A Review on Advanced Processes of Biohydrogen Generation from Lignocellulosic Biomass with Special Emphasis on Thermochemical Conversion
Abstract
:1. Introduction
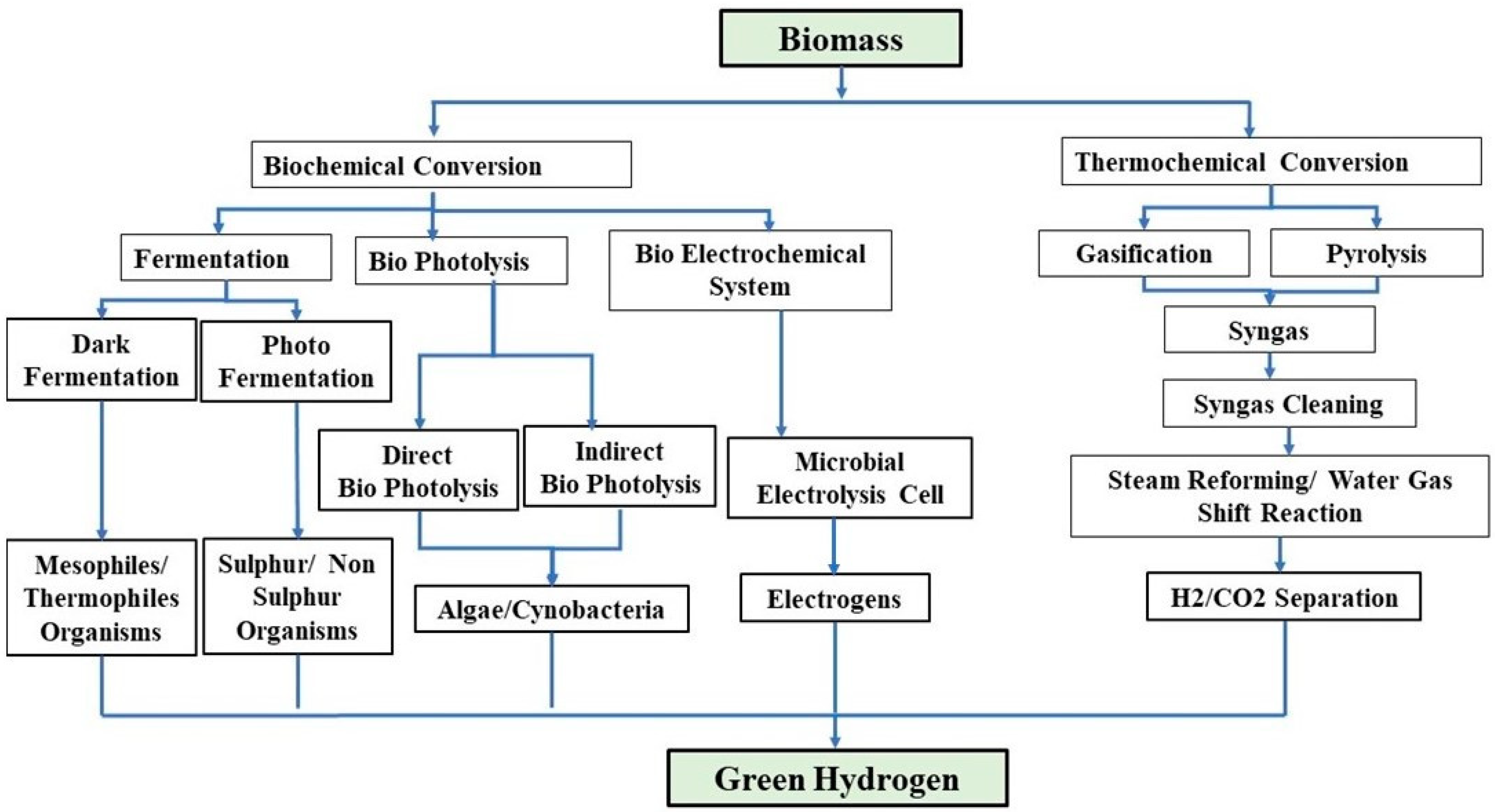
2. Biohydrogen Production Technologies
2.1. Fermentation
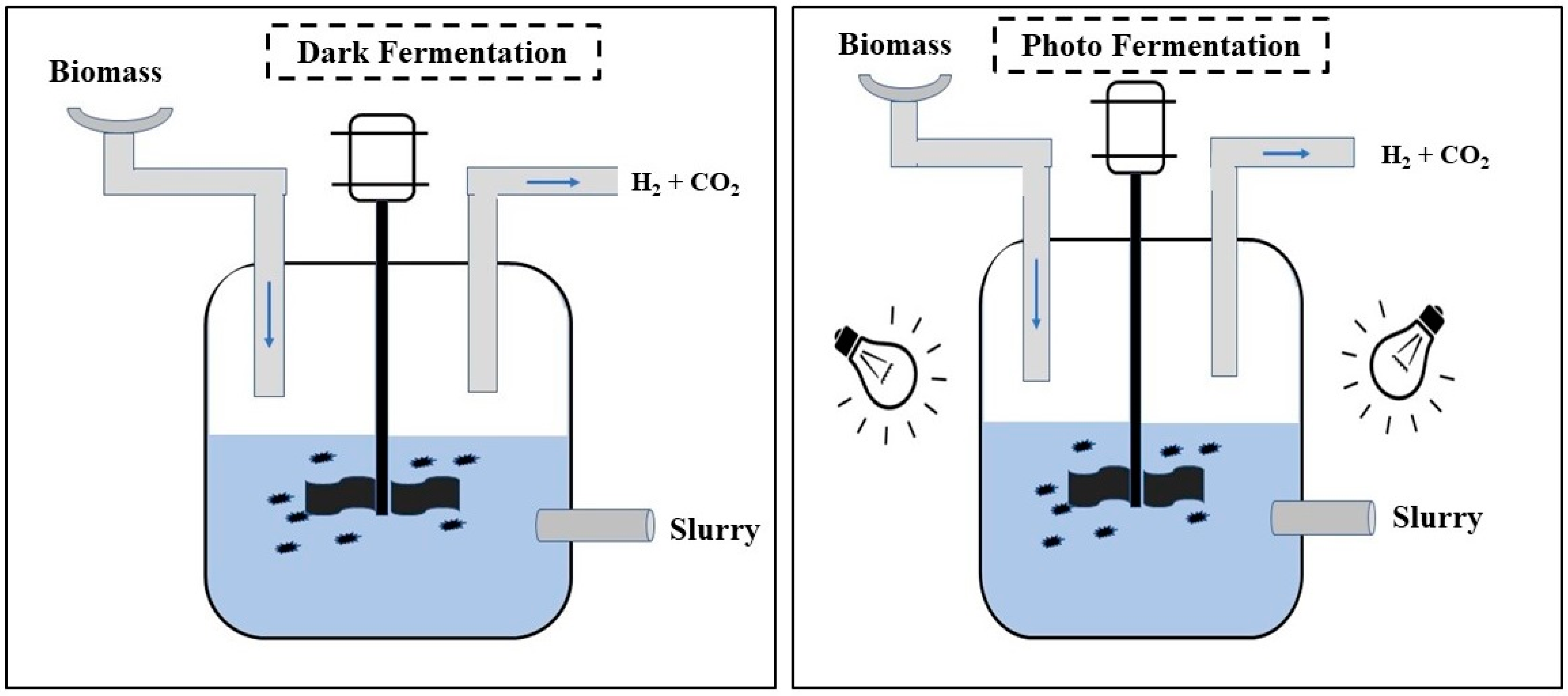
2.1.1. Dark Fermentation
2.1.2. Photofermentation
2.2. Biophotolysis
2.2.1. Direct Biophotolysis
2.2.2. Indirect Biophotolysis
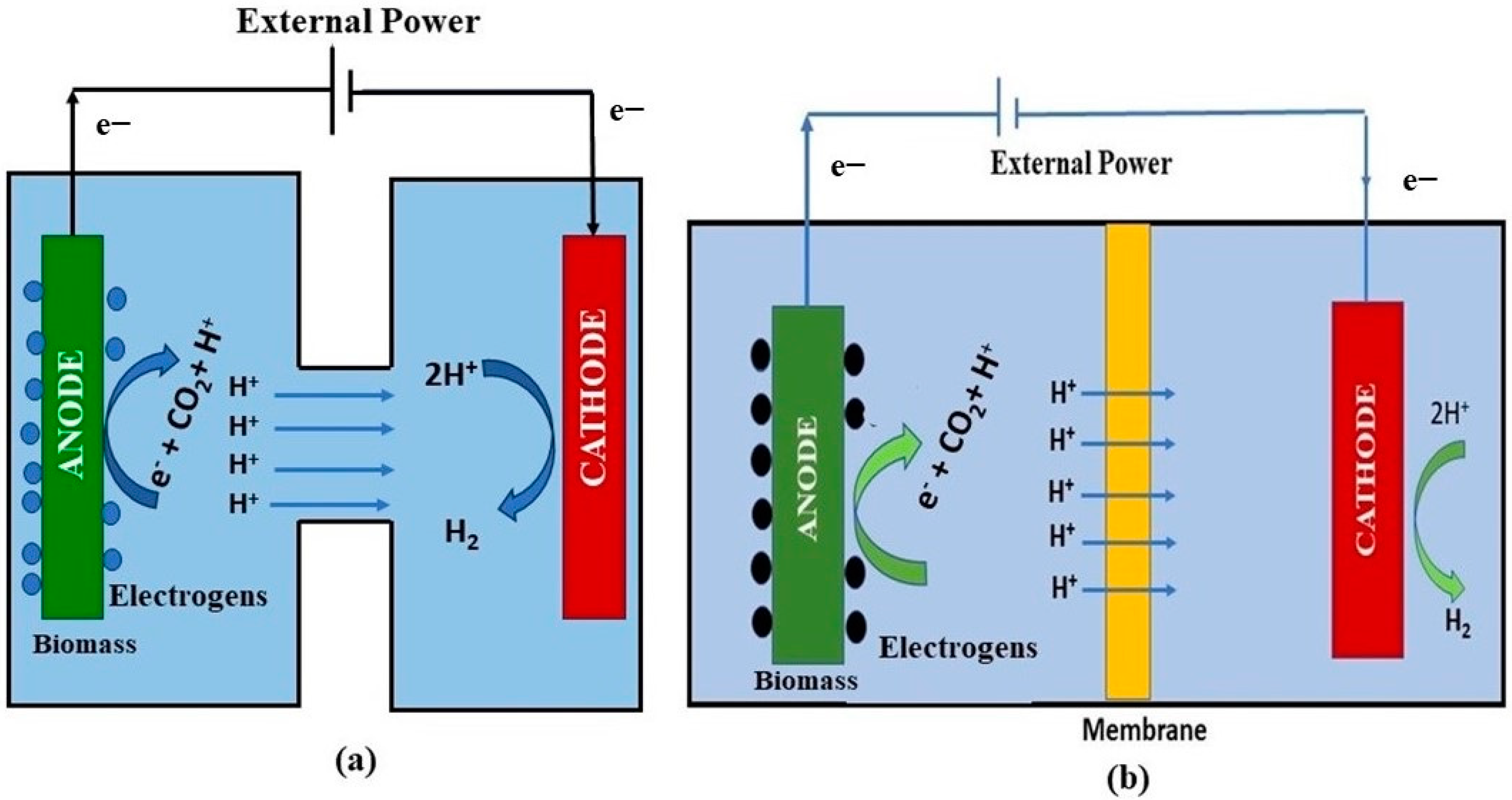
2.3. Biohydrogen Production using Microbial Electrolysis Cells (MECs)
2.4. Thermochemical Conversion of Biomass
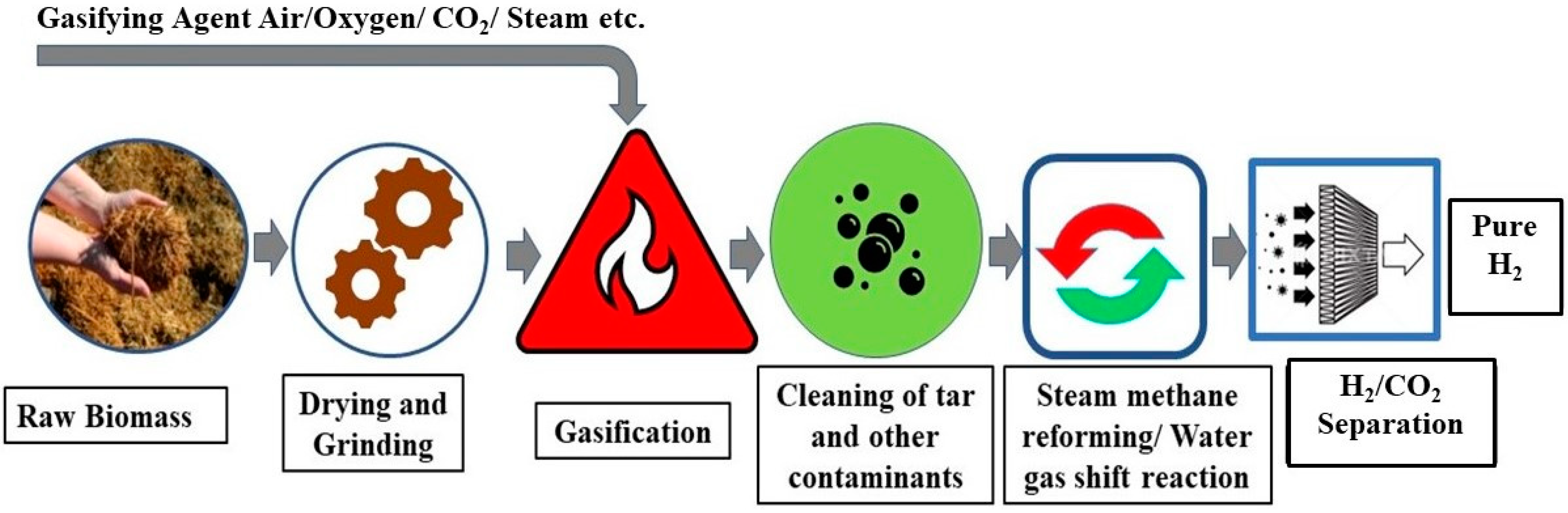
2.4.1. Gasification
Air Gasification
Oxy-Blown Gasification
Steam-Blown Gasification
Supercritical Water Gasification
3. Cleaning and Processing the Gas
3.1. Producer Gas Reforming
3.1.1. Steam Methane Reforming
3.1.2. Dry Methane Reforming
3.1.3. Tri-Methane Reforming
3.1.4. Water–Gas Shift Reaction
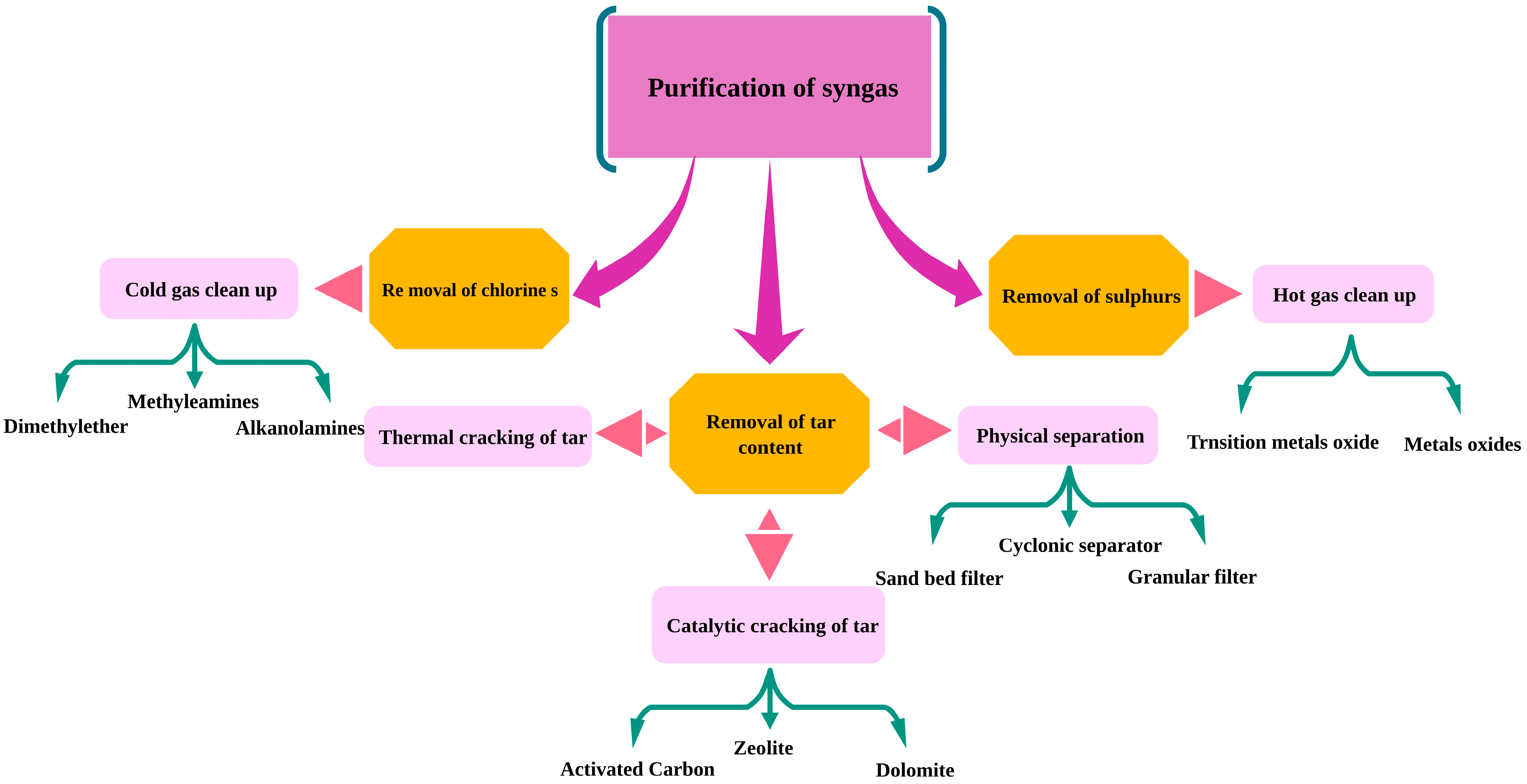
3.2. Separation and Purification of Hydrogen
3.2.1. Removal of Tars
Removal of Tar Using Catalysts
Removal of Tars via Physical Methods
3.2.2. Removal of Sulfur
3.2.3. Removal of Chlorine
4. Energy Efficiency and Green House Gas Emission Footprints of Different Hydrogen Production Routes
5. Comparison between Different Biohydrogen Production Methods
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, R.K.; Bhattacharya, T.K.; Kumain, A.; Chand, P.; Mandal, S.; Azad, D. Energy Use Pattern in Wheat Crop Production System among Different Farmer Groups of the Himalayan Tarai Region. Curr. Sci. 2020, 118, 448. [Google Scholar] [CrossRef]
- Mandal, S.; Sharma, R.K. Application of Gasification Technology in Agriculture for Power Generation. In Handbook of Energy Management in Agriculture; Springer Nature: Singapore, 2023; pp. 1–22. [Google Scholar]
- Sharma, R.K.; Singh, T.P.; Mandal, S.; Azad, D.; Kumar, S. Chemical Treatments for Biochar Modification: Opportunities, Limitations and Advantages. In Engineered Biochar; Springer Nature: Singapore, 2022; pp. 65–84. [Google Scholar]
- Mandal, S.; Sharma, R.K.; Bhattacharya, T.K.; Tanna, H.; Haydary, J. Charring of Pine Needles Using a Portable Drum Reactor. Chem. Pap. 2022, 76, 1239–1252. [Google Scholar] [CrossRef]
- Mandal, S.; Sharma, R.K.; Bhattacharya, T.K. Deriving Fuel from Pine Needles through Pyrolysis, Charring and Briquetting and Their GHG Emission Potential. Curr. Sci. 2023, 124, 1210–1215. [Google Scholar]
- Chen, G.; Yao, J.; Liu, J.; Yan, B.; Shan, R. Biomass to Hydrogen-Rich Syngas via Catalytic Steam Gasification of Bio-Oil/Biochar Slurry. Bioresour. Technol. 2015, 198, 108–114. [Google Scholar] [CrossRef]
- Shen, R.; Liu, Z.; He, Y.; Zhang, Y.; Lu, J.; Zhu, Z.; Si, B.; Zhang, C.; Xing, X.-H. Microbial Electrolysis Cell to Treat Hydrothermal Liquefied Wastewater from Cornstalk and Recover Hydrogen: Degradation of Organic Compounds and Characterization of Microbial Community. Int. J. Hydrogen Energy 2016, 41, 4132–4142. [Google Scholar] [CrossRef]
- Mandal, S.; Haydary, J.; Bhattacharya, T.K.; Tanna, H.R.; Husar, J.; Haz, A. Valorization of Pine Needles by Thermal Conversion to Solid, Liquid and Gaseous Fuels in a Screw Reactor. Waste Biomass Valorization 2019, 10, 3587–3599. [Google Scholar] [CrossRef]
- Chang, A.C.C.; Chang, H.-F.; Lin, F.-J.; Lin, K.-H.; Chen, C.-H. Biomass Gasification for Hydrogen Production. Int. J. Hydrogen Energy 2011, 36, 14252–14260. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, K.S. Hydrogen Production from Steam Gasification of Biomass: Influence of Process Parameters on Hydrogen Yield—A Review. Renew. Energy 2014, 66, 570–579. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H.; Sumathy, K. An Overview of Hydrogen Production from Biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Tomasik, P.; Horton, D. Enzymatic Conversions of Starch. Adv. Carbohydr. Chem. Biochem. 2012, 68, 59–436. [Google Scholar] [PubMed]
- Rizwan, M.; Shah, S.H.; Mujtaba, G.; Mahmood, Q.; Rashid, N.; Shah, F.A. Ecofuel Feedstocks and Their Prospect. In Advanced Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–16. [Google Scholar]
- Mona, S.; Kumar, S.S.; Kumar, V.; Parveen, K.; Saini, N.; Deepak, B.; Pugazhendhi, A. Green Technology for Sustainable Biohydrogen Production (Waste to Energy): A Review. Sci. Total Environ. 2020, 728, 138481. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A Review on Dark Fermentative Biohydrogen Production from Organic Biomass: Process Parameters and Use of by-Products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Hallenbeck, P.C. Fermentative Hydrogen Production: Principles, Progress, and Prognosis. Int. J. Hydrogen Energy 2009, 34, 7379–7389. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Nanda, S. Biohydrogen Production through Dark Fermentation. Chem. Eng. Technol. 2020, 43, 601–612. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and Evaluation of Hydrogen Production Methods for Better Sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Tian, H.; Li, J.; Yan, M.; Tong, Y.W.; Wang, C.-H.; Wang, X. Organic Waste to Biohydrogen: A Critical Review from Technological Development and Environmental Impact Analysis Perspective. Appl. Energy 2019, 256, 113961. [Google Scholar] [CrossRef]
