The Condensation Characteristics of Propane in Binary and Ternary Mixtures on a Vertical Plate
Abstract
:1. Introduction
2. Model and Methods
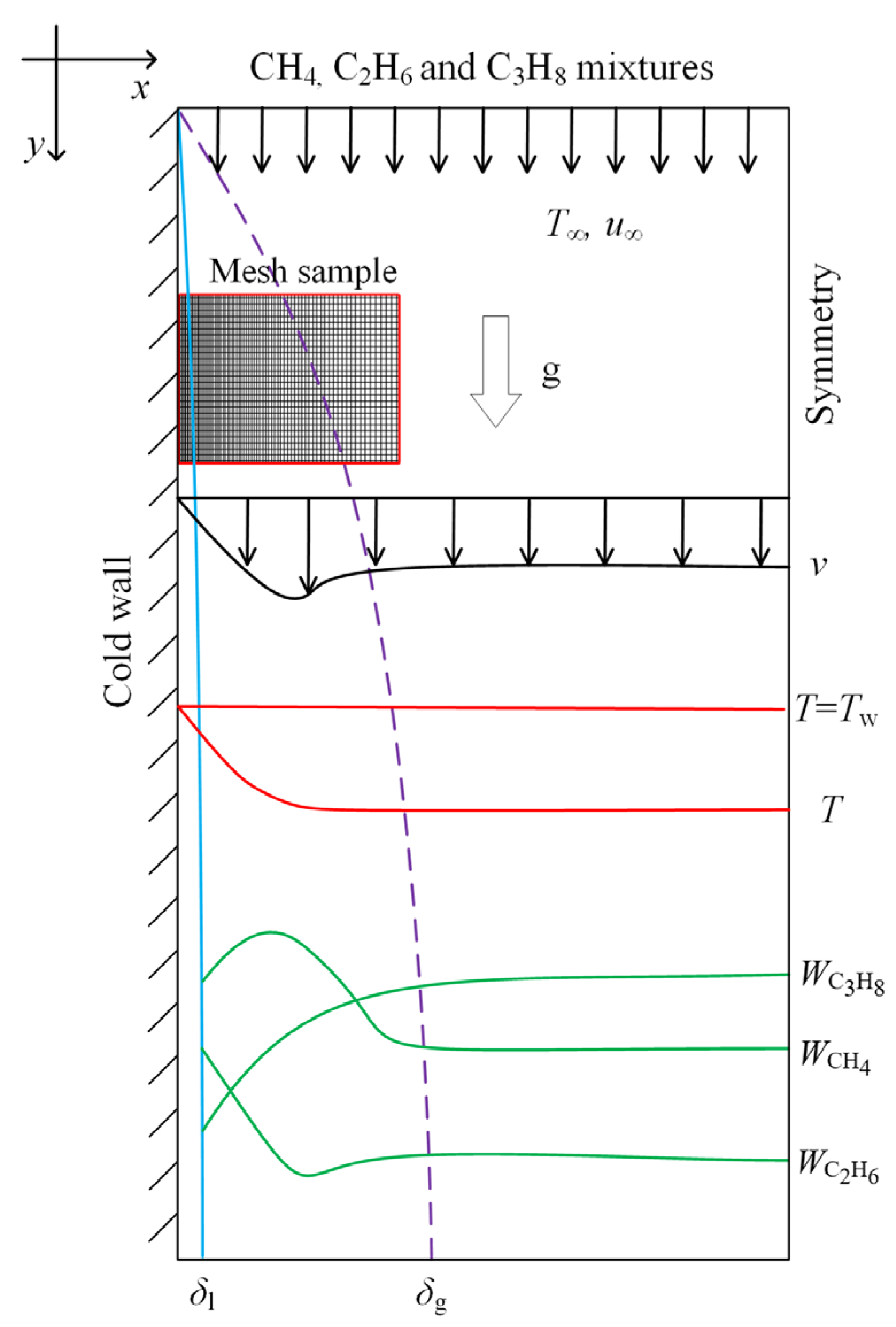
2.1. Physical Model
2.2. Governing Equations
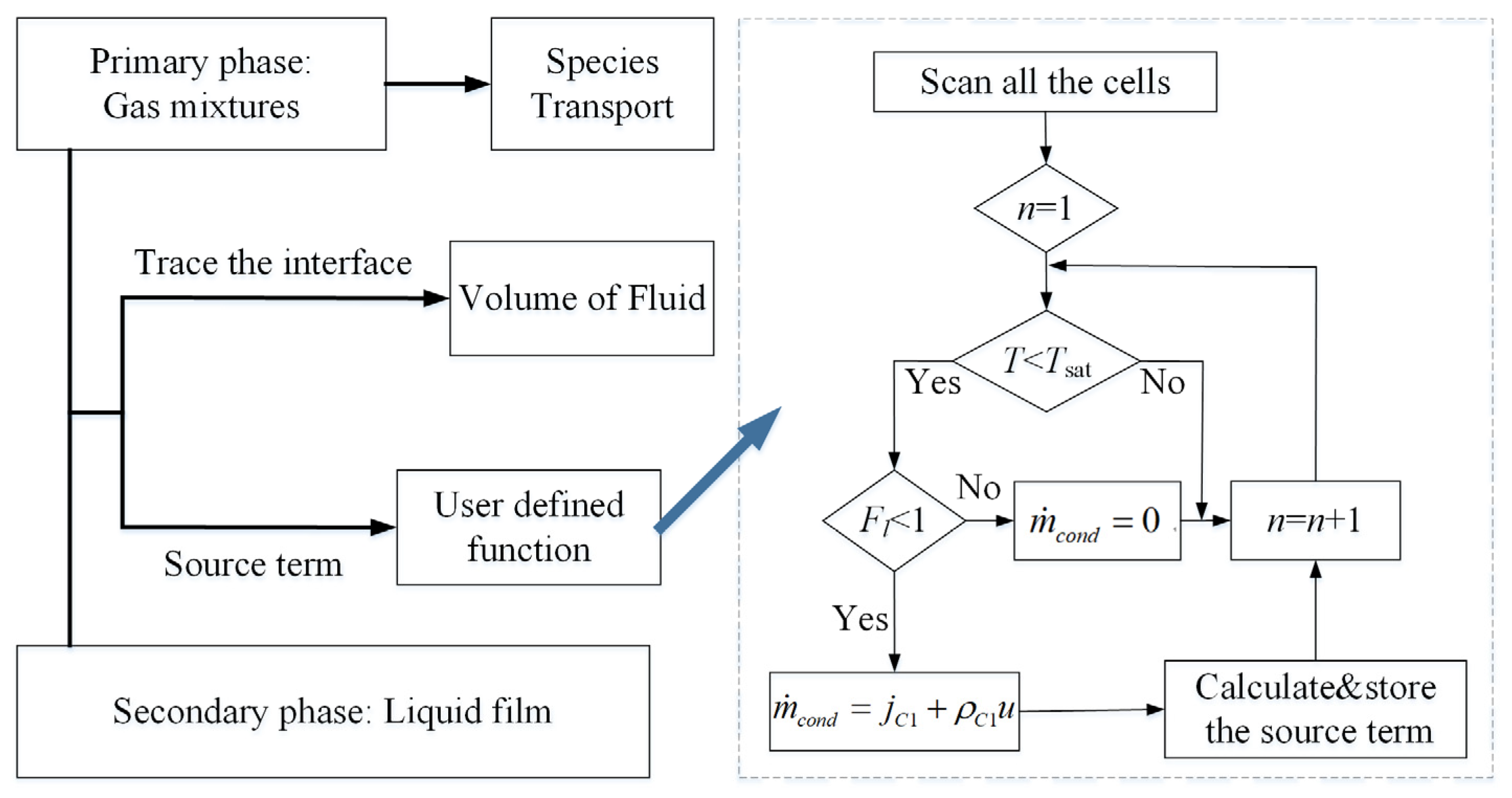
