossRef]
- Miao, Y.; Wang, Y.; Zhu, X.; Chen, W.; He, Z.; Yu, L.; Li, J. Minimizing the Effect of Oxygen on Supported Polyamine for Direct Air Capture. Sep. Purif. Technol. 2022, 298, 121583. [Google Scholar] [CrossRef]
- Barzagli, F.; Giorgi, C.; Mani, F.; Peruzzini, M. Screening Study of Different Amine-Based Solutions as Sorbents for Direct CO2 Capture from Air. ACS Sustain. Chem. Eng. 2020, 8, 14013–14021. [Google Scholar] [CrossRef]
- Kiani, A.; Jiang, K.; Feron, P. Techno-Economic Assessment for CO2 Capture From Air Using a Conventional Liquid-Based Absorption Process. Front. Energy Res. 2020, 8, 92. [Google Scholar] [CrossRef]
- Brennecke, J.F.; Maginn, E.J. Ionic Liquids: Innovative Fluids for Chemical Processing. AIChE J. 2001, 47, 2384–2389. [Google Scholar] [CrossRef]
- Wang, T.; Jens, K.J. Possible Pathways for Oxidative Degradation of β-Hydroxyl Alkanolamine for Post-Combustion CO2 Capture. Energy Procedia 2014, 51, 259–266. [Google Scholar] [CrossRef]
- Hospital-Benito, D.; Moya, C.; Gazzani, M.; Palomar, J. Direct Air Capture Based on Ionic Liquids: From Molecular Design to Process Assessment. Chem. Eng. J. 2023, 468, 143630. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, X.; Bai, L.; Zhang, X.; Wang, H.; Wang, J.; Bao, D.; Li, M.; Liu, X.; Zhang, S. Ionic-Liquid-Based CO2 Capture Systems: Structure, Interaction and Process. Chem. Rev. 2017, 117, 9625–9673. [Google Scholar] [CrossRef] [PubMed]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A Systematic Review on CO2 Capture with Ionic Liquids: Current Status and Future Prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Ramdin, M.; De Loos, T.W.; Vlugt, T.J.H. State-of-the-Art of CO2 Capture with Ionic Liquids. Ind. Eng. Chem. Res. 2012, 51, 8149–8177. [Google Scholar] [CrossRef]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [PubMed]
- Krupiczka, R.; Rotkegel, A.; Ziobrowski, Z. Comparative Study of CO2 Absorption in Packed Column Using Imidazolium Based Ionic Liquids and MEA Solution. Sep. Purif. Technol. 2015, 149, 228–236. [Google Scholar] [CrossRef]
- Moitra, D.; Mokhtari-Nori, N.; Siniard, K.M.; Qiu, L.; Fan, J.; Dong, Z.; Hu, W.; Liu, H.; Jiang, D.; Lin, H.; et al. High-Performance CO2 Capture from Air by Harnessing the Power of CaO- and Superbase-Ionic-Liquid-Engineered Sorbents. ChemSusChem 2023, e202300808. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, Q.; Wang, Y.; Gao, H.; Zhang, N.; Zhou, J.; Zhang, L.; Yang, Q.; Yang, Q.; Lu, Z. Atomically Dispersed Lewis Acid Sites Meet Poly(Ionic Liquid)s Networks for Solvent-Free and Co-Catalyst-Free Conversion of CO2 to Cyclic Carbonates. Appl. Catal. B 2022, 313, 121463. [Google Scholar] [CrossRef]
- Ding, M.; Jiang, H.L. Incorporation of Imidazolium-Based Poly(Ionic Liquid)s into a Metal-Organic Framework for CO2 Capture and Conversion. ACS Catal. 2018, 8, 3194–3201. [Google Scholar] [CrossRef]
- Qiu, L.; Peng, L.; Moitra, D.; Liu, H.; Fu, Y.; Dong, Z.; Hu, W.; Lei, M.; Jiang, D.; Lin, H.; et al. Harnessing the Hybridization of a Metal-Organic Framework and Superbase-Derived Ionic Liquid for High-Performance Direct Air Capture of CO2. Small 2023, 2302708. [Google Scholar] [CrossRef] [PubMed]
- Valentine, J.; Zoelle, A.; Homsy, S.; Mantripragada, H.; Woods, M.; Roy, N.; Kilstofte, A.; Sturdivan, M.; Steutermann, M.; Fout, T. Direct Air Capture Case Studies: Sorbent System; National Energy Technology Laboratory (NETL): Pittsburgh, PA, USA, 2022. [CrossRef]
- McQueen, N.; Gomes, K.V.; McCormick, C.; Blumanthal, K.; Pisciotta, M.; Wilcox, J. A Review of Direct Air Capture (DAC): Scaling up Commercial Technologies and Innovating for the Future. Prog. Energy 2021, 3, 032001. [Google Scholar] [CrossRef]
- Barkakaty, B.; Sumpter, B.G.; Ivanov, I.N.; Potter, M.E.; Jones, C.W.; Lokitz, B.S. Emerging Materials for Lowering Atmospheric Carbon. Environ. Technol. Innov. 2017, 7, 30–43. [Google Scholar] [CrossRef]
- Zagho, M.M.; Hassan, M.K.; Khraisheh, M.; Al-Maadeed, M.A.A.; Nazarenko, S. A Review on Recent Advances in CO2 Separation Using Zeolite and Zeolite-like Materials as Adsorbents and Fillers in Mixed Matrix Membranes (MMMs). Chem. Eng. J. Adv. 2021, 6, 100091. [Google Scholar] [CrossRef]
- Thakkar, H.; Issa, A.; Rownaghi, A.A.; Rezaei, F. CO2 Capture from Air Using Amine-Functionalized Kaolin-Based Zeolites. Chem. Eng. Technol. 2017, 40, 1999–2007. [Google Scholar] [CrossRef]
- Wilson, S.M.W.; Tezel, F.H. Direct Dry Air Capture of CO2 Using VTSA with Faujasite Zeolites. Ind. Eng. Chem. Res. 2020, 59, 8783–8794. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.J. Chemically Modified Carbonaceous Adsorbents for Enhanced CO2 Capture: A Review. J. Clean. Prod. 2021, 290, 125776. [Google Scholar] [CrossRef]
- Kua, H.W.; Pedapati, C.; Lee, R.V.; Kawi, S. Effect of Indoor Contamination on Carbon Dioxide Adsorption of Wood-Based Biochar—Lessons for Direct Air Capture. J. Clean. Prod. 2019, 210, 860–871. [Google Scholar] [CrossRef]
- Kishibayev, K.K.; Serafin, J.; Tokpayev, R.R.; Khavaza, T.N.; Atchabarova, A.A.; Abduakhytova, D.A.; Ibraimov, Z.T.; Sreńscek-Nazzal, J. Physical and Chemical Properties of Activated Carbon Synthesized from Plant Wastes and Shungite for CO2 Capture. J. Environ. Chem. Eng. 2021, 9, 106798. [Google Scholar] [CrossRef]
