Platinum–Nickel Electrocatalysts for a Proton-Exchange Membrane Fuel Cell Cathode: Their Synthesis, Acid Treatment, Microstructure and Electrochemical Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the PtNi/C Catalyst with a Core–Shell NP Structure
2.3. Preparation of the De-Alloyed Catalysts
2.4. Methods to Study the Catalysts’ Composition and Structure
2.5. Electrochemical Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NPs | Nanoparticles |
| FC | Fuel cell |
| PEMFCs | Proton-exchange membrane fuel cells |
| ORR | Oxygen reduction reaction |
| TEA | Triethylamine, N,N-Diethylethanamine |
| OLEA | Oleic acid, (9Z)-Octadec-9-enoic acid |
| TOA | Trioctylamine, N,N-Di(octyl)octan-1-amine |
| DMF | Dimethylformamide, N,N-Dimethylformamide |
| XRD | X-ray powder diffraction |
| TEM | Transmission electron microscopy |
| EDX | Energy-dispersive X-ray spectrometry |
| ECSA | Electrochemically active surface area |
| CV | Cyclic voltammetry |
| LSV | Linear sweep voltammetry |
| STEM | Scanning transmission electron microscopy |
| RDE | Rotating disk electrode |
| RHE | Reversible hydrogen electrode |
| MEA | Membrane electrode assembly |
| FCC | Face-centered cubic |
References
- Olabi, A.G.; Sayed, E.T. Developments in Hydrogen Fuel Cells. Energies 2023, 16, 2431. [Google Scholar] [CrossRef]
- Pourrahmani, H.; Yavarinasab, A.; Siavashi, M.; Matian, M.; Van herle, J. Progress in the Proton Exchange Membrane Fuel Cells (PEMFCs) Water/Thermal Management: From Theory to the Current Challenges and Real-Time Fault Diagnosis Methods. Energy Rev. 2022, 1, 100002. [Google Scholar] [CrossRef]
- Pedicini, R.; Romagnoli, M.; Santangelo, P.E. A Critical Review of Polymer Electrolyte Membrane Fuel Cell Systems for Automotive Applications: Components, Materials, and Comparative Assessment. Energies 2023, 16, 3111. [Google Scholar] [CrossRef]
- Kodama, K.; Nagai, T.; Kuwaki, A.; Jinnouchi, R.; Morimoto, Y. Challenges in Applying Highly Active Pt-Based Nanostructured Catalysts for Oxygen Reduction Reactions to Fuel Cell Vehicles. Nat. Nanotechnol. 2021, 16, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Chen, M.; Zhao, Z.; Zhang, Z.; Ye, S.; Xu, S.; Wang, H.; Li, H. Bridging the Gap between Highly Active Oxygen Reduction Reaction Catalysts and Effective Catalyst Layers for Proton Exchange Membrane Fuel Cells. Nat. Energy 2021, 6, 475–486. [Google Scholar] [CrossRef]
- Si, F.; Zhang, Y.; Yan, L.; Zhu, J.; Xiao, M.; Liu, C.; Xing, W.; Zhang, J. Electrochemical Oxygen Reduction Reaction. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts; Elsevier: Amsterdam, The Netherlands, 2014; pp. 133–170. [Google Scholar] [CrossRef]
