The Potential Role of Ammonia for Hydrogen Storage and Transport: A Critical Review of Challenges and Opportunities
Abstract
:1. Introduction
1.1. Hydrogen and Ammonia Characteristics
2. Ammonia Production
2.1. Energy and Emissions
2.2. Economic Aspects
3. Ammonia Transport and Distribution
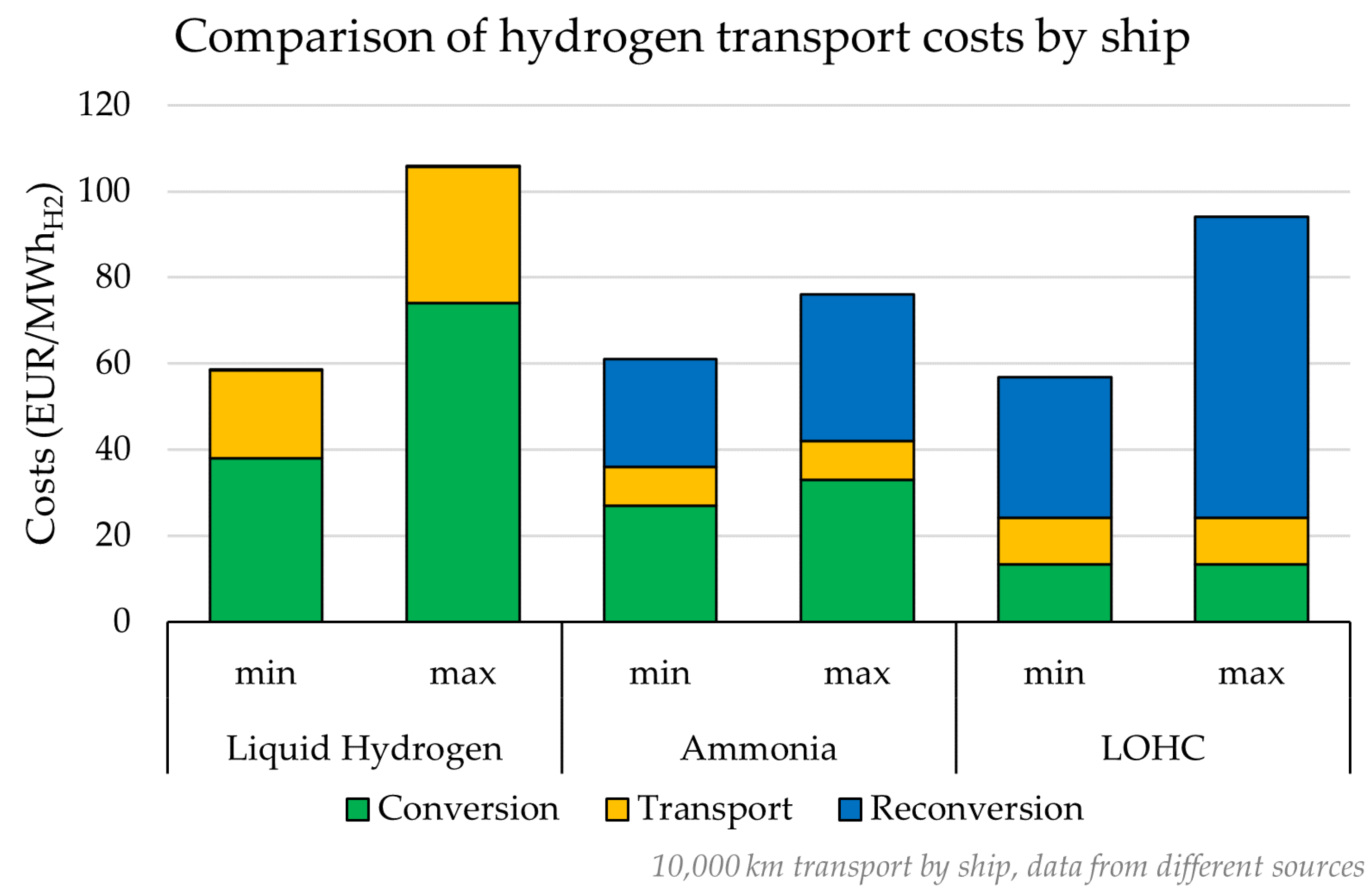
3.1. Shipping Transport
- Liquid hydrogen: In this case, the hydrogen is liquefied, transported via shipping and regasified. The residual energy content of hydrogen along the chain compared to the initial amount is estimated at around 73–79%. The phase with the highest energy loss is the liquefaction process.
- Ammonia carrier: In this case, the hydrogen is sent to the Haber–Bosch process for the production of ammonia, which is then transported via shipment and subjected to the cracking process. The final energy content of hydrogen is estimated to be around 63–64% of the initial amount. The phase with the highest energy loss is the ammonia cracking process.