- Sun, N.; Tang, Z.; Wei, W.; Snape, C.E.; Sun, Y. Solid Adsorbents for Low-Temperature CO2 Capture with Low-Energy Penalties Leading to More Effective Integrated Solutions for Power Generation and Industrial Processes. Front. Energy Res. 2015, 3, 123870. [Google Scholar] [CrossRef]
- Kamran, U.; Choi, J.R.; Park, S.J. A Role of Activators for Efficient CO2 Affinity on Polyacrylonitrile-Based Porous Carbon Materials. Front. Chem. 2020, 8, 558899. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.K.; Balbuena, P.B.; Zhou, H.C. Carbon Dioxide Capture-Related Gas Adsorption and Separation in Metal-Organic Frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Jeong, C.; Ansari, M.Z.; Hakeem Anwer, A.; Kim, S.H.; Nasar, A.; Shoeb, M.; Mashkoor, F. A Review on Metal-Organic Frameworks for the Removal of Hazardous Environmental Contaminants. Sep. Purif. Technol. 2023, 305, 122416. [Google Scholar] [CrossRef]
- Ding, M.; Flaig, R.W.; Jiang, H.L.; Yaghi, O.M. Carbon Capture and Conversion Using Metal–Organic Frameworks and MOF-Based Materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, T.; Dong, H.; Liu, W. Modified Metal–Organic Framework by a Novel Coordinatively Unsaturated Amine Grafting Mechanism for Direct Air Capture of CO2. Chem. Eng. J. 2023, 454, 140431. [Google Scholar] [CrossRef]
- Alexandratos, S.D. Ion-Exchange Resins: A Retrospective from Industrial and Engineering Chemistry Research. Ind. Eng. Chem. Res. 2009, 48, 388–398. [Google Scholar] [CrossRef]
- Lackner, K.S. Capture of Carbon Dioxide from Ambient Air. Eur. Phys. J. Spec. Top. 2009, 176, 93–106. [Google Scholar] [CrossRef]
- Chen, H.; Dong, H.; Shi, Z.; SenGupta, A.K. Direct Air Capture (DAC) and Sequestration of CO2: Dramatic Effect of Coordinated Cu(II) onto a Chela Weak Base Ion Exchanger. Sci. Adv. 2023, 9, eadg1956. [Google Scholar] [CrossRef]
- Wang, T.; Liu, J.; Huang, H.; Fang, M.; Luo, Z. Preparation and Kinetics of a Heterogeneous Sorbent for CO2 Capture from the Atmosphere. Chem. Eng. J. 2016, 284, 679–686. [Google Scholar] [CrossRef]
- Wang, X.; Song, J.; Chen, Y.; Xiao, H.; Shi, X.; Liu, Y.; Zhu, L.; He, Y.L.; Chen, X. CO2 Absorption over Ion Exchange Resins: The Effect of Amine Functional Groups and Microporous Structures. Ind. Eng. Chem. Res. 2020, 59, 16507–16515. [Google Scholar] [CrossRef]
- Drechsler, C.; Agar, D.W. Simulation and Optimization of a Novel Moving Belt Adsorber Concept for the Direct Air Capture of Carbon Dioxide. Comput. Chem. Eng. 2019, 126, 520–534. [Google Scholar] [CrossRef]
- Jahandar Lashaki, M.; Khiavi, S.; Sayari, A. Stability of Amine-Functionalized CO2 Adsorbents: A Multifaceted Puzzle. Chem. Soc. Rev. 2019, 48, 3320–3405. [Google Scholar] [CrossRef] [PubMed]
- Subraveti, S.G.; Pai, K.N.; Rajagopalan, A.K.; Wilkins, N.S.; Rajendran, A.; Jayaraman, A.; Alptekin, G. Cycle Design and Optimization of Pressure Swing Adsorption Cycles for Pre-Combustion CO2 Capture. Appl. Energy 2019, 254, 113624. [Google Scholar] [CrossRef]
- Siqueira, R.M.; Freitas, G.R.; Peixoto, H.R.; Nascimento, J.F.D.; Musse, A.P.S.; Torres, A.E.B.; Azevedo, D.C.S.; Bastos-Neto, M. Carbon Dioxide Capture by Pressure Swing Adsorption. Energy Procedia 2017, 114, 2182–2192. [Google Scholar] [CrossRef]
- Sreenivasulu, B.; Gayatri, D.V.; Sreedhar, I.; Raghavan, K.V. A Journey into the Process and Engineering Aspects of Carbon Capture Technologies. Renew. Sustain. Energy Rev. 2015, 41, 1324–1350. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, L.; Deng, S.; Song, C.; He, J.; Shao, Y.; Li, S. A Comparative Study on CO2 Capture Performance of Vacuum-Pressure Swing Adsorption and Pressure-Temperature Swing Adsorption Based on Carbon Pump Cycle. Energy 2017, 137, 495–509. [Google Scholar] [CrossRef]
- Beuttler, C.; Charles, L.; Wurzbacher, J. The Role of Direct Air Capture in Mitigation of Anthropogenic Greenhouse Gas Emissions. Front. Clim. 2019, 1, 10. [Google Scholar] [CrossRef]
- Wang, T.; Liu, J.; Fang, M.; Luo, Z. A Moisture Swing Sorbent for Direct Air Capture of Carbon Dioxide: Thermodynamic and Kinetic Analysis. Energy Procedia 2013, 37, 6096–6104. [Google Scholar] [CrossRef]
- Wang, T.; Lackner, K.S.; Wright, A. Moisture Swing Sorbent for Carbon Dioxide Capture from Ambient Air. Environ. Sci. Technol. 2011, 45, 6670–6675. [Google Scholar] [CrossRef]
- Lackner, K.S.; Brennan, S.; Matter, J.M.; Park, A.H.A.; Wright, A.; Van Der Zwaan, B. The Urgency of the Development of CO2 Capture from Ambient Air. Proc. Natl. Acad. Sci. USA 2012, 109, 13156–13162. [Google Scholar] [CrossRef]
- Muroyama, A.P.; Pătru, A.; Gubler, L. Review—CO2 Separation and Transport via Electrochemical Methods. J. Electrochem. Soc. 2020, 167, 133504. [Google Scholar] [CrossRef]
- Shu, Q.; Legrand, L.; Kuntke, P.; Tedesco, M.; Hamelers, H.V.M. Electrochemical Regeneration of Spent Alkaline Absorbent from Direct Air Capture. Environ. Sci. Technol. 2020, 54, 8990–8998. [Google Scholar] [CrossRef] [PubMed]
- Wynveen, R.A.; Schubert, F.H.; Powell, J.D. One-Man, Self-Contained CO2 Concentrating System; NASA: Washington, DC, USA, 1972.
