Anaerobic Digestion of Spoiled Maize, Lucerne and Barley Silage Mixture with and without Cow Manure: Methane Yields and Kinetic Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Kinetic Modelling
2.3. Theoretical Methane Yield
2.4. Statistical Analysis
2.5. Synergistic Effect
2.6. Analytical Methods
3. Results and Discussion
3.1. Chemical Composition of Substrates and Inoculum
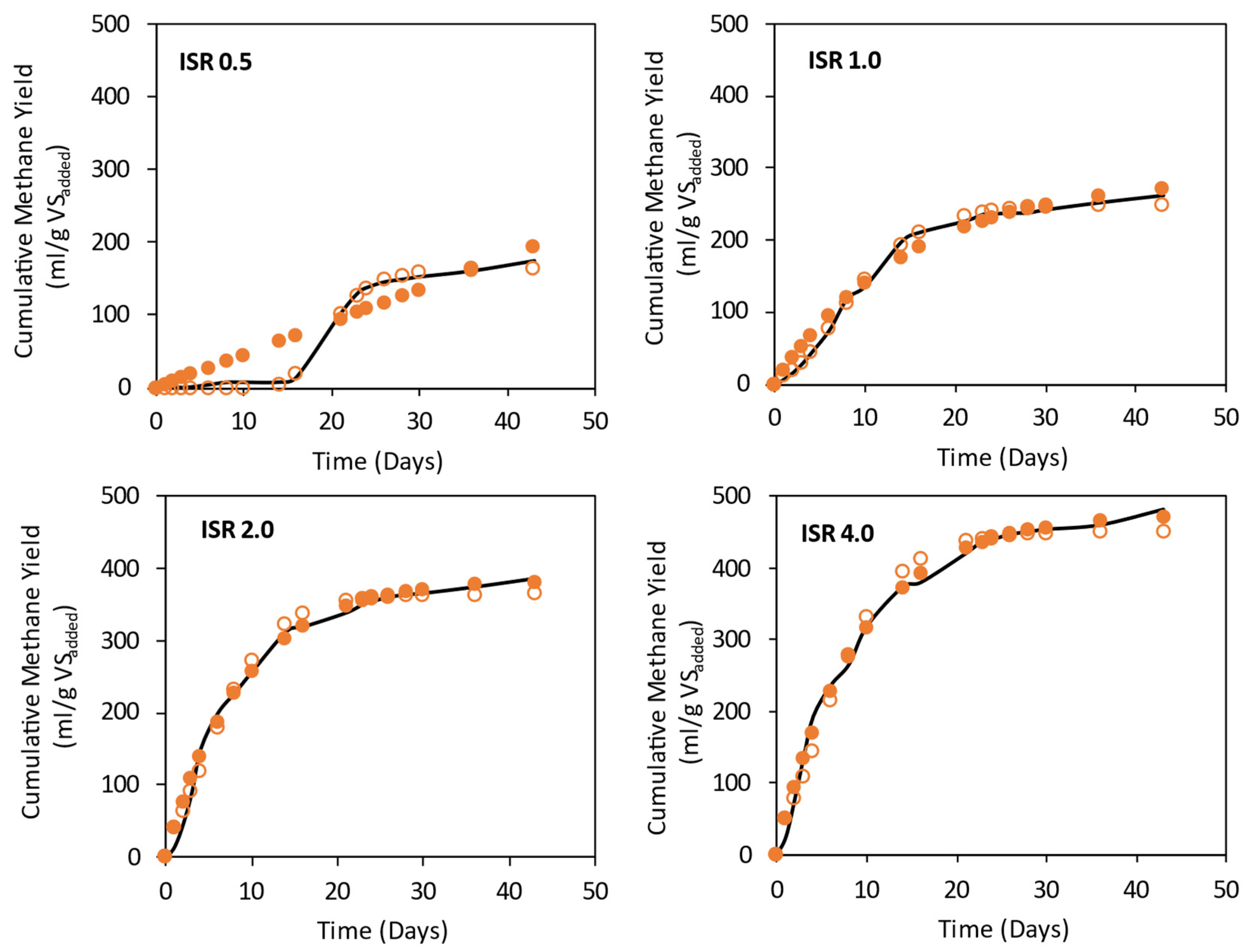
