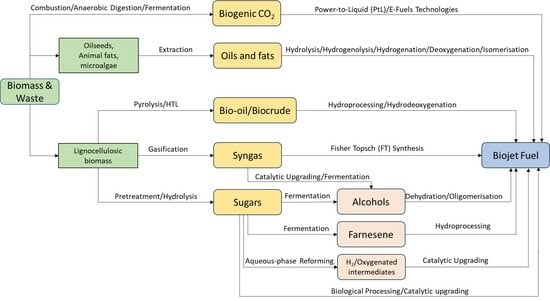
A Review of Current and Emerging Production Technologies for Biomass-Derived Sustainable Aviation Fuels
Abstract
1. Introduction
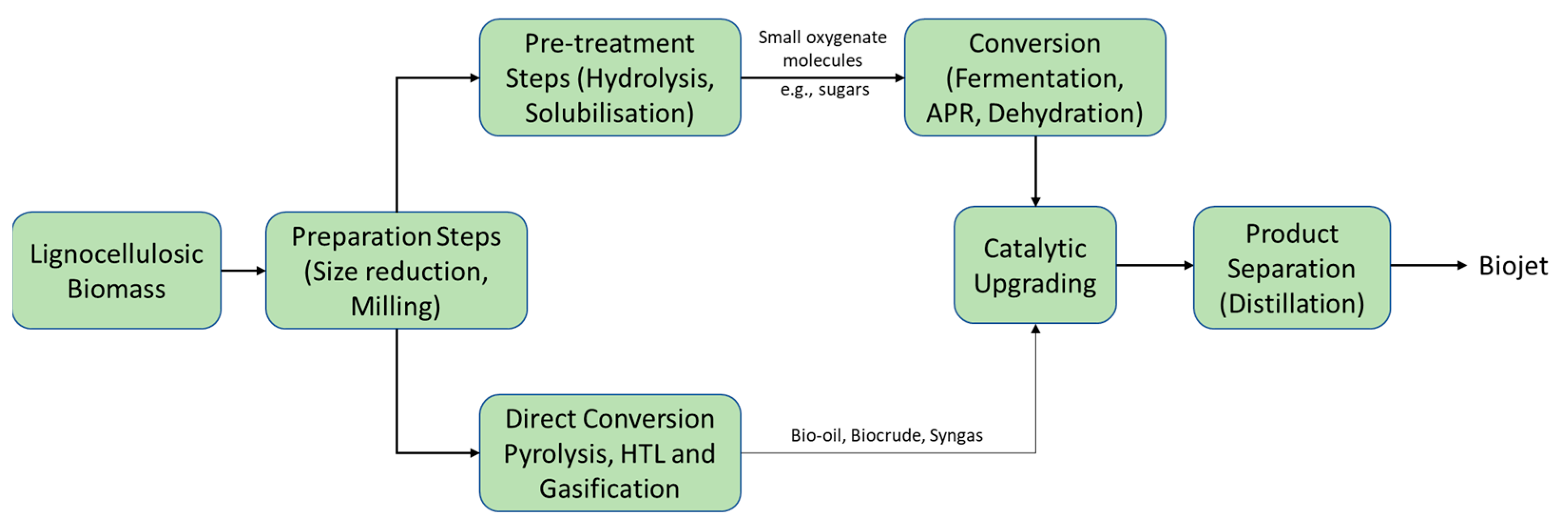
2. Biojet Fuel Technologies
| Biojet Fuel Technology | Year of Approval | Feedstock Type | Production Company | Airline Company | Blends (%) | Ref. |
|---|---|---|---|---|---|---|
| Gasification and Fischer–Tropsch (FT) D 7566 Annex 1 | 2009 | Woody and lignocellulosic biomass | Syntroleum (Tulsa, OK, USA), SynFuels International (Dallas, TX, USA), Rentech (Los Angeles, CA, USA), Shell (London, UK), Solena (Gilroy, CA, USA), Coskata (Warrenville, IL, USA), INEOS (London, UK), Bio/Lanza Tech (Skokie, IL, USA), Swedish Biofuels (Lidingö, Sweden), Fulcrum BioEnergy (Pleasanton, CA, USA), Red Rock Biofuels (Fort Collins, CO, USA), Velocys (Harwell, UK) | Qatar Airways, United Airlines, Air bus, British Airways, Virgin Atlantic, Southwest Airlines | 50 | [12,13,20,21,22,23] |
| Hydroprocessed Esters and Fatty Acids (HEFA) D 7566 Annex 2 | 2011 | Plant oils, food industry waste oils, algal oil, animal fats | HoneyWell UOP (Des Plaines, IL, USA), SG Biofuels (San Diego, CA, USA), AltAir Fuels (Paramount, CA, USA), Agrisoma Biosciences (Gatineau, QC, Canada), Neste Oil (Espoo, Finland), Petrochina (Beijing, China), Sapphire Energy (San Diego, CA, USA), Syntroleum (Tulsa, OK, USA)/Tyson Food (Springdale, AR, USA), PEMEX (Mexico City, Mexico), ASA, Renewable Energy Group (Ames, IA, USA), ENI (Rome, Italy), UPM (Helsinki, Finland) Valero Energy Corp. and Darling Ingredients Inc (Norco, CA, USA), World Energy (Boston, MA, USA) | Boeing, Lufthansa, Virgin Atlantic, Virgin Blue, GE Aviation, Air New Zealand, Rolls-Royce, Continental, CFM, JAL, Airbus, KLM, Pratt & Whitney, Air China, TAM Airlines, Jet Blue Airways, IAE, United Airlines, Air France, Finnair, Air Mexico, Thomson Airways, Porter Airlines, Alaska Airlines, Horizon Air, Etihad Airways, Romanian Air, Bombardier, DHL Express, Amazon (Cargo), SG Preston | 50 | [12,13,24,25] |
| Synthesised Iso-Paraffin (SIP) D 7566 Annex 3 | 2014 | Sugars, cellulosic materials | Amyris (Emeryville, CA, USA)/Total (Courbevoie, France), Solazyme (South San Francisco, CA, USA), LS9 (South San Francisco, CA, USA) | Boeing, Embraer, Azul Airlines, GE, Trip Airlines | 10 | [12,13,27] |
| * Fischer–Tropsch Synthethic Paraffinic Kerosene with Aromatics (FT-SPK/A) D 7566 Annex 4 | 2015 | Wastes (MSW, etc.), coal, gas, sawdust | Shell (London, UK), Sasol (Johannesburg, South Africa) | Boeing, Embraer, Azul Airlines, GE, Trip Airlines | 50 | [12,13,20,21,22,23] |
| Alcohol-To-Jet (ATJ) D 7566 Annex 5 | 2016 | Sugars, starches, alcohol | Terrabon (Houston, TX, USA)/Advanced BioFuels (Frederick, MD, USA) LanzaJet (Skokie, IL, USA), LanzaJet/LanzaTech (Skokie, IL, USA), Coskata (Warrenville, IL, USA), Gevo (Englewood, CO, USA), Byogy (San Jose, CA, USA), Albemarle (Charlotte, NC, USA)/Cobalt (Mountain View, CA, USA), Solazyme (South San Francisco, CA, USA), HoneyWell UOP (Des Plaines, IL, USA), Nova Pangea (Redcar and Cleveland, UK), Swedish Biofuels (Stockholm, Sweden) | Airbus, Boeing, Virgin Atlantic, Continental Airlines, United Airlines, British Airways, Air New Zealand, Delta Airlines | 50 | [12,13,14,15,16,17,18,19] |
| Co-hydroprocessing of esters and fatty acids D1655 Annex 1 | 2018 | Fischer–Tropsch hydrocarbons co-processed with petroleum | - | - | 5 | [12,13] |
| Co-hydroprocessing of Fischer–Tropsch hydrocarbons D1655 Annex A1 | ||||||
| Catalytic Hydrothermolysis (CH) D 7566 Annex 6 | 2020 | Plant oils, food industry waste oils, algal oil, animal fats | Applied Research Association (Albuquerque, NM, USA), Aemetis (Cupertino, CA, USA)/Chevron Lummus Global (Rio De Janeiro, Brazil) | Rolls-Royce, Pratt & Whitney | 50 | [12,13,26] |
| Hydroprocessed Hydrocarbons Hydroprocessed Esters and Fatty Acids (HH-SPK or HC-HEFA) D 7566 Annex 7 | 2020 | Algae (Botryococcus braunii) | Applied Research Association (Albuquerque, NM, USA) | - | 10 | [12,13] |
| Biojet Technology | Company | Feedstocks | Capacity L/year | Status |
|---|---|---|---|---|
| HEFA/HRJ | Neste (Espoo, Finland) | Veg. oil, WCO, animal fat | 2 B | Operational |
| ENI (Rome, Italy) | Veg. oil | 155 M | Operational | |
| Valero Energy Corp. and Darling Ingredients Inc. (Norco, CA, USA) | Veg. oil, WCO, animal fat | 2.13 B | Operational | |
| World Energy (Boston, MA, USA), AltAir Fuels (Paramount, CA, USA) | Non-edible oil, waste oil | 150 B | Operational | |
| Total (Courbevoie, France) | WCO, Veg. oil | 453 M | Operational | |
| UPM (Helsinki, Finland) | Crude tall oil | 120 M | Operational | |
| Renewable Energy Group (Ames, IA, USA) | High and low free fatty acid feedstocks | 284 M | Operational | |
| FT | Fulcrum Bioenergy (Pleasanton, CA, USA) | MSW | 1.8 B | Planned |
| Red Rock Biofuels (Fort Collins, CO, USA) | Wood | 909.2 M | Planned | |
| ATJ | Swedish Biofuel Technology (Stockholm, Sweden) | Ethanol | 10 M | Operational |
| Biochemtex (Ortona, Italy) | Lignocellulosic biomass | <10 M | Operational | |
| LanzaJet (Skokie, IL, USA) | Ethanol | 180 B | Operational |
2.1. Biojet Fuel Technology Based on Lipid Feedstocks
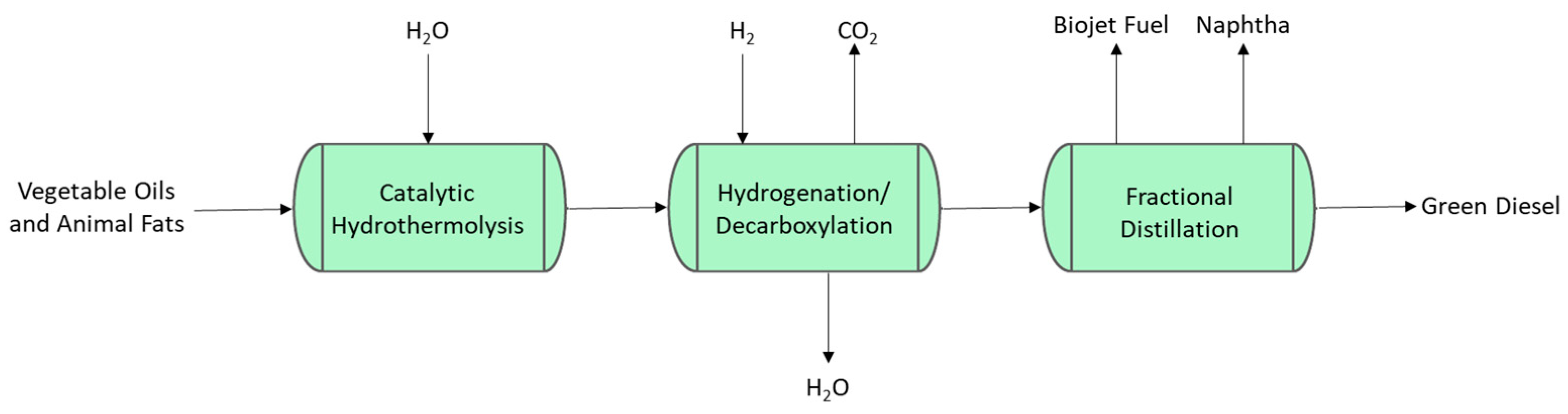
2.1.1. Hydroprocessed Esters and Fatty Acids (HEFAs)
- Higher Heating Value: HEFA fuel exhibits a higher heating value than biodiesel. This means it contains more energy per unit volume, resulting in increased fuel efficiency and improved overall performance in aviation engines. The higher energy content allows aircraft to achieve better fuel economy and potentially extend their flight range.
- Superior Energy Density: HEFA fuel boasts a higher energy density, which means it can store a greater amount of energy per unit mass. This characteristic is highly desirable for aviation fuel, as it allows for longer flights without the need for frequent refuelling. The higher energy density of HRJ fuel contributes to increased aircraft endurance and reduces the need for additional fuel stops.