- Muroyama, A.P.; Beard, A.; Pribyl-Kranewitter, B.; Gubler, L. Separation of CO2 from Dilute Gas Streams Using a Membrane Electrochemical Cell. ACS EST Eng. 2021, 1, 905–916. [Google Scholar] [CrossRef]
- Renfrew, S.E.; Starr, D.E.; Strasser, P. Electrochemical Approaches toward CO2 Capture and Concentration. ACS Catal. 2020, 10, 13058–13074. [Google Scholar] [CrossRef]
- Voskian, S.; Hatton, T.A. Faradaic Electro-Swing Reactive Adsorption for CO2 Capture. Energy Environ. Sci. 2019, 12, 3530–3547. [Google Scholar] [CrossRef]
- Seo, H.; Rahimi, M.; Hatton, T.A. Electrochemical Carbon Dioxide Capture and Release with a Redox-Active Amine. J. Am. Chem. Soc. 2022, 144, 2164–2170. [Google Scholar] [CrossRef]
- Seo, H.; Hatton, T.A. Electrochemical Direct Air Capture of CO2 Using Neutral Red as Reversible Redox-Active Material. Nat. Commun. 2023, 14, 313. [Google Scholar] [CrossRef]
- Kemp, K.W.; Ostericher, A.L.; Olmstead, D.E. Systems and Methods for Capturing Carbon Dioxide and Regenerating a Capture Solution. U.S. Patent 17/735,943, 17 November 2022. [Google Scholar]
- Sharifian, R.; Boer, L.; Wagterveld, R.M.; Vermaas, D.A. Oceanic Carbon Capture through Electrochemically Induced in Situ Carbonate Mineralization Using Bipolar Membrane. Chem. Eng. J. 2022, 438, 135326. [Google Scholar] [CrossRef]
- Pärnamäe, R.; Mareev, S.; Nikonenko, V.; Melnikov, S.; Sheldeshov, N.; Zabolotskii, V.; Hamelers, H.V.M.; Tedesco, M. Bipolar Membranes: A Review on Principles, Latest Developments, and Applications. J. Memb. Sci. 2021, 617, 118538. [Google Scholar] [CrossRef]
- Sabatino, F.; Mehta, M.; Grimm, A.; Gazzani, M.; Gallucci, F.; Kramer, G.J.; Van, M.; Annaland, S. Evaluation of a Direct Air Capture Process Combining Wet Scrubbing and Bipolar Membrane Electrodialysis. ACS Publ. 2020, 59, 7007–7020. [Google Scholar] [CrossRef]
- Huang, C.; Xu, T. Electrodialysis with Bipolar Membranes for Sustainable Development. Environ. Sci. Technol. 2006, 40, 5233–5243. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Global Warming of 1.5 °C: IPCC Special Report on Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels in Context of Strengthening Response to Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2022. [Google Scholar] [CrossRef]
- Pant, D.; Shah, K.K.; Sharma, S.; Bhatta, M.; Tripathi, S.; Pandey, H.P.; Tiwari, H.; Shrestha, J.; Bhat, A.K. Soil and Ocean Carbon Sequestration, Carbon Capture, Utilization, and Storage as Negative Emission Strategies for Global Climate Change. J. Soil. Sci. Plant Nutr. 2023, 23, 1421–1437. [Google Scholar] [CrossRef]
- Hitchon, B. Aquifer Disposal of Carbon Dioxide: Hydrodynamic and Mineral Trapping—Proof of Concept; Alberta Research Council: Devon, AB, Canada, 1996. [Google Scholar]
- Insights Series 2015—Storing CO2 through Enhanced Oil Recovery—Analysis—IEA. Available online: https://www.iea.org/reports/storing-co2-through-enhanced-oil-recovery (accessed on 16 July 2023).
- Wang, X.; Yuan, Y.; Du, Y.; Golsanami, N.; Malozyomov, B.V.; Martyushev, N.V.; Kukartsev, V.V.; Tynchenko, V.S.; Bukhtoyarov, V.V.; Wu, X.; et al. Overview of Methods for Enhanced Oil Recovery from Conventional and Unconventional Reservoirs. Energies 2023, 16, 4907. [Google Scholar] [CrossRef]
- Technology Roadmap Carbon Capture and Storage in Industrial Applications; Global CCS Institute: Melbourne, Australia, 2011.
- Net Zero by 2050—Analysis—IEA. Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 5 February 2023).