3.2. Effect of ISR on Methane Yields for Mono-Digestion of Spoiled Silage Mixture
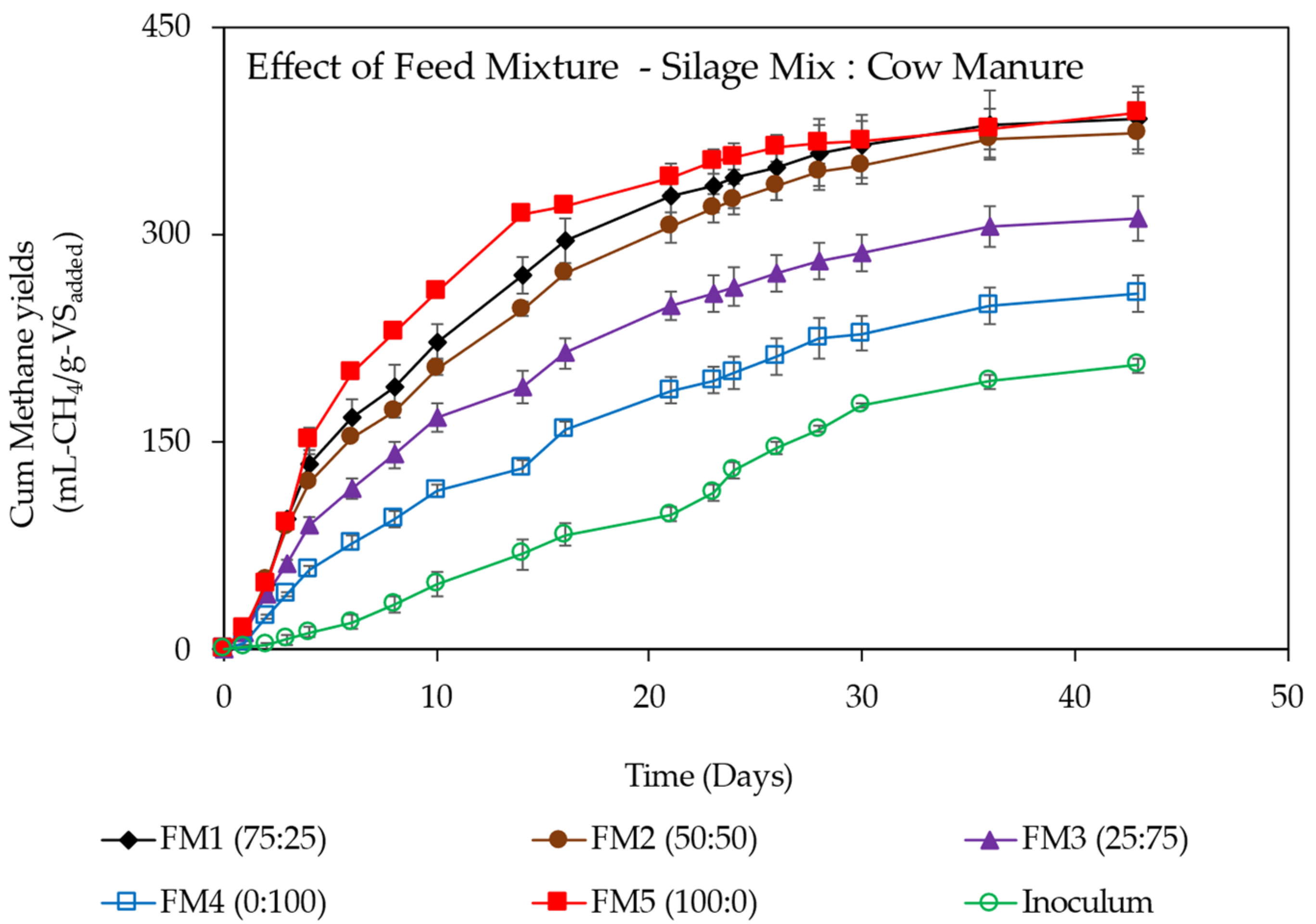
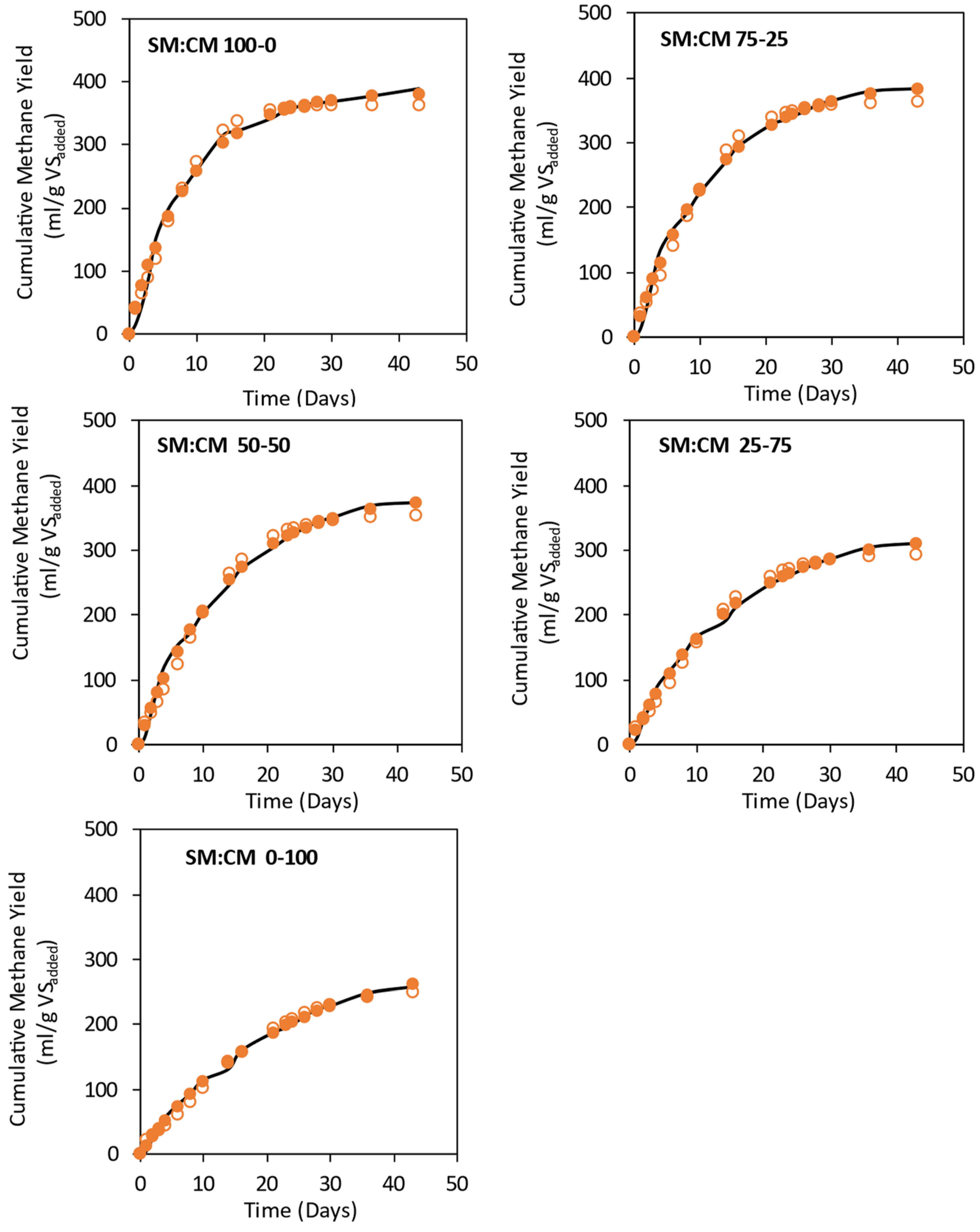
3.3. Effect of Feed Mix Ratios on Methane Yields for Co-Digestion of Spoiled Silage Mix with Cow Manure
3.4. Effect of ISR and Feed Mix Ratio on Chemical Composition of Digestates
3.5. VS Removal
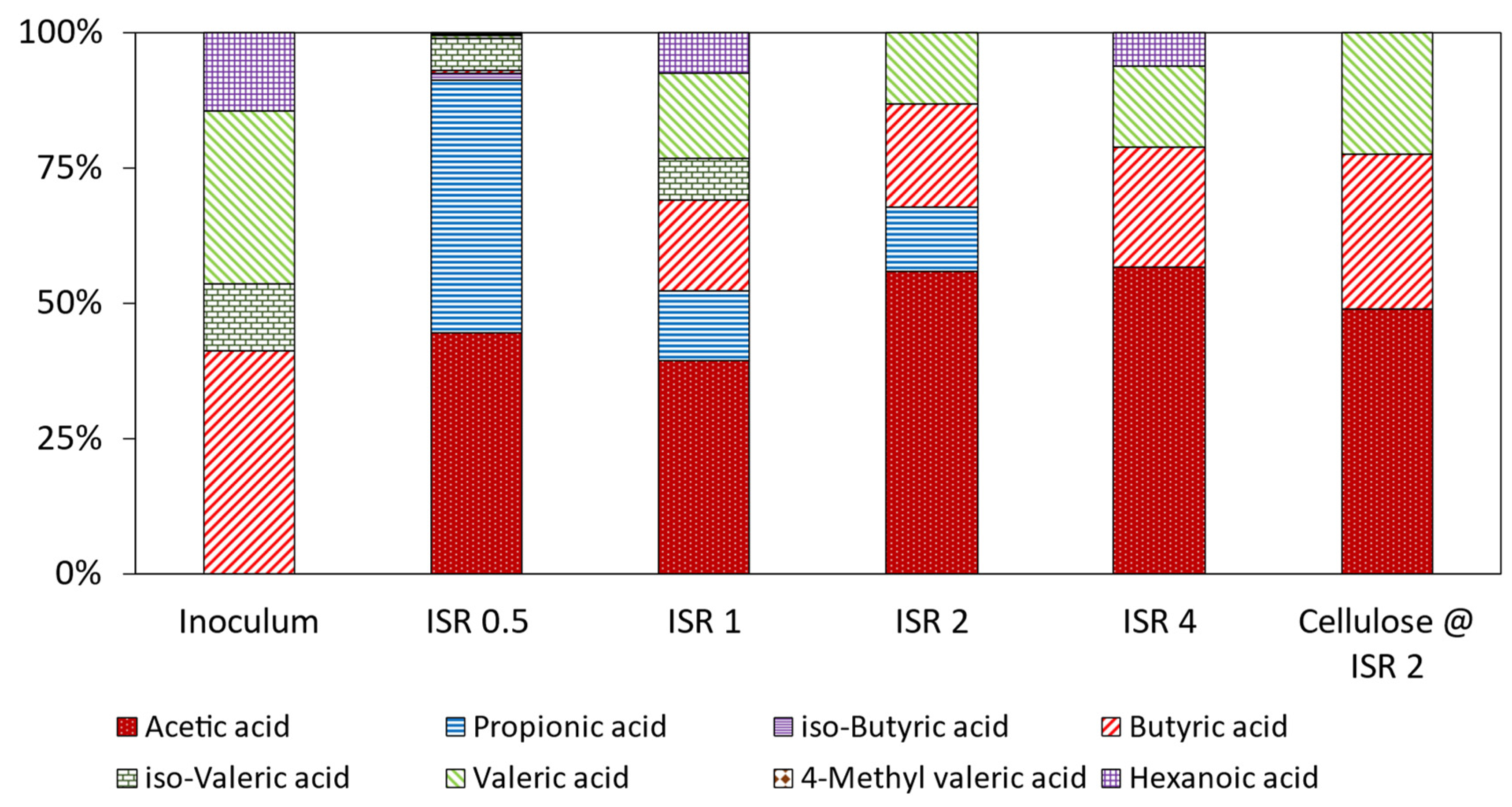
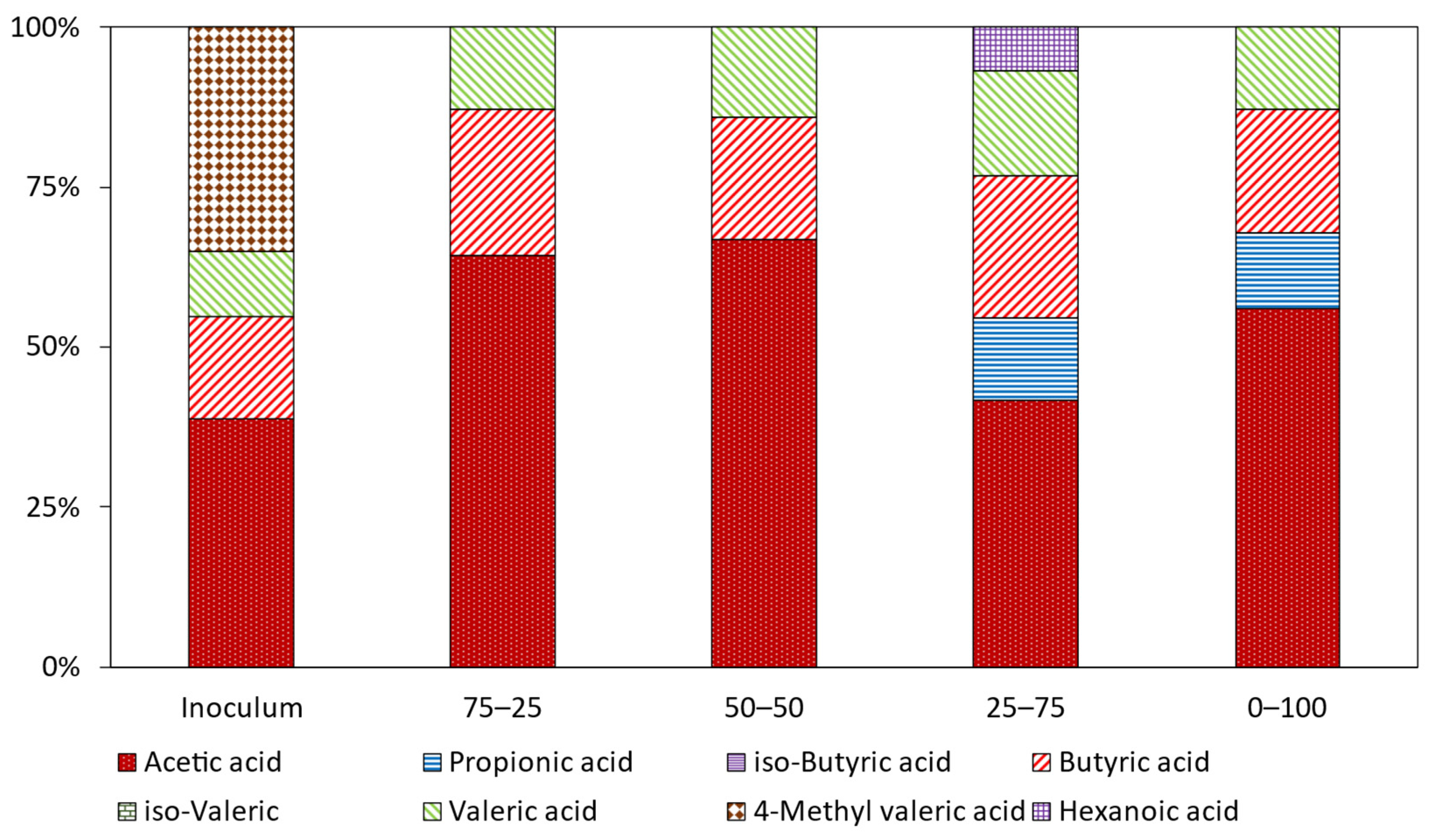
3.6. Volatile Fatty Acids Profile in the Digestates
4. Effect of ISR and Feed Ratio on Methane Production Kinetics
Synergistic and Antagonistic Effect
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Australian Bureau of Statistics. Australia Agricultural Commities. 2023. Available online: https://www.abs.gov.au/statistics/industry/agriculture/agricultural-commodities-australia/latest-release (accessed on 24 June 2023).