- Alibardi, L.; Cossu, R. Effects of Carbohydrate, Protein and Lipid Content of Organic Waste on Hydrogen Production and Fermentation Products. Waste Manag. 2016, 47, 69–77. [Google Scholar] [CrossRef]
- Xing, Y.; Li, Z.; Fan, Y.; Hou, H. Biohydrogen Production from Dairy Manures with Acidification Pretreatment by Anaerobic Fermentation. Environ. Sci. Pollut. Res. 2010, 17, 392–399. [Google Scholar] [CrossRef]
- Rafieenia, R.; Lavagnolo, M.C.; Pivato, A. Pre-Treatment Technologies for Dark Fermentative Hydrogen Production: Current Advances and Future Directions. Waste Manag. 2018, 71, 734–748. [Google Scholar] [CrossRef]
- Mamimin, C.; Singkhala, A.; Kongjan, P.; Suraraksa, B.; Prasertsan, P.; Imai, T.; O-Thong, S. Two-Stage Thermophilic Fermentation and Mesophilic Methanogen Process for Biohythane Production from Palm Oil Mill Effluent. Int. J. Hydrogen Energy 2015, 40, 6319–6328. [Google Scholar] [CrossRef]
- Kumar, G.; Zhen, G.; Sivagurunathan, P.; Bakonyi, P.; Nemestóthy, N.; Bélafi-Bakó, K.; Kobayashi, T.; Xu, K.-Q. Biogenic H2 Production from Mixed Microalgae Biomass: Impact of PH Control and Methanogenic Inhibitor (BESA) Addition. Biofuel Res. J. 2016, 3, 470–474. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Lee, Y.-J.; Lee, D.-W. Biohydrogen Production: Strategies to Improve Process Efficiency through Microbial Routes. Int. J. Mol. Sci. 2015, 16, 8266–8293. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Venkata Mohan, S. Bio-Electrohydrolysis as a Pretreatment Strategy to Catabolize Complex Food Waste in Closed Circuitry: Function of Electron Flux to Enhance Acidogenic Biohydrogen Production. Int. J. Hydrogen Energy 2014, 39, 11411–11422. [Google Scholar] [CrossRef]
- Tsygankov, A.A.; Tekucheva, D.N. (Eds.) Integration of Biological H2 Producing Processes. In State of the Art and Progress in Production of Biohydrogen; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012; pp. 78–93. [Google Scholar]
- Colombo, B.; Villegas Calvo, M.; Pepè Sciarria, T.; Scaglia, B.; Savio Kizito, S.; D’Imporzano, G.; Adani, F. Biohydrogen and Polyhydroxyalkanoates (PHA) as Products of a Two-Steps Bioprocess from Deproteinized Dairy Wastes. Waste Manag. 2019, 95, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Ding, L.; Liu, M.; Cheng, J.; Zhou, J.; Li, Y.-Y. Improving Biohydrogen Production through Dark Fermentation of Steam-Heated Acid Pretreated Alternanthera Philoxeroides by Mutant Enterobacter Aerogenes ZJU1. Sci. Total Environ. 2020, 716, 134695. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Wang, Q.; Rupani, P.F.; Krishnan, S.; Ahmad, F.; Rezania, S.; Rashid, M.A.; Sha, C.; Md Din, M.F. Biohydrogen Production via Thermophilic Fermentation: A Prospective Application of Thermotoga Species. Energy 2020, 197, 117199. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Enhanced Hydrogen Production from Sewage Sludge by Co-Fermentation with Forestry Wastes. Energy Fuels 2017, 31, 9633–9641. [Google Scholar] [CrossRef]
- Alemahdi, N.; Che Man, H.; Abd Rahman, N.; Nasirian, N.; Yang, Y. Enhanced Mesophilic Bio-Hydrogen Production of Raw Rice Straw and Activated Sewage Sludge by Co-Digestion. Int. J. Hydrogen Energy 2015, 40, 16033–16044. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Kushwaha, D.; Gupta, V.K.; Manikanta, A.; Ramteke, P.W.; Mishra, P.K. Efficient Dark Fermentative Hydrogen Production from Enzyme Hydrolyzed Rice Straw by Clostridium Pasteurianum (MTCC116). Bioresour. Technol. 2017, 238, 552–558. [Google Scholar] [CrossRef]
- Rai, P.K.; Singh, S.P.; Asthana, R.K.; Singh, S. Biohydrogen Production from Sugarcane Bagasse by Integrating Dark- and Photo-Fermentation. Bioresour. Technol. 2014, 152, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Soto, L.R.; Byrne, E.; van Niel, E.W.J.; Sayed, M.; Villanueva, C.C.; Hatti-Kaul, R. Hydrogen and Polyhydroxybutyrate Production from Wheat Straw Hydrolysate Using Caldicellulosiruptor Species and Ralstonia Eutropha in a Coupled Process. Bioresour. Technol. 2019, 272, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Monroy, I.; Buitrón, G. Production of Polyhydroxybutyrate by Pure and Mixed Cultures of Purple Non-Sulfur Bacteria: A Review. J. Biotechnol. 2020, 317, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Das, D. Advances in Biohydrogen Production Processes: An Approach towards Commercialization. Int. J. Hydrogen Energy 2009, 34, 7349–7357. [Google Scholar] [CrossRef]
- Mishra, P.; Krishnan, S.; Rana, S.; Singh, L.; Sakinah, M.; Ab Wahid, Z. Outlook of Fermentative Hydrogen Production Techniques: An Overview of Dark, Photo and Integrated Dark-Photo Fermentative Approach to Biomass. Energy Strategy Rev. 2019, 24, 27–37. [Google Scholar] [CrossRef]
- Cai, J.; Zhao, Y.; Fan, J.; Li, F.; Feng, C.; Guan, Y.; Wang, R.; Tang, N. Photosynthetic Bacteria Improved Hydrogen Yield of Combined Dark- and Photo-Fermentation. J. Biotechnol. 2019, 302, 18–25. [Google Scholar] [CrossRef]