- Improved Cold Point Qualities: HEFA fuel possesses superior cold point qualities when compared to biodiesel. It exhibits enhanced low-temperature flow properties, ensuring that the fuel remains in a liquid state and flows smoothly even in cold climates or high altitudes. This characteristic is of particular importance during aircraft take-off and landing in colder regions, as it helps maintain optimal fuel flow and prevents fuel line blockages caused by cold temperatures. The two important parameters in this context are viscosity at low temperatures (−20 °C and −40 °C) and the freeze point. These properties play a crucial role in determining the fuel’s ability to perform under cold conditions, especially during aircraft take-off and landing in colder regions [9,43].

Commercialisation Challenges of HEFA
2.1.2. Catalytic Hydrothermolysis (CH)
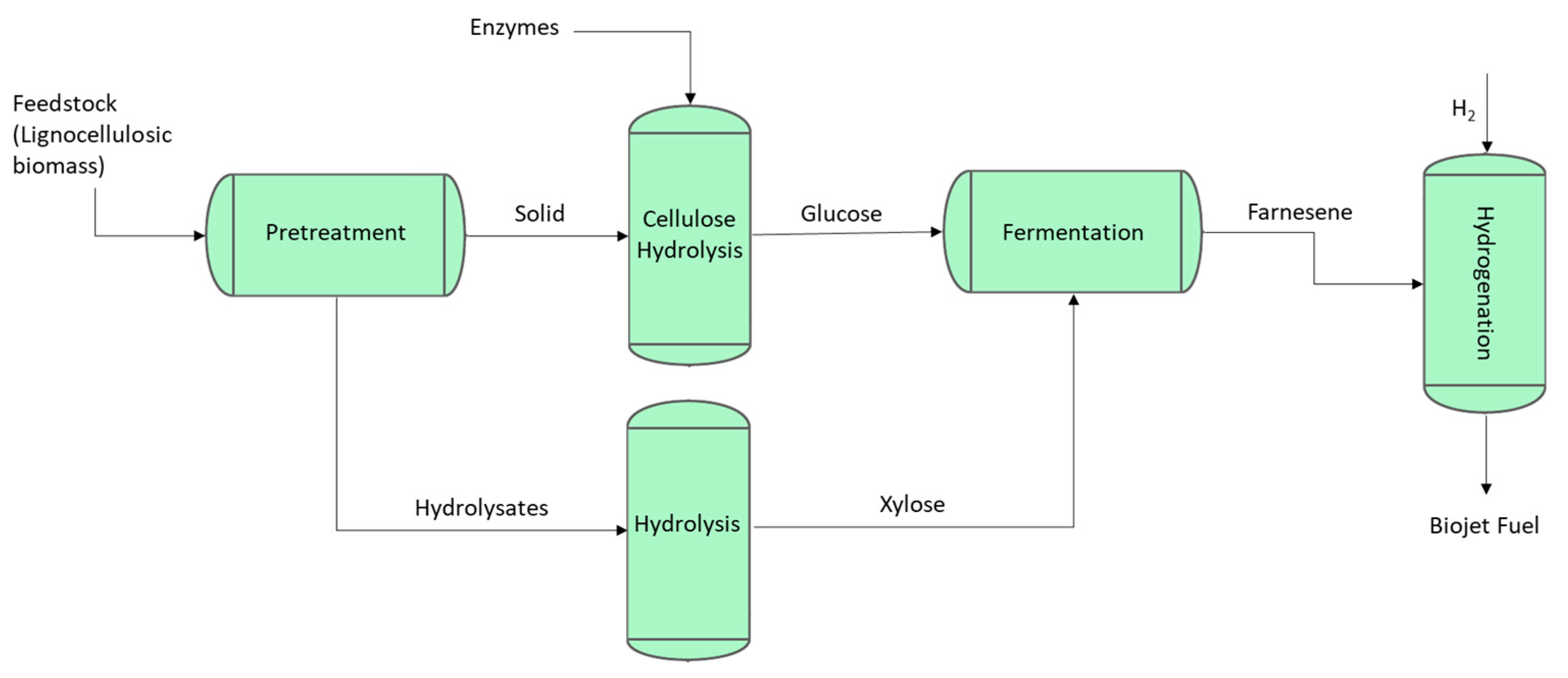
2.2. Biojet Production from Lignocellulosic Biomass
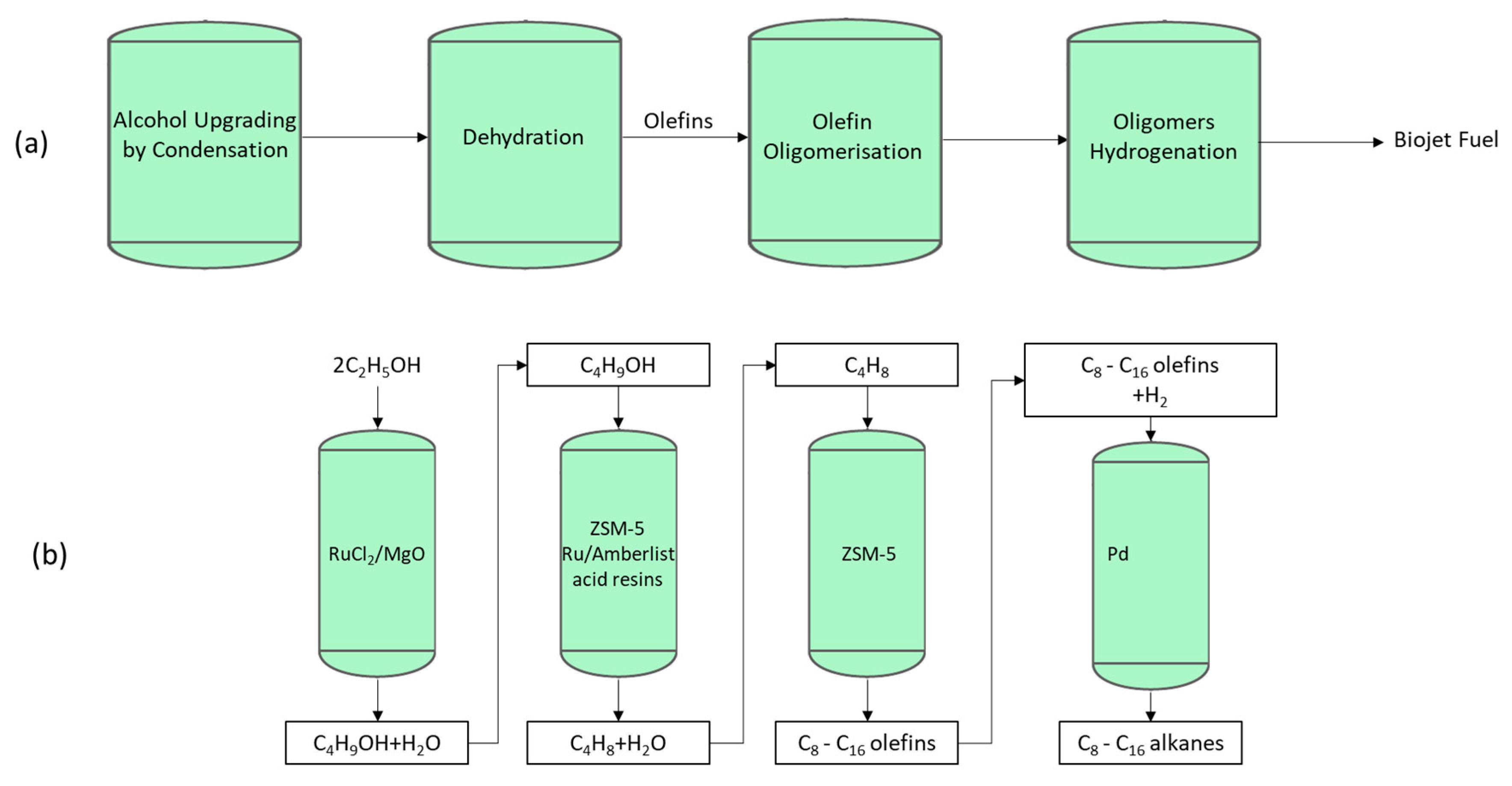
Biojet Production Routes Based on Alcohol Feedstocks (Alcohol to Jet)
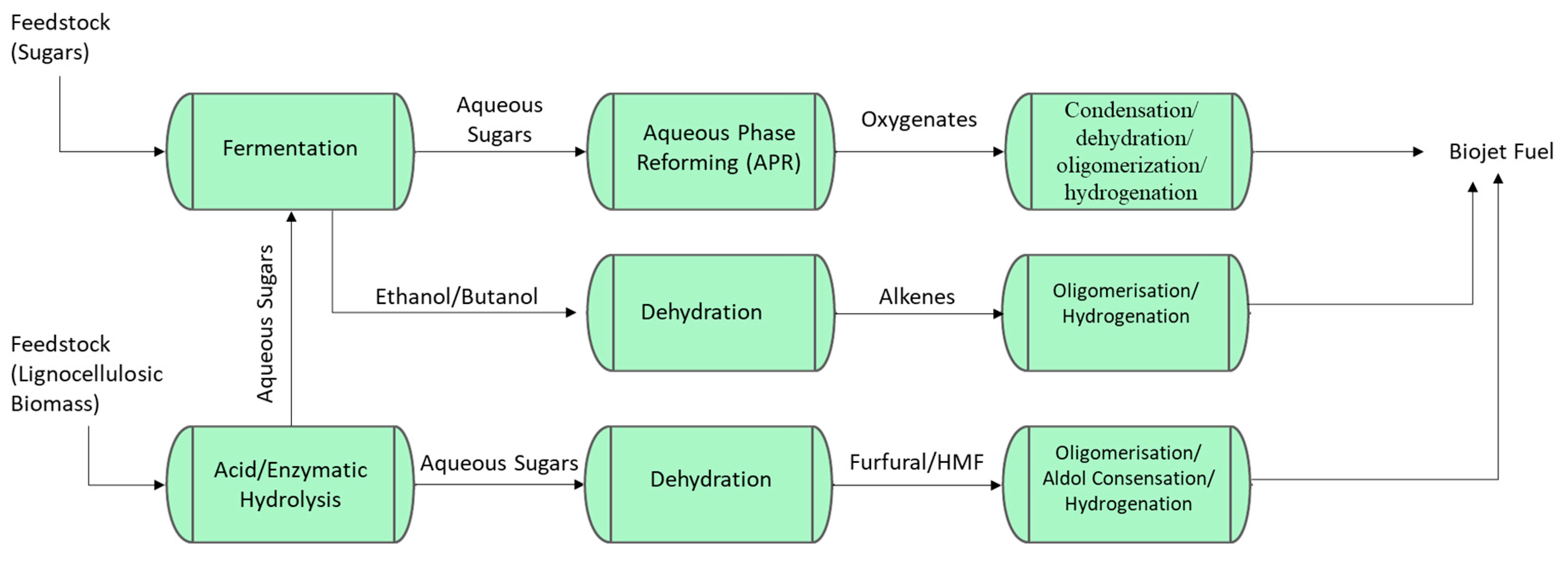
2.3. Biojet Fuel Production Routes Based on Sugar Feedstocks
2.3.1. Synthesised Iso-Paraffins (SIP) (Formerly Direct Sugar to Hydrocarbon (DSHC))
2.3.2. Thermocatalytic Routes for Sugar-to-Biojet
Aqueous Phase Reforming (APR) for Biojet Fuel
Furanics-to-Biojet Fuel
- limited selectivity due to poor control over side reactions;
- the limited control associated with glucose the feedstock;
- the extra steps required to isomerise glucose into fructose as fructose gives higher yields of HMF with better selectivity and rates as seen in Table 4.