- Stockdale, C. Wastage of conserved fodder when feeding livestock. Anim. Prod. Sci. 2010, 50, 400–404. [Google Scholar] [CrossRef]
- Driehuis, F.; Elferink, S.O. The impact of the quality of silage on animal health and food safety: A review. Vet. Q. 2000, 22, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.; Idler, C.; Heiermann, M. Improving aerobic stability and biogas production of maize silage using silage additives. Bioresour. Technol. 2015, 197, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.; Davies, D. The aerobic stability of silage: Key findings and recent developments. Grass Forage Sci. 2013, 68, 1–19. [Google Scholar] [CrossRef]
- Tabacco, E.; Righi, F.; Quarantelli, A.; Borreani, G. Dry matter and nutritional losses during aerobic deterioration of corn and sorghum silages as influenced by different lactic acid bacteria inocula. J. Dairy Sci. 2011, 94, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Pakarinen, O.; Lehtomäki, A.; Rissanen, S.; Rintala, J. Storing energy crops for methane production: Effects of solids content and biological additive. Bioresour. Technol. 2008, 99, 7074–7082. [Google Scholar] [CrossRef]
- Robinson, N.E.; Derksen, F.; Olszewski, M.; Buechner-Maxwell, V. The pathogenesis of chronic obstructivepulmonary disease of horses. Br. Vet. J. 1996, 152, 283–306. [Google Scholar] [CrossRef]
- Wang, J. Decentralized biogas technology of anaerobic digestion and farm ecosystem: Opportunities and challenges. Front. Energy Res. 2014, 2, 10. [Google Scholar] [CrossRef]
- Villadsen, S.N.; Fosbøl, P.L.; Angelidaki, I.; Woodley, J.M.; Nielsen, L.P.; Møller, P. The potential of biogas; the solution to energy storage. ChemSusChem 2019, 12, 2147–2153. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Wang, S.; Wang, Z.; Liu, Y.; Hu, Z.; Zhan, X. Environmental sustainability assessment of pig manure mono-and co-digestion and dynamic land application of the digestate. Renew. Sustain. Energy Rev. 2021, 137, 110476. [Google Scholar] [CrossRef]
- Dechrugsa, S.; Kantachote, D.; Chaiprapat, S. Effects of inoculum to substrate ratio, substrate mix ratio and inoculum source on batch co-digestion of grass and pig manure. Bioresour. Technol. 2013, 146, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Beausang, C.; McDonnell, K.; Murphy, F. Assessing the environmental sustainability of grass silage and cattle slurry for biogas production. J. Clean. Prod. 2021, 298, 126838. [Google Scholar] [CrossRef]
- Wall, D.M.; Allen, E.; Straccialini, B.; O’Kiely, P.; Murphy, J.D. Optimisation of digester performance with increasing organic loading rate for mono-and co-digestion of grass silage and dairy slurry. Bioresour. Technol. 2014, 173, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, T.; Santos, E.M. Advances in Silage Production and Utilization; BoD–Books on Demand: McFarland, WI, USA, 2016. [Google Scholar]
- Sun, C.; Cao, W.; Banks, C.J.; Heaven, S.; Liu, R. Biogas production from undiluted chicken manure and maize silage: A study of ammonia inhibition in high solids anaerobic digestion. Bioresour. Technol. 2016, 218, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Klang, J.; Theuerl, S.; Szewzyk, U.; Huth, M.; Tölle, R.; Klocke, M. Dynamic variation of the microbial community structure during the long-time mono-fermentation of maize and sugar beet silage. Microb. Biotechnol. 2015, 8, 764–775. [Google Scholar] [CrossRef]
- Gallegos, D.; Wedwitschka, H.; Moeller, L.; Weinrich, S.; Zehnsdorf, A.; Nelles, M.; Stinner, W. Mixed silage of Elodea and wheat straw as a substrate for energy production in anaerobic digestion plants. Energy Sustain. Soc. 2018, 8, 7. [Google Scholar] [CrossRef]
- Kintl, A.; Elbl, J.; Vítěz, T.; Brtnický, M.; Skládanka, J.; Hammerschmiedt, T.; Vítězová, M. Possibilities of using white sweetclover grown in mixture with maize for biomethane production. Agronomy 2020, 10, 1407. [Google Scholar] [CrossRef]
- Bhatnagar, N.; Ryan, D.; Murphy, R.; Enright, A. Effect of co-digestion ratio and enzyme treatment on biogas production from grass silage and chicken litter. Waste Biomass Valoriz. 2019, 10, 3271–3277. [Google Scholar] [CrossRef]
- Arici, Ş.; Koçar, G. The effect of adding maize silage as a co-substrate for anaerobic animal manure digestion. Int. J. Green Energy 2015, 12, 453–460. [Google Scholar] [CrossRef]
- Filer, J.; Ding, H.H.; Chang, S. Biochemical methane potential (BMP) assay method for anaerobic digestion research. Water 2019, 11, 921. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Pererva, Y.; Miller, C.D.; Sims, R.C. Existing empirical kinetic models in biochemical methane potential (BMP) testing, their selection and numerical solution. Water 2020, 12, 1831. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.G.; Kuthiala, S.; Angelidaki, I. Co-digestion and model simulations of source separated municipal organic waste with cattle manure under batch and continuously stirred tank reactors. Energy Convers. Manag. 2018, 159, 1–6. [Google Scholar] [CrossRef]
- Association, A.P.H.; Association, A.P.H. Standard methods for the Examination of Water and Wastewater, APHA. In American Water Works Association and Water Environment Federation, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Latif, M.A.; Mehta, C.M.; Batstone, D.J. Influence of low pH on continuous anaerobic digestion of waste activated sludge. Water Res. 2017, 113, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Sarojam, P. Analysis of Wastewater for Metals Using ICP-OES; Perkin Elmer Instruments: Shelton, CT, USA, 2010; p. 11. Available online: https://resources.perkinelmer.com/lab-solutions/resources/docs/app_metalsinwastewater.pdf (accessed on 17 July 2023).
