Abstract
In this study, the corrosion behavior of X52 pipeline steel affected by H2O content in supercritical CO2 streams containing O2, H2S, SO2 and NO2 impurities was investigated by the weight loss test and surface characterization. The corrosion differences of the steel in impure supercritical CO2 streams containing different H2O contents were analyzed. The influence of the variation of H2O content on the corrosion mechanism of steel in the complex impurity-containing supercritical CO2 streams was discussed. The results show that the H2O content limit is 100 ppmv in supercritical CO2 streams containing 200 ppmv O2, 200 ppmv H2S, 200 ppmv SO2 and 200 ppmv NO2 at 10 MPa and 50 °C. The impurities and their interactions significantly promote the formation of corrosive aqueous phase, thereby exacerbating the corrosion of X52 steel. The corrosion process of X52 steel in the environment with a low H2O content is controlled by the products of impurity reactions, whereas the impurities and the products of impurity reactions jointly control the corrosion process of the steel in the environment with a high H2O content.
1. Introduction
Carbon capture, utilization and storage (CCUS) is an important technique to combat climate change [1,2]. A large number of CCUS projects have been successfully applied worldwide, and the success of these projects relies heavily on the safety, reliability, and cost effectiveness of the CCUS process [3,4]. In CCUS projects, CO2 pipeline, as the primary transport manner of CO2, is a key component in ensuring the safe and efficient transport of CO2 from the capture site to its destination [5]. However, the inevitable presence of corrosive impurities such as H2O, O2, SO2, NO2 and H2S in the supercritical CO2 streams transported by pipelines can pose a serious threat to the service safety of carbon steel pipelines [2,6,7,8,9,10,11,12,13].
It is generally accepted that CO2 itself is not corrosive and does not cause the corrosion of pipeline steel when transported as pure CO2 [14]. However, the presence of impurity components in the captured gas is inevitable. For the particular system of supercritical CO2 transportation pipelines, H2O in supercritical CO2 streams usually exists in a dissolved state, and this form of H2O is largely unlikely to cause corrosion of pipeline steel [2,15,16,17]. However, the presence of other impurity gases, such as O2, SO2, NO2 and H2S, can significantly reduce the solubility of H2O in supercritical CO2 and induce the precipitation of free aqueous phase [8,17,18,19,20]. In addition, there are also complex chemical reactions among these impurity components, which can not only react to generate H2O but also form strong corrosive substances such as H2SO4 and HNO3, thereby exacerbating the corrosion of pipeline steel [6]. Therefore, for the application of CCUS, it is of great interest to clarify the threshold of H2O content that does not cause significant corrosion for impurity-laden supercritical CO2 transport environments. At present, numerous studies have been carried out to explore the possible threshold of H2O content in CO2 transportation environments [6,8,21,22]. The H2O content thresholds obtained vary in the range of 40 ppmv to 2500 ppmv, which is closely related to the types of impurities, the concentration of impurities and the pipeline operating parameters. Although some progress has been achieved, the knowledge on the influence of H2O content on corrosion is still very limited, and most of the research work only involves a small number of impurity combinations. However, in the actual operation of the pipelines, it is inevitable that there will be a complex corrosive environment in which various impurities such as O2, H2S, SO2 and NO2 coexist. In this case, it not only raises a higher requirement for the limitation of H2O content, but also causes great changes in the corrosion law and mechanism of pipeline steel. Therefore, it is currently difficult for us to determine the H2O content limit in CO2 transportation environments with the coexistence of all possibly corrosive impurities of O2, H2S, SO2 and NO2.
In view of this, this study investigated the effect of H2O content variation on the corrosion rate, corrosion morphology and corrosion film characteristics of X52 pipeline steel in a supercritical CO2 environment where H2O, O2, H2S, SO2 and NO2 impurities coexisted, and explored the influence mechanism of multiple impurity interactions on the corrosion of X52 steel under different H2O content conditions. Compared with the knowledge achieved in this domain, this study is expected to provide novel insights into the corrosion mechanism of pipeline steel in a complex impurity-containing CO2 transportation environment, and also provide a scientific basis for the limitation of H2O content when the impurities of O2, H2S, SO2 and NO2 coexist in supercritical CO2 transportation pipelines.
2. Materials and Methods
The specimen used for the corrosion test was machined to a size of 40 mm × 15 mm × 3 mm from a commercial X52 pipeline steel. Four parallel specimens were prepared for each group of tests to ensure the repeatability of test results. The chemical compositions of X52 steel were 0.10% C, 0.17% Si, 1.10% Mn, 0.011% P, 0.006% S, 0.006% Mo, 0.020% Cr, 0.009% Ni, 0.062% Al, 0.016% Cu, 0.002% V and Fe balance. Figure 1 shows the microstructure of X52 steel, which consisted of ferrite and pearlite. Prior to the tests, the surface of the specimen was successively ground with 240, 600, 800 and 1000 grit SiC paper to ensure the same surface roughness. After that, the specimen was cleaned with deionized (DI) water, dewatered with alcohol and dried with cold air. The original weight (W1) of the prepared specimen was measured by an electronic balance with a metering precision of 0.0001 g.