- Ghiat, I.; Al-Ansari, T. A Review of Carbon Capture and Utilisation as a CO2 Abatement Opportunity within the EWF Nexus. J. CO2 Util. 2021, 45, 101432. [Google Scholar] [CrossRef]
- Li, Z.; Das, S.; Hongmanorom, P.; Dewangan, N.; Wai, M.H.; Kawi, S. Silica-Based Micro- and Mesoporous Catalysts for Dry Reforming of Methane. Catal. Sci. Technol. 2018, 8, 2763–2778. [Google Scholar] [CrossRef]
- Jang, W.J.; Shim, J.O.; Kim, H.M.; Yoo, S.Y.; Roh, H.S. A Review on Dry Reforming of Methane in Aspect of Catalytic Properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Mustafa, A.; Lougou, B.G.; Shuai, Y.; Wang, Z.; Tan, H. Current Technology Development for CO2 Utilization into Solar Fuels and Chemicals: A Review. J. Energy Chem. 2020, 49, 96–123. [Google Scholar] [CrossRef]
- Ranjbar, A.; Irankhah, A.; Aghamiri, S.F. Reverse Water Gas Shift Reaction and CO2 Mitigation: Nanocrystalline MgO as a Support for Nickel Based Catalysts. J. Environ. Chem. Eng. 2018, 6, 4945–4952. [Google Scholar] [CrossRef]
- Kattel, S.; Liu, P.; Chen, J.G. Tuning Selectivity of CO2 Hydrogenation Reactions at the Metal/Oxide Interface. J. Am. Chem. Soc. 2017, 139, 9739–9754. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yan, B.; Chen, J.G. Catalytic Reduction of CO2 by H2 for Synthesis of CO, Methanol and Hydrocarbons: Challenges and Opportunities. Energy Environ. Sci. 2016, 9, 62–73. [Google Scholar] [CrossRef]
- Onishi, N.; Laurenczy, G.; Beller, M.; Himeda, Y. Recent Progress for Reversible Homogeneous Catalytic Hydrogen Storage in Formic Acid and in Methanol. Coord. Chem. Rev. 2018, 373, 317–332. [Google Scholar] [CrossRef]
- Dang, S.; Yang, H.; Gao, P.; Wang, H.; Li, X.; Wei, W.; Sun, Y. A Review of Research Progress on Heterogeneous Catalysts for Methanol Synthesis from Carbon Dioxide Hydrogenation. Catal. Today 2019, 330, 61–75. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Jiang, X.; Zhu, J.; Liu, Z.; Guo, X.; Song, C. A Short Review of Recent Advances in CO2 Hydrogenation to Hydrocarbons over Heterogeneous Catalysts. RSC Adv. 2018, 8, 7651–7669. [Google Scholar] [CrossRef] [PubMed]
- Patricio, J.; Angelis-Dimakis, A.; Castillo-Castillo, A.; Kalmykova, Y.; Rosado, L. Method to Identify Opportunities for CCU at Regional Level—Matching Sources and Receivers. J. CO2 Util. 2017, 22, 330–345. [Google Scholar] [CrossRef]
- Veselovskaya, J.V.; Parunin, P.D.; Netskina, O.V.; Okunev, A.G. A Novel Process for Renewable Methane Production: Combining Direct Air Capture by K2CO3/Alumina Sorbent with CO2 Methanation over Ru/Alumina Catalyst. Top. Catal. 2018, 61, 1528–1536. [Google Scholar] [CrossRef]
- Kiani, A.; Lejeune, M.; Li, C.; Patel, J.; Feron, P. Liquefied Synthetic Methane from Ambient CO2 and Renewable H2—A Technoeconomic Study. J. Nat. Gas. Sci. Eng. 2021, 94, 104079. [Google Scholar] [CrossRef]
- Gür, T.M. Carbon Dioxide Emissions, Capture, Storage and Utilization: Review of Materials, Processes and Technologies. Prog. Energy Combust. Sci. 2022, 89, 100965. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, S.Y.; Li, H.; Cai, J.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent Advances in Carbon Dioxide Utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799. [Google Scholar] [CrossRef]
- Doucet: Scoping Study on CO2 Mineralization Technologies—Google Scholar. Available online: https://scholar.google.com/scholar?cluster=12645457356333919173&hl=en&oi=scholarr (accessed on 27 July 2023).
- Kelemen, P.B.; McQueen, N.; Wilcox, J.; Renforth, P.; Dipple, G.; Vankeuren, A.P. Engineered Carbon Mineralization in Ultramafic Rocks for CO2 Removal from Air: Review and New Insights. Chem. Geol. 2020, 550, 119628. [Google Scholar] [CrossRef]
- Hanifa, M.; Agarwal, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. A Review on CO2 Capture and Sequestration in the Construction Industry: Emerging Approaches and Commercialised Technologies. J. CO2 Util. 2023, 67, 102292. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Ling, T.C.; Juan, J.C.; Lee, D.J.; Chang, J.S.; Show, P.L. Biorefineries of Carbon Dioxide: From Carbon Capture and Storage (CCS) to Bioenergies Production. Bioresour. Technol. 2016, 215, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Cheah, W.Y.; Show, P.L.; Chang, J.S.; Ling, T.C.; Juan, J.C. Biosequestration of Atmospheric CO2 and Flue Gas-Containing CO2 by Microalgae. Bioresour. Technol. 2015, 184, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Negoro, M.; Shioji, N.; Miyamoto, K.; Micira, Y. Growth of Microalgae in High CO2 Gas and Effects of SOX and NOX. Appl. Biochem. Biotechnol. 1991, 28–29, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, J.; Chen, P.; Ji, C.; Kang, Q.; Lu, B.; Li, K.; Liu, J.; Ruan, R. Bio-Mitigation of Carbon Dioxide Using Microalgal Systems: Advances and Perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1163–1175. [Google Scholar] [CrossRef]
- Wilcox, J.; Psarras, P.C.; Liguori, S. Assessment of Reasonable Opportunities for Direct Air Capture. Environ. Res. Lett. 2017, 12, 065001. [Google Scholar] [CrossRef]
- Yu, J. Bio-Based Products from Solar Energy and Carbon Dioxide. Trends Biotechnol. 2014, 32, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Pikaar, I.; de Vrieze, J.; Rabaey, K.; Herrero, M.; Smith, P.; Verstraete, W. Carbon Emission Avoidance and Capture by Producing In-Reactor Microbial Biomass Based Food, Feed and Slow Release Fertilizer: Potentials and Limitations. Sci. Total Environ. 2018, 644, 1525–1530. [Google Scholar] [CrossRef]
- Leonzio, G.; Fennell, P.; Shah, N. Analysis of Technologies for Carbon Dioxide Capture from the Air. Appl. Sci. 2022, 12, 8321. [Google Scholar] [CrossRef]
- RoCo. Available online: https://roco.global/ (accessed on 26 July 2023).