- Gómez-Marín, A.M.; Ticianelli, E.A. A Reviewed Vision of the Oxygen Reduction Reaction Mechanism on Pt-Based Catalysts. Curr. Opin. Electrochem. 2018, 9, 129–136. [Google Scholar] [CrossRef]
- Escudero-Escribano, M.; Jensen, K.D.; Jensen, A.W. Recent Advances in Bimetallic Electrocatalysts for Oxygen Reduction: Design Principles, Structure-Function Relations and Active Phase Elucidation. Curr. Opin. Electrochem. 2018, 8, 135–146. [Google Scholar] [CrossRef]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-Strain Control of the Activity in Dealloyed Core–Shell Fuel Cell Catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Chattot, R.; Martens, I.; Scohy, M.; Herranz, J.; Drnec, J.; Maillard, F.; Dubau, L. Disclosing Pt-Bimetallic Alloy Nanoparticle Surface Lattice Distortion with Electrochemical Probes. ACS Energy Lett. 2020, 5, 162–169. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; ALOthman, Z.A.; Mola, G.T. Novel Development of Nanoparticles to Bimetallic Nanoparticles and Their Composites: A Review. J. King Saud Univ. Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Martínez-Hincapié, R.; Čolić, V. Electrocatalysts for the Oxygen Reduction Reaction: From Bimetallic Platinum Alloys to Complex Solid Solutions. ChemEngineering 2022, 6, 19. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in Electrocatalysis on Extended and Nanoscale Pt-Bimetallic Alloy Surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Yang, D.; Lv, H.; Zhang, C. Preparation Optimization and Single Cell Application of PtNi/C Octahedral Catalyst with Enhanced ORR Performance. Electrochim. Acta 2018, 288, 126–133. [Google Scholar] [CrossRef]
- Fan, C.; Wang, G.; Zou, L.; Fang, J.; Zou, Z.; Yang, H. Composition- and Shape-Controlled Synthesis of the PtNi Alloy Nanotubes with Enhanced Activity and Durability toward Oxygen Reduction Reaction. J. Power Sources 2019, 429, 1–8. [Google Scholar] [CrossRef]
- Gong, W.; Jiang, Z.; Huang, L.; Shen, P.K. PtNi Alloy Hyperbranched Nanostructures with Enhanced Catalytic Performance towards Oxygen Reduction Reaction. Int. J. Hydrogen Energy 2018, 43, 18436–18443. [Google Scholar] [CrossRef]
- Nie, Y.; Deng, J.; Chen, S.; Wei, Z. Promoting Stability and Activity of PtNi/C for Oxygen Reduction Reaction via Polyaniline-Confined Space Annealing Strategy. Int. J. Hydrogen Energy 2019, 44, 5921–5928. [Google Scholar] [CrossRef]