Figure 1.
Microstructure of X52 pipeline steel.
Corrosion simulation tests were carried out in a high-temperature and high-pressure reactor with a 3 L volume. The test device was described in a previous study [23]. The conditions of corrosion simulation tests are shown in Table 1, where the H2O content of 4333 ppmv was the saturation solubility of H2O in supercritical CO2 at 50 °C and 10 MPa (at a H2O content of up to 4333 ppmv, H2O will be completely dissolved in supercritical CO2, i.e., there is no free water phase in the initial corrosion environment). The concentrations of O2, H2S, SO2 and NO2 impurities were determined based on the commonly used CO2 quality specifications [21,24,25,26]. Among various impurities, the maximum concentration of H2S was limited to 200 ppmv due to health and safety considerations [21,24]. Therefore, a concentration of 200 ppmv was selected in this study mainly according to the limitation on H2S. In order to keep a consistent concentration of each impurity, the concentrations of O2, SO2 and NO2 impurities were also fixed at 200 ppmv. Prior to the test, the specimens were placed on a Teflon fixture and the required amount of H2O (de-oxygenated DI water) was added to the reactor. In order to remove the air left in the reactor during the installation, a continuous flow of high-purity CO2 was introduced into the reactor for 2 h after closing the reactor. The reactor was preferentially heated to 50 °C. The impurities of O2, H2S, SO2 and NO2 were added to the reactor in their respective required concentrations. Finally, CO2 was added to reach 10 MPa. The test duration was 72 h.

Table 1.
Test conditions.
At the end of the test, the specimens were taken out, photographed using a digital camera and then placed in a vacuum drying chamber for natural dehydration. A total of 1 L pickling solution was prepared using 100 mL of hydrochloric acid (the density was 1.19 g/mL), 5 g of hexamethyltetramine and DI water [27]. Three corroded specimens were placed in the above solution to remove the corrosion products. The weight (W2) of each specimen was measured again after drying. The corrosion rate of the specimen was calculated using the weight loss method [27]:
where VCR is the corrosion rate, mm/y; W1 and W2 are the weight of the specimen before and after corrosion, g; S is the exposed area of the specimen, cm2; ρ is the density of the specimen, g/cm3; t is the corrosion time, h; 8.76 × 104 is the unit conversion constant. The corrosion rates with error bars reported in this study were the average values calculated from three parallel specimens.
The surface and cross-sectional morphologies of the corroded specimens were observed using scanning electron microscopy (SEM). The elemental compositions of the corrosion products and the elemental distributions in the cross-section of corrosion product film were analyzed by energy dispersive spectroscopy (EDS). The phase compositions of the corrosion products were determined by X-ray diffraction (XRD, Cu target, 40 kV, 40 mA). The chemical valences of the corrosion products were analyzed by X-ray photoelectron spectroscopy (XPS, Al target, hv = 1486.6 eV).
3. Results and Discussion
3.1. Corrosion Rate
Figure 2 exhibits the variation of corrosion rate of X52 steel with H2O content exposed to supercritical CO2 steams containing the impurities of O2, H2S, SO2 and NO2 at 10 MPa and 50 °C. At a H2O content of 20 ppmv, the corrosion rate of X52 steel is 0.0199 mm/y. The rate increases to 0.0234 mm/y when the H2O content reaches 100 ppmv. However, in the range of 100–2000 ppmv H2O content, the corrosion rate of X52 steel dramatically increases as the H2O content rises. Upon reaching the 2000 ppmv H2O content, X52 steel has a corrosion rate of 0.2671 mm/y. After the H2O content exceeds 2000 ppmv, the increment trend of corrosion rate slows down, reaching a value of 0.2838 mm/y at the saturated solubility of 4333 ppmv. Obviously, the corrosion rate of X52 steel is strongly associated with the variation of H2O content. Given that the remarkable increase in corrosion rate when the H2O content exceeds 100 ppmv, it is reasonably inferred that the H2O content limit should be 100 ppmv in supercritical CO2 streams containing 200 ppmv O2, 200 ppmv H2S, 200 ppmv SO2 and 200 ppmv NO2 at 10 MPa and 50 °C.

Figure 2.
Variations of corrosion rate of X52 steel with H2O content exposed to supercritical CO2 steams containing the impurities of O2, H2S, SO2 and NO2 at 10 MPa and 50 °C for 72 h.
