Abstract
The quest for sustainable and environmentally friendly fuel feedstocks has led to the exploration of green solvents for the extraction of bio-oil from various biomass sources. This review paper provides a comprehensive analysis of the challenges and future research outlooks for different categories of green extraction solvents, including bio-based solvents, water-based solvents, supercritical fluids, and deep eutectic solvents (DES). The background of each solvent category is discussed, highlighting their potential advantages and limitations. Challenges such as biomass feedstock sourcing, cost fluctuations, solvent properties variability, limited compatibility, solute solubility, high costs, and potential toxicity are identified and examined in detail. To overcome these challenges, future research should focus on alternative and abundant feedstock sources, the development of improved solubility and separation techniques, optimization of process parameters, cost-effective equipment design, standardization of DES compositions, and comprehensive toxicological studies. By addressing these challenges and advancing research in these areas, the potential of green extraction solvents can be further enhanced, promoting their widespread adoption and contributing to more sustainable and environmentally friendly industrial processes.
1. Introduction
Recognizing the urgent need to move away from fossil fuels and reduce greenhouse gas emissions, societies around the world have been steadily increasing their demand for renewable energy sources in recent years [1]. Climate change, energy security, and the depletion of conventional energy sources are all contributing factors to this increase in demand [2,3]. Consequently, there has been a lot of focus on investigating renewable energy sources such as biomass-based biofuels.
The growing demand for sustainable energy sources has spurred interest in the production of bio-oil from biomass as a renewable alternative to fossil fuels [4]. Bio-crude extraction involves the conversion of biomass feedstocks into valuable liquid fuels through various thermochemical and biochemical processes [5,6,7]. The versatility of bio-crude extraction allows the use of various biomass sources such as agricultural residues, energy crops, and forestry waste, providing a sustainable alternative to fossil fuels. The significance of bio-crude lies in its potential to reduce reliance on finite fossil fuel resources and mitigate climate change by reducing carbon dioxide emissions [7,8]. As a renewable energy source, bio-crude offers a pathway towards achieving energy security, diversification, and a transition to a low-carbon economy. Furthermore, bio-crude production can contribute to waste management by utilizing agricultural and forestry residues that would otherwise go to waste [9,10,11,12,13,14].
In recent years, there has been a significant focus on developing efficient and environmentally friendly methods for bio-crude extraction, with particular attention given to the selection of solvents used in the extraction process [15,16,17]. Conventional methods for bio-crude extraction typically involve the use of organic solvents, such as, hexane [14], acetone [18], dichloromethane (DCM) [19], methanol [20], and toluene [21]. While these solvents have been widely employed due to their effectiveness in extracting bio-oil, they come with certain limitations that hinder their sustainability and environmental compatibility [22].
Firstly, conventional organic solvents are often derived from fossil fuels, contributing to carbon emissions and the depletion of finite resources. Additionally, the production and use of these solvents can have adverse environmental impacts, including air pollution, water contamination, and potential health risks [23,24]. For example, DCM, a volatile organic compound (VOC), contributes to air pollution, ozone depletion, and environmental contamination. Methanol can contaminate water bodies and harm aquatic life. Improper disposal or accidental release of hexane and toluene can lead to soil and water pollution [19,20,21,23,24]. Furthermore, the disposal of spent solvents presents challenges in terms of waste management and potential ecological harm. Therefore, there is an urgent need to develop and adopt sustainable solvents that minimize environmental impacts and promote the ecological viability of bio-crude extraction.
Green solvents, such as bio-based (derived from renewable sources), water-based (dissolved in water), supercritical fluids (above their critical point), and deep eutectic solvents (formed by mixing two or more components), offer alternatives to conventional organic solvents for bio-oil extraction. These solvents are characterized by being non-toxic, non-volatile, recyclable, and biodegradable. Ideally, they are made from easily accessible materials, and their synthesis is cost-effective [25,26]. They can enhance the efficiency and sustainability of the extraction process, contributing to the overall environmental profile of bio-crude production. Their use aligns with the growing demand for renewable and eco-friendly solutions in the energy sector, making them essential players in the transition towards a more sustainable future [27]. Green solvents offer a promising approach to enhance the sustainability of bio-crude extraction by minimizing the environmental impact of solvents. Additionally, green solvents can reduce carbon emissions and the overall ecological footprint associated with the extraction process [25,26,27]. However, not all green solvents are without limitations. Challenges include cost: The production and implementation of green solvents on a large scale can be economically challenging. Many bio-based and environmentally friendly solvents may have higher production costs compared to conventional organic solvents, which can affect their widespread adoption in industrial applications, and compatibility issues with green solvents can vary depending on the type of solvent used. For example, bio-based green solvents derived from renewable biomass sources, may have different interactions with various biomasses during extraction processes. Similarly, water-based green solvents, or DES, may have unique interactions with biomasses, affecting their effectiveness in extracting bio-oil components. Some green solvents may have limitations in terms of their solubility or extraction efficiency for certain biomass feedstocks, which need to be addressed for their widespread implementation in the bio-crude production industry [26,28].
Therefore, the main objective of this review is to compare the advancements from conventional solvents to green solvents for bio-oil extraction and evaluate their potential. It will provide a detailed analysis of the challenges, opportunities, and future research directions in the development and usage of green solvents, including bio-based solvents, water-based solvents, supercritical fluids, and DES for bio-oil extraction. The paper aims to highlight the advantages and limitations of green solvents, discuss their environmental and health impacts, and propose strategies to optimize their performance, scalability, and overall sustainability. Through this thorough comparison with conventional solvents commonly used in bio-oil extraction processes, the review will underscore the advancements achieved by utilizing green solvents, emphasizing their potential in enhancing the bio-oil extraction process while minimizing environmental impact.
2. Traditional Solvents vs. Green Solvents
Oil is typically extracted using one of three traditional methods: hydraulic pressing, expeller pressing, or solvent extraction (SE). SE is one such technique that has seen extensive use due to its low cost and numerous practical applications.
2.1. Solvent Extraction with Traditional Organic Solvents
In SE, traditional solvents commonly used in bio-crude extraction processes include petroleum-based solvents such as hexane, toluene, and chloroform. These solvents have been traditionally favored due to their ability to efficiently extract bio-crude from biomass [29]. Organic solvent efficiency is determined by molecular structure-based selectivity for desired compounds and chemicals [30]. The polarity and classification of the solvent used have a significant impact on the extracted or fractionated chemicals from bio-oil. It functions on the principle of the difference in solubility between two mutually incompatible liquids or phases. Organic solvent extraction relies on the solubility of organic compounds in the solvent, which allows for their dispersion and subsequent separation into a denser organic fraction via settling. Figure 1 is a diagrammatic representation of the SE.
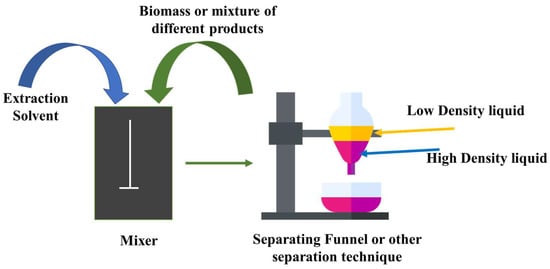
Figure 1.
Schematic diagram of the SE.
After that, the organic solvent will evaporate, leaving behind the extracted materials. Physical and chemical solvents have been studied and reported for use in the extraction and fractionation of bio-oil through solubility properties [31].
Table 1 provides a summary of recent research on organic solvents used in bio-oil fractionation and extraction. These research works looked at different solvents and how they could be optimized for extraction to establish the base knowledge and viability of organic solvent extraction.

Table 1.
Bio-oil extraction using different conventional organic solvents.
2.2. Environmental and Health Concerns Associated with Traditional Solvents
Traditional organic solvents, commonly used in various industries such as manufacturing, painting, cleaning, and pharmaceuticals, have been associated with several environmental and health concerns. These solvents, often based on petroleum-derived compounds, pose risks to both ecosystems and human well-being. Solvents, also known as volatile organic compounds (VOCs), are also found in a wide variety of building materials, including paints, paint strippers, thinners, and glues [53,54].
The primary reasons why these compounds are classified as VOCs are because of their low boiling point and high volatility. Furthermore, the United States Environmental Protection Agency (EPA) and the World Health Organization (WHO) classify VOCs into three distinct groups based on their boiling points. The terms “very volatile organic compounds VVOCs” (or “wicked volatile organic compounds”), “volatile organic compounds”, and “semi-volatile organic compounds (SVOCs)” are used to describe these substances [55]. However, the US EPA does not make a distinction between VOCs and VVOCs, and instead uses a wide range of definitions, including:
“VOC means any compound of carbon, excluding carbon monoxide, carbon dioxide, carbonic acid, metallic carbides or carbonates, and ammonium carbonate, which participates in atmospheric photochemical reactions, except those designated by EPA as having negligible photochemical reactivity”.
“VOCs, are organic chemical compounds whose composition makes it possible for them to evaporate under normal indoor atmospheric conditions of temperature and pressure”[56].
Certain solids and liquids release VOCs as gases into the environment. Some of the chemicals that make up VOCs have been linked to both short- and long-term health problems. The concentrations of many VOCs are reliably higher (up to ten times higher) inside than they are outside [57]. However, SVOCs are slowly released from their sources and then segregate into the gas phase, airborne particle phase, and dust phase. The most common persistent organic compounds (SVOCs) found indoors are phthalates and flame retardants (FRs). Asthma and other allergy-related health problems have been linked to phthalate exposure, with consistent evidence coming from both epidemiological and experimental studies [58].
Table 2 displays the full catalog of organic extraction solvents, categorizing them according to their potential hazards to human and environmental health.

Table 2.
Classification and Environmental and Human health Hazards associated with traditional organic solvents.
The abovementioned environmental and health concerns associated with traditional organic solvents, such as petroleum-based solvents, emphasize the urgent need for green solvents in various applications [106]. Conventional solvents have been linked to air pollution, water contamination, soil degradation, and potential health hazards, posing risks to both the environment and human well-being [107]. In response to these challenges, the adoption of green solvents has gained prominence. Green solvents are designed to be more environmentally friendly, sustainable, and less harmful to human health. Green solvents encompass a range of compounds, some of which are derived from renewable resources, while others are synthesized using sustainable methods. They generally have lower toxicity profiles and provide a safer alternative to conventional solvents [108]. By transitioning to green solvents, industries can reduce their ecological footprint, improve worker safety, comply with stringent regulations, and contribute to a more sustainable future. Additionally, the development of green solvents drives innovation and research in solvent chemistry, leading to the discovery of new solvents with improved performance and efficiency in applications such as bio-crude extraction [106]. The need for green solvents is thus evident, as they offer a path towards a more sustainable and responsible approach to solvent usage, mitigating the environmental and health concerns associated with traditional organic solvents.
2.3. Introduction to Green Solvents and Their Advantages
Green solvents, also known as environmentally friendly or sustainable solvents, have emerged as a viable alternative to traditional organic solvents due to their reduced environmental impact and improved safety profiles. Green solvents are designed to minimize or eliminate the use of hazardous and toxic substances, making them more sustainable and less harmful to human health and the environment. They offer a range of advantages, including lower toxicity, lower volatility, and higher biodegradability compared to conventional solvents [109].
One of the key features of green solvents is their reduced environmental impact. These solvents are typically derived from renewable resources, such as biomass, agricultural waste, or bio-based feedstocks, which helps reduce the dependence on fossil fuels and promote a more sustainable approach to solvent usage [106,109]. Green solvents also exhibit lower vapor pressures, which reduces their emission into the atmosphere, thereby minimizing air pollution and the formation of ground-level ozone [109].
In addition to their environmental advantages, green solvents prioritize the safety and well-being of workers and end-users. Many traditional solvents are associated with health risks, including respiratory irritation, skin sensitization, and potential carcinogenicity. Green solvents, on the other hand, are designed to have lower toxicity profiles, minimizing the potential harm to human health [106,109]. Furthermore, green solvents contribute to the overall sustainability and efficiency of industrial processes. They can enhance process efficiency by reducing the need for energy-intensive operations, such as distillation or solvent recovery, due to their lower boiling points and increased recyclability [109]. These solvents also offer opportunities for waste reduction and resource conservation, as they can be easily recovered and recycled, reducing waste generation and promoting a circular economy [106].
The development and utilization of green solvents have gained significant attention in various industries, including pharmaceuticals, chemicals, and biofuels. Regulatory bodies and industry initiatives are driving the adoption of green solvents to meet sustainability goals and reduce the environmental footprint of chemical processes [109].
3. Classification of Green Solvents
Green solvents can be classified into different categories based on their properties and origins. One common classification scheme divides green solvents into four main categories: bio-based solvents, water-based solvents, supercritical fluids, and deep eutectic solvents [110], as shown in Figure 2.
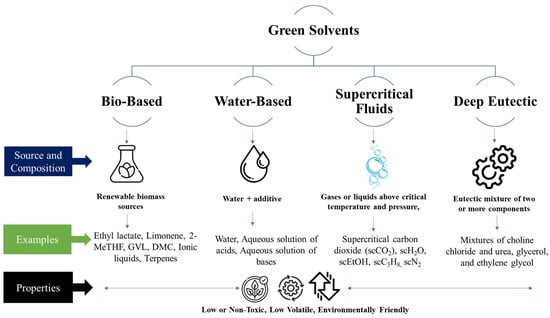
Figure 2.
The scheme of green solvent classification.
Bio-based Solvents:
- Composition: Bio-based solvents are derived from renewable biomass sources, such as plants, algae, or microorganisms.
- Properties: Bio-based solvents are typically non-toxic and biodegradable, and have low VOC content. They offer favorable health and environmental profiles compared to petroleum-based solvents.
- Applications: Bio-based solvents find applications in various industries, including extraction processes, cleaning agents, coatings, and personal care products [110,111,112,113,114].
Water-based Solvents:
- Composition: Water is the primary component of water-based solvents, often supplemented with small amounts of other solvents or additives.
- Properties: Water-based solvents are non-toxic, non-flammable, and have low VOC emissions. They are readily available, inexpensive, and have high heat capacity.
- Applications: Water-based solvents are extensively used in industries such as paints and coatings, adhesives, cleaning products, and textile manufacturing [111,112,113,115].
Supercritical Fluids:
- Composition: Supercritical fluids are typically gases or liquids that are above their critical temperature and pressure, resulting in a distinct supercritical state.
- Properties: Supercritical fluids are a hybrid between a gas and a liquid. Their solvating power is adjustable, they have low viscosity, and high diffusivity. The most popular choice for supercritical fluids is supercritical carbon dioxide (CO2).
- Applications: Supercritical fluids are widely employed in extraction processes, such as herbal extraction, decaffeination, and essential oil extraction. They are also utilized in various fields, including pharmaceuticals, food processing, and materials science [110,111,112,113,116].
Deep Eutectic Solvents (DES):
- Composition: DES are liquid solvents composed of a eutectic mixture of two or more components, typically a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA) [117]. These components can include both bio-based and non-bio-based compounds.
- Properties: They often have low toxicity, low volatility, and high thermal stability. DES can be tailored to possess specific characteristics, such as tunable polarity, viscosity, and solubility, by selecting different combinations of HBD and HBA components [118].
- Applications: DES find applications across various industries and processes. They have been used as green solvents in extraction processes for natural products, such as extraction of bioactive compounds from plant materials. DES have also shown promise in catalysis and electrochemistry, and as reaction media for organic synthesis. In addition, DES have been explored for their potential in industrial applications such as metal processing, biomass conversion, and separation processes [119].
Listed below is an in-depth description of each class of environmentally friendly solvents.
3.1. Bio-Based Solvents
The term “bio-based extraction solvents” refers to solvents that are produced from renewable biomass sources. These solvents are utilized in extraction processes to separate desired compounds or components from various materials. Bio-based extraction solvents offer several advantages over conventional solvents, including lower environmental impact, reduced toxicity, and potential biodegradability [112,113]. There are several bio-based extraction solvents, such as ethyl lactate [120], limonene [121], 2-methyltetrahydrofuran (2-MeTHF) [122], γ-valerolactone (GVL) [123], dimethyl carbonate (DMC) [124], Ionic liquids (ILs) [125], terpenes (e.g., pinene, terpinene, etc.) [126] which are reported in literature. The detailed explanation about these solvents is discussed below.
3.1.1. Ethyl Lactate
Ethyl lactate is primarily composed of two isomers: L-lactic acid and D-lactic acid, which are produced through the fermentation of renewable resources. The esterification of lactic acid with ethanol yields ethyl lactate. The schematic diagram of the ethyl lactate production is shown in Figure 3.
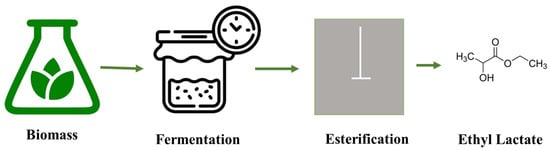
Figure 3.
The production of ethyl lactate process from renewable sources.
This solvent exhibits a unique combination of desirable properties, making it suitable for various extraction processes. Ethyl lactate possesses excellent solvating power and can dissolve a wide range of organic compounds, including natural products, essential oils, and pharmaceutical compounds. It is commonly used as a solvent in the extraction of natural products, such as plant-derived bioactive compounds, due to its ability to selectively extract target compounds while leaving behind unwanted impurities. One of the advantages of ethyl lactate is its low toxicity compared to traditional solvents. It has a relatively low vapor pressure and does not contribute significantly to volatile organic compound (VOC) emissions. This makes it safer for workers and reduces potential harm to the environment. Additionally, ethyl lactate is biodegradable, further enhancing its sustainability profile [120].