2.3. Phase Change and Mass Transfer Model
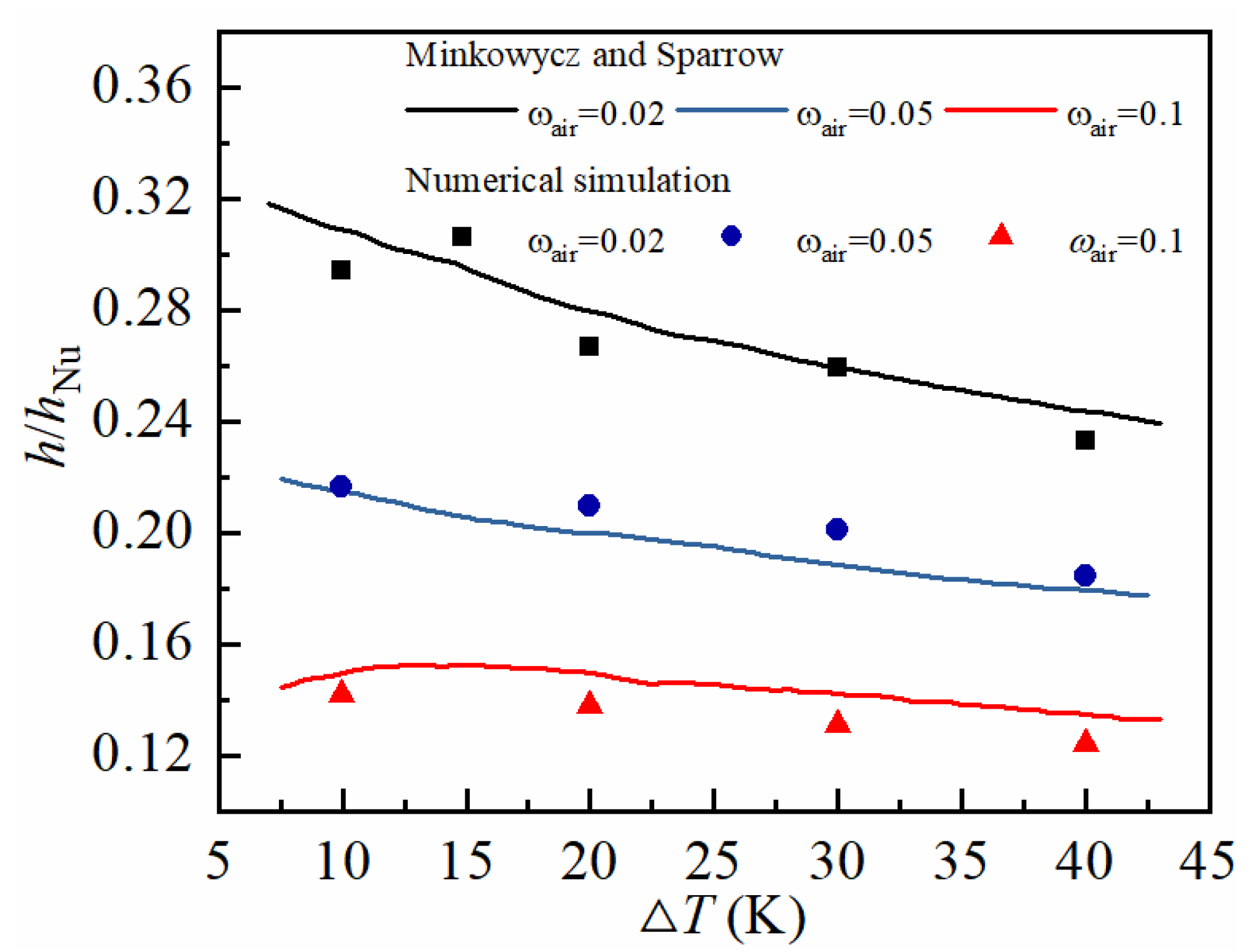
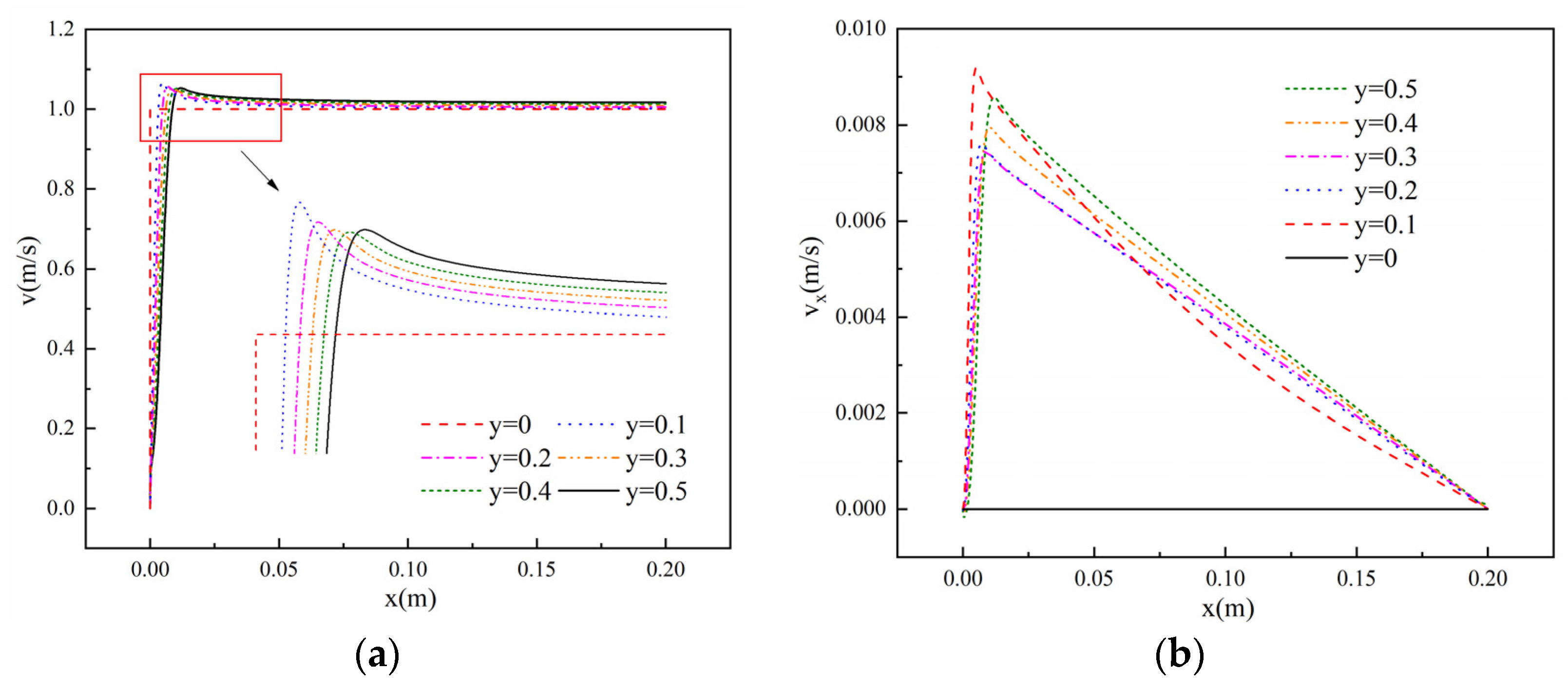
2.4. Numerical Setup and Model Validation
3. Results
3.1. Characteristics of the Filmwise Condensation
