Recent Developments in Ceria-Driven Solar Thermochemical Water and Carbon Dioxide Splitting Redox Cycle
Abstract
:1. Introduction
2. Advances in Ceria-Based Solar Thermochemical Fuel Production since 2017
2.1. Year 2017
2.2. Year 2018
2.3. Year 2019
2.4. Year 2020
2.5. Year 2021
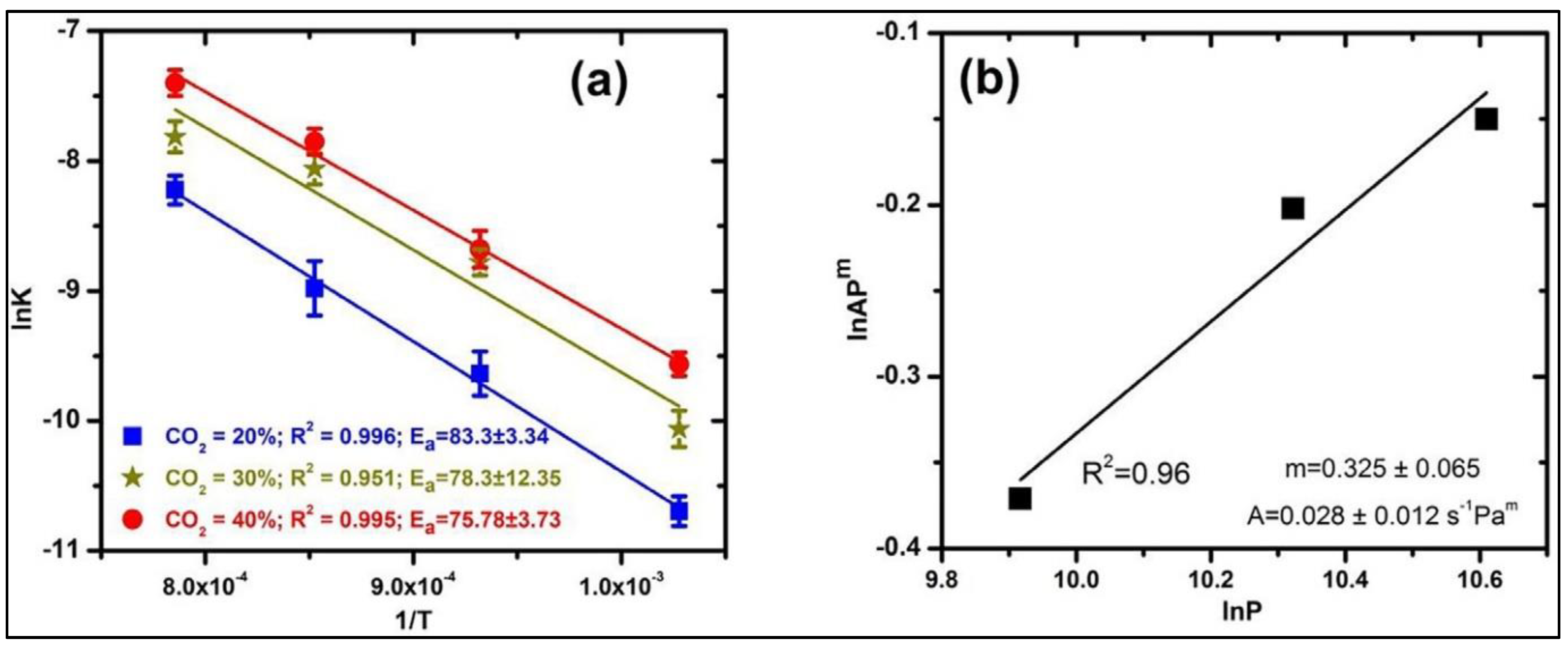
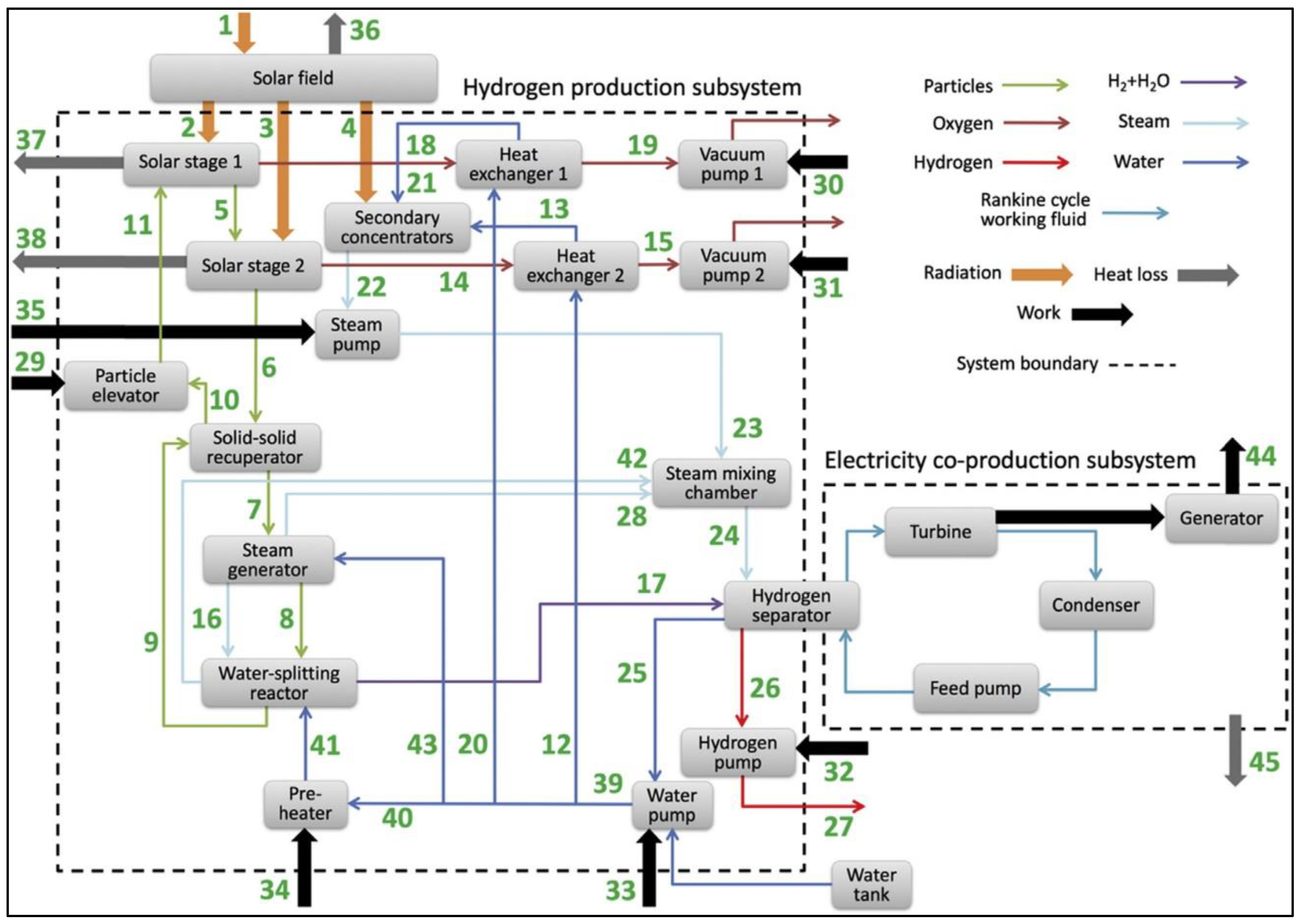