- Liquid organic hydrogen carrier (LOHC): Hydrogen is used for the production of LOHC, which is then shipped and dehydrogenated back to hydrogen. In this case, the final energy content of hydrogen is estimated to be around 57–59%. The phase with the highest energy loss is the dehydrogenation process back to hydrogen.
3.2. Pipeline Transport
4. Final Energy Uses of Ammonia
4.1. Ammonia for Engine Applications
4.2. Ammonia for Fuel Cell Applications
4.3. Ammonia Used in Other Energy Technologies
4.4. Cracking Process of Ammonia
5. Open Issues
5.1. Health and Safety Issues
5.2. Environmental Impacts
5.3. Competition between E-Fuels and Fertilizers Markets
5.4. Comparison with Other Hydrogen Storage Solutions
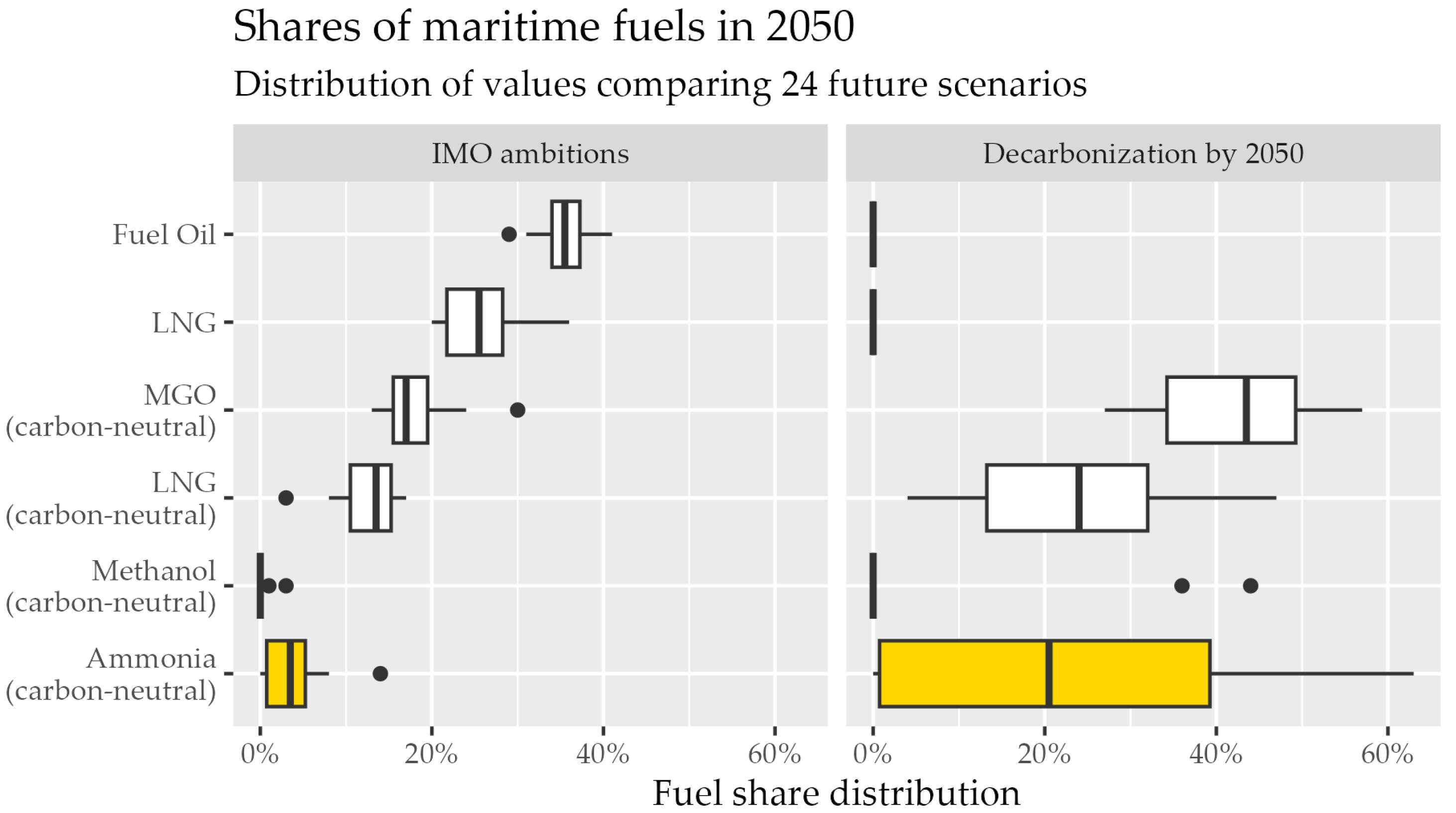
5.5. Ammonia as an Alternative Maritime Fuel
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATR | auto-thermal reforming |
| BAT | best available technology |
| CCS | carbon capture and storage |
| CI | compression ignition |
| EGR | exhaust gas recirculation |
| ESS | energy storage system |
| EU | European Union |
| FC | fuel cell |
| GHG | greenhouse gas |
| HFO | heavy fuel oil |
| HHV | high heating value |
| IEA | International Energy Agency |
| IMO | International Maritime Organization |
| LCOA | levelized cost of ammonia |
| LHV | low heating value |
| LNG | liquefied natural gas |
| LOHC | liquid organic hydrogen carrier |
| MDO | maritime diesel oil |
| MGO | marine gas oil |
| PEM | proton exchange membrane |
| RES | renewable energy source |
| RFNBO | renewable fuel of non-biological origin |
| SCR | selective catalytic reduction |
| SI | spark ignition |
| SMR | steam methane reforming |
| SOFC | solid oxide fuel cell |
| UK | United Kingdom |
References
- IPCC AR6 WGI. Climate Change 2021 the Physical Science Basis. 2021. Available online: https://www.ipcc.ch/report/ar6/wg1/ (accessed on 23 June 2023).