3.1.2. Limonene
Limonene is obtained through the steam distillation or cold-press extraction of citrus peels. It is composed of two isomers: d-limonene and l-limonene. D-limonene is the more common and commercially available isomer. Due to its natural origin, limonene is considered a sustainable and renewable solvent option. Limonene exhibits excellent solvency power and can dissolve a wide range of organic compounds. It is particularly effective in extracting non-polar compounds, such as essential oils, terpenes, and hydrocarbons. This makes it a suitable solvent for various applications, including natural product extraction, flavor and fragrance production, and cleaning formulations. One of the notable advantages of limonene is its low toxicity and environmentally friendly characteristics. It has low volatility and low vapor pressure, reducing the risk of inhalation exposure. The United States Food and Drug Administration (FD) has classified limonene as a “Generally Recognized as Safe (GRAS)” substance, meaning it is safe for use in food and cosmetics. Moreover, limonene is readily biodegradable, which means it can break down naturally in the environment without causing long-term harm. This biodegradability contributes to its reduced environmental impact compared to petroleum-based solvents [121].
3.1.3. 2-Methyltetrahydrofuran (2-MeTHF)
2-MeTHF is typically produced through the hydrogenation of levulinic acid, as depicted in Figure 4. The production process involves several steps. First, biomass feedstock, such as lignocellulosic materials or waste streams, is subjected to pretreatment to break down the complex polymers into simpler sugars. These sugars are then fermented to produce levulinic acid through microbial or enzymatic processes. Once levulinic acid is obtained, it undergoes hydrogenation in the presence of a catalyst to convert it into 2-MeTHF.
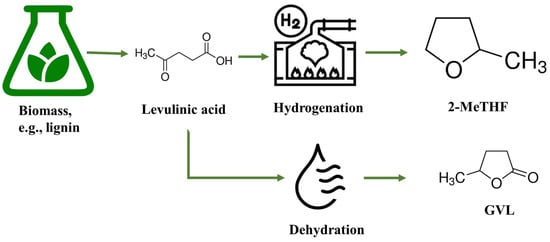
Figure 4.
The schematic diagram of 2-MeTHF and GVL production from biomass.
This synthesis route allows for the utilization of renewable feedstocks, reducing reliance on fossil resources. One of the key advantages of 2-MeTHF is its excellent solvency power for a wide range of organic compounds. It is useful in many extraction processes because it dissolves both polar and non-polar substances. It has been used in the extraction of bioactive compounds, natural products, and chemicals from biomass. Compared to its predecessor, THF, 2-MeTHF offers improved stability and lower flammability, making it a safer alternative. It has a higher boiling point, wider liquid range, and is less prone to form peroxides, enhancing its handling and storage characteristics. 2-MeTHF also exhibits lower toxicity compared to other commonly used solvents. It has a lower vapor pressure, reducing the risk of inhalation exposure. Studies have shown that 2-MeTHF is less toxic to aquatic organisms and has a lower impact on the environment compared to traditional solvents [122,127].
3.1.4. γ-Valerolactone (GVL)
GVL can be made by dehydrating levulinic acid derived from biomass in the presence of an acid catalyst (as shown in Figure 4). It can also be obtained as a co-product of the sugar platform, where glucose is converted into levulinic acid and GVL in the presence of acid catalysts. This synthesis route allows for the utilization of renewable resources and offers a sustainable alternative to petroleum-based solvents. One of the significant advantages of GVL is its versatile solvency power. It is effective at dissolving both polar and non-polar organic compounds. This makes it suitable for various extraction processes, such as the extraction of lignin, cellulose, and other valuable compounds from biomass. GVL exhibits a high boiling point, which allows for its efficient recovery and recycling during extraction processes. It has a lower vapor pressure compared to traditional solvents, reducing the risk of inhalation exposure and improving workplace safety. Furthermore, GVL has been recognized for its low toxicity compared to other commonly used solvents. It has a lower impact on human health and the environment, making it a more sustainable option. GVL is biodegradable, which means it can naturally break down in the environment, minimizing its long-term ecological impact [123,128].
3.1.5. Dimethyl Carbonate (DMC)
Dimethyl carbonate (DMC) is a bio-based extraction solvent that has gained attention as a green solvent alternative to traditional petroleum-based solvents. It is a clear, colorless liquid with a pleasant odor. DMC is produced through the reaction of methanol with carbon dioxide (CO2), making it a sustainable option as it utilizes CO2 as a feedstock and reduces reliance on fossil resources. DMC offers several advantages as a green solvent. It has a low toxicity profile and is considered safe for use in various applications. It has a low vapor pressure and low volatility, reducing the risk of inhalation exposure. DMC also exhibits good solvating power for a wide range of organic compounds and can dissolve both polar and non-polar substances effectively.
One of the significant advantages of DMC is its environmentally friendly nature. It is biodegradable, meaning it can naturally break down in the environment without causing long-term harm. Additionally, DMC has a low impact on air quality and does not contribute significantly to VOCs emissions. These properties make DMC a desirable option for industries seeking to reduce their environmental footprint. DMC finds application as a solvent in various fields, including pharmaceuticals, coatings, adhesives, and cleaning products. It is also used as a reactant in the synthesis of chemicals and as a fuel additive due to its high oxygen content. Its compatibility with existing infrastructure and processes further supports its potential as a green solvent alternative [124,129,130].
3.1.6. Ionic Liquids (ILs)
The special properties of ionic liquids (ILs) have made them a popular choice as a green solvent for bio-based extraction processes. ILs are composed of ions, typically consisting of organic cations and inorganic or organic anions. Their name, “ionic liquids”, comes from the fact that they are liquids at or near room temperature. Advantages of ILs include high solubility for a wide variety of compounds, low volatility, and the ability to modify the IL’s properties. They have low vapor pressure, reducing the risk of inhalation exposure and making them safer to handle. Additionally, ILs have high thermal stability, allowing them to be used at elevated temperatures without significant degradation.
One of the notable advantages of ILs is their tunable nature. The combination of different cations and anions provides the ability to tailor the physical and chemical properties of ILs to suit specific applications. This includes adjusting their solvating power, polarity, viscosity, and other characteristics to meet the requirements of different extraction processes. ILs exhibit high solubility for a wide range of compounds, including polar and non-polar substances. This property makes them suitable for the extraction of various target compounds from different sources, such as natural products, pharmaceuticals, and biofuels. ILs can selectively dissolve specific compounds, allowing for efficient separation and purification. Moreover, ILs are considered environmentally friendly due to their negligible volatility and low toxicity. They have a reduced impact on air quality, as they do not readily evaporate into the atmosphere. ILs are also biodegradable, and some ILs derived from renewable sources offer a sustainable alternative to traditional solvents [125,131,132].
3.1.7. Terpenes
Terpenes are naturally occurring compounds found in the essential oils of plants. They are typically extracted from plant material through methods such as steam distillation or cold-press extraction. Pinene and terpinene are examples of terpenes that have been investigated for their potential as green solvents. Terpenes offer several advantages as extraction solvents. They have low toxicity profiles, making them safer to handle compared to traditional solvents. They have excellent solvating power and can dissolve many different kinds of organic compounds, both polar and non-polar. That is why they are great for obtaining the desired results when it comes to natural remedies, essential oils, and bioactive compounds.
One of the notable features of terpenes is their pleasant aroma. Their characteristic scents can be beneficial in applications where odor is a consideration, such as the formulation of fragrances, cosmetics, and personal care products. Terpenes can also contribute to the sensory experience of food and beverage products. In addition to their low toxicity and biodegradability, terpenes are derived from renewable plant sources, making them a sustainable alternative to petroleum-based solvents. The use of plant sources for their production is consistent with green chemistry and aids in the transition to less resource-intensive methods [126].
3.2. Water-Based Solvents
Water-based green solvents refer to solvents where water acts as the primary solvent, providing a sustainable and environmentally friendly alternative to traditional organic solvents. These solvents leverage the unique properties of water, such as its abundance, low cost, low toxicity, and high boiling point, making them suitable for various applications while minimizing the environmental impact. Water-based green solvents offer several advantages over organic solvents, including reduced flammability, reduced health and safety risks, and lower emissions of VOCs. They are widely used in green chemistry practices, as they align with the principles of sustainability, waste reduction, and hazard minimization [115,133]. There are several water-based solvents such as water [115,134], aqueous solution of acids (e.g., acetic acid, citric acid) [135,136], aqueous solution of bases (e.g., sodium hydroxide, ammonia) [137,138], aqueous alcohol solution (e.g., ethanol, methanol) [137,139,140,141] and aqueous organic solvents (e.g., water/ethanol, water/acetone) [138,142] which are reported in the literature and a brief description is given below.
3.2.1. Water
Water-based extraction involves utilizing water as the primary solvent for extracting target compounds from various sources, such as plants, food products, and natural materials. Water acts as a polar solvent, making it particularly effective in extracting polar compounds, including sugars, organic acids, phenolic compounds, and hydrophilic bioactive compounds. One of the advantages of water as an extraction solvent is its low toxicity. Compared to organic solvents, water is generally considered safe for human consumption and has minimal health risks. This is especially important in applications where the extracted compounds are intended for use in food, pharmaceuticals, or cosmetics. Water-based extraction methods can be performed under mild conditions, reducing the need for high temperatures or harsh chemical reagents. This not only saves energy but also helps preserve the integrity and bioactivity of the extracted compounds. Water-based extraction is often preferred for sensitive compounds that may degrade under harsher extraction conditions. Furthermore, water is a sustainable and readily available solvent. Its abundance and low cost contribute to its attractiveness as an extraction solvent, providing economic benefits and reducing reliance on non-renewable resources [115,134].
3.2.2. Aqueous Solution of Acids
Aqueous solutions of acids are commonly used as extraction solvents due to their ability to selectively dissolve and extract specific compounds. The acid component provides acidic conditions, which can influence the solubility and extraction efficiency of different substances. Acids such as acetic acid and citric acid are particularly popular due to their low toxicity, wide availability, and compatibility with food and pharmaceutical applications. In the case of acetic acid, its presence in water forms acetic acid–water mixtures that can enhance the solubility of polar and semi-polar compounds. Acetic acid–water mixtures can be used for the extraction of various natural products, including organic acids, polyphenols, and pigments.
Similarly, citric acid, a weak organic acid, can form aqueous solutions with desirable extraction properties. Citric acid solutions are known for their chelating abilities and pH-dependent extraction selectivity. These solutions can be used for the extraction of metal ions, alkaloids, and other compounds that exhibit pH-dependent solubility characteristics. The pH of the acid solution plays a crucial role in the extraction process, as it can influence the solubility and interaction of the target compounds. By adjusting the pH of the acid solution, specific compounds can be selectively extracted or precipitated, providing a means to isolate desired components from complex mixtures [135,136].
3.2.3. Aqueous Solutions of Bases
Aqueous solutions of bases are commonly used in extraction processes due to their ability to alter pH and enhance solubility of target compounds. Sodium hydroxide and ammonia are frequently utilized bases in aqueous solutions for extraction purposes. Sodium hydroxide (NaOH) is a strong base that can increase the pH of an aqueous solution, resulting in alkaline conditions. Alkaline solutions can enhance the extraction efficiency of certain compounds, particularly those that are acidic in nature. Alkaline conditions can deprotonate acidic functional groups, thereby increasing their solubility. This is especially useful for the extraction of organic acids, phenolic compounds, and other acid-sensitive substances.
Ammonia (NH3) is a weak base that can also be used in aqueous solutions for extraction purposes. Similar to sodium hydroxide, ammonia can modify pH and solubility of compounds. Ammonia-based solutions are particularly effective in the extraction of nitrogen-containing compounds, such as alkaloids and amines. The alkaline conditions provided by ammonia can facilitate the solubility and extraction of these nitrogen-rich compounds. By adjusting the concentration of the base and the pH of the solution, aqueous solutions of bases allow for selective extraction of specific compounds. The pH-dependent solubility of various substances can be exploited to selectively extract desired components from complex mixtures [137,138].
3.2.4. Aqueous Alcohol Solutions
Aqueous alcohol solutions combine the properties of water and alcohol, providing an effective solvent system for extraction. Ethanol and methanol are commonly used alcohols in aqueous solutions for extraction purposes. The presence of alcohol in the aqueous solution can enhance the solubility of both polar and non-polar compounds. Alcohols can disrupt intermolecular interactions, allowing for the extraction of a wider range of compounds compared to water alone. This makes aqueous alcohol solutions particularly suitable for the extraction of various natural products, including bioactive compounds, phenolic compounds, and essential oils.
Ethanol, as a polar alcohol, can effectively solubilize polar and semi-polar compounds. It is commonly used in extraction processes for its ability to extract a diverse range of compounds, including flavonoids, alkaloids, and terpenes. Ethanol also acts as a co-solvent with water, further expanding its solubilizing power. Methanol, another commonly used alcohol, is highly polar and can efficiently dissolve both polar and non-polar compounds. Methanol-based extraction solvents are often employed in the extraction of bioactive compounds, natural products, and metabolites.
The use of aqueous alcohol solutions allows for the adjustment of the polarity of the solvent system by changing the concentration of alcohol. This flexibility provides control over the extraction selectivity and efficiency, allowing for the extraction of specific compounds or classes of compounds from complex mixtures [137,139,140,141].
3.2.5. Aqueous Organic Solvents
Aqueous organic solvents are solvent systems that consist of a combination of water and an organic solvent, such as ethanol or acetone. These mixtures provide a versatile extraction solvent option, leveraging the solubilizing power of both water and organic solvents.
Water/ethanol mixtures are commonly used as extraction solvents due to their ability to solubilize a wide range of compounds. Ethanol, as an organic solvent, can dissolve both polar and non-polar compounds, including bioactive compounds, flavonoids, and phenolic compounds. The addition of water to ethanol improves the solubility of polar compounds, expands the range of compounds that can be extracted, and provides a more environmentally friendly solvent system.
Water/acetone mixtures are also utilized as extraction solvents in various applications. Acetone, an organic solvent, is particularly effective at dissolving non-polar compounds and lipophilic substances. The addition of water to acetone enhances its solubility for polar compounds and expands its extraction capabilities.
Aqueous organic solvent systems allow for control over the polarity and solubility characteristics of the solvent system by adjusting the ratio of water to organic solvent. This flexibility enables tailored extraction selectivity and efficiency, making these solvent systems suitable for the extraction of specific compounds or classes of compounds from complex mixtures. The use of aqueous organic solvents offers several advantages, including enhanced extraction efficiency, expanded solubility range, and reduced reliance on purely organic solvents. These solvents also align with the principles of green chemistry by reducing the use of hazardous organic solvents and promoting sustainable extraction processes [137,141,142].
3.3. Supercritical Fluids
The properties of both liquids and gases are present in supercritical fluids, which are substances that are kept above their critical temperature and pressure. In the context of green solvents, supercritical fluids, particularly supercritical carbon dioxide (scCO2), are considered environmentally friendly solvents for various applications. Supercritical fluids, including scCO2, offer several advantages as green solvents. They have low toxicity and are non-flammable, making them safer to handle compared to traditional organic solvents. Additionally, supercritical fluids can be easily separated from the extracted compounds, allowing for their efficient recovery and recycling.
Both polar and non-polar compounds are soluble in supercritical fluids due to their exceptional solvating properties. By adjusting the temperature and pressure, the solvating power of supercritical fluids can be fine-tuned, enabling selective extraction of target compounds while leaving impurities behind. One of the key environmental benefits of supercritical fluids is their low environmental impact. Supercritical carbon dioxide is abundant, non-toxic, and non-polluting, making it a sustainable alternative to organic solvents derived from fossil fuels. Furthermore, the use of supercritical fluids eliminates or significantly reduces the need for VOCs, leading to reduced emissions and air pollution [110,111,112,113,116,142].
3.3.1. Supercritical Carbon Dioxide (scCO2)
Supercritical carbon dioxide (scCO2) is carbon dioxide above its critical temperature (31.1 °C) and critical pressure (74 bar), where it possesses properties of both a gas and a liquid. These conditions enable scCO2 to function as an efficient and selective extraction solvent. One of the major advantages of scCO2 is its low critical temperature, which allows for the extraction of temperature-sensitive compounds without degradation. This makes it particularly suitable for extracting heat-sensitive bioactive compounds and natural products, such as essential oils and flavors. scCO2’s solvating power is so high that it can dissolve both polar and non-polar compounds. Extraction conditions, such as pressure, temperature, and the addition of a co-solvent, can be adjusted to maximize its solvating power. This versatility enables the selective extraction of specific compounds from complex matrices [143,144].
Another advantage of scCO2 is its environmental friendliness. Carbon dioxide is a byproduct of many manufacturing procedures, so it is abundant, safe, and nonflammable. It can replace dangerous organic solvents in the extraction process, lowering both the environmental and health risks. scCO2 also offers advantages in terms of easy solvent removal and product recovery. The extracted compounds and scCO2 can be easily separated by lowering the pressure after extraction. This facilitates downstream processing and minimizes the need for extensive post-extraction purification steps [142,143,144].