- Leteba, G.M.; Wang, Y.C.; Slater, T.J.A.; Cai, R.; Byrne, C.; Race, C.P.; Mitchell, D.R.G.; Levecque, P.B.J.; Young, N.P.; Holmes, S.M.; et al. Oleylamine Aging of PtNi Nanoparticles Giving Enhanced Functionality for the Oxygen Reduction Reaction. Nano Lett. 2021, 21, 3989–3996. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, D.; Niu, Z.; Chen, P.; Zhou, G.; Li, Y.; Wu, Y.E.; Wang, D.S.; Niu, Z.Q.; Li, Y.D.; et al. A Strategy for Designing a Concave Pt–Ni Alloy through Controllable Chemical Etching. Angew. Chem. Int. Ed. 2012, 51, 12524–12528. [Google Scholar] [CrossRef] [PubMed]
- Oezaslan, M.; Hasché, F.; Strasser, P. Pt-Based Core–Shell Catalyst Architectures for Oxygen Fuel Cell Electrodes. J. Phys. Chem. Lett. 2013, 4, 3273–3291. [Google Scholar] [CrossRef]
- Luo, M.; Wei, L.; Wang, F.; Han, K.; Zhu, H. Gram-Level Synthesis of Core–Shell Structured Catalysts for the Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. J. Power Sources 2014, 270, 34–41. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, H.; Liu, S.; Zhang, B.; Zhong, H.; Su, D.S. Facile Synthesis of Supported Pt-Cu Nanoparticles with Surface Enriched Pt as Highly Active Cathode Catalyst for Proton Exchange Membrane Fuel Cells. Int. J. Hydrogen Energy 2012, 37, 17978–17983. [Google Scholar] [CrossRef]
- Gamler, J.T.L.; Ashberry, H.M.; Skrabalak, S.E.; Koczkur, K.M. Random Alloyed versus Intermetallic Nanoparticles: A Comparison of Electrocatalytic Performance. Adv. Mater. 2018, 30, 1801563. [Google Scholar] [CrossRef]
- Jayasayee, K.; Van Veen, J.A.R.; Manivasagam, T.G.; Celebi, S.; Hensen, E.J.M.; de Bruijn, F.A. Oxygen Reduction Reaction (ORR) Activity and Durability of Carbon Supported PtM (Co, Ni, Cu) Alloys: Influence of Particle Size and Non-Noble Metals. Appl. Catal. B 2012, 111–112, 515–526. [Google Scholar] [CrossRef]
- Zhao, X.; Sasaki, K. Advanced Pt-Based Core-Shell Electrocatalysts for Fuel Cell Cathodes. Acc. Chem. Res. 2022, 55, 1226–1236. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, Z.; Yang, F.; Liu, Y.; Chen, S. Ni-Pt Core-Shell Nanoparticles as Oxygen Reduction Electrocatalysts: Effect of Pt Shell Coverage. J. Phys. Chem. C 2011, 115, 24073–24079. [Google Scholar] [CrossRef]
- Alekseenko, A.A.; Pavlets, A.S.; Mikheykin, A.S.; Belenov, S.V.; Guterman, E.V. The Integrated Approach to Studying the Microstructure of De-Alloyed PtCu/C Electrocatalysts for PEMFCs. Appl. Surf. Sci. 2023, 631, 157539. [Google Scholar] [CrossRef]
- Falina, I.; Pavlets, A.; Alekseenko, A.; Titskaya, E.; Kononenko, N. Influence of PtCu/C Catalysts Composition on Electrochemical Characteristics of Polymer Electrolyte Fuel Cell and Properties of Proton Exchange Membrane. Catalysts 2021, 11, 1063. [Google Scholar] [CrossRef]