- European Union. Clean energy for all Europeans package. 2019. Available online: https://energy.ec.europa.eu/topics/energy-strategy/clean-energy-all-europeans-package_en (accessed on 23 June 2023).
- EU. REPowerEU: A Plan to Rapidly Reduce Dependence on Russian Fossil Fuels and Fast Forward the Green Transition. 2022. Available online: https://ec.europa.eu/commission/presscorner/detail/en/IP_22_3131 (accessed on 23 June 2023).
- Council of the European Union. Council and Parliament Reach Provisional Deal on Renewable Energy Directive. 2023. Available online: https://www.consilium.europa.eu/en/press/press-releases/2023/03/30/council-and-parliament-reach-provisional-deal-on-renewable-energy-directive/ (accessed on 10 July 2023).
- Mucci, S.; Mitsos, A.; Bongartz, D. Power-to-X processes based on PEM water electrolyzers: A review of process integration and flexible operation. Comput. Chem. Eng. 2023, 175, 108260. [Google Scholar] [CrossRef]
- Wang, A.; Jens, J.; Mavins, D.; Moultak, M.; Schimmel, M.; van der Leun, K.; Peters, D.; Buseman, M. European Hydrogen Backbone: Analysing Future Demand, Supply, and Transport of Hydrogen. 2021. Available online: https://hydrogen-central.com/2021-european-hydrogen-backbone-demand-supply-transport-hydrogen/ (accessed on 23 June 2023).
- Hydrogens. Available online: https://energy.ec.europa.eu/topics/energy-systems-integration/hydrogen_en (accessed on 23 June 2023).
- Atchison, J. Hydrogen Europe: The Role of Clean Ammonia. 2023. Available online: https://www.ammoniaenergy.org/articles/hydrogen-europe-the-role-of-clean-ammonia/ (accessed on 20 June 2023).
- International Energy Agency. The Future of Hydrogen; International Energy Agency: Paris, France, 2019. [Google Scholar] [CrossRef]
- Bermudez, J.M.; Evangelopoulou, S.; Pavan, F. Hydrogen Report. 2021. Available online: https://www.iea.org/reports/hydrogen (accessed on 20 June 2023).
- European Commission. A Hydrogen Strategy for a Climate-Neutral Europe. 2020. Available online: https://knowledge4policy.ec.europa.eu/publication/communication-com2020301-hydrogen-strategy-climate-neutral-europe_en (accessed on 10 July 2023).
- Noussan, M.; Raimondi, P.P.; Scita, R.; Hafner, M. The Role of Green and Blue Hydrogen in the Energy Transition—A Technological and Geopolitical Perspective. Sustainability 2021, 13, 298. [Google Scholar] [CrossRef]
- Liponi, A.; Pasini, G.; Baccioli, A.; Ferrari, L. Hydrogen from renewables: Is it always green? The Italian scenario. Energy Convers. Manag. 2023, 276, 116525. [Google Scholar] [CrossRef]
- Ramanan, A. Five Reasons to Be Concerned about Green Hydrogen. 2021. Available online: https://www.cleanegroup.org/wp-content/uploads/Five-Reasons-to-be-Concerned-About-Green-Hydrogen.pdf (accessed on 30 June 2023).