- Direct Air Capture|Carbon Blade, Distributed Direct Air Capture Solution. Available online: https://www.carbon-blade.com/ (accessed on 9 July 2023).
- Nulwala, H. Direct Air Capture of CO2 Using Leaf-like Layered Contactor Coupled with Electro Dialysis Bipolar Membrane Regeneration. 2022. Available online: https://patentimages.storage.googleapis.com/53/78/26/384c32c879f713/WO2022192501A1.pdf (accessed on 22 May 2023).
- Fueling the Circular Carbon Economy|CHEManager. Available online: https://www.chemanager-online.com/en/news/fueling-circular-carbon-economy (accessed on 25 May 2023).
- Greenlyte Carbon Technologies. Available online: https://www.greenlyte.tech/ (accessed on 25 May 2023).
- Projects Selected for Phase 2 of the Direct Air Capture and Greenhouse Gas Removal Programme—GOV.UK. Available online: https://www.gov.uk/government/publications/direct-air-capture-and-other-greenhouse-gas-removal-technologies-competition/projects-selected-for-phase-2-of-the-direct-air-capture-and-greenhouse-gas-removal-programme (accessed on 1 June 2023).
- Gobaille-Shaw, G.; Ghosh, S.; Chadwick, N. Method of Capturing a Target Species from a Gas. 2022. Available online: https://patentimages.storage.googleapis.com/bb/2e/8a/ff248227651116/WO2022195299A1.pdf (accessed on 31 May 2023).
- Not Boring Founders: Nicholas Chadwick, Mission Zeries Technologies—YouTube. Available online: https://www.youtube.com/watch?v=Ny0bJ0Ex99U (accessed on 26 July 2023).
- Our Technology—Mission Zero Technologies—Home & News. Available online: https://www.missionzero.tech/our-technology (accessed on 1 June 2023).
- Achieve Net Zero Targets with Climeworks Direct Air Capture. Available online: https://climeworks.com/ (accessed on 5 February 2023).
- Direct Action: Carbon Capture Gears up for Climate Battle. Available online: https://www.theengineer.co.uk/content/in-depth/direct-action-carbon-capture-gears-up-for-climate-battle (accessed on 9 July 2023).
- Racing against the Clock to Decarbonise the Planet—FT Channels. Available online: https://channels.ft.com/en/rethink/racing-against-the-clock-to-decarbonise-the-planet/ (accessed on 11 July 2023).
- Climeworks Confirms Expansion of U.S. Team in Coming Year to Meet Increasing Carbon Removal Industry Demand. Available online: https://climeworks.com/news/expanding-our-us-team (accessed on 21 August 2023).
- Khunsupat, R.; Jones, C.W.; Bali, S. Supported Poly(Allyl)Amine and Derivatives for CO2 Capture from Flue Gas or Ultra-Dilute Gas Streams Such as Ambient Air or Admixtures Thereof. U.S. Patent 20220040669A1, 10 February 2022. [Google Scholar]
- Eisenberger, P.; Ping, E.W.; Sakwa-Novak, M. Systems and Methods for Carbon Dioxide Capture. U.S. Patent 17/912,798, 18 May 2023. [Google Scholar]
- Eisenberger, P. Rotating Multi-Monolith Bed Movement System for Removing CO2 from the Atmosphere. U.S. Patent 9,925,488, 27 March 2018. [Google Scholar]
- Chichilnisky, G.; Moesler, F. An Improved System for Direct Air Capture of Carbon Dioxide without Movement. 2022. Available online: https://patentimages.storage.googleapis.com/66/db/b1/5530b3f310d42a/WO2022104252A1.pdf (accessed on 21 July 2023).
- “We Are Focused on Getting Direct Air Capture Deployments up and Running as Quickly as Possible”, Nicholas Eisenberger, President Global Thermostat. Available online: https://carbonherald.com/direct-air-capture-nicholas-eisenberger-president-global-thermostat/ (accessed on 11 July 2023).
- A Buzzy New Carbon Removal Plant Is Catching and Releasing CO2|Canary Media. Available online: https://www.canarymedia.com/articles/carbon-capture/a-buzzy-new-carbon-removal-plant-is-catching-and-releasing-co2 (accessed on 11 July 2023).
- Affordable Carbon Capture with a Soda on the Side|Grist. Available online: https://grist.org/article/direct-air-carbon-capture-global-thermostat/ (accessed on 11 July 2023).
- Why This New Plant Is Capturing Carbon Dioxide Just to Let It Back Out Again—The Verge. Available online: https://www.theverge.com/2023/4/6/23669582/american-climate-tech-company-colorado-global-thermostat-direct-air-capture (accessed on 11 July 2023).
- Vázquez, F.V.; Koponen, J.; Ruuskanen, V.; Bajamundi, C.; Kosonen, A.; Simell, P.; Ahola, J.; Frilund, C.; Elfving, J.; Reinikainen, M.; et al. Power-to-X Technology Using Renewable Electricity and Carbon Dioxide from Ambient Air: SOLETAIR Proof-of-Concept and Improved Process Concept. J. CO2 Util. 2018, 28, 235–246. [Google Scholar] [CrossRef]
- Lampinen, M.; Anttila, T.; Rauhala, K. Filtration Method and a Filter Device for Removing Impurities from the Air of a Limited Space and an Apparatus for Removing Carbon Dioxide from the Air of an Air-Raid Shelter. 2004. Available online: https://patentimages.storage.googleapis.com/b6/a6/49/f1a1db48580478/US7601189.pdf (accessed on 29 June 2023).
- Direct Air Capture (DAC) Appliances—Hydrocell Oy. Available online: https://hydrocell.fi/en/air-cleaners-carbon-dioxide-filters-and-dac-appliances/dac-appliances/ (accessed on 5 February 2023).