3.2. Distribution of the Non-Condensable Components
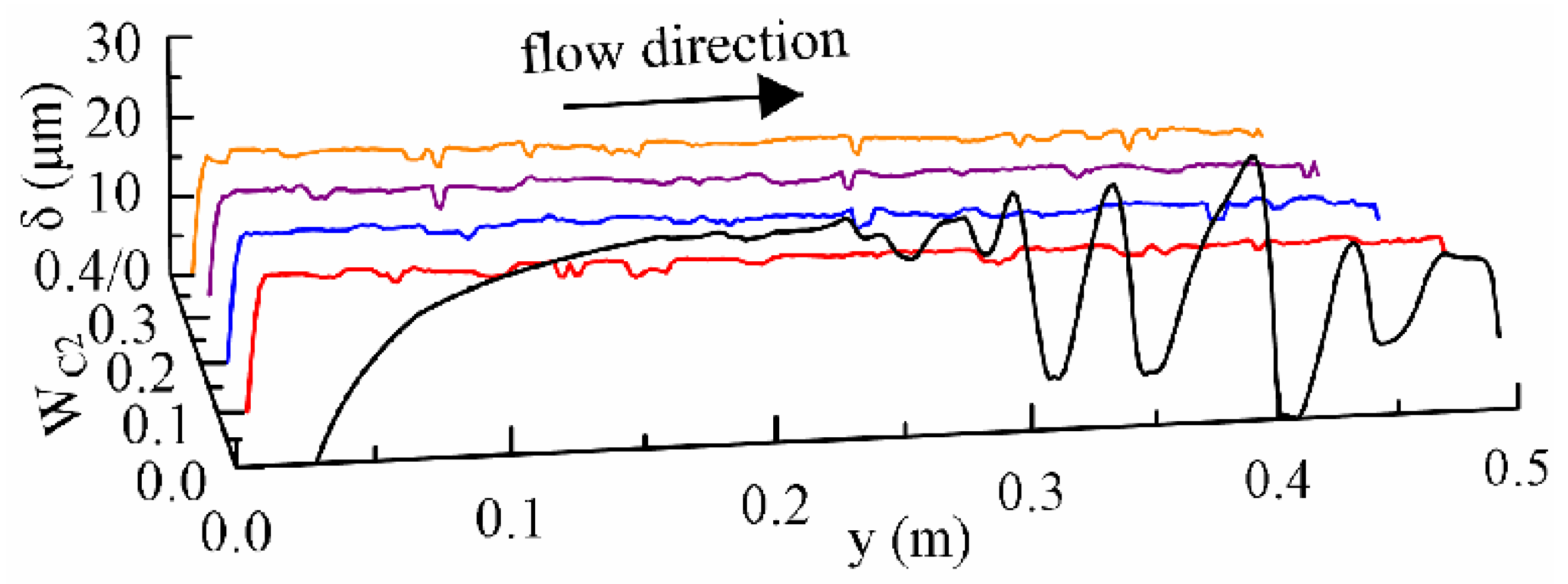
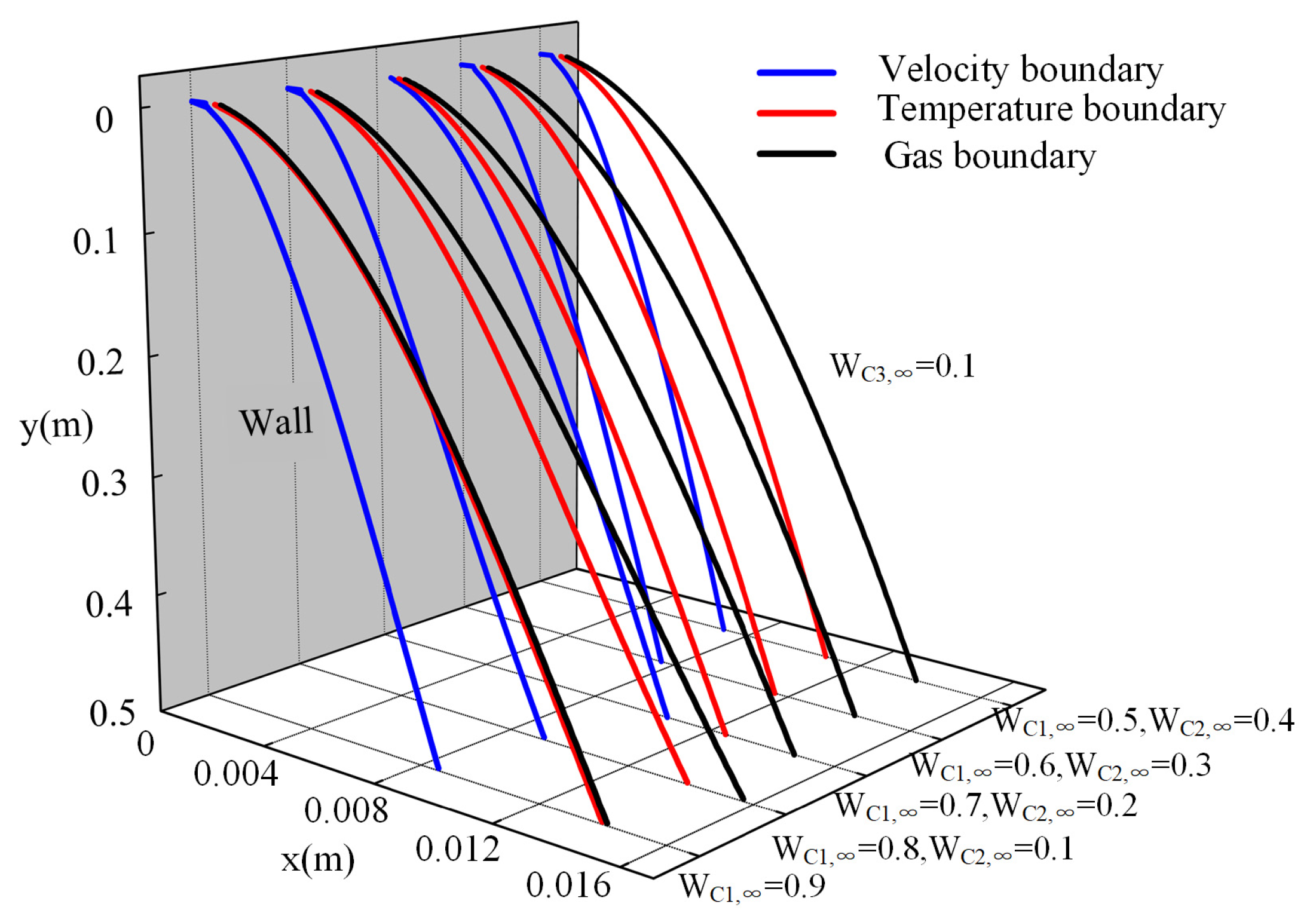
3.3. The Distribution of the Liquid Film and Boundary Layers
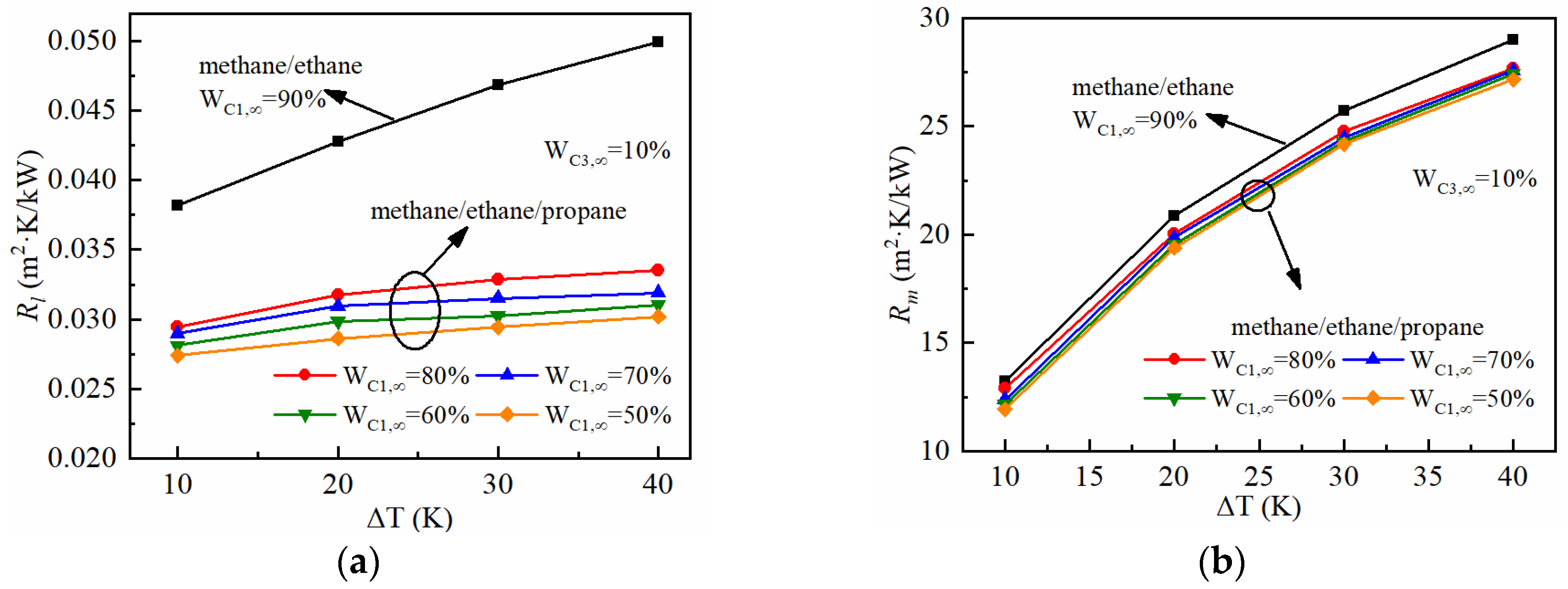
3.4. The Condensation Heat Transfer Characteristics