3.3.2. Supercritical Water (scH2O)
Supercritical water (scH2O) offers several advantages as an extraction solvent. Firstly, its high temperature and pressure (p > 22.064 MPa, T > 373.946 °C) enhance the solubility and diffusivity of solutes, allowing for efficient extraction of various compounds. scH2O is especially effective at extracting polar and ionic compounds due to its high polarity in the supercritical state. One of the notable features of scH2O is its unique ability to undergo ionization, resulting in the generation of reactive hydroxyl radicals. These radicals can promote the degradation of organic pollutants or complex molecules, making scH2O a potential solvent for waste treatment and environmental remediation applications [145,146].
Furthermore, scH2O offers benefits in terms of selectivity. The solubility of different compounds in scH2O varies with temperature and pressure, allowing for the selective extraction of specific target compounds from complex matrices. This selectivity can be fine-tuned by adjusting the extraction conditions, offering control over the extraction process. Organic pollutants, natural products, and bioactive compounds can all be extracted using supercritical water. Various heat-sensitive substances, including essential oils and phenolic compounds, have been extracted using this method [147,148].
3.3.3. Supercritical Ethanol (scEtOH)
When ethanol is heated and compressed beyond its critical temperature (243 °C) and critical pressure (6.38 MPa), it changes phase and takes on characteristics of both a gas and a liquid, giving rise to the term “supercritical ethanol” (scEtOH). In this state, ethanol has enhanced solvating power and can efficiently extract various compounds from different matrices. Supercritical ethanol has a higher solvating power compared to subcritical ethanol due to its increased density and diffusion coefficient. This facilitates the removal of polar and non-polar compounds, expanding its usefulness. Ethanol is known for its ability to extract a variety of bioactive compounds, including natural products, pharmaceuticals, and flavors. In its supercritical state, ethanol can further enhance its extraction capabilities, improving the extraction efficiency and selectivity [149,150].
Extracting essential oils, bioactive compounds from plant material, and other compounds from natural products are just some of the many applications of supercritical ethanol in the extraction process. The specific conditions of temperature and pressure can be adjusted to optimize the extraction process and target specific compounds of interest [151,152]. While the use of supercritical ethanol as an extraction solvent is not as extensively documented as supercritical carbon dioxide, it shows potential for various applications and can be further explored in research and development [153].
3.3.4. Supercritical Propane (scC3H8)
Propane that has been subjected to conditions above its critical temperature (96.7 °C) and critical pressure (42.5 bar) is said to be supercritical, or scC3H8 for short. In this state, propane can possess unique solvating properties and may be explored as an extraction solvent in certain applications. Propane is known for its non-polarity and low critical temperature, which may limit its solvating power compared to other supercritical fluids. However, it can still potentially be utilized for the extraction of non-polar compounds and lipophilic substances [154,155]. The use of supercritical propane as an extraction solvent may have applications in specific industries or processes where its solvating properties and unique characteristics can provide advantages over other solvents. Supercritical propane shows promise as an extraction solvent, but there is a lack of data on its use so far; more study is needed to fully understand its capabilities.
3.3.5. Supercritical Nitrogen (scN2)
Supercritical nitrogen (scN2) is nitrogen that has been subjected to conditions above its critical temperature (−147.1 °C) and critical pressure (33.5 bar), at which it becomes a hybrid between a gas and a liquid. In this state, nitrogen may possess unique solvating properties and can potentially be used as an extraction solvent in specific applications. Nitrogen is an inert gas and generally considered nonpolar, which may limit its solvating power for polar compounds compared to other supercritical fluids. However, it can still potentially be used for the extraction of nonpolar or low-polarity compounds [156,157].
The use of supercritical nitrogen as an extraction solvent may have applications in certain industries or processes where its inertness and specific solvating properties can provide advantages over other solvents. However, it is important to note that the literature on supercritical nitrogen as an extraction solvent is limited, and further research and development would be necessary to explore its full potential.
3.4. Deep Eutectic Solvents
Solvents that fall into the category of “deep eutectic solvents” (DES) are eutectic mixtures of two or more components, typically a hydrogen bond donor (HBD) and an acceptor (HBA). These solvents exhibit unique properties that make them attractive alternatives to conventional organic solvents. DES can be composed of bio-based or non-bio-based components, and they possess characteristics such as low toxicity, low volatility, high thermal stability, and tunable physical properties. The formation of DES occurs when the mixture of HBD and HBA components forms a eutectic system, characterized by a lower melting point than the individual components alone. The hydrogen bond interactions between the components contribute to the unique properties of DES, including their ability to dissolve a wide range of solutes.
Many different fields, such as catalysis, extraction, electrochemistry, and separation processes, have taken an interest in DES because of their potential utility. These solvents offer advantages such as high solubility for polar and non-polar compounds, tunable properties through the selection of different HBD-HBA combinations, and their ability to serve as reaction media for a variety of chemical reactions. The mixtures of choline chloride and urea, glycerol, and ethylene glycol are all examples of DES. Applications utilizing these DES have included biomass pretreatment, metal extraction, and organic synthesis [117,118,119].
3.4.1. Choline Chloride–Urea
Choline chloride–urea DES is made by reacting the quaternary ammonium salt choline chloride with the hydrogen bond acceptor urea. This DES exhibits a unique eutectic behavior, characterized by a lower melting point compared to the individual components. Choline chloride–urea DES has been extensively studied for its excellent solubility power, which makes it effective against a wide variety of organic and inorganic compounds in need of dissolution. It has been explored in various applications, such as biomass processing, metal extraction, and catalysis. In biomass pretreatment, choline chloride–urea DES has been shown to effectively dissolve cellulose, aiding in the extraction of valuable components [158,159].
3.4.2. Choline Chloride–Glycerol
When choline chloride and the hydrogen bond donor glycerol are mixed together, they form choline chloride–glycerol DES. This DES has many positive characteristics that make it an attractive choice, such as its low volatility, high thermal stability, and high solvating power. Choline chloride–glycerol DES has been widely investigated for its potential applications in extraction processes, synthesis of nanoparticles, and organic transformations. Because of its versatility as a solvent, it has been put to use in the environmentally friendly extraction of natural products such as bioactive compounds from plants [160].
3.4.3. Choline Chloride–Ethylene Glycol
The reaction between choline chloride and ethylene glycol produces choline chloride–ethylene glycol DES. The viscosity, solubility, and thermal stability of this DES are just a few of its remarkable characteristics. Choline chloride–ethylene glycol DES has been investigated for various applications, including solvent extraction, catalysis, and biomass processing. Its potential as a solvent for removing valuable compounds from plants such as essential oils and bioactive molecules has been demonstrated. The ability of choline chloride–ethylene glycol DES to dissolve and extract target compounds efficiently makes it a promising solvent in green extraction processes [161].
The overall production flow of chemical reactions for DES production has been summarized in Figure 5. The choline chloride salt (HBA) contributes a hydrogen ion, which binds with the hydrogen of the hydroxyl group (HBD) in the DES to form a hydrogen bond.
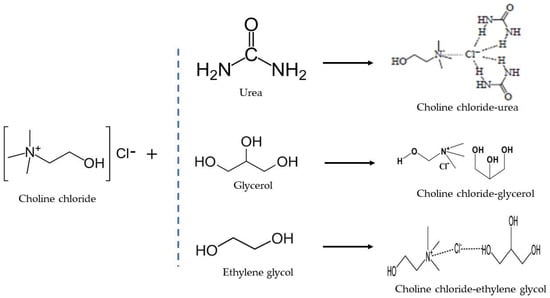
Figure 5.
Overall Reactions mechanism for DES Production.
3.5. Properties of Green Solvents
Green solvents, as opposed to traditional solvents, are safer for human health and the environment because they are made from renewable resources. The unique properties and characteristics of these solvents make them desirable for a wide range of uses. Green solvents’ key properties and characteristics are illustrated and explained in Figure 6 [158,162,163,164].
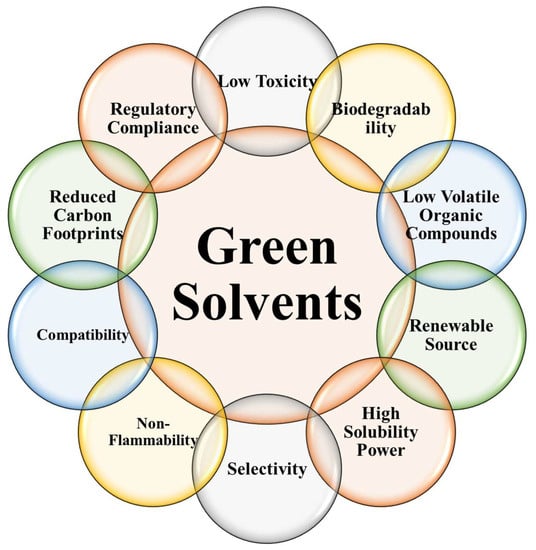
Figure 6.
Key properties of the green solvents.
Renewable Source: Renewable plant materials, biomass, or food waste are the building blocks of green solvents. They are more environmentally friendly than solvents made from fossil fuels, which deplete natural resources and increase greenhouse gas production.
Low Toxicity: In comparison to traditional solvents, green solvents are safer to use. They pose less of a threat to human health and the environment because of their lower volatility and lower levels of hazardous components.
Biodegradability: Green solvents are biodegradable, meaning they can be broken down by microbes or enzymes in the environment. This feature lessens their ability to persist in ecosystems and the damage they do to groundwater, surface water, and atmospheric chemistry.
Low VOCs Content: Chemical compounds that can evaporate into the air are called VOCs. Cleaner indoor and outdoor air, less smog, and fewer health risks are all benefits of using green solvents because of their typically low VOC content.
High Solubility Power: Many environmentally friendly solvents have high solubility and can be used to dissolve a wide variety of organic and inorganic compounds. Because of this quality, they can be used in a wide range of contexts, such as extraction, synthesis, and formulation.
Selectivity: Some environmentally friendly solvents can be selective toward a given compound or group of compounds, allowing for more precise extraction and separation. This selectivity can improve process efficiency by zeroing in on useful components and eliminating unnecessary purification steps.
Non-Flammability: In comparison to traditional solvents, many eco-friendly options are less flammable. This quality makes handling, storage, and transport less likely to result in fires.
Compatibility: Most environmentally friendly solvents work well with a wide variety of substrates, from metals to plastics to even natural fibers. Because of this compatibility, they can be used in many different fields without posing a threat to machinery or degrading raw materials.
Reduced Carbon Footprint: Green solvents help cut down on pollution and carbon dioxide emissions. Carbon dioxide (CO2) emissions from their production and use are lower than those of traditional solvents, lending support to climate change mitigation efforts.
Regulatory Compliance: The standards for health, safety, and environmental protection that go into making green solvents are rigorous. To guarantee their usefulness in particular contexts and their conformity with applicable rules, they are put through extensive testing and evaluation.
Some of the physiochemical properties of green solvents are listed in detail in Table 3. Green solvents are a more sustainable and environmentally friendly alternative to traditional solvents due to their unique properties and characteristics.

Table 3.
Physicochemical Properties of Green solvents.
4. Performance Evaluation of Green Solvents
Use of green solvents is on the rise as businesses try to reduce their environmental impact. Efficiency, selectivity, safety, and overall sustainability can only be grasped through a thorough assessment of green solvents’ performance. In order to better understand the performance evaluation factors for green solvents, this study will focus on the most important considerations and methodologies. As can be seen in Figure 7, a number of criteria have been used to evaluate green solvents’ performance.
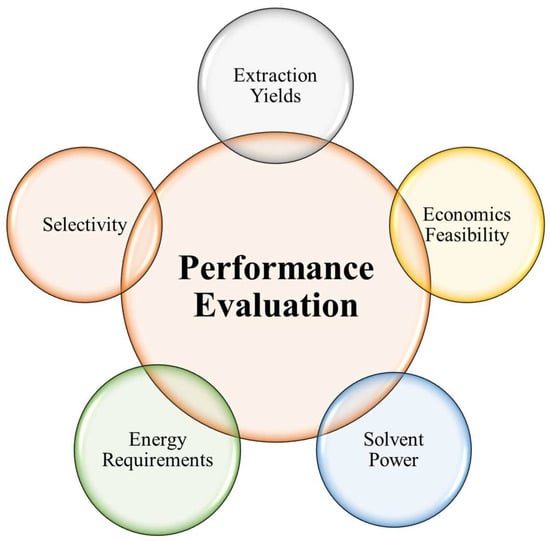
Figure 7.
Various aspects for the Performance Evaluation of the green solvents.
4.1. Solvent Power
A solvent’s “solvent power” is its capacity to dissolve and extract desired compounds from a wide range of matrices. It is an essential metric for gauging the efficacy of eco-friendly solvents. The effectiveness of a solvent can be evaluated based on its solubilization, extraction, and selective properties.
4.1.1. Solubility Parameters
Solubility parameters are quantitative measures used to describe the solvating power of solvents and their compatibility with different solutes. These parameters take into account various intermolecular forces, such as dispersion forces, dipole–dipole interactions, and hydrogen bonding. Understanding solubility parameters is essential for predicting solute–solvent interactions and solubility behavior. Hansen solubility parameters (HSP) are widely used in the field of green solvents. The HSP approach divides solubility parameters into three components: δD (dispersion forces), δP (polar forces), and δH (hydrogen bonding forces) [193]. By comparing the HSP values of solvents and solutes, one can predict the solubility or compatibility between them. When the HSP values of a solvent and solute are similar, they are more likely to be miscible and form a homogeneous solution.
The Hildebrand solubility parameter is another commonly used parameter that represents the cohesive energy density of solvents and solutes, providing an overall measure of intermolecular forces [194]. The Hildebrand parameter (δ) is calculated as the square root of the cohesive energy density. Kamlet–Taft parameters are a set of solubility parameters that provide information about solvents’ polarity, hydrogen-bonding ability, and dipolarity. These parameters, including the polarity parameter (π*), the hydrogen-bonding acidity parameter (α), and the hydrogen-bonding basicity parameter (β), help assess solute–solvent interactions and predict solubility behavior [195].
By utilizing solubility parameters, researchers and chemists can select green solvents that have compatible solubility characteristics with their target solutes. This approach aids in the development of efficient and sustainable processes while reducing the use of harmful or less environmentally friendly solvents.
Interactions with Different Solute Classes
The solubility power of green solvents can vary depending on the nature of the solute. Different solute classes, such as polar compounds, non-polar compounds, and amphiphilic molecules, exhibit varying degrees of solubility in green solvents. Polar compounds, which possess dipole moments or hydrogen-bonding capabilities, tend to dissolve well in polar green solvents. Examples of polar green solvents include water, alcohols (e.g., ethanol), and certain bio-based solvents. The polar nature of these solvents enables them to interact with polar solutes through dipole–dipole interactions or hydrogen bonding, facilitating solubility. Non-polar compounds, on the other hand, have limited solubility in polar solvents but are more soluble in non-polar or low-polarity green solvents. Non-polar solvents, such as supercritical carbon dioxide (scCO2) or hydrocarbons (e.g., limonene), can dissolve non-polar compounds effectively due to their similar intermolecular forces, including dispersion forces.
Amphiphilic molecules, which possess both polar and non-polar regions, may exhibit solubility in a wider range of green solvents. These solutes can interact with both polar and non-polar solvents, depending on the specific composition and structure. Intermolecular interactions, such as hydrogen bonding, π-π stacking, and dipole–dipole interactions, also play a significant role in solute–solvent interactions and solubility. The compatibility of solute and solvent based on these interactions determines the solubility behavior and the ability of green solvents to effectively dissolve specific solutes [196].
4.1.2. Bio-Oil Extraction Yields Using Green Solvents
Green solvents’ efficiency in extracting target compounds from biomass or raw materials can be evaluated in large part by measuring the yield of their solvent power, or extraction. It measures how much of the target compounds were successfully extracted, thus providing an indication of the solvent’s efficiency. Several factors affect the extraction yield. These include the extraction conditions, the extraction methods used, and the interactions between the solute and the solvent [140,142]. Table 4 summarizes data from relevant studies to provide a comprehensive overview of the performance of green solvents in extracting desired compounds and further illustrates the extraction yields achieved using these solvents.

Table 4.
Bio-oil extraction yields using green and conventional extraction solvents.
The quantity of bio-crude obtained from biomass during extraction is highly sensitive to the solvent used. Green solvents can produce high yields of bio-crude oil (Table 4). The components of biomass are more easily soluble in these solvents, leading to more effective extraction and larger quantities of bio-crude. The extraction of bio-crude has traditionally relied on the use of traditional solvents, many of which are derived from petroleum. However, their non-renewable quality and potential toxicity raise environmental concerns. It is possible that the extraction yields and bio-crude quality would suffer if conventional solvents such as hexane or dichloromethane were used.
Green solvents perform as well as, or even better than, conventional solvents when comparing bio-crude extraction yields. Increasing bio-crude yields is possible through the use of green solvents, which are in line with sustainable practices. The extraction process can be fine-tuned to obtain bio-crude with desirable properties by choosing the appropriate green solvent.
4.1.3. Selectivity
Solvents made from renewable biomass have unique solvation properties that allow for selective extraction of target compounds. Green solvent selectivity is affected by properties such as chemical make-up, polarity, and interactions with biomass components. These solvents can have varying degrees of affinity for different biomass components, facilitating selective extraction of the compounds of most interest.