- Paulose, P.; Kaparaju, P. Anaerobic mono-digestion of sugarcane trash and bagasse with and without pretreatment. Ind. Crops Prod. 2021, 170, 113712. [Google Scholar] [CrossRef]
- Beckett, N.M.; Cresswell, S.L.; Grice, D.I.; Carter, J.F. Isotopic profiling of seized benzylpiperazine and trifluoromethylphenylpiperazine tablets using δ13C and δ15N stable isotopes. Sci. Justice 2015, 55, 51–56. [Google Scholar] [CrossRef]
- Krotz, L.; Giazzi, G. Elemental Analysis: CHNS/O characterization of Carbon Black Compliance with ASTM D5373 Method; Thermo Fisher Scientific: Milan, Italy, 2017; Available online: https://assets.thermofisher.com/TFS-Assets/CMD/Application-Notes/AN-42238-OEA-CHNSO-Carbon-Black-AN42238-EN.pdf (accessed on 21 July 2023).
- Sambusiti, C.; Ficara, E.; Malpei, F.; Steyer, J.; Carrère, H. Benefit of sodium hydroxide pretreatment of ensiled sorghum forage on the anaerobic reactor stability and methane production. Bioresour. Technol. 2013, 144, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.C.; Cheng, Y.W.; Ishak, S.; Lam, M.K.; Lim, J.W.; Tan, I.S.; Show, P.L.; Lee, K.T. Anaerobic digestate as a low-cost nutrient source for sustainable microalgae cultivation: A way forward through waste valorization approach. Sci. Total Environ. 2022, 803, 150070. [Google Scholar] [CrossRef]
- Yan, Z.; Song, Z.; Li, D.; Yuan, Y.; Liu, X.; Zheng, T. The effects of initial substrate concentration, C/N ratio, and temperature on solid-state anaerobic digestion from composting rice straw. Bioresour. Technol. 2015, 177, 266–273. [Google Scholar] [CrossRef]
- Rodriguez, C.; Alaswad, A.; El-Hassan, Z.; Olabi, A.G. Waste paper and macroalgae co-digestion effect on methane production. Energy 2018, 154, 119–125. [Google Scholar] [CrossRef]
- Moset, V.; Al-zohairi, N.; Møller, H.B. The impact of inoculum source, inoculum to substrate ratio and sample preservation on methane potential from different substrates. Biomass Bioenergy 2015, 83, 474–482. [Google Scholar] [CrossRef]
- Zeng, S.; Yuan, X.; Shi, X.; Qiu, Y. Effect of inoculum/substrate ratio on methane yield and orthophosphate release from anaerobic digestion of Microcystis spp. J. Hazard. Mater. 2010, 178, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Raposo, F.; Fernández-Cegrí, V.; De la Rubia, M.; Borja, R.; Béline, F.; Cavinato, C.; Demirer, G.; Fernández, B.; Fernández-Polanco, M.; Frigon, J. Biochemical methane potential (BMP) of solid organic substrates: Evaluation of anaerobic biodegradability using data from an international interlaboratory study. J. Chem. Technol. Biotechnol. 2011, 86, 1088–1098. [Google Scholar] [CrossRef]
- Lallement, A.; Siaud, A.; Peyrelasse, C.; Kaparaju, P.; Schraauwers, B.; Maunas, S.; Monlau, F. Impact of operational factors, inoculum origin, and feedstock preservation on the biochemical methane potential. Bioengineering 2021, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Neves, L.; Oliveira, R.; Alves, M. Influence of inoculum activity on the bio-methanization of a kitchen waste under different waste/inoculum ratios. Process Biochem. 2004, 39, 2019–2024. [Google Scholar] [CrossRef]
- Jensen, M.B.; De Jonge, N.; Dolriis, M.D.; Kragelund, C.; Fischer, C.H.; Eskesen, M.R.; Noer, K.; Møller, H.B.; Ottosen, L.D.M.; Nielsen, J.L. Cellulolytic and xylanolytic microbial communities associated with lignocellulose-rich wheat straw degradation in anaerobic digestion. Front. Microbiol. 2021, 12, 645174. [Google Scholar] [CrossRef]
- Raposo, F.; Borja, R.; Rincon, B.; Jimenez, A. Assessment of process control parameters in the biochemical methane potential of sunflower oil cake. Biomass Bioenergy 2008, 32, 1235–1244. [Google Scholar] [CrossRef]
- Wu, X.; Yao, W.; Zhu, J. Biogas and CH4 Productivity by Co-Digesting Swine Manure with Three Crop Residues as an External Carbon Source. Bioresour. Technol. 2010, 101, 4042–4047. [Google Scholar] [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Kaparaju, P.; Rintala, J. Anaerobic co-digestion of potato tuber and its industrial by-products with pig manure. Resour. Conserv. Recycl. 2005, 43, 175–188. [Google Scholar] [CrossRef]
- Ma, G.; Ndegwa, P.; Harrison, J.H.; Chen, Y. Methane yields during anaerobic co-digestion of animal manure with other feedstocks: A meta-analysis. Sci. Total Environ. 2020, 728, 138224. [Google Scholar] [CrossRef]
- Xie, S.; Lawlor, P.G.; Frost, J.; Hu, Z.; Zhan, X. Effect of pig manure to grass silage ratio on methane production in batch anaerobic co-digestion of concentrated pig manure and grass silage. Bioresour. Technol. 2011, 102, 5728–5733. [Google Scholar] [CrossRef]
- Akyol, Ç.; Ozbayram, E.G.; Ince, O.; Kleinsteuber, S.; Ince, B. Anaerobic co-digestion of cow manure and barley: Effect of cow manure to barley ratio on methane production and digestion stability. Environ. Prog. Progress Sustain. Energy 2016, 35, 589–595. [Google Scholar] [CrossRef]