- Azwar, M.Y.; Hussain, M.A.; Abdul-Wahab, A.K. Development of Biohydrogen Production by Photobiological, Fermentation and Electrochemical Processes: A Review. Renew. Sustain. Energy Rev. 2014, 31, 158–173. [Google Scholar] [CrossRef]
- Mirza, S.S.; Qazi, J.I.; Liang, Y.; Chen, S. Growth Characteristics and Photofermentative Biohydrogen Production Potential of Purple Non Sulfur Bacteria from Sugar Cane Bagasse. Fuel 2019, 255, 115805. [Google Scholar] [CrossRef]
- García-Sánchez, R.; Ramos-Ibarra, R.; Guatemala-Morales, G.; Arriola-Guevara, E.; Toriz-González, G.; Corona-González, R.I. Photofermentation of Tequila Vinasses by Rhodopseudomonas Pseudopalustris to Produce Hydrogen. Int. J. Hydrogen Energy 2018, 43, 15857–15869. [Google Scholar] [CrossRef]
- Laurinavichene, T.; Tekucheva, D.; Laurinavichius, K.; Tsygankov, A. Utilization of Distillery Wastewater for Hydrogen Production in One-Stage and Two-Stage Processes Involving Photofermentation. Enzym. Microb. Technol. 2018, 110, 1–7. [Google Scholar] [CrossRef]
- Machado, R.G.; Moreira, F.S.; Batista, F.R.X.; Ferreira, J.S.; Cardoso, V.L. Repeated Batch Cycles as an Alternative for Hydrogen Production by Co-Culture Photofermentation. Energy 2018, 153, 861–869. [Google Scholar] [CrossRef]
- Keskin, T.; Hallenbeck, P.C. Hydrogen Production from Sugar Industry Wastes Using Single-Stage Photofermentation. Bioresour. Technol. 2012, 112, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Li, Y.; Zhu, S.; Zhang, H.; Jing, Y.; Jiang, D.; He, C.; Zhang, Z. Evaluation of Biohydrogen Yield Potential and Electron Balance in the Photo-Fermentation Process with Different Initial PH from Starch Agricultural Leftover. Bioresour. Technol. 2020, 305, 122900. [Google Scholar] [CrossRef]
- Adessi, A.; Venturi, M.; Candeliere, F.; Galli, V.; Granchi, L.; De Philippis, R. Bread Wastes to Energy: Sequential Lactic and Photo-Fermentation for Hydrogen Production. Int. J. Hydrogen Energy 2018, 43, 9569–9576. [Google Scholar] [CrossRef]
- Guo, S.; Lu, C.; Wang, K.; Wang, J.; Zhang, Z.; Jing, Y.; Zhang, Q. Enhancement of PH Values Stability and Photo-Fermentation Biohydrogen Production by Phosphate Buffer. Bioengineered 2020, 11, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Kim, D.-H.; Cha, J.; Lee, J.K. Effect of Carbon and Nitrogen Sources on Photo-Fermentative H2 Production Associated with Nitrogenase, Uptake Hydrogenase Activity, and PHB Accumulation in Rhodobacter Sphaeroides KD131. Bioresour. Technol. 2012, 116, 179–183. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Li, Y.; Lu, C.; Zhu, S.; He, C.; Ai, F.; Zhang, Q. Investigation of the Interaction between Lighting and Mixing Applied during the Photo-Fermentation Biohydrogen Production Process from Agricultural Waste. Bioresour. Technol. 2020, 312, 123570. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, Z.; Zhang, H.; Jing, Y.; Li, Y.; Zhang, Q. Rheological Properties of Corn Stover Hydrolysate and Photo-Fermentation Bio-Hydrogen Producing Capacity under Intermittent Stirring. Int. J. Hydrogen Energy 2020, 45, 3721–3728. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, D.; Zhang, H.; Lee, D.J.; Zhang, Z.; Zhang, Q.; Jing, Y.; Zhang, Y.; Xia, C. Effects of Different Pretreatment Methods on the Structural Characteristics, Enzymatic Saccharification and Photo-Fermentative Bio-Hydrogen Production Performance of Corn Straw. Bioresour. Technol. 2020, 304, 122999. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Lee, D.J.; Zhang, T.; Jiang, D.; Zhang, Z.; Zhang, Q. Effect of Enzymolysis Time on Biohydrogen Production from Photo-Fermentation by Using Various Energy Grasses as Substrates. Bioresour. Technol. 2020, 305, 123062. [Google Scholar] [CrossRef]
- Nagakawa, H.; Takeuchi, A.; Takekuma, Y.; Noji, T.; Kawakami, K.; Kamiya, N.; Nango, M.; Furukawa, R.; Nagata, M. Efficient Hydrogen Production Using Photosystem I Enhanced by Artificial Light Harvesting Dye. Photochem. Photobiol. Sci. 2019, 18, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Fakhimi, N.; Tavakoli, O. Improving Hydrogen Production Using Co-Cultivation of Bacteria with Chlamydomonas reinhardtii Microalga. Mater. Sci. Energy Technol. 2019, 2, 1–7. [Google Scholar] [CrossRef]
- Kossalbayev, B.D.; Tomo, T.; Zayadan, B.K.; Sadvakasova, A.K.; Bolatkhan, K.; Alwasel, S.; Allakhverdiev, S.I. Determination of the Potential of Cyanobacterial Strains for Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 2627–2639. [Google Scholar] [CrossRef]
- Hoshino, T.; Johnson, D.J.; Scholz, M.; Cuello, J.L. Effects of Implementing PSI-Light on Hydrogen Production via Biophotolysis in Chlamydomonas reinhardtii Mutant Strains. Biomass Bioenergy 2013, 59, 243–252. [Google Scholar] [CrossRef]
- Nyberg, M.; Heidorn, T.; Lindblad, P. Hydrogen Production by the Engineered Cyanobacterial Strain Nostoc PCC 7120 ΔhupW Examined in a Flat Panel Photobioreactor System. J. Biotechnol. 2015, 215, 35–43. [Google Scholar] [CrossRef]
- Volgusheva, A.; Kukarskikh, G.; Krendeleva, T.; Rubin, A.; Mamedov, F. Hydrogen Photoproduction in Green Algae Chlamydomonas reinhardtii under Magnesium Deprivation. RSC Adv. 2015, 5, 5633–5637. [Google Scholar] [CrossRef]