Selectivity toward various classes of bioactive compounds has been demonstrated by bio-based solvents such as ethanol, ethyl lactate, and terpenes. For instance, ethanol has seen extensive use in the pharmaceutical industry for the isolation of phenolic compounds, flavonoids, and alkaloids from plants [218,219]. Selectivity in ethyl lactate extraction of lipids and fatty acids from microalgae for bio-crude production has been demonstrated [198,203]. Terpenes, such as limonene and pinene, exhibit selectivity towards essential oils and other volatile compounds found in plants [198,202,220]. These bio-based solvents offer targeted extraction of specific compounds, reducing the need for additional purification steps and improving the overall efficiency of the bio-crude extraction process.
DES also exhibit remarkable selectivity due to their unique molecular interactions. The combination of HBD and HBA components in DES forms a eutectic mixture with tailored properties. The choice of HBD-HBA components can influence the selectivity of DES towards different types of compounds. For example, choline chloride–ethylene glycol DES has shown selectivity in extracting bioactive compounds, such as essential oils, from various plant materials [221,222,223]. Choline chloride–urea DES has been found to be selective for the extraction of cellulose and lignin from biomass [224,225,226]. DES’s ability to modulate its selectivity through the choice of components makes them versatile solvents for targeted extraction of specific biomolecules.
Compared to conventional solvents, green solvents exhibit superior selectivity due to their unique interactions with biomass components. Conventional solvents, such as hexane or dichloromethane, are often less selective and extract a broader range of compounds, including undesired impurities. This can lead to the need for additional purification steps, reducing the overall efficiency of the bio-crude extraction process.
The selectivity of green solvents contributes to the production of high-quality bio-crude with a targeted composition, reducing the need for extensive downstream processing. Moreover, the selectivity of green solvents minimizes the extraction of non-desirable components, resulting in cleaner and more environmentally friendly extraction processes.
4.2. Energy Requirements
When comparing green solvents for extraction processes, it is important to take into account energy needs and optimize the process. Because of their reduced negative effects on the environment and longer lifespan, green solvents such as bio-based solvents and deep DES are increasingly popular. The extraction process must be optimized for energy efficiency after determining the energy needs of the target population.
4.2.1. Reduced Energy Consumption
Compared to traditional solvents, green solvents may require less energy to operate, making them an attractive option for use in extraction processes. Because of their special properties, green solvents help reduce energy consumption, making them a more sustainable and cost-effective option. Several studies, including those depicted in Figure 8, have brought attention to the fact that eco-friendly solvents require less energy to use.
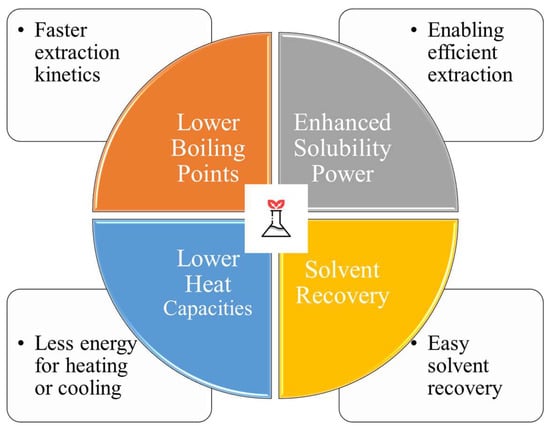
Figure 8.
Different parameters or causes of green solvents for the reduced energy consumption.
Lower Boiling Points: When compared to traditional solvents, green solvents typically have lower boiling points, which results in faster extraction kinetics and less energy required during the heating phase [227,228]. For example, the use of green solvents such as ethanol or supercritical carbon dioxide (scCO2) with lower boiling points, for instance, can drastically cut down on the amount of energy required for heating and evaporation.
Enhanced Solubility Power: The high solubility power of green solvents facilitates the rapid and complete removal of desired compounds. This quality allows for more rapid mass transfer, which shortens the extraction process and saves on energy [163]. Ionic liquids and DES are two examples of green solvents that have shown promising results in a variety of extraction processes due to their high solubility power [164].
Lower Heat Capacities: Typically, green solvents have lower heat capacities than traditional solvents. Therefore, less energy is needed for heating or cooling during extraction [162]. Green solvents’ lower heat capacities mean less energy is required to bring them up to the desired temperature and the process can be completed more quickly.
Solvent Recovery: Due to their distinct properties, green solvents such as supercritical fluids and some ionic liquids provide the added benefit of simple solvent recovery. Solvent recovery and reuse greatly improve process sustainability by lowering energy consumption [162,227].
4.2.2. Economics Feasibility and Scalability
When considering green extraction solvents, it is important to look at more than just their efficiency and impact on the environment. The practicality and commercial viability of these solvents depend critically on their cost-effectiveness and ability to be scaled. However, related research, analysis, and data have not been reported just yet.
The price of the environmentally friendly extraction solvent is a major factor in the budget. To be economically viable, the price must be comparable to that of traditional solvents. Raw material costs, production methods, and the relationship between supply and demand all play a role. It is important to carefully evaluate potential problems with sourcing and price fluctuations when using green solvents made from renewable resources [229].
The cost-effectiveness of extracting with environmentally friendly solvents is directly related to the efficiency and yield achieved. If the extraction process is more effective, more of the desired compounds will be recovered from the starting material, which could cut down on expenses. Improving economic results is one of the benefits of optimizing extraction conditions and methods [230].
Economic viability depends on the ease with which solvents can be recovered and reused. Recoverable and reusable green solvents reduce the frequency with which new solvents must be purchased, saving money in the long run. The effectiveness, cost, and effect on solvent quality of recovery technologies such as distillation, adsorption, and membrane separation should be assessed [231].
The viability and widespread use of green extraction solvents depends on their ability to be scaled up. It can be difficult to scale up laboratory or pilot-scale processes for full-scale industrial use. To ensure scalability without compromising economic feasibility, factors such as raw material availability, process efficiency, equipment design, and energy needs must be carefully considered [230].
5. Environmental Concerns: Challenges and Limitations
Given their lower toxicity and longer shelf life, green solvents have emerged as a potentially useful alternative for bio-crude extraction. The use of these materials in extraction processes, however, is not without difficulties. Detailed information about the challenges is discussed below.
5.1. Safety, Health and Environmental Issues
Since green extraction solvents are safer for both people and the environment, they are increasingly being used instead of traditional solvents. However, it is crucial to keep in mind the potential threats to health, safety, and the environment that their use poses. The “CHEM21 selection guide for classical and less classical solvents” was used to generate Table 5, which displays the outcomes of environmental concerns associated with green solvents [232,233]. The order of the solvents is as follows:
Recommended (or Preferred): Assuming there are no chemical incompatibilities with the process conditions, these solvents are recommended as the first choice for testing in a screening exercise.
Problematic: These solvents are suitable for use in the lab or the kilolab. However, particular measures may be necessary or substantial energy consumption may result from their implementation in pilot plants or at production scale.
Hazardous: Scalability issues are particularly problematic for these types of solvents. During the R&D phase of a process, replacing these solvents should be a top priority.
Highly Hazardous: Even in a laboratory setting, it is best to avoid using these solvents.
To ensure safe and efficient operations, it is crucial to take the rankings into account and choose solvents that are compatible with the requirements and constraints of the extraction process.
A comparison to other resources is used to determine the order of prevalence of the most frequently used solvents. In order to determine which new-generation and bio-derived solvents are desirable, a set of criteria must be established before they can be included in the CHEM21 guide. This evaluation needs to be in sync with the standing of “Registration, Evaluation, Authorization, and Restriction of Chemicals (REACh)” -registered solvents that are widely used in industry [234]. Safety, Health, and Environment (SH&E) criteria are typically prioritized in solvent selection guides, with additional Industrial or Regulatory constraints considered as an option. The criteria have been streamlined and combined to reduce their number from five to three, with one each for safety, health, and the environment. Each criterion receives a score between 1 and 10, with 10 being the highest possible risk. A preliminary ranking can be obtained by combining these three SH&E scores, with solvents being classified as either recommended, problematic, or hazardous. The difference between hazardous and highly hazardous solvents cannot yet be determined; however, a solvent with a score of 10 in any category is likely to fall into the latter category [232].

Table 5.
Safety, Health and Environmental concerns related to green extraction solvents.
Table 5.
Safety, Health and Environmental concerns related to green extraction solvents.
| Solvents | Safety Score | Health Score | Environmental Score | Ranking by Default | Ranking by Discussion | Overall Health and Environmental Concerns | |
|---|---|---|---|---|---|---|---|
| Water- and Bio-Based solvents | Water | 1 | 1 | 1 | Recommended | Recommended | Water and bio-based green extraction solvents offer reduced toxicity and environmental impact. Concerns include water pollution, human health effects (inhalation and skin contact), ecological impacts on aquatic ecosystems, and sustainability of sourcing and production. Proper waste management, safety measures, and research are essential for safe and responsible use. |
| Ethanol | 4 | 3 | 3 | Problematic | Recommended | ||
| EA | 5 | 3 | 3 | Recommended | Recommended | ||
| Diethyl ether | 10 | 3 | 7 | Hazardous | Highly Hazardous | ||
| MTBE | 8 | 3 | 5 | Hazardous | Hazardous | ||
| MeTHF | 6 | 5 | 3 | Problematic | Problematic | ||
| DME | 7 | 10 | 3 | Hazardous | Hazardous | ||
| Cyclohexane | 6 | 3 | 7 | Problematic | Problematic | ||
| Benzene | 6 | 10 | 3 | Hazardous | Highly Hazardous | ||
| Carbon disulfide | 9 | 7 | 7 | Hazardous | Highly Hazardous | ||
| Glycerol | 1 | 1 | 7 | Problematic | Problematic | ||
| GVL | 1 | 5 | 7 | Problematic | Problematic | ||
| CPME | 7 | 2 | 5 | Problematic | Problematic | ||
| D-limonene | 4 | 2 | 7 | Problematic | Problematic | ||
| p-cymene | 4 | 5 | 5 | Problematic | Problematic | ||
| DMC | 4 | 1 | 3 | Recommended | Recommended | ||
| Ethyl lactate | 3 | 4 | 5 | Problematic | Problematic | Deep Eutectic Solvents (DES) extraction solvents present low toxicity and biodegradability. | |
| DES Solvents | ChCl (HBA) | - | - | - | Not a hazardous substance [235] | ||
| Gly (HBD1) | - | - | - | ||||
| ChCl/Gly | - | - | - | All DESs were toxic [236] | |||
Table 5 shows that among various bio- and water-based green solvents, only water, EA, and DMC are recommended. DES, on the other hand, are a novel class of solvent that has garnered attention from scientists thanks to its eco-friendly characteristics and its adaptability to a wide variety of uses. There are a wide variety of described DES, making it difficult to provide a definitive characterization or broad generalization of their properties. This is especially crucial when considering the (eco)toxicity data available for these compounds [236,237].
5.2. Other Challenges and Limitations
Green solvents, including bio-based solvents, water-based solvents, supercritical fluids, and DES, offer promising alternatives to conventional solvents for extraction processes. However, these green solvents are not without their challenges and limitations. Some examples are:
Bio-based Solvents [229]:
- Sourcing and availability of biomass feedstock;
- Cost fluctuations and economic feasibility;
- Variability in solvent properties due to biomass composition;
- Limited compatibility with certain solute classes.
Water-based Solvents:
- Limited solubility for hydrophobic compounds;
- Potential formation of emulsions in biphasic systems;
- Difficulty in extracting polar compounds;
- Challenges in separating solvent from water after extraction.
Supercritical Fluids [143]:
- High energy requirements for maintaining supercritical conditions;
- Limited solubility for certain compounds;
- High capital costs associated with supercritical fluid extraction equipment;
- Challenges in recovering and recycling supercritical fluids.
DES [118]:
- Limited understanding of long-term environmental impacts;
- Challenges in optimizing DES properties for specific applications;
- Potential for high viscosity and reduced mass transfer rates;
- Limited availability of DES with desired properties.
5.2.1. Bio-Based Solvents Challenges
Bio-based solvents, derived from renewable biomass sources, offer a more sustainable alternative to conventional solvents. However, they also present certain challenges that need to be addressed for their widespread adoption. One key challenge is the availability and consistency of feedstock for the production of bio-based solvents. The reliance on agricultural crops or waste biomass as raw materials can be influenced by factors such as seasonal variations, land availability, and competition with food production [238].
Another challenge is the cost-effectiveness and competitiveness of bio-based solvents compared to their conventional counterparts. The production processes for bio-based solvents often involve additional steps such as biomass pretreatment, enzymatic hydrolysis, or fermentation, which can increase production costs [239]. Achieving cost parity with conventional solvents remains a significant hurdle that needs to be overcome for wider adoption.
Additionally, the performance and functionality of bio-based solvents can vary compared to conventional solvents. Bio-based solvents may have different solvency power, boiling points, or viscosities, which can impact their compatibility with existing industrial processes and equipment [240]. Adapting and optimizing processes to accommodate the specific characteristics of bio-based solvents can require additional research and development efforts.
Furthermore, the sustainability of bio-based solvents should be carefully assessed throughout their life cycle. This includes evaluating the environmental impacts associated with feedstock cultivation, solvent production, and disposal or recycling processes. It is important to ensure that bio-based solvents deliver genuine sustainability benefits, such as reduced greenhouse gas emissions, minimized resource depletion, and reduced toxicity compared to conventional solvents [241].
5.2.2. Water-Based Solvents Challenges
Water-based green solvents, also known as aqueous solvents, offer several advantages such as low toxicity, environmental friendliness, and ease of availability [242]. However, they also present specific challenges that need to be addressed for their successful application in various processes. Some of the key challenges associated with water-based green solvents include:
Limited Solubility: Water has limited solubility for many non-polar and hydrophobic compounds. This can pose challenges when trying to extract or dissolve certain target compounds that are not readily soluble in water. Strategies such as the addition of co-solvents or surfactants can be employed to enhance the solubility of non-polar solutes in water [224,243,244].
Extraction Efficiency: Compared to organic solvents, water-based green solvents may have lower extraction efficiencies for certain compounds. The polar nature of water may hinder the extraction of non-polar or low-polarity compounds. Optimization of extraction parameters such as temperature, pH, and extraction time can help improve the extraction efficiency of water-based solvents [245].
Stability and Shelf Life: Water-based green solvents can be susceptible to microbial growth and degradation over time, which can impact their stability and shelf life. Contamination by microorganisms can affect solvent quality and introduce variability in performance. Proper storage conditions and the use of preservatives or additives can help maintain the stability and extend the shelf life of water-based green solvents [131].
Corrosion and Compatibility: Water-based solvents can exhibit corrosive properties, especially in the presence of certain ions or corrosive compounds. Compatibility with equipment and materials used in industrial processes can be a concern. Understanding the corrosion behavior and compatibility of water-based solvents with various materials is crucial to prevent equipment damage and ensure safe and effective operation [246].
Energy Consumption: Water-based extraction processes may require additional energy input compared to organic solvent-based processes. For example, the use of high temperatures or the need for extensive purification steps to remove impurities can increase energy consumption. Optimization of extraction conditions and the development of energy-efficient processes are important considerations for minimizing energy requirements [245].
5.2.3. Supercritical Fluid Challenges
Supercritical fluids, such as supercritical carbon dioxide (scCO2), offer unique properties that make them attractive as green solvents for various extraction processes. However, they also present specific challenges that need to be addressed for their effective application. Some of the key challenges associated with supercritical fluid green solvents include:
Limited Solubility: Supercritical fluids have limited solubility for polar and high-molecular-weight compounds. This can pose challenges when trying to extract target compounds that are highly polar or have large molecular sizes. Modifying the extraction conditions, such as adjusting pressure and temperature, or adding co-solvents, can help enhance solubility and improve extraction efficiency [247].
High Equipment and Operational Costs: Supercritical fluid extraction requires specialized equipment that can handle high pressures and temperatures, which can be expensive to acquire and maintain. The high equipment and operational costs associated with supercritical fluid extraction can hinder its widespread adoption, particularly for small-scale applications [248].
Process Optimization and Scalability: The optimization of extraction parameters, such as pressure, temperature, and flow rate, is crucial to achieve desired extraction efficiencies and selectivity. Scaling up supercritical fluid extraction processes from laboratory to industrial scale can be challenging due to the need for efficient heat transfer, precise control of parameters, and equipment design considerations [249].
Safety Concerns: Supercritical fluids, especially those used at high pressures, can present safety risks if not handled properly. The high pressure and temperature conditions require appropriate safety measures to prevent accidents. Safety protocols, training, and adherence to guidelines and regulations are essential to ensure the safe operation of supercritical fluid extraction systems [247].
Environmental Impact: While supercritical fluids are considered environmentally friendly compared to conventional solvents, their production and disposal can still have environmental implications. The energy-intensive nature of supercritical fluid extraction processes and the management of waste solvents require careful consideration to minimize the overall environmental impact [248].
5.2.4. DES Challenges
DES are a class of green solvents that have gained attention for their unique properties and potential applications in various fields, including extraction processes. However, DES also present specific challenges that need to be addressed for their effective utilization. Some of the key challenges associated with DES as green solvents include:
Limited Solubility: DES may have limited solubility for certain solutes or classes of compounds. The selection of suitable DES components and their proportions is crucial to achieve the desired solubility and extraction efficiency. Additionally, the solubility of solutes in DES can be influenced by factors such as temperature, pressure, and the presence of co-solvents or additives [250,251].
Viscosity and Mass Transfer Limitations: DES often exhibit high viscosities compared to conventional solvents, which can hinder mass transfer rates and extraction efficiencies. The high viscosity of DES may restrict the diffusion of solutes and slow down the extraction process. Strategies such as optimizing temperature, selecting appropriate DES components, or utilizing assisted extraction techniques can help overcome these limitations and improve mass transfer rates [252].