- Zhang, W.; Kong, T.; Xing, W.; Li, R.; Yang, T.; Yao, N.; Lv, D. Links between carbon/nitrogen ratio, synergy and microbial characteristics of long-term semi-continuous anaerobic co-digestion of food waste, cattle manure and corn straw. Bioresour. Technol. 2022, 343, 126094. [Google Scholar] [CrossRef]
- Isaksson, S. Biogas Production at High Ammonia Levels: The Importance of Temperature and Trace Element Supplementation on Microbial Communities. Student Thesis, Faculty of Science and Technology, Uppsala University, Uppsala, Sweden, 2018. Available online: https://www.diva-portal.org/smash/get/diva2:1203787/FULLTEXT01.pdf (accessed on 12 July 2023).
- McCarty, P. Anaerobic waste treatment fundamentals: Part III. Toxic Materials and their Control. Public Works. 1964, 95, 91–94. [Google Scholar]
- Yangin-Gomec, C.; Ozturk, I. Effect of maize silage addition on biomethane recovery from mesophilic co-digestion of chicken and cattle manure to suppress ammonia inhibition. Energy Convers. Manag. 2013, 71, 92–100. [Google Scholar] [CrossRef]
- Alavi-Borazjani, S.A.; Capela, I.; Tarelho, L.A. Over-acidification control strategies for enhanced biogas production from anaerobic digestion: A review. Biomass Bioenergy 2020, 143, 105833. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, Y.; Ma, X.; Guan, W.; Gao, M.; Li, Y.-Y.; Wang, Q.; Wu, C. Effect of zero-valent iron addition on the biogas fermentation of food waste after anaerobic preservation. J. Environ. Chem. Eng. 2021, 9, 106013. [Google Scholar] [CrossRef]
- Egwu, U.; Onyelowe, K.; Tabraiz, S.; Johnson, E.; Mutshow, A.D. Investigation of the effect of equal and unequal feeding time intervals on process stability and methane yield during anaerobic digestion grass silage. Renew. Sustain. Energy Rev. 2022, 158, 112092. [Google Scholar] [CrossRef]
- Babel, S.; Fukushi, K.; Sitanrassamee, B. Effect of acid speciation on solid waste liquefaction in an anaerobic acid digester. Water Res. 2004, 38, 2417–2423. [Google Scholar] [CrossRef]
- Gyenge, L.; Crognale, S.; Lányi, S.; Abraham, B.; Ráduly, B. Anaerobic digestion of corn-DDGS: Effect of pH-control, agitation and batch repetition. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2014, 76, 163–172. [Google Scholar]
- Siegert, I.; Banks, C. The effect of volatile fatty acid additions on the anaerobic digestion of cellulose and glucose in batch reactors. Process Biochem. 2005, 40, 3412–3418. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, R.; Li, K.; Ma, R. A review of crop straw pretreatment methods for biogas production by anaerobic digestion in China. Renew. Sustain. Energy Rev. 2019, 107, 51–58. [Google Scholar] [CrossRef]
- Fathya, S.; Assia, K.; Hamza, M. Influence of inoculums/substrate ratios (ISRs) on the mesophilic anaerobic digestion of slaughterhouse waste in batch mode: Process stability and biogas production. Energy Procedia 2014, 50, 57–63. [Google Scholar]
- Zhou, Y.; Huang, K.; Jiao, X.; Stanisavljevic, N.; Li, L.; Vujovic, S.; Peng, X.; Wang, X. Anaerobic co-digestion of organic fractions of municipal solid waste: Synergy study of methane production and microbial community. Biomass Bioenergy 2021, 151, 106137. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Macé, S.; Llabres, P. Anaerobic digestion of organic solid wastes. An overview of research achievements and perspectives. Bioresour. Technol. 2000, 74, 3–16. [Google Scholar] [CrossRef]
- Marcos, A.; Al-Kassir, A.; López, F.; Cuadros, F.; Brito, P. Environmental treatment of slaughterhouse wastes in a continuously stirred anaerobic reactor: Effect of flow rate variation on biogas production. Fuel Process. Technol. 2012, 103, 178–182. [Google Scholar] [CrossRef]
- Donoso-Bravo, A.; Ortega, V.; Lesty, Y.; Bossche, H.V.; Olivares, D. Addressing the synergy determination in anaerobic co-digestion and the inoculum activity impact on BMP test. Water Sci. Technol. 2019, 80, 387–396. [Google Scholar] [CrossRef]
- Kouas, M.; Torrijos, M.; Sousbie, P.; Harmand, J.; Sayadi, S. Modeling the anaerobic co-digestion of solid waste: From batch to semi-continuous simulation. Bioresour. Technol. 2019, 274, 33–42. [Google Scholar] [CrossRef]
- Kreuger, E.; Nges, I.A.; Björnsson, L. Ensiling of crops for biogas production: Effects on methane yield and total solids determination. Biotechnol. Biofuels 2011, 4, 1–8. [Google Scholar] [CrossRef]
- Wilkinson, J.; Garnsworthy, P. Dietary options to reduce the environmental impact of milk production. J. Agric. Sci. 2017, 155, 334–347. [Google Scholar] [CrossRef]
- Aybek, A.; Solak, M.; Ekinci, K. Co-digestion of spoiled maize silage with cattle manure. Mediterr. Agric. Sci. 2021, 34, 287–291. [Google Scholar] [CrossRef]
- Vellinga, T.V.; Blonk, H.; Marinussen, M.; Van Zeist, W.; Starmans, D. Methodology used in Feedprint: A Tool Quantifying Greenhouse Gas Emissions of Feed Production and Utilization; 1570-8616; Wageningen UR Livestock Research: Wageningen, The Netherlands, 2013. [Google Scholar]
- AS 4564-2011; Specification for General Purpose Natural Gas. Australian Standard: Sydney, Australia, 2011.