- Batyrova, K.; Gavrisheva, A.; Ivanova, E.; Liu, J.; Tsygankov, A. Sustainable Hydrogen Photoproduction by Phosphorus-Deprived Marine Green Microalgae Chlorella sp. Int. J. Mol. Sci. 2015, 16, 2705–2716. [Google Scholar] [CrossRef]
- Shi, X.-Y.; Yu, H.-Q. Simultaneous Metabolism of Benzoate and Photobiological Hydrogen Production by Lyngbya sp. Renew. Energy 2016, 95, 474–477. [Google Scholar] [CrossRef]
- Oncel, S.; Kose, A. Comparison of Tubular and Panel Type Photobioreactors for Biohydrogen Production Utilizing Chlamydomonas reinhardtii Considering Mixing Time and Light Intensity. Bioresour. Technol. 2014, 151, 265–270. [Google Scholar] [CrossRef]
- Kosourov, S.; Seibert, M.; Ghirardi, M.L. Effects of Extracellular PH on the Metabolic Pathways in Sulfur-Deprived, H2-Producing Chlamydomonas reinhardtii Cultures. Plant Cell Physiol. 2003, 44, 146–155. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Abdeshahian, P.; Azman, N.F.; Chandrasekhar, K.; Kalil, M.S. A Comprehensive Review of Microbial Electrolysis Cells (MEC) Reactor Designs and Configurations for Sustainable Hydrogen Gas Production. Alex. Eng. J. 2016, 55, 427–443. [Google Scholar] [CrossRef]
- Yossan, S.; Xiao, L.; Prasertsan, P.; He, Z. Hydrogen Production in Microbial Electrolysis Cells: Choice of Catholyte. Int. J. Hydrogen Energy 2013, 38, 9619–9624. [Google Scholar] [CrossRef]
- Luo, S.; Jain, A.; Aguilera, A.; He, Z. Effective Control of Biohythane Composition through Operational Strategies in an Innovative Microbial Electrolysis Cell. Appl. Energy 2017, 206, 879–886. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Edwards, S.R.; Dolfing, J.; Cotterill, S.E.; Curtis, T.P. Performance of a Pilot Scale Microbial Electrolysis Cell Fed on Domestic Wastewater at Ambient Temperatures for a 12 Month Period. Bioresour. Technol. 2014, 173, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Sun, D.; Cao, G.; Wang, H.; Ren, N.; Wu, W.-M.; Logan, B.E. Integrated Hydrogen Production Process from Cellulose by Combining Dark Fermentation, Microbial Fuel Cells, and a Microbial Electrolysis Cell. Bioresour. Technol. 2011, 102, 4137–4143. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Jiang, Y.; Ge, Z.; Lu, J.; Zhang, Y.; Liu, Z.; Ren, Z.J. Microbial Electrolysis Treatment of Post-Hydrothermal Liquefaction Wastewater with Hydrogen Generation. Appl. Energy 2018, 212, 509–515. [Google Scholar] [CrossRef]
- Wagner, R.C.; Regan, J.M.; Oh, S.-E.; Zuo, Y.; Logan, B.E. Hydrogen and Methane Production from Swine Wastewater Using Microbial Electrolysis Cells. Water Res. 2009, 43, 1480–1488. [Google Scholar] [CrossRef]
- Escapa, A.; Gil-Carrera, L.; García, V.; Morán, A. Performance of a Continuous Flow Microbial Electrolysis Cell (MEC) Fed with Domestic Wastewater. Bioresour. Technol. 2012, 117, 55–62. [Google Scholar] [CrossRef]
- Cusick, R.D.; Kiely, P.D.; Logan, B.E. A Monetary Comparison of Energy Recovered from Microbial Fuel Cells and Microbial Electrolysis Cells Fed Winery or Domestic Wastewaters. Int. J. Hydrogen Energy 2010, 35, 8855–8861. [Google Scholar] [CrossRef]
- Lu, L.; Xing, D.; Liu, B.; Ren, N. Enhanced Hydrogen Production from Waste Activated Sludge by Cascade Utilization of Organic Matter in Microbial Electrolysis Cells. Water Res. 2012, 46, 1015–1026. [Google Scholar] [CrossRef]
- Dhar, B.R.; Elbeshbishy, E.; Hafez, H.; Lee, H.-S. Hydrogen Production from Sugar Beet Juice Using an Integrated Biohydrogen Process of Dark Fermentation and Microbial Electrolysis Cell. Bioresour. Technol. 2015, 198, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Feng, H.; Huang, L.; Ying, X.; Shen, D.; Chen, T.; Shen, X.; Zhou, Y.; Xu, Y. Continuous Hydrogen Production from Food Waste by Anaerobic Digestion (AD) Coupled Single-Chamber Microbial Electrolysis Cell (MEC) under Negative Pressure. Waste Manag. 2020, 103, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Liang, D.-W.; Bai, Y.-X.; Fan, Y.-T.; Hou, H.-W. Enhanced H2 Production from Corn Stalk by Integrating Dark Fermentation and Single Chamber Microbial Electrolysis Cells with Double Anode Arrangement. Int. J. Hydrogen Energy 2014, 39, 8977–8982. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, Y.; Zhang, Y. Enhancement of Sludge Decomposition and Hydrogen Production from Waste Activated Sludge in a Microbial Electrolysis Cell with Cheap Electrodes. Environ. Sci. 2015, 1, 761–768. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, L.; Zularisam, A.W. Exoelectrogens: Recent Advances in Molecular Drivers Involved in Extracellular Electron Transfer and Strategies Used to Improve It for Microbial Fuel Cell Applications. Renew. Sustain. Energy Rev. 2016, 56, 1322–1336. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Cheng, K.Y.; Selvam, A.; Bose, A.; Wong, J.W.C. Bioelectrohydrogenesis and Inhibition of Methanogenic Activity in Microbial Electrolysis Cells—A Review. Biotechnol. Adv. 2017, 35, 758–771. [Google Scholar] [CrossRef]
- Mandal, S.; Prasanna Kumar, G.V.; Bhattacharya, T.K.; Tanna, H.R.; Jena, P.C. Briquetting of Pine Needles (Pinus roxburgii) and Their Physical, Handling and Combustion Properties. Waste Biomass Valorization 2019, 10, 2415–2424. [Google Scholar] [CrossRef]