Limited Stability and Reusability: Some DES may have limited stability, especially under certain extraction conditions or prolonged use. Factors such as thermal stability, chemical reactivity, and water content can affect the stability of DES. Continuous research is being conducted to develop more stable DES systems and enhance their reusability, thereby improving the economic and environmental sustainability of their use [117].
Toxicity and Safety Considerations: Although DES are generally considered greener and safer alternatives to conventional solvents, their toxicity and safety profiles need to be thoroughly evaluated. Some DES components may still exhibit toxicity, and proper handling, storage, and disposal practices should be followed to minimize risks. Comprehensive risk assessments and adherence to safety guidelines are essential when working with DES [250].
Process Optimization and Scalability: Optimizing DES extraction processes and scaling them up to industrial levels can be challenging. The selection of suitable DES components, determination of optimal operating conditions, and consideration of equipment design and process efficiency are important factors for successful scale-up. Continuous efforts are being made to develop efficient and scalable DES extraction technologies [226,253].
6. Future Research and Outlook
The field of green solvents continues to evolve, and future research holds great potential for further advancements. Numerous areas of focus can drive the development and implementation of green solvents in various industries. Future research can explore the synthesis of novel green solvents with improved properties and performance, such as enhanced selectivity, efficiency, and stability. Additionally, efforts can be directed towards optimizing extraction processes to improve the overall sustainability and economic feasibility of green solvents. Further investigations into the environmental impact and life cycle assessment of green solvents can provide valuable insights for decision-making and guide the development of more sustainable extraction techniques. Furthermore, exploring the compatibility of green solvents with a broader range of solutes and materials can expand their applicability in different extraction processes. By addressing these research areas, future studies can contribute to the continued advancement and adoption of green solvents, promoting sustainable and environmentally friendly practices across various industries. The detailed future outlook for the green solvents is described below.
6.1. Bio-Based Solvents
Given the challenges and limitations associated with bio-based green extraction solvents, future research can focus on addressing these issues and exploring new avenues for improvement. Here are some potential research outlooks:
Biomass feedstock optimization: Further research can be conducted to identify alternative biomass feedstocks that are more readily available, sustainable, and cost-effective. This includes exploring non-food feedstocks, waste materials, or biomass from non-arable land.
Process optimization and cost reduction: Developing more efficient and cost-effective extraction processes is crucial for the economic feasibility of bio-based solvents. Research can focus on optimizing extraction conditions, improving solvent recovery methods, and reducing energy consumption to minimize overall costs.
Solvent property standardization: Due to the variability in biomass composition, the properties of bio-based solvents can vary, which may affect their performance and applicability. Future research can focus on standardizing the properties of bio-based solvents to ensure consistency and reliability in extraction processes.
Compatibility with solute classes: Bio-based solvents may have limited compatibility with certain solute classes, restricting their applicability in certain extraction processes. Further research can investigate ways to enhance the compatibility of bio-based solvents with a wider range of solutes, expanding their utility.
Life cycle assessment and sustainability analysis: Conducting comprehensive life cycle assessments (LCAs) and sustainability analyses can provide a holistic understanding of the environmental impact and sustainability of bio-based green extraction solvents. This research can help identify potential improvements and guide decision-making towards more sustainable extraction processes.
Development of novel bio-based solvents: Research efforts can be directed towards the development of novel bio-based solvents with improved properties and performance. This can involve exploring new biomass sources, employing innovative synthesis techniques, and tailoring solvent structures to enhance selectivity, efficiency, and compatibility.
6.2. Water-Based Solvents
Considering the challenges and discussions surrounding water-based green extraction solvents, several future research directions can be pursued to enhance their effectiveness and overcome limitations. Firstly, research can focus on developing new water-based solvents or modifying existing ones to improve their solubility and selectivity for a wider range of solutes. This can involve the exploration of innovative solvent formulations and the integration of additives or modifiers to enhance their performance. Additionally, efforts can be directed towards optimizing extraction conditions, such as temperature, pressure, and pH, to maximize the efficiency of water-based solvents and improve extraction yields. Moreover, the development of efficient and cost-effective methods for solvent recovery and recycling can significantly enhance the sustainability and economic feasibility of water-based extraction processes. Further studies can also investigate the environmental impact of water-based solvents, including their potential for water pollution or ecosystem disruption, and propose mitigation strategies to minimize any adverse effects. Additionally, exploring the integration of water-based solvents with other green technologies, such as ultrasound-assisted or microwave-assisted extraction, can potentially enhance the extraction efficiency and reduce extraction times. By addressing these research directions, future studies can contribute to the continuous improvement and broader adoption of water-based green extraction solvents, promoting sustainable and environmentally friendly practices in the field of extraction processes.
6.3. Supercritical Fluids
Supercritical Fluid Sources and Availability:
- Exploration of alternative sources for supercritical fluids with high availability and sustainability.
- Development of innovative methods for extracting and producing supercritical fluids from renewable resources.
Process Optimization and Cost Reduction:
- Optimization of supercritical fluid extraction processes to enhance efficiency and reduce energy consumption.
- Development of cost-effective methods for supercritical fluid generation and recovery.
Solvent Property Enhancement:
- Modification of supercritical fluid properties through the addition of modifiers or co-solvents to improve selectivity and extraction efficiency.
- Investigation of new solvents or solvent blends with tailored properties for specific extraction applications.
Environmental Impact and Sustainability:
- Evaluation of the environmental impact and carbon footprint of supercritical fluid extraction processes.
- Implementation of sustainable practices in supercritical fluid production, such as using renewable energy sources and reducing waste generation.
Scale-up and Industrial Application:
- Investigation of scale-up strategies for supercritical fluid extraction processes to facilitate commercial production.
- Application of supercritical fluid extraction in various industries, such as pharmaceuticals, food processing, and natural product extraction.
Process Integration and Optimization:
- Integration of supercritical fluid extraction with other green technologies to enhance overall process efficiency and sustainability.
- Development of innovative extraction techniques and equipment to improve the performance and versatility of supercritical fluid extraction.
6.4. DES Solvents
DES Composition and Properties:
- Exploration of novel DES compositions to expand the range of solvents available for different extraction applications.
- Investigation of the influence of DES composition and structure on extraction efficiency, selectivity, and solute compatibility.
Sustainability and Environmental Impact:
- Development of environmentally friendly and sustainable methods for synthesizing DES.
- Assessment of the environmental impact and biodegradability of DES to ensure their safe and sustainable use.
Process Optimization and Efficiency:
- Optimization of DES extraction processes through the study of extraction parameters, such as temperature, pressure, and solvent-to-feed ratio.
- Development of innovative extraction techniques and equipment to improve the efficiency and scalability of DES-based extraction processes.
Solvent Recovery and Recycling:
- Development of efficient and cost-effective methods for the recovery and recycling of DES to minimize waste generation.
- Exploration of techniques for regenerating and reusing DES to enhance the economic feasibility and sustainability of the extraction process.
Solute Compatibility and Selectivity:
- Investigation of the solute–solvent interactions in DES-based extraction processes to enhance selectivity and improve the extraction of target compounds.
- Understanding the influence of DES composition on solute compatibility and the extraction of different classes of compounds.
Scale-up and Industrial Application:
- Evaluation of the scalability of DES-based extraction processes for industrial applications.
- Demonstration of the feasibility and economic viability of DES extraction on a larger scale in various industries, such as pharmaceuticals, biotechnology, and natural product extraction.
These future research outlooks aim to address the challenges associated with bio-based, water-based, supercritical fluid, and DES green extraction solvents and provide a pathway for their practical implementation. By focusing on these research areas, scientists and engineers can further optimize the performance, sustainability, and economic feasibility of DES-based extraction processes.
7. Conclusions
In conclusion, this review paper highlights the significance of green solvents in bio-oil extraction, focusing on bio-based solvents, water-based solvents, supercritical fluids, and deep eutectic solvents. These green solvents offer environmentally friendly and sustainable alternatives to conventional solvents. To fully realize their potential, addressing challenges in biomass feedstock sourcing, economic feasibility, solvent properties variability, compatibility, and scalability is crucial. By advancing research in biomass supply chains, process optimization, and solvent design, the adoption of green solvents can pave the way for a greener and more sustainable future in extraction processes.
Author Contributions
Conceptualization, M.U.; methodology, M.U.; validation, J.S.C.; investigation, M.U.; writing—original draft preparation, M.U.; writing—review and editing, S.C., S.B. and J.S.C.; supervision, J.S.C.; project administration, J.S.C.; funding acquisition, J.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge the Japanese Government (Ministry of Education, Culture, Sports, Science, and Technology (Monbukagakusho): MEXT) Scholarship for providing financial support and Tokyo Institute of Technology (Tokyo Tech) for funding and providing research facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IPCC Global Warming of 1.5 °C. Available online: https://www.ipcc.ch/sr15/ (accessed on 8 July 2023).
- BP Statistical Review of World Energy 2020. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2020-full-report.pdf (accessed on 8 July 2023).
- Wu, L.; Wei, W.; Chen, Z.; Chen, X.; Ni, B.-J. Long-Chain Alcohol Production in Open Culture Anaerobic Fermentation. Chem. Eng. J. 2023, 452, 139225. [Google Scholar] [CrossRef]
- Zhou, Y.-W.; Kumar, V.; Harirchi, S.; Vigneswaran, V.S.; Murphy, J.D.; Sharma, P.; Tong, Y.W.; Binod, P.; Sindhu, R.; Sarsaiya, S.; et al. Recovery of Value-Added Products from Biowaste: A Review. Bioresour. Technol. 2022, 360, 127565. [Google Scholar] [CrossRef]
- Akindolire, M.A.; Rama, H.; Roopnarain, A. Psychrophilic Anaerobic Digestion: A Critical Evaluation of Microorganisms and Enzymes to Drive the Process. Renew. Sustain. Energy Rev. 2022, 161, 112394. [Google Scholar] [CrossRef]
- Wang, K.; Yun, S.; Xing, T.; Li, B.; Abbas, Y.; Liu, X. Binary and Ternary Trace Elements to Enhance Anaerobic Digestion of Cattle Manure: Focusing on Kinetic Models for Biogas Production and Digestate Utilization. Bioresour. Technol. 2021, 323, 124571. [Google Scholar] [CrossRef]
- Manikandan, S.; Vickram, S.; Sirohi, R.; Subbaiya, R.; Krishnan, R.A.; Karmegam, N.; Sumathijones, C.; Rajagopal, R.; Chang, S.W.; Ravindran, B.; et al. Critical Review of Biochemical Pathways to Transformation of Waste and Biomass into Bioenergy. Bioresour. Technol. 2023, 372, 128679. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Cheng, S.; Cross, J.S. Biomass Feedstocks for Liquid Biofuels Production in Hawaii & Tropical Islands: A Review. Int. J. Renew. Energy Dev. 2021, 11, 111–132. [Google Scholar] [CrossRef]
- Alipour, Z.; Borugadda, V.B.; Wang, H.; Dalai, A.K. Syngas Production through Dry Reforming: A Review on Catalysts and Their Materials, Preparation Methods and Reactor Type. Chem. Eng. J. 2023, 452, 139416. [Google Scholar] [CrossRef]
- Haghighi, M.; Zare, L.; Ghiasi, M. Biodiesel Production from Spirulina Algae Oil over [Cu(H2PDC)(H2O)2] Complex Using Transesterification Reaction: Experimental Study and DFT Approach. Chem. Eng. J. 2022, 430, 132777. [Google Scholar] [CrossRef]
- Kwon, G.; Kim, K.-H.; Kwon, E.E.; Rinklebe, J.; Wang, H.; Kim, K.-H. Beneficial Use of Fe-Impregnated Bentonite as a Catalyst for Pyrolysis of Grass Cut into Syngas, Bio-Oil and Biochar. Chem. Eng. J. 2022, 448, 137502. [Google Scholar] [CrossRef]
- Leng, L.; Zhang, W.; Peng, H.; Li, H.; Jiang, S.; Huang, H. Nitrogen in Bio-Oil Produced from Hydrothermal Liquefaction of Biomass: A Review. Chem. Eng. J. 2020, 401, 126030. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Hu, X.; Gholizadeh, M. Progress in Application of the Pyrolytic Lignin from Pyrolysis of Biomass. Chem. Eng. J. 2021, 419, 129560. [Google Scholar] [CrossRef]
- Usman, M.; Cheng, S.; Cross, J.S. Biodiesel Production from Wet Sewage Sludge and Reduced CO2 Emissions Compared to Incineration in Tokyo, Japan. Fuel 2023, 341, 127614. [Google Scholar] [CrossRef]
- Usman, M.; Cheng, S.; Boonyubol, S.; Cross, J.S. The Future of Aviation Soars with HTL-Based SAFs: Exploring Potential and Overcoming Challenges Using Organic Wet Feedstocks. Sustain. Energy Fuels 2023. [Google Scholar] [CrossRef]
- Moon, M.; Park, W.-K.; Lee, S.-Y.; Hwang, K.-R.; Lee, S.; Kim, M.-S.; Kim, B.; Oh, Y.-K.; Lee, J.Y. Utilization of Whole Microalgal Biomass for Advanced Biofuel and Biorefinery Applications. Renew. Sustain. Energy Rev. 2022, 160, 112269. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Vickram, S.; Dey, N.; Gulothungan, G.; Subbaiya, R.; Govarthanan, M.; Karmegam, N.; Kim, W. The Urge of Algal Biomass-Based Fuels for Environmental Sustainability against a Steady Tide of Biofuel Conflict Analysis: Is Third-Generation Algal Biorefinery a Boon? Fuel 2022, 317, 123494. [Google Scholar] [CrossRef]
- Zhu, Z.; Rosendahl, L.; Toor, S.; Yu, D.; Chen, G. Hydrothermal Liquefaction of Barley Straw to Bio-Crude Oil: Effects of Reaction Temperature and Aqueous Phase Recirculation. Appl. Energy 2015, 137, 183–192. [Google Scholar] [CrossRef]
- Rahman, T.; Jahromi, H.K.; Roy, P.; Adhikari, S.; Hassani, E.; Oh, T.-S. Hydrothermal Liquefaction of Municipal Sewage Sludge: Effect of Red Mud Catalyst in Ethylene and Inert Ambiences. Energy Convers. Manag. 2021, 245, 114615. [Google Scholar] [CrossRef]
- Thomsen, L.; Carvalho, P.; Passos, J.S.D.; Anastasakis, K.; Bester, K.; Biller, P. Hydrothermal Liquefaction of Sewage Sludge; Energy Considerations and Fate of Micropollutants during Pilot Scale Processing. Water Res. 2020, 183, 116101. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Y.; Zhang, J.; Schideman, L.; Yu, G.P.; Zhang, P.; Minarick, M. Co-Liquefaction of Swine Manure and Mixed-Culture Algal Biomass from a Wastewater Treatment System to Produce Bio-Crude Oil. Appl. Energy 2014, 128, 209–216. [Google Scholar] [CrossRef]