- Jung, J.Y.; Kim, D.G.; Jang, I.; Kim, N.D.; Yoo, S.J.; Kim, P. Synthesis of Hollow Structured PtNi/Pt Core/Shell and Pt-Only Nanoparticles via Galvanic Displacement and Selective Etching for Efficient Oxygen Reduction Reaction. J. Ind. Eng. Chem. 2022, 111, 300–307. [Google Scholar] [CrossRef]
- Alekseenko, A.A.; Guterman, V.E.; Belenov, S.V.; Menshikov, V.S.; Tabachkova, N.Y.; Safronenko, O.I.; Moguchikh, E.A. Pt/C Electrocatalysts Based on the Nanoparticles with the Gradient Structure. Int. J. Hydrogen Energy 2018, 43, 3676–3687. [Google Scholar] [CrossRef]
- Kim, D.G.; Sohn, Y.; Jang, I.; Yoo, S.J.; Kim, P. Formation Mechanism of Carbon-Supported Hollow PtNi Nanoparticles via One-Step Preparations for Use in the Oxygen Reduction Reaction. Catalysts 2022, 12, 513. [Google Scholar] [CrossRef]
- Mardle, P.; Du, S. Annealing Behaviour of Pt and PtNi Nanowires for Proton Exchange Membrane Fuel Cells. Materials 2018, 11, 1473. [Google Scholar] [CrossRef]
- Alia, S.M.; Ngo, C.; Shulda, S.; Ha, M.A.; Dameron, A.A.; Weker, J.N.; Neyerlin, K.C.; Kocha, S.S.; Pylypenko, S.; Pivovar, B.S. Exceptional Oxygen Reduction Reaction Activity and Durability of Platinum-Nickel Nanowires through Synthesis and Post-Treatment Optimization. ACS Omega 2017, 2, 1408–1418. [Google Scholar] [CrossRef]
- Paperzh, K.O.; Pavlets, A.S.; Alekseenko, A.A.; Pankov, I.V.; Guterman, V.E. The Integrated Study of the Morphology and the Electrochemical Behavior of Pt-Based ORR Electrocatalysts during the Stress Testing. Int. J. Hydrogen Energy 2023, 48, 22401–22414. [Google Scholar] [CrossRef]
- Alekseenko, A.A.; Pavlets, A.S.; Belenov, S.V.; Safronenko, O.I.; Pankov, I.V.; Guterman, V.E. The Electrochemical Activation Mode as a Way to Exceptional ORR Performance of Nanostructured PtCu/C Materials. Appl. Surf. Sci. 2022, 595, 153533. [Google Scholar] [CrossRef]
- Paperzh, K.; Alekseenko, A.; Danilenko, M.; Pankov, I.; Guterman, V.E. Advanced Methods of Controlling the Morphology, Activity, and Durability of Pt/C Electrocatalysts. ACS Appl. Energy Mater. 2022, 5, 9530–9541. [Google Scholar] [CrossRef]
- Shinozaki, K.; Zack, J.W.; Richards, R.M.; Pivovar, B.S.; Kocha, S.S. Oxygen Reduction Reaction Measurements on Platinum Electrocatalysts Utilizing Rotating Disk Electrode Technique: I. Impact of Impurities, Measurement Protocols and Applied Corrections. J. Electrochem. Soc. 2015, 162, F1144. [Google Scholar] [CrossRef]
- Inaba, M.; Quinson, J.; Bucher, J.R.; Arenz, M. On the Preparation and Testing of Fuel Cell Catalysts Using the Thin Film Rotating Disk Electrode Method. J. Vis. Exp. 2018, 2018, e57105. [Google Scholar] [CrossRef]