| Feedstock | Solvent | Catalyst | Temp. (°C) | Time (min) | Yield (%) | Reference |
|---|---|---|---|---|---|---|
| Fructose | CHClO | Malonic acid | 80 | 60 | 41 | [51] |
| CHClO | Oxalic acid | 80 | 60 | 62 | [51] | |
| Water | HCl (aq) | 95 | 90 | 68 | [145] | |
| Water-acetone | Dowex-50wx8-100 | 150 | 15 | 73 | [145] | |
| CHClO | Citric acid | 80 | 60 | 76.3 | [53] | |
| 1:1 water-DMSO/7:3 MIBK/2-BuOH | HCl | 170 | 4 | 85 | [145] | |
| DMSO | CNT-PSSA | 120 | 30 | 89 | [53] | |
| [HexylMIM]Cl | SO42−/ZrO2 | 100 | 30 | 89 | [145] | |
| [BMIM]Cl | LS | 100 | 10 | 94.3 | [146] | |
| [BMIM]Cl | NHC/CrCl2 | 100 | 360 | 96 | [145] | |
| 1:7 DMSO/MIBK | Acidic ion exchange resin | 76 | - | 97 | [145] | |
| DMSO | NH4Cl | 100 | 45 | 100 | [145] | |
| DMSO | Amberlyst-15 powder | 120 | 120 | 100 | [145] | |
| Glucose | Water | TiO2/ZrO2 | 250 | 5 | 29 | [145] |
| [EMIM]Cl | Boric acid | 120 | 180 | 41 | [147] | |
| Water | H3PO4/Nb2O5 | 120 | 180 | 52 | [145] | |
| DMSO | CNT-PSSA | 140 | 60 | 57 | [145] | |
| 1:2.25 Water-MIBK | AgPW12O40 | 130 | 240 | 76 | [145] | |
| Cellulose | Water | HCL | 300 | 30 | 21 | [145] |
| 1:5 Water/MIBK | TiO2 | 270 | 2 | 30 | [145] | |
| [EMIM]Cl | Boric acid | 120 | 480 | 32 | [147] | |
| Water | Cr[(DS)H2PW12O40]3 | 150 | 120 | 53 | [145] | |
| [EMIM]Cl | CrCl2 | 120 | 360 | 89 | [145] |
2.4. Biojet Fuel Production Routes Based on Whole Biomass
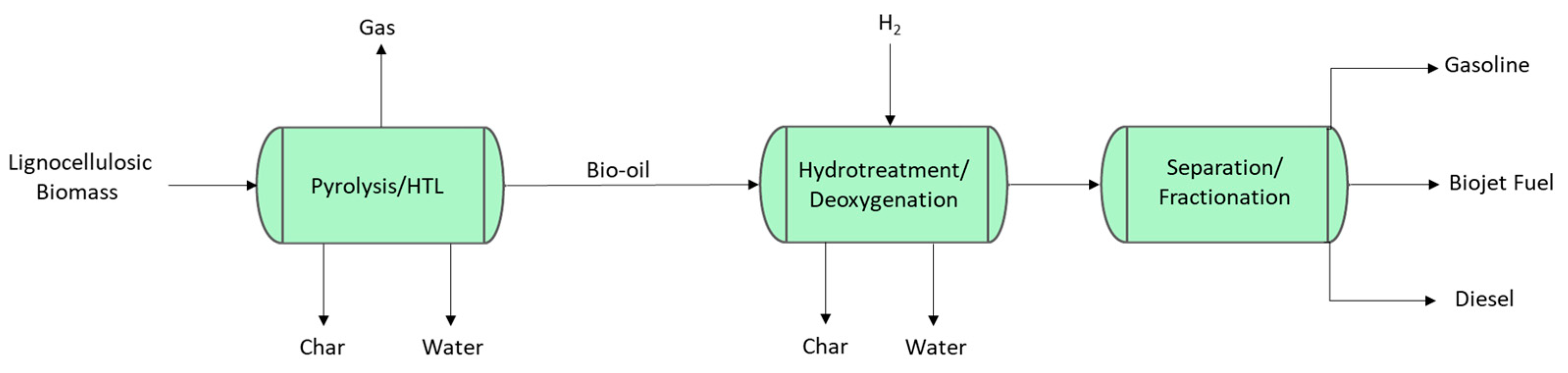
2.4.1. Biomass Pyrolysis and Hydrothermal Liquefaction (HTL) to Jet Fuel
| Properties | Bio-Oil | Crude Oil |
|---|---|---|
| Density (kg/m3 @ 15 °C) | 818.4–923.6 | 772.1–936.0 |
| Total acid number (mgKOH/g) | 116.2–207.5 | 0.0–2.0 |
| Aromatics (%) | 20.4–60.5 | 32.6–53.0 |
| C (%) | 55–65 | 83–86 |
| H (%) | 5–7 | 11–14 |
| O (%) | 28–50 | <1 |
| Water (%) | 15–30 | 0.1 |
| Heating value (MJ/Kg) | 16–19 | 44 |
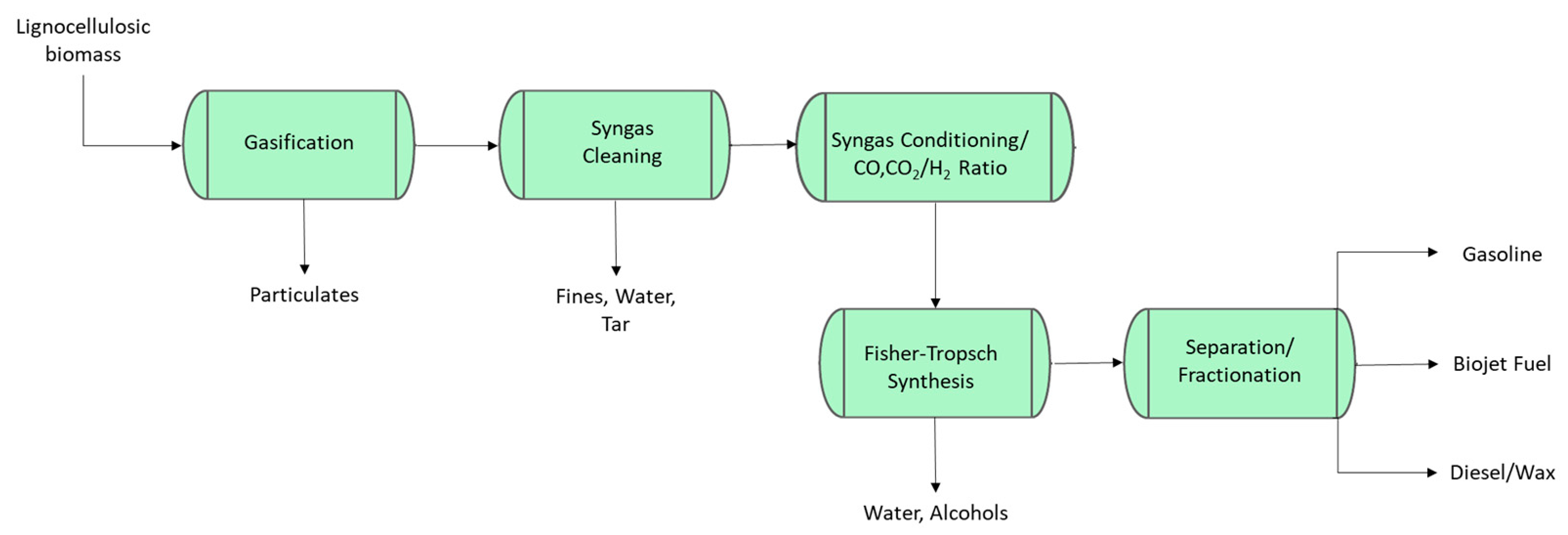
2.4.2. Biomass Gasification—Fischer–Tropsch to Biojet (Gas-to-Jet)
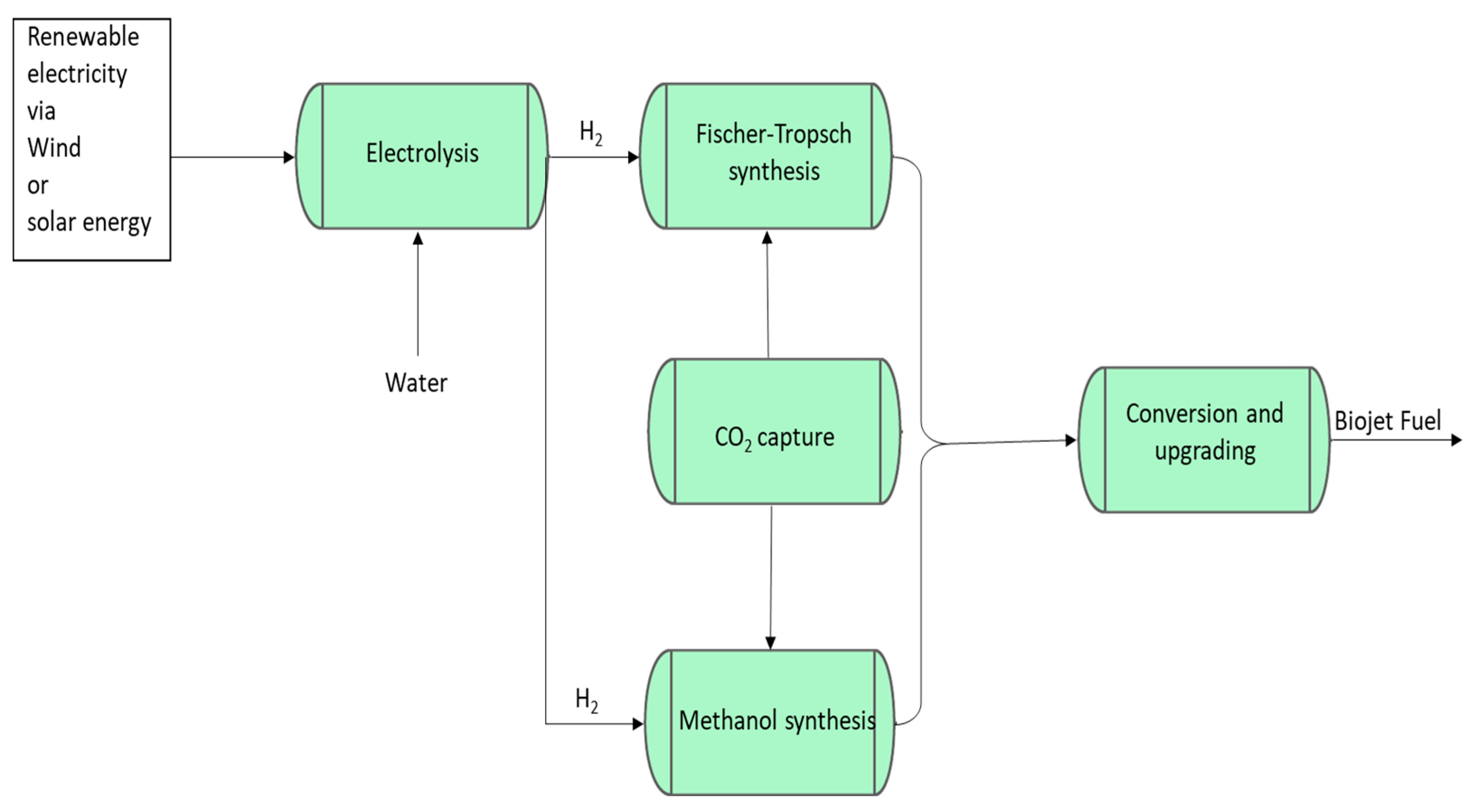
2.5. Biojet Fuel Production from Biogenic CO2 as Part of Power-to-Liquid Pathway
Power-to-Liquids Fuel
2.6. Comparison of Yields and Properties of Biojet Fuels from Different Routes/Pathways
2.7. Comparative Life Cycle GHG Emissions of Approved Bio-Based SAF
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Air Transport Association. Sustainable Aviation Fuel Fact Sheet. 2023. Available online: https://www.iata.org/en/iata-repository/pressroom/fact-sheets/fact-sheet---alternative-fuels/ (accessed on 2 June 2023).
- Sustainable Aviation Fuel Grand Challenge. Energy.gov. 2023. Available online: https://www.energy.gov/eere/bioenergy/sustainable-aviation-fuel-grand-challenge (accessed on 6 August 2023).
- Luman, R.; Zhang, C. Climate Targets Expedite the Take-Off of Sustainable Aviation Fuels. 2023. Available online: https://think.ing.com/articles/climate-targets-push-the-take-off-of-sustainable-aviation-fuels/ (accessed on 2 August 2023).
- United Airlines. United Becomes First U.S. Airline to Begin Using Sustainable Aviation Biofuel for Regularly Scheduled Flights. 2018. Available online: https://www.united.com/ual/en/us/fly/company/global-citizenship/environment/sustainable-aviation-fuel.html (accessed on 6 June 2023).
- European Commission. Proposal for a Directive of the European Parliament and of the Council Amending Directive (EU) 2018/2001 on the Promotion of the Use of Energy from Renewable Sources (Recast). 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52020PC0197&from=EN (accessed on 6 June 2023).
- Delta Air Lines. Delta, Airbus, and Air BP Partner to Advance Aviation’s Net-Zero Ambitions. 2021. Available online: https://news.delta.com/delta-airbus-and-air-bp-partner-advance-aviations-net-zero-ambitions (accessed on 6 June 2023).
- Clarkson, N. Virgin Atlantic’s Historic 100% Sustainable Aviation Fuel Transatlantic Flight Closer to Takeoff: Virgin, Virgin.com. 2023. Available online: https://www.virgin.com/about-virgin/latest/virgin-atlantics-historic-net-zero-transatlantic-flight-closer-to-takeoff (accessed on 2 August 2023).
- Aviation’s Long Haul to Replace Jet Fuel. Airlines. 2023. Available online: https://airlines.iata.org/2022/06/09/aviations-long-haul-replace-jet-fuel (accessed on 2 August 2023).