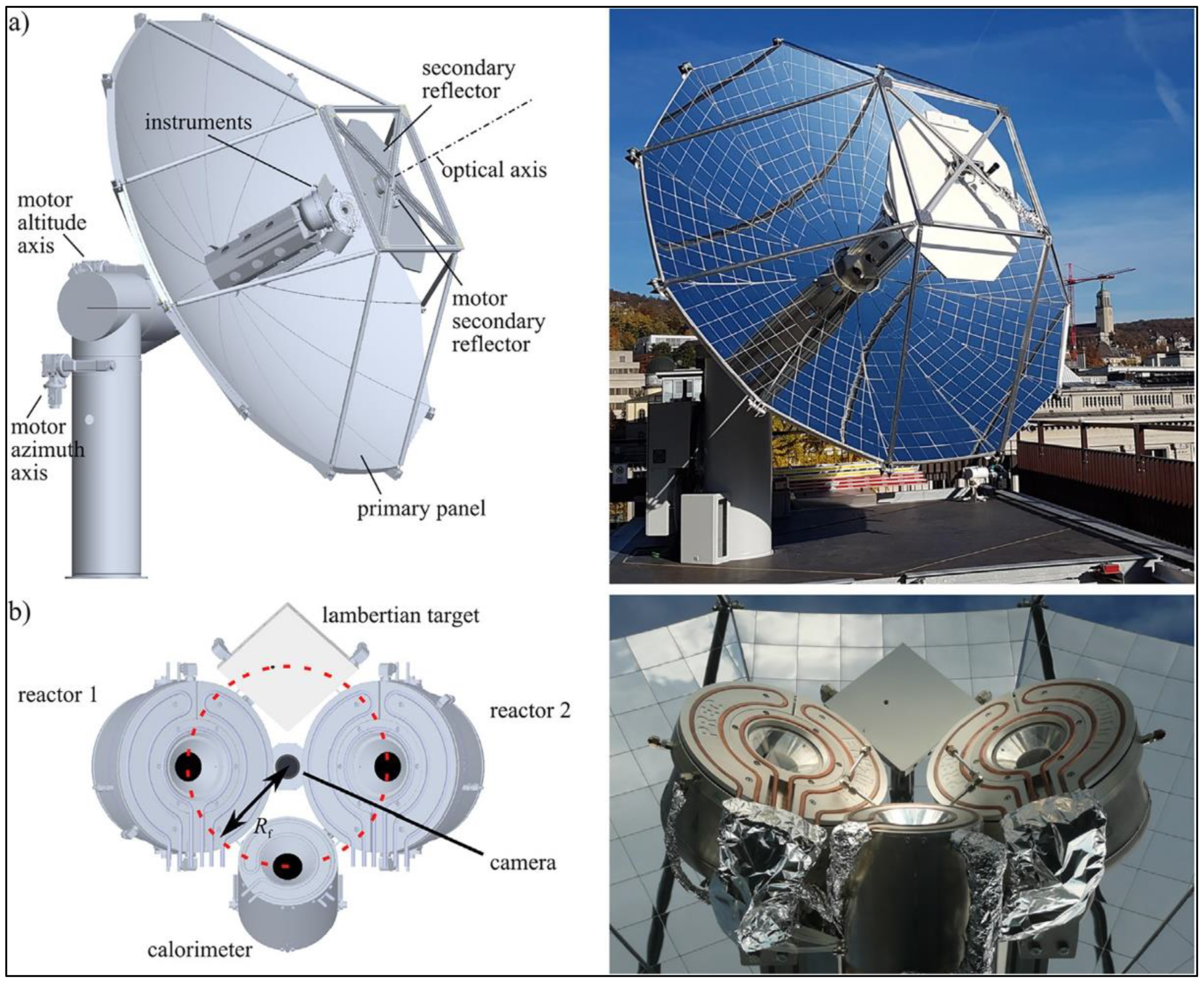
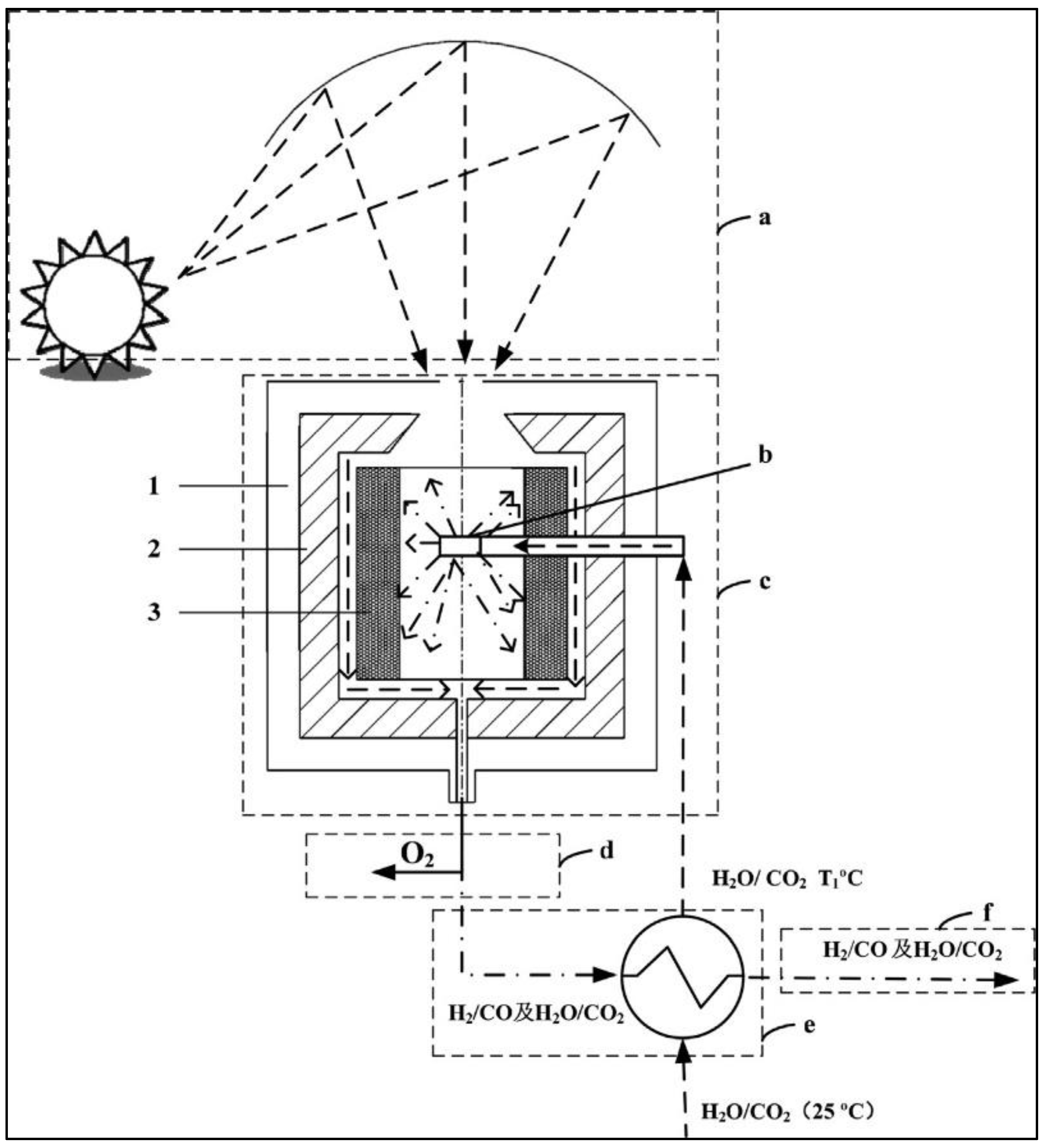
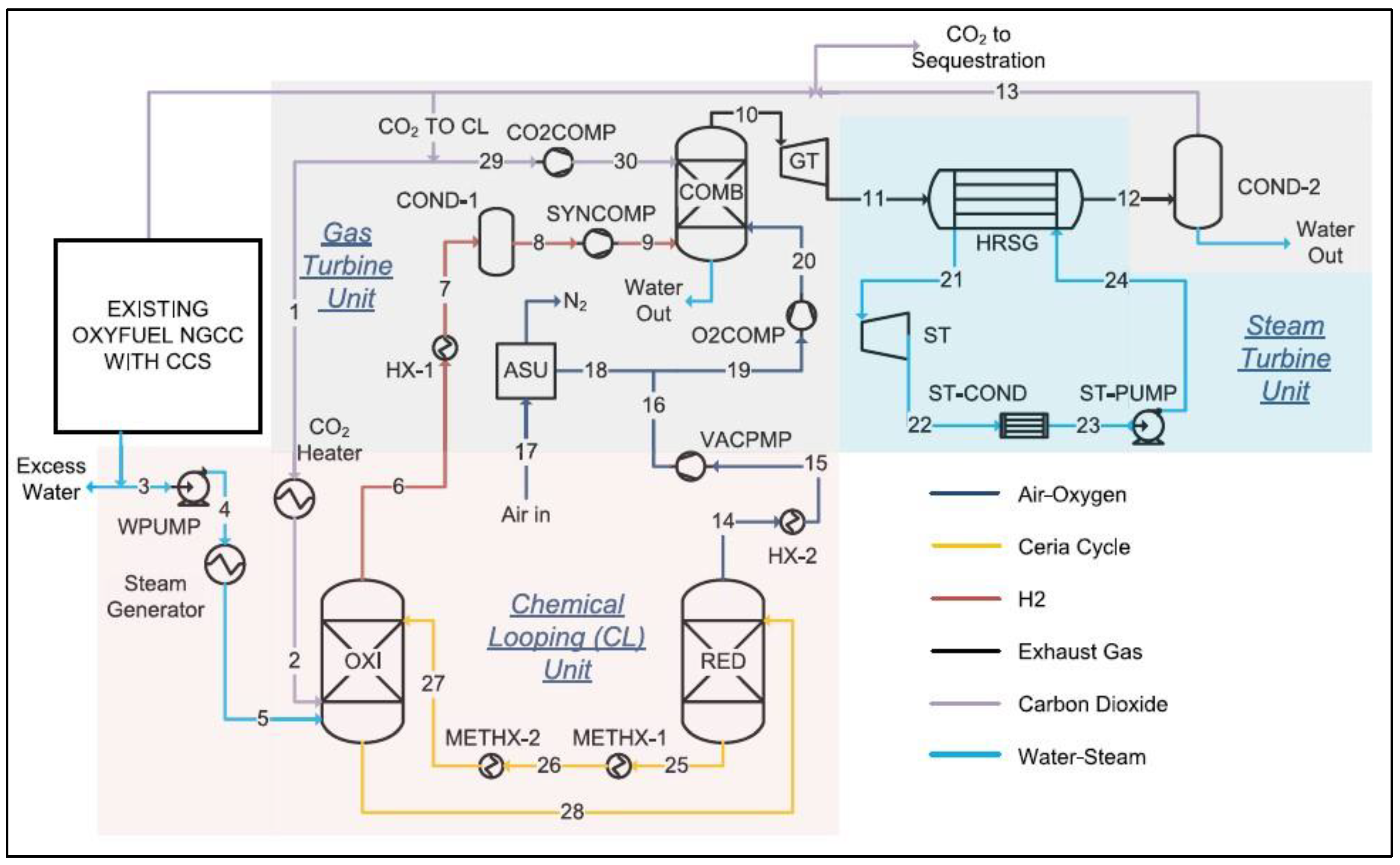
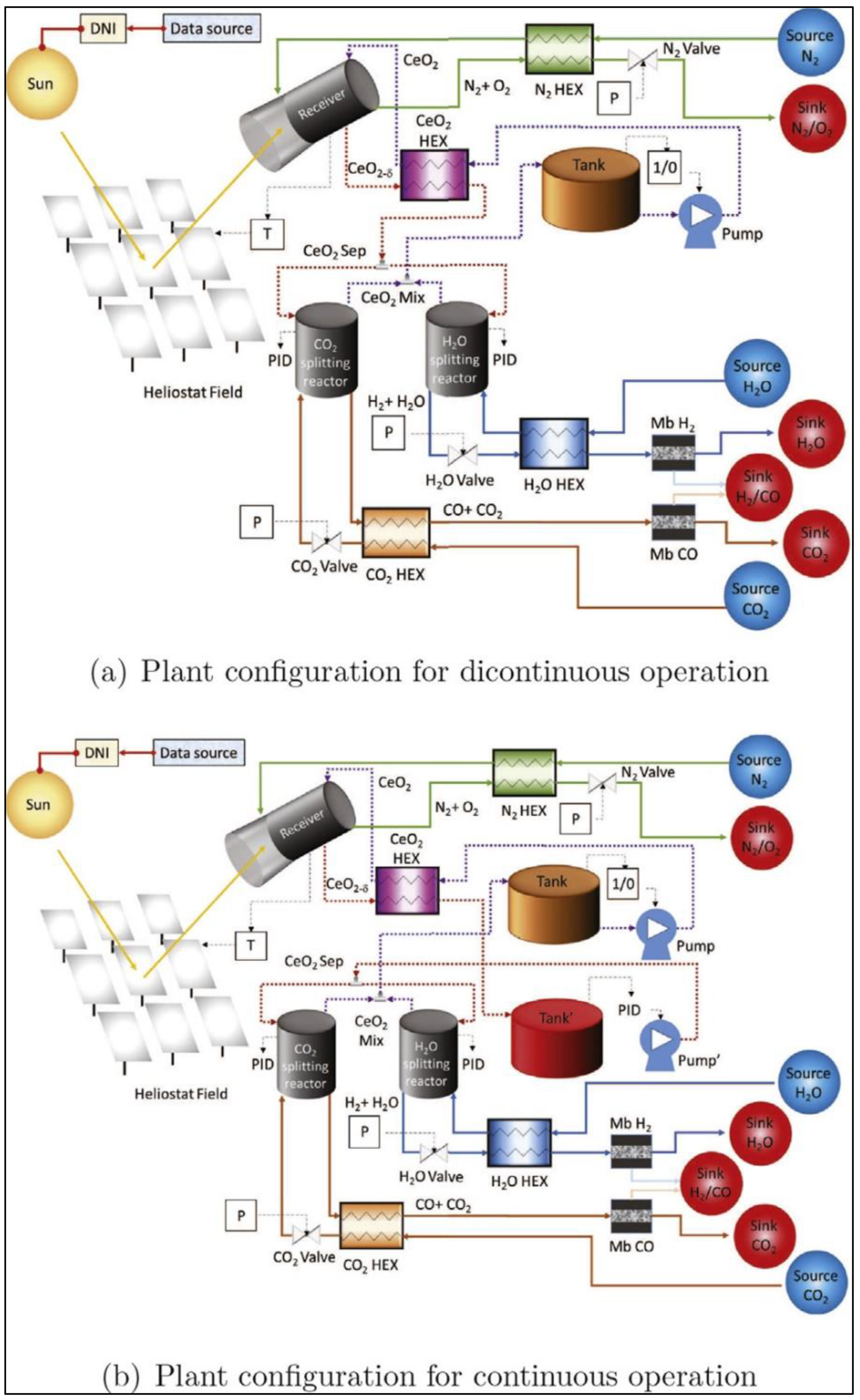
2.6. Year 2022
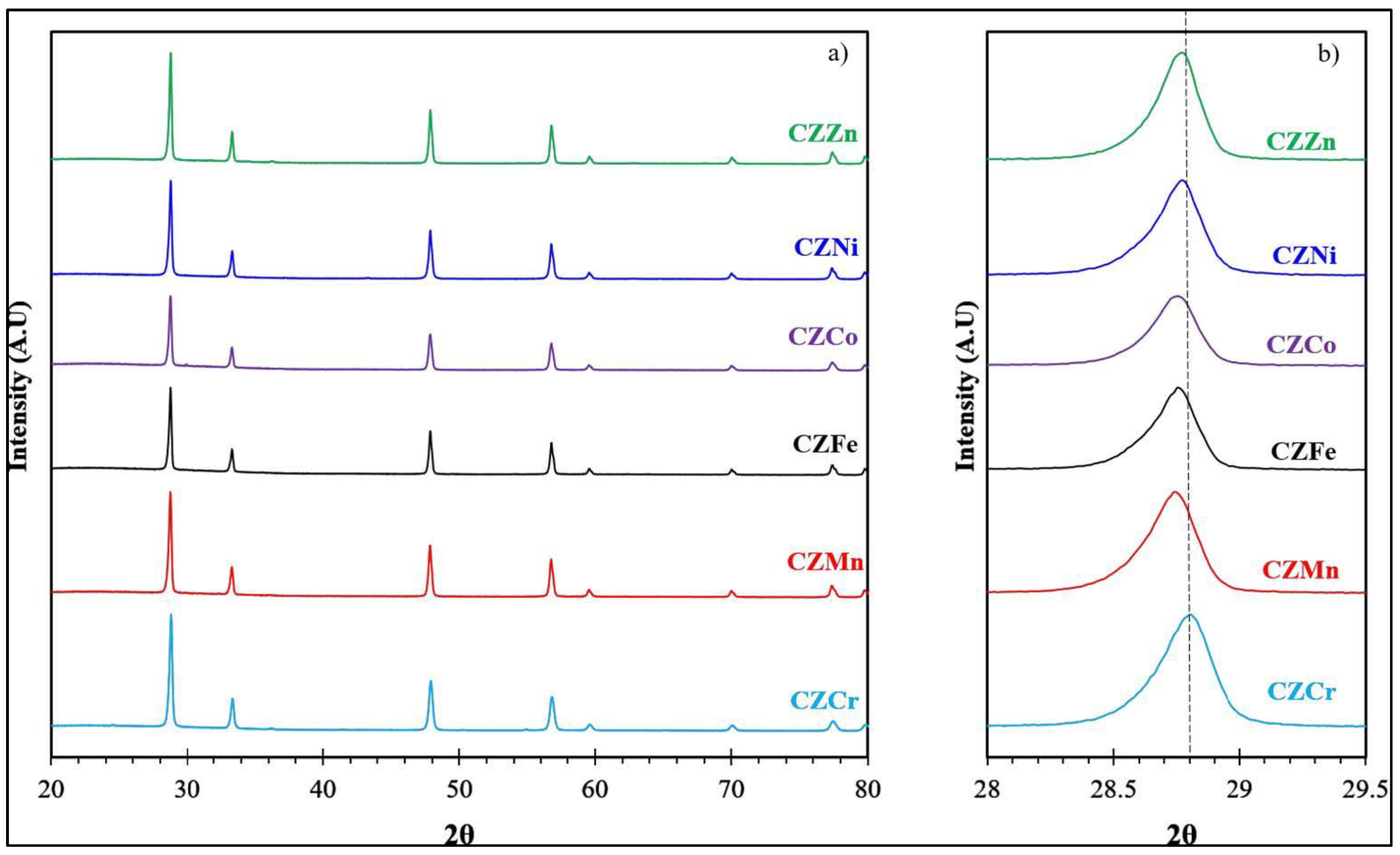
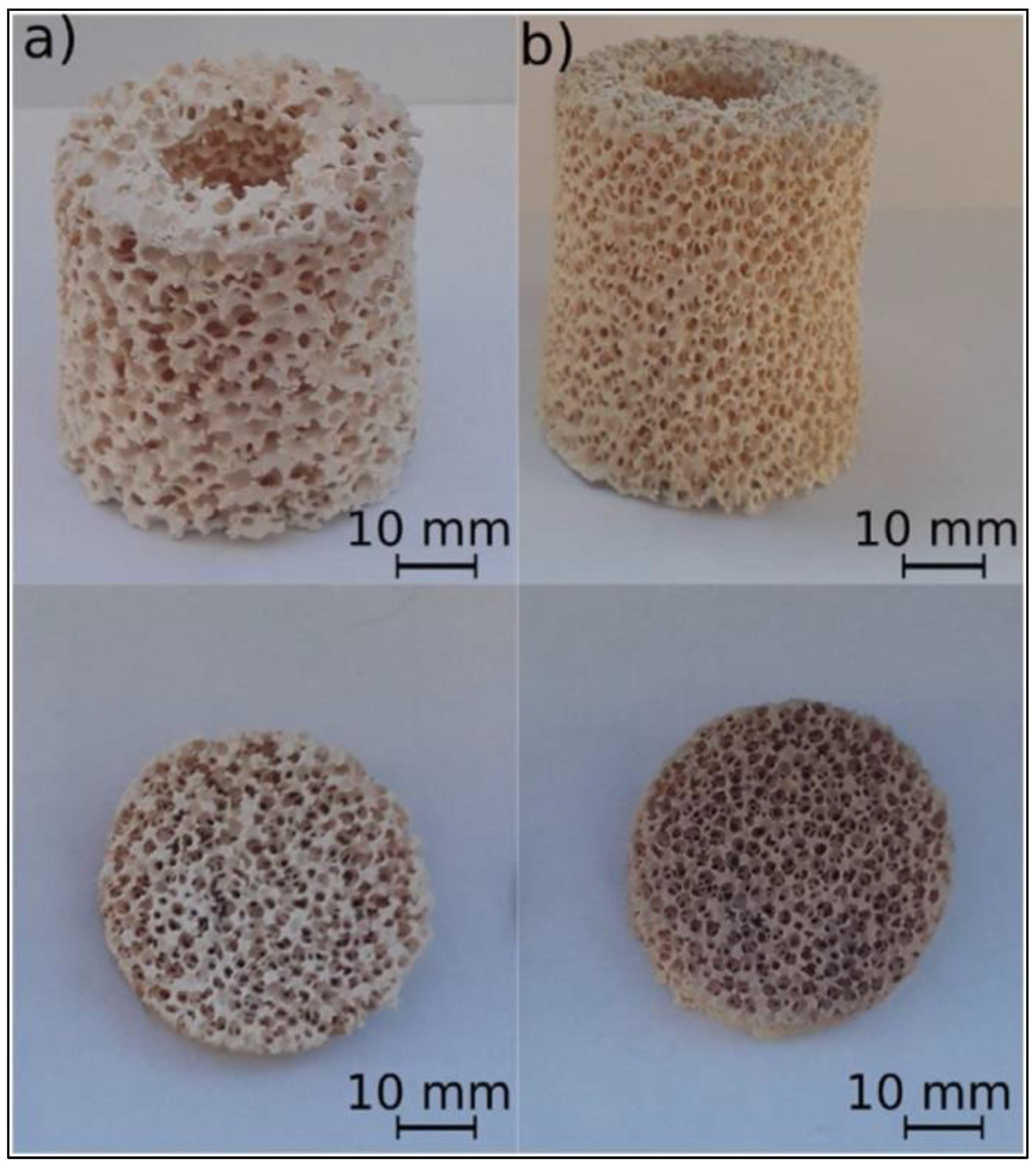
2.7. Year 2023
3. Summary
- Couple the ceria materials with perovskites (chemically and mechanically) to utilize the advantage of both redox materials.
- Use CH4 instead of an inert gas as the reducing agent to decrease the cycle temperatures and achieve fuel production in thermal reduction and oxidation steps.
- Invest more into developing the pilot scale reactor system under concentrated solar power.