- Soletair Demo Plant Produces Renewable Hydrocarbon Fuel from CO2 Captured from the Air—Green Car Congress. Available online: https://www.greencarcongress.com/2017/06/20170609-soletair.html (accessed on 28 June 2023).
- Soletair Power|Turning Buildings into Carbon Sinks. Available online: https://www.soletairpower.fi/ (accessed on 28 June 2023).
- Beaumont, M.; Thirkettle, A. Method and Device for the Reversible Adsorption of Carbon Dioxide. 2014. Available online: https://patentimages.storage.googleapis.com/6e/37/dd/26634ec14db31b/US10722835.pdf (accessed on 20 July 2023).
- Skytree|Scalable Carbon Removal Solutions. Available online: https://skytree.eu/ (accessed on 5 February 2023).
- Skytree Launches First On-Site Carbon Capture Unit at Growy Farms in the Netherlands. Available online: https://www.prweb.com/releases/2022/7/prweb18795808.htm (accessed on 9 July 2023).
- First “MechanicalTree” Installed on ASU’s Tempe Campus|ASU News. Available online: https://news.asu.edu/20220415-solutions-first-mechanicaltree-installed-asu-carbon-collect-tempe (accessed on 8 July 2023).
- Lackner, K.; Page, R.; Cirucci, J.; Green, M. Enhanced Capture Structures for Direct Air Capture. U.S. Patent 17/729,296, 27 October 2022. [Google Scholar]
- Choodamani, V.; Kedia, S.; Lackner, K.; Page, R. Device, System, and Method for Passive Collection of Atmospheric Carbon Dioxide. U.S. Patent 17/288,481, 16 December 2021. [Google Scholar]
- Carbon Capture Program R&D: Compendium of Carbon Capture Technology 2022; Department of Energy: Washington, DC, USA, 2022.
- Lackner’s Carbon-Capture Technology Moves to Commercialization|ASU News. Available online: https://news.asu.edu/20190429-solutions-lackner-carbon-capture-technology-moves-commercialization (accessed on 9 July 2023).
- Carbon Collect—Radical Breakthrough in Carbon Capture. Available online: https://carboncollect.com/ (accessed on 8 July 2023).
- Can an Army of Mechanical Trees Save the Planet?—LeafScore. Available online: https://www.leafscore.com/blog/can-an-army-of-mechanical-trees-save-the-planet/ (accessed on 8 July 2023).
- The World’s First Mechanical Tree Prototype Is to Be Built at ASU Next Year—The Arizona State Press. Available online: https://www.statepress.com/article/2020/10/spbiztech-the-worlds-first-mechanical-tree-is-to-be-built-at-asu-by-next-year# (accessed on 9 July 2023).
- Rio Tinto Invests in Mining Carbon Dioxide from the Air—E&E News. Available online: https://www.eenews.net/articles/rio-tinto-invests-in-mining-carbon-dioxide-from-the-air/ (accessed on 25 June 2023).
- CarbonCapture Inc. Announces Five Megaton Direct Air Capture and Storage Project in Wyoming|Business Wire. Available online: https://www.businesswire.com/news/home/20220908005446/en/CarbonCapture-Inc.-Announces-Five-Megaton-Direct-Air-Capture-and-Storage-Project-in-Wyoming (accessed on 25 June 2023).
- What We Do|Carbon Capture Inc. Available online: https://www.carboncapture.com/what-we-do (accessed on 25 June 2023).
- Besarati, S.; Gross, W.T.; Colbert, E.W.; Fang, D. Temperature Vacuum Swing Adsorption Process Suited for Carbon Capture to Regenerate Sorbents Using the CO2 Product Gas as the Heat Transfer Medium. 2022. Available online: https://patentimages.storage.googleapis.com/e4/b3/01/1875136d236e76/US11654393.pdf (accessed on 25 June 2023).
- Holman, B.J.; Gross, W.T.; Pedretti, A.; Besarati, S.; Welch, A.; Fang, D. Continuous Processes and Systems to Reduce Energy Requirements of Using Zeolites for Carbon Capture under Humid Conditions. 2022. Available online: https://patentimages.storage.googleapis.com/66/56/00/b1201b5534b45a/US20230073553A1.pdf (accessed on 25 June 2023).
- Verdox Captures $80M to Develop Novel Electric Carbon Removal Technology|Business Wire. Available online: https://www.businesswire.com/news/home/20220201006125/en/Verdox-Captures-80M-to-Develop-Novel-Electric-Carbon-Removal-Technology (accessed on 3 June 2023).
- Hydro Invests in Carbon Capture Company Verdox to Eliminate Emissions from Aluminium Production. Available online: https://www.hydro.com/en/media/news/2022/hydro-invests-in-carbon-capture-company-verdox-to-eliminate-emissions-from-aluminium-production/ (accessed on 29 July 2023).
- A New Approach to Carbon Capture|MIT News|Massachusetts Institute of Technology. Available online: https://news.mit.edu/2020/new-approach-to-carbon-capture-0709 (accessed on 3 June 2023).
- TerraFixing—Technology. Available online: https://www.terrafixing.com/technology (accessed on 13 June 2023).
- TerraFixing’s Direct Air Capture (DAC) Technology Summary—Climate Solutions Advancement Network (ClimateSAN). Available online: https://climatesan.org/terrafixings-direct-air-capture-dac-technology-summary/ (accessed on 13 June 2023).
- Sean Michael Wynn WILSON Direct Air Capture and Concentration of CO2 Using Adsorbents. Available online: https://patents.google.com/patent/WO2022109746A1/en (accessed on 14 June 2023).
- Steiner, M. Capturing Carbon One Cooling Tower at a Time|Greenbiz. Available online: https://www.greenbiz.com/article/capturing-carbon-one-cooling-tower-time (accessed on 26 May 2023).
- Shieber, J. Noya Labs Turns Cooling Towers into Direct Air Capture Devices for CO2 Emissions|TechCrunch. Available online: https://techcrunch.com/2021/02/24/noya-labs-turns-cooling-towers-into-direct-air-capture-devices-for-co2-emissions/ (accessed on 26 May 2023).
- De Chant, T. Noya Pivots to Embrace Modular Direct Air Capture, Lands $11M Series A|TechCrunch. Available online: https://techcrunch.com/2023/04/17/noya-series-a-modular-direct-air-capture/ (accessed on 26 May 2023).