4. Conclusions
- (1)
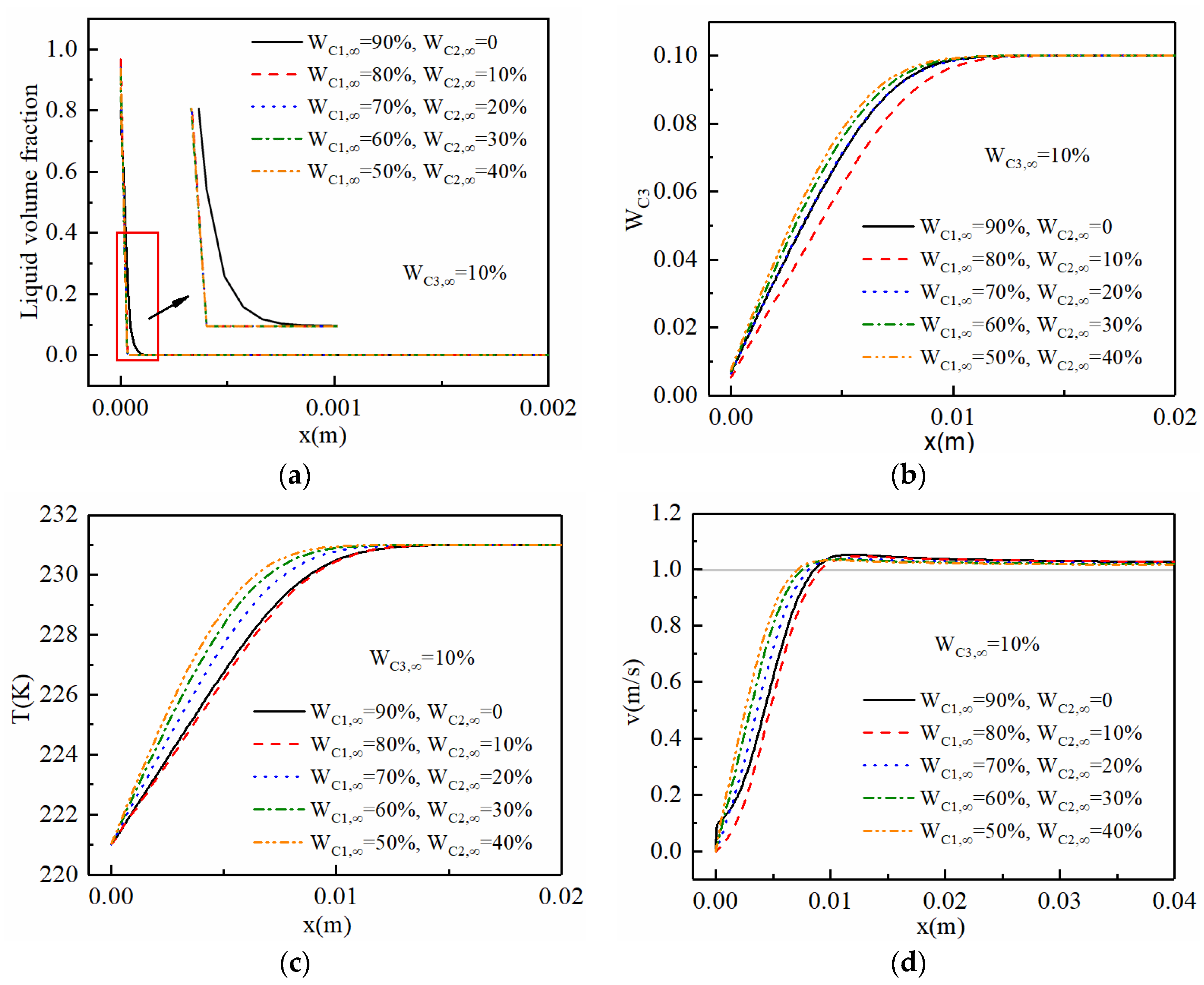
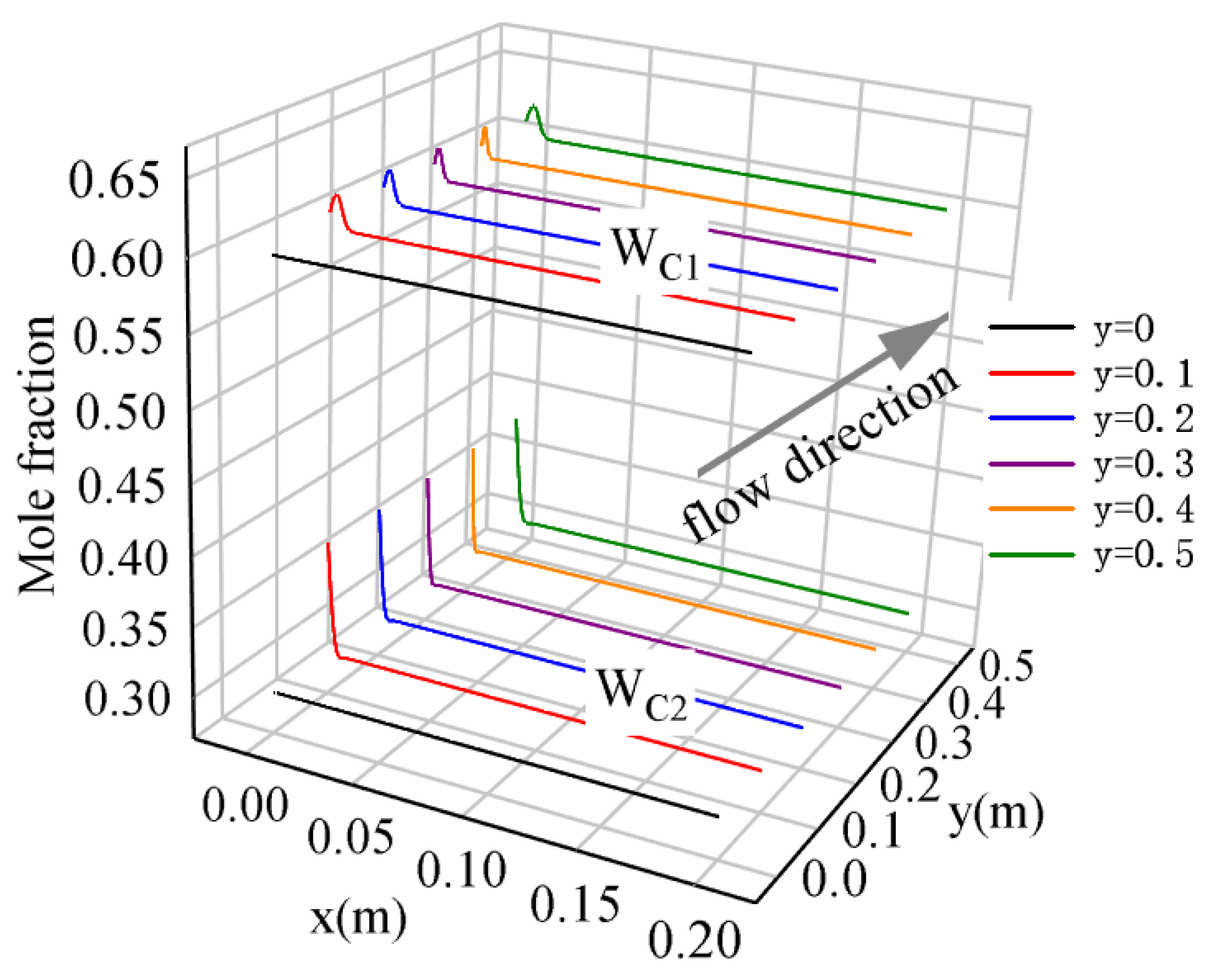
- Different from the monotonic distribution of the non-condensable gas concentration in a binary mixture, there were peak and valley values, respectively, in the profile of the methane and ethane concentration along the x-direction in the ternary mixture. The increase in the wall subcooled temperature promotes the accumulation of methane and ethane gas near the gas–liquid interface.