3.2. Morphological Observation of Corrosion Product Film
3.2.1. H2O Content of between 20 and 100 ppmv
Figure 3 shows the macroscopic and SEM surface morphology, cross-sectional backscattered electron images and elemental distributions in cross-section of X52 steel in supercritical CO2-H2O-impurity environment with H2O content ranging from 20 to 100 ppmv, where the results of EDS analysis of corrosion products at regions A and B are shown in Table 2. It can be seen that at a H2O content of 20 ppmv, a thin layer of yellowish corrosion products is deposited unevenly on the surface of the specimen, and the polishing traces due to the pretreatment are still observed on the local areas of the specimen. A small amount of spherical corrosion products is distributed on the surface of the specimen, and the matrix below is slightly corroded. With the increase in H2O content, the coverage of the corrosion products on the specimen surface increases significantly, and the corrosion product film exhibits an obvious oxidation color. It can be seen from SEM images that a large number of spherical products and a small number of worm-like products are alternately distributed on the specimen surface. The results of the EDS analysis show that in the range of 20–100 ppmv, the corrosion products are mainly composed of Fe and O, indicating that the corrosion products are mainly oxygen-containing compounds. In addition, the increase in the coverage of corrosion product film and the corrosion rate of the steel also shows that even when the H2O content is far less than the saturation solubility (4333 ppmv) of H2O in supercritical CO2 streams, the free aqueous phase can still precipitate from the CO2 streams, thereby resulting in the corrosion of X52 steel. The increased corrosion rate strongly supports that the precipitation of the free aqueous phase increases with the increase in the H2O content in impure supercritical CO2 streams.

Figure 3.
(a1,b1) Macroscopic morphologies, (a2,b2) SEM surface morphologies, (a3,b3) cross-sectional backscattered electron images and (a4,b4) distributions of main elements in cross-section of X52 steels after corrosion for 72 h in supercritical CO2-H2O-O2-H2S-SO2-NO2 environment with different H2O contents at 10 MPa and 50 °C: (a1–a4) 20 ppmv H2O; (b1–b4) 100 ppmv H2O. (A and B are the regions where EDS analysis is performed, the blue box region is the magnified image of the region denoted by the arrow, and the corrosion products are located in the region between the dotted lines.)

Table 2.
Main elements in corrosion products on X52 steel denoted by A-B in Figure 3 (at%).
3.2.2. H2O Content of between 500 and 2000 ppmv
Figure 4 shows the macroscopic and SEM surface morphology, cross-sectional backscattered electron images and elemental distributions in cross-section of X52 steel in the environment with a H2O content of 500 to 2000 ppmv. It can be seen that when the H2O content is 500 ppmv, the surface of the specimen is uniformly covered with a layer of spherical corrosion products with a thickness of about 1 μm. When the H2O content increases to 1000 ppmv, a large number of worm-like corrosion products are formed on the specimen surface, and the thickness of the corrosion product film reaches about 10 μm. However, when the H2O content increases to 2000 ppmv, the macro- and micro-morphologies of the corrosion product film change significantly. The corrosion products gradually become grey-black and present a mud-like morphology. Moreover, the cracks caused by dehydration can be observed. Compared to the uniform deposition of corrosion products at 500 and 1000 ppmv H2O contents, the corrosion product film at 2000 ppmv H2O content shows varying degrees of bulging and more pronounced corrosion of the matrix beneath it. The EDS results show that at the H2O content of 500 ppmv, the corrosion products are mainly composed of Fe and O elements, while at the H2O content of 1000 ppmv or 2000 ppmv, the corrosion products mainly contain Fe, O and S elements. Apparently, the increase in H2O content significantly promotes the formation of sulfur-containing products in the corrosion product film. This implies that the increase in H2O content causes the changes not only in corrosion rate and corrosion morphology, but also possibly in corrosion mechanism, i.e., the influence of sulfur-containing substances (e.g., H2S and SO2) on the corrosion of X52 steel intensifies with the increase in H2O content.

Figure 4.
(a1–c1) Macroscopic morphologies, (a2–c2) SEM surface morphologies, (a3–c3) cross-sectional backscattered electron images and (a4–c4) distributions of main elements in cross-section of X52 steels after corrosion for 72 h in supercritical CO2-H2O-O2-H2S-SO2-NO2 environment with different H2O contents at 10 MPa and 50 °C: (a1–a4) 500 ppmv H2O; (b1–b4) 1000 ppmv H2O; (c1–c4) 2000 ppmv H2O. (The blue box region is the magnified image of the region denoted by the arrow, and t the corrosion products are located in the region between the dotted lines.)