- Convert the powdered samples into porous structures, which can attain the highest possible mass and heat transfer rates with minimum resistance.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhosale, R.R.; Mahajani, V.V. Kinetics of Absorption of Carbon Dioxide in Aqueous Solution of Ethylaminoethanol Modified with N-methyl-2-pyrolidone. Sep. Sci. Technol. 2013, 48, 2324–2337. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Kumar, A.; AlMomani, F.; Ghosh, U.; AlNouss, A.; Scheffe, J.; Gupta, R.B. CO2 Capture Using Aqueous Potassium Carbonate Promoted by Ethylaminoethanol: A Kinetic Study. Ind. Eng. Chem. Res. 2016, 55, 5238–5246. [Google Scholar] [CrossRef]
- Scheffe, J.R.; Steinfeld, A. Oxygen exchange materials for solar thermochemical splitting of H2O and CO2: A review. Mater. Today 2014, 17, 341–348. [Google Scholar] [CrossRef]
- Scheffe, J.R.; Weibel, D.; Steinfeld, A. Lanthanum–Strontium–Manganese Perovskites as Redox Materials for Solar Thermochemical Splitting of H2O and CO2. Energy Fuels 2013, 27, 4250–4257. [Google Scholar] [CrossRef]
- Loutzenhiser, P.G.; Meier, A.; Steinfeld, A. Review of the Two-Step H2O/CO2-Splitting Solar Thermochemical Cycle Based on Zn/ZnO Redox Reactions. Materials 2010, 3, 4922–4938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haeussler, A.; Abanades, S.; Jouannaux, J.; Julbe, A. Non-Stoichiometric Redox Active Perovskite Materials for Solar Thermochemical Fuel Production: A Review. Catalysts 2018, 8, 611. [Google Scholar] [CrossRef] [Green Version]
- Bhosale, R.R.; Kumar, A.; AlMomani, F.; Ghosh, U.; Sutar, P.; Takalkar, G.; Ashok, A.; Alxneit, I. Effectiveness of Ni incorporation in iron oxide crystal structure towards thermochemical CO2 splitting reaction. Ceram. Int. 2017, 43, 5150–5155. [Google Scholar] [CrossRef]
- Bhosale, R.R. Thermodynamic efficiency analysis of zinc oxide based solar driven thermochemical H2O splitting cycle: Effect of partial pressure of O2, thermal reduction and H2O splitting temperatures. Int. J. Hydrogen Energy 2018, 43, 14915–14924. [Google Scholar] [CrossRef]
- Koepf, E.; Alxneit, I.; Wieckert, C.; Meier, A. A review of high temperature solar driven reactor technology: 25years of experience in research and development at the Paul Scherrer Institute. Appl. Energy 2017, 188, 620–651. [Google Scholar] [CrossRef]
- Steinfeld, A. Solar hydrogen production via a two-step water-splitting thermochemical cycle based on Zn/ZnO redox reactions. Int. J. Hydrogen Energy 2002, 27, 611–619. [Google Scholar] [CrossRef]
- Abanades, S.; Charvin, P.; Lemont, F.; Flamant, G. Novel two-step SnO2/SnO water-splitting cycle for solar thermochemical production of hydrogen. Int. J. Hydrogen Energy 2008, 33, 6021–6030. [Google Scholar] [CrossRef]
- Abanades, S. CO2 and H2O reduction by solar thermochemical looping using SnO2/SnO redox reactions: Thermogravimetric analysis. Int. J. Hydrogen Energy 2012, 37, 8223–8231. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Kumar, A.; Sutar, P. Thermodynamic analysis of solar driven SnO2/SnO based thermochemical water splitting cycle. Energy Convers. Manag. 2017, 135, 226–235. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Kumar, A.; AlMomani, F.; Alxneit, I. Propylene oxide assisted sol–gel synthesis of zinc ferrite nanoparticles for solar fuel production. Ceram. Int. 2016, 42, 2431–2438. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Zygogianni, A.; Pagkoura, C.; Kostoglou, M.; Konstandopoulos, A.G. Hydrogen production via solar-aided water splitting thermochemical cycles with nickel ferrite: Experiments and modeling. AIChE J. 2013, 59, 1213–1225. [Google Scholar] [CrossRef]
- Shende, R.V.; Puszynski, J.A.; Opoku, M.K.; Bhosale, R.R. Synthesis of novel ferrite foam material for water-splitting application. In Proceedings of the NSTI Nanotech Conference & Expo., Houston, TX, USA, 3–7 May 2009; pp. 201–204. [Google Scholar]
- Scheffe, J.R.; Li, J.; Weimer, A.W. A spinel ferrite/hercynite water-splitting redox cycle. Int. J. Hydrogen Energy 2010, 35, 3333–3340. [Google Scholar] [CrossRef]
- Bhosale, R.R. Thermodynamic analysis of Ni-ferrite based solar thermochemical H2O splitting cycle for H2 production. Int. J. Hydrogen Energy 2019, 44, 61–71. [Google Scholar] [CrossRef]
- Amar, V.S.; Puszynski, J.A.; Shende, R.V. H2generation from thermochemical water-splitting using yttria stabilized NiFe2O4core-shell nanoparticles. J. Renew. Sustain. Energy 2015, 7, 023113. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, C.-K.; Haile, S.M. Ceria–Zirconia Solid Solutions (Ce1–xZrxO2−δ, x ≤ 0.2) for Solar Thermochemical Water Splitting: A Thermodynamic Study. Chem. Mater. 2014, 26, 6073–6082. [Google Scholar] [CrossRef]
- Furler, P.; Scheffe, J.; Gorbar, M.; Moes, L.; Vogt, U.; Steinfeld, A. Solar Thermochemical CO2 Splitting Utilizing a Reticulated Porous Ceria Redox System. Energy Fuels 2012, 26, 7051–7059. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Kumar, A.; AlMomani, F.; Alxneit, I. Sol–gel derived CeO2–Fe2O3 nanoparticles: Synthesis, characterization and solar thermochemical application. Ceram. Int. 2016, 42, 6728–6737. [Google Scholar] [CrossRef]
- Takalkar, G.; Bhosale, R.R. Solar thermocatalytic conversion of CO2 using PrxSr(1−x)MnO3−δ perovskites. Fuel 2019, 254, 115624. [Google Scholar] [CrossRef]
- Takalkar, G.; Bhosale, R.R.; AlMomani, F.; Rashid, S.; Qiblawey, H.; Saleh Saad, M.A.; Khraisheh, M.; Kumar, G.; Gupta, R.B.; Shende, R.V. Thermochemical splitting of CO2 using solution combustion synthesized lanthanum–strontium–manganese perovskites. Fuel 2021, 285, 119154. [Google Scholar] [CrossRef]
- Dey, S.; Rao, C.N.R. Splitting of CO2 by Manganite Perovskites to Generate CO by Solar Isothermal Redox Cycling. ACS Energy Lett. 2016, 1, 237–243. [Google Scholar] [CrossRef]