- (2)
- The addition of ethane to the binary mixture (methane/propane) separated the temperature boundary layer and gas boundary layer. The separation distance becomes larger with the increase in inlet molar fraction of ethane. The ethane gas is more likely to accumulate near the wall compared with the lighter methane. Meanwhile, the increase in ethane concentration lowers the accumulation of methane near the wall.
- (3)
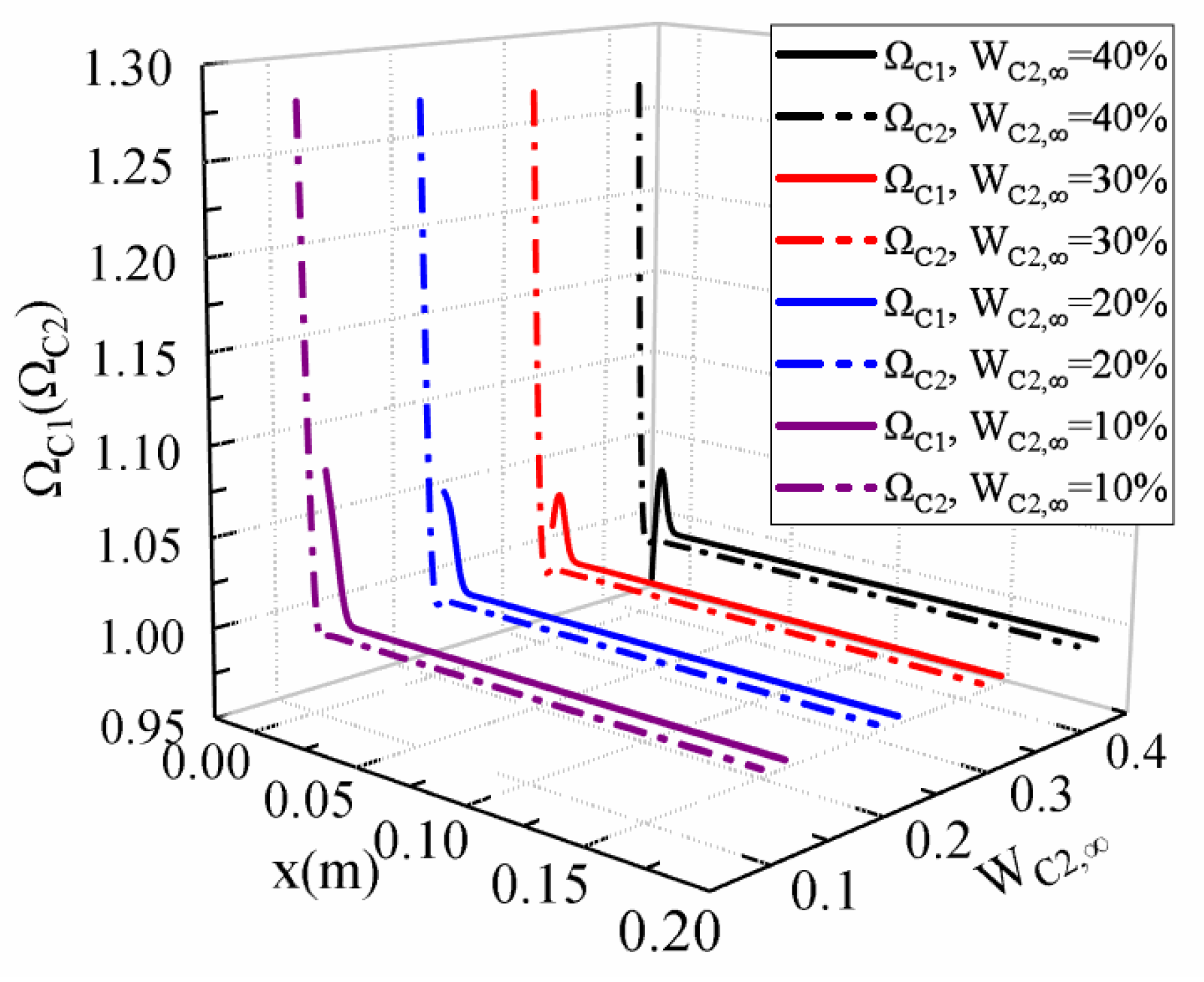
- In the condensation process of the propane vapor with a high non-condensable gas molar fraction of 90%, the thermal resistance of the gas boundary layer is one hundred times higher than that of the liquid film. The effect of the wall sub-cooled temperature is more significant on the gas boundary layer than that of the liquid film.