3.2.3. H2O Content of over 2000 ppmv
Figure 5 shows the macroscopic and SEM surface morphology, cross-sectional backscattered electron images and elemental distributions in cross-section of X52 steel in the environment with a H2O content of 4333 ppmv. It can be seen that at the saturation solubility (4333 ppmv) of H2O in supercritical CO2 streams, the corrosion product film on the surface of the specimen is black in color and the corrosion products with droplet-shaped protrusions can be observed in local areas. SEM morphology shows that the raised areas of the corrosion products exhibit the same characteristics as the overall corrosion product film, all of which present the mud-like morphology. In addition, corrosion is more evident in the matrix below the raised areas of the corrosion products. The results of EDS line scanning analysis show that the corrosion products in different areas are mainly composed of Fe, O and S elements. Therefore, the formation of localized droplet-like corrosion products on the surface of the corrosion product film may be related to the deposition state of the aqueous phase on the surface of the specimen at this H2O content.
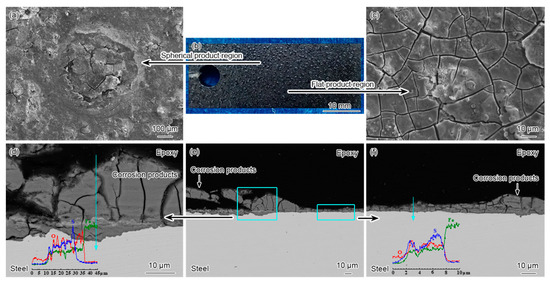
Figure 5.
(a,c) SEM surface morphologies, (b) macroscopic morphologies, and (d–f) cross-sectional backscattered electron images and EDS line scanning analysis along blue arrow in cross-section of corrosion products on X52 steels after corrosion for 72 h in supercritical CO2-H2O-O2-H2S-SO2-NO2 environment with 4333 ppmv H2O content at 10 MPa and 50 °C. ((d,f) are the magnified images of the regions denoted by the blue frame and arrow in (e).)
Obviously, the increased H2O content can not only cause the change in corrosion rate, but also lead to the significant change in the characteristics of corrosion product film on the specimen surface. At a high H2O content, the content of S element in the corrosion products increases significantly, and the corrosion product film on the surface of X52 steel gradually transforms from Fe-O product-dominated film to Fe-O-S mixed product film.
3.3. Analysis of Corrosion Products
3.3.1. XRD Analysis
Figure 6 shows the XRD patterns of the corrosion product film on the surface of X52 steel in supercritical CO2-H2O-impurity environment with different H2O contents. The diffraction peak of Fe is detected in the XRD pattern at a H2O content of 500 ppmv. As the average thickness of the corrosion product film formed on the surface of X52 steel is only about 2–3 μm (Figure 4(a3)), X-rays can penetrate this thin corrosion product film and excite the diffraction peaks of Fe in the steel matrix, which in turn masks the diffraction peaks of the corrosion products. The thickness of the corrosion film formed on the surface of X52 steel increases with the increase in H2O content. Correspondingly, the intensity of the diffraction peak of the corrosion products increases and appears in the XRD pattern. At H2O contents of 1000 and 2000 ppmv, XRD results show that the corrosion products are both mainly composed of FeOOH and FeSO4. Combined with the previous EDS results, it can be deduced that the corrosion products have a low FeSO4 content at 1000 ppmv H2O content and a high FeSO4 content at 2000 and 4333 ppmv H2O contents.

Figure 6.
XRD patterns of corrosion products on X52 steel after corrosion for 72 h in supercritical CO2-H2O-O2-H2S-SO2-NO2 environment with different H2O contents at 10 MPa and 50 °C.
3.3.2. XPS Analysis
When the H2O content is below 500 ppmv, there are obvious corrosion products on the surface of the specimen (Figure 3). However, it is difficult to detect these products by XRD due to the fact that the depth that X-rays can penetrate is generally between ten and dozens of micrometers. For the thin corrosion product film on the surface of the specimen, the diffraction peaks of the corrosion products are easily masked by the high-intensity diffraction peaks of Fe derived from the matrix [28]. When the H2O content is higher than 500 ppmv, the thickness of the corrosion product film on the surface of the specimen increases significantly, and the products of FeOOH and FeSO4·4H2O can be detected by XRD, but the high-intensity diffraction peak of Fe from the matrix still masks some details of the composition of the corrosion products. In addition, some amorphous products are also difficult to characterize with XRD [14]. Different from XRD, XPS, as a high-resolution surface analysis technology, can extract the chemical information from 0 to 10 nm on the surface of the material, and amorphous or low-content corrosion products are easily detected by XPS [8]. Therefore, XPS was used to further determine the chemical state of the corrosion products on the surface of X52 steel formed at different H2O contents in order to eliminate the influence of the matrix.