- Amin, M.; Butt, A.S.; Ahmad, J.; Lee, C.; Azam, S.U.; Mannan, H.A.; Naveed, A.B.; Farooqi, Z.U.R.; Chung, E.; Iqbal, A. Issues and challenges in hydrogen separation technologies. Energy Rep. 2023, 9, 894–911. [Google Scholar] [CrossRef]
- Hydrogen Production, Distribution, Storage and Power Conversion in a Hydrogen Economy—A Technology Review. Chem. Eng. J. Adv. 2021, 8, 100172. [CrossRef]
- Klopčič, N.; Grimmer, I.; Winkler, F.; Sartory, M.; Trattner, A. A review on metal hydride materials for hydrogen storage. J. Energy Storage 2023, 72, 108456. [Google Scholar] [CrossRef]
- International Energy Agency. Global Hydrogen Review 2022. Available online: https://www.iea.org/reports/global-hydrogen-review-2022 (accessed on 23 June 2023).
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.; Bowen, P. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C. Combustion Characteristics of Ammonia in a Modern Spark-Ignition Engine. In Proceedings of the Conference on Sustainable Mobility, Catania, Italy, 4–7 October 2022; Available online: https://saemobilus.sae.org/content/2019-24-0237/ (accessed on 15 June 2023).
- Marbán, G.; Valdés-Solís, T. Towards the hydrogen economy? Int. J. Hydrogen Energy 2007, 32, 1625–1637. [Google Scholar] [CrossRef]
- Reuß, M.; Grube, T.; Robinius, M.; Preuster, P.; Wasserscheid, P.; Stolten, D. Seasonal storage and alternative carriers: A flexible hydrogen supply chain model. Appl. Energy 2017, 200, 290–302. [Google Scholar] [CrossRef]
- Aslam, R.; Müller, K.; Müller, M.; Koch, M.; Wasserscheid, P.; Arlt, W. Measurement of Hydrogen Solubility in Potential Liquid Organic Hydrogen Carriers. J. Chem. Eng. Data 2016, 61, 643–649. [Google Scholar] [CrossRef]
- Dias, V.; Pochet, M.; Contino, F.; Jeanmart, H. Energy and Economic Costs of Chemical Storage. Front. Mech. Eng. 2020, 6, 21. [Google Scholar] [CrossRef]
- Forsythe, R.C.; Cox, C.P.; Wilsey, M.K.; Müller, A.M. Pulsed Laser in Liquids Made Nanomaterials for Catalysis. Chem. Rev. 2021, 121, 7568–7637. [Google Scholar] [CrossRef]
- International Energy Agency. Ammonia Technology Roadmap. 2021. Available online: https://www.iea.org/reports/ammonia-technology-roadmap (accessed on 6 June 2023).
- Khaselev, O.; Bansal, A.; Turner, J. High-efficiency integrated multijunction photovoltaic/electrolysis systems for hydrogen production. Int. J. Hydrogen Energy 2001, 26, 127–132. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, F.; Zhang, Y.; Djire, A. Recent development in electrocatalysts for hydrogen production through water electrolysis. Int. J. Hydrogen Energy 2021, 46, 32284–32317. [Google Scholar] [CrossRef]
- Feng, D.; Liu, X.Y.; Ye, R.; Huang, W.; Tong, Y. Carbon-encapsulated Co2P/P-modified NiMoO4 hierarchical heterojunction as superior pH-universal electrocatalyst for hydrogen production. J. Colloid Interface Sci. 2023, 634, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Energy and environmental assessment of hydrogen from biomass sources: Challenges and perspectives. Biomass Bioenergy 2022, 165, 106556. [CrossRef]
- IEA. Net Zero by 2050: A Roadmap for the Global Energy Sector. 2021. Available online: https://iea.blob.core.windows.net/assets/063ae08a-7114-4b58-a34e-39db2112d0a2/NetZeroby2050-ARoadmapfortheGlobalEnergySector.pdf (accessed on 30 June 2023).
- Cesaro, Z.; Ives, M.; Nayak-Luke, R.; Mason, M.; Bañares-Alcántara, R. Ammonia to power: Forecasting the levelized cost of electricity from green ammonia in large-scale power plants. Appl. Energy 2021, 282, 116009. [Google Scholar] [CrossRef]
- Bouaboula, H.; Ouikhalfan, M.; Saadoune, I.; Chaouki, J.; Zaabout, A.; Belmabkhout, Y. Addressing sustainable energy intermittence for green ammonia production. Energy Rep. 2023, 9, 4507–4517. [Google Scholar] [CrossRef]
- IRENA. A Pathway to Decarbonise the Shipping Sector by 2050. 2021. Available online: https://www.irena.org/publications/2021/Oct/A-Pathway-to-Decarbonise-the-Shipping-Sector-by-2050 (accessed on 30 June 2023).