- Abdoulmoumine, N.; Adhikari, S.; Kulkarni, A.; Chattanathan, S. A Review on Biomass Gasification Syngas Cleanup. Appl. Energy 2015, 155, 294–307. [Google Scholar] [CrossRef]
- Liao, C.; Wu, C.; Yan, Y. The Characteristics of Inorganic Elements in Ashes from a 1 MW CFB Biomass Gasification Power Generation Plant. Fuel Process. Technol. 2007, 88, 149–156. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An Overview of Advances in Biomass Gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef]
- Sansaniwal, S.K.; Rosen, M.A.; Tyagi, S.K. Global Challenges in the Sustainable Development of Biomass Gasification: An Overview. Renew. Sustain. Energy Rev. 2017, 80, 23–43. [Google Scholar] [CrossRef]
- Trippe, F.; Fröhling, M.; Schultmann, F.; Stahl, R.; Henrich, E. Techno-Economic Assessment of Gasification as a Process Step within Biomass-to-Liquid (BtL) Fuel and Chemicals Production. Fuel Process. Technol. 2011, 92, 2169–2184. [Google Scholar] [CrossRef]
- Pio, D.T.; Tarelho, L.A.C.; Nunes, T.F.V.; Baptista, M.F.; Matos, M.A.A. Co-Combustion of Residual Forest Biomass and Sludge in a Pilot-Scale Bubbling Fluidized Bed. J. Clean. Prod. 2020, 249, 119309. [Google Scholar] [CrossRef]
- Inayat, M.; Sulaiman, S.A.; Bhayo, B.A.; Shahbaz, M. Application of Response Surface Methodology in Catalytic Co-Gasification of Palm Wastes for Bioenergy Conversion Using Mineral Catalysts. Biomass Bioenergy 2020, 132, 105418. [Google Scholar] [CrossRef]
- Niu, M.; Huang, Y.; Jin, B.; Wang, X. Oxygen Gasification of Municipal Solid Waste in a Fixed-Bed Gasifier. Chin. J. Chem. Eng. 2014, 22, 1021–1026. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Bhattacharya, A.; Datta, A. Modeling of Hydrogen Production Process from Biomass Using Oxygen Blown Gasification. Int. J. Hydrogen Energy 2012, 37, 18782–18790. [Google Scholar] [CrossRef]
- Weiland, F.; Hedman, H.; Marklund, M.; Wiinikka, H.; Öhrman, O.; Gebart, R. Pressurized Oxygen Blown Entrained-Flow Gasification of Wood Powder. Energy Fuels 2013, 27, 932–941. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, W.; Blasiak, W. Energy and Exergy Analysis of High Temperature Agent Gasification of Biomass. Energies 2014, 7, 2107–2122. [Google Scholar] [CrossRef]
- Siedlecki, M.; de Jong, W. Biomass Gasification as the First Hot Step in Clean Syngas Production Process—Gas Quality Optimization and Primary Tar Reduction Measures in a 100 KW Thermal Input Steam–Oxygen Blown CFB Gasifier. Biomass Bioenergy 2011, 35, S40–S62. [Google Scholar] [CrossRef]
- Lopez, G.; Cortazar, M.; Alvarez, J.; Amutio, M.; Bilbao, J.; Olazar, M. Assessment of a Conical Spouted with an Enhanced Fountain Bed for Biomass Gasification. Fuel 2017, 203, 825–831. [Google Scholar] [CrossRef]
- Hu, F.-X.; Yang, G.-H.; Ding, G.-Z.; Li, Z.; Du, K.-S.; Hu, Z.-F.; Tian, S.-R. Experimental Study on Catalytic Cracking of Model Tar Compounds in a Dual Layer Granular Bed Filter. Appl. Energy 2016, 170, 47–57. [Google Scholar] [CrossRef]
- Luo, S.; Xiao, B.; Hu, Z.; Liu, S.; Guo, X.; He, M. Hydrogen-Rich Gas from Catalytic Steam Gasification of Biomass in a Fixed Bed Reactor: Influence of Temperature and Steam on Gasification Performance. Int. J. Hydrogen Energy 2009, 34, 2191–2194. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.Z.; Xu, D.H.; Gong, Y.M.; Ma, H.H.; Tang, X.Y. Review of Catalytic Supercritical Water Gasification for Hydrogen Production from Biomass. Renew. Sustain. Energy Rev. 2010, 14, 334–343. [Google Scholar] [CrossRef]
- Calzavara, Y.; Joussot-Dubien, C.; Boissonnet, G.; Sarrade, S. Evaluation of Biomass Gasification in Supercritical Water Process for Hydrogen Production. Energy Convers. Manag. 2005, 46, 615–631. [Google Scholar] [CrossRef]
- Safari, F.; Javani, N.; Yumurtaci, Z. Hydrogen Production via Supercritical Water Gasification of Almond Shell over Algal and Agricultural Hydrochars as Catalysts. Int. J. Hydrogen Energy 2018, 43, 1071–1080. [Google Scholar] [CrossRef]
- Gao, N.; Li, A.; Quan, C.; Gao, F. Hydrogen-Rich Gas Production from Biomass Steam Gasification in an Updraft Fixed-Bed Gasifier Combined with a Porous Ceramic Reformer. Int. J. Hydrogen Energy 2008, 33, 5430–5438. [Google Scholar] [CrossRef]
- Sui, M.; Li, G.; Guan, Y.; Li, C.; Zhou, R.; Zarnegar, A.-M. Hydrogen and Syngas Production from Steam Gasification of Biomass Using Cement as Catalyst. Biomass Convers. Biorefin. 2020, 10, 119–124. [Google Scholar] [CrossRef]
- TURN, S. An Experimental Investigation of Hydrogen Production from Biomass Gasification. Int. J. Hydrogen Energy 1998, 23, 641–648. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Williams, P.T. Hydrogen and Methane Selectivity during Alkaline Supercritical Water Gasification of Biomass with Ruthenium-Alumina Catalyst. Appl. Catal. B 2013, 132–133, 70–79. [Google Scholar] [CrossRef]
- Antal, M.J.; Allen, S.G.; Schulman, D.; Xu, X.; Divilio, R.J. Biomass Gasification in Supercritical Water. Ind. Eng. Chem. Res. 2000, 39, 4040–4053. [Google Scholar] [CrossRef]
- ISO 14687: 2019; Hydrogen Fuel Quality—Product Specification. International Organization for Standardization: Geneva, Switzerland, 2019.