- Nagai, T.; Jahn, C.; Jia, H. Improved Accelerated Stress Tests for ORR Catalysts Using a Rotating Disk Electrode. J. Electrochem. Soc. 2019, 166, F3111–F3115. [Google Scholar] [CrossRef]
- Nalawade, P.; Mukherjee, P.; Kapoor, S. Triethylamine Induced Synthesis of Silver and Bimetallic (Ag/Au) Nanoparticles in Glycerol and Their Antibacterial Study. J. Nanostruct. Chem. 2014, 4, 113. [Google Scholar] [CrossRef]
- Yang, D.; Gu, J.; Liu, X.; He, H.; Wang, M.; Wang, P.; Zhu, Y.; Fan, Q.; Huang, R. Monodispersed Pt3Ni Nanoparticles as a Highly Efficient Electrocatalyst for PEMFCs. Catalysts 2019, 9, 588. [Google Scholar] [CrossRef]
- Shen, Z.; Yamada, M.; Miyake, M. Preparation of Single-Crystalline Platinum Nanowires with Small Diameters under Mild Conditions. Chem. Commun. 2007, 3, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Pryadchenko, V.V.; Srabionyan, V.V.; Kurzin, A.A.; Bulat, N.V.; Shemet, D.B.; Avakyan, L.A.; Belenov, S.V.; Volochaev, V.A.; Zizak, I.; Guterman, V.E.; et al. Bimetallic PtCu Core-Shell Nanoparticles in PtCu/C Electrocatalysts: Structural and Electrochemical Characterization. Appl. Catal. A Gen. 2016, 525, 226–236. [Google Scholar] [CrossRef]
- Srabionyan, V.V.; Pryadchenko, V.V.; Kurzin, A.A.; Belenov, S.V.; Avakyan, L.A.; Guterman, V.E.; Bugaev, L.A. Atomic Structure of PtCu Nanoparticles in PtCu/C Catalysts from EXAFS Spectroscopy Data. Phys. Solid State 2016, 58, 752–762. [Google Scholar] [CrossRef]
- Lagrow, A.P.; Knudsen, K.R.; Alyami, N.M.; Anjum, D.H.; Bakr, O.M. Effect of Precursor Ligands and Oxidation State in the Synthesis of Bimetallic Nano-Alloys. Chem. Mater. 2015, 27, 4134–4141. [Google Scholar] [CrossRef]
- Pavlets, A.; Alekseenko, A.; Kozhokar, E.; Pankov, I.; Alekseenko, D.; Guterman, V. Efficient Pt-Based Nanostructured Electrocatalysts for Fuel Cells: One-Pot Preparation, Gradient Structure, Effect of Alloying, Electrochemical Performance. Int. J. Hydrogen Energy 2023, 48, 22379–22388. [Google Scholar] [CrossRef]
- Du, K.; Ernst, F.; Pelsozy, M.C.; Barthel, J.; Tillmann, K. Expansion of Interatomic Distances in Platinum Catalyst Nanoparticles. Acta Mater. 2010, 58, 836–845. [Google Scholar] [CrossRef]
- Garsany, Y.; Ge, J.; St-Pierre, J.; Rocheleau, R.; Swider-Lyons, K.E. Analytical Procedure for Accurate Comparison of Rotating Disk Electrode Results for the Oxygen Reduction Activity of Pt/C. J. Electrochem. Soc. 2014, 161, F628–F640. [Google Scholar] [CrossRef]
- Garsany, Y.; Singer, I.L.; Swider-Lyons, K.E. Impact of Film Drying Procedures on RDE Characterization of Pt/VC Electrocatalysts. J. Electroanal. Chem. 2011, 662, 396–406. [Google Scholar] [CrossRef]