- Hine, L. Ammonia Swings into Frame as a Potential Future Marine Fuel. 2019. Available online: https://www.tradewindsnews.com/gas/ammonia-swings-into-frame-as-a-potential-future-marine-fuel/2-1-523024 (accessed on 6 June 2023).
- Reuters. Explainer: Why a Clean Energy Transition Is so Important to G7 Chair Japan. 2023. Available online: https://www.reuters.com/business/environment/why-clean-energy-transition-is-so-important-g7-chair-japan-2023-04-12/ (accessed on 6 June 2023).
- Reuters. Japanese Gov’t Awards $1.62 bln to Japan-Australia Hydrogen Energy Supply JV. 2023. Available online: https://www.reuters.com/business/energy/japanese-govt-awards-162-bln-japan-australia-hydrogen-energy-supply-jv-2023-03-07/ (accessed on 6 June 2023).
- Reuters. Japan Receives First Low-Carbon Ammonia Cargo from Saudi Arabia. 2023. Available online: https://www.reuters.com/business/sustainable-business/japan-receives-first-low-carbon-ammonia-cargo-saudi-arabia-2023-04-21/ (accessed on 6 June 2023).
- Zawya Projects. Saudi Arabia Ships World’s First Accredited Low-Carbon Blue Ammonia to South Korea. 2022. Available online: https://www.zawya.com/en/projects/industry/saudi-arabia-ships-worlds-first-accredited-low-carbon-blue-ammonia-to-south-korea-bj3dz936 (accessed on 6 June 2023).
- Hayek, S. First Low-Carbon Ammonia Shipment from the UAE to Germany. 2022. Available online: https://www.hydrogen-worldexpo.com/industry_news/first-low-carbon-ammonia-shipment-from-the-uae-to-germany/ (accessed on 6 June 2023).
- TÜV Rheinland. Certified Product: Production of Blue Ammonia, ID 0000083801. 2022. Available online: https://www.certipedia.com/quality_marks/0000083801?locale=en&certificate_number=C01-2022-07-21256370 (accessed on 6 June 2023).
- TÜV Rheinland. Certified Product: Production of Blue Ammonia, ID 0000083803. 2022. Available online: https://www.certipedia.com/quality_marks/0000083803?locale=en&certificate_number=C02-2022-07-21256370 (accessed on 6 June 2023).
- Giddey, S.; Badwal, S.P.S.; Munnings, C.; Dolan, M. Ammonia as a Renewable Energy Transportation Media. ACS Sustain. Chem. Eng. 2017, 5, 10231–10239. [Google Scholar] [CrossRef]
- Gerlitz, L.; Mildenstrey, E.; Prause, G. Ammonia as Clean Shipping Fuel for the Baltic Sea Region. Transp. Telecommun. J. 2022, 23, 102–112. [Google Scholar] [CrossRef]
- Al-Breiki, M.; Bicer, Y. Technical assessment of liquefied natural gas, ammonia and methanol for overseas energy transport based on energy and exergy analyses. Int. J. Hydrogen Energy 2020, 45, 34927–34937. [Google Scholar] [CrossRef]
- IRENA. Hydrogen: A Renewable Energy Perspective. 2019. Available online: https://www.irena.org/publications/2019/Sep/Hydrogen-A-renewable-energy-perspective (accessed on 10 July 2023).
- Cihlar, J.; Villar Lejarreta, A.; Wang, A.; Melgar, F.; Jens, J.; Rio, P. Hydrogen Generation in Europe: Overview of Costs and Key Benefits; European Commission and Directorate-General for Energy Publications Office: Brussel, Belgium, 2020. [Google Scholar] [CrossRef]
- BNEF. Hydrogen Economy Outlook. 2020. Available online: https://data.bloomberglp.com/professional/sites/24/BNEF-Hydrogen-Economy-Outlook-Key-Messages-30-Mar-2020.pdf (accessed on 10 July 2023).
- Papadias, D.; Ahluwalia, R.K.; Connelly, E.; Devlin, P. Total Cost of Ownership (TCO) Analysis for Hydrogen Fuel Cells in Maritime Applications—Preliminary Results. 2019. Available online: https://www.energy.gov/sites/prod/files/2019/10/f68/fcto-h2-at-ports-workshop-2019-viii5-ahluwalia.pdf (accessed on 10 July 2023).
- The Royal Society. Ammonia: Zero-Carbon Fertiliser, Fuel and Energy Store; The Royal Society: London, UK, 2020. [Google Scholar]
- Reuters. Russian Forces Repeatedly Shell Ammonia Pipeline in Ukraine’s Kharkiv Region, Governor Says. 2023. Available online: https://www.reuters.com/world/europe/russian-forces-repeatedly-shell-ammonia-pipeline-ukraines-kharkiv-region-2023-06-06/ (accessed on 5 July 2023).