Based on the results of EDS and XRD analyses, it can be concluded that the corrosion products mainly contain Fe, O and S elements. Therefore, these elements were selected for analysis in the XPS test. Figure 7 shows the high-resolution XPS spectra of the different elements in the corrosion products for H2O contents of 20 ppmv, 1000 ppmv and 4333 ppmv, respectively. The high-resolution XPS spectra of Fe 2p in Figure 7a show that the main peaks of Fe 2p3/2 and Fe 2p1/2 are located at the binding energies of 711.3 eV and 725.1 eV, respectively, indicating that Fe is in the oxidation states (Fe2+ and/or Fe3+) [14,29]. As the Fe-related compounds have similar binding energies, it is difficult to determine their specific compositions based on Fe 2p spectra alone. Therefore, this study determines the corrosion products mainly based on the fitting results of the split peak of O 1s and S 2p spectra. As shown in Figure 7b, the O 1s peak can be decomposed into three characteristic peaks at different H2O contents, the characteristic peaks at 530.1 eV and 531.6 eV correspond to hydroxyl oxides and the characteristic peak at 532.2 eV corresponds to sulphates [14,29,30]. According to the peak splitting results of S 2p spectra (Figure 7c), one S 2p3/2 peak at 168.6 eV indicates the presence of sulfate at a H2O content of 20 ppmv [14,30]; two S 2p3/2 peaks are present at 164.0 eV and 168.6 eV, which are ascribed to elemental sulfur and sulfate, respectively, at a H2O content of 1000 ppmv [14,30]; and at a H2O content of 4333 ppmv, three S 2p3/2 characteristic peaks are present at 164.0 eV, 166.8 eV and 168.6 eV, corresponding to elemental sulfur, sulfite and sulfate, respectively [14,30]. Taking into account the chemical state of each element, it can be determined that at a H2O content of 20 ppmv, the corrosion products are mainly FeOOH and a small amount of FeSO4; at a H2O content of 1000 ppmv, the corrosion products are mainly FeOOH, FeSO4 and S; at a H2O content of 4333 ppmv, the corrosion products are mainly FeOOH, FeSO3, FeSO4 and S. According to the results of EDS, XRD and XPS analyses, it can be concluded that the sulfur-containing products in the corrosion product film gradually increase as the H2O content increases.

Figure 7.
XPS spectra of (a) Fe 2p, (b) O 1s and (c) S 2p for the corrosion products on X52 steels after corrosion for 72 h in supercritical CO2-H2O-O2-H2S-SO2-NO2 environment with different H2O contents at 10 MPa and 50 °C.
3.4. Analysis of Corrosion Mechanism
In the environment of supercritical CO2 transportation containing impurities, although supercritical CO2 is the main body, the previous analysis results show that the chemical composition of the corrosion product film on the surface of X52 steel under different H2O contents is mainly FeOOH and FeSO4 and a small amount of S or FeSO3, without detecting the typical product of CO2 corrosion (FeCO3). This phenomenon indicates that the corrosion process and film formation of X52 steel are mainly controlled by impurity components. Related studies have shown that impurity components in the supercritical CO2 streams can reduce the solubility of H2O in supercritical CO2 and promote the formation of the aqueous phase [8,17,18,19,20]. Furthermore, the complex chemical reactions among various impurity components such as O2, H2S, SO2 and NO2 can generate additional corrosive substances such as H2SO4, HNO3, elemental S and H2O [6]. Therefore, the obvious corrosion of X52 steel at conditions well below the saturation solubility of H2O is probably related to the additional corrosive substances, which are generated by the reaction between the impurities and exert influence on the amount of aqueous phase formation and the chemical environment of the aqueous phase. To further prove the above inference, hydrochemical simulations of supercritical CO2 streams containing impurities were carried out with the aid of the Stream Analyzer module of the OLI Analyzer Studio software. The basic compositions of the streams used for the above calculation are 990 g CO2 and 10 g H2O, with O2, H2S, H2SO3, H2SO4 and HNO3, with the content (mass fraction %) ranging from 0 to 0.03%, at a temperature of 50 °C and pressure of 10 MPa, respectively, the results of which are shown in Figure 8.

Figure 8.
Influence of impurities on aqueous phase formation and pH value of aqueous phase formed in supercritical CO2 streams at 10 MPa and 50 °C (calculated by OLI Analyzer Studio).