- ASTM D7566; Standard Specification for Aviation Turbine Fuel Containing Synthesized Hydrocarbons. ASTM International: West Conshohocken, PA, USA, 2019.
- Holladay, J.; Abdullah, Z.; Heyne, J. Sustainable Aviation Fuel: Review of Technical Pathways; Department of Energy: Washington, DC, USA, 2020. [Google Scholar] [CrossRef]
- Kramer, S.; Andac, G.; Heyne, J.; Ellsworth, J.; Herzig, P.; Lewis, K.C. Perspectives on Fully Synthesized Sustainable Aviation Fuels: Direction and Opportunities. Front. Energy Res. 2022, 9, 782823. [Google Scholar] [CrossRef]
- IATA Fact Sheet 2—IATA. Available online: https://www.iata.org/contentassets/d13875e9ed784f75bac90f000760e998/saf-technical-certifications.pdf (accessed on 2 August 2023).
- ICAO. Conversion Processes. 2023. Available online: https://www.icao.int/environmental-protection/GFAAF/Pages/Conversion-processes.aspx (accessed on 3 August 2023).
- Brooks, K.; Snowden-Swan, L.; Jones, S.; Butcher, M.; Lee, G.; Anderson, D.; Frye, J.; Holladay, J.; Owen, J.; Harmon, L.; et al. Chapter 6-Low-Carbon Aviation Fuel through the Alcohol to Jet Pathway. In Biofuels for Aviation; Academic Press: Cambridge, MA, USA, 2016; pp. 109–150. [Google Scholar]
- Kelly, S. US Ethanol Industry Expands Focus to Lower-Carbon Aviation Sector. Reuters. 2023. Available online: https://www.reuters.com/business/sustainable-business/us-ethanol-industry-expands-focus-lower-carbon-aviation-sector-2023-04-27/ (accessed on 2 August 2023).
- Surgenor, C.; Harrington, T. British Airways Moves into Alcohol-to-Jet Fuels Following an Offtake and Investment Agreement with LanzaJet. GreenAir News. 2023. Available online: https://www.greenairnews.com/?p=637 (accessed on 2 August 2023).
- Nilson, P. Airbus Partners with LanzaJet for SAF Production. Airport Technology. 2023. Available online: https://www.airport-technology.com/news/airbus-partners-with-lanzajet-for-saf-production/ (accessed on 2 August 2023).
- Honeywell. Honeywell Revolutionizes Ethanol-to-Jet Fuel Technology to Meet Rising Demand for Sustainable Aviation Fuel, PR Newswire: Press Release Distribution, Targeting, Monitoring and Marketing. 2022. Available online: https://www.prnewswire.com/news-releases/honeywell-revolutionizes-ethanol-to-jet-fuel-technology-to-meet-rising-demand-for-sustainable-aviation-fuel-301644079.html (accessed on 2 August 2023).
- LanzaJet and LanzaTech Selected by Air New Zealand and New Zealand Government to Undertake Study for Domestic Sustainable Aviation Fuel Production in New Zealand. LanzaTech. 2023. Available online: https://ir.lanzatech.com/news-releases/news-release-details/lanzajet-and-lanzatech-selected-air-new-zealand-and-new-zealand (accessed on 2 August 2023).
- Geleynse, S.; Brandt, K.; Garcia-Perez, M.; Wolcott, M.; Zhang, X. The Alcohol-to-Jet Conversion Pathway for Drop-In Biofuels: Techno-Economic Evaluation. Chemsuschem 2018, 11, 3728–3741. [Google Scholar] [CrossRef]
- O’Rear, E.G.; Jones, W.; Hiltbrand, G.; Wimberger, E.; Larsen, J.; Sustainable Aviation Fuels: The Key to Decarbonizing Aviation. Rhodium Group. 2022. Available online: https://rhg.com/research/sustainable-aviation-fuels/ (accessed on 3 August 2023).
- Krause, H. Sustainable Aviation Fuel Agencies Should Track Progress. 2023. Available online: https://www.gao.gov/assets/gao-23-105300.pdf (accessed on 3 August 2023).
- Michaga, M.F.R.; Michailos, S.; Hughes, K.J.; Ingham, D.; Pourkashanian, M. Techno-economic and life cycle assessment review of sustainable aviation fuel produced via biomass gasification. In Sustainable Biofuels; Academic Press: Cambridge, MA, USA, 2021; pp. 269–303. [Google Scholar] [CrossRef]
- Kurzawska, P. Overview of Sustainable Aviation Fuels including emission of particulate matter and harmful gaseous exhaust gas compounds. Transp. Res. Procedia 2021, 59, 38–45. [Google Scholar] [CrossRef]
- Van Dyk, S.; Ebadian, M.; Su, J.; Larock, F.; Zhang, Y.; Monnier, J.; Wang, H.; Santosa, D.M.; Olarte, M.V.; Neuenschwander, G.; et al. 2019. Available online: http://task39.sites.olt.ubc.ca/files/2019/11/GARDN-NEC-21-ATM-project-final-report-public-release.pdf (accessed on 6 August 2023).
- Wang, W.; Tao, L.; Markham, J.; Zhang, Y.; Tan, E.; Batan, L.; Warner, E.; Biddy, M. Review of Biojet Fuel Conversion Technologies. 2020. Available online: https://www.nrel.gov/docs/fy16osti/66291.pdf (accessed on 7 June 2023).
- Bosch, J.; de Jong, S.; Hoefnagels, D.; Slade, D. 2020. Available online: https://www.imperial.ac.uk/media/imperial-college/grantham-institute/public/publications/briefing-papers/BP-23-Aviation-Biofuels.pdf (accessed on 23 June 2023).
- Kojima, M.; Oyama, T. Life-cycle greenhouse gas emissions of biojet fuels made from forestry residues and waste cooking oil: A case study of Japan. Environ. Sci. Technol. 2020, 54, 9612–9621. [Google Scholar]
- Virgin Atlantic. Virgin Atlantic Operates First Commercial Flight Using Pioneering low-Carbon Fuel. 2018. Available online: https://www.virginatlantic.com/gb/en/footer/media-centre/press-releases/virgin-atlantic-operates-first-commercial-flight-using-pioneering-low-carbon-fuel.html (accessed on 3 March 2023).
- Boeing. Sustainable Aviation Fuel. 2023. Available online: https://www.boeing.com/environment/sustainable-aviation-fuel.page (accessed on 7 July 2023).
- ICAO. Sustainable Aviation Fuels. 2019. Available online: https://www.icao.int/environmental-protection/Documents/EnvironmentalReports/2019/ENVReport2019_pg171-173.pdf (accessed on 2 August 2023).
- University of Bristol. Scientists Make Jet Fuel from Seawater-Tolerant Plant. 2022. Available online: https://www.bristol.ac.uk/news/2022/january/plant-jet-fuel.html (accessed on 23 June 2023).
- Grimme, W. The Introduction of Sustainable Aviation Fuels—A Discussion of Challenges, Options and Alternatives. Aerospace 2023, 10, 218. [Google Scholar] [CrossRef]
- Erkul Kaya, N. World Jet Fuel Demand Could Drop By 70% Due to COVID-19. 2020. Available online: https://www.aa.com.tr/en/economy/world-jet-fuel-demand-could-drop-by-70-due-to-covid-19/1778544# (accessed on 31 July 2023).
- Atsonios, K.; Li, J.; Inglezakis, V.J. Process analysis and comparative assessment of advanced thermochemical pathways for e-kerosene production. Energy 2023, 278, 127868. [Google Scholar] [CrossRef]
- Sustainable Aviation. 2023. Available online: https://www.sustainableaviation.co.uk/wp-content/uploads/2023/04/SA9572_2023CO2RoadMap_Brochure_v4.pdf (accessed on 6 August 2023).
- Neste Corporation. Neste to Supply Sustainable Aviation Fuel to United Airlines Flights Departing San Francisco International Airport. 2023. Available online: https://energydigital.com/sustainability/plans-arise-for-neste-and-ua-sustainable-aviation-fuel-usage (accessed on 4 August 2023).
- Daly, T. Eni Plants Seeds of Biofuel Revolution. Energy Intelligence. 2023. Available online: https://www.energyintel.com/00000186-953d-d045-afd6-bf3dcaa60000 (accessed on 4 August 2023).
- IRVING. Diamond Green Diesel (DGD) Approves a Sustainable Aviation Fuel Project at Port Arthur, Texas, Darling Ingredients | Investors. 2023. Available online: https://ir.darlingii.com/2023-01-31-Diamond-Green-Diesel-DGD-Approves-a-Sustainable-Aviation-Fuel-Project-at-Port-Arthur,-Texas (accessed on 4 August 2023).
- Pinatel, B. LA Mède: A Multipurpose Facility for the Energies of Tomorrow. TotalEnergies.com. 2022. Available online: https://totalenergies.com/energy-expertise/projects/bioenergies/la-mede-a-forward-looking-facility (accessed on 4 August 2023).
- Early, C. The Sustainable Aviation Fuel Entrepreneurs Poised for Takeoff. Reuters. 2023. Available online: https://www.reuters.com/sustainability/climate-energy/sustainable-aviation-fuel-entrepreneurs-poised-takeoff-2023-06-07/ (accessed on 4 August 2023).
- RENO. Fulcrum Bioenergy Ships first Fuel by Railcar from Sierra Biofuels Plant. Fulcrum BioEnergy. 2023. Available online: https://www.fulcrum-bioenergy.com/news-resources/first-fuel-railcar (accessed on 4 August 2023).
- Chu, P.L.; Vanderghem, C.; MacLean, H.L.; Saville, B.A. Process modeling of hydrodeoxygenation to produce renewable jet fuel and other hydrocarbon fuels. Fuel 2017, 196, 298–305. [Google Scholar] [CrossRef]
- Costa, C.Z.; Sousa-Aguiar, E.F.; Couto, M.A.P.G.; de Carvalho Filho, J.F.S. Hydrothermal Treatment of Vegetable Oils and Fats Aiming at Yielding Hydrocarbons: A Review. Catalysts 2020, 10, 843. [Google Scholar] [CrossRef]
- Scaldaferri, C.A.; Pasa, V.M.D. Production of jet fuel and green diesel range biohydrocarbons by hydroprocessing of soybean oil over niobium phosphate catalyst. Fuel 2019, 245, 458–466. [Google Scholar] [CrossRef]
- Mousavi-Avval, S.H.; Shah, A. Techno-economic analysis of hydroprocessed renewable jet fuel production from pennycress oilseed. Renew. Sustain. Energy Rev. 2021, 149, 111340. [Google Scholar] [CrossRef]
- Wang, W.-C.; Hsieh, C.-H. Hydro-processing of biomass-derived oil into straight-chain alkanes. Chem. Eng. Res. Des. 2019, 153, 63–74. [Google Scholar] [CrossRef]
- Vásquez, M.C.; Silva, E.E.; Castillo, E.F. Hydrotreatment of vegetable oils: A review of the technologies and its developments for jet biofuel production. Biomass-Bioenergy 2017, 105, 197–206. [Google Scholar] [CrossRef]
- Martinez-Hernandez, E.; Ramírez-Verduzco, L.F.; Amezcua-Allieri, M.A.; Aburto, J. Process simulation and techno-economic analysis of bio-jet fuel and green diesel production—Minimum selling prices. Chem. Eng. Res. Des. 2019, 146, 60–70. [Google Scholar] [CrossRef]
- Doll, K.M.; Moser, B.R.; Knothe, G. Decarboxylation of oleic acid using iridium catalysis to form products of increased aromatic content compared to ruthenium systems. Int. J. Sustain. Eng. 2021, 14, 2018–2024. [Google Scholar] [CrossRef]
- Hu, W.; Wang, H.; Lin, H.; Zheng, Y.; Ng, S.; Shi, M.; Zhao, Y.; Xu, R. Catalytic Decomposition of Oleic Acid to Fuels and Chemicals: Roles of Catalyst Acidity and Basicity on Product Distribution and Reaction Pathways. Catalysts 2019, 9, 1063. [Google Scholar] [CrossRef]
- Jeništová, K.; Hachemi, I.; Mäki-Arvela, P.; Kumar, N.; Peurla, M.; Čapek, L.; Wärnå, J.; Murzin, D.Y. Hydrodeoxygenation of stearic acid and tall oil fatty acids over Ni-alumina catalysts: Influence of reaction parameters and kinetic modelling. Chem. Eng. J. 2017, 316, 401–409. [Google Scholar] [CrossRef]
- Cheah, K.W.; Yusup, S.; Kyriakou, G.; Ameen, M.; Taylor, M.J.; Nowakowski, D.J.; Bridgwater, A.V.; Uemura, Y. In-situ hydrogen generation from 1,2,3,4-tetrahydronaphthalene for catalytic conversion of oleic acid to diesel fuel hydrocarbons: Parametric studies using Response Surface Methodology approach. Int. J. Hydrogen Energy 2019, 44, 20678–20689. [Google Scholar] [CrossRef]
- Krobkrong, N.; Itthibenchapong, V.; Khongpracha, P.; Faungnawakij, K. Deoxygenation of oleic acid under an inert atmosphere using molybdenum oxide-based catalysts. Energy Convers. Manag. 2018, 167, 1–8. [Google Scholar] [CrossRef]
- Popov, S.; Kumar, S. Rapid Hydrothermal Deoxygenation of Oleic Acid over Activated Carbon in a Continuous Flow Process. Energy Fuels 2015, 29, 3377–3384. [Google Scholar] [CrossRef]
- Çakman, G.; Ceylan, S.; Balci, S. Catalytic Deoxygenation of Oleic Acid over Synthesized Ni@CMK-3 Catalyst using Analytical Py-GC/MS and TG-FTIR. J. Porous Mater. 2022, 30, 899–909. [Google Scholar] [CrossRef]
- Smoljan, C.S.; Crawford, J.M.; Carreon, M.A. Mesoporous microspherical NiO catalysts for the deoxygenation of oleic acid. Catal. Commun. 2020, 143, 106046. [Google Scholar] [CrossRef]
- Cheah, K.W.; Taylor, M.J.; Osatiashtiani, A.; Beaumont, S.K.; Nowakowski, D.J.; Yusup, S.; Bridgwater, A.V.; Kyriakou, G. Monometallic and bimetallic catalysts based on Pd, Cu and Ni for hydrogen transfer deoxygenation of a prototypical fatty acid to diesel range hydrocarbons. Catal. Today 2020, 355, 882–892. [Google Scholar] [CrossRef]
- Hossain, Z.; Chowdhury, M.B.I.; Jhawar, A.K.; Xu, W.Z.; Biesinger, M.C.; Charpentier, P.A. Continuous Hydrothermal Decarboxylation of Fatty Acids and Their Derivatives into Liquid Hydrocarbons Using Mo/Al2O3 Catalyst. ACS Omega 2018, 3, 7046–7060. [Google Scholar] [CrossRef] [PubMed]
- Irena. 2017. Available online: https://www.irena.org/documentdownloads/publications/irena_biofuels_for_aviation_2017.pdf (accessed on 30 March 2023).