- Abanades, S.; Flamant, G. Thermochemical hydrogen production from a two-step solar-driven water-splitting cycle based on cerium oxides. Sol. Energy 2006, 80, 1611–1623. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Jouannaux, J.; Drobek, M.; Ayral, A.; Julbe, A. Recent progress on ceria doping and shaping strategies for solar thermochemical water and CO2 splitting cycles. AIMS Mater. Sci. 2019, 6, 657–684. [Google Scholar] [CrossRef]
- Yadav, D.; Banerjee, R. A review of solar thermochemical processes. Renew. Sustain. Energy Rev. 2016, 54, 497–532. [Google Scholar] [CrossRef]
- Carrillo, R.J.; Scheffe, J.R. Advances and trends in redox materials for solar thermochemical fuel production. Sol. Energy 2017, 156, 3–20. [Google Scholar] [CrossRef]
- Budama, V.K.; Rincon Duarte, J.P.; Roeb, M.; Sattler, C. Potential of solar thermochemical water-splitting cycles: A review. Sol. Energy 2023, 249, 353–366. [Google Scholar] [CrossRef]
- Abanades, S. Redox Cycles, Active Materials, and Reactors Applied to Water and Carbon Dioxide Splitting for Solar Thermochemical Fuel Production: A Review. Energies 2022, 15, 7061. [Google Scholar] [CrossRef]
- Warren, K.J.; Weimer, A.W. Solar thermochemical fuels: Present status and future prospects. Sol. Compass 2022, 1, 100010. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Takalkar, G.; Sutar, P.; Kumar, A.; AlMomani, F.; Khraisheh, M. A decade of ceria based solar thermochemical H2O/CO2 splitting cycle. Int. J. Hydrogen Energy 2019, 44, 34–60. [Google Scholar] [CrossRef]
- Muhich, C.; Steinfeld, A. Principles of doping ceria for the solar thermochemical redox splitting of H2O and CO2. J. Mater. Chem. A Mater. 2017, 5, 15578–15590. [Google Scholar] [CrossRef] [Green Version]
- Hoes, M.; Muhich, C.L.; Jacot, R.; Patzke, G.R.; Steinfeld, A. Thermodynamics of paired charge-compensating doped ceria with superior redox performance for solar thermochemical splitting of H2O and CO2. J. Mater. Chem. A Mater. 2017, 5, 19476–19484. [Google Scholar] [CrossRef] [Green Version]
- Tou, M.; Michalsky, R.; Steinfeld, A. Solar-Driven Thermochemical Splitting of CO2 and In Situ Separation of CO and O2 across a Ceria Redox Membrane Reactor. Joule 2017, 1, 146–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackermann, S.; Takacs, M.; Scheffe, J.; Steinfeld, A. Reticulated porous ceria undergoing thermochemical reduction with high-flux irradiation. Int. J. Heat Mass Transf. 2017, 107, 439–449. [Google Scholar] [CrossRef] [Green Version]
- Jacot, R.; Moré, R.; Michalsky, R.; Steinfeld, A.; Patzke, G.R. Trends in the phase stability and thermochemical oxygen exchange of ceria doped with potentially tetravalent metals. J. Mater. Chem. A 2017, 5, 19901–19913. [Google Scholar] [CrossRef] [Green Version]
- Marxer, D.; Furler, P.; Takacs, M.; Steinfeld, A. Solar thermochemical splitting of CO2 into separate streams of CO and O2 with high selectivity, stability, conversion, and efficiency. Energy Environ. Sci. 2017, 10, 1142–1149. [Google Scholar] [CrossRef] [Green Version]
- Rothensteiner, M.; Bonk, A.; Vogt, U.F.; Emerich, H.; van Bokhoven, J.A. Structural changes in equimolar ceria–hafnia materials under solar thermochemical looping conditions: Cation ordering, formation and stability of the pyrochlore structure. RSC Adv. 2017, 7, 53797–53809. [Google Scholar] [CrossRef] [Green Version]
- Takacs, M.; Ackermann, S.; Bonk, A.; Neises-von Puttkamer, M.; Haueter, P.; Scheffe, J.R.; Vogt, U.F.; Steinfeld, A. Splitting CO2 with a ceria-based redox cycle in a solar-driven thermogravimetric analyzer. AIChE J. 2017, 63, 1263–1271. [Google Scholar] [CrossRef] [Green Version]
- Naghavi, S.S.; Emery, A.A.; Hansen, H.A.; Zhou, F.; Ozolins, V.; Wolverton, C. Giant onsite electronic entropy enhances the performance of ceria for water splitting. Nat. Commun. 2017, 8, 285. [Google Scholar] [CrossRef] [Green Version]
- Pappacena, A.; Rancan, M.; Armelao, L.; Llorca, J.; Ge, W.; Ye, B.; Lucotti, A.; Trovarelli, A.; Boaro, M. New Insights into the Dynamics That Control the Activity of Ceria–Zirconia Solid Solutions in Thermochemical Water Splitting Cycles. J. Phys. Chem. C 2017, 121, 17746–17755. [Google Scholar] [CrossRef] [Green Version]
- Ruan, C.; Tan, Y.; Li, L.; Wang, J.; Liu, X.; Wang, X. A novel CeO2 − xSnO2/Ce2Sn2O7 pyrochlore cycle for enhanced solar thermochemical water splitting. AIChE J. 2017, 63, 3450–3462. [Google Scholar] [CrossRef]
- Grobbel, J.; Brendelberger, S.; Sattler, C.; Pitz-Paal, R. Heat transfer in a directly irradiated ceria particle bed under vacuum conditions. Sol. Energy 2017, 158, 737–745. [Google Scholar] [CrossRef]
- Mostrou, S.; Büchel, R.; Pratsinis, S.E.; van Bokhoven, J.A. Improving the ceria-mediated water and carbon dioxide splitting through the addition of chromium. Appl. Catal. A Gen. 2017, 537, 40–49. [Google Scholar] [CrossRef]
- Takalkar, G.; Bhosale, R.; Kumar, A.; AlMomani, F.; Khraisheh, M.; Shakoor, R.; Gupta, R. Transition metal doped ceria for solar thermochemical fuel production. Sol. Energy 2018, 172, 204–211. [Google Scholar] [CrossRef]
- Costa Oliveira, F.A.; Barreiros, M.A.; Abanades, S.; Caetano, A.P.; Novais, R.M.; Pullar, R.C. Solar thermochemical CO2 splitting using cork-templated ceria ecoceramics. J. CO2 Util. 2018, 26, 552–563. [Google Scholar] [CrossRef]
- Muhich, C.L.; Blaser, S.; Hoes, M.C.; Steinfeld, A. Comparing the solar-to-fuel energy conversion efficiency of ceria and perovskite based thermochemical redox cycles for splitting H2O and CO2. Int. J. Hydrogen Energy 2018, 43, 18814–18831. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Y.; Li, F. Reactivity of Ni, Cr and Zr doped ceria in CO2 splitting for CO production via two-step thermochemical cycle. Int. J. Hydrogen Energy 2018, 43, 13754–13763. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Y. Reactivity and Efficiency of Ceria-Based Oxides for Solar CO2 Splitting via Isothermal and Near-Isothermal Cycles. Energy Fuels 2018, 32, 736–746. [Google Scholar] [CrossRef]