- (4)
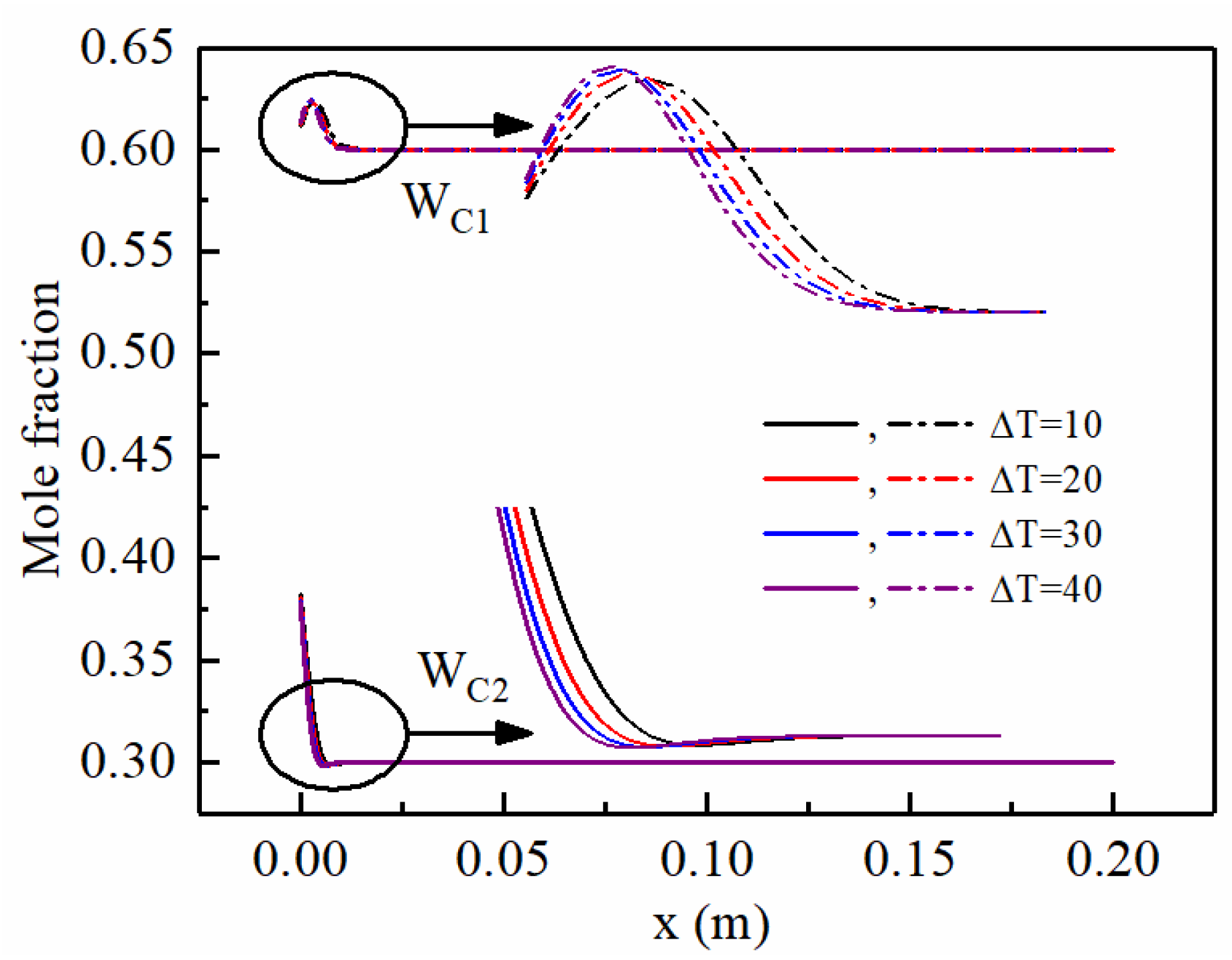
- The addition of ethane to the propane and methane mixture increases the heat transfer coefficient by about 11% (at ΔT = 10 K) and 7% (at ΔT = 40 K), as the molar fraction of ethane increases from 0 to 40%. Under the calculated conditions of WC2,∞ = 10%, the condensation heat transfer coefficient decreases by 53~56% as the wall sub-cooled temperature increases from 10 K to 40 K.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| A | Area (m2) |
| cp | Specific heat capacity (J/(kg⋅K)) |
| D | Diffusion coefficient (m2/s) |
| F | Volume fraction |
| g | Gravity acceleration (m/s2) |
| h | Heat transfer coefficient (W/(m2⋅K)) |
| hfg | Latent heat (J/kg) |
| j | Diffusion flux (kg/(m2⋅s)) |
| k | Thermal conductivity (W/(m⋅K)) |
| Mass flux (kg/(m2⋅s)) | |
| M | Molecular mass (kg/mol) |
| u | Velocity (m/s) |
| w | Mass fraction |
| W | Mole fraction |
| p | Pressure (Pa) |
| q | Heat flux (W/m2) |
| R | Heat transfer resistance (m2⋅K/W) |
| S | Source term (kg/(m⋅s) or W/m) |
| T | Temperature (K) |
| x | Horizontal coordinate axis (m) |
| y | Longitudinal coordinate axis (m) |
| Greek symbols | |
| δ | Liquid film thickness (m) |
| ρ | Density (kg/m3) |
| σ | Surface tension coefficient (N/m) |
| μ | Dynamic viscosity (Pa⋅s) |
| ν | Kinematic viscosity (m2/s) |
| Subscripts | |
| l | Liquid |
| m | Gas mixture |
| i | Gas–liquid interface |
| ∞ | Inlet (bulk flow) |
| C1 | Methane |
| C2 | Ethane |
| C3 | Propane |
| cond | Condensation |
| conv | Convection |
| Acronyms | |
| VOF | Volume of Fluid method |
| LNG | Liquefied natural gas |
| PISO | Pressure-Implicit with Splitting of Operators |
| PRESTO | Pressure Staggering Option |
References
- Zhang, L.; Zhang, G.; Mao, W.; Zhang, Y.; Zhang, J. Experimental and numerical study on filmwise condensation of pure propane and propane/methane mixture. Int. J. Heat Mass Transf. 2020, 156, 119744. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, G.; Zhang, Y.; Tian, M.; Zhang, J. A 2D numerical study on the condensation characteristics of three non-azeotropic binary hydrocarbon vapor mixtures on a vertical plate. Chin. J. Chem. Eng. 2020, 28, 2746–2757. [Google Scholar] [CrossRef]
- Maxwell, J.C. On the Dynamical Theory of Gases. In Kinetic Theory; Brush, S.G., Ed.; Pergamon: Oxford, UK, 1867; Volume 157, pp. 49–88. [Google Scholar]
- Toor, H. Diffusion in three-component gas mixtures. AIChE J. 1957, 3, 198–207. [Google Scholar] [CrossRef]
- Krishna, R.; Wesselingh, J.A. The Maxwell-Stefan approach to mass transfer. Chem. Eng. Sci. 1997, 52, 861–911. [Google Scholar] [CrossRef]
- Jackson, R. Diffusion in Ternary Mixtures with and without Phase Boundaries. Ind. Eng. Chem. Fundam. 1977, 16, 304–306. [Google Scholar] [CrossRef]
- Broeke, L.J.P.V.D.; Nijhuis, S.A.; Krishna, R. Monte Carlo Simulations of Diffusion in Zeolites and Comparison with the Generalized Maxwell-Stefan Theory. J. Catal. 1992, 136, 463–477. [Google Scholar]
- Kerkhof, P.J.A.M. A modified Maxwell-Stefan model for transport through inert membranes: The binary friction model. Chem. Eng. J. Biochem. Eng. J. 1996, 64, 319–343. [Google Scholar] [CrossRef] [Green Version]
- Minkowycz, W.J.; Sparrow, E.M. Condensation heat transfer in the presence of noncondensables, interfacial resistance, superheating, variable properties, and diffusion. Int. J. Heat Mass Transf. 1966, 9, 1125–1144. [Google Scholar] [CrossRef]
- Dehbi, A.A. The Effects of Noncondensable Gases on Steam Condensation under Turbulent Natural Convection Conditions. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 1991. [Google Scholar]
- Colburn, A.P.; Hougen, O.A. Design of Cooler Condensers for Mixtures of Vapors with Noncondensing Gases. Ind. Eng. Chem. 1934, 26, 1178–1182. [Google Scholar] [CrossRef]
- Maheshwari, N.K.; Saha, D.; Sinha, R.K.; Aritomi, M. Investigation on condensation in presence of a noncondensable gas for a wide range of Reynolds number. Nucl. Eng. Des. 2004, 227, 219–238. [Google Scholar] [CrossRef]