- Icao.int. 2017. Available online: https://www.icao.int/environmental-protection/knowledge-sharing/Docs/Sustainable%20Aviation%20Fuels%20Guide_vf.pdf (accessed on 30 March 2023).
- Yilmaz, N.; Atmanli, A.; Vigil, F.M.; Donaldson, B. Comparative Assessment of Polycyclic Aromatic Hydrocarbons and Toxicity in a Diesel Engine Powered by Diesel and Biodiesel Blends with High Concentrations of Alcohols. Energies 2022, 15, 8523. [Google Scholar] [CrossRef]
- Wang, W.-C. Techno-economic analysis for evaluating the potential feedstocks for producing hydro-processed renewable jet fuel in Taiwan. Energy 2019, 179, 771–783. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; dos Santos, I.A.; Arcanjo, M.R.A.; Cavalcante, C.L.; de Luna, F.M.T.; Fernandez-Lafuente, R.; Vieira, R.S. Production of Jet Biofuels by Catalytic Hydroprocessing of Esters and Fatty Acids: A Review. Catalysts 2022, 12, 237. [Google Scholar] [CrossRef]
- Gorlova, A.M.; Komova, O.V.; Netskina, O.V.; Bulavchenko, O.A.; Lipatnikova, I.L.; Simagina, V.I. Hydrogen for Fuel Cells: Effect of Copper and Iron Oxides on the Catalytic Hydrolysis and Hydrothermolysis of Ammonia Borane. Russ. J. Electrochem. 2020, 56, 170–173. [Google Scholar] [CrossRef]
- Eswaran, S.; Subramaniam, S.; Geleynse, S.; Brandt, K.; Wolcott, M.; Zhang, X. Techno-economic analysis of catalytic hydrothermolysis pathway for jet fuel production. Renew. Sustain. Energy Rev. 2021, 151, 111516. [Google Scholar] [CrossRef]
- McGarvey, E.; Tyner, W.E. A stochastic techno-economic analysis of the catalytic hydrothermolysis aviation biofuel technology. Biofuels Bioprod. Biorefining 2018, 12, 474–484. [Google Scholar] [CrossRef]
- Elkelawy, M.; Bastawissi, H.A.-E.; Radwan, A.M.; Ismail, M.T.; El-Sheekh, M. Biojet fuels production from algae: Conversion technologies, characteristics, performance, and process simulation. In Handbook of Algal Biofuels; Elsevier: Amsterdam, Netherlands, 2022; pp. 331–361. [Google Scholar] [CrossRef]
- Hashmi, S.F.; Meriö-Talvio, H.; Hakonen, K.J.; Ruuttunen, K.; Sixta, H. Hydrothermolysis of organosolv lignin for the production of bio-oil rich in monoaromatic phenolic compounds. Fuel Process. Technol. 2017, 168, 74–83. [Google Scholar] [CrossRef]
- Wang, W.C.; Tao, L. Bio-jet fuel conversion technologies. Renew. Sustain. Energy Rev. 2016, 53, 801–822. [Google Scholar] [CrossRef]
- Li, L.; Coppola, E.; Rine, J.; Miller, J.L.; Walker, D. Catalytic Hydrothermal Conversion of Triglycerides to Non-ester Biofuels. Energy Fuels 2010, 24, 1305–1315. [Google Scholar] [CrossRef]
- Grau, J.M.; Parera, J. Conversion of heavy n-alkanes into light isomers over H-mordenite, platinum/H-mordenite, platinum/alumina and composite catalysts. Appl. Catal. A Gen. 1993, 106, 27–49. [Google Scholar] [CrossRef]
- Fu, J.; Yang, C.; Wu, J.; Zhuang, J.; Hou, Z.; Lu, X. Direct production of aviation fuels from microalgae lipids in water. Fuel 2015, 139, 678–683. [Google Scholar] [CrossRef]
- Li, T.; Cheng, J.; Huang, R.; Zhou, J.; Cen, K. Conversion of waste cooking oil to jet biofuel with nickel-based mesoporous zeolite Y catalyst. Bioresour. Technol. 2015, 197, 289–294. [Google Scholar] [CrossRef]
- Chen, L.; Li, H.; Fu, J.; Miao, C.; Lv, P.; Yuan, Z. Catalytic hydroprocessing of fatty acid methyl esters to renewable alkane fuels over Ni/HZSM-5 catalyst. Catal. Today 2016, 259, 266–276. [Google Scholar] [CrossRef]
- Choi, I.-H.; Lee, J.-S.; Kim, C.-U.; Kim, T.-W.; Lee, K.-Y.; Hwang, K.-R. Production of bio-jet fuel range alkanes from catalytic deoxygenation of Jatropha fatty acids on a WOx/Pt/TiO2 catalyst. Fuel 2018, 215, 675–685. [Google Scholar] [CrossRef]
- Silva, L.N.; Fortes, I.C.; de Sousa, F.P.; Pasa, V.M. Biokerosene and green diesel from macauba oils via catalytic deoxygenation over Pd/C. Fuel 2016, 164, 329–338. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, Z.; Zhang, X.; Fan, Z.; Liu, J.; Zhou, J. Continuous hydroprocessing of microalgae biodiesel to jet fuel range hydrocarbons promoted by Ni/hierarchical mesoporous Y zeolite catalyst. Int. J. Hydrogen Energy 2019, 44, 11765–11773. [Google Scholar] [CrossRef]
- Shim, J.-O.; Jeon, K.-W.; Jang, W.-J.; Na, H.-S.; Cho, J.-W.; Kim, H.-M.; Lee, Y.-L.; Jeong, D.-W.; Roh, H.-S.; Ko, C.H. Facile production of biofuel via solvent-free deoxygenation of oleic acid using a CoMo catalyst. Appl. Catal. B Environ. 2018, 239, 644–653. [Google Scholar] [CrossRef]
- de Sousa, F.P.; Cardoso, C.C.; Pasa, V.M. Producing hydrocarbons for green diesel and jet fuel formulation from palm kernel fat over Pd/C. Fuel Process. Technol. 2016, 143, 35–42. [Google Scholar] [CrossRef]
- Itthibenchapong, V.; Srifa, A.; Kaewmeesri, R.; Kidkhunthod, P.; Faungnawakij, K. Deoxygenation of palm kernel oil to jet fuel-like hydrocarbons using Ni-MoS2/γ-Al2O3 catalysts. Energy Convers. Manag. 2017, 134, 188–196. [Google Scholar] [CrossRef]
- Cheng, J.; Li, T.; Huang, R.; Zhou, J.; Cen, K. Optimizing catalysis conditions to decrease aromatic hydrocarbons and increase alkanes for improving jet biofuel quality. Bioresour. Technol. 2014, 158, 378–382. [Google Scholar] [CrossRef]
- Rabaev, M.; Landau, M.V.; Vidruk-Nehemya, R.; Koukouliev, V.; Zarchin, R.; Herskowitz, M. Conversion of vegetable oils on Pt/Al2O3/SAPO-11 to diesel and jet fuels containing aromatics. Fuel 2015, 161, 287–294. [Google Scholar] [CrossRef]
- Morgan, T.; Santillan-Jimenez, E.; Harman-Ware, A.E.; Ji, Y.; Grubb, D.; Crocker, M. Catalytic deoxygenation of triglycerides to hydrocarbons over supported nickel catalysts. Chem. Eng. J. 2012, 189–190, 346–355. [Google Scholar] [CrossRef]
- Veriansyah, B.; Han, J.Y.; Kim, S.K.; Hong, S.-A.; Kim, Y.J.; Lim, J.S.; Shu, Y.-W.; Oh, S.-G.; Kim, J. Production of renewable diesel by hydroprocessing of soybean oil: Effect of catalysts. Fuel 2012, 94, 578–585. [Google Scholar] [CrossRef]
- Wang, M.; He, M.; Fang, Y.; Baeyens, J.; Tan, T. The Ni-Mo/γ-Al2O3 catalyzed hydrodeoxygenation of FAME to aviation fuel. Catal. Commun. 2017, 100, 237–241. [Google Scholar] [CrossRef]
- El-Sawy, M.S.; Hanafi, S.A.; Ashour, F.; Aboul-Fotouh, T.M. Co-hydroprocessing and hydrocracking of alternative feed mixture (vacuum gas oil/waste lubricating oil/waste cooking oil) with the aim of producing high quality fuels. Fuel 2020, 269, 117437. [Google Scholar] [CrossRef]
- LMC International. LMC Lipid Feedstock Outlook to 2030. 2021. Available online: https://advancedbiofuelsassociation.com/wp-content/uploads/2021/11/LMC-Lipid-Feedstocks-Outlook-2030-CONCLUSIONS-Nov-2021.pdf (accessed on 10 August 2023).
- U.S. Energy Information Administration (EIA). 2020a.Annual Energy Outlook 2020. Table 47, Air Travel Energy Use. Available online: https://www.eia.gov/outlooks/aeo/data/browser/#/?id=57-AEO2020&cases=ref2020&sourcekey=0 (accessed on 10 August 2023).
- Popp, J.; Kovács, S.; Oláh, J.; Divéki, Z.; Balázs, E. Bioeconomy: Biomass and biomass-based energy supply and demand. New Biotechnol. 2020, 60, 76–84. [Google Scholar] [CrossRef]
- Beckham, G.T.; Linger, J.G.; Vardon, D.R.; Guarnieri, M.T.; Karp, E.M.; Franden, M.A.; Johnson, C.W.; Strathmann, T.J.; Pienkos, P.T. Lignin Conversion to Fuels, Chemicals and Materials. US10266852B2, 23 April 2019. [Google Scholar]
- Fu, C.; Li, Z.; Jia, C.; Zhang, W.; Zhang, Y.; Yi, C.; Xie, S. Recent advances on bio-based isobutanol separation. Energy Convers. Manag. X 2021, 10, 100059. [Google Scholar] [CrossRef]
- Hansen, S.; Mirkouei, A.; Diaz, L.A. A comprehensive state-of-technology review for upgrading bio-oil to renewable or blended hydrocarbon fuels. Renew. Sustain. Energy Rev. 2020, 118, 109548. [Google Scholar] [CrossRef]
- Geleynse, S.; Jiang, Z.; Brandt, K.; Garcia-Perez, M.; Wolcott, M.; Zhang, X. Pulp mill integration with alcohol-to-jet conversion technology. Fuel Process. Technol. 2020, 201, 106338. [Google Scholar] [CrossRef]
- Atsonios, K.; Kougioumtzis, M.-A.; Panopoulos, K.D.; Kakaras, E. Alternative thermochemical routes for aviation biofuels via alcohols synthesis: Process modeling, techno-economic assessment and comparison. Appl. Energy 2015, 138, 346–366. [Google Scholar] [CrossRef]
- Tao, L.; Markham, J.N.; Haq, Z.; Biddy, M.J. Techno-economic analysis for upgrading the biomass-derived ethanol-to-jet blendstocks. Green Chem. 2017, 19, 1082–1101. [Google Scholar] [CrossRef]
- Aldrett, S.; Worstell, J.H. Improved Ethylene Oligomerization Modeling Using Aspentech’s Polymers Plus; Semantic Scholar: Seattle, WA, USA, 2003. [Google Scholar]
- Gevo Inc. Renewable Compositions. US8193402B2, 5 June 2008. [Google Scholar]
- Wright, M.E. Process for the Dehydration of Aqueous Bio-Derived Terminal Alcohols to Terminal Alkenes. US9242226B2, 26 January 2016. [Google Scholar]
- Wright, D. Biomass to Alcohol to Jet/Diesel. 2012. Available online: AUS_BRIEF_2012_final.pdf (accessed on 4 August 2023).