| Substrate | TS | VS | VS/TS | C | N | C/N | TKN | TKP |
|---|---|---|---|---|---|---|---|---|
| % | % | %TS | %TS | gN/kgTS | gN/kgTS | |||
| Cow Manure | 14.64 | 11.64 | 0.79 | 43.45 | 2.81 | 15.49 | 25.16 | 3.25 |
| Lucerne Silage | 87.77 | 75.98 | 0.87 | 43.71 | 4.17 | 10.47 | 35.71 | 3.32 |
| Maize Silage | 88.08 | 81.94 | 0.93 | 46.80 | 1.62 | 28.96 | 17.21 | 2.85 |
| Barley Silage | 84.03 | 76.23 | 0.91 | 47.39 | 2.40 | 19.73 | 22.25 | 12.23 |
| Parameters | Silage Mixture | Cow Manure |
|---|---|---|
| Carbon (% of TS) | 39.75 | 43.45 |
| Nitrogen (% of TS) | 2.51 | 2.81 |
| Hydrogen (% of TS) | 5.22 | 5.31 |
| Oxygen (% of TS) | 29.25 | 41.25 |
| Sulphur (% of TS) | 0 | 1 |
| pH | TS (%) | VS (%) | VS/TS | C (%TS) | N (%TS) | C/N | NH4–N (mg/L) | TKN (gN/kgTS) | TKP (gP/kgTS) | VS Removal (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect of ISR | |||||||||||
| Inoculum | 7.57 | 1.70 | 1.18 | 0.69 | 34.64 | 5.65 | 6.13 | 1494.8 | 53.04 | 28.30 | 19.93 |
| ISR 0.5 | 7.20 | 3.51 | 2.56 | 0.73 | 36.19 | 4.62 | 7.83 | 1605.9 | 17.50 | 8.11 | 52.50 |
| ISR 1 | 7.42 | 2.36 | 1.69 | 0.72 | 35.22 | 4.87 | 7.23 | 1414.0 | 43.14 | 22.60 | 65.08 |
| ISR 2 | 7.45 | 2.01 | 1.39 | 0.69 | 34.97 | 5.10 | 6.86 | 1323.1 | 47.78 | 25.52 | 75.10 |
| ISR 4 | 7.45 | 1.87 | 1.30 | 0.70 | 35.48 | 5.27 | 6.73 | 1272.6 | 61.93 | 33.11 | 66.46 |
| Cellulose @ ISR 2 | 7.37 | 1.78 | 1.24 | 0.70 | 35.46 | 6.02 | 5.89 | 1191.8 | 63.83 | 33.99 | 92.25 |
| Effect of Feed Mix | |||||||||||
| Inoculum | 7.60 | 1.77 | 1.21 | 0.68 | 35.41 | 5.78 | 6.13 | 1494.8 | 55.75 | 0.02 | 17.78 |
| 100–0 | 7.45 | 2.01 | 1.39 | 0.69 | 34.97 | 5.10 | 6.86 | 1323.1 | 47.78 | 25.52 | 75.10 |
| 75–25 | 7.42 | 2.06 | 1.41 | 0.68 | 34.21 | 4.59 | 7.46 | 1313.0 | 46.06 | 25.31 | 72.18 |
| 50–50 | 7.39 | 2.19 | 1.52 | 0.69 | 35.63 | 4.63 | 7.70 | 1292.8 | 48.83 | 28.63 | 58.19 |
| 25–75 | 7.40 | 2.22 | 1.51 | 0.68 | 35.40 | 4.47 | 7.92 | 1272.6 | 43.44 | 26.19 | 58.58 |
| 0–100 | 7.38 | 2.30 | 1.60 | 0.70 | 35.11 | 4.65 | 7.56 | 1262.5 | 45.04 | 25.93 | 47.60 |
| Acetic Acid | Propionic Acid | Iso-Butyric Acid | Butyric Acid | Iso-Valeric Acid | Valeric Acid | 4-Methyl Valeric Acid | Hexanoic Acid | Total VFA | |
|---|---|---|---|---|---|---|---|---|---|
| Effect of ISR | |||||||||
| Inoculum | 0.00 | 0.0 | 0.0 | 2.3 | 0.7 | 1.6 | 0.0 | 0.8 | 5.6 |
| ISR 0.5 | 1250 | 1310.9 | 35.4 | 15.2 | 164.2 | 16.1 | 10.5 | 2.9 | 2805.9 |
| ISR 1 | 8.2 | 2.7 | 0.0 | 3.6 | 1.6 | 3.4 | 0.0 | 1.6 | 21.5 |
| ISR 2 | 7.1 | 1.5 | 0.0 | 2.4 | 0.0 | 1.7 | 0.0 | 0.0 | 12.8 |
| ISR 4 | 6 | 0.0 | 0.0 | 2.4 | 0.0 | 1.6 | 0.0 | 0.7 | 10.8 |
| Cellulose @ ISR 2 | 4.1 | 0.0 | 0.0 | 2.4 | 0.0 | 1.9 | 0.0 | 0.0 | 8.5 |
| Effect of Feed ratio | |||||||||
| Inoculum | 7.2 | 0,0 | 0.0 | 2.3 | 0.0 | 2.3 | 0.0 | 0.0 | 11.8 |
| 75–25 | 6.3 | 0.0 | 0.0 | 2.6 | 0.0 | 1.7 | 5.8 | 0.0 | 16.5 |
| 50–50 | 7.8 | 0.0 | 0.0 | 2.8 | 0.0 | 1.6 | 0.0 | 0.0 | 12.2 |
| 25–75 | 7.7 | 0.0 | 0.0 | 2.2 | 0.0 | 1.6 | 0.0 | 0.0 | 11.5 |
| 0–100 | 4.5 | 1.4 | 0.0 | 2.4 | 0.0 | 1.8 | 0.0 | 0.8 | 10.9 |
| 100–0 | 7.1 | 1.5 | 0.0 | 2.4 | 0.0 | 1.7 | 0.0 | 0.0 | 12.8 |
| Experimental Methane Yields | First-Order Kinetic Model | Modified Gompertz Model | Chen and Hashimoto Model | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (NmL/gVSadded) | Khyd (Day−1) | B0 (NmL/gVSadded) | R2 | RMSE (%) | % Difference | λ (Day) | RMax (NmL/gVS.Day) | B90 (NmL/gVSadded) | T90 (Days) | Teff (Days) | R2 | RMSE (%) | % Difference | KCH | µm (Day−1) | HRTCritical (Day) | |