- Bacquart, T.; Moore, N.; Storms, W.; Chramosta, N.; Morris, A.; Murugan, A.; Gozlan, B.; Lescornez, Y.; Férat, S.; Pinte, G.; et al. Hydrogen Fuel Quality for Transport—First Sampling and Analysis Comparison in Europe on Hydrogen Refuelling Station (70 MPa) According to ISO 14687 and EN 17124. Fuel Commun. 2021, 6, 100008. [Google Scholar] [CrossRef]
- Ren, Q.; Zhao, C.; Wu, X.; Liang, C.; Chen, X.; Shen, J.; Wang, Z. Formation of NOx Precursors during Wheat Straw Pyrolysis and Gasification with O2 and CO2. Fuel 2010, 89, 1064–1069. [Google Scholar] [CrossRef]
- Xu, C.; Donald, J.; Byambajav, E.; Ohtsuka, Y. Recent Advances in Catalysts for Hot-Gas Removal of Tar and NH3 from Biomass Gasification. Fuel 2010, 89, 1784–1795. [Google Scholar] [CrossRef]
- Chiang, K.Y.; Lu, C.H.; Lin, M.H.; Chien, K.L. Reducing Tar Yield in Gasification of Paper-Reject Sludge by Using a Hot-Gas Cleaning System. Energy 2013, 50, 47–53. [Google Scholar] [CrossRef]
- Ngo, T.N.L.T.; Chiang, K.Y.; Liu, C.F.; Chang, Y.H.; Wan, H.P. Hydrogen Production Enhancement Using Hot Gas Cleaning System Combined with Prepared Ni-Based Catalyst in Biomass Gasification. Int. J. Hydrogen Energy 2021, 46, 11269–11283. [Google Scholar] [CrossRef]
- Halabi, M.H.; De Croon, M.H.J.M.; Van Der Schaaf, J.; Cobden, P.D.; Schouten, J.C. Low Temperature Catalytic Methane Steam Reforming over Ceria–Zirconia Supported Rhodium. Appl. Catal. A Gen. 2010, 389, 68–79. [Google Scholar] [CrossRef]
- McFarlan, A.; Maffei, N. Assessing a Commercial Steam Methane Reforming Catalyst for Tar Removal in Biomass Gasification. Bioresour. Technol. Rep. 2022, 17, 100968. [Google Scholar] [CrossRef]
- Swierczynski, D.; Courson, C.; Kiennemann, A. Study of Steam Reforming of Toluene Used as Model Compound of Tar Produced by Biomass Gasification. Chem. Eng. Process. Process Intensif. 2008, 47, 508–513. [Google Scholar] [CrossRef]
- Tanios, C.; Labaki, M. Catalytic Reforming: A Sustainable Technology for Hydrogen Production. In Recent Advances in Renewable Energy Technologies; Elsevier: Amsterdam, The Netherlands, 2022; pp. 199–247. [Google Scholar]
- Gupta, S.; Deo, G. Effect of Metal Amount on the Catalytic Performance of Ni–Al2O3 Catalyst for the Tri-Reforming of Methane. Int. J. Hydrogen Energy 2023, 48, 5478–5492. [Google Scholar] [CrossRef]
- Ammal, S.C.; Heyden, A. Origin of the Unique Activity of Pt/TiO2 Catalysts for the Water–Gas Shift Reaction. J. Catal. 2013, 306, 78–90. [Google Scholar] [CrossRef]
- Pang, S. Recent Advances in Thermochemical Conversion of Woody Biomass for Production of Green Hydrogen and CO2 Capture: A Review. J. Bioresour. Bioprod. 2023. [Google Scholar] [CrossRef]
- Teh, J.S.; Teoh, Y.H.; How, H.G.; Le, T.D.; Jason, Y.J.J.; Nguyen, H.T.; Loo, D.L. The Potential of Sustainable Biomass Producer Gas as a Waste-to-Energy Alternative in Malaysia. Sustainability 2021, 13, 3877. [Google Scholar] [CrossRef]
- Baraj, E.; Ciahotný, K.; Hlinčík, T. The Water Gas Shift Reaction: Catalysts and Reaction Mechanism. Fuel 2021, 288, 119817. [Google Scholar] [CrossRef]
- Chen, W.H.; Chen, C.Y. Water Gas Shift Reaction for Hydrogen Production and Carbon Dioxide Capture: A Review. Appl. Energy 2020, 258, 114078. [Google Scholar] [CrossRef]
- Lee, D.W.; Lee, M.S.; Lee, J.Y.; Kim, S.; Eom, H.J.; Moon, D.J.; Lee, K.Y. The Review of Cr-Free Fe-Based Catalysts for High-Temperature Water-Gas Shift Reactions. Catal. Today 2013, 210, 2–9. [Google Scholar] [CrossRef]
- Patra, T.K.; Sheth, P.N. Biomass Gasification Coupled with Producer Gas Cleaning, Bottling and HTS Catalyst Treatment for H2-Rich Gas Production. Int. J. Hydrogen Energy 2019, 44, 11602–11616. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Rajamehala, M.; Karthigadevi, G.; Praveenkumar, R.; Bharathiraja, B. A Review on Feedstock, Pretreatment Methods, Influencing Factors, Production and Purification Processes of Bio-Hydrogen Production. Case Stud. Chem. Environ. Eng. 2020, 2, 100038. [Google Scholar] [CrossRef]
- Noichi, H.; Uddin, A.; Sasaoka, E. Steam Reforming of Naphthalene as Model Biomass Tar over Iron–Aluminum and Iron–Zirconium Oxide Catalyst Catalysts. Fuel Process. Technol. 2010, 91, 1609–1616. [Google Scholar] [CrossRef]
- Sutton, D.; Kelleher, B.; Ross, J.R.H. Review of Literature on Catalysts for Biomass Gasification. Fuel Process. Technol. 2001, 73, 155–173. [Google Scholar] [CrossRef]
- Anis, S.; Zainal, Z.A. Tar Reduction in Biomass Producer Gas via Mechanical, Catalytic and Thermal Methods: A Review. Renew. Sustain. Energy Rev. 2011, 15, 2355–2377. [Google Scholar] [CrossRef]
- Delgado, J.; Aznar, M.P.; Corella, J. Biomass Gasification with Steam in Fluidized Bed: Effectiveness of CaO, MgO, and CaO−MgO for Hot Raw Gas Cleaning. Ind. Eng. Chem. Res. 1997, 36, 1535–1543. [Google Scholar] [CrossRef]
- Pallozzi, V.; Di Carlo, A.; Bocci, E.; Carlini, M. Combined Gas Conditioning and Cleaning for Reduction of Tars in Biomass Gasification. Biomass Bioenergy 2018, 109, 85–90. [Google Scholar] [CrossRef]
- Orío, A.; Corella, J.; Narváez, I. Performance of Different Dolomites on Hot Raw Gas Cleaning from Biomass Gasification with Air. Ind. Eng. Chem. Res. 1997, 36, 3800–3808. [Google Scholar] [CrossRef]
- Pinto, F.; André, R.N.; Carolino, C.; Miranda, M.; Abelha, P.; Direito, D.; Dohrup, J.; Sørensen, H.R.; Girio, F. Effects of Experimental Conditions and of Addition of Natural Minerals on Syngas Production from Lignin by Oxy-Gasification: Comparison of Bench- and Pilot Scale Gasification. Fuel 2015, 140, 62–72. [Google Scholar] [CrossRef]
- Roche, E.; De Andrés, J.M.; Narros, A.; Rodríguez, M.E. Air and Air-Steam Gasification of Sewage Sludge. The Influence of Dolomite and Throughput in Tar Production and Composition. Fuel 2014, 115, 54–61. [Google Scholar] [CrossRef]
- Richardson, Y.; Blin, J.; Julbe, A. A Short Overview on Purification and Conditioning of Syngas Produced by Biomass Gasification: Catalytic Strategies, Process Intensification and New Concepts. Prog. Energy Combust. Sci. 2012, 38, 765–781. [Google Scholar] [CrossRef]
- Simell, P.A.; Leppälahti, J.K.; Bredenberg, J.B. son Catalytic Purification of Tarry Fuel Gas with Carbonate Rocks and Ferrous Materials. Fuel 1992, 71, 211–218. [Google Scholar] [CrossRef]
- Taralas, G.; Vassilatos, V.; Sjöström, K.; Delgado, J. Thermal and Catalytic Cracking of n -Heptane in Presence of CaO, MgO and Calcined Dolomites. Can. J. Chem. Eng. 1991, 69, 1413–1419. [Google Scholar] [CrossRef]
- Taralas, G. Catalytic Steam Cracking of n -Heptane with Special Reference to the Effect of Calcined Dolomite. Ind. Eng. Chem. Res. 1996, 35, 2121–2126. [Google Scholar] [CrossRef]
- Tuomi, S.; Kurkela, E.; Simell, P.; Reinikainen, M. Behaviour of Tars on the Filter in High Temperature Filtration of Biomass-Based Gasification Gas. Fuel 2015, 139, 220–231. [Google Scholar] [CrossRef]
- Simeone, E.; Siedlecki, M.; Nacken, M.; Heidenreich, S.; De Jong, W. High Temperature Gas Filtration with Ceramic Candles and Ashes Characterisation during Steam–Oxygen Blown Gasification of Biomass. Fuel 2013, 108, 99–111. [Google Scholar] [CrossRef]
- Rapagnà, S.; Gallucci, K.; Foscolo, P.U. Olivine, Dolomite and Ceramic Filters in One Vessel to Produce Clean Gas from Biomass. Waste Manag. 2018, 71, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Savuto, E.; Di Carlo, A.; Steele, A.; Heidenreich, S.; Gallucci, K.; Rapagnà, S. Syngas Conditioning by Ceramic Filter Candles Filled with Catalyst Pellets and Placed inside the Freeboard of a Fluidized Bed Steam Gasifier. Fuel Process. Technol. 2019, 191, 44–53. [Google Scholar] [CrossRef]