- Kolosz, B.W.; Luo, Y.; Xu, B.; Maroto-Valer, M.; Andresen, J.M. Life cycle environmental analysis of ‘drop in’ alternative aviation fuels: A Review. Sustain. Energy Fuels 2020, 4, 3229–3263. [Google Scholar] [CrossRef]
- Phillips, S.D.; Jones, S.B.; Meyer, P.A.; Snowden-Swan, L.J. Techno-economic analysis of cellulosic ethanol conversion to fuel and chemicals. Biofuels Bioprod. Biorefining 2022, 16, 640–652. [Google Scholar] [CrossRef]
- Fivga, A.; Speranza, L.G.; Branco, C.M.; Ouadi, M.; Hornung, A. A review on the current state of the art for the production of advanced liquid biofuels. AIMS Energy 2019, 7, 46–76. [Google Scholar] [CrossRef]
- Wyman, C.E.; Bain, R.L.; Hinman, N.D.; Stevens, D.J. Chapter 21—Ethanol and Methanol from Cellulosic Materials. In Renewable Energy: Sources for Fuels and Electricity; Johansson, T.B., Kelly, H., Reddy, A.K.N., Williams, R.H., Burnham, L., Eds.; Island Press: Washington, DC, USA, 1993. [Google Scholar]
- Ayodele, B.V.; Alsaffar, M.A.; Mustapa, S.I. An overview of integration opportunities for sustainable bioethanol production from first- and second-generation sugar-based feedstocks. J. Clean. Prod. 2020, 245, 118857. [Google Scholar] [CrossRef]
- de Vries, S.C.; van de Ven, G.W.J.; van Ittersum, M.K.; Giller, K.E. Resource use efficiency and environmental performance of nine major biofuel crops, processed by first-generation conversion techniques. Biomass Bioenergy 2010, 34, 588–601. [Google Scholar] [CrossRef]
- Ahmad Dar, R.; Ahmad Dar, E.; Kaur, A.; Gupta Phutela, U. Sweet sorghum-a promising alternative feedstock for biofuel production. Renew. Sustain. Energy Rev. 2018, 82, 4070–4090. [Google Scholar] [CrossRef]
- Lennartsson, P.R.; Erlandsson, P.; Taherzadeh, M.J. Integration of the first and second generation bioethanol processes and the importance of by-products. Bioresour. Technol. 2014, 165, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Fava, F.; Totaro, G.; Diels, L.; Reis, M.; Duarte, J.; Carioca, O.B.; Poggi-Varaldo, H.M.; Ferreira, B.S. Biowaste biorefinery in Europe: Opportunities and research & development needs. New Biotechnol. 2015, 32, 100–108. [Google Scholar] [CrossRef]
- Lee, J. Biological conversion of lignocellulosic biomass to ethanol. J. Biotechnol. 1997, 56, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Amyris Inc. Total, Amyris to Market Renewable Jet Fuel from Commercial Flights. Biomass Magazine, 17 June 2014.
- Sundstrom, E.; Yaegashi, J.; Yan, J.; Masson, F.; Papa, G.; Rodriguez, A.; Mirsiaghi, M.; Liang, L.; He, Q.; Tanjore, D.; et al. Demonstrating a separation-free process coupling ionic liquid pretreatment, saccharification, and fermentation with Rhodosporidium toruloides to produce advanced biofuels. Green Chem. 2018, 20, 2870–2879. [Google Scholar] [CrossRef]
- Klein-Marcuschamer, D.; Turner, C.; Allen, M.; Gray, P.; Dietzgen, R.G.; Gresshoff, P.M.; Hankamer, B.; Heimann, K.; Scott, P.T.; Stephens, E.; et al. Technoeconomic analysis of renewable aviation fuel from microalgae, Pongamia pinnata, and sugarcane. Biofuels Bioprod. Biorefining 2013, 7, 416–428. [Google Scholar] [CrossRef]
- Kang, A.; Mendez-Perez, D.; Goh, E.-B.; Baidoo, E.E.K.; Benites, V.T.; Beller, H.R.; Keasling, J.D.; Adams, P.D.; Mukhopadhyay, A.; Lee, T.S. Optimization of the IPP-bypass mevalonate pathway and fed-batch fermentation for the production of isoprenol in Escherichia coli. Metab. Eng. 2019, 56, 85–96. [Google Scholar] [CrossRef]
- Karatzos, S.; McMillan, J.D.; Saddler, J.N. The Potential and Challenges of Drop-in Biofuels; IEA Bioenergy: Paris, France, 2014. [Google Scholar]
- Gray, D.; Sato, S.; Garcia, F.; Eppler, R.; Cherry, J.; Amyris, Inc. Integrated Biorefinery Project Summary Final Report—Public Version; Amyris Inc.: Emeryville, CA, USA, 2014. [Google Scholar]
- Tepelus, A.; Dragomir, R.E.; Rosca, P. Biojet from Sugar Derivatives. Rev. Roum. Chim. 2021, 66, 313–320. [Google Scholar]
- Amyris Inc. Total and Amyris Renewable Jet Fuel Ready for Use in Commercial Aviation. 2014. Available online: https://totalenergies.com/media/news/press-releases/total-and-amyris-renewable-jet-fuel-ready-use-commercial-aviation (accessed on 16 August 2023).
- Steen, E.J.; Kang, Y.; Bokinsky, G.; Hu, Z.; Schirmer, A.; McClure, A.; del Cardayre, S.B.; Keasling, J.D. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 2010, 463, 559–562. [Google Scholar] [CrossRef]
- Davis, R.; Tao, L.; Scarlata, C.; Tan, E.C.D.; Ross, J.; Lukas, J.; Sexton, D. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbons: Dilute-Acid and Enzymatic Deconstruction of Biomass to Sugars and Catalytic Conversion of Sugars to Hydrocarbons; National Renewable Energy Laboratory: Golden, CO, USA, 2015. Available online: https://www.nrel.gov/docs/fy14osti/60223.pdf (accessed on 10 August 2023).
- Whited, G.M.; Feher, F.J.; Benko, D.A.; Cervin, M.A.; Chotani, G.K.; McAuliffe, J.C.; LaDuca, R.J.; Ben-Shoshan, E.A.; Sanford, K.J. Technology update: Development of a gas-phase bioprocess for isoprene-monomer production using metabolic pathway engineering. Ind. Biotechnol. 2010, 6, 152–163. [Google Scholar] [CrossRef]
- Yang, J.; Xian, M.; Su, S.; Zhao, G.; Nie, Q.; Jiang, X.; Zheng, Y.; Liu, W. Enhancing Production of Bio-Isoprene Using Hybrid MVA Pathway and Isoprene Synthase in E. coli. PLOS ONE 2012, 7, e33509. [Google Scholar] [CrossRef] [PubMed]
- Chotani, G.K.; Kirshner, B.; Latone, J.; Pucci, J.P. Production of Isoprene under Reduced Oxygen Inlet Levels. Danisco US Inc. 2013. Available online: https://patents.google.com/patent/US8865442B2/en (accessed on 10 August 2023).
- Woodroffe, J.-D.; Harvey, B.G. Thermal cyclodimerization of isoprene for the production of high-performance sustainable aviation fuel. Energy Adv. 2022, 1, 338–343. [Google Scholar] [CrossRef]
- Walls, L.E.; Rios-Solis, L. Sustainable Production of Microbial Isoprenoid Derived Advanced Biojet Fuels Using Different Generation Feedstocks: A Review. Front. Bioeng. Biotechnol. 2020, 8, 599560. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.-K.; Liu, Q.; Zhang, J.-A.; Li, J.-P.; Xu, J.-M.; Wang, G.-H. Improved 2,3-butanediol production from corncob acid hydrolysate by fed-batch fermentation using Klebsiella oxytoca. Process. Biochem. 2010, 45, 613–616. [Google Scholar] [CrossRef]
- Adhikari, S.P.; Zhang, J.; Guo, Q.; Unocic, K.A.; Tao, L.; Li, Z. A hybrid pathway to biojet fuel via 2,3-butanediol. Sustain. Energy Fuels 2020, 4, 390. [Google Scholar] [CrossRef]
- Díaz-Pérez, M.A.; Serrano-Ruiz, J.C. Catalytic Production of Jet Fuels from Biomass. Molecules 2020, 25, 802. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Dumesic, J.A. Catalytic routes for the conversion of biomass into liquid hydrocarbon transportation fuels. Energy Environ. Sci. 2011, 4, 83–99. [Google Scholar] [CrossRef]
- Cortright, R. Cellulosic Biomass Sugars to Advantaged Jet Fuel—Catalytic Conversion of Corn Stover to Energy Dense, Low Freeze Point Paraffins and Naphthenes; Virent, Inc.: Madison, WI, USA, 2015. [Google Scholar] [CrossRef]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-Phase Catalytic Processing of Biomass-Derived Oxygenated Hydrocarbons to Fuels and Chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef]
- Tanzil, A.H.; Brandt, K.; Zhang, X.; Wolcott, M.; Lora, E.E.S.; Stockle, C.; Garcia-Perez, M. Evaluation of bio-refinery alternatives to produce sustainable aviation fuels in a sugarcane mill. Fuel 2022, 321, 123992. [Google Scholar] [CrossRef]
- Barbaro, P.; Liguori, F.; Moreno-Marrodan, C. Selective direct conversion of C5 and C6 sugars to high added-value chemicals by a bifunctional, single catalytic body. Green Chem. 2016, 18, 2935–2940. [Google Scholar] [CrossRef]
- Pipitone, G.; Zoppi, G.; Pirone, R.; Bensaid, S. Sustainable aviation fuel production using in-situ hydrogen supply via aqueous phase reforming: A techno-economic and life-cycle greenhouse gas emissions assessment. J. Clean. Prod. 2023, 418, 138141. [Google Scholar] [CrossRef]
- Kunkes, E.L.; Simonetti, D.A.; West, R.M.; Serrano-Ruiz, J.C.; Gartner, C.A.; Dumesic, J.A. Catalytic Conversion of Biomass to Monofunctional Hydrocarbons and Targeted Liquid-Fuel Classes. Science 2008, 322, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.-H.; Hwang, K.-R.; Choi, H.-Y.; Lee, J.-S. Catalytic deoxygenation of waste soybean oil over hybrid catalyst for production of bio-jet fuel: In situ supply of hydrogen by aqueous-phase reforming (APR) of glycerol. Res. Chem. Intermed. 2018, 44, 3713–3722. [Google Scholar] [CrossRef]
- Sutton, A.D.; Waldie, F.D.; Wu, R.; Schlaf, M.; Silks, L.A.; Gordon, J.C. The hydrodeoxygenation of bioderived furans into alkanes. Nat. Chem. 2013, 5, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Zoppi, G.; Tito, E.; Bianco, I.; Pipitone, G.; Pirone, R.; Bensaid, S. Life cycle assessment of the biofuel production from lignocellulosic biomass in a hydrothermal liquefaction—aqueous phase reforming integrated biorefinery. Renew. Energy 2023, 206, 375–385. [Google Scholar] [CrossRef]
- Wang, T.; Tan, J.; Qiu, S.; Zhang, Q.; Long, J.; Chen, L.; Ma, L.; Li, K.; Liu, Q.; Zhang, Q. Liquid Fuel Production by Aqueous Phase Catalytic Transformation of Biomass for Aviation. Energy Procedia 2014, 61, 432–435. [Google Scholar] [CrossRef]
- Weng, Y.; Qiu, S.; Ma, L.; Liu, Q.; Ding, M.; Zhang, Q.; Zhang, Q.; Wang, T. Jet-Fuel Range Hydrocarbons from Biomass-Derived Sorbitol over Ni-HZSM-5/SBA-15 Catalyst. Catalysts 2015, 5, 2147–2160. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Y.; Li, Y.; Zhang, H.; Li, H.; Yang, S. Advances in Diels–Alder/aromatization of biomass furan derivatives towards renewable aromatic. Catal. Sci. Technol. 2022, 12, 1902–1921. [Google Scholar] [CrossRef]
- Malinowski, A.; Wardzińska, D. Catalytic Conversion of Furfural towards Fuel Biocomponents. 2012. Available online: https://www.researchgate.net/publication/281835425_Catalytic_conversion_of_furfural_towards_fuel_biocomponents/stats (accessed on 28 April 2020).