- DAC Startup Noya Secures $11M from USV, Collab Fund. Available online: https://www.axios.com/pro/climate-deals/2023/04/11/dac-startup-noya-secures-11m-usv-collab-fund (accessed on 13 June 2023).
- McQueen, N.; Ghoussoub, M.; Mills, J.; Scholten, M. A Scalable Direct Air Capture Process Based on Accelerated Weathering of Calcium Hydroxide; Heirloom Carbon Technologies: San Francisco, CA, USA, 2022. [Google Scholar]
- Heirloom Blog—Electric Kilns: How an Old Technology Is Key to Our Climate Future. Available online: https://www.heirloomcarbon.com/news/electric-kilns-how-an-old-technology-is-key-to-our-climate-future (accessed on 9 June 2023).
- Heirloom Blog—Announcing Heirloom. Available online: https://www.heirloomcarbon.com/news/announcing-heirloom (accessed on 9 June 2023).
- Heirloom Blog—It’s Getting Hot in Here: A Tale of Temperature and Energy. Available online: https://www.heirloomcarbon.com/news/its-getting-hot-in-here-a-tale-of-temperature-and-energy-2 (accessed on 9 June 2023).
- Battelle, Climeworks, Heirloom Carbon, Gulf Coast Sequestration Bid on Direct Air Capture Hub for Department of Energy. Available online: https://www.battelle.org/insights/newsroom/press-release-details/battelle-climeworks-heirloom-carbon-gulf-coast-sequestration-bid-on-direct-air-capture-hub-for-department-of-energy (accessed on 9 June 2023).
- Gupta, R. Carbon Reimagined Our Direct Air Capture Solution. Available online: https://netl.doe.gov/sites/default/files/netl-file/22CM_CDR16_Gupta.pdf (accessed on 3 June 2023).
- How Direct Air Capture Can Help Solve Kenya’s Energy Access Problem. Available online: https://illuminem.com/illuminemvoices/how-direct-air-capture-can-help-solve-kenyas-energy-access-problem (accessed on 13 June 2023).
- Sguazzin, A. First Southern Hemisphere Direct Air Capture Plant Planned in Kenya Rift Valley—Bloomberg. Available online: https://www.bloomberg.com/news/articles/2023-07-19/first-southern-hemisphere-direct-air-capture-plant-planned#xj4y7vzkg (accessed on 24 July 2023).
- Why We Invested: Octavia Carbon Is the Global South’s First Direct Air Capture (DAC) Company, Building the World’s Lowest-Cost DAC Hub—BFA Global. Available online: https://bfaglobal.com/catalyst-fund/insights/why-we-invested-octavia-carbon/ (accessed on 13 June 2023).
- Heidel, K.R.; Keith, D.W.; Ritchie, J.A.; Vollendorf, N.; Fessler, E. Recovering a Caustic Solution via Calcium Carbonate Crystal Aggregates. 2020. Available online: https://patentimages.storage.googleapis.com/b8/af/f9/f9bfa400c9fdd0/US11014823.pdf (accessed on 19 July 2023).
- Keith, D.; Mahmoudkhani, M.; Biglioli, A.; Hart, B.; Heidel, K.R.; Foniok, M. Carbon Dioxide Capture Method and Facility. 2019. Available online: https://patentimages.storage.googleapis.com/b7/d7/f0/d5e4b8eb6f8bc4/US11504667.pdf (accessed on 19 July 2023).
- Behr, P. Process and Absorbent for Absorbing Carbon Dioxide from the Air. 2022. Available online: https://patentimages.storage.googleapis.com/5d/ba/e9/5727384e5facea/WO2022184840A1.pdf (accessed on 26 May 2023).
- Gebald, C.; Repond, N.; Ruesch, T.; Wurzbacher, J.A. Low-Pressure Drop Structure of Particle Adsorbent Bed for Adsorption Gas Separation Process. 2017. Available online: https://patentimages.storage.googleapis.com/73/1c/13/402470e32b5a27/US10427086.pdf (accessed on 20 July 2023).
- Gebald, C.; Zimmermann, T.; Tingaut, P. Porous Adsorbent Structure for Adsorption of CO2 from a Gas Mixture. 2012. Available online: https://patentimages.storage.googleapis.com/0e/11/67/bbb80d42e81f36/WO2012168346A1.pdf (accessed on 20 July 2023).
- Gebald, C.; Repond, N.; Wurzbacher, J.A. Steam Assisted Vacuum Desorption Process for Carbon Dioxide Capture. 2017. Available online: https://patentimages.storage.googleapis.com/53/80/57/85c40c96a53f8a/EP3166708B1.pdf (accessed on 20 July 2023).
- Piispanen, A. Method and Apparatus for Separating Carbon Dioxide and for Utilizing Carbon Dioxide. 2021. Available online: https://patentimages.storage.googleapis.com/c3/7d/8c/cd2bf3545db166/US20210205755A1.pdf (accessed on 19 July 2023).
- Voskian, S.; Thomas-Alyea, K.; Burns, E.G.; Popere, B.C. Electroswing Adsorption Cell with Patterned Electrodes for Separation of Gas Components. 2021. Available online: https://patentimages.storage.googleapis.com/63/4b/2c/14f328e22ca526/US20210387139A1.pdf (accessed on 20 July 2023).
- Voskian, S.; Rogers, C. Composite for Electrochemical Gas Separation. 2022. Available online: https://patentimages.storage.googleapis.com/34/3f/7c/3f601947ad94db/WO2022104000A1.pdf (accessed on 20 July 2023).
- Rogers, C.; Voskian, S. Quinone-Containing Polymer, Methods for the Manufacture Thereof, and Use for Electrochemical Gas Separation. 2023. Available online: https://patentimages.storage.googleapis.com/8d/92/31/adc1dbeeb43368/WO2023096955A1.pdf (accessed on 20 July 2023).
- Santos-Heard, J.I.; Cavero Rodriguez, D.; Petitjean, L.; Welch, A.J. Systems and Methods for Capturing Carbon Dioxide. 2022. Available online: https://patentimages.storage.googleapis.com/05/ad/d4/d0368f87a72e05/WO2023009858A1.pdf (accessed on 26 May 2023).