- Hasler, P.; Nussbaumer, T. Gas Cleaning for IC Engine Applications from Fixed Bed Biomass Gasification. Biomass Bioenergy 1999, 16, 385–395. [Google Scholar] [CrossRef]
- Nakamura, S.; Kitano, S.; Yoshikawa, K. Biomass Gasification Process with the Tar Removal Technologies Utilizing Bio-Oil Scrubber and Char Bed. Appl. Energy 2016, 170, 186–192. [Google Scholar] [CrossRef]
- De Jong, W.; Ünal, Ö.; Andries, J.; Hein, K.R.G.; Spliethoff, H. Biomass and Fossil Fuel Conversion by Pressurised Fluidised Bed Gasification Using Hot Gas Ceramic Filters as Gas Cleaning. Biomass Bioenergy 2003, 25, 59–83. [Google Scholar] [CrossRef]
- Van Paasen, S.V.B.; Rabou, L.P.L.M.; Bär, R. TAR Removal with A Wet Electrostatic Precipitator (ESP); A Parametric Study. In Proceedings of the 2nd World Conference and Technology on Biomass for Energy, Industry and Climate Protection, Rome, Italy, 10–14 May 2004. [Google Scholar]
- Ma, L.; Verelst, H.; Baron, G.V. Integrated High Temperature Gas Cleaning: Tar Removal in Biomass Gasification with a Catalytic Filter. Catal Today 2005, 105, 729–734. [Google Scholar] [CrossRef]
- Engelen, K.; Zhang, Y.; Draelants, D.J.; Baron, G.V. A Novel Catalytic Filter for Tar Removal from Biomass Gasification Gas: Improvement of the Catalytic Activity in Presence of H2S. Chem. Eng. Sci. 2003, 58, 665–670. [Google Scholar] [CrossRef]
- Khummongkol, D.; Tangsathitkulchai, C. A Model for Tar-Removal Efficiency from Biomass-Produced Gas Impinging on a Water Surface. Energy 1989, 14, 113–121. [Google Scholar] [CrossRef]
- Prabhansu; Karmakar, M.K.; Chandra, P.; Chatterjee, P.K. A Review on the Fuel Gas Cleaning Technologies in Gasification Process. J. Environ. Chem. Eng. 2015, 3, 689–702. [Google Scholar] [CrossRef]
- Bhave, A.G.; Vyas, D.K.; Patel, J.B. A Wet Packed Bed Scrubber-Based Producer Gas Cooling–Cleaning System. Renew. Energy 2008, 33, 1716–1720. [Google Scholar] [CrossRef]
- Cheah, S.; Carpenter, D.L.; Magrini-Bair, K.A. Review of Mid- to High-Temperature Sulfur Sorbents for Desulfurization of Biomass- and Coal-Derived Syngas. Energy Fuels 2009, 23, 5291–5307. [Google Scholar] [CrossRef]
- Husmann, M.; Zuber, C.; Maitz, V.; Kienberger, T.; Hochenauer, C. Comparison of Dolomite and Lime as Sorbents for In-Situ H2S Removal with Respect to Gasification Parameters in Biomass Gasification. Fuel 2016, 181, 131–138. [Google Scholar] [CrossRef]
- Gupta, R.P.; O’Brien, W.S. Desulfurization of Hot Syngas Containing Hydrogen Chloride Vapors Using Zinc Titanate Sorbents. Ind. Eng. Chem. Res. 2000, 39, 610–619. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Maiti, A. Adsorption and Decomposition of H 2 S on MgO(100), NiMgO(100), and ZnO(0001) Surfaces: A First-Principles Density Functional Study. J. Phys. Chem. B 2000, 104, 3630–3638. [Google Scholar] [CrossRef]
- Slimane, R.B.; Abbasian, J. Copper-Based Sorbents for Coal Gas Desulfurization at Moderate Temperatures. Ind. Eng. Chem. Res. 2000, 39, 1338–1344. [Google Scholar] [CrossRef]
- Zeng, Y.; Kaytakoglu, S.; Harrison, D.P. Reduced Cerium Oxide as an Efficient and Durable High Temperature Desulfurization Sorbent. Chem. Eng. Sci. 2000, 55, 4893–4900. [Google Scholar] [CrossRef]
- Meng, X.; de Jong, W.; Pal, R.; Verkooijen, A.H.M. In Bed and Downstream Hot Gas Desulphurization during Solid Fuel Gasification: A Review. Fuel Process. Technol. 2010, 91, 964–981. [Google Scholar] [CrossRef]
- Lew, S.; Sarofim, A.F.; Flytzani-Stephanopoulos, M. Sulfidation of Zinc Titanate and Zinc Oxide Solids. Ind. Eng. Chem. Res. 1992, 31, 1890–1899. [Google Scholar] [CrossRef]
- Fan, H.-L.; Li, C.-H.; Li, C.-H. Testing of Iron Oxide Sorbent for High-Temperature Coal Gas Desulfurization. Energy Sources 2005, 27, 245–250. [Google Scholar] [CrossRef]
- Yrjas, K.P.; Zevenhoven, C.A.P.; Hupa, M.M. Hydrogen Sulfide Capture by Limestone and Dolomite at Elevated Pressure. 1. Sorbent Performance. Ind. Eng. Chem. Res. 1996, 35, 176–183. [Google Scholar] [CrossRef]
- Ephraim, A.; Ngo, L.; Pham Minh, D.; Lebonnois, D.; Peregrina, C.; Sharrock, P.; Nzihou, A. Valorization of Waste-Derived Inorganic Sorbents for the Removal of HCl in Syngas. Waste Biomass Valorization 2019, 10, 3435–3446. [Google Scholar] [CrossRef]
- Verdone, N.; De Filippis, P. Reaction Kinetics of Hydrogen Chloride with Sodium Carbonate. Chem. Eng. Sci. 2006, 61, 7487–7496. [Google Scholar] [CrossRef]
- Ohtsuka, Y.; Tsubouchi, N.; Kikuchi, T.; Hashimoto, H. Recent Progress in Japan on Hot Gas Cleanup of Hydrogen Chloride, Hydrogen Sulfide and Ammonia in Coal-Derived Fuel Gas. Powder Technol. 2009, 190, 340–347. [Google Scholar] [CrossRef]
- Krishnan, G.; Gupta, R. Development of Disposable Sorbents for Chloride Removal from High Temperature Coal-Derived Gases; United States Department of Energy: Pittsburgh, PA, USA; Morgantown, WV, USA, 1999.
- Dou, B.; Wang, C.; Chen, H.; Song, Y.; Xie, B.; Xu, Y.; Tan, C. Research Progress of Hot Gas Filtration, Desulphurization and HCl Removal in Coal-Derived Fuel Gas: A Review. Chem. Eng. Res. Des. 2012, 90, 1901–1917. [Google Scholar] [CrossRef]
- Ren, X.; Rokni, E.; Liu, Y.; Levendis, Y.A. Reduction of HCl Emissions from Combustion of Biomass by Alkali Carbonate Sorbents or by Thermal Pretreatment. J. Energy Eng. 2018, 144. [Google Scholar] [CrossRef]
- Baek, J.-I.; Eom, T.H.; Lee, J.B.; Jegarl, S.; Ryu, C.K.; Park, Y.C.; Jo, S.-H. Cleaning of Gaseous Hydrogen Chloride in a Syngas by Spray-Dried Potassium-Based Solid Sorbents. Korean J. Chem. Eng. 2015, 32, 845–851. [Google Scholar] [CrossRef]
- Fremaux, S.; Beheshti, S.M.; Ghassemi, H.; Shahsavan-Markadeh, R. An Experimental Study on Hydrogen-Rich Gas Production via Steam Gasification of Biomass in a Research-Scale Fluidized Bed. Energy Convers. Manag. 2015, 91, 427–432. [Google Scholar] [CrossRef]
- Manish, S.; Banerjee, R. Comparison of Biohydrogen Production Processes. Int. J. Hydrogen Energy 2008, 33, 279–286. [Google Scholar] [CrossRef]
- Megía, P.J.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production Technologies: From Fossil Fuels toward Renewable Sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Jiang, D.; Ge, X.; Zhang, T.; Liu, H.; Zhang, Q. Photo-Fermentative Hydrogen Production from Enzymatic Hydrolysate of Corn Stalk Pith with a Photosynthetic Consortium. Int. J. Hydrogen Energy 2016, 41, 16778–16785. [Google Scholar] [CrossRef]
- Jiang, D.; Ge, X.; Lin, L.; Zhang, T.; Liu, H.; Hu, J.; Zhang, Q. Continuous Photo-Fermentative Hydrogen Production in a Tubular Photobioreactor Using Corn Stalk Pith Hydrolysate with a Consortium. Int. J. Hydrogen Energy 2020, 45, 3776–3784. [Google Scholar] [CrossRef]
- Osman, A.I.; Deka, T.J.; Baruah, D.C.; Rooney, D.W. Critical Challenges in Biohydrogen Production Processes from the Organic Feedstocks. Biomass Convers. Biorefin. 2023, 13, 8383–8401. [Google Scholar] [CrossRef]
| Feedstock | Preparation of Feedstock | Microorganism | pH | Temperature (°C) | H2 Yield (mL/g VS) | Refs. |
|---|---|---|---|---|---|---|
| Dairy manure | Treatment with hydrochloric acid (0.2% concentration), boiling, and exposure to infrared radiation. | Mixed culture | 5.0 | 36.0 ± 1 | 31.5 | [22] |
| Poplar residue with sewage sludge | - | - | - | - | 20.8 | [32] |