- Egerer, J.; Grimm, V.; Niazmand, K.; Runge, P. The economics of global green ammonia trade—“Shipping Australian wind and sunshine to Germany”. Appl. Energy 2023, 334, 120662. [Google Scholar] [CrossRef]
- Rodríguez, C.G.; Lamas, M.I.; Rodríguez, J.d.D.; Abbas, A. Possibilities of Ammonia as Both Fuel and NOx Reductant in Marine Engines: A Numerical Study. J. Mar. Sci. Eng. 2022, 10, 43. [Google Scholar] [CrossRef]
- Gross, C.W.; Kong, S.C. Performance characteristics of a compression-ignition engine using direct-injection ammonia–DME mixtures. Fuel 2013, 103, 1069–1079. [Google Scholar] [CrossRef]
- Mørch, C.; Bjerre, A.; Gøttrup, M.; Sorenson, S.; Schramm, J. Ammonia/hydrogen mixtures in an SI-engine: Engine performance and analysis of a proposed fuel system. Fuel 2011, 90, 854–864. [Google Scholar] [CrossRef]
- Hansson, J.; Brynolf, S.; Fridell, E.; Lehtveer, M. The Potential Role of Ammonia as Marine Fuel—Based on Energy Systems Modeling and Multi-Criteria Decision Analysis. Sustainability 2020, 12, 3265. [Google Scholar] [CrossRef]
- Lan, R.; Tao, S. Ammonia as a suitable fuel for fuel cells. Front Energy Res 2014, 2, 35. [Google Scholar] [CrossRef]
- Kim, K.; Roh, G.; Kim, W.; Chun, K. A Preliminary Study on an Alternative Ship Propulsion System Fueled by Ammonia: Environmental and Economic Assessments. J. Mar. Sci. Eng. 2020, 8, 183. [Google Scholar] [CrossRef]
- Julia, H.; Erik, F.; Selma, B. Ammonia as Fuel for Shipping—A Synthesis of Knowledge; Lighthouse: Bandar Lampung, Indonesia, 2020; 20p, Available online: https://fudinfo.trafikverket.se/fudinfoexternwebb/Publikationer/Publikationer_004101_004200/Publikation_004185/FS2_2019_The%20potential%20of%20ammonia%20as%20fuel%20for%20shipping.pdf (accessed on 5 July 2023).
- Schönborn, A. Aqueous solution of ammonia as marine fuel. Proc. Inst. Mech. Eng. Part M J. Eng. Marit. Environ. 2021, 235, 142–151. [Google Scholar] [CrossRef]
- Bilgili, L. A systematic review on the acceptance of alternative marine fuels. Renew. Sustain. Energy Rev. 2023, 182, 113367. [Google Scholar] [CrossRef]
- Dimitriou, P.; Javaid, R. A review of ammonia as a compression ignition engine fuel. Int. J. Hydrogen Energy 2020, 45, 7098–7118. [Google Scholar] [CrossRef]
- Together in Safety. Future Fuels Risk Assessment—Together in Safety. 2022. Available online: https://togetherinsafety.info/wp-content/uploads/2022/06/Future-Fuels-Report.pdf (accessed on 30 June 2023).
- Reiter, A.J.; Kong, S.C. Diesel Engine Operation Using Ammonia as a Carbon-Free Fuel. In Proceedings of the 2010 Internal Combustion Engine Division Fall Technical Conference, San Antonio, TX, USA, 12–15 September 2010. [Google Scholar] [CrossRef]
- Reiter, A.J.; Kong, S.C. Combustion and emissions characteristics of compression-ignition engine using dual ammonia-diesel fuel. Fuel 2011, 90, 87–97. [Google Scholar] [CrossRef]
- Gill, S.; Chatha, G.; Tsolakis, A.; Golunski, S.; York, A. Assessing the effects of partially decarbonising a diesel engine by co-fuelling with dissociated ammonia. Int. J. Hydrogen Energy 2012, 37, 6074–6083. [Google Scholar] [CrossRef]
- Zero Carbon Shipping. Managing Emissions from Ammonia-Fueled Vessels. 2023. Available online: https://www.zerocarbonshipping.com/publications/managing-emissions-from-ammonia-fueled-vessels/ (accessed on 30 June 2023).
- EMSA. Study on the Use of Fuel Cells in Shipping. 2017. Available online: https://www.emsa.europa.eu/publications/item/2921-emsa-study-on-the-use-of-fuel-cells-in-shipping.html (accessed on 30 June 2023).