- Taitel, Y.; Tamir, A. Film condensation of multicomponent mixtures. Int. J. Multiph. Flow 1974, 1, 697–714. [Google Scholar] [CrossRef]
- Nusselt, W. The surface condensation of water vapour. Z. Des Vereines Dtsch. Ingenieure 1916, 60, 541–546. [Google Scholar]
- Sage, F.E.; Estrin, J. Film condensation from a ternary mixture of vapors upon a vertical surface. Int. J. Heat Mass Transf. 1976, 19, 323–333. [Google Scholar] [CrossRef]
- Peterson, P.F. Diffusion Layer Modeling for Condensation with Multicomponent Noncondensable Gases. J. Heat Transf. 2000, 122, 716. [Google Scholar] [CrossRef]
- Ganguli, A.; Patel, A.G.; Maheshwari, N.K.; Pandit, A.B. Theoretical modeling of condensation of steam outside different vertical geometries (tube, flat plates) in the presence of noncondensable gases like air and helium. Nucl. Eng. Des. 2008, 238, 2328–2340. [Google Scholar] [CrossRef]
- Karkoszka, K.; Anglart, H. Multidimensional Effects in Laminar Filmwise Condensation of Water Vapour in Binary and Ternary Mixtures with Noncondensable Gases. Nucl. Eng. Des. 2008, 238, 1373–1381. [Google Scholar] [CrossRef]
- Karkoszka, K.; Anglart, H. Laminar filmwise condensation of vapor in presence of multi-component mixture of non-condensable gases. In Proceedings of the 12th International Topical Meeting on Nuclear Reactor Thermal Hydraulics, Pittsburgh, PA, USA, 30 September–4 October 2007. [Google Scholar]
- Liu, H.; Todreas, N.E.; Driscoll, M.J. An experimental investigation of a passive cooling unit for nuclear plant containment. Nucl. Eng. Des. 2000, 199, 243–255. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Sun, Z.; Ding, M.; Fan, G. Analysis of experiments for the effect of noncondensable gases on steam condensation over a vertical tube external surface under low wall subcooling. Nucl. Eng. Des. 2014, 278, 644–650. [Google Scholar] [CrossRef]
- Xu, H.Q.; Gu, H.F.; Sun, Z.N. Forced convection condensation of steam in the presence of multicomponent noncondensable gases inside a horizontal tube. Int. J. Heat Mass Transf. 2017, 104, 1110–1119. [Google Scholar] [CrossRef]
- Park, I.W.; Yang, S.H.; Lee, Y.-G. Effect of light gas on condensation heat transfer of steam–air mixture and gas stratification. Int. J. Heat Mass Transf. 2021, 179, 121716. [Google Scholar] [CrossRef]
- Park, I.W.; Yang, S.H.; Lee, Y.-G. Degradation of condensation heat transfer on a vertical cylinder by a light noncondensable gas mixed with air-steam mixtures. Int. Commun. Heat Mass Transf. 2022, 130, 105779. [Google Scholar] [CrossRef]
- Duncan, J.B.; Toor, H.L. An experimental study of three component gas diffusion. AIChE J. 1962, 8, 38–41. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, G.; Tian, M.; Zhang, J.; Zhang, Y. Modeling of laminar filmwise condensation of methane with nitrogen on an isothermal vertical plate. Int. Commun. Heat Mass Transf. 2019, 105, 10–18. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Cui, Y.; Mao, W.; Sheng, X.; Zhang, G. The Condensation Characteristics of Propane in Binary and Ternary Mixtures on a Vertical Plate. Energies 2023, 16, 5873. https://doi.org/10.3390/en16165873
Zhang L, Cui Y, Mao W, Sheng X, Zhang G. The Condensation Characteristics of Propane in Binary and Ternary Mixtures on a Vertical Plate. Energies. 2023; 16(16):5873. https://doi.org/10.3390/en16165873
Chicago/Turabian StyleZhang, Lili, Yongzhang Cui, Wenlong Mao, Xiangzhuo Sheng, and Guanmin Zhang. 2023. "The Condensation Characteristics of Propane in Binary and Ternary Mixtures on a Vertical Plate" Energies 16, no. 16: 5873. https://doi.org/10.3390/en16165873
APA StyleZhang, L., Cui, Y., Mao, W., Sheng, X., & Zhang, G. (2023). The Condensation Characteristics of Propane in Binary and Ternary Mixtures on a Vertical Plate. Energies, 16(16), 5873. https://doi.org/10.3390/en16165873