| Rice straw | Drying at 80–100 °C | Activated sewage sludge | 4.0–5.5 | 35.0 | 14.5 + 0.3 | [33] |
| Rice straw | Size reduction of less than 2 mm, 1.0% alkali pre-treatment, cellulose hydrolysis | Clostridium pasteurianum | 7.5 | 37.0 ± 2 | 2.6 (47.6 mL/g released sugar) | [34] |
| Sugarcane bagasse | Pre-treatment with H2SO4 | Enterobacter aerogenes | 6.8 | 30.0 | 1000.0 | [35] |
| Wheat straw | Acetic acid pre-treatment followed by steam exposure at 190 °C for 10 min and enzymatic hydrolysis lasting 72 h | Caldicellulosiruptor saccharolyticus | 6.5 ± 0.1 | 70.0 | 134.0 | [36] |
| Feedstock | Microorganisms | Enzyme | H2 Yield | Refs. |
|---|---|---|---|---|
| Potato residue | Rhodospirillum rubrum, Rhodobacter capsulatus and Rhodopseudomonas palustris | Alpha-amylase | 642 mL /(L h) | [47] |
| Bread waste | R. palustris | - | 3.1 mol H2/mol | [48] |
| Corn stalk | Rhodospirillum rubrum, R. capsulata, R. pulastris, Rhodobacter sphaeroides, Rhodobacter capsulatus | Cellulose | 23.96 mL/h H2 | [49] |
| Fermented Waste food | Rhodobacter sphaeoides KD131 | - | 24% Substrate conversion efficiency (%) | [50] |
| Corncob | Rhodospirillum rubrum, Rhodobacter capsulatus, Rhodopseudomonas palustri | Cellulase | 84.7 mL H2/g TS | [51] |
| Corn stover | HAU-M1 | Cellulase | 57.63 mL/g VS | [52] |
| Corn straw | Rhodospirillum rubrum, Rhodopseudomonas capsulate, Rhodopseudomonas palustris, Rhodobacter sphaeroides and Rhodobacter capsulatus | Cellulase | 137.76 mL H2/g TS | [53] |
| Energy grass | Rhodospirillum rubrum, R. capsulata, R. pulastris, Rhodobacter sphaeroides, Rhodoba | Cellulase | 5.53 mL H2/(h g TS) | [54] |
| Microalgae/Cyanobacteria | Process Condition | Light Intensity (W/m2) | H2 Production | Refs. |
|---|---|---|---|---|
| Nostoc PCC 7120 | BG110 medium, supplied with a mixture of red and white light, altering 100% Ar and Ar/N2 (20/80) | 18.8 | 6.2 mL/L/h | [59] |
| C. reinhardtii cbn 1–48 | Tris-acetate-phosphate medium, 5% CO2, dark anaerobic adaptation | 426.6 | 40.2 mL/kg | [58] |
| C. reinhardtii Dang 137+ | TAP (Tris-acetate-phosphate) medium | 34.1 | 6.0 mmol/L | [60] |
| Chlorella sp. IOAC707S | TAP-seawater medium | 10.7 | 38.0 mL/L | [61] |
| yngby asp. (benzoate as a carbon source) | Basal medium, 600 mg/L benzoate at late exponential phase | 31.6 | 17.1 μmol H2/g Chl a/h | [62] |
| C. reinhardtii (CC124) | sulfur-free TAP medium | 64.0 | 1.3 ± 0.1 mL/L/h | [63] |
| C. reinhardtii CC-425 strain | TAP medium, TAP-sulfur | 121.6 | 0.8 μmol/mg Chl /h | [64] |
| Type of Waste | Type of MEC Reactor | Temperature (°C) | pH | External Voltage (V) | H2 Yield (L/L/d) | Refs. |
|---|---|---|---|---|---|---|
| Swine manure + waste water | Two-chamber | 25.0 ± 2 | 7.0 | 1.2 | 5.1 | [70] |
| Waste-activated sludge | Single-chamber | 20.0 | 7.0 ± 0.2 | 0.6 | 90.6 | [78] |
| Waste of sugar beet juice | Two-chamber | 25.0 | 7.2 | 0.4 | 306.0 | [75] |
| Cornstalk wastewater | Two-chamber | 25.0 ± 2 | 7.0 | 1.0 | 3.9 | [7] |
| Type of Biomass | Type of Gasification | Operating Conditions | H2 Yield | Refs. |
|---|---|---|---|---|
| Pine sawdust | Steam-blown | S/B = 1.05–3.47 Temperature: 800–950 °C | 55.87% volume | [100] |
| Wood chips | Steam-blown | S/B = 0.18–1.32 Temperature: 800–950 °C | 50.3% volume | [101] |
| Sawdust | Steam- and oxy-blown | S/B = 1.1–4.7 ER = 0–0.37 Temperature: 750–950 °C | 57.4% volume | [102] |
| Lignocellulosic biomass | Air | ER = 0.20–0.34 Temperature: 600–1000 °C | 29.54% volume | [3] |
| Sawdust | Supercritical water | Temperature: 550 °C Pressure: 36–40 MPa | 10.40 mol/kg | [103] |
| Corn starch | Supercritical water | Temperature: 745 °C Pressure: 280 bar | 55% volume | [104] |
| Standard | ISO 14687-2019 SAE J2719-202003 [105] |
|---|---|
| Purity of hydrogen | 99.97% |
| Total non-hydrogen gases | 300 ppm |
| H2O | 5 ppm |
| hydrocarbons without CH4 | 2 ppm |
| CH4 | 100 ppm |
| O2 | 5 ppm |
| He | 300 ppm |
| N2 | 300 ppm |
| Ar | 300 ppm |
| CO2 | 2 ppm |
| CO | 0.2 ppm |
| S.No | Technology | Type of Cleaning Method | Tar Removal Efficiency (%) | Operational Temperature (°C) | Rank | [Ref.] |
|---|---|---|---|---|---|---|
| 1. | Cyclonic separator | Dry | 30–70 | 100–900 | 10 | [140] |
| 2. | Fabric filter | Dry | 0–50 | Up to 600 | 12 | [140] |
| 3. | Sand bed filter | Dry | 50–90 | 20 | 6 | [140] |
| 4. | Bio-oil scrubber | Wet | 60 | 50 | 11 | [141] |
| 5. | Quartz filter | Dry | 75–95 | 650–770 | 5 | [142] |
| 6. | Activated carbon as adsorbent | Dry | 80 | 20 | 4 | [141] |
| 7. | Electrostatic precipitator | Wet | 40–70 | 20–30 | 9 | [143] |
| 8. | Permeable catalytic filter disk (aluminum oxide (2.5 wt%); nickel (1.0 wt%); magnesium (0.5 wt%)) | Dry | 77–99 | 800 900 | 3 | [144] |
| 9. | Permeable catalytic filter disk (nickel (1 wt%)/calcium oxide (0.5 wt%)) | Dry | 96–98 | 900 | 1 | [145] |
| 10. | Impinger | Wet | 70 | 50 | 8 | [146] |
| 11. | Three impingers in series | Wet | >95 | 50 | 2 | [146] |
| 12. | Washing tower | Wet | 10–25 | 50–60 | 14 | [140] |
| 13. | Venturi scrubber | Wet | 50–90 | 20–100 | 6 | [147] |
| 14. | Packed bed scrubber | Wet | 75 | 300 | 7 | [148] |
| 15. | Water scrubber | Wet | 22 | 20–100 | 13 | [147] |
| Sorbent | Ideal Sorption Capacity (g S/g Sorbent) | Operating Temperature (°C) | Rank | Ref. |
|---|---|---|---|---|
| Cerium oxide | 0.093 | 500–700 | 7 | [155] |
| Copper oxide | 0.224 | 540–700 | 6 | [155] |
| Zinc copper ferrite | 0.398 | 540–680 | 3 | [155] |
| Zinc oxide | 0.395 | 450–650 | 4 | [156] |
| Manganese oxide | 0.400 | 400–900 | 2 | [155] |
| Iron oxide | 0.245 | 450–700 | 5 | [157] |
| Lime powder | 0.571 | 815–980 | 1 | [158] |
| Pathway | Conventional Energy Use (MJ) | Energy Efficiency (%) | GHGs Emission (kg CO2 eq) | Ref. |
|---|---|---|---|---|
| Thermochemical Conversion | 256.8 | 43–70 | 2.14 | [166] |
| Dark Fermentation | 61.7 | 1–10 | −87 | [12] |
| Photofermentation | 40.1 | 1–25 | −21.9 | [12] |
| Dark + Photofermentation | 39.3 | 27.2 | −19.5 | [167] |
| Microbial Electrolysis | 64.8 | 6–26 | −17.5 | [167] |
| H2 Production Processes | Advantages | Constraints | Refs. |
|---|---|---|---|
| Dark fermentation |
|
| [35] |
| Photofermentation |
|
| [35,169,170] |
| Biophotolysis |
|
| [168,171] |
| MEC |
|
| [171] |
| Gasification |
|
| [85,168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, R.K.; Nazari, M.A.; Haydary, J.; Singh, T.P.; Mandal, S. A Review on Advanced Processes of Biohydrogen Generation from Lignocellulosic Biomass with Special Emphasis on Thermochemical Conversion. Energies 2023, 16, 6349. https://doi.org/10.3390/en16176349
Sharma RK, Nazari MA, Haydary J, Singh TP, Mandal S. A Review on Advanced Processes of Biohydrogen Generation from Lignocellulosic Biomass with Special Emphasis on Thermochemical Conversion. Energies. 2023; 16(17):6349. https://doi.org/10.3390/en16176349
Chicago/Turabian StyleSharma, Rajat Kumar, Mohammad Ali Nazari, Juma Haydary, Triveni Prasad Singh, and Sandip Mandal. 2023. "A Review on Advanced Processes of Biohydrogen Generation from Lignocellulosic Biomass with Special Emphasis on Thermochemical Conversion" Energies 16, no. 17: 6349. https://doi.org/10.3390/en16176349
APA StyleSharma, R. K., Nazari, M. A., Haydary, J., Singh, T. P., & Mandal, S. (2023). A Review on Advanced Processes of Biohydrogen Generation from Lignocellulosic Biomass with Special Emphasis on Thermochemical Conversion. Energies, 16(17), 6349. https://doi.org/10.3390/en16176349