- Jeerh, G.; Zhang, M.; Tao, S. Recent progress in ammonia fuel cells and their potential applications. J. Mater. Chem. A 2021, 9, 727–752. [Google Scholar] [CrossRef]
- Karabeyoglu, A.; Evans, B.; Stevens, J.; Cantwell, B.; Micheletti, D. Development of Ammonia Based Fuels for Environmentally Friendly Power Generation. In Proceedings of the 10th International Energy Conversion Engineering Conference, Atlanta, GA, USA, 30 July–1 August 2012; Available online: http://xxx.lanl.gov/abs/https://arc.aiaa.org/doi/pdf/10.2514/6.2012-4055 (accessed on 25 June 2023).
- Fallah, M.; Mahmoudi, S.M.S.; Yari, M.; Akbarpour Ghiasi, R. Advanced exergy analysis of the Kalina cycle applied for low temperature enhanced geothermal system. Energy Convers. Manag. 2016, 108, 190–201. [Google Scholar] [CrossRef]
- European Commission, Ortiz Cebolla, R.; Dolci, F.; Weidner, E. Assessment of Hydrogen Delivery Options: Feasibility of Transport of Green Hydrogen within EUROPE; Ufficio delle Pubblicazioni dell’Unione Europea: Brussels, Belgium, 2022. [Google Scholar] [CrossRef]
- Morlanés, N.; Katikaneni, S.P.; Paglieri, S.N.; Harale, A.; Solami, B.; Sarathy, S.M.; Gascon, J. A technological roadmap to the ammonia energy economy: Current state and missing technologies. Chem. Eng. J. 2021, 408, 127310. [Google Scholar] [CrossRef]
- Wan, Z.; Tao, Y.; Shao, J.; Zhang, Y.; You, H. Ammonia as an effective hydrogen carrier and a clean fuel for solid oxide fuel cells. Energy Convers. Manag. 2021, 228, 113729. [Google Scholar] [CrossRef]
- Shim, C.; Han, J.; Henze, D.K.; Shephard, M.W.; Zhu, L.; Moon, N.; Kharol, S.K.; Dammers, E.; Cady-Pereira, K. Impact of NH3 Emissions on Particulate Matter Pollution in South Korea: A Case Study of the Seoul Metropolitan Area. Atmosphere 2022, 13, 1227. [Google Scholar] [CrossRef]
- Boero, A.J.; Kardux, K.; Kovaleva, M.; Salas, D.A.; Mooijer, J.; Mashruk, S.; Townsend, M.; Rouwenhorst, K.; Valera-Medina, A.; Ramirez, A.D. Environmental Life Cycle Assessment of Ammonia-Based Electricity. Energies 2021, 14, 6721. [Google Scholar] [CrossRef]
- Leach, A.M.; Galloway, J.N.; Bleeker, A.; Erisman, J.W.; Kohn, R.; Kitzes, J. A nitrogen footprint model to help consumers understand their role in nitrogen losses to the environment. Environ. Dev. 2012, 1, 40–66. [Google Scholar] [CrossRef]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.; Bleeker, A.; Howard, C.; Bekunda, M.; Grizzetti, B.; de Vries, W.; van Grinsven, H.; Abrol, Y.; Adhya, T.; Billen, G.; et al. Our Nutrient World: The Challenge to Produce More Food and Energy with Less Pollution; NERC/Centre for Ecology & Hydrology: Edinburgh, UK, 2013. [Google Scholar]
- McKinlay, C.J.; Turnock, S.R.; Hudson, D.A. Route to zero emission shipping: Hydrogen, ammonia or methanol? Int. J. Hydrogen Energy 2021, 46, 28282–28297. [Google Scholar] [CrossRef]
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Prussi, M.; Scarlat, N.; Acciaro, M.; Kosmas, V. Potential and limiting factors in the use of alternative fuels in the European maritime sector. J. Clean. Prod. 2021, 291, 125849. [Google Scholar] [CrossRef]
- Department for Transport. Clean Maritime Plan. 2019. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/815664/clean-maritime-plan.pdf (accessed on 30 June 2023).
- Global Maritime Fuel. Mapping of Zero Emission Pilots and Demonstration Projects. 2022. Available online: https://www.globalmaritimeforum.org/content/2022/03/Mapping-of-zero-emission-pilots-and-demonstration-projects_third-edition.pdf (accessed on 30 June 2023).
- Gray, N.; McDonagh, S.; O’Shea, R.; Smyth, B.; Murphy, J.D. Decarbonising ships, planes and trucks: An analysis of suitable low-carbon fuels for the maritime, aviation and haulage sectors. Adv. Appl. Energy 2021, 1, 100008. [Google Scholar] [CrossRef]
- SEA-LNG. Comparison of Alternative Marine Fuels. 2019. Available online: https://sea-lng.org/wp-content/uploads/2020/04/Alternative-Marine-Fuels-Study_final_report_25.09.19.pdf (accessed on 6 June 2023).