- Perrier, A.; Delsart, C.; Boussetta, N.; Grimi, N.; Citeau, M.; Vorobiev, E. Effect of Ultrasound and Green Solvents Addition on the Oil Extraction Efficiency from Rapeseed Flakes. Ultrason. Sonochem. 2017, 39, 58–65. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.P.; Chen, F.; Davis, M.M.; Davison, B.D.; Dixon, R.A.; Gilna, P.; Keller, M.B.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Clark, J.H.; Deswarte, F.E.I.; Farmer, T.W. The Integration of Green Chemistry into Future Biorefineries. Biofuels Bioprod. Biorefin. 2009, 3, 72–90. [Google Scholar] [CrossRef]
- De Jesus, S.S.; Filho, R.M. Recent Advances in Lipid Extraction Using Green Solvents. Renew. Sustain. Energy Rev. 2020, 133, 110289. [Google Scholar] [CrossRef]
- Janicka, P.; Płotka-Wasylka, J.; Szczepańska, N.; Chabowska, A.M.; Fares, M.Y.; Andruch, V.; Kaykhaii, M.; Gębicki, J. Trends in the New Generation of Green Solvents in Extraction Processes. Curr. Opin. Green Sustain. Chem. 2022, 37, 100670. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, S.; Banerjee, R.; Agarwal, D.C.; Kulkarni, K.S.; Ramesh, K.P. Green Solvents and Technologies for Oil Extraction from Oilseeds. Chem. Cent. J. 2017, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Af, T. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, E.; Ma, F.; Jiang, K. An Integrated Biorefinery Process for Co-Production of Xylose and Glucose Using Maleic Acid as Efficient Catalyst. Bioresour. Technol. 2021, 325, 124698. [Google Scholar] [CrossRef] [PubMed]
- Stephan, C.; Dicko, M.; Stringari, P.; Coquelet, C. Liquid-Liquid Equilibria of Water + Solutes (Acetic Acid/Acetol/Furfural/Guaiacol/Methanol/Phenol/Propanal) + Solvents (Isopropyl Acetate/Toluene) Ternary Systems for Pyrolysis Oil Fractionation. Fluid Phase Equilibria 2018, 468, 49–57. [Google Scholar] [CrossRef]
- Kanaujia, P.K.; Naik, D.V.; Tripathi, D.; Singh, R.; Poddar, M.K.; Konathala, L.N.S.; Sharma, Y. Pyrolysis of Jatropha Curcas Seed Cake Followed by Optimization of Liquid-liquid Extraction Procedure for the Obtained Bio-Oil. J. Anal. Appl. Pyrolysis 2016, 118, 202–224. [Google Scholar] [CrossRef]
- Kumar, S.; Lange, J.-P.; Van Rossum, G.; Kersten, S.R.A. Bio-Oil Fractionation by Temperature-Swing Extraction: Principle and Application. Biomass Bioenergy 2015, 83, 96–104. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, X.; Jiang, X.; Liu, S.; Lin, J.; Lin, X.; Zhang, Y.; Shi, C.; Chaohua, Z.; Yang, J. Application of Polysaccharide-Rich Solution Derived from Waste Macroalgae Enteromorpha Prolifera in Cherry Tomato Preservation and Utilizing Post-Extraction Residue for Crude Bio-Oil Production. Food Chem. 2023, 409, 135301. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.; Xu, D.; Wang, Y.; Wang, Y.; Kapusta, K.; Guo, Y. Catalytic Hydrothermal Liquefaction of Municipal Sludge for Biocrude Production over Non-Noble Bimetallic Catalyst in Ethanol Solvent. Fuel 2023, 331, 125812. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, H.; Xu, S.; Qian, L.-X.; Li, H.; Gao, J.; Zhao, G.; Ray, M.B.; Xu, C. Influence of Extraction Solvents on the Recovery Yields and Properties of Bio-Oils from Woody Biomass Liquefaction in Sub-Critical Water, Ethanol or Water–Ethanol Mixed Solvent. Fuel 2022, 307, 121930. [Google Scholar] [CrossRef]
- Kumar, R.; Strezov, V.; Lu, X.; Kan, T.; Weldekidan, H.; He, J.; Dastjerdi, B.; Scott, J. Bio-Oil Upgrading with Catalytic Pyrolysis of Biomass Using Copper/Zeolite-Nickel/Zeolite and Copper-Nickel/Zeolite Catalysts. Bioresour. Technol. 2019, 279, 404–409. [Google Scholar] [CrossRef]
- Li, B.; Song, M.; Xie, X.; Wei, J.; Xu, D.; Ding, K.; Huang, Y.; Zhang, S.; Hu, X.; Zhang, S.; et al. Oxidative Fast Pyrolysis of Biomass in a Quartz Tube Fluidized Bed Reactor: Effect of Oxygen Equivalence Ratio. Energy 2023, 270, 126987. [Google Scholar] [CrossRef]
- Aravind, S.V.; Ahmed, G.; Kishore, N. Pyrolysis of Delonix Regia Using Metal Oxide Catalysts and Solvent Effect on Fuel Fraction of Bio-Oil. Results Eng. 2023, 17, 100876. [Google Scholar] [CrossRef]
- Iliopoulou, E.F.; Pachatouridou, E.; Marianou, A.A.; Michailof, C.; Kalogiannis, K.K.; Lappas, A.A. Catalytic Pyrolysis of Olive Mill Wastes towards Advanced Bio-Fuels and Bio-Chemicals Using Metal Oxide Catalysts. Catal. Today 2023, 420, 114151. [Google Scholar] [CrossRef]
- Andrade, Y.; Schneider, J.K.; Farrapeira, R.; Lucas, A.N.L.; Da Mota, I.D.P.; Bjerk, T.R.; Krause, L.C.; Caramão, E.B.; Hynek, R. Chromatographic Analysis of N-compounds from the Pyrolysis of Spent Coffee Grounds. SSC Plus 2022, 6, 2200057. [Google Scholar] [CrossRef]
- Li, H.; Xia, S.; Li, Y.; Ma, P.; Zhao, C. Stability Evaluation of Fast Pyrolysis Oil from Rice Straw. Chem. Eng. Sci. 2015, 135, 258–265. [Google Scholar] [CrossRef]
- Li, H.; Xia, S.; Ma, P. Upgrading Fast Pyrolysis Oil: Solvent–Anti-Solvent Extraction and Blending with Diesel. Energy Convers. Manag. 2016, 110, 378–385. [Google Scholar] [CrossRef]
- Tsirigka, A.; Ntoula, M.; Kontogiannopoulos, K.N.; Karabelas, A.J.; Patsios, S.I. Optimization of Solvent Extraction of Lipids from Yarrowia Lipolytica towards Industrial Applications. Fermentation 2022, 9, 35. [Google Scholar] [CrossRef]
- Shafi, A.; Farooq, U.; Akram, K.; Majeed, H.; Hakim, A.; Jayasinghe, M. Cucumis Melo Seed Oil: Agro-food By-product with Natural Anti-hyperlipidemic Potential. J. Sci. Food Agric. 2022, 103, 1644–1650. [Google Scholar] [CrossRef]
- Özcan, M.M.; Öztürk, Ö.; Lemiasheuski, V. Quality Properties, Fatty Acid Composition, and Mineral Contents of Some Citrus Seeds and Oils Extracted by Solvent Extraction. Erwerbs-Obstbau 2022, 65, 127–132. [Google Scholar] [CrossRef]
- Abreu-Jaureguí, C.; Reynel-Ávila, H.E.; Bonilla-Petriciolet, A. Biodiesel Production from Wastewater Scum of Dairy Industry: Lipid Extraction Studies and Reaction Routes. Fuel 2023, 342, 127868. [Google Scholar] [CrossRef]
- Usman, M.; Shou, C.; Cross, J.S. Biodiesel Production from Urban and Suburban Municipal Sewage Sludges in Tokyo, Japan. In Proceedings of the 2022 International Conference and Utility Exhibition on Energy, Environment and Climate Change (ICUE), Pattaya, Thailand, 26–28 October 2022. [Google Scholar] [CrossRef]
- Patiño, Y.; Mantecón, L.; Polo, S.M.T.; Faba, L.; Díaz, E.; Ordóñez, S. Effect of Sludge Features and Extraction-Esterification Technology on the Synthesis of Biodiesel from Secondary Wastewater Treatment Sludges. Bioresour. Technol. 2018, 247, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Dancs, G.; Kakucska, G.; Dobrányi, S.; Ecker, J.; Fülöp, L. Efficient Method for the Determination of the Neutral Lipid Content of Oil-Producing Microalgae Strains Required for Biodiesel. Fuel 2023, 331, 125831. [Google Scholar] [CrossRef]
- Eswaramoorthi, Y.; Pandian, S.; Sahadevan, R. Kinetic studies on the extraction of oil from a new feedstock (Chukrasia tabularis L. seed) for biodiesel production using a heterogeneous catalyst. Environ. Sci. Pollut. Res. 2022, 30, 14565–14579. [Google Scholar] [CrossRef]
- Ntalikwa, J.W. Solvent Extraction of Jatropha Oil for Biodiesel Production: Effects of Solvent-to-Solid Ratio, Particle Size, Type of Solvent, Extraction Time, and Temperature on Oil Yield. J. Renew. Energy 2021, 2021, 9221168. [Google Scholar] [CrossRef]
- Kesharvani, S.; Dwivedi, G.; Verma, T.N.; Verma, P. Sustainable Alternative Fuel Derived from Different Feedstocks and Its Comparative Life Cycle Assessment. Sustain. Energy Technol. Assess. 2023, 57, 103159. [Google Scholar] [CrossRef]
- HSE Solvents—Controlling Hazardous Substances in Construction. Available online: https://www.hse.gov.uk/construction/healthrisks/hazardous-substances/solvents.htm (accessed on 8 July 2023).
- European Commission Extraction Solvents. Available online: https://food.ec.europa.eu/safety/food-improvement-agents/extraction-solvents_en (accessed on 8 July 2023).
- Rury, M. The Hidden Dangers of Organic Solvents. Biotage 2023. Available online: https://www.biotage.com/blog/the-hidden-dangers-of-organic-solvents (accessed on 8 July 2023).
- EPA Indoor Air Quality (IAQ) | US EPA. Available online: https://www.epa.gov/indoor-air-quality-iaq (accessed on 8 July 2023).
- EPA What Are Volatile Organic Compounds (VOCs)? | US EPA. Available online: https://www.epa.gov/indoor-air-quality-iaq/what-are-volatile-organic-compounds-vocs (accessed on 8 July 2023).
- Bamai, Y.A. Semi-Volatile Organic Compounds (SVOCs): Phthalates and Phosphorous Frame Retardants and Health Risks. In Current Topics in Environmental Health and Preventive Medicine; Springer Nature: Berlin/Heidelberg, Germany, 2019; pp. 159–178. [Google Scholar]
- Salthammer, T. Very Volatile Organic Compounds: An Understudied Class of Indoor Air Pollutants. Indoor Air 2014, 26, 25–38. [Google Scholar] [CrossRef]
- EPA Methanol. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/methanol.pdf (accessed on 8 July 2023).
- ATSDR Methanol, Toxicological Profiles | ATSDR. Available online: https://www.atsdr.cdc.gov/toxprofiledocs/index.html (accessed on 8 July 2023).
- ECHA Substance Information—ECHA. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.028.773 (accessed on 8 July 2023).
- CDC CDC—NIOSH Pocket Guide to Chemical Hazards—Methyl Alcohol. Available online: https://www.cdc.gov/niosh/npg/npgd0397.html (accessed on 8 July 2023).
- DCCEEW Methanol. Available online: https://www.dcceew.gov.au/environment/protection/npi/substances/fact-sheets/methanol#:~:text=Methanol%20may%20affect%20animals%2C%20birds,affect%20their%20appearance%20or%20behaviour (accessed on 8 July 2023).
- Bell-Young, L. What Is Dichloromethane? ReAgent Chemical Services. 2021. Available online: https://www.chemicals.co.uk/blog/what-is-dichloromethane (accessed on 8 July 2023).
- CDC Methylene Chloride | Toxicological Profile | ATSDR. Available online: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=234&tid=42 (accessed on 8 July 2023).
- ECHA Dichloromethane: Substance Information—ECHA. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.000.391 (accessed on 8 July 2023).
- EPA Methylene Chloride (Dichloromethane). Available online: https://www.epa.gov/sites/default/files/2016-09/documents/methylene-chloride.pdf (accessed on 8 July 2023).
- DCCEEW Ethanol (Ethyl Alcohol)—DCCEEW. Available online: https://www.dcceew.gov.au/environment/protection/npi/substances/fact-sheets/ethanol-ethyl-alcohol (accessed on 8 July 2023).
- US EPA TLVs and BEIs: Based on the Documentation of the Threshold Limit Values for Chemical Substances and Physical Agents & Biological Exposure Indices. Available online: https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/7310932 (accessed on 8 July 2023).
- CDC CDC—NIOSH Pocket Guide to Chemical Hazards—Endrin. Available online: https://www.cdc.gov/niosh/npg/npgd0252.html (accessed on 8 July 2023).
- EPA Ethanol. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/ethanol.pdf (accessed on 8 July 2023).
- EPA Chloroform. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/chloroform.pdf (accessed on 8 July 2023).
- DCCEEW Chloroform (Trichloromethane). Available online: https://www.dcceew.gov.au/environment/protection/npi/substances/fact-sheets/chloroform-trichloromethane (accessed on 8 July 2023).
- CDC Chloroform | Toxicological Profile | ATSDR. Available online: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=53&tid=16 (accessed on 8 July 2023).
- IARC IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Available online: https://publications.iarc.fr/_publications/media/download/2931/d7a4e802483b1374482768a36a7c78e1b33aa1c8.pdf (accessed on 8 July 2023).
- EPA Hexane. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/hexane.pdf (accessed on 8 July 2023).
- National Academies Press (US) N-Hexane: Acute Exposure Guideline Levels. Available online: https://www.ncbi.nlm.nih.gov/books/NBK201488/#:~:text=In%20humans%2C%20n%2Dhexane%20is,n%2Dhexane%20or%20commercial%20hexane (accessed on 8 July 2023).
- CDC N-Hexane | Toxicological Profile | ATSDR. Available online: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=393&tid=68 (accessed on 8 July 2023).
- CDC NIOSH Pocket Guide to Chemical Hazards-Hexafluoroacetone. Available online: https://www.cdc.gov/niosh/npg/npgd0319.html (accessed on 8 July 2023).
- NJ Diethyl Ether. Available online: https://nj.gov/health/eoh/rtkweb/documents/fs/0701.pdf (accessed on 8 July 2023).
- HS Toxicological Summary for: Ethyl Ether. Available online: https://www.health.state.mn.us/communities/environment/risk/docs/guidance/gw/ethylether.pdf (accessed on 8 July 2023).
- CDC—NIOSH Pocket Guide to Chemical Hazards—Dichlorvos. Available online: https://www.cdc.gov/niosh/npg/npgd0202.html (accessed on 8 July 2023).
- EPA Ethyl Ether. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/ethyl-ether.pdf (accessed on 8 July 2023).
- Acetone: General Information. Available online: https://www.gov.uk/government/publications/acetone-properties-and-incident-management/acetone-general-information (accessed on 8 July 2023).
- DCCEEW Acetone. Available online: https://www.dcceew.gov.au/environment/protection/npi/substances/fact-sheets/acetone#:~:text=It%20can%20move%20from%20the,can%20be%20degraded%20within%20days (accessed on 8 July 2023).
- CDC Acetone | Toxicological Profile | ATSDR. Available online: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=5&tid=1 (accessed on 8 July 2023).
- CDC NIOSH Pocket Guide to Chemical Hazards -Acetylene Tetrabromide. Available online: https://www.cdc.gov/niosh/npg/npgd0009.html (accessed on 8 July 2023).
- CDC Acetone. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/acetone.pdf (accessed on 8 July 2023).
- Benzene—DCCEEW. Available online: https://www.dcceew.gov.au/environment/protection/npi/resource/student/benzene#:~:text=What%20effect%20does%20benzene%20have,to%20contaminate%20water%20and%20soil (accessed on 8 July 2023).
- Duarte-Davidson, R.; Courage, C.; Rushton, L.; Levy, L. Benzene in the environment: An assessment of the potential risks to the health of the population. Occup. Environ. Med. 2001, 58, 2–13. [Google Scholar] [CrossRef]
- CDC Benzene | Toxicological Profile | ATSDR. Available online: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=40&tid=14 (accessed on 8 July 2023).
- EPA Toxicological Profile for Benzene | US EPA. Available online: https://www.epa.gov/foia/toxicological-profile-benzene (accessed on 8 July 2023).
- Iarc Benzene. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Benzene-2018 (accessed on 8 July 2023).
- EPA Benzene. Available online: https://www.epa.gov/sites/default/files/2020-07/documents/benzene.pdf (accessed on 8 July 2023).
- NJ Furfural-Hazardous Substance Fact Sheet. Available online: https://nj.gov/health/eoh/rtkweb/documents/fs/0953.pdf (accessed on 8 July 2023).
- CDC Fluorides, Hydrogen Fluoride, and Fluorine | Toxicological Profile | ATSDR. Available online: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=212&tid=38 (accessed on 8 July 2023).
- CDC CDC—NIOSH Pocket Guide to Chemical Hazards—Grain Dust (Oat, Wheat, Barley). Available online: https://www.cdc.gov/niosh/npg/npgd0305.html (accessed on 8 July 2023).
- EPA Furfural (Furan-2-Carbaldehyde). Available online: https://www.epa.gov/sites/default/files/2016-09/documents/furfural.pdf (accessed on 8 July 2023).
- Morales-Gonzalez, O.M.; Zhang, C.; Li, S.; Hessel, V. Solvent Impact Assessment for the “One-Flow Functional Solvent Factory”. Chem. Eng. Sci. X 2019, 3, 100024. [Google Scholar] [CrossRef]
- CDC CDC—NIOSH Pocket Guide to Chemical Hazards—Acetylsalicylic Acid. Available online: https://www.cdc.gov/niosh/npg/npgd0010.html (accessed on 8 July 2023).
- EPA Acetic Acid. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/acetic-acid.pdf (accessed on 8 July 2023).
- Atsdr Toxicological Profile for Stoddard Solvent. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp79.pdf (accessed on 8 July 2023).
- CDC NIOSH Pocket Guide to Chemical Hazards-Phosgene. Available online: https://www.cdc.gov/niosh/npg/npgd0504.html (accessed on 8 July 2023).
- EPA Guidance for Hazardous Waste Combustion Units—Petroleum Refining Industry. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/petroleum-refining-industry.pdf (accessed on 8 July 2023).
- Sheldon, R.A. Green Solvents for Sustainable Organic Synthesis: State of the Art. Green Chem. 2005, 7, 267. [Google Scholar] [CrossRef]
- Conelli, D.; Margiotta, N.; Grisorio, R.; Suranna, G.P. Implementation of Sustainable Solvents in Green Polymerization Approaches. Macromol. Chem. Phys. 2020, 222, 2000382. [Google Scholar] [CrossRef]
- Marteel-Parrish, A.E.; Abraham, M.A. Green Chemistry and Engineering: A Pathway to Sustainability. 2013. Available online: https://www.perlego.com/book/997542/green-chemistry-and-engineering-a-pathway-to-sustainability-pdf (accessed on 8 July 2023).
- Imperato, G.; König, B.; Chiappe, C. Ionic Green Solvents from Renewable Resources. Eur. J. Org. Chem. 2007, 2007, 1049–1058. [Google Scholar] [CrossRef]
- Warner, P.T.A.J.C. Green Chemistry: Theory and Practice. CiNii Books. 1998. Available online: https://www.amazon.com/Green-Chemistry-Practice-Paul-Anastas/dp/0198506988 (accessed on 8 July 2023).