As shown in Figure 8a, an increase in O2 or H2S content does not have a significant effect on the amount of aqueous phase formed in supercritical CO2 streams. However, the amount of aqueous phase formed in supercritical CO2 streams increases with an increase in H2SO3 (associated with SO2), H2SO4 (associated with O2 and SO2) or HNO3 (associated with NO2). For supercritical CO2 streams containing H2SO3, H2SO4 or HNO3, the amount of aqueous phase formation increases significantly with an increase in their concentrations. It also implies that the presence of even a small amount of impurities in the environment where multiple impurities exist can significantly promote the formation of aqueous phase, leading to the formation of acid-rich aqueous phases in low-H2O-content environments. Whereas these acid-rich aqueous phases usually have lower pH values (Figure 8b), the higher H+ concentration in the aqueous phase promotes hydrogen evolution reactions at the cathode, which in turn intensifies the corrosion of the steel. This should be an important reason why the corrosion rate of up to 0.0199 mm/y can be achieved at the H2O content of only 20 ppmv.
When the H2O content is in the range of 20–100 ppmv, the corrosion products of X52 steel are mainly FeOOH and a small amount of FeSO4. This suggests that the reaction between impurities is able to form at least the corrosive H2SO4 [31,32], thus producing the characteristic product of FeSO4. Based on the results of this study, it is impossible to determine the exact FeOOH formation pathway. However, related studies have shown that FeOOH is a common corrosion product in environments containing strong oxidizing impurities of O2 or NO2 [31,33,34]. The amount of aqueous phase formed in low-H2O-content environments is relatively small compared to high-H2O-content environments. This is because the corrosion products formed on the steel surface can be oxidized in full contact with oxidants in supercritical CO2 streams. For example, FeSO4 can be oxidized by O2 into FeOOH [8,35]:
4FeSO4 + 6H2O + O2→4FeOOH + H2SO4
As a result, the corrosion product film on the surface of X52 steel in the low-H2O-content environment shows an obvious oxidation color (Figure 3(a1,b1)), with the corrosion products being dominated by iron oxides. It is worth noting that the oxidation reaction between the primary products of corrosion and impurities also results in the cyclic regeneration of corrosive substances, which continues to cause corrosion of the steel matrix [8,35]. This may also be one of the reasons for the high corrosion rate of X52 steel in an extremely low-H2O-content environment.
When the H2O content is in the range of 500–2000 ppmv, the amount of aqueous phase condensed on the surface of X52 steel under the interaction of impurities increases considerably due to the increased H2O content in the corrosion system, which also provides more electrolytes for the corrosion reaction, resulting in a significant increase in the corrosion rate. Moreover, the deposition of the aqueous phase on the corrosion products can prevent to some extent the oxidation of the corrosion products caused by the oxidizing agent in the supercritical CO2 streams. This also leads to a gradual increase in the content of FeSO4 in the corrosion products within this H2O content range (Figure 4(a4–c4)), which also corresponds to a shift in the macroscopic morphological characteristics of X52 steel (Figure 4(a1–c1)).
When the H2O content is above 2000 ppmv, a small amount of FeSO3 can be detected in the corrosion products of X52 steel in addition to FeOOH and FeSO4 (Figure 7), indicating that SO2 has been involved in the film formation reaction. This shows that in addition to the chemical reaction products of impurities involved in the corrosion process, the impurities themselves can also be involved in the corrosion process. However, although the results of this study demonstrate that the reaction between impurities can form elemental S, it cannot be proved whether elemental S is involved in the corrosion process of X52 steel. But related studies have shown that elemental S formed in the supercritical CO2 transport environment containing impurities can cause elemental sulfur corrosion of pipeline steel, which is one of the important reasons for the aggravation of pipeline steel corrosion in the environment where multiple impurities coexist [23]. As a result, X52 steel corrodes more severely in a high-H2O-content environment under the combined effect of impurities and chemical reaction products between impurities, with corrosion rates exceeding 0.25 mm/y (Figure 2).
4. Conclusions
In summary, we explored the effect of H2O content in changing the corrosion behavior of X52 steel in a supercritical CO2 environment containing the impurities of 200 ppmv O2, 200 ppmv H2S, 200 ppmv SO2 and 200 ppmv NO2 at 10 MPa and 50 °C. The main conclusions are drawn as follows:
- (1)
- The corrosion rate of X52 steel increases from 0.0199 mm/y to 0.2838 mm/y as the H2O content increases from 20 ppmv to saturation solubility (4333 ppmv), while the critical H2O content that causes a significant change in the corrosion rate is 100 ppmv.
- (2)
- O2, H2S, SO2 and NO2 impurities and their interactions jointly promote the formation of corrosive aqueous phases and aggravate the corrosion of X52 steel. With the increase in H2O content, the corrosion product film of X52 steel gradually changes from FeOOH-dominated film to FeSO4 and FeOOH mixed film. Correspondingly, the corrosion process of X52 steel, which is controlled by the products of impurity reactions at a low H2O content, is transformed to be under the joint control of impurities and the products of impurity reactions at a high H2O content.
- (3)
- The change in the corrosion rate of X52 steel strongly depends on the amount of aqueous phase precipitation in the environment and the amount of corrosive aqueous phase generated by the chemical reactions between impurities. The increase in H2O content and the direct participation of impurities in the corrosion process greatly aggravate the corrosion of X52 steel.