- Korberg, A.; Brynolf, S.; Grahn, M.; Skov, I. Techno-economic assessment of advanced fuels and propulsion systems in future fossil-free ships. Renew. Sustain. Energy Rev. 2021, 142, 110861. [Google Scholar] [CrossRef]
- Böck, H. Here’s What You Need to Know: Ammonia and Methanol 101 Pros and Cons. 2023. Available online: https://fathom.world/ammonia-methanol-101/ (accessed on 6 June 2023).
- DNV. Maritime Forecast to 2050—Energy Transition Outlook 2022. 2022. Available online: https://www.dnv.com/maritime/publications/maritime-forecast-2022/ (accessed on 31 July 2023).

| Hydrogen | Ammonia | |
|---|---|---|
| Boiling point [°C] | −252.7 | −33.34 |
| Melting point [°C] | −259 | −77.73 |
| Gas density [kg/m3] | 0.089 | 0.769 |
| Liquid density [kg/L] | 0.071 | 0.6819 |
| LHV [MJ/kg] | 119.9 | 18.6 |
| LHV as liquid [MJ/L] | 8.5 | 12.7 |
| HHV [MJ/kg] | 141.9 | 22.5 |
| HHV as liquid [MJ/L] | 10.1 | 15.3 |
| Auto ignition [°C] | 585 | 651 |
| Flammability/air [-] | 4–75% | 15–28% |
| Production Route | Energy Intensity (GJ/t) | t CO2/t | |||||
|---|---|---|---|---|---|---|---|
| Feedstock | Fuel | Electricity | Steam | Gross | Net | ||
| Natural gas SMR | 21.0 | 11.1 | 0.3 | −4.8 | 32.4 | 27.6 | 1.8 |
| Natural gas ATR | 25.8 | 2.1 | 1.0 | 0.0 | 28.9 | 28.9 | 1.6 |
| Coal gasification | 18.6 | 15.1 | 3.7 | −1.3 | 37.4 | 36.1 | 3.2 |
| SMR with CCS | 21.0 | 11.1 | 1.0 | −3.1 | 33.1 | 30.0 | 0.1 |
| ATR with CCS | 25.8 | 2.1 | 1.5 | 0.0 | 29.4 | 29.4 | 0.1 |
| Coal with CCS | 18.6 | 15.1 | 4.9 | 2.6 | 38.6 | 41.2 | 0.2 |
| Electrolysis | 0.0 | 0.0 | 36.0 | −1.6 | 36.0 | 34.4 | 0.0 |
| Biomass gasification | 18.6 | 16.5 | 1.4 | 0.0 | 36.5 | 36.5 | 0.0 |
| Methane pyrolysis | 40.5 | 0.0 | 8.4 | −1.6 | 48.9 | 47.3 | 0.0 |
| Temperature | Pressure | Gravimetric H2 Content | Volumetric H2 Content | |
|---|---|---|---|---|
| K | MPa | wt% | kWh/dm3 | |
| Compressed hydrogen | ambient | 35 | 100% | 0.8 |
| Compressed hydrogen | ambient | 70 | 100% | 1.3 |
| Liquefied hydrogen | −253 | ambient | 100% | 2.2 |
| Ammonia | ambient | 1 | 18% | 4.0 |
| Methanol | ambient | ambient | 13% | 3.3 |
| Metal hydrides | 252–285 | 0.2–0.8 | 2% | 4–4.1 |
| Complex hydrides | 473–573 | n.a. | 8–19% | 3.2–4.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negro, V.; Noussan, M.; Chiaramonti, D. The Potential Role of Ammonia for Hydrogen Storage and Transport: A Critical Review of Challenges and Opportunities. Energies 2023, 16, 6192. https://doi.org/10.3390/en16176192
Negro V, Noussan M, Chiaramonti D. The Potential Role of Ammonia for Hydrogen Storage and Transport: A Critical Review of Challenges and Opportunities. Energies. 2023; 16(17):6192. https://doi.org/10.3390/en16176192
Chicago/Turabian StyleNegro, Viviana, Michel Noussan, and David Chiaramonti. 2023. "The Potential Role of Ammonia for Hydrogen Storage and Transport: A Critical Review of Challenges and Opportunities" Energies 16, no. 17: 6192. https://doi.org/10.3390/en16176192
APA StyleNegro, V., Noussan, M., & Chiaramonti, D. (2023). The Potential Role of Ammonia for Hydrogen Storage and Transport: A Critical Review of Challenges and Opportunities. Energies, 16(17), 6192. https://doi.org/10.3390/en16176192