- Huber, G.W.; Chheda, J.N.; Barrett, C.J.; Dumesic, J.A. Production of Liquid Alkanes by Aqueous-Phase Processing of Biomass-Derived Carbohydrates. Science 2005, 308, 1446–1450. [Google Scholar] [CrossRef]
- Sayed, M.S.A.; Warlin, N.; Hulteberg, C.; Munslow, I.; Lundmark, S.; Pajalic, O.; Tunå, P.; Zhang, B.; Pyo, S.-H.; Hatti-Kaul, R. 5-Hydroxymethylfurfural from fructose: An efficient continuous process in a water-dimethyl carbonate biphasic system with high yield product recovery. Green Chem. 2020, 22, 5402–5413. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, Z.K.; Wang, Q. Catalytic Conversion of Inulin and Fructose into 5-Hydroxymethylfurfural by Lignosulfonic Acid in Ionic Liquids. Chemsuschem 2012, 5, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Ståhlberg, T.; Rodriguez-Rodriguez, S.; Fristrup, P.; Riisager, A. Metal-Free Dehydration of Glucose to 5-(Hydroxymethyl)furfural in Ionic Liquids with Boric Acid as a Promoter. Chem. A Eur. J. 2011, 17, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.W.; Sutton, A.D.; Robichaud, D.J. Chapter 8—Pyrolysis of biomass for Aviation Fuel. In Biofuels for Aviation; Academic Press: Cambridge, MA, USA, 2016; pp. 191–215. [Google Scholar]
- Park, L.K.-E.; Liu, J.; Yiacoumi, S.; Borole, A.P.; Tsouris, C. Contribution of acidic components to the total acid number (TAN) of bio-oil. Fuel 2017, 200, 171–181. [Google Scholar] [CrossRef]
- Banks, S.; Bridgwater, A. Catalytic Fast Pyrolysis for Improved Liquid Quality; Woodhead Publishing: Sawston, UK, 2016; pp. 391–429. Handbook of Biofuels Production. [Google Scholar] [CrossRef]
- Ramirez, J.A.; Brown, R.J.; Rainey, T.J. A Review of Hydrothermal Liquefaction Bio-Crude Properties and Prospects for Upgrading to Transportation Fuels. Energies 2015, 8, 6765–6794. [Google Scholar] [CrossRef]
- Yildiz, G.; Lathouwers, T.; Toraman, H.E.; van Geem, K.M.; Marin, G.B.; Ronsse, F.; van Duren, R.; Kersten, S.R.A.; Prins, W. Catalytic Fast Pyrolysis of Pine Wood: Effect of Successive Catalyst Regeneration. Energy Fuels 2014, 28, 4560–4572. [Google Scholar] [CrossRef]
- Kumar, R.; Strezov, V.; Kan, T.; Weldekidan, H.; He, J.; Jahan, S. Investigating the Effect of Mono- and Bimetallic/Zeolite Catalysts on Hydrocarbon Production during Bio-oil Upgrading from Ex Situ Pyrolysis of Biomass. Energy Fuels 2019, 34, 389–400. [Google Scholar] [CrossRef]
- Westerhof, R.J.M.; Brilman, D.W.F.; Garcia-Perez, M.; Wang, Z.; Oudenhoven, S.R.G.; Kersten, S.R.A. Stepwise Fast Pyrolysis of Pine Wood. Energy Fuels 2012, 26, 7263–7273. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A.V. Overview of Applications of Biomass Fast Pyrolysis Oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Mythili, R.; Venkatachalam, P.; Subramanian, P.; Uma, D. Characterization of bioresidues for biooil production through pyrolysis. Bioresour. Technol. 2013, 138, 71–78. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Grunwaldt, J.-D.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A Gen. 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Beims, R.F.; Bertoli, S.L.; Botton, V.; Ender, L.; Simionatto, E.L.; Meier, H.F.; Wiggers, V.R. Co-Processing of Thermal Cracking Bio-Oil at Petroleum Refineries. Braz. J. Pet. Gas 2017, 11, 99–113. [Google Scholar] [CrossRef][Green Version]
- Shah, Z.; Veses, R.C.; Vaghetti, J.C.P.; Amorim, V.D.A.; da Silva, R. Preparation of jet engine range fuel from biomass pyrolysis oil through hydrogenation and its comparison with aviation kerosene. Int. J. Green Energy 2019, 16, 350–360. [Google Scholar] [CrossRef]
- Shi, W.; Gao, Y.; Song, S.; Zhao, Y. One-Pot Conversion of Bio-oil to Diesel- and Jet-Fuel-Range Hydrocarbons in Supercritical Cyclohexane. Ind. Eng. Chem. Res. 2014, 53, 11557–11565. [Google Scholar] [CrossRef]
- Wang, J.; Bi, P.; Zhang, Y.; Xue, H.; Jiang, P.; Wu, X.; Liu, J.; Wang, T.; Li, Q. Preparation of jet fuel range hydrocarbons by catalytic transformation of bio-oil derived from fast pyrolysis of straw stalk. Energy 2015, 86, 488–499. [Google Scholar] [CrossRef]
- Duan, D.; Zhang, Y.; Lei, H.; Qian, M.; Villota, E.; Wang, C.; Wang, Y.; Ruan, R. A novel production of phase-divided jet-fuel-range hydrocarbons and phenol-enriched chemicals from catalytic co-pyrolysis of lignocellulosic biomass with low-density polyethylene over carbon catalysts. Sustain. Energy Fuels 2020, 4, 3687–3700. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Alam, T.; Krishna, B.B.; Bhaskar, T.; Perkins, G. A review on the production of renewable aviation fuels from the gasification of biomass and residual wastes. Bioresour. Technol. 2020, 312, 123596. [Google Scholar] [CrossRef]
- Boger, T.; Heibel, A.K.; Sorensen, C.M. Monolithic Catalysts for the Chemical Industry. Ind. Eng. Chem. Res. 2004, 43, 4602–4611. [Google Scholar] [CrossRef]
- Dry, M.E. Fischer–Tropsch reactions and the environment. Appl. Catal. A Gen. 1999, 189, 185–190. [Google Scholar] [CrossRef]
- Rushdi, A.I.; Simoneit, B.R.T. Lipid Formation by Aqueous Fischer-Tropsch-Type Synthesis over a Temperature Range of 100 to 400 °C. Space Life Sci. 2001, 31, 103–118. [Google Scholar] [CrossRef]
- Bolder, F.H.A. Dehydrogenation of Alcohol Mixtures to Esters and Ketones. Ind. Eng. Chem. Res. 2008, 47, 7496–7500. [Google Scholar] [CrossRef]
- Wang, Y.; Kazumi, S.; Gao, W.; Gao, X.; Li, H.; Guo, X.; Yoneyama, Y.; Yang, G.; Tsubaki, N. Direct conversion of CO2 to aromatics with high yield via a modified Fischer-Tropsch synthesis pathway. Appl. Catal. B Environ. 2020, 269, 118792. [Google Scholar] [CrossRef]
- Swanson, R.M.; Platon, A.; Satrio, J.A.; Brown, R.C. Techno-economic analysis of biomass-to-liquids production based on gasification. Fuel 2010, 89, S11–S19. [Google Scholar] [CrossRef]
- Goh, B.H.H.; Chong, C.T.; Ong, H.C.; Seljak, T.; Katrašnik, T.; Józsa, V.; Ng, J.-H.; Tian, B.; Karmarkar, S.; Ashokkumar, V. Recent advancements in catalytic conversion pathways for synthetic jet fuel produced from bioresources. Energy Convers. Manag. 2022, 251, 114974. [Google Scholar] [CrossRef]
- Griffin, D.W.; Schultz, M.A. Fuel and chemical products from biomass syngas: A comparison of gas fermentation to thermochemical conversion routes. Environ. Prog. Sustain. Energy 2012, 31, 219–224. [Google Scholar] [CrossRef]
- Weber, D. Scholarcommons.usf.edu. 2018. Available online: https://scholarcommons.usf.edu/cgi/viewcontent.cgi?article=8440&context=etd (accessed on 17 August 2023).
- Dagle, V.L.; Smith, C.; Flake, M.; Albrecht, K.O.; Gray, M.J.; Ramasamy, K.K.; Dagle, R.A. Integrated process for the catalytic conversion of biomass-derived syngas into transportation fuels. Green Chem. 2016, 18, 1880–1891. [Google Scholar] [CrossRef]
- IEA. CO2 Emissions in 2022—Analysis, IEA. 2022. Available online: https://www.iea.org/reports/co2-emissions-in-2022 (accessed on 6 August 2023).
- Schmidt, P.; Batteiger, V.; Roth, A.; Weindorf, W.; Raksha, T. Power-to-Liquids as Renewable Fuel Option for Aviation: A Review. Chem. Ing. Tech. 2018, 90, 127–140. [Google Scholar] [CrossRef]
- Yang, F.; Meerman, J.; Faaij, A. Carbon capture and biomass in industry: A techno-economic analysis and comparison of negative emission options. Renew. Sustain. Energy Rev. 2021, 144, 111028. [Google Scholar] [CrossRef]
- Haertel, C.J.J.; McNutt, M.; Ozkan, M.; Aradóttir, E.S.; Valsaraj, K.T.; Sanberg, P.R.; Talati, S.; Wilcox, J. The promise of scalable direct air capture. Chem. 2021, 7, 2831–2834. [Google Scholar] [CrossRef]
- Ozkan, M. Direct air capture of CO2: A response to meet the global climate targets. MRS Energy Sustain. 2021, 8, 51–56. [Google Scholar] [CrossRef]
- Newsroom Engie. Engie and Infinium Unveil a Partnership to Develop an Industrial Hub on an EUR. 2022. Available online: https://en.newsroom.engie.com/news/engie-and-infinium-unveil-a-partnership-to-develop-an-industrial-hub-on-an-european-scale-to-produce-synthetic-fuel-in-dunkirk-a098-314df.html (accessed on 6 August 2023).
- Porsche Newsroom. Construction Begins on World’s First Integrated Commercial Plant for Producing eFuels. 2021. Available online: https://newsroom.porsche.com/en/2021/company/porsche-construction-begins-commercial-plant-production-co2-neutral-fuel-chile-25683.html (accessed on 6 August 2023).
- Reals, K. 2023 DLR to Set up ‘World’s Largest’ Power-to-Liquid Jet Fuel Research Facility. Available online: https://www.flightglobal.com/aerospace/dlr-to-set-up-worlds-largest-power-to-liquid-jet-fuel-research-facility/152615.article (accessed on 6 August 2023).
- Store, J. Fit for 55: Council Adopts Key Pieces of Legislation Delivering on 2030 Climate Targets. 2023. Available online: https://www.consilium.europa.eu/en/press/press-releases/2023/04/25/fit-for-55-council-adopts-key-pieces-of-legislation-delivering-on-2030-climate-targets/ (accessed on 6 August 2023).
- Elhaj, H.F.A.; Lang, A. The Worldwide Production of bio-Jet Fuels—The Current Developments Regarding Technologies and Feedstocks, and Innovative New R&D Developments. 2014. Available online: https://www.researchgate.net/publication/273765924_The_worldwide_production_of_bio-jet_fuels_-_The_current_developments_regarding_technologies_and_feedstocks_and_innovative_new_RD_developments (accessed on 17 August 2023). [CrossRef]
- Yang, J.; Xin, Z.; He, Q.; Corscadden, K.; Niu, H. An overview on performance characteristics of bio-jet fuels. Fuel 2019, 237, 916–936. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H.; Zhu, L.; Wei, Y.; Liu, Y.; Yadavalli, G.; Yan, D.; Wu, J.; Chen, S. Production of renewable jet fuel range alkanes and aromatics via integrated catalytic processes of intact biomass. Fuel 2015, 160, 375–385. [Google Scholar] [CrossRef]
- Yeboah, I.; Feng, X.; Rout, K.R.; Chen, D. Versatile One-Pot Tandem Conversion of Biomass-Derived Light Oxygenates into High-Yield Jet Fuel Range Aromatics. Ind. Eng. Chem. Res. 2021, 60, 15095–15105. [Google Scholar] [CrossRef]
- Green, S.K.; Patet, R.E.; Nikbin, N.; Williams, C.L.; Chang, C.-C.; Yu, J.; Gorte, R.J.; Caratzoulas, S.; Fan, W.; Vlachos, D.G.; et al. Diels–Alder cycloaddition of 2-methylfuran and ethylene for renewable toluene. Appl. Catal. B Environ. 2015, 180, 487–496. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, P.; Jin, F.; Liu, J.; Zhang, Y.; Zhu, L.; Xia, T.; Shao, K.; Wang, T.; Li, Q. Production of jet fuel range biofuels by catalytic transformation of triglycerides based oils. Fuel 2017, 188, 205–211. [Google Scholar] [CrossRef]
- Ganda, E.T.; Isa, Y.M. Production of bio-aromatics from ethanol-waste cooking oil mixtures over ZSM-5 catalyst material. Catal. Sustain. Energy 2016, 5, 19–27. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Zhang, J.; Zhang, Y.; Li, X.; Xia, H.; Wang, F. Catalytic Pyrolysis of Nonedible Oils for the Production of Renewable Aromatics Using Metal-Modified HZSM-5 Catalysts. ACS Omega 2022, 7, 18953–18968. [Google Scholar] [CrossRef]
- ICAO. CORSIA Supporting Document: CORSIA Eligible Fuels-Life Cycle Assessment Methodology. 2019. Available online: https://www.icao.int/environmental-protection/CORSIA/Documents/CORSIA_Eligible_Fuels/CORSIA_Supporting_Document_CORSIA%20Eligible%20Fuels_LCA_Methodology_V5.pdf (accessed on 17 August 2023).