- Arifin, D.; Weimer, A.W. Kinetics and mechanism of solar-thermochemical H2 and CO production by oxidation of reduced CeO2. Sol. Energy 2018, 160, 178–185. [Google Scholar] [CrossRef]
- Muhich, C.; Hoes, M.; Steinfeld, A. Mimicking tetravalent dopant behavior using paired charge compensating dopants to improve the redox performance of ceria for thermochemically splitting H2O and CO2. Acta Mater. 2018, 144, 728–737. [Google Scholar] [CrossRef]
- Falter, C.; Pitz-Paal, R. Energy analysis of solar thermochemical fuel production pathway with a focus on waste heat recuperation and vacuum generation. Sol. Energy 2018, 176, 230–240. [Google Scholar] [CrossRef]
- Jacot, R.; Naik, J.M.; Moré, R.; Michalsky, R.; Steinfeld, A.; Patzke, G.R. Reactive stability of promising scalable doped ceria materials for thermochemical two-step CO2 dissociation. J. Mater. Chem. A Mater. 2018, 6, 5807–5816. [Google Scholar] [CrossRef]
- Farooqui, A.; Pica, A.M.; Marocco, P.; Ferrero, D.; Lanzini, A.; Fiorilli, S.; Llorca, J.; Santarelli, M. Assessment of kinetic model for ceria oxidation for chemical-looping CO2 dissociation. Chem. Eng. J. 2018, 346, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Budama, V.K.; Johnson, N.G.; McDaniel, A.; Ermanoski, I.; Stechel, E.B. Thermodynamic development and design of a concentrating solar thermochemical water-splitting process for co-production of hydrogen and electricity. Int. J. Hydrogen Energy 2018, 43, 17574–17587. [Google Scholar] [CrossRef]
- Arribas, L.; González-Aguilar, J.; Romero, M. Solar-Driven Thermochemical Water-Splitting by Cerium Oxide: Determination of Operational Conditions in a Directly Irradiated Fixed Bed Reactor. Energies 2018, 11, 2451. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Wheeler, V.M.; Kreider, P.B.; Lipiński, W. Thermodynamic Analyses of Fuel Production via Solar-Driven Non-stoichiometric Metal Oxide Redox Cycling. Part 1. Revisiting Flow and Equilibrium Assumptions. Energy Fuels 2018, 32, 10838–10847. [Google Scholar] [CrossRef]
- Li, S.; Wheeler, V.M.; Kreider, P.B.; Bader, R.; Lipiński, W. Thermodynamic Analyses of Fuel Production via Solar-Driven Non-stoichiometric Metal Oxide Redox Cycling. Part 2. Impact of Solid–Gas Flow Configurations and Active Material Composition on System-Level Efficiency. Energy Fuels 2018, 32, 10848–10863. [Google Scholar] [CrossRef]
- Roberts, S.J.; Carr, N.G.; McLaughlin, J.; Hagelin-Weaver, H.E. Iron precipitated onto ceria-zirconia nanoparticle mixtures for the production of hydrogen via two-step thermochemical water splitting. Int. J. Hydrogen Energy 2018, 43, 12970–12984. [Google Scholar] [CrossRef]
- Dähler, F.; Wild, M.; Schäppi, R.; Haueter, P.; Cooper, T.; Good, P.; Larrea, C.; Schmitz, M.; Furler, P.; Steinfeld, A. Optical design and experimental characterization of a solar concentrating dish system for fuel production via thermochemical redox cycles. Sol. Energy 2018, 170, 568–575. [Google Scholar] [CrossRef]
- Bhosale, R.; Takalkar, G. Nanostructured co-precipitated Ce0.9Ln0.1O2 (Ln = La, Pr, Sm, Nd, Gd, Tb, Dy, or Er) for thermochemical conversion of CO2. Ceram. Int. 2018, 44, 16688–16697. [Google Scholar] [CrossRef]
- Kong, H.; Kong, X.; Wang, H.; Wang, J. A strategy for optimizing efficiencies of solar thermochemical fuel production based on nonstoichiometric oxides. Int. J. Hydrogen Energy 2019, 44, 19585–19594. [Google Scholar] [CrossRef]
- Hoes, M.; Ackermann, S.; Theiler, D.; Furler, P.; Steinfeld, A. Additive-Manufactured Ordered Porous Structures Made of Ceria for Concentrating Solar Applications. Energy Technol. 2019, 7, 1900484. [Google Scholar] [CrossRef] [Green Version]
- Takalkar, G.; Bhosale, R.; AlMomani, F. Evaluation of redox performance of silver and transition metal-doped ternary ceria oxides for thermochemical splitting of CO2. Int. J. Energy Res. 2019, 43, 3616–3627. [Google Scholar] [CrossRef]
- Tou, M.; Jin, J.; Hao, Y.; Steinfeld, A.; Michalsky, R. Solar-driven co-thermolysis of CO2 and H2O promoted by in situ oxygen removal across a non-stoichiometric ceria membrane. React. Chem. Eng. 2019, 4, 1431–1438. [Google Scholar] [CrossRef] [Green Version]
- Farooqui, A.; Bose, A.; Ferrero, D.; Llorca, J.; Santarelli, M. Simulation of two-step redox recycling of non-stoichiometric ceria with thermochemical dissociation of CO2/H2O in moving bed reactors—Part II: Techno-economic analysis and integration with 100 MW oxyfuel power plant with carbon capture. Chem. Eng. Sci. 2019, 205, 358–373. [Google Scholar] [CrossRef]
- Li, S.; Kreider, P.B.; Wheeler, V.M.; Lipiński, W. Thermodynamic Analyses of Fuel Production Via Solar-Driven Ceria-Based Nonstoichiometric Redox Cycling: A Case Study of the Isothermal Membrane Reactor System. J. Sol. Energy Eng. 2019, 141, 021012. [Google Scholar] [CrossRef] [Green Version]
- Carrillo, R.J.; Warren, K.J.; Scheffe, J.R. Experimental Framework for Evaluation of the Thermodynamic and Kinetic Parameters of Metal-Oxides for Solar Thermochemical Fuel Production. J. Sol. Energy Eng. 2019, 141, 021007. [Google Scholar] [CrossRef]
- Zoller, S.; Koepf, E.; Roos, P.; Steinfeld, A. Heat Transfer Model of a 50 kW Solar Receiver–Reactor for Thermochemical Redox Cycling Using Cerium Dioxide. J. Sol. Energy Eng. 2019, 141, 021014. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Scheffe, J.R. Characterization of a Laser-Based Heating System Coupled With In Operando Raman Spectroscopy for Studying Solar Thermochemical Redox Cycles. J. Sol. Energy Eng. 2019, 141, 021013. [Google Scholar] [CrossRef]