Author Contributions
Conceptualization, J.L. and D.Y.; Investigation, J.L., K.C., C.W. and C.S.; Writing—original draft preparation, J.L. and C.S.; Writing—review and editing, C.S., H.P. and F.M.; Supervision, B.C. and L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the PetroChina Scientific Research and Technology Development Project (2021ZZ01-02) and the National Natural Science Foundation of China (No. 52001328).
Data Availability Statement
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical and time limitations. Concurrently, the data also form part of an ongoing study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jones, A.C.; Lawson, A.J. Carbon Capture and Sequestration (CCS) in the United States; Congressional Research Service: Washington, DC, USA, 2021.
- Kairy, S.K.; Zhou, S.; Turnbull, A.; Hinds, G. Corrosion of pipeline steel in dense phase CO2 containing impurities: A critical review of test methodologies. Corros. Sci. 2023, 214, 110986. [Google Scholar] [CrossRef]
- Yao, J.; Han, H.; Yang, Y.; Song, Y.; Li, G. A review of recent progress of carbon capture, utilization, and storage (CCUS) in China. Appl. Sci. 2023, 13, 1169. [Google Scholar] [CrossRef]
- Peletiri, S.P.; Rahmanian, N.; Mujtaba, I.M. CO2 Pipeline design: A review. Energies 2018, 11, 2184. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Garamanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Barker, R.; Hua, Y.; Neville, A. Internal corrosion of carbon steel pipelines for dense-phase CO2 transport in carbon capture and storage (CCS)—A review. Int. Mater. Rev. 2017, 62, 1–31. [Google Scholar] [CrossRef]
- Halseid, M.; Dugstad, A.; Morland, B. Corrosion and bulk phase reactions in CO2 transport pipelines with impurities: Review of recent published studies. Energy Procedia 2014, 63, 2557–2569. [Google Scholar] [CrossRef]
- Sun, C.; Sun, J.B.; Liu, S.B.; Wang, Y. Effect of water content on the corrosion behavior of X65 pipeline steel in supercritical CO2-H2O-O2-H2S-SO2 environment as relevant to CCS application. Corros. Sci. 2018, 137, 151–162. [Google Scholar] [CrossRef]
- Morland, B.H.; Tadesse, A.; Svenningsen, G.; Springer, R.D.; Anderko, A. Nitric and sulfuric acid solubility in dense phase CO2. Ind. Eng. Chem. Res. 2019, 58, 22924–22933. [Google Scholar] [CrossRef]
- Xiang, Y.; Xu, M.H.; Choi, Y.S. State-of-the-art overview of pipeline steel corrosion in impure dense CO2 for CCS transportation: Mechanisms and models. Corros. Eng. Sci. Technol. 2017, 52, 485–509. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Y.; Sun, J.B.; Lin, X.Q.; Li, X.D.; Liu, H.F.; Cheng, X.K. Effect of impurity on the corrosion behavior of X65 steel in water-saturated supercritical CO2 system. J. Supercrit. Fluids 2016, 116, 70–82. [Google Scholar] [CrossRef]
- Zhao, G.X.; Wang, Y.C.; Zhang, S.Q.; Song, Y. Influence mechanism of H2S/CO2-charging on corrosion of J55 steel in an artificial solution. J. Chin. Soc. Corros. Prot. 2022, 42, 785–790. [Google Scholar]
- Sun, C.; Liu, J.X.; Sun, J.B.; Li, H.; Zhao, Z.; Lin, X.Q.; Wang, Y. Corrosion behaviors of X65 steel in gaseous CO2 environment containing impurities. J. China Univ. Petrol. 2022, 43, 129–139. [Google Scholar]
- Li, C.; Xiang, Y.; Song, C.C.; Ji, Z.L. Assessing the corrosion product scale formation characteristics of X80 steel in supercritical CO2-H2O binary systems with flue gas and NaCl impurities relevant to CCUS technology. J. Supercrit. Fluids 2019, 146, 107–119. [Google Scholar] [CrossRef]
- Cui, G.; Yang, J.G.; Liu, J.G.; Li, Z.L. A comprehensive review of metal corrosion in a supercritical CO2 environment. Int. J. Greenh. Gas. Control 2019, 90, 102814. [Google Scholar] [CrossRef]
- Jiang, X.; Qu, D.R.; Song, X.L.; Liu, X.H.; Zhang, Y.L. Critical water content for corrosion of X65 mild steel in gaseous, liquid and supercritical CO2 stream. Int. J. Greenh. Gas. Control 2019, 85, 11–22. [Google Scholar] [CrossRef]