| ISR | |||||||||||||||||
| 0.5 | 174.49± 9.29 | 0.000018 | 240,578.3 | 0.92 | 27.16 | NA | 15.29 | 18.38 | 157.04 | 33.33 | 18.04 | 0.99 | 4.71 | 6.17 | 0.9 | 0.18 | 5.43 |
| 1.0 | 262.18 ± 14.96 | 0.06 | 287.46 | 0.99 | 14.07 | 8.79 | 1.72 | 17.68 | 235.95 | 21.68 | 19.95 | 0.99 | 5.39 | 5.32 | 0.69 | 0.28 | 3.48 |
| 2.0 | 387.77 ± 14.43 | 0.11 | 384.81 | 0.99 | 11.29 | 0.76 | 0 | 29.97 | 348.99 | 22.39 | 22.39 | 0.98 | 14.76 | 6.07 | 0.5 | 0.28 | 3.47 |
| 4.0 | 482.23 ± 38.47 | 0.1 | 474.41 | 0.99 | 10.39 | 1.61 | 0 | 13.69 | 434.00 | 22.40 | 22.40 | 0.98 | 20.71 | 6.74 | 5.84 | 3.06 | 0.32 |
| Feed Mix ratio (SM:CM) | |||||||||||||||||
| 100–0 | 387.77 ± 14.43 | 0.11 | 384.81 | 0.99 | 11.29 | 0.76 | 0 | 29.97 | 348.99 | 22.39 | 22.39 | 0.98 | 14.76 | 6.07 | 0.5 | 0.28 | 3.47 |
| 75–25 | 382.86 ± 24.63 | 0.08 | 392.59 | 0.99 | 8.10 | 2.47 | 0 | 23.64 | 344.57 | 25.08 | 25.08 | 0.99 | 16.54 | 5.09 | 0.48 | 0.24 | 4.13 |
| 50–50 | 373.19 ± 11.79 | 0.07 | 388.21 | 0.99 | 7.58 | 3.86 | 0 | 20.70 | 335.87 | 26.09 | 26.09 | 0.99 | 16.74 | 4.83 | 0.61 | 0.25 | 4.04 |
| 25–75 | 311.26 ± 16.49 | 0.06 | 327.85 | 0.99 | 5.46 | 5.06 | 0 | 15.84 | 280.13 | 27.86 | 27.86 | 0.99 | 13.39 | 5.14 | 0.83 | 0.26 | 3.8 |
| 0–100 | 257.40 ± 12.76 | 0.04 | 302.82 | 0.99 | 4.48 | 14.99 | 0 | 10.17 | 231.66 | 31.00 | 31.00 | 0.99 | 9.08 | 0.64 | 0.96 | 0.25 | 4.07 |
| Feed Mix Ratio (SM:CM) | Theoretical Methane Yield (NmL CH4/gVSadded) | BI (%) | Weighted Methane Yield (NmL CH4/gVSadded) | Experimental Methane Yields (NmL CH4/gVSadded) | % Difference | CPI | Synergistic or Antagonistic Effect |
|---|---|---|---|---|---|---|---|
| 100–0 | 516.60 | 75.06 | 387.77 | 387.77 ± 14.43 | 0.00 | 1.00 | No Effect |
| 75–25 | 493.43 | 77.42 | 354.60 | 382.86 ± 24.63 | 7.17 | 1.07 | No Effect |
| 50–50 | 468.26 | 79.70 | 322.20 | 373.19 ± 11.79 | 13.66 | 1.15 | Synergistic |
| 25–75 | 443.09 | 70.25 | 289.80 | 311.26 ± 16.49 | 6.89 | 1.07 | No Effect |
| 0–100 | 417.93 | 61.59 | 257.40 | 257.40 ± 12.76 | 0.00 | 1.00 | No Effect |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarode, S.D.; Kumar, D.; Mathias, D.; McNeill, D.; Kaparaju, P. Anaerobic Digestion of Spoiled Maize, Lucerne and Barley Silage Mixture with and without Cow Manure: Methane Yields and Kinetic Studies. Energies 2023, 16, 6179. https://doi.org/10.3390/en16176179
Sarode SD, Kumar D, Mathias D, McNeill D, Kaparaju P. Anaerobic Digestion of Spoiled Maize, Lucerne and Barley Silage Mixture with and without Cow Manure: Methane Yields and Kinetic Studies. Energies. 2023; 16(17):6179. https://doi.org/10.3390/en16176179
Chicago/Turabian StyleSarode, Shubham Dilip, Deepak Kumar, Divya Mathias, David McNeill, and Prasad Kaparaju. 2023. "Anaerobic Digestion of Spoiled Maize, Lucerne and Barley Silage Mixture with and without Cow Manure: Methane Yields and Kinetic Studies" Energies 16, no. 17: 6179. https://doi.org/10.3390/en16176179
APA StyleSarode, S. D., Kumar, D., Mathias, D., McNeill, D., & Kaparaju, P. (2023). Anaerobic Digestion of Spoiled Maize, Lucerne and Barley Silage Mixture with and without Cow Manure: Methane Yields and Kinetic Studies. Energies, 16(17), 6179. https://doi.org/10.3390/en16176179