- Dicks, A.P.; Hent, A. Green Chemistry Metrics: A Guide to Determining and Evaluating Process Greenness; Springer: Berlin/Heidelberg, Germany, 2014; Available online: https://link.springer.com/book/10.1007/978-3-319-10500-0 (accessed on 8 July 2023).
- Sheldon, R.A. Metrics of Green Chemistry and Sustainability: Past, Present, and Future. ACS Sustain. Chem. Eng. 2017, 6, 32–48. [Google Scholar] [CrossRef]
- Erythropel, H.C.; Zimmerman, J.B.; De Winter, T.M.; Petitjean, L.; Melnikov, F.; Lam, C.B.I.; Lounsbury, A.W.; Mellor, K.E.; Janković, N.; Tu, Q.; et al. The Green ChemisTREE: 20 Years after Taking Root with the 12 Principles. Green Chem. 2018, 20, 1929–1961. [Google Scholar] [CrossRef]
- Pereira, C.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl Lactate as a Solvent: Properties, Applications and Production Processes—A Review. Green Chem. 2011, 13, 2658. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Kim, K.-H. Green Synthesis of Metal–Organic Frameworks: A State-of-the-Art Review of Potential Environmental and Medical Applications. Coord. Chem. Rev. 2020, 420, 213407. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical Fluid Extraction and Fractionation of Natural Matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.B.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Smith, E.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.; Looi, C.Y. Applications of Deep Eutectic Solvents in Biotechnology and Bioengineering—Promises and Challenges. Biotechnol. Adv. 2017, 35, 105–134. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.Z.; Hussein, H.A.; Alshajrawi, O.M.S. Ethyl Lactate as a Green Solvent in the Pharmaceutical Industry. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 185–194. [Google Scholar] [CrossRef]
- Virot, M.; Tomao, V.; Ginies, C.; Chemat, F. Total Lipid Extraction of Food Using D-Limonene as an Alternative to n-Hexane. Chromatographia 2008, 68, 311–313. [Google Scholar] [CrossRef]
- Soszka, E.; Jȩdrzejczyk, M.; Keller, N.; Ruppert, A.M. High Yield Production of 2-Methyltetrahydrofuran Biofuel with Reusable Ni-Co Catalysts. Fuel 2023, 332, 126118. [Google Scholar] [CrossRef]
- Raj, T.; Chandrasekhar, K.; Banu, J.R.; Rene, E.R.; Aslam, M.; Kim, S.H. Synthesis of γ-Valerolactone (GVL) and Their Applications for Lignocellulosic Deconstruction for Sustainable Green Biorefineries. Fuel 2021, 303, 121333. [Google Scholar] [CrossRef]
- Pyo, S.-H.; Park, J.-W.; Chang, T.-S.; Hatti-Kaul, R. Dimethyl Carbonate as a Green Chemical. Curr. Opin. Green Sustain. Chem. 2017, 5, 61–66. [Google Scholar] [CrossRef]
- Lim, J.H.; Chua, L.S.; Mustaffa, A.A. Ionic Liquids as Green Solvent and Their Applications in Bioactive Compounds Extraction from Plants. Process Biochem. 2022, 122, 292–306. [Google Scholar] [CrossRef]
- Da Silva, R.V.; Rocha-Santos, T.; Duarte, A.C. Supercritical Fluid Extraction of Bioactive Compounds. Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Talibi, M.; Hellier, P.; Ladommatos, N. Investigating the Combustion and Emissions Characteristics of Biomass-Derived Platform Fuels as Gasoline Extenders in a Single Cylinder Spark-Ignition Engine; SAE International: Beijing, China, 2017. [Google Scholar]
- Teoh, K.-S.; Melchiorre, M.; Kreth, F.A.; Bothe, A.; Köps, L.; Ruffo, F.; Balducci, A. Γ-Valerolactone as Sustainable and Low-Toxic Solvent for Electrical Double Layer Capacitors. ChemSusChem 2022, 16, e202201845. [Google Scholar] [CrossRef]
- Fiorani, G.; Perosa, A.; Selva, M. Dimethyl Carbonate: A Versatile Reagent for a Sustainable Valorization of Renewables. Green Chem. 2018, 20, 288–322. [Google Scholar] [CrossRef]
- O’Neill, M.; Sankar, M.; Hintermair, U. Sustainable Synthesis of Dimethyl- and Diethyl Carbonate from CO2 in Batch and Continuous Flow—Lessons from Thermodynamics and the Importance of Catalyst Stability. ACS Sustain. Chem. Eng. 2022, 10, 5243–5257. [Google Scholar] [CrossRef]
- Choi, Y.D.; Verpoorte, R. Green Solvents for the Extraction of Bioactive Compounds from Natural Products Using Ionic Liquids and Deep Eutectic Solvents. Curr. Opin. Food Sci. 2019, 26, 87–93. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, S.; Ma, P. Use of Ionic Liquids as ‘Green’ Solvents for Extractions. J. Chem. Technol. Biotechnol. 2005, 80, 1089–1096. [Google Scholar] [CrossRef]
- Kong, X.-J.; Li, J.-R. An Overview of Metal–Organic Frameworks for Green Chemical Engineering. Engineering 2021, 7, 1115–1139. [Google Scholar] [CrossRef]
- Souza, M.M.V.M.; Santos, M.; Sumere, B.R.; Da Silva, L.C.; Cunha, D.; Martínez, J.; Barbero, G.F. Isolation of Gallic Acid, Caffeine and Flavonols from Black Tea by on-Line Coupling of Pressurized Liquid Extraction with an Adsorbent for the Production of Functional Bakery Products. Lebensm.-Wiss. Technol. 2020, 117, 108661. [Google Scholar] [CrossRef]
- Patra, A.; Abdullah, S.; Pradhan, R.C. Review on the Extraction of Bioactive Compounds and Characterization of Fruit Industry By-Products. Bioresour. Bioprocess. 2022, 9, 14. [Google Scholar] [CrossRef]
- Mahindrakar, K.V.; Rathod, V.K. Ultrasonic Assisted Aqueous Extraction of Catechin and Gallic Acid from Syzygium Cumini Seed Kernel and Evaluation of Total Phenolic, Flavonoid Contents and Antioxidant Activity. Chem. Eng. Process. 2020, 149, 107841. [Google Scholar] [CrossRef]
- Da Silva, L.C.; Souza, M.M.V.M.; Sumere, B.R.; Silva, L.F.; Da Cunha, D.T.; Barbero, G.F.; Bezerra, R.M.N.; Martínez, J. Simultaneous Extraction and Separation of Bioactive Compounds from Apple Pomace Using Pressurized Liquids Coupled On-Line with Solid-Phase Extraction. Food Chem. 2020, 318, 126450. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, H.; Chua, B.L.; Mah, S.H.; Chow, Y.H. An Insight into Extraction, Isolation, Identification and Quantification of Bioactive Compounds from Crataegus monogyna Plant Extract. Rev. Agric. Sci. J.-STAGE 2022, 10, 304–327. [Google Scholar] [CrossRef]
- Muhamad, I.I.; Hassan, N.A.; Mamat, S.S.; Nawi, N.M.; Rashid, W.; Tan, N.A.H. Extraction Technologies and Solvents of Phytocompounds from Plant Materials: Physicochemical Characterization and Identification of Ingredients and Bioactive Compounds from Plant Extract Using Various Instrumentations. In Ingredients Extraction by Physicochemical Methods in Food; Academic Press: London, UK, 2017; pp. 523–560. [Google Scholar]
- Alternative Solvents for Natural Products Extraction; Springer Nature: Berlin/Heidelberg, Germany, 2014; Available online: https://link.springer.com/book/10.1007/978-3-662-43628-8 (accessed on 8 July 2023).
- Chemat, F.; Strube, J. Green Extraction of Natural Products: Theory and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2016; Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9783527676828 (accessed on 8 July 2023).
- Zhou, F.; Hearne, Z.; Li, C.-J. Water—the Greenest Solvent Overall. Curr. Opin. Green Sustain. Chem. 2019, 18, 118–123. [Google Scholar] [CrossRef]
- McHugh, M.; Krukonis, V. Supercritical Fluid Extraction: Principles and Practice; Elsevier: Amsterdam, The Netherlands, 2013; Available online: https://www.sciencedirect.com/book/9780080518176/supercritical-fluid-extraction (accessed on 8 July 2023).
- Ray, A.; Dubey, K.; Marathe, S.J.; Singhal, R. Supercritical Fluid Extraction of Bioactives from Fruit Waste and Its Therapeutic Potential. Food Biosci. 2023, 52, 102418. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, H.; Xu, Y.; Hu, M.; Hu, Z.-T.; Wang, J.; Pan, Z. Reactor for Biomass Conversion and Waste Treatment in Supercritical Water: A Review. Renew. Sustain. Energy Rev. 2023, 171, 113031. [Google Scholar] [CrossRef]
- Koschinsky, A.; Garbe-Schönberg, D.; Sander, S.G.; Schmidt, K.; Gennerich, H.-H.; Strauss, H. Hydrothermal Venting at Pressure-Temperature Conditions above the Critical Point of Seawater, 5 °S on the Mid-Atlantic Ridge. Geology 2008, 36, 615. [Google Scholar] [CrossRef]
- Al-Muntaser, A.A.; Varfolomeev, M.A.; Suwaid, M.A.; Feoktistov, D.A.; Yuan, C.; Klimovitskii, A.E.; Gareev, B.I.; Djimasbe, R.; Nurgaliev, D.K.; Kudryashov, S.I.; et al. Hydrogen Donating Capacity of Water in Catalytic and Non-Catalytic Aquathermolysis of Extra-Heavy Oil: Deuterium Tracing Study. Fuel 2021, 283, 118957. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, D.; Hao, B.; Guo, S.; Guo, Y.; Wang, S. Chemical Reactions of Organic Compounds in Supercritical Water Gasification and Oxidation. Water Res. 2021, 190, 116634. [Google Scholar] [CrossRef]
- Subramani, V.B.; Atanda, L.; Doherty, W.O.S.; Rackemann, D.W.; Moghaddam, L. Co-Liquefaction of Cotton Gin Trash and Low-Density Polyethylene Wastes via Supercritical Ethanolysis for Hydrocarbon-Rich Oil. Energy Convers. Manag. 2023, 290, 117216. [Google Scholar] [CrossRef]
- Wang, X.; Xu, W.; Zhang, D.; Li, X.; Shi, J. Structural Characteristics–Reactivity Relationships for Catalytic Depolymerization of Lignin into Aromatic Compounds: A Review. Int. J. Mol. Sci. 2023, 24, 8330. [Google Scholar] [CrossRef] [PubMed]
- Brand, S.; Hardi, F.; Kim, J.; Suh, D.I. Effect of Heating Rate on Biomass Liquefaction: Differences between Subcritical Water and Supercritical Ethanol. Energy 2014, 68, 420–427. [Google Scholar] [CrossRef]
- Furusjö, E.; Akalin, M.; Karagöz, S. Experimental Design for Extraction of Bio-Oils from Flax Seeds under Supercritical Ethanol Conditions. Clean Technol. Environ. Policy 2015, 18, 461–471. [Google Scholar] [CrossRef]
- Zeb, H.; Choi, J.; Kim, Y.; Kim, J. A New Role of Supercritical Ethanol in Macroalgae Liquefaction (Saccharina japonica): Understanding Ethanol Participation, Yield, and Energy Efficiency. Energy 2017, 118, 116–126. [Google Scholar] [CrossRef]
- Kuo, T.M.; Gardner, H. Lipid Biotechnology; CRC Press: Boca Raton, FL, USA, 2002; Available online: https://www.amazon.com/Lipid-Biotechnology-Tsung-Min-Kuo/dp/0824706196 (accessed on 8 July 2023).
- King, J.W. Supercritical Fluid Technology for Lipid Extraction, Fractionation, and Reactions. In Lipid Biotechnology; Taylor and Francis: Abingdon, UK, 2002; pp. 656–680. [Google Scholar] [CrossRef]
- Sun, X.; Li, X.; Tan, X.; Zheng, W.; Zhu, G.; Cai, J.; Zhang, Y.-Y. Pyrolysis of Heavy Oil in Supercritical Multi-Thermal Fluid: An Effective Recovery Agent for Heavy Oils. J. Pet. Sci. Eng. 2021, 196, 107784. [Google Scholar] [CrossRef]
- Ma, C. Injection Production Process of Fluids Produced by Supercritical Water. Oxidation. Patent CN102322248A, 18 January 2021. [Google Scholar]
- Prabhune, A.; Dey, R. Green and Sustainable Solvents of the Future: Deep Eutectic Solvents. J. Mol. Liq. 2023, 379, 121676. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.-L.; Chen, C.-T.A.; Sun, P.; Patel, A.K.; Singhania, R.R.; Nargotra, P.; Dong, C. Deep Eutectic Solvents as Promising Pretreatment Agents for Sustainable Lignocellulosic Biorefineries: A Review. Bioresour. Technol. 2022, 360, 127631. [Google Scholar] [CrossRef] [PubMed]
- Atilhan, M.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep Eutectic Solvents: Physicochemical Properties and Gas Separation Applications. Energy Fuels 2015, 29, 2616–2644. [Google Scholar] [CrossRef]
- El-Deen, A.K.; Shimizu, K. Deep Eutectic Solvents as Promising Green Solvents in Dispersive Liquid–Liquid Microextraction Based on Solidification of Floating Organic Droplet: Recent Applications, Challenges and Future Perspectives. Molecules 2021, 26, 7406. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Welton, T. Solvents and Sustainable Chemistry. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015, 471, 20150502. [Google Scholar] [CrossRef]
- Oklu, N.K.; Matsinha, L.C.; Makhubela, B.C.E. Bio-Solvents: Synthesis, Industrial Production and Applications. In Solvents, Ionic Liquids and Solvent Effects; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- PubChem Water. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/962 (accessed on 8 July 2023).
- The Drive to Make Things Happen. Available online: https://vrchemistry.chem.ox.ac.uk/potential/text/solutions3.htm (accessed on 8 July 2023).
- PubChem Hazardous Substances Data Bank (HSDB): 82. Available online: https://pubchem.ncbi.nlm.nih.gov/source/hsdb/82#section=FDA-Requirements-(Complete) (accessed on 8 July 2023).
- Sierra-Amor, R.I. CRC Handbook of Laboratory Safety, 5th ed.; Keith Furr, A., Ed.; CRC Press LCC: Boca Raton, FL, USA, 2000; p. 774. ISBN 0-8493-2523-4. [Google Scholar] [CrossRef]
- Vedantu Ethyl Acetate. VEDANTU. 2022. Available online: https://www.vedantu.com/chemistry/ethyl-acetate (accessed on 8 July 2023).
- LSU Ethyl Acetate Solvent Properties. Available online: https://macro.lsu.edu/howto/solvents/ethylacetate.htm (accessed on 8 July 2023).
- LSU Ethyl Ether Solvent Properties. Available online: https://macro.lsu.edu/howto/solvents/ether.htm (accessed on 8 July 2023).
- PubChem Diethyl Ether. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Diethyl-Ether#section=Computed-Descriptors (accessed on 8 July 2023).
- ICSC 1164—Methyl Tert-Butyl Ether. Available online: https://inchem.org/documents/icsc/icsc/eics1164.htm (accessed on 8 July 2023).
- GESTIS-Stoffdatenbank. Available online: https://gestis.dguv.de/data?name=510639&lang=en (accessed on 8 July 2023).
- Pace, V.; Holzer, W.; Hoyos, P.; Hernáiz, M.J.; Alcántara, A.R. 2-Methyltetrahydrofuran. In Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 1–6. [Google Scholar] [CrossRef]
- GESTIS-Stoffdatenbank. Available online: https://gestis.dguv.de/data?name=025460&lang=en (accessed on 8 July 2023).
- Dimethylether. Available online: https://web.archive.org/web/20211106032850/https://encyclopedia.airliquide.com/dimethylether (accessed on 8 July 2023).
- PubChem Cyclohexane. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cyclohexane#section=Structures (accessed on 8 July 2023).
- DCCEEW Cyclohexane. Available online: https://www.dcceew.gov.au/environment/protection/npi/substances/fact-sheets/cyclohexane (accessed on 8 July 2023).
- Admin Benzene (C6H6)—Definition, Discovery, Structure, Resonance, Aromaticity & Uses of Benzene (C6H6). BYJUS 2023. Available online: https://byjus.com/chemistry/benzene/ (accessed on 8 July 2023).
- DCCEEW Carbon Disulfide. Available online: https://www.dcceew.gov.au/environment/protection/npi/substances/fact-sheets/carbon-disulfide#:~:text=Pure%20carbon%20disulfide%20is%20a,at%200.016%20to%200.42%20ppm (accessed on 8 July 2023).
- Worldofchemicals. Glycerol: Properties, Production and Uses. 2017. Available online: worldofchemicals.com (accessed on 8 July 2023).
- Wikipedia. Wikipedia Contributors National Institute for Occupational Safety and Health. 2023. Available online: https://en.wikipedia.org/wiki/National_Institute_for_Occupational_Safety_and_Health (accessed on 8 July 2023).