- Sun, C.; Sun, J.B.; Luo, J.-L. Unlocking the impurity-induced pipeline corrosion based on phase behavior of impure CO2 streams. Corros. Sci. 2020, 165, 108367. [Google Scholar] [CrossRef]
- Morland, B.; Norby, T.; Tjelta, M.; Sevenningsen, G. Effect of SO2, O2, NO2, and H2O concentrations on chemical reactions and corrosion of carbon steel in dense phase CO2. Corrosion 2019, 75, 1327–1338. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Hassani, S.; Vu, T.N.; Nešić, S.; Abas, A.Z.B. Effect of H2S on the corrosion behavior of pipeline steels in supercritical and liquid CO2 environments. Corrosion 2016, 72, 999–1009. [Google Scholar] [CrossRef]
- Sun, J.; Sun, C.; Zhang, G.; Li, X.; Zhao, W.; Jiang, T.; Liu, H.; Cheng, X.; Wang, Y. Effcet of O2 and H2S impurities on the corrosion behavior of X65 steel in water-saturated supercritical CO2 system. Corros. Sci. 2016, 107, 31–40. [Google Scholar] [CrossRef]
- De Visser, E.; Hendriks, C.; Barrio, M.; Molnvik, M.J.; de Koeijer, G.; Liljemark, S.; Le Gallo, Y. Dynamis CO2 quality recommendations. Int. J. Greenh. Gas. Control 2008, 2, 478–484. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, Z.; Yang, X.X.; Zheng, L.; Ni, W.D. The upper limit of moisture content for supercritical CO2 pipeline transport. J. Supercrit. Fluids 2012, 67, 14–21. [Google Scholar] [CrossRef]
- Sun, C.; Sun, J.B.; Wang, Y.; Lin, X.Q.; Li, X.D.; Cheng, X.K.; Liu, H.F. Synergistic effect of O2, H2S and SO2 impurities on the corrosion behavior of X65 steel in water-saturated supercritical CO2 system. Corros. Sci. 2016, 107, 193–203. [Google Scholar] [CrossRef]
- ISO 27913:2016; Carbon Dioxide Capture, Transportation and Geological Storage—Pipeline Transportation Systems. International Organization for Standardization: Geneva, Switzerland, 2016.
- Shirley, P.; Myles, P. Quality Guidelines for Energy System Studies: CO2 Impurity Design Parameters; National Energy Technology Laboratory: Pittsburgh, PA, USA, 2019; pp. 12–18.
- Brown, J.; Graver, B.; Gulbrandsen, E.; Dugstad, A.; Morland, B. Update of DNV recommended practice RP-J202 with focus on CO2 corrosion with impurities. Energy Procedia 2014, 63, 2432–2441. [Google Scholar] [CrossRef]
- ASTM G1-03:2011; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2011.
- Liu, J.; Saw, R.E.; Kiang, Y.-H. Calculation of effective penetration depth in X-ray diffraction for pharmaceutical solids. J. Pharm. Sci. 2010, 99, 3807–3814. [Google Scholar] [CrossRef]
- Heuer, J.K.; Stubbins, J.F. An XPS characterization of FeCO3 films from CO2 corrosion. Corros. Sci. 1999, 41, 1231–1243. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, Z.; Xu, C.; Zhou, C.; Li, Z.; Ni, W. Impact of SO2 concentration on the corrosion rate of X70 steel and iron in water-saturated supercritical CO2 mixed with SO2. J. Supercrit. Fluids 2011, 58, 286–294. [Google Scholar] [CrossRef]
- Dugstad, A.; Halseid, M.; Morland, B. Effect of SO2 and NO2 on corrosion and solid formation in dense phase CO2 pipelines. Energy Procedia 2013, 37, 2877–2887. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Q.; Yang, X.X.; Wang, Z.; Liu, J.; Li, Z. Impact of surface roughness and humidity on X70 steel corrosion in supercritical CO2 mixture with SO2, H2O, and O2. J. Supercrit. Fluids 2016, 107, 286–297. [Google Scholar] [CrossRef]
- Sim, S.; Cole, I.S.; Choi, Y.-S.; Birbilis, N. A review of the protection strategies against internal corrosion for the safe transport of supercritical CO2 via steel pipelines for CCS purposes. Int. J. Greenh. Gas. Con. 2014, 29, 185–199. [Google Scholar] [CrossRef]
- Mclntire, G.; Lippert, J.; Yedelson, J. The effect of dissolved CO2 and O2 on the corrosion of iron. Corrosion 1990, 46, 91–95. [Google Scholar] [CrossRef]
- Choi, Y.S.; Nešić, S.; Young, D. Effect of impurities on the corrosion behavior of CO2 transmission pipeline steel in supercritical CO2-water environments. Environ. Sci. Technol. 2010, 44, 9233–9238. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).