- ICAO. CORSIA Default Life Cycle Emissions Values for CORSIA Eligible Fuels. 2021. Available online: https://www.icao.int/environmental-protection/CORSIA/Documents/ICAO%20document%2006%20-%20Default%20Life%20Cycle%20Emissions%20-%20March%202021.pdf (accessed on 17 August 2023).
- Prussi, M.; Lee, U.; Wang, M.; Malina, R.; Valin, H.; Taheripour, F.; Velarde, C.; Staples, M.D.; Lonza, L.; Hileman, J.I. CORSIA: The first internationally adopted approach to calculate life-cycle GHG emissions for aviation fuels. Renew. Sustain. Energy Rev. 2021, 150, 111398. [Google Scholar] [CrossRef]
- Jing, L.; El-Houjeiri, H.M.; Monfort, J.-C.; Littlefield, J.; Al-Qahtani, A.; Dixit, Y.; Speth, R.L.; Brandt, A.R.; Masnadi, M.S.; MacLean, H.L.; et al. Understanding variability in petroleum jet fuel life cycle greenhouse gas emissions to inform aviation decarbonization. Nat. Commun. 2022, 13, 7853. [Google Scholar] [CrossRef]
- Oehmichen, K.; Majer, S.; Müller-Langer, F.; Thrän, D. Comprehensive LCA of Biobased Sustainable Aviation Fuels and JET A-1 Multiblend. Appl. Sci. 2022, 12, 3372. [Google Scholar] [CrossRef]
- Yoo, E.; Lee, U.; Wang, M. Life-Cycle Greenhouse Gas Emissions of Sustainable Aviation Fuel through a Net-Zero Carbon Biofuel Plant Design. ACS Sustain. Chem. Eng. 2022, 10, 8725–8732. [Google Scholar] [CrossRef]
- Zemanek, D.; Champagne, P.; Mabee, W. Review of life-cycle greenhouse-gas emissions assessments of hydroprocessed renewable fuel (HEFA) from oilseeds. Biofuels Bioprod. Biorefining 2020, 14, 935–949. [Google Scholar] [CrossRef]

| Feedstock | Process Route | Operating Conditions | Catalysts | Yields | Reference |
|---|---|---|---|---|---|
| Algal oil | Decarboxylation | T = 360 °C | Pt/C | 53.63% heptadecane | [73] |
| Solvent = water Residence time = 45 min | |||||
| Castor oil | Hydrodeoxygenation | T = 300 °C– 360 °C | NiAg/SAPO-11 | 87.12% C8–C15 hydrocarbons | [74] |
| P = 3 MPa (H2) WHSV = 2 h−1 | |||||
| FAME | Deoxygenation | T = 300 °C | Ni/HZSM-5 | 26.90% C8–C16 alkane yield | [75] |
| P = 0.8 MPa (H2) | |||||
| LHSV = 4 h−1 | |||||
| H2/oil molar ratio = 15 | |||||
| Jatropha oil | Deoxygenation without H2 | T = 200 °C | WOx/Pt/TiO2 | 75% C8–C16 hydrocarbons | [76] |
| P = 4 MPa (N2) | |||||
| LHSV = 1.33 h−1 | |||||
| Macauba oil | Decarboxylation | T = 300 °C | Pd/C | 33% C9–C16 hydrocarbons | [77] |
| P = 1 MPa (H2) Residence time = 5 h | |||||
| Microalgae biodiesel | Deoxygenation | T = 275 °C | Ni/meso-Y zeolite | 48.6% alkanes | [78] |
| Injection rate= 0.02 mL/min | 2.7% aromatics | ||||
| 0.18% alkenes | |||||
| Oleic acid | Deoxygenation without H2 | T = 300 °C | CoMo | 20.1% C9–C16 hydrocarbons | [79] |
| P = 1 atm. (N2) Residence time = 3 h | |||||
| Palm oil | Hydrodeoxygenation | T = 300 °C | Pd/C | 90% C9–C15 hydrocarbons | [80] |
| P = 1 MPa (H2) Residence time = 5 h | |||||
| Hydrodeoxygenation | T = 330 °C | Ni-MoS2/ɣ-Al2O3 | 60% C10–C12 hydrocarbons | [81] | |
| P = 5 MPa (H2) LHSV = 1 h−1 | |||||
| H2/oil = 1000 Ncm3/cm3 | |||||
| Soybean oil | Decarbonylation | T = 390 °C | Ni-Mo/HY | 30% aromatics | [82] |
| P = 1 MPa (H2) Residence time = 8 h | 30% alkanes | ||||
| Hydrodeoxygenation | T = 370–385 °C P = 3 MPa (H2) LHSV = 1 h−1 | Pt/Al2O3/SAPO-11 | 15% aromatics | [83] | |
| H2/oil = 800 NL/L | |||||
| Decarboxylation | T = 350 °C | NbOPO4 | 62% C9–C16 hydrocarbons | [46] | |
| P = 1 MPa (H2) Residence time = 5 h | |||||
| Deoxygenation | T = 350 °C | Ni-Al2O3 | 80% C8–C17 hydrocarbons | [84] | |
| P = 0.7 MPa (N2) Residence time = 4 h | |||||
| Deoxygenation | T = 400 °C | CoMo-Al2O3 | 13.5% biojet fuel range hydrocarbons | [85] | |
| P = 9.2 MPa (H2) Residence time = 1 h | |||||
| Waste cooking oil | Decarbonylation | T = 400 °C | Ni/Meso-Y | 37.5% alkanes | [74] |
| P = 3 MPa (H2) Residence time = 8 h | 10% aromatics | ||||
| Hydrodeoxygenation | T = 300 °C | Ni-Mo/ɣ-Al2O3 | 80% alkanes | [86] | |
| P = 4 MPa (H2) Residence time = 7.5 h | 3% alkenes | ||||
| 6% aromatics | |||||
| Waste cooking oil + waste lubricating oil + vacuum gas oil | Deoxygenation | T = 380 °C | Ni-Mo/Al2O3 | 65% kerosene range hydrocarbons | [87] |
| P = 7 MPa (H2) LHSV = 1.5 h−1 | |||||
| H2/oil = 400/400 Nm3/m3 | Ni-W/SiO2/Al2O3 |
| Pyrolysis Type | Residence Time | Heating Rate | Temperature (°C) | Major Product |
|---|---|---|---|---|
| Carbonisation | h–days | very low | 400 | char |
| Conventional | 10 s–10 min | low–moderate | <600 | gas, char, liquid |
| Flash (liquid) | <1 s | high | <600 | liquid |
| Flash (gas) | <1 s | high | >700 | gas, char, liquid |
| Ultra | <0.5 s | very high | 1000 | gas, chemicals |
| Vacuum | 2–30 s | moderate | 400 | liquid |
| HTL | ≤60 min | moderate | 300–350 | liquid |
| Catalyst | Reactor Type | Time (h) | P (bar) | T (°C) | Degree of Deoxygenation (%) | Upgraded Oil Yield (%) |
|---|---|---|---|---|---|---|
| Hydrodeoxygenation (HDO) | ||||||
| CoMoS2/Al2O3 | Batch | 4 | 200 | 350 | 81 | 26 |
| CoMoS2/Al2O3 | Continuous | 4 | 300 | 370 | 100 | 33 |
| NiMoS2/Al2O3 | Batch | 4 | 200 | 350 | 74 | 28 |
| NiMoS2/Al2O3 | Continuous | 0.5 | 85 | 400 | 28 | 84 |
| Pd/C | Batch | 4 | 200 | 350 | 85 | 65 |
| Pd/C | Continuous | 4 | 140 | 85 | 64 | 48 |
| Pt/Al2O3/SiO2 | Continuous | 0.5 | 85 | 400 | 45 | 81 |
| Ru/Al2O3 | Batch | 4 | 200 | 350 | 78 | 36 |
| Ru/C | Continuous | 0.2 | 230 | 350–400 | 73 | 38 |
| Ru/C | Batch | 4 | 200 | 350 | 86 | 53 |
| Ru/TiO2 | Batch | 4 | 200 | 350 | 77 | 67 |
| Zeolite cracking | ||||||
| HZSM-5 | Continuous | 0.32 | 1 | 380 | 50 | 24 |
| HZSM-5 | Continuous | 0.91 | 1 | 500 | 53 | 12 |
| Properties | JET-A | HEFA | ATJ | FT | SIP |
|---|---|---|---|---|---|
| Acid n°. (mgKOH/g) | 0.10 max. | 0.02 max. | 0.02 max. | 0.02 max. | 0.02 max. |
| Flash point (°C) | 38 min. | 38 min. | 48 min. | 38 min. | 100 min. |
| Freezing point (°C) | −47 max. | −40 max. | −80 max. | −40 max. | −60 max. |
| Density @ 15 °C (kg/m3) | 775–840 | 730–770 | 763 | 730–770 | 765–780 |
| Net heat of combustion (MJ/Kg) | 42.8 min. | 42.8 min. | 43.2 min. | 42.8 min. | 43.5 min. |
| Additive antioxidants (mg/L) | 24.0 max. | 17 min. | 17.2 min. | 17 min. | 17 min. |
| 24 max. | 24 max. | 24 max. | 24 max. | ||
| Aromatics (%) | 25 max. | 0.5 max. | - | 0.5 max. | 0.5 max. |
| Sulphur content (ppm) | 0.30 max. | 15 max. | 10 max. | 15 max. | 2 max. |
| Core LCA Values (gCO2e/MJ) | ILUC LCA Values (gCO2e/MJ) | ||||
|---|---|---|---|---|---|
| Biojet Technology | Lowest | Highest | GHG Emissions Savings Based on Core LCA Values) | Lowest | Highest |
| FT-SPK | 7.7 | 12.2 | 86.3–91.3% | −12.6 | 8.6 |
| HEFA | 13.9 | 60 | 32.5–84.4% | 13.4 | 26 |
| SIP | 32.4 | 32.8 | 63.1–63.6% | 11.1 | 11.2 |
| FT-SPK/A * | 5.2 | 86.2 | 3.04–94.2% | N/A | N/A |
| ATJ | 23.8 | 65.7 | 26.1–73.2% | −23.6 | 34.9 |
| Co-processing bio-oils with petroleum | N/A | N/A | N/A | N/A | N/A |
| CHJ | N/A | N/A | N/A | N/A | N/A |
| HC-HEFA | 16.7 | 40.7 | 54.2–81.2% | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, M.A.; Alves, C.T.; Onwudili, J.A. A Review of Current and Emerging Production Technologies for Biomass-Derived Sustainable Aviation Fuels. Energies 2023, 16, 6100. https://doi.org/10.3390/en16166100
Peters MA, Alves CT, Onwudili JA. A Review of Current and Emerging Production Technologies for Biomass-Derived Sustainable Aviation Fuels. Energies. 2023; 16(16):6100. https://doi.org/10.3390/en16166100
Chicago/Turabian StylePeters, Morenike Ajike, Carine Tondo Alves, and Jude Azubuike Onwudili. 2023. "A Review of Current and Emerging Production Technologies for Biomass-Derived Sustainable Aviation Fuels" Energies 16, no. 16: 6100. https://doi.org/10.3390/en16166100
APA StylePeters, M. A., Alves, C. T., & Onwudili, J. A. (2023). A Review of Current and Emerging Production Technologies for Biomass-Derived Sustainable Aviation Fuels. Energies, 16(16), 6100. https://doi.org/10.3390/en16166100