- De la Calle, A.; Bayon, A. Annual performance of a thermochemical solar syngas production plant based on non-stoichiometric CeO2. Int. J. Hydrogen Energy 2018, 44, 1409–1424. [Google Scholar] [CrossRef]
- Takalkar, G.; Bhosale, R.R.; AlMomani, F. Thermochemical splitting of CO2 using Co-precipitation synthesized Ce0.75Zr0.2M0.05O2−δ (M = Cr, Mn, Fe, CO, Ni, Zn) materials. Fuel 2019, 256, 115834. [Google Scholar] [CrossRef]
- Luciani, G.; Landi, G.; Imparato, C.; Vitiello, G.; Deorsola, F.A.; Di Benedetto, A.; Aronne, A. Improvement of splitting performance of Ce0.75Zr0.25O2 material: Tuning bulk and surface properties by hydrothermal synthesis. Int. J. Hydrogen Energy 2019, 44, 17565–17577. [Google Scholar] [CrossRef]
- Shi, H.; Luo, J.; Wang, F.; Pu, Y.; Yang, J.; Xiao, F.; Zhao, N.; Song, Q.; Chen, Z. Synthesis of CeO2-ZrO2 Solid Solutions for Thermochemical CO2 Splitting. Energy Technol. 2019, 7, 1800890. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Julbe, A.; Jouannaux, J.; Cartoixa, B. Solar thermochemical fuel production from H2O and CO2 splitting via two-step redox cycling of reticulated porous ceria structures integrated in a monolithic cavity-type reactor. Energy 2020, 201, 117649. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Julbe, A.; Jouannaux, J.; Drobek, M.; Ayral, A.; Cartoixa, B. Remarkable performance of microstructured ceria foams for thermochemical splitting of H2O and CO2 in a novel high–temperature solar reactor. Chem. Eng. Res. Des. 2020, 156, 311–323. [Google Scholar] [CrossRef]
- Costa Oliveira, F.A.; Barreiros, M.A.; Haeussler, A.; Caetano, A.P.F.; Mouquinho, A.I.; Oliveira e Silva, P.M.; Novais, R.M.; Pullar, R.C.; Abanades, S. High performance cork-templated ceria for solar thermochemical hydrogen production via two-step water-splitting cycles. Sustain. Energy Fuels 2020, 4, 3077–3089. [Google Scholar] [CrossRef]
- Takalkar, G.; Bhosale, R.R.; Rashid, S.; Almomani, F.; Shakoor, R.A.; Al Ashraf, A. Application of Li-, Mg-, Ba-, Sr-, Ca-, and Sn-doped ceria for solar-driven thermochemical conversion of carbon dioxide. J. Mater. Sci. 2020, 55, 11797–11807. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Costa Oliveira, F.A.; Barreiros, M.A.; Caetano, A.P.F.; Novais, R.M.; Pullar, R.C. Solar Redox Cycling of Ceria Structures Based on Fiber Boards, Foams, and Biomimetic Cork-Derived Ecoceramics for Two-Step Thermochemical H2O and CO2 Splitting. Energy Fuels 2020, 34, 9037–9049. [Google Scholar] [CrossRef]
- Takalkar, G.; Bhosale, R.R. Investigation of Zr-doped ceria for solar thermochemical valorization of CO2. Int. J. Energy Res. 2020, 44, 12284–12294. [Google Scholar] [CrossRef]
- Naghavi, S.S.; He, J.; Wolverton, C. CeTi2O6—A Promising Oxide for Solar Thermochemical Hydrogen Production. ACS Appl. Mater. Interfaces 2020, 12, 21521–21527. [Google Scholar] [CrossRef] [PubMed]
- Rawadieh, S.E.; Altarawneh, M.; Altarawneh, I.S.; Batiha, M.A.; Al-Makhadmeh, L.A. A kinetic model for evolution of H2 and CO over Zr-doped ceria. Mol. Catal. 2020, 498, 111256. [Google Scholar] [CrossRef]
- Arifin, D.; Ambrosini, A.; Wilson, S.A.; Mandal, B.; Muhich, C.; Weimer, A.W. Investigation of Zr, Gd/Zr, and Pr/Zr—Doped ceria for the redox splitting of water. Int. J. Hydrogen Energy 2020, 45, 160–174. [Google Scholar] [CrossRef]
- Hao, Y.; Jin, J.; Jin, H. Thermodynamic analysis of isothermal CO2 splitting and CO2-H2O co-splitting for solar fuel production. Appl. Therm. Eng. 2020, 166, 113600. [Google Scholar] [CrossRef]
- Luciani, G.; Landi, G.; Di Benedetto, A. Syngas Production Through H2O/CO2 Thermochemical Splitting Over Doped Ceria-Zirconia Materials. Front. Energy Res. 2020, 8, 204. [Google Scholar] [CrossRef]
- Takalkar, G.; Bhosale, R.R.; AlMomani, F.; Rashid, S. Co-precipitation synthesized nanostructured Ce0.9Ln0.05Ag0.05O2−δ materials for solar thermochemical conversion of CO2 into fuels. J. Mater. Sci. 2020, 55, 9748–9761. [Google Scholar] [CrossRef] [Green Version]
- Farooqui, A.; Bose, A.; Ferrero, D.; Llorca, J.; Santarelli, M. Simulation of two-step redox recycling of non-stoichiometric ceria with thermochemical dissociation of CO2/H2O in moving bed reactors—Part I: Model development with redox kinetics and sensitivity analysis. Chem. Eng. Sci. 2020, 226, 114873. [Google Scholar] [CrossRef]
- Abanades, S.; Haeussler, A. Two-step thermochemical cycles using fibrous ceria pellets for H2 production and CO2 reduction in packed-bed solar reactors. Sustain. Mater. Technol. 2021, 29, e00328. [Google Scholar] [CrossRef]
- Yang, S.; Li, L.; Wang, B.; Li, S.; Wang, J.; Lund, P.; Lipiński, W. Thermodynamic Analysis of a Conceptual Fixed-Bed Solar Thermochemical Cavity Receiver–Reactor Array for Water Splitting Via Ceria Redox Cycling. Front. Energy Res. 2021, 9, 565761. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S. Additive manufacturing and two-step redox cycling of ordered porous ceria structures for solar-driven thermochemical fuel production. Chem. Eng. Sci. 2021, 246, 116999. [Google Scholar] [CrossRef]
- Abanades, S.; Haeussler, A.; Julbe, A. Thermochemical solar-driven reduction of CO2 into separate streams of CO and O2 via an isothermal oxygen-conducting ceria membrane reactor. Chem. Eng. J. 2021, 422, 130026. [Google Scholar] [CrossRef]
- Chen, J.; Ma, J.; Alford, M.B.; Sun, Y.; Tong, J.; Peng, F. Porous Zr-Doped Ceria Microspheres for Thermochemical Splitting of Carbon Dioxide. ACS Appl. Energy Mater. 2021, 4, 10451–10458. [Google Scholar] [CrossRef]
- Sediva, E.; Carrillo, A.J.; Halloran, C.E.; Rupp, J.L.M. Evaluating the Redox Behavior of Doped Ceria for Thermochemical CO2 Splitting Using Time-Resolved Raman Spectroscopy. ACS Appl. Energy Mater. 2021, 4, 1474–1483. [Google Scholar] [CrossRef]