- Takahashi, I.; Kocha, S.S. Examination of the Activity and Durability of PEMFC Catalysts in Liquid Electrolytes. J. Power Sources 2010, 195, 6312–6322. [Google Scholar] [CrossRef]
- Shinozaki, K.; Zack, J.W.; Pylypenko, S.; Pivovar, B.S.; Kocha, S.S. Oxygen Reduction Reaction Measurements on Platinum Electrocatalysts Utilizing Rotating Disk Electrode Technique: II. Influence of Ink Formulation, Catalyst Layer Uniformity and Thickness. J. Electrochem. Soc. 2015, 162, F1384. [Google Scholar] [CrossRef]
- Yunker, P.J.; Still, T.; Lohr, M.A.; Yodh, A.G. Suppression of the Coffee-Ring Effect by Shape-Dependent Capillary Interactions. Nature 2011, 476, 308–311. [Google Scholar] [CrossRef] [PubMed]

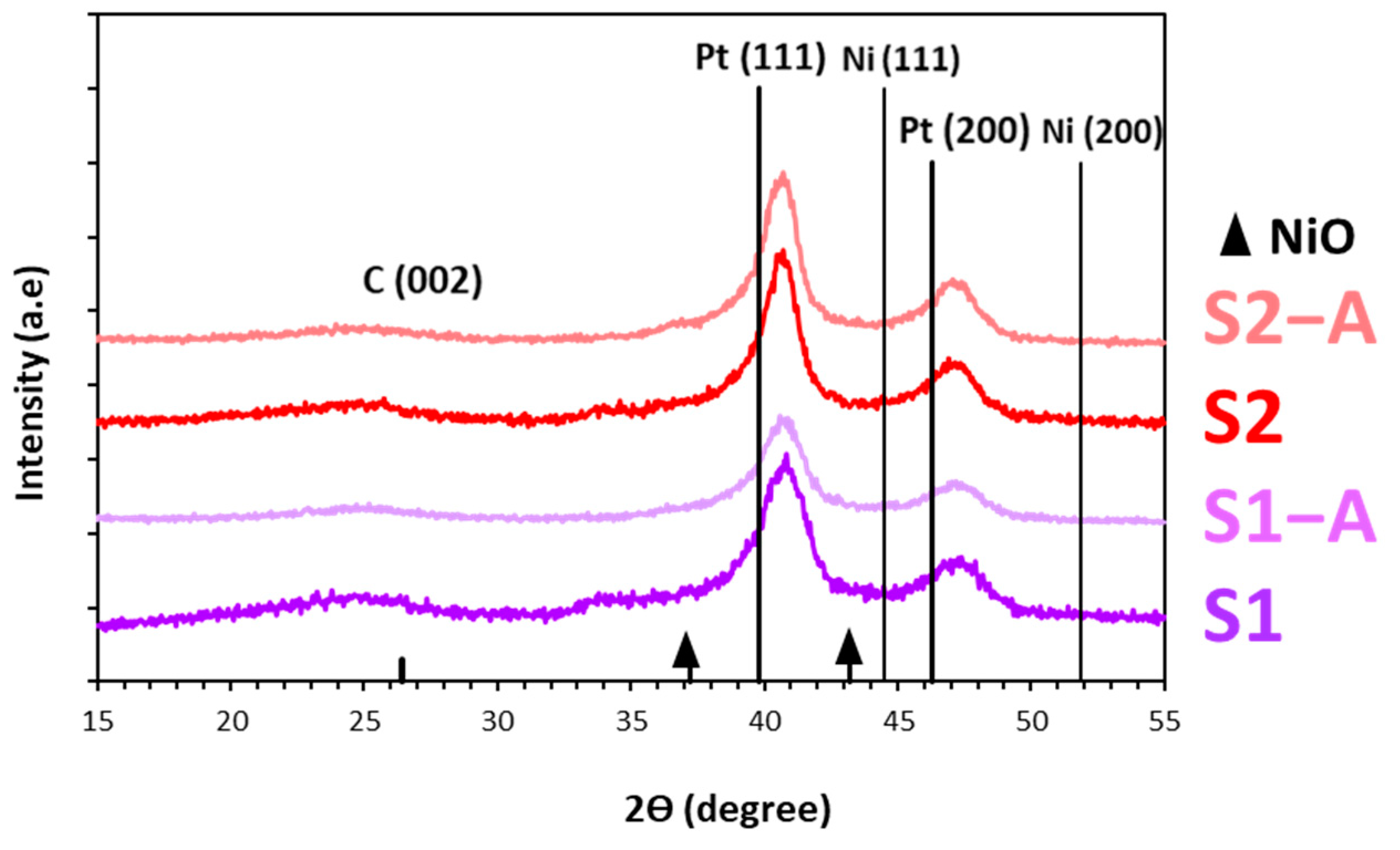




| Sample | ω(Pt+NiO), % Mass. | Average Crystallite Diameter, DAv, nm (XRD) | Average Diameter of NPs, nm (TEM) | Composition According to the Vegard’s Law, PtNix |
|---|---|---|---|---|
| S1 | 41.6 | 4.0 | 4.9 | 0.25 |
| S2 | 38.7 | 5.6 | 6.6 | 0.21 |
| S1–A | 26.0 | 4.6 | 6.4 | 0.24 |
| S2–A | 30.6 | 5.9 | 6.7 | 0.21 |
| Sample | ECSA (Hads), m2 gPt−1 | Ik, mA | Imass, A gPt−1 | E1/2, V |
|---|---|---|---|---|
| S1 | 20 | 0.8 | 157 | 0.90 |
| S1–A | 33 | 1.6 | 320 | 0.91 |
| S2 | 26 | 1.5 | 317 | 0.92 |
| S2–A | 30 | 2.0 | 389 | 0.92 |
| JM20 | 78 | 1.2 | 248 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozhokar, E.; Pavlets, A.; Pankov, I.; Alekseenko, A. Platinum–Nickel Electrocatalysts for a Proton-Exchange Membrane Fuel Cell Cathode: Their Synthesis, Acid Treatment, Microstructure and Electrochemical Behavior. Energies 2023, 16, 6078. https://doi.org/10.3390/en16166078
Kozhokar E, Pavlets A, Pankov I, Alekseenko A. Platinum–Nickel Electrocatalysts for a Proton-Exchange Membrane Fuel Cell Cathode: Their Synthesis, Acid Treatment, Microstructure and Electrochemical Behavior. Energies. 2023; 16(16):6078. https://doi.org/10.3390/en16166078
Chicago/Turabian StyleKozhokar, Ekaterina, Angelina Pavlets, Ilya Pankov, and Anastasia Alekseenko. 2023. "Platinum–Nickel Electrocatalysts for a Proton-Exchange Membrane Fuel Cell Cathode: Their Synthesis, Acid Treatment, Microstructure and Electrochemical Behavior" Energies 16, no. 16: 6078. https://doi.org/10.3390/en16166078
APA StyleKozhokar, E., Pavlets, A., Pankov, I., & Alekseenko, A. (2023). Platinum–Nickel Electrocatalysts for a Proton-Exchange Membrane Fuel Cell Cathode: Their Synthesis, Acid Treatment, Microstructure and Electrochemical Behavior. Energies, 16(16), 6078. https://doi.org/10.3390/en16166078