- MCQUEEN, N.; Samala, S.; Dubel, A. Systems and Methods of Carbon Capture from Cement Production Process. 2022. Available online: https://patentimages.storage.googleapis.com/f8/e1/77/337e6e8afac065/WO2023122540A1.pdf (accessed on 12 July 2023).
- Gupta, R.P.; Sanderson, C.E.; Zhou, S.J.; Agarwal, S.; Lanigan, A.T.; Toppo, A.C.; Shen, J.-P. Direct Air Capture CO2 Removal System and Process. 2022. Available online: https://patentimages.storage.googleapis.com/e8/cc/17/d11b1b14210d62/WO2022192408A2.pdf (accessed on 20 July 2023).
- Lanigan-Atkins, T.L.; Shen, J.P.; Gupta, R.P.; Sanderson, C.E. Systems and Processes for Removal of Carbon Dioxide (CO2) from CO2-Containing Gases Using Alkali Metal Adsorbents. 2022. Available online: https://patentimages.storage.googleapis.com/38/b0/a1/a0250233a3a837/WO2022235664A2.pdf (accessed on 20 July 2023).
- McQueen, N.; Psarras, P.; Pilorgé, H.; Liguori, S.; He, J.; Yuan, M.; Woodall, C.M.; Kian, K.; Pierpoint, L.; Jurewicz, J.; et al. Cost Analysis of Direct Air Capture and Sequestration Coupled to Low-Carbon Thermal Energy in the United States. Environ. Sci. Technol. 2020, 54, 7542–7551. [Google Scholar] [CrossRef]
- Sinha, A.; Realff, M.J. A Parametric Study of the Techno-Economics of Direct CO2 Air Capture Systems Using Solid Adsorbents. AIChE J. 2019, 65, e16607. [Google Scholar] [CrossRef]
- Sadiq, M.M.; Konstas, K.; Falcaro, P.; Hill, A.J.; Suzuki, K.; Hill, M.R. Engineered Porous Nanocomposites That Deliver Remarkably Low Carbon Capture Energy Costs. Cell Rep. Phys. Sci. 2020, 1, 100070. [Google Scholar] [CrossRef]
- Mazzotti, M.; Baciocchi, R.; Desmond, M.J.; Socolow, R.H. Direct Air Capture of CO2 with Chemicals: Optimization of a Two-Loop Hydroxide Carbonate System Using a Countercurrent Air-Liquid Contactor. Clim. Change 2013, 118, 119–135. [Google Scholar] [CrossRef]
- Evans, S. The Swiss Company Hoping to Capture 1% of Global CO2 Emissions by 2025—Carbon Brief. Available online: https://www.carbonbrief.org/swiss-company-hoping-capture-1-global-co2-emissions-2025/ (accessed on 24 July 2023).
- Electricity Prices to Homes and Industry in the Nordic Countries 2013–2022. PxWeb. Available online: https://px.hagstofa.is/pxen/pxweb/en/Umhverfi/Umhverfi__4_orkumal__1_orkuverdogkostnadur/IDN02303.px/table/tableViewLayout2/ (accessed on 16 July 2023).
- CER—Manitoba. Available online: https://www.cer-rec.gc.ca/en/data-analysis/energy-commodities/electricity/report/canadian-residential-electricity-bill/manitoba.html (accessed on 16 July 2023).
- McQueen, N.; Desmond, M.J.; Socolow, R.H.; Psarras, P.; Wilcox, J. Natural Gas vs. Electricity for Solvent-Based Direct Air Capture. Front. Clim. 2021, 2, 618644. [Google Scholar] [CrossRef]
- Direct Air Capture of CO2 with Chemicals Direct Air Capture of CO2 with Chemicals a Technology Assessment for the APS Panel on Public Affairs; American Physical Society: College Park, MD, USA, 2011.
- Kulkarni, A.R.; Sholl, D.S. Analysis of Equilibrium-Based TSA Processes for Direct Capture of CO2 from Air. Ind. Eng. Chem. Res. 2012, 51, 8631–8645. [Google Scholar] [CrossRef]
- Brilman, D.W.F.; Veneman, R. Capturing Atmospheric CO2 Using Supported Amine Sorbents. Energy Procedia 2013, 37, 6070–6078. [Google Scholar] [CrossRef]
- Zeman, F. Reducing the Cost of Ca-Based Direct Air Capture of CO2. Environ. Sci. Technol. 2014, 48, 11730–11735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.; Sun, C.; Drage, T.C.; Snape, C.E. Capturing CO2 from Ambient Air Using a Polyethyleneimine-Silica Adsorbent in Fluidized Beds. Chem. Eng. Sci. 2014, 116, 306–316. [Google Scholar] [CrossRef]
- Broehm, M.; Strefler, J.; Bauer, N. Techno-Economic Review of Direct Air Capture Systems for Large Scale Mitigation of Atmospheric CO2 Techno-Economic Review of Direct Air Capture Systems for Large Scale Mitigation of Atmospheric CO2. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2665702 (accessed on 28 July 2023).
- Sinha, A.; Darunte, L.A.; Jones, C.W.; Realff, M.J.; Kawajiri, Y. Systems Design and Economic Analysis of Direct Air Capture of CO2 through Temperature Vacuum Swing Adsorption Using MIL-101(Cr)-PEI-800 and Mmen-Mg2(Dobpdc) MOF Adsorbents. Ind. Eng. Chem. Res. 2017, 56, 750–764. [Google Scholar] [CrossRef]
- Sabatino, F.; Grimm, A.; Gallucci, F.; van Sint Annaland, M.; Kramer, G.J.; Gazzani, M. A Comparative Energy and Costs Assessment and Optimization for Direct Air Capture Technologies. Joule 2021, 5, 2047–2076. [Google Scholar] [CrossRef]
- Smith, S.M.; Geden, O.; Nemet, G.; Gidden, M.; Lamb, W.F.; Powis, C.; Bellamy, R.; Callaghan, M.; Cowie, A.; Cox, E.; et al. The State of Carbon Dioxide Removal—1st Edition. State Carbon Dioxide Remov. 2023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).