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone—A Sustainable Liquid for Energy and Carbon-Based Chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Baird, Z.S.; Uusi-Kyyny, P.; Pokki, J.-P.; Pedegert, E.; Alopaeus, V. Vapor Pressures, Densities, and PC-SAFT Parameters for 11 Bio-Compounds. Int. J. Thermophys. 2019, 40, 102. [Google Scholar] [CrossRef]
- 5614-37-9 CAS | Cyclopentyl Methyl Ether (CPME) | High Purity Solvents | Article No. 03139. Available online: https://www.lobachemie.com/High-Purity-Solvents-03139/CYCLOPENTYL-METHYL-ETHER-CPME-CASNO-5614-37-9.aspx (accessed on 8 July 2023).
- Cyclopentyl Methyl Ether (CPME) | Specialty Solvents | Zeon Corporation. Available online: https://www.zeon.co.jp/en/business/enterprise/special/solvent-cpme/ (accessed on 8 July 2023).
- Gov, N.O. of R. and R.U. D-LIMONENE | CAMEO Chemicals | NOAA. Available online: https://cameochemicals.noaa.gov/chemical/20568 (accessed on 8 July 2023).
- Sonu, K.S.; Mann, B.; Sharma, R.; Kumar, R.; Singh, R. Physico-Chemical and Antimicrobial Properties of d-Limonene Oil Nanoemulsion Stabilized by Whey Protein–Maltodextrin Conjugates. J. Food Sci. Technol. 2018, 55, 2749–2757. [Google Scholar] [CrossRef]
- P-Cymene | 99-87-6. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB9262508.htm (accessed on 8 July 2023).
- Dimethyl Carbonate | 616-38-6. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB8853983.htm (accessed on 8 July 2023).
- Ethyl L(-)-Lactate. Available online: https://www.chembk.com/en/chem/Ethyl%20L(-)-lactate (accessed on 8 July 2023).
- Hansen, C. Hansen Solubility Parameters; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Hildebrand, B.J.H. The Solubility of Non-Electrolytes. CiNii Books 1936. Available online: http://ci.nii.ac.jp/ncid/BA2379215X (accessed on 8 July 2023).
- Panayiotou, C.; Zuburtikudis, I.; Khalifeh, H.A.; Hatzimanikatis, V. Linear Solvation–Energy Relationships (LSER) and Equation-of-State Thermodynamics: On the Extraction of Thermodynamic Information from the LSER Database. Liquids 2023, 3, 66–89. [Google Scholar] [CrossRef]
- Reichardt, C.L.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2010. [Google Scholar] [CrossRef]
- Sicaire, A.-G.; Vian, M.A.; Fine, F.; Joffre, F.; Carré, P.; Tostain, S.; Chemat, F. Alternative Bio-Based Solvents for Extraction of Fat and Oils: Solubility Prediction, Global Yield, Extraction Kinetics, Chemical Composition and Cost of Manufacturing. Int. J. Mol. Sci. 2015, 16, 8430–8453. [Google Scholar] [CrossRef]
- Breil, C.; Meullemiestre, A.; Vian, M.A.; Chemat, F. Bio-Based Solvents for Green Extraction of Lipids from Oleaginous Yeast Biomass for Sustainable Aviation Biofuel. Molecules 2016, 21, 196. [Google Scholar] [CrossRef] [PubMed]
- Mamidipally, P.K.; Liu, S.X. First Approach on Rice Bran Oil Extraction Using Limonene. Eur. J. Lipid Sci. Technol. 2004, 106, 122–125. [Google Scholar] [CrossRef]
- Rebey, I.B.; Bourgou, S.; Detry, P.; Wannes, W.A.; Kenny, T.; Ksouri, R.; Sellami, I.H.; Fauconnier, M.-L. Green Extraction of Fennel and Anise Edible Oils Using Bio-Based Solvent and Supercritical Fluid: Assessment of Chemical Composition, Antioxidant Property, and Oxidative Stability. Food Bioprocess Technol. 2019, 12, 1798–1807. [Google Scholar] [CrossRef]
- Bourgou, S.; Rebey, I.B.; Dakhlaoui, S.; Msaada, K.; Tounsi, M.S.; Ksouri, R.; Fauconnier, M.-L.; Hamrouni-Sellami, I. Green Extraction of Oil FromCarum Carviseeds Using Bio-based Solvent and Supercritical Fluid: Evaluation of Its Antioxidant and Anti-inflammatory Activities. Phytochem. Anal. 2019, 31, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Tanzi, C.D.; Vian, M.A.; Ginies, C.; Elmaataoui, M.; Chemat, F. Terpenes as Green Solvents for Extraction of Oil from Microalgae. Molecules 2012, 17, 8196–8205. [Google Scholar] [CrossRef]
- Mahmood, W.M.F.W.; Theodoropoulos, C.; Gonzalez-Miquel, M. Enhanced Microalgal Lipid Extraction Using Bio-Based Solvents for Sustainable Biofuel Production. Green Chem. 2017, 19, 5723–5733. [Google Scholar] [CrossRef]
- Li, P.; Kanda, H.; Makino, H. Simultaneous Production of Bio-Solid Fuel and Bio-Crude from Vegetal Biomass Using Liquefied Dimethyl Ether. Fuel 2014, 116, 370–376. [Google Scholar] [CrossRef]
- Li, P.; Makino, H. Liquefied Dimethyl Ether: An Energy-Saving, Green Extraction Solvent. In Alternative Solvents for Natural Products Extraction; Springer: Berlin/Heidelberg, Germany, 2014; pp. 91–106. [Google Scholar] [CrossRef]
- De Jesus, S.S.; Ferreira, G.K.; Moreira, L.S.; Maciel, M.R.W.; Filho, R.M. Comparison of Several Methods for Effective Lipid Extraction from Wet Microalgae Using Green Solvents. Renew. Energy 2019, 143, 130–141. [Google Scholar] [CrossRef]
- Akretche-K, S.; Ferhat, Z.; Amiali, M. Vegetable and Nut Oils Extraction by D-Limonene as Alternative Solvent. Int. J. Agric. Res. 2017, 12, 82–87. [Google Scholar] [CrossRef]
- Ben-Youssef, S.; Fakhfakh, J.; Breil, C.; Vian, M.A.; Chemat, F.; Allouche, N. Green Extraction Procedures of Lipids from Tunisian Date Palm Seeds. Ind. Crops Prod. 2017, 108, 520–525. [Google Scholar] [CrossRef]
- Xiao, Z.; Qingdan, W.; Zheng, X.; Zhang, L.; Zou, D.; Chen, B.; Wang, B.; Liu, F. Thermochemical Liquefaction of Brassica Napus Straw: Effect of Liquefaction Parameters on Biocrude. Ind. Crops Prod. 2022, 188, 115564. [Google Scholar] [CrossRef]
- Yan, W.; Duan, P.; Wang, F.; Xu, Y. Composition of the Bio-Oil from the Hydrothermal Liquefaction of Duckweed and the Influence of the Extraction Solvents. Fuel 2016, 185, 229–235. [Google Scholar] [CrossRef]
- Jiang, J.; Savage, P.E. Using Solvents to Reduce the Metal Content in Crude Bio-Oil from Hydrothermal Liquefaction of Microalgae. Ind. Eng. Chem. Res. 2019, 58, 22488–22496. [Google Scholar] [CrossRef]
- Patil, P.D.; Dandamudi, K.P.R.; Wang, J.; Deng, Q.; Deng, S. Extraction of Bio-Oils from Algae with Supercritical Carbon Dioxide and Co-Solvents. J. Supercrit. Fluids 2018, 135, 60–68. [Google Scholar] [CrossRef]
- Babiker, E.E.; Al-Juhaimi, F.; Choi, Y. Supercritical Fluid Extraction of Phenolic Compounds and Antioxidants from Grape (Vitis labrusca B). Seeds. Plant Foods Hum. Nutr. 2012, 67, 407–414. [Google Scholar] [CrossRef]
- Abdelmoez, W.; Abdelfatah, R.M.; Tayeb, A.M.; Yoshida, H. Extraction of Cottonseed Oil Using Subcritical Water Technology. AIChE J. 2010, 57, 2353–2359. [Google Scholar] [CrossRef]
- Abdelmoez, W.; Abdelhamid, M.I.; Yoshida, H. Extraction of Jojoba Oil Using Subcritical Water Technology. Recent Pat. Chem. Eng. 2012, 5, 63–70. [Google Scholar] [CrossRef]
- Halim, N.D.A.; Abidin, Z.Z.; Izhar, S.; Hean, C.G.; Harun, M. Optimization Studies and Compositional Analysis of Subcritical Water Extraction of Essential Oil from Citrus hystrix DC. leaves. J. Supercrit. Fluids 2021, 178, 105384. [Google Scholar] [CrossRef]
- Alhassan, Y.; Kumar, R.; Bugaje, I.M. Hydrothermal Liquefaction of De-Oiled Jatropha Curcas Cake Using Deep Eutectic Solvents (DESs) as Catalysts and Co-Solvents. Bioresour. Technol. 2016, 199, 375–381. [Google Scholar] [CrossRef]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Aziz, M.F.A.; Sazili, A.Q. Green Extraction of Bioactive Compounds from Plant Biomass and Their Application in Meat as Natural Antioxidant. Antioxidants 2021, 10, 1465. [Google Scholar] [CrossRef]
- Lainez-Cerón, E.; Ramírez-Corona, N.; Jiménez-Munguía, M.T.; Palou, E.; López-Malo, A. Extraction of Bioactive Compounds from Plants by Means of New Environmentally Friendly Solvents. In Research and Technological Advances in Food Science; Academic Press: Cambridge, MA, USA, 2022; pp. 301–332. [Google Scholar] [CrossRef]
- Endara, M.J.; Soule, A.J.; Forrister, D.L.; Dexter, K.G.; Pennington, R.T.; Nicholls, J.A.; Loiseau, O.; Kursar, T.A.; Coley, P.D. The Role of Plant Secondary Metabolites in Shaping Regional and Local Plant Community Assembly. J. Ecol. 2021, 110, 34–45. [Google Scholar] [CrossRef]
- Ozturk, B.; Esteban, J.; Gonzalez-Miquel, M. Deterpenation of Citrus Essential Oils Using Glycerol-Based Deep Eutectic Solvents. J. Chem. Eng. Data 2018, 63, 2384–2393. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Hayyan, M.; AlSaadi, M.A.; Akib, S.; Hayyan, A.; Hashim, M.A. Glycerol-Based Deep Eutectic Solvents: Physical Properties. J. Mol. Liq. 2016, 215, 98–103. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, P.; Zheng, W.; Yu, G.-W.; Li, Z.; She, Y.; Lee, M.-R. Three-Stage Microwave Extraction of Cumin (Cuminum cyminum L.) Seed Essential Oil with Natural Deep Eutectic Solvents. Ind. Crops Prod. 2019, 140, 111660. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, Z.; Chen, J.; Yang, G.; He, M. Facile Sulfation of Cellulose via Recyclable Ternary Deep Eutectic Solvents for Low-Cost Cellulose Nanofibril Preparation. Nanoscale Adv. 2023, 5, 356–360. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.-J. Lignocellulosic Biomass Pretreatment by Deep Eutectic Solvents on Lignin Extraction and Saccharification Enhancement: A Review. Bioresour. Technol. 2021, 339, 125587. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ragauskas, A.J.; Wan, C. Lignin Extraction and Upgrading Using Deep Eutectic Solvents. Ind. Crops Prod. 2020, 147, 112241. [Google Scholar] [CrossRef]
- Lomba, L.; Giner, B.; Bandrés, I.; Lafuente, C.; Pino, M.R. Physicochemical Properties of Green Solvents Derived from Biomass. Green Chem. 2011, 13, 2062. [Google Scholar] [CrossRef]
- Nanda, B.; Sailaja, M.; Mohapatra, P.K.; Pradhan, R.; Nanda, B.B. Green Solvents: A Suitable Alternative for Sustainable Chemistry. Mater. Today Proc. 2021, 47, 1234–1240. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology Development for the Production of Biobased Products from Biorefinery Carbohydrates—The US Department of Energy’s “Top 10” Revisited. Green Chem. 2010, 12, 539. [Google Scholar] [CrossRef]
- Schuur, B. Selection and Design of Ionic Liquids as Solvents in Extractive Distillation and Extraction Processes. Chem. Pap. 2015, 69, 245–253. [Google Scholar] [CrossRef]
- Zhou, H.; Shaoyuan, B.; Zhang, Y.-N.; Xu, D.; Wang, M.L. Recent Advances in Ionic Liquids and Ionic Liquid-Functionalized Graphene: Catalytic Application and Environmental Remediation. Int. J. Environ. Res. Public Health 2022, 19, 7584. [Google Scholar] [CrossRef]
- CHEM21 Solvent Selection Guide: CHEM21. Available online: http://www.chem21.eu/project/chem21-solvent-selection-guide/ (accessed on 8 July 2023).
- Prat, D.; Wells, A.; Hayler, J.D.; Sneddon, H.F.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.O. CHEM21 Selection Guide of Classical- and Less Classical-Solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- Regulation (EC) No 1907/2006—Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) | Safety and Health at Work EU-OSHA. Available online: https://osha.europa.eu/en/legislation/directives/regulation-ec-no-1907-2006-of-the-european-parliament-and-of-the-council (accessed on 8 July 2023).
- Nejrotti, S.; Antenucci, A.; Pontremoli, C.; Gontrani, L.; Barbero, N.; Carbone, M.; Dini, D. Critical Assessment of the Sustainability of Deep Eutectic Solvents: A Case Study on Six Choline Chloride-Based Mixtures. ACS Omega 2022, 7, 47449–47461. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.; Hayyan, A.; Al-Saadi, M.; AlNashef, I.M.; Mirghani, M.E.S.; Saheed, O.K. Are Deep Eutectic Solvents Benign or Toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef] [PubMed]
- Lomba, L.; Ribate, M.P.; Sangüesa, E.; Concha, J.; Garralaga, M.A.; Errazquin, D.; García, C.; Giner, B. Deep Eutectic Solvents: Are They Safe? Appl. Sci. 2021, 11, 10061. [Google Scholar] [CrossRef]
- Winterton, N. The Green Solvent: A Critical Perspective. Clean Technol. Environ. Policy 2021, 23, 2499–2522. [Google Scholar] [CrossRef] [PubMed]
- Meshksar, M.; Afshariani, F.; Rahimpour, M.R. Industrial Applications of Green Solvents for Sustainable Development of Technologies in Organic Synthesis. In Nanotechnology in the Life Sciences; Springer: Cham, Switzerland, 2020; pp. 435–455. [Google Scholar]
- Occhiuzzi, J.; Politano, G.G.; D’Olimpio, G.; Politano, A. The Quest for Green Solvents for the Sustainable Production of Nanosheets of Two-Dimensional (2D) Materials, a Key Issue in the Roadmap for the Ecology Transition in the Flatland. Molecules 2023, 28, 1484. [Google Scholar] [CrossRef] [PubMed]
- Häckl, K.; Kunz, W. Some Aspects of Green Solvents. Comptes Rendus Chim. 2018, 21, 572–580. [Google Scholar] [CrossRef]
- Iqbal, M.; Tao, Y.; Xie, S.; Zhu, Y.; Chen, D.; Wang, X.; Huang, L.; Peng, D.; Sattar, A.; Shabbir, M.A.B.; et al. Aqueous Two-Phase System (ATPS): An Overview and Advances in Its Applications. Biol. Proced. Online 2016, 18, 18. [Google Scholar] [CrossRef]
- Singla, M.; Sit, N. Theoretical Aspects and Applications of Aqueous Two-Phase Systems. ChemBioEng 2022, 10, 65–80. [Google Scholar] [CrossRef]
- Teixeira, A.G.; Agarwal, R.; Ko, K.R.; Grant-Burt, J.; Leung, B.M.; Frampton, J. Emerging Biotechnology Applications of Aqueous Two-Phase Systems. Adv. Healthc. Mater. 2017, 7, 1701036. [Google Scholar] [CrossRef]
- Unido Extraction Technologies for Medicinal and Aromatic Plants. Available online: https://www.unido.org/sites/default/files/2009-10/Extraction_technologies_for_medicinal_and_aromatic_plants_0.pdf (accessed on 8 July 2023).
- Anastas, P.T.; Kirchhoff, M.M. Origins, Current Status, and Future Challenges of Green Chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef]
- Brunner, G. Supercritical Fluids: Technology and Application to Food Processing. J. Food Eng. 2005, 67, 21–33. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Girotra, P.; Singh, S.; Nagpal, K. Supercritical Fluid Technology: A Promising Approach in Pharmaceutical Research. Pharm. Dev. Technol. 2012, 18, 22–38. [Google Scholar] [CrossRef]
- Tang, B.; Zhang, H.; Row, K.H. Application of Deep Eutectic Solvents in the Extraction and Separation of Target Compounds from Various Samples. J. Sep. Sci. 2015, 38, 1053–1064. [Google Scholar] [CrossRef]
- Farooq, M.; Abbasi, N.M.; Anderson, J.L. Deep Eutectic Solvents in Separations: Methods of Preparation, Polarity, and Applications in Extractions and Capillary Electrochromatography. J. Chromatogr. A 2020, 1633, 461613. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, W.; Zhang, N.; Soladoye, O.P.; Zhang, Y.; Fu, Y. Deep Eutectic Solvents as New Media for Green Extraction of Food Proteins: Opportunity and Challenges. Food Res. Int. 2022, 161, 111842. [Google Scholar] [CrossRef] [PubMed]
- Scelsi, E.; Angelini, A.; Frison, N. Deep Eutectic Solvents for the Valorisation of Lignocellulosic Biomasses towards Fine Chemicals. Biomass 2021, 1, 29–59. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).