- Orfila, M.; Sanz, D.; Linares, M.; Molina, R.; Sanz, R.; Marugán, J.; Botas, J. H2 production by thermochemical water splitting with reticulated porous structures of ceria-based mixed oxide materials. Int. J. Hydrogen Energy 2021, 46, 17458–17471. [Google Scholar] [CrossRef]
- Li, S.; Wheeler, V.M.; Kumar, A.; Venkataraman, M.B.; Muhich, C.L.; Hao, Y.; Lipiński, W. Thermodynamic Guiding Principles for Designing Nonstoichiometric Redox Materials for Solar Thermochemical Fuel Production: Ceria, Perovskites, and Beyond. Energy Technol. 2021, 10, 2000925. [Google Scholar] [CrossRef]
- Ben-Arfa, B.A.E.; Abanades, S.; Salvado, I.M.M.; Ferreira, J.M.F.; Pullar, R.C. Robocasting of 3D printed and sintered ceria scaffold structures with hierarchical porosity for solar thermochemical fuel production from the splitting of CO2. Nanoscale 2022, 14, 4994–5001. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wei, R.; Vafai, K. A dual-scale transport model of the porous ceria based on solar thermochemical cycle water splitting hydrogen production. Energy Convers. Manag. 2022, 272, 116363. [Google Scholar] [CrossRef]
- Thanda, V.; Fend, T.; Laaber, D.; Lidor, A.; von Storch, H.; Säck, J.; Hertel, J.; Lampe, J.; Menz, S.; Piesche, G.; et al. Experimental investigation of the applicability of a 250 kW ceria receiver/reactor for solar thermochemical hydrogen generation. Renew. Energy 2022, 198, 389–398. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Dai, Y.; Wang, C.-H. Thermodynamic analysis of an epitrochoidal rotary reactor for solar hydrogen production via a water-splitting thermochemical cycle using nonstoichiometric ceria. Energy Convers. Manag. 2022, 268, 115968. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, Q.; An, H.; Zhang, J.; Hao, S.; Li, X.; Cai, L.; Yu, W.; You, K.; Zhu, X.; et al. Platinum Group Metal Catalyst (RuOx, PtOx, and IrOx)-Decorated Ceria-Zirconia Solid Solution as High Active Oxygen Carriers for Solar Thermochemical CO2 Splitting. ACS Catal. 2022, 12, 7719–7736. [Google Scholar] [CrossRef]
- Bai, W.; Huang, H.; Suter, C.; Haussener, S.; Lin, M. Enhanced Solar-to-Fuel Efficiency of Ceria-Based Thermochemical Cycles via Integrated Electrochemical Oxygen Pumping. ACS Energy Lett. 2022, 7, 2711–2716. [Google Scholar] [CrossRef]
- Onigbajumo, A.; Swarnkar, P.; Will, G.; Sundararajan, T.; Taghipour, A.; Couperthwaite, S.; Steinberg, T.; Rainey, T. Techno-economic evaluation of solar-driven ceria thermochemical water-splitting for hydrogen production in a fluidized bed reactor. J. Clean. Prod. 2022, 371, 133303. [Google Scholar] [CrossRef]
- Zoller, S.; Koepf, E.; Nizamian, D.; Stephan, M.; Patané, A.; Haueter, P.; Romero, M.; González-Aguilar, J.; Lieftink, D.; de Wit, E.; et al. A solar tower fuel plant for the thermochemical production of kerosene from H2O and CO2. Joule 2022, 6, 1606–1616. [Google Scholar] [CrossRef]
- Lee, K.; Knoblauch, N.; Agrafiotis, C.; Pein, M.; Roeb, M.; Sattler, C. Oxidation kinetics of La and Yb incorporated Zr-doped ceria for solar thermochemical fuel production in the context of dopant ionic radius and valence. Open Ceram. 2022, 10, 100269. [Google Scholar] [CrossRef]
- Lampe, J.; Menz, S.; Akinci, K.; Böhm, K.; Seeger, T.; Fend, T. Optimizing the operational strategy of a solar-driven reactor for thermochemical hydrogen production. Int. J. Hydrogen Energy 2022, 47, 14453–14468. [Google Scholar] [CrossRef]
- Lee, K.; Knoblauch, N.; Agrafiotis, C.; Pein, M.; Roeb, M.; Schmücker, M.; Sattler, C. Strategic co-doping of ceria for improved oxidation kinetics in solar thermochemical fuel production. Mater. Today Energy 2023, 35, 101321. [Google Scholar] [CrossRef]
- Uxa, D.; Dörrer, L.; Schulz, M.; Knoblauch, N.; Fielitz, P.; Roeb, M.; Schmücker, M.; Borchardt, G. Investigation of CO2 Splitting on Ceria-Based Redox Materials for Low-Temperature Solar Thermochemical Cycling with Oxygen Isotope Exchange Experiments. Processes 2022, 11, 109. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Zhu, X.; Wang, Y.; Tian, T.; Dai, Y.; Wang, C.-H. An epitrochoidal rotary reactor for solar-driven hydrogen production based on the redox cycling of ceria: Thermodynamic analysis and geometry optimization. Energy 2023, 270, 126833. [Google Scholar] [CrossRef]
- Ma, J.; Chen, J.; Geng, X.; Alford, B.; Zhang, Z.; Xiao, H.; Tong, J.; Peng, F. Sol-gel fabrication of porous ceria microspheres for thermochemical carbon dioxide (CO2) splitting. Nucl. Anal. 2023, 2, 100063. [Google Scholar] [CrossRef]
- Razmgar, K.; Shittu, T.; Oluwoye, I.; Khaleel, A.; Senanayake, G.; Altarawneh, M. Thermochemical activation of CO2 into syngas over ceria-supported niobium oxide catalyst: An integrated experimental-DFT study. J. CO2 Util. 2023, 67, 102339. [Google Scholar] [CrossRef]







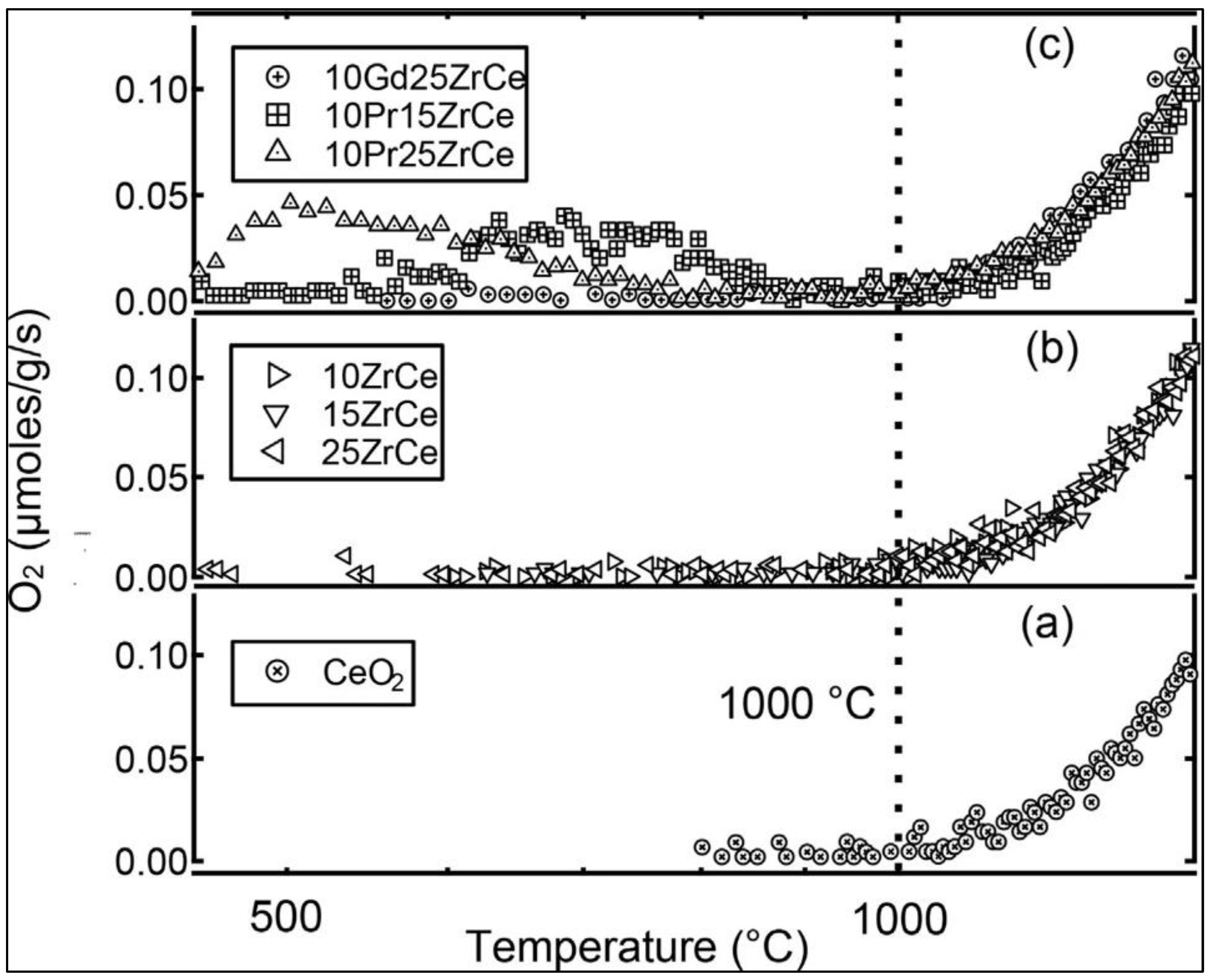
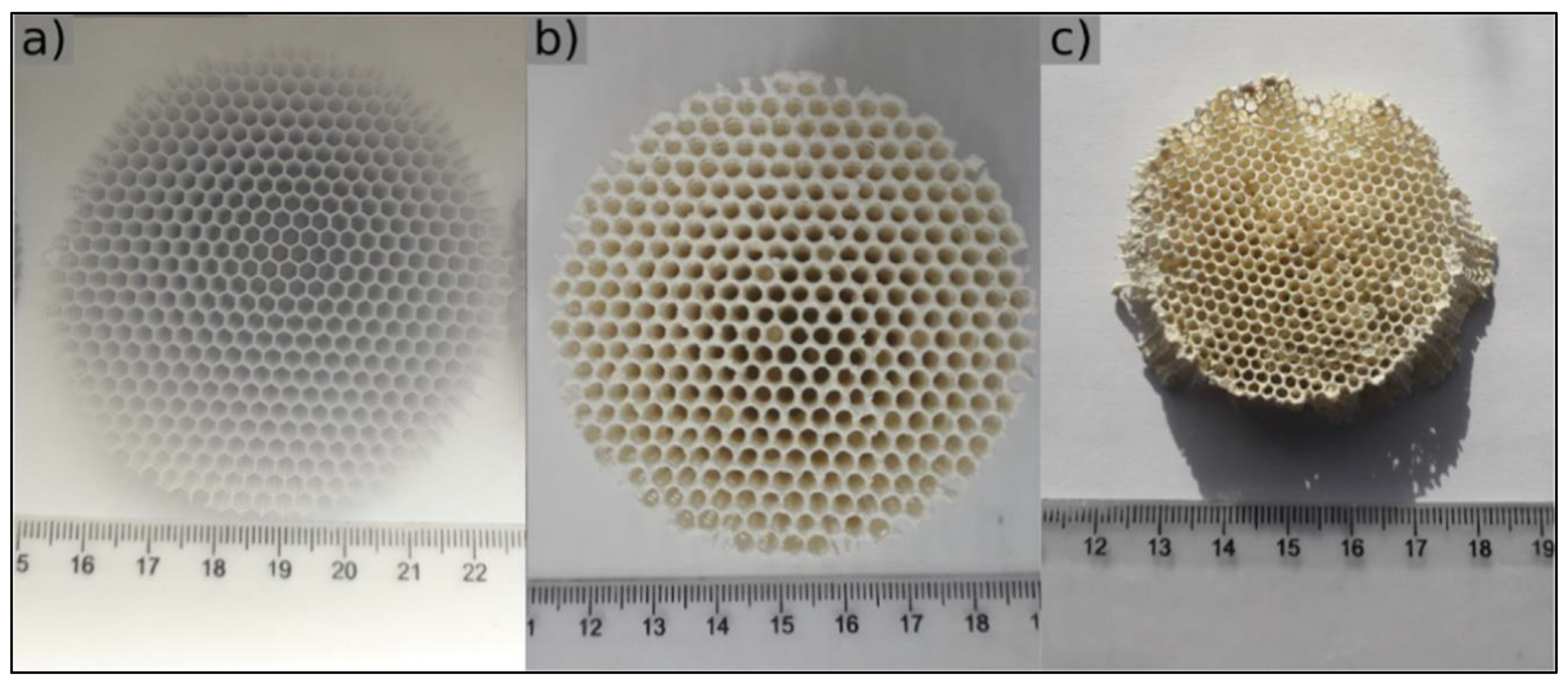
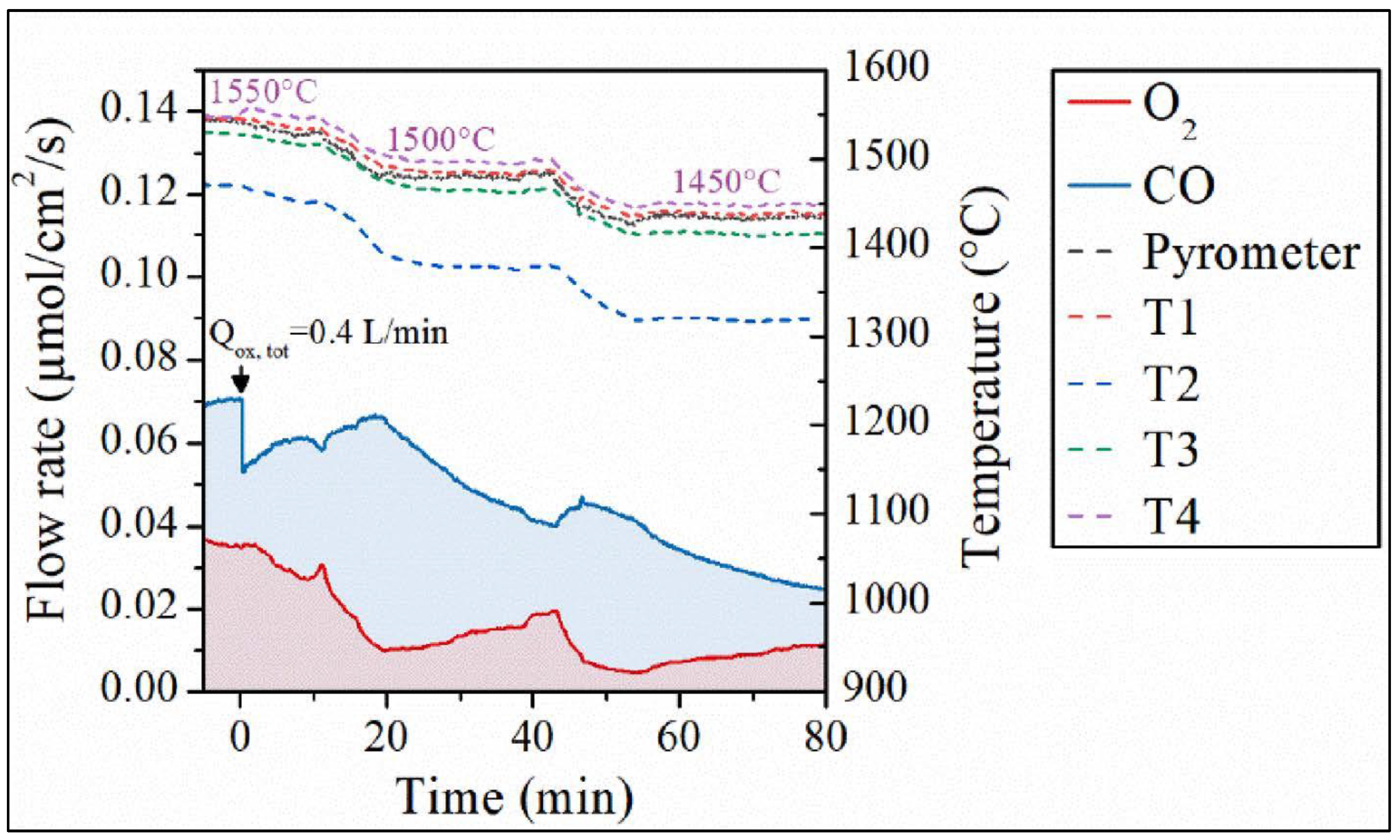
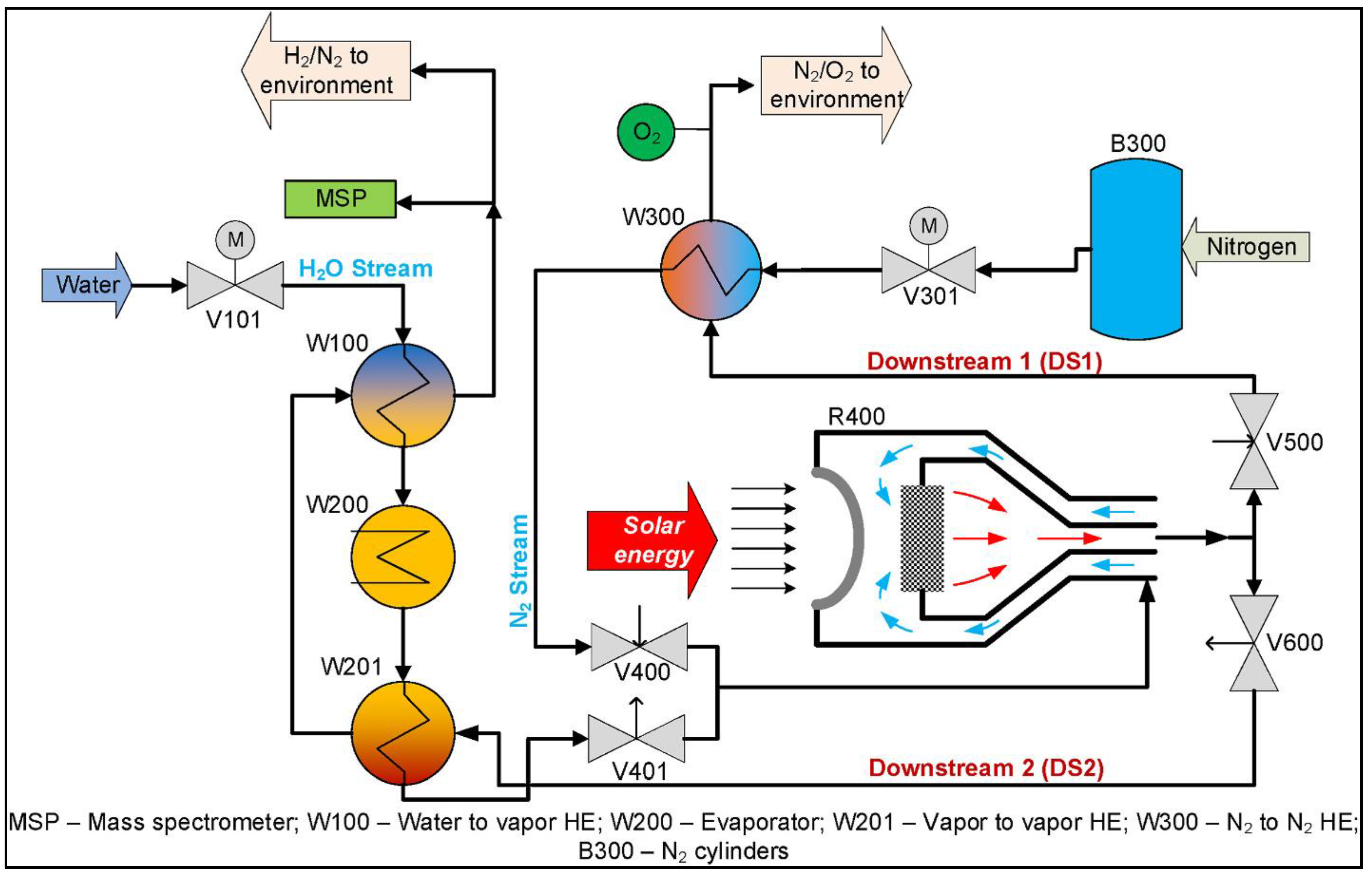
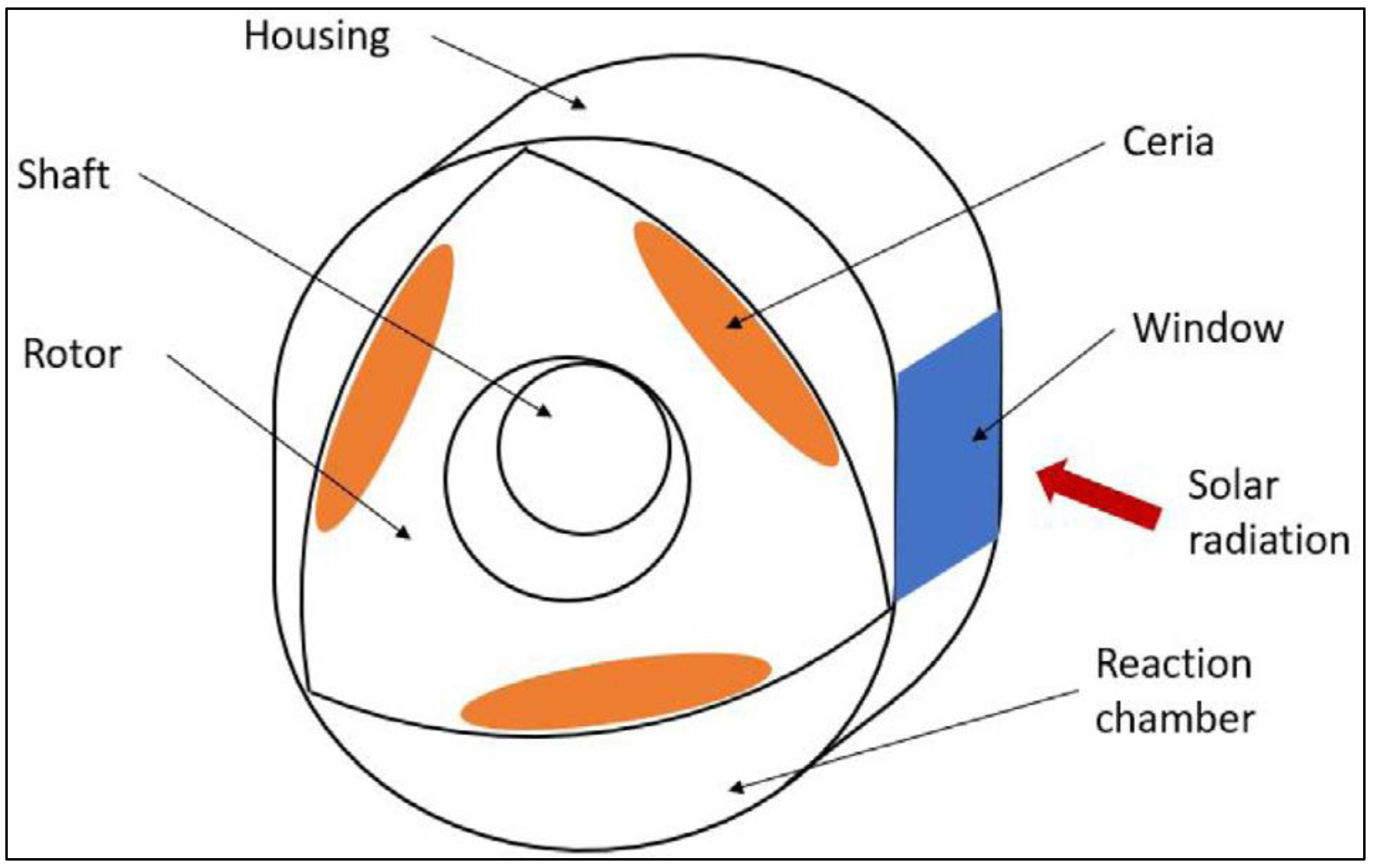
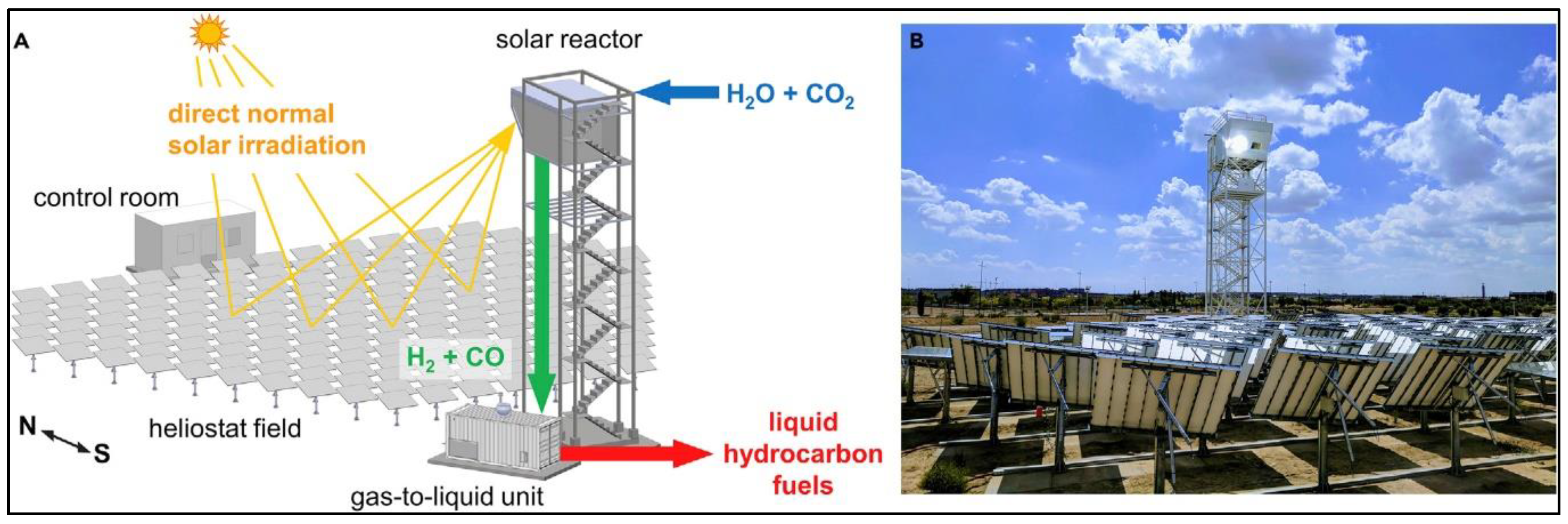
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhosale, R.R. Recent Developments in Ceria-Driven Solar Thermochemical Water and Carbon Dioxide Splitting Redox Cycle. Energies 2023, 16, 5949. https://doi.org/10.3390/en16165949
Bhosale RR. Recent Developments in Ceria-Driven Solar Thermochemical Water and Carbon Dioxide Splitting Redox Cycle. Energies. 2023; 16(16):5949. https://doi.org/10.3390/en16165949
Chicago/Turabian StyleBhosale, Rahul R. 2023. "Recent Developments in Ceria-Driven Solar Thermochemical Water and Carbon Dioxide Splitting Redox Cycle" Energies 16, no. 16: 5949. https://doi.org/10.3390/en16165949
APA StyleBhosale, R. R. (2023). Recent Developments in Ceria-Driven Solar Thermochemical Water and Carbon Dioxide Splitting Redox Cycle. Energies, 16(16), 5949. https://doi.org/10.3390/